Abstract
Purpose
To compare TSH levels of hypothyroid patients treated with liquid LT4 at breakfast or 30 min before breakfast.
Patients and methods
Subjects, aged 18–75 years old, were eligible if they presented hypothyroidism, due to Hashimoto’s thyroiditis or after thyroidectomy for proven benign goiter. Seven hundred ninety-eight patients were recruited and enrolled in the study. Thirty-seven subjects withdrew from the trial. A total of 761 patients (mean age 46.2 ± 10.8 years) completed the study. The starting dose of LT4 was determined through clinical judgment, taking into account TSH levels, estimated residual thyroid function, age, body weight and comorbidities. All patients underwent TSH, fT4, and fT3 evaluation to verify achievement of euthyroidism with their initial fasting state assumption of LT4 after 8 weeks of therapy. If euthyroidism was not achieved, an appropriately adjusted LT4 dose was administered for 8 weeks, after which thyroid function parameters were checked again. If euthyroidism was achieved, the patients were asked to take LT4 at breakfast and hormone levels were checked again after 6 months.
Results
At the end of the study period, no significant differences in serum TSH level were observed whether LT4 was ingested at breakfast or 30 min prior in a fasting state: 2.61 ± 1.79 vs. 2.54 ± 1.86 mIU/L, respectively (p = 0.455).
Conclusions
This study confirms in a large set of patients that a liquid LT4 formulation can be taken directly at breakfast and potentially improve therapeutic compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypothyroidism is a widespread chronic disorder, with prevalence rates in up to 2% of the population [1,2,3]. Therapeutic mainstay is represented by levothyroxine (LT4) administration to maintain serum thyrotropin (TSH) concentration within a narrow range, which itself depicts a successful treatment [4, 5]. However, some patients may still feel “continuously tired” and, more generally “dissatisfied” with an appropriate L-T4 replacement. Even if the routine use of LT4 + LT3 combination therapy is not recommended in adult hypothyroid patients, it may be considered in few selected subjects with autoimmune hypothyroidism and persistent non-specific symptoms during LT4 monotherapy [6].
Even if the management of hypothyroidism is generally considered straightforward, several cross-sectional surveys of patients receiving levothyroxine (LT4) showed that a large percentage of the aforementioned patients (40–48%) are either over- or undertreated [2, 7].
In addition, given that many factors may affect LT4 intestinal absorption (such as food ingestion, dietary fiber, coffee, drugs, gastric or intestinal resection, and diseases), the current guidelines recommend LT4 to be administered in a fasting state [4, 8].
Conversely, compliance to medical recommendations has been identified as one of the most challenging tasks in the field of drug therapies [9], and a significant number of patients showed sub-optimal adherence to LT4 therapy, as they had to postpone their breakfast of at least 30 min [10]. Thus, despite fasting state assumption is recommended by the current guidelines, this prescription often leads to poor therapeutic compliance.
Over the last few years, pharmaceutical companies have introduced, in a few countries, new non-tablet LT4 formulations, such as liquid and soft gel capsules. These “alternative” formulations have shown to be more efficacious in many cases of refractory hypothyroidism, such as many gastrointestinal diseases including Helicobacter pylori infection, celiac disease, atrophic body gastritis, but also some medications (i.e. proton-pump inhibitors, sucralfate) [11].
Randomized, placebo-controlled trials, such as our previous TICO Study [12], demonstrated that hypothyroid patients easily maintained euthyroidism despite taking liquid LT4 at breakfast; this shows that the administration of the same dose of oral liquid LT4 either at breakfast or in a fasting state—a condition that potentially improves therapeutic compliance—has indistinguishable effects on the thyroid hormonal profile.
The purpose of this study was to verify, in a large cohort of patients, any difference in serum TSH levels when ingesting liquid LT4 at breakfast compared to when ingested 30 min earlier (fasting state).
Materials and methods
The study was approved by an independent Institutional Review Board and conducted in compliance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonisation. All participants provided prior written informed consent.
Patients and Design
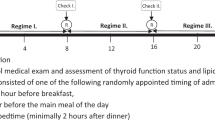
Individuals, aged 18–75 years old, were eligible if they had hypothyroidism due to Hashimoto’s thyroiditis, or after thyroidectomy for proven benign goiter. Subjects with elevated plasma thyroid peroxidase and thyroglobulin antibody with thyroid parenchyma heterogeneity associated with reduced echogenicity, were considered as HT patients [13]. Subjects with congestive heart failure (NYHA III–IV), coronary heart disease, severe hypertension, uncontrolled diabetes mellitus (HbA1c > 64 mmol/mol), or untreated dyslipidemia were excluded. Pregnant or lactating women or women who could possibly become pregnant at any time during the entire study were also excluded.
The starting dose of LT4 was determined through clinical judgment, taking into account TSH levels, estimated residual thyroid function, age, body weight and comorbidities [4]. All patients underwent TSH, fT4, and fT3 evaluation to verify achievement of euthyroidism (0.2 ≤ TSH ≤ 4.2 mIU/L) with their initial fasting state assumption of LT4 after 8 weeks of therapy. If euthyroidism was not achieved, an appropriately adjusted LT4 dose was administered for 8 weeks, after which thyroid function parameters were checked again. If euthyroidism was achieved, the patients were asked to take LT4 at breakfast and hormone levels were checked again after 6 months.
All participants were required to maintain the same breakfast habits throughout the study. The introduction of any additional drugs had to be reported to the researchers.
Adherence to protocol requirements—regularity and timing of administration, unchanged eating habits at breakfast—was assessed by a physician via personal interviews at the end of each regimen period. At the end of the study, all patients were formally asked whether they would prefer their daily LT4 treatment directly at breakfast or 30–60 min before.
Hormone assays
Serum concentrations of fT4 (normal range 8.0 ± 19.0 pg/mL, analytical sensitivity 1 pg/mL), fT3 (normal range 2.4 ± 4.7 pg/mL; analytical sensitivity 0.35 pg/mL), and TSH (normal range 0.4 ± 4.5 mIU/L, analytical sensitivity 0.004 mIU/L) were measured using a fully automated Architect i2000 analyzer (Abbott Diagnostics, Abbott Park, IL, USA) using chemiluminescent magnetic immunoassays.
Statistical analysis
Data are presented as mean ± standard deviations for parameters with normal distribution. Normal distribution was checked via Shapiro–Wilk test; when normality was rejected, data are presented as medians and 95% confidence interval (CI). Comparisons between continuous variables were performed by paired samples t test or related samples via Wilcoxon signed-rank test, as appropriate. Categorical variables were compared using the Chi-square test. In addition, a multiple variable regression model was built in order to assess independent correlation of covariates with TSH variation, which was defined as: [TSH levels after “at breakfast assumption”] − [TSH levels after “fasting state assumption”]. Two-tailed p values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics for Windows v17.0 software (SPSS, Inc., Chicago, IL).
Results
Out of 798 patients, 37 withdrew from the study: 31 due to the introduction of metformin during the study spam, whilst the remaining 6 for unspecified personal reasons. A total of 761 patients (mean age 46.2 ± 10.8 years; 72.9% being female) completed the study. Of those, 498 patients (65.4%) were in replacement therapy for Hashimoto thyroiditis, whilst 263 (34.6%) after thyroidectomy for the removal of histologically proven benign goiter. Baseline demographic and clinical characteristics are shown in Table 1.
The median dose of LT4 ingested by the 761 patients at the end of the first regimen sequence (i.e. in fasting state) was 75 μg daily (95% CI 75–100 μg; range 25–200 μg), and not changed when assumed at breakfast as foreseen by the protocol study.
At the end of the study period, no significant differences in serum TSH level were observed whether LT4 was ingested at breakfast or 30 min before in a fasting state: 2.61 ± 1.79 vs. 2.54 ± 1.86 mIU/L, respectively (p = 0.455; Fig. 1). In addition, even cumulative frequency distributions of the two groups did not result significantly different (p = 0.531; Fig. 2).
A sub-analysis was performed on 202 patients assuming a concomitant drug (such as proton pump inhibitors, calcium or iron supplements) or using fiber and soy milk products at breakfast. Again, no difference in TSH levels was observed: 2.69 ± 1.96 vs. 2.63 ± 1.53 mIU/L; p = 0.732 (Fig. 3).
In conclusion, median TSH variation (defined as: TSH levels after “at breakfast assumption” − TSH levels after “fasting state assumption”) was + 0.06 mIU/L (95% CI -0.03 to +0.17 mIU/L), and ranged from − 3.53 to + 3.78 mIU/L. A multivariable regression analysis was performed to identify factors independently correlated with such variation (Table 2).
The regression model showed acceptable goodness of fit (R2 0.45), and significance level (p = 0.046); and resulted that only age (p = 0.0007) and LT4 dosage (p = 0.047) were significantly correlated with the magnitude of TSH variation.
No specific complaints were reported by the patients. In addition, no adverse events were observed by the investigators; none of the patients noticed changes in the taste of their breakfast, and almost all patients said they would prefer to take their daily LT4 treatment directly at breakfast.
Discussion
This study extends the results from those of TICO’s [10] in a large cohort of hypothyroid patients confirming that liquid LT4 can be easily assumed at breakfast.
As known, the guidelines of the American Thyroid Association (ATA) clearly indicated that LT4 should be ingested in fasting state at least 30 min before breakfast, or at bedtime, at least three hours after the evening meal in order to simplify optimal and consistent absorption [4]. This because the concomitant ingestion of LT4 with food [14,15,16,17], coffee [15], or fiber and soy products [18, 19] is associated to a lower absorption of levothyroxine.
The new LT4 formulations—either liquid or soft gel—apparently almost if not completely circumvent this problem [20]. Vita et al. [10], have in fact showed that treatment with a soft gel preparation of LT4 (Tiche capsules, IBSA Switzerland) is not associated with a reduced absorption of the drug by coffee [15] and proton pump inhibitors [20]. Recently, our group previously reported important data from a randomized trial investigating TSH differences when a liquid LT4 formulation (Tirosint, IBSA Italy) was administered “at” or “before” breakfast [12]. The latter, named TICO Study, demonstrated the absence of any significant difference in terms of hormone levels when liquid LT4 ingestion was either at breakfast or 30 min before breakfast, in a fasting state [12].
The results of this study, performed on a very large cohort of patients, are consistent with those of TICO’s [12], and clearly indicate that the administration of the same dose of oral liquid LT4 either at breakfast or in a fasting state, 30 min before breakfast, has indistinguishable effects on the thyroid hormonal profile. One important feature of this study is its real-life approach in which patients’ breakfast habits and intake of drugs and supplements are completely respected. In fact, we did not exclude any patient on concomitant drug treatment (including proton pump inhibitors, sucralfate, calcium or iron supplements) or patients taking fibre and soy-milk products at breakfast as foreseen by the protocol of the study. The only subjects excluded were those that introduced metformin during the study spam in view of the TSH-lowering effect of metformin [21, 22]. For this reason, the lack of influence exerted by breakfast composition or morning co-treatment (including proton pump inhibitors) on TSH levels was another important result. The last data confirm the results of a recent prospective study obtained by Vita et al. [23]. The authors showed that liquid LT4 overcomes the concurrent interference exerted by the ingestion of multiple interfering drugs, thus it allows targeting TSH in few weeks after switching from tablets [23]. In addiction, a recent retrospective Italian survey showed that the use of oral liquid LT4 is not associated with increased in prescribed daily dosages, compared to tablets formulation, during exposure to potential drug–drug interactions [24].
This finding, as well as the unanimous preference expressed by patients for taking the medication directly at breakfast, may represent a significant advantage for the liquid LT4 formulation (compared to traditional LT4 tablets) in order to maximize patients’ therapeutic compliance.
A critical issue that has not been directly addressed by this study is whether liquid LT4 may have distinct advantages over tablet preparations in terms of clinical outcomes, beyond timing of treatment. Indeed, two previous studies showed a significant reduction in the variability of TSH values both in adult and in elderly subjects [25, 26]. This is interesting due to the well-recognized increased risk of heart disease, osteoporosis, bone fracture, and cognitive impairment among subclinical hyperthyroid elderly subjects [26].
The main limitation of this study is that, as the liquid formulation is currently available only in Italy, all clinical studies have been conducted on an homogeneous ethnic group with similar breakfast habits. Indeed, the U.S. Food and Drug Administration (FDA) has recently approved liquid oral solution (Tirosint-SOL, IBSA Switzerland) for replacement therapy in primary (thyroidal), secondary (pituitary), tertiary (hypothalamic), congenital or acquired hypothyroidism, and pituitary thyrotropin suppression in thyroid cancer (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/206977Orig1s000TOC.cfm 2017/6/28).
Therefore, we hope that we will soon have data on liquid levothyroxine therapy assumed at breakfast even in different populations with different breakfast habits.
In conclusion, this study confirms, in a large set of patients, that a liquid LT4 formulation can be ingested directly at breakfast and thus potentially improve therapeutic compliance. This observation is of considerable clinical relevance as the fasting state administration is the leading cause of therapeutic non-adherence, and because subjects who do not comply with LT4 therapy requirements are more likely to show variability in their TSH concentrations.
References
Vanderpump PJ, Tunbrldge WM, French JM, Appleton D, Bates D et al (1995) The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol 43:55–68
Canaris GJ, Manowitz NR, Mayor G, Ridgway EC (2000) The Colorado thyroid disease prevalence study. Arch Internal Med 160:526–534
Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L et al (2007) Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 17:1211–1223
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR et al (2014) American Thyroid Association Task Force on Thyroid Hormone Replacement 2014 Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid 24:1670–1751
Vaidya B, Pearce SH (2008) Management of hypothyroidism in adults. BMJ 337:a801
Biondi B, Bartalena L, Chiovato L, Lenzi A, Mariotti S, Pacini F et al (2016) Recommendations for treatment of hypothyroidism with levothyroxine and levotriiodothyronine: a 2016 position statement of the Italian Society of Endocrinology and the Italian Thyroid Association. J Endocrinol Invest 39:1465–1474
Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC (1993) Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract 43:107–109
Liwanpo L, Hershman JM (2009) Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab 23:781–792
Dusing R, Lottermoser K, Mengden T (2001) Compliance with drug therapy-new answers to an old question. Nephrol Dial Transplant 16:1317–1321
Vita R, Saraceno G, Trimarchi F, Benvenga S (2013) A novel formulation of l-thyroxine (LT4) reduces the problem of LT4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 43:154–160
Centanni M, Benvenga S, Sachmechi I (2017) Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest 40:1289–1301
Cappelli C, Pirola I, Daffini L, Formenti A, Iacobello C et al (2016) A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid 26:197–202
Esposito D, Rotondi M, Accardo G, Vallone G, Conzo G, Docimo G et al (2017) Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: clinical results of a blinded placebo-controlled randomized prospective trial. J Endocrinol Invest 40:83–89
Wenzel KW, Kirschsieper HE (1997) Aspects of the absorption of oral l-thyroxine in normal man. Metabolism 26:1–8
Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D et al (2008) Altered intestinal absorption of l-thyroxine caused by coffee. Thyroid 18:293–301
Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J (2009) Timing of levothyroxine administration affects serum thyrotropin concentration. JCEM 94:3905–3912
Perez CL, Araki FS, Graf H, de Carvalho GA (2013) Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid 23:779–784
Liel Y, Harman-Boehm I, Shany S (1996) Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab 81:857–885
Bell DS, Ovalle F (2001) Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 7:193–194
Vita R, Fallahi P, Antonelli A, Benvenga S (2014) The administration of l-thyroxine as soft gel capsule or liquid solution. Expert Opin Drug Deliv 11:1103–1111
Cappelli C, Rotondi M, Pirola I, Agosti B, Formenti A, Zarra E et al (2012) Thyreotropin levels in diabetic patients on metformin treatment. Eur J Endocrinol 167:261–265
Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U et al (2009) TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care 32:1589–1590
Vita R, Di Bari F, Benvenga S (2017) Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv 14:467–472
Ferrara R, Ientile V, Arcoraci V, Ferrajolo C, Piccinni C et al (2017) Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009-2015. Endocrine 58(1):143–152
Negro R, Valcavi R, Agrimi D, Toulis KA (2014) Levothyroxine liquid solution versus tablet for replacement treatment in hypothyroid patients. Endocr Pract 1:1–20
Cappelli C, Pirola I, Daffini L, Gandossi E, Agosti B et al (2014) Thyroid hormonal profile in elderly patients treated with two different levothyroxine formulations: a single institute survey. Eur Geriatr Med 5:382–385
Acknowledgements
We thank the patients who volunteered to participate in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Any no funds have been provided for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pirola, I., Gandossi, E., Brancato, D. et al. TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 min before breakfast. J Endocrinol Invest 41, 1301–1306 (2018). https://doi.org/10.1007/s40618-018-0867-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0867-3