Abstract
Lipases are enzymes that catalyze the ester bond hydrolysis in triglycerides with the release of fatty acids, mono- and diglycerides, and glycerol. The microbial lipases account for $400 million market size in 2017 and it is expected to reach $590 million by 2023. Many biotechnological processes are expedited at high temperatures and hence much research is dealt with thermostable enzymes. Cold active lipases are now gaining importance in the detergent, synthesis of chiral intermediates and frail/fragile compounds, and food and pharmaceutical industries. In addition, they consume less energy since they are active at low temperatures. These cold active lipases have not been commercially exploited so far compared to mesophilic and thermophilc lipases. Cold active lipases are distributed in microbes found at low temperatures. Only a few microbes were studied for the production of these enzymes. These cold-adapted enzymes show increased flexibility of their structures in response to freezing effect of the cold habitats. This review presents an update on cold-active lipases from microbial sources along with some structural features justifying high enzyme activity at low temperature. In addition, recent achievements on their use in various industries will also be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans have been continuously searching novel materials from the natural environment and using them for their survival. Organic chemists are probably more familiar with the organometallic catalysis because they have no experience with the biocatalysis. Though much information is available on chemical catalysis, both chemical catalysts and biocatalysts are required to develop greener technologies [1]. Myriad of products were produced by chemical and allied industries for well-being of our society. Copious amounts of waste generated by chemical processes was not an important issue until 1980s. However, negative effects of many chemicals on human health and natural environment presented a problem. Therefore, it was necessary to look for alternative resource-efficient and cleaner technologies which minimize or eliminate waste. As a result, emphasis gradually shifted to waste prevention as opposed to waste remediation [1]. The US Pollution Prevention act of 1990 focused much attention on waste prevention that eliminated the cost of waste treatment and also reduced the environmental pollution. Waste prevention not only led to efficient use of raw materials but also strengthened the economic competitiveness. This fundamental shift from waste generation to waste prevention led to the emergence of the term “Green Chemistry” at the US Environmental Protection Agency in 1990. The Green Chemistry utilizes renewable resources efficiently, minimizes or eliminates waste generation in the manufacture and application of chemical products. The principle of Green Chemistry is to design the environmentally benign processes for production of chemical products [2]. Biological tools are becoming increasingly important which may replace harsh chemical tools of processing materials that generate copious amounts of waste [3]. Biocatalysis has been integrated in to the mainstream of organic synthesis making the industrial processes greener and sustainable. Modern approaches in molecular biology, system biology, and directed evolution enabled the previously unimaginative insights in to processes with promising industrial applications. The developments in these approaches helped to expand the boundary of biocatalysis in organic chemistry.
Majority of the Earth’s crust and atmosphere (85%) experiences perennially cold (< 5°C) environments that harbor psychrophilic microorganisms that include archaea bacteria, yeast, fungi and algae [4]. The ability of these microbes to thrive at such cold environment requires several adaptation strategies that enable them to grow and perform metabolic activity at low temperature. These adaptation strategies developed by psychrophiles along with their underlying mechanisms have been very well reviewed [5, 6]. Adaptation to cold habitats leads to change in membrane fluidity, expression of cold-shock proteins involved in transcription and translational and posttranslational processes. The cell membrane of cold-adapted microbes reveals the presence of unsaturated fatty acids with a greater number of double bonds. The role of these unsaturated fatty acids in cold adaptation is very well understood. The long-chain polyunsaturated fatty acids are presumed to be involved in maintaining membrane fluidity during cold adaptation [6]. Psychrophiles are subjected to freeze-thaw cycles in frozen environments and face harmful crystallization stress leading to cell damage. In response to these freezing-related detrimental challenges, psychrophiles produce novel compounds such as ice-binding proteins, biosurfactants, and extracellular polysaccharides (EPS) [7, 8]. Psychrophilic organisms produce ice-binding proteins (Antifreeze proteins) that bind to ice and prevent ice growth and recrystallization. These AFPs are believed to stabilize the cell membranes and protect the cells from damage [8]. Interestingly, cold-adapted organisms produce biosurfactant [9, 10] but their potential role in cold adaptation is not understood. Hence, further studies are required to elucidate their precise role and mechanism in cold adaptation. Further studies will also clarify whether they constitute part of the cells toolkit to help in cold adaptation. Similarly, pigment production (especially carotenoids) is common in psychrophiles isolated from ice cores and glaciers [11] and high-altitude soils [12]. It is presumed that carotenoids might have some role in modulation of membrane fluidity and especially polar carotenoids are believed to enhance membrane rigidity leading to stabilization of membrane [13]. Cold-adapted bacteria are known to produce high concentrations of EPS especially at freezing temperatures and their role in cold adaptation is well documented [14, 15].
The cold active enzymes produced by psychrophiles are now being explored for industrial and biotechnological applications. They have a huge market potential due to their inevitable applications in various industries because of their high catalytic activity and increased affinity towards substrates at low temperatures. In addition, the reduced energy consumption and diverse adaptation of these enzymes make them to perform well in unfavorable conditions in industrial processes [16]. Among the group of cold active enzymes, cold active lipases are gaining importance because of their use in fine chemical synthesis, bioremediation, food processing and mainly as detergent supplement. Very few reports are available on the isolation of cold-active lipases as compared to mesophilic and thermophilic lipases [17, 18]. Particularly, not much attention has been paid to the bacterial lipases from extremely cold environments [19]. This review provides, in particular the recent information of cold-active lipases with their potential industrial applications.
Lipases and Their General Structure
Lipases (triacylglycerol acyl hydrolases, EC 3.1.1.3) have emerged as potential enzymes accounting for approximately 30% of the world’s enzyme market. The microbial lipase market was $400 million in 2017 and it is expected to reach $590 million by 2023 (https://www.marketsandmarkets.com/Market-Reports/microbial-lipase-market-248464055.html). Lipases play a major role in metabolism of fats and lipids in plants, animals, humans and microorganisms. In humans and animals, the role of lipases is to control the digestion, absorption and reconstitution of fat and lipoprotein metabolism. Plant lipases are involved in metabolism of oil reserves during seed germination. They provide energy required for the synthesis of carbon source (glucose) and nitrogen source (amino acids) necessary for embryonic growth [20]. Lipases catalyze the hydrolysis of esters especially water-insoluble long chain fatty acid esters such as triglycerides.
The three-dimensional structure of lipase of Mucor miehei [21] was first revealed using X-ray crystallography followed by the three-dimensional structure of human pancreatic lipase [22]. Very recently, Rhizomucor meihei, was known to possess two structures of lipase, mature lipase and its prolipase. In the prolipase structure, the mature domain is in the closed inactive form which is stabilized by a region of the propeptide sitting on the top of the active site which prevents its opening. They also demonstrated that the propeptide inhibits the lipase activity in standard lipase assay [23]. The active site of lipases was shown to possess classical catalytic triad composed of Ser-His-Asp with involvement of nucleophilic Serine for catalytic activity. These enzymes exhibited same structural architecture, α/β hydrolase fold, and were known to be the members of this folding family [24, 25]. The α/β hydrolase fold is made up of central β-sheet of eight parallel β-strands with β2 strand antiparallel with respect to other β-strands. The central β-sheet is connected by α-helices packed on both the sides of β-sheet. The active site located in the β sheet is made up of highly conserved catalytic triad. The catalytic triad consists of serine or cysteine (nucleophilic residue), aspartic or glutamic acid (catalytic acid residue) and histidine residue. In lipases, serine always acts as nucleophile [26]. The serine residue is located in a highly conserved pentapeptide Gly-X-Ser-X-Gly. Glycine in the pentapeptide may sometimes be substituted by alanine, threonine, serine, or valine. The pentapeptide forms a sharp γ-like turn (nucleophilic elbow) between β5 strand and α helix strand. Aspartic acid situated in the reverse loop after the β7 strand interacts with highly conserved catalytic histidine via hydrogen bond. The catalytic histidine is situated in a loop positioned after β8 strand [27]. Most of the lipases operate at lipid-water interphase enabled by the mobile amphiphilic region called “Lid” that covers the active site and regulates the lipase activity [28, 29]. The lids have been classified in to larger lids and smaller lids based on structures of lid domains. Larger lid domains were observed in thermophilic lipases with two or more helices and smaller lid domains were found in mesophilic lipases in the form of a loop or a helix [29]. Various efforts such as modifications of lid domain using site-directed mutagenesis [30], lid swapping [31], and computational approaches [32, 33] have been employed to modify the activity and thermostability of lipases. Further advancement in the bioinformatics tools will help to predict the accurate function of amino acids present near the lid region of lipases. Protein engineering of lid may provide an opportunity for better understanding of the structural basis of the lipase property. There is a possibility of using these protein engineered thermostable lipases as industrial enzymes at high temperatures.
Cold active lipases (CLPs) are structurally similar to other mesophilic/thermophilic lipases. They also possess α/β hydrolase fold and the catalytic triad at their active site with serine as nucleophilic residue, histidine as basic residue, and aspartic/glutamic acid as acidic residue. CLPs showed high activities at low temperatures and also molecular activities are much increased at 0–10o C. CLPs are structurally modified and show increased molecular flexibility that helps to accommodate the substrates. The studies on psychrophilic Pseudomonas immobilis lipase suggest that the high catalytic and molecular activities at lower temperature and its low thermostability were due molecular flexibility of an active site of the enzyme [34]. In addition, the 3D-model of psychrophilic P. immobilis lipase revealed several other features which include low proportion of arginine compared to lysine, low content of proline residues, small hydrophobic region and aromatic-aromatic interactions. All these features are typical of cold-adapted enzymes and are responsible for more flexible structure, low activation energy and low thermal stability. The efforts were made to understand molecular basis of cold adaptation by Wintrode et al. [35] in which mesophilic subtilisin-like protease was converted to its psychrophilic counterpart using directed evolution. The identified three variants exhibited improved protease activity compared to wild type at low temperature. These studies particularly suggest that mesophilic to psychrophilic conversion of enzyme was achieved by single point mutation. Such single amino acid substitutions were also reported that confer psychrophilic characteristic to mesophilic enzymes [36].
Interfacial Activation of Lipases
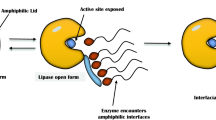
Holwerda et al. [37] observed the phenomenon of interfacial activation for the first time followed by its confirmation by Schonheyder and Volowartz [38]. The natural substrates of lipases are the glycerides that have low solubility in water leading to formation of insoluble droplets. Lipases act on these insoluble droplets using a peculiar mechanism of action, known as interfacial activation which allows the lipases to adsorb on the hydrophobic surface of the glyceride droplets and act at the interface [39, 40]. This interfacial activation is based on the formation of a large hydrophobic pocket surrounding the active center of the enzyme. The lipase with this large hydrophobic pocket has low solubility in water which makes the enzyme unstable. The active site is isolated from aqueous medium by a polypeptide chain called lid which covers the hydrophobic pocket and this is referred as the closed form of the lipase which is inactive. The internal hydrophobic region of the lid interacts with the hydrophobic regions of the active site while external hydrophilic region interacts with the reaction medium [41, 42]. The huge hydrophobic regions formed by shifting of the lid causes exposure of the active site to the medium which results in open/active form of the lipase. In the active form of lipase, the hydrophilic phase interacts with the protein surface. Both the active and inactive forms of the lipase remain in equilibrium. However, when lipase comes in contact with oil droplets, the open form of the lipase is adsorbed on hydrophobic surface of the droplets shifting the equilibrium towards open form which attacks the glycerides.
Modifications of Lipase Structure for Cold Adaptation
Major studies have been focused on psychrophilic microbes at molecular level particularly on cold active enzymes [43, 44]. These cold-adapted enzymes are characterized by increased turnover and high catalytic efficiency at low temperatures due to their flexile structures, reduced activation enthalpy and negative entropy of activation compared to mesophilic enzymes. However, cold active lipases are heat unstable and rapidly inactivated even at moderate temperatures. The thermal denaturation leads to enzyme inactivation in industrial applications and therefore industrial enzymes need to be thermostable [45]. Many strategies have been proposed to improve the thermostability of the cold active enzymes including lipases which include the use of soluble additives, immobilization, protein engineering, and chemical modification [46, 47]. Recent developments focused on the construction of smaller libraries by combining the rational design and directed evolution procedures [48]. The rational design requires the knowledge of relationships of structure, sequence, function, and catalytic mechanisms of protein. This knowledge is necessary for prediction of amino acid residues needed for mutation. These mutations are introduced at specific site of gene encoding protein by site directed mutagenesis approaches. Directed evolution involves the generation of random mutant library followed by screening of required mutant using a suitable screening system. Smaller libraries are produced by combining rational protein design and directed evolution procedures. These strategies have been used to alter the enzyme structure by changing the one or more amino acids in the protein sequence leading to improvement of enzyme properties. For example, the change in the amino acid sequences of the lid region of lipase led to remarkable improvement in substrate specificity and enantioselectivity [49]. The use of these methods requires information on enzyme structure and target specific regions on them in each revolution cycle. The approaches such as saturation mutagenesis and iterative saturation mutagenesis were introduced as efficient methods for directed evolution of functional enzymes. The goals and various strategies for lipase improvements have been well discussed by Bassegoda et al. [50]. Sharma et al. [51] used error-prone-PCR method for increasing the thermostability of lipase from metagenomic library and the mutant enzyme showed 144-fold enhanced thermostability at 60° C. This ep-PCR approach was also employed to enhance the thermostability of cold active lipase from Pseudomonas fragi leading to 5-fold increase in its half-life at 42° C [52]. The directed evolution proved to be efficient technique for the engineering Staphylococcus epidermidis AT2 cold-adapted lipase in a single round of error-prone PCR. This random mutation replaced non-polar glycine residue with polar cysteine residue in the lid region of the lipase which most probably provided rigidity to the structure thereby increasing the thermostability of lipase [53, 54]. The lid 1 structure of lipase of Pseudomonas fluorescens AM8 is rigid and was stabilized by 17 hydrogen bond linkages leading to low hydrophobicity. The change in Thr-52 and Gly-55 present in the lid 1 region to aromatic tyrosine resulted in achieving higher solvent accessible surface area and longer half-life at 25 to 37°C in 0.5% toluene [55]. Efforts were made to improve thermostability of cold active lipase of Penicillium cyclopium by addition of disulfide bridge using software Disulfide (Design version 1.2) and the recombinant lipases were found to show enhanced thermostability and catalytic efficiency [56]. Similar studies were performed to demonstrate that the rationally designed point mutations and introduction of disulfide bonds could effectively reduce the number of screened colonies to enhance the thermostability of Rhizomucor miehei lipase [57]. Detection of lipase positive clones/mutants from a library of thousands of transformants requires high-throughput screening. Zhang et al. [58] developed an easy screening method for detection of thermostable lipase activity based on its synthetic activity. They obtained a double mutant (Asn120Lys/Lys131Phe) from Rhizomucor miehei lipase saturation mutated library that was based on amino acid residue B factors. The screening method involved the use of pH-indicator in the screening medium on a colony plate which facilitated the high-throughput screening for lipase synthetic activity. The lipase thermostability was further evaluated by heating the colony plate before the addition of synthetic substrate and visualizing the halo zone around the colony which is indicative of lipase synthetic activity. Such high-throughput screening approaches need to be developed for detecting the desired strains from the libraries containing thousands of transformants. Lipases stable in organic solvents are versatile biocatalysts to be employed in their applications in food, pharmaceuticals, and green products manufacturing industries [59]. The inherent structural flexibility of cold active enzymes causes their denaturation by heat and organic solvents and very few reports are available on cold-adapted solvent tolerant lipases [60]. Cold-adapted lipases, PML and LipS showed increased conformation flexibility with increasing concentrations of organic solvent [61]. Majority of the studies employed directed evolution to improve the tolerance of enzymes in organic solvents [62]. Pseudomonas aeruginosa lipase, LST-03, has high stability in various organic solvents. The use of directed evolution approach was found to enhance organic solvent stability of LST-03 lipase. The mutation in the Lip9 on the surface of lipase through directed evolution increased stability (9-11-fold) in cyclohexane and n-decane [63].
Sources of Cold Active Lipases
Cold active lipases of microbial origin are being paid due attention for biotechnological applications because of their increasing use in synthesis of chiral intermediates, fine chemicals, biodiesel, and biopolymers [64, 65]. In addition, the high activity at low temperatures favors the production of frail/fragile compounds. Many cold active lipase producing microbes have been isolated but very few bacteria and yeast received attention for commercial exploitation. Most of the psychrophilic bacteria have been isolated from Antarctic and polar regions exhibiting colder habitats (<5o C). A list of various cold active lipase producing bacteria is presented in Table 1. Recently, B. cereus isolated from marine habitat was reported to produce highest lipase activity (285 U/mL) at 10o C [67]. Kumar et al [86, 87] reported the existence of psychrophilic bacteria harboring cold active enzymes in Sikkim Himalaya using genome-based predictions. Cold-adapted and broad temperature active alkalophilic lipase was purified from one of the isolates, Chryseobacterium polytrichastri ERMR1:04 [88]. Many psychrophilic yeasts have been isolated which produce extracellular or intracellular lipases. However, cold active lipases A and B (CALA and CALB) from Candida antarctica have been paid great attention. CALB was immobilized on a resin Lewatit VP OC1600 via interfacial activation which is commercially available as Novozyme 435. It is most widely used commercial lipase in both academy and industry [89]. Majority of reports on cold active lipases has been focused on bioprospection on bacteria and yeasts from Antarctic or polar region but very few reports are available on fungal cold active lipases. The recent review provides the information on production of cold-adapted enzymes including lipases by fungi from terrestrial and marine Antarctic environments [44]. Table 2 gives the list of various yeast and fungi producing cold active lipases.
Recombinant Expression of Lipases in Heterologous Hosts
Fermentation at low temperatures is advantageous since it prevents and limits the risk of contamination with mesophilic organisms especially in continuous fermentation. However, cold-adapted microbes are not appropriate candidates to be exploited for large-scale fermentations because of requirement of energy consuming cooling systems. The other drawback is the low production level of cold-adapted wild strains. These disadvantages can be overcome by overexpressing genes encoding for cold active enzymes in mesophilic hosts using appropriately designed efficient expression systems which leads to obtaining sufficient yields of enzymes for commercial exploitation. Mesophilic hosts are most commonly used for heterologous expression of genes encoding for cold active enzymes. The problem with use of mesophilic hosts is their optimal growth temperature which is not compatible with the temperature required for proper folding of the cold active enzymes to retain their structure and functional activity [105]. This issue can be circumvented by incubating the host organisms at temperatures lower than their optimal growth temperatures after induction to solve the folding issues for obtaining functional enzymes. However, the growth of mesophilic hosts at lower temperatures leads to decreased growth rate and also reduced synthesis of enzymes. Table 3 summarizes the heterologous expression of genes coding for cold active lipases in commonly used mesophilic hosts. Table also provides the information on heterologous mesophilic hosts and vectors used for expression, temperature optimum of recombinant lipase and residual activity at specific lower temperature. Several strategies have been suggested for expression of cold active enzymes including lipases with proper folding in heterologous host to retain structure and functional activity. These strategies include the use of molecular chaperones, cold active promoters, fusion partners, and psychrophilic hosts and these have been well covered in previous reviews [114].
Applications of Cold Active Lipases
Unusual specificities and high activity at low temperature offer great opportunities for cold active lipases to be exploited and applied in versatile industrial fields. Lipases from psychrophilic organisms are promising enzymes that can replace the conventional processes used in biotechnological industries. These enzymes are mainly of interest in organic synthesis of fragile chiral compounds, as additives in food industry (cheese manufacture, bakery), as detergents as well as bioremediations, biomedicines, or molecular biology approaches [115]. The manufacturing of multi-ton scale biotechnological products presently employs fungal lipases from H. lanuginosa lipase and Rhizopus niveus. These lipases are used especially for the manufacture of detergents and cocobutter substitutes [116]. C. antarctica lipase B has been one of the cold active lipases which has seen the commercial success [89, 117]. Number of papers and patents on cold active lipases for their use in various industries are available. However, they have not been exploited much at industrial level due to their high price and low thermostability. Sometimes, low thermostability is desirable property of cold active lipases in biotransformation processes in which biocatalytic reaction can be terminated by heat inactivation of the enzymes. The following section discusses on the potential applications of cold active lipases in various areas of commercial importance.
Synthesis of Fine Chemicals and Pharmaceuticals
Novel synthetic methods are in great demand essentially to produce new classes of organic compounds required for biomedical research. Enzyme-catalyzed transformations for synthesizing new compounds present as alternative and convenient solutions to intractable chemical synthesis [118]. The synthesis of single enantiomer is very important in pharma industry. Though chemical synthesis is one of the approaches to synthesize such enantiomers, biocatalysis route has several advantages over chemical catalysis. Biocatalysis are enzyme-catalyzed reactions that are chemo-selective, stereoselective and region-selective and performed at ambient temperature and pressure [119]. The cold-adapted lipases and their application in fine chemical synthesis have been discussed in many reviews [18, 120].
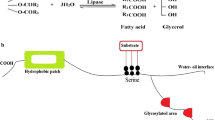
The 4-(R)-Hydroxycyclopent-2-en-1-(S)-acetate is an important intermediate in the synthesis of cyclopentanoid natural products such as prostaglandins, prostacyclins, thromboxanes and some anti-HIV drugs. Trichosporon beigelii NCIM 3326 cells having a pro-R preference were evaluated for the hydrolysis of mesodiacetate; but the enantio-selectivity was poor. Addition of 10% v/v ethanol was found to enhance the enantioselectivity of the enzyme affording 4-(R)-hydroxycyclopent-2-en-1-(S)-acetate with 85% optical purity and 83% yield. Further exploration of inherent consecutive kinetic resolutions to the desymmetrization afforded 4-(R)-hydroxycyclopent-2-en-1-(S)-acetate of more than 98% optical purity with 74% chemical yield [121, 122]. The chiral product is then converted to 4-(R)-t-butyldimethylsilyloxycyclopent-2-en-1-one which is the precursor for synthesis of cyclopentanoid natural products (Fig. 1). Lavandulol is a constituent of essential oils and also an important additive in perfumery and cosmetic industry. Y. lipolytica NCIM 3639 was isolated from refrigerated Tween 80 samples in our laboratory which produced both extracellular and cell bound cold active lipases at 20o C. Cell bound and extracellular cold active lipases preferentially hydrolyzed the racemic lavandulyl acetate at 25o C to corresponding (R)- and (S)- lavandulol respectively confirming that extracellular and cell bound lipases are different (Fig. 2a, b) [123]. Such differences in the enantioselectivity of the extra- and intracellular lipases are not very commonly found in microbes. The (R)-1-Arylallyl alcohols were synthesized with excellent enantioselectivities via kinetic resolution of the corresponding acetates using immobilized Candida antarctica lipase B. Very recently, the chitosan immobilized Candida antarctica lipase B was used for kinetic resolution of some racemic heteroarylethanols through transesterification with vinyl acetate. The reaction was carried out at 45oC in n-hexane which resulted in 50% conversion in 3-16 h and >96% enantiomeric excess [124]. The immobilized preparation remained active even after 10 recycles proving its potential as promising candidate for getting higher productivities. Ascorbyl fatty acid esters are good antioxidants and surfactants which are prepared by acylation of ascorbic acid (vitamin C) with different acyl donors using lipases. Novozyme 435 and Pseudomonas stutzeri lipase immobilized on silica octyl were evaluated for synthesis of ascorbyl palmitate in 2-methyl-2-butanol as solvent. Novozyme 435 was found to give highest yield (81%) of ascorbyl palmitate at 55o C due to its high reactivity and stability in 2-methyl-2-butanol. [125]. The flavor esters are in high demands especially in food, cosmetic and pharma industries and are produced by commercial lipases (Novozyme 435) using esterification or transesterification reactions [126]. Ethyl hexanoate, an apple-pineapple flavor ester, was synthesized by the esterification of hexanoic acid and ethanol using free and chitosan based immobilized cold active lipase of Pseudomonas sp. AMS8 [127]. The reaction was performed at 20o C in toluene as a solvent and the immobilized lipase showed higher conversion of ester (80%) compared with free enzyme (59%). Formate esters, especially phenethyl formate, is a fragrance ester found in cosmetics, perfumes, shampoos and toiletries with its annual requirement of about 1–10 tons and it was recently GRASS cleared for its use as food additive [128]. Phenethyl formate was synthesized by esterification of formic acid and phenethyl alcohol using Novozyme 435 with 96% conversion of formic acid in 1,2-dichloroethane as solvent. However, Novozyme 435 was denaturated in 1,2-dichloroethane. When the reaction was carried out in toluene at 40o C, Novozyme 435 gave 92% conversion and the enzyme was recycled for 20 reactions with the same conversion efficiency [129]. The pure (S)-4-methoxymandelonitrile benzoate was synthesized from 4-anisaldehyde using concurrent bienzymatic cascade. The first enzyme, hydroxynitrile lyase from Manihot esculenta, catalyzed the hydrocyanation of 4-anisaldehyde followed by benzoylation of the (S)-cyanohydrin by Candida antarctica lipase A in organic solvent [130]. Both hydrocyanation and benzoylation reactions were carried out at 10o C which afforded excellent enantioselectivity with satisfactory reaction rate. The bienzymatic cascade resulted in 81% conversion of (S)-4-anisaldehyde to benzoyl cyanohydrins with 98%ee. The benzoyl cyanohydrins are further converted via single hydrogenation step to (S)-terbamide having antiviral (HIV) and hypoglycemic activity. Acylated cyanohydrins (especially O-acylated) have wide applications in agriculture since pyrethroids insecticides have such structures. Xanthene’s are biologically active compounds and derivatives of 2,2’-arylmethylene dicyclohexane-1,3-dione serve as key intermediates in the synthesis of xanthenes. These intermediates are synthesized via the Knoevenagel condensation and Michael addition of aromatic aldehydes with 1,3-cyclohexanedione or 1,3-cyclic diketones using HClO4-SiO2 as catalyst [131], CsF as catalyst [132] or urea as catalyst [133]. These chemical methods suffer from low yields and harsh reaction conditions. Jiang et al. [134] developed efficient method for synthesis of these derivatives via cascade reactions catalyzed by Amano lipase DF with high yields (94%) fulfilling the requirements of green chemistry and simplified production process. Amano lipase DF is a dietary supplementary grade lipase of Rhizopus oryzae manufactured by Amano enzyme, USA.
Applications in Food Industry
Food preservation at ambient temperature is necessary to prevent its spoilage and deterioration and also to minimize unwanted changes in the quality of food. The chemical or physical changes in the food usually occur when it is stored at high temperature. From the nutritional point of view, the use of cold active lipases in food and feed are known to prevent spoilage and undesirable changes in the composition of substrates utilized during food processing [135]. The other major requirement of food industry is the improvement in the organoleptic properties of food or the finished products [136]. Flavors and emulsifiers synthesized by lipases improve such organoleptic properties of food products. Short-chain flavor esters include predominantly ethyl acetate, ethyl caproate, ethyl lactate, and butyl butyrate, which are commonly used in food, cosmetic, chemical and pharma industries. They are commonly found in several plant species and the global annual market exceeds $US 22 billion. [137]. Esters produced by action of lipases seem to be the preferred alternative to their synthesis by chemical routes which are not only harsh but require chemical catalysts and high energy consumption resulting in environmentally non-friendly processes [137]. Psychrophilic lipases have been used for synthesis of model esters such as butyl caprylate in non-aqueous media. Cold-adapted lipase from Pseudomonas AMS8 exhibited high specific activity and catalytic activity at low temperatures (0–20° C) and immobilized preparation of this enzyme was used for synthesis of ethyl hexanoate by esterification in toluene as solvent [127]. This immobilized preparation could be potentially used as cost–effective lipase in the food industry. Cold active lipase (CALB) was immobilized on to iron magnetic nanoparticles and evaluated for synthesis of methyl and ethyl butyrates using heptane as a solvent system. The optimum conditions for both esters were achieved at 25o C using heptane as solvent which resulted in more than 90% substrate conversion in 8 h of reaction [138]. Sometimes, some organic solvents are not used for safety concerns and hence ester synthesis using solvent-free systems are preferred [139]. A novel lipase from A. niger was identified with capability of synthesizing flavor esters in a soybean-oil-solvent free system. The lipase was active at 20o C and showed tolerance to acid and alkaline conditions exhibiting efficient esterification ability for synthesis of ethyl lactate, butyl butyrate, and ethyl caprylate in soybean-oil-solvent system [140]. Phenolic acid esters and alkyl hydroxybenzoates display beneficial effect on health due to their antioxidant property. Such esters have been isolated in small amounts from plants [141]. Though chemical methods for preparation of phenolic esters are available, the enzymatic synthesis of such esters may be advantageous particularly for food use. Long chain alkyl (hydroxyl) benzoates and (hydroxyl) benzyl alkenoates were synthesized by esterification and transesterification reactions using immobilized Candida antarctica lipase B [89, 142]. A recombinant Saccharomyces cerevisiae was constructed by Han et al. [143] which showed C. antarctica lipase B activity on its cell surface. This whole cell recombinant yeast was used to synthesize ethyl hexanoate by esterification of hexanoic acid and ethanol in n-heptane with yield of 98% within 12 h. Polyunsaturated fatty acids (PUFAs) such as omega-3 fatty acid are usually obtained by hydrolysis/alcoholysis of fish oil by lipases [144]. PUFAs such as Omega-3 fatty acid, has great market demand as dietary supplement and nutraceuticals since they have vital role in brain and retina improvement in young people. In addition, they protect the adults and aging people from cardiovascular diseases and improve the muscle function in older women [145, 146]. Although the mesophilic enzymes are employed in food industry, many cold active enzymes have been patented and ready for commercial exploitation [147].
Detergent Industry
Normally, the fabrics with lipid stains are washed using hot/warm water to remove lipid stains. The fabrics which cannot withstand higher temperature gets damaged due to wear and tear during washing with hot water. In such cases, cold active lipases offer great advantages in detergent industry since their use lowers the washing temperature, improves the energy conservation and minimizes the wear and tear [148]. The effectiveness of these enzymes has been proven beyond doubt in cleaning formulations used for laundry and dishwashing purposes. The use of such enzymes reduces environmental pollution and also helps in biodegradation of undesirable chemicals in detergents. Novozymes produced cold active lipase “Lipoclean” which is active at low temperature and was used to remove lipid stains. The detergent formulations blended with such lipases have been applied to porous building materials to clean the surfaces causing no material damages normally caused by the use of other standard formulations [149]. Approximately 1000 tons of lipases make their way into detergents that account for only 5% of the detergent market (https://www.enzymeinnovation.com/lipase-detergent-everything-you-need-know). Cold active lipases in detergents remove oil stains by converting them into more hydrophilic substances without altering the texture and the quality of the fabrics [150]. The most suitable cold active lipases to be used as detergent additives should have the stability at alkaline pH and also should withstand oxidizing and chelating agents [148]. In addition, the enzyme should be effective at low concentration in presence surfactants. Li et al. [151] reported high detergent performance cold-adapted extracellular lipase from Pseudomonas stutzeri (isolated from Daing oil fields, China) which was stable in surfactants, solvents and oxidizing agents. These properties of lipase exhibited its potential applications as commercial additive in detergents. Recently, alkaline cold active lipases from Penicillium canesense and Pseudogymnoascus roseus were found to exhibit 90% stability for 1 h when incubated in commercial detergents at 20o C proving their potential in detergent application [152]. Fusarium solani N4-2, isolated from Soda Lake produced alkaline cold active lipase active 10o C with retention of 82% of its original activity [153]. The properties such as stability in surfactants, metal ions, commercial detergents and oxidizing agents makes it most suitable for commercial use as additive in detergent formulations. Very recently, Chryseobacterium polytrichastri isolated from Sikkim Himalaya was reported to produce cold-adapted lipase with activity over the temperature range of 5-65o C. Its compatibility with commercial detergents and wide temperature and substrate range renders it a potential candidate for detergent formulations [88]. Such cold active lipases are in high demand and hence considerable research inputs are necessary to look for novel cold active lipases with unique properties from the untapped microbial sources.
Environmental Application
Due to the increasing demands for fossil fuels, spillage of petroleum products is causing threat to the environment where the environmental cleaners are required to effectively degrade the pollutants in the cold climate. Cold-adapted microbes can act as environmental cleaners for bioremediation and waste water treatment in cold climates that help in reducing the amounts of toxic compounds earlier considered as non-degradable [154, 155]. Cold-adapted lipase producing organisms play a significant role in bioremediation of fat contaminated wastewater and in lowering of toxic compounds such as hydrocarbons, heavy metals, and diesel oils in the cold environment [18, 156]. Very few reports are available on cold-adapted pyrethroid degrading enzymes with high catalytic activity at low temperatures which can be used for pyrethroid-contaminated vegetables. A novel pyrethroid-hydrolyzing esterase was identified from Mao-tofu metagenome [157]. The esterase was immobilized on a matrix of mesoporous silica to improve its thermostability. This immobilized enzyme was found to efficiently hydrolyze (90%) pyrethroids on contaminated cucumbers in short time. Recently, the use of CAL produced by B. cereus HSS was reported to reduce BOD, TSS and oil and grease with highest efficiency proving its potentiality in biological waste water treatment [67]. Alkaline lipase from Pseudomonas aeruginosa HFE733 was used in removal of oil in lipid rich food waste water showing its promise for biodegradation of food wastewater treatment [158]. Butyl esters of long chain fatty acids are widely used as biofuels to reduce environmental pollution. Novozyme 435 is regarded as very efficient biocatalyst for biodiesel production using jatropha oil, sunflower oil, soybean oil and waste cooking oil [89]. Novel lipase isolated from Halocynthiibacter arcticus catalyzed the formation of oleic acid esters using methanol, ethanol, and butanol suggesting its possibility to prepare fatty acid methyl esters biodiesels [159].
Conclusions and Future Perspectives
Cold-adapted microbes are regarded as potential sources for lipases exploited for numerous biotechnological applications due to their ability to withstand the permanently cold habitats. The cold active lipases represent versatile extracellular enzymes gaining importance mainly in food, detergent industries due to their features like easy to handle and activity at low temperatures. These enzymes display high catalytic efficiency with low thermal stability differentiating them from their mesophilic and thermophilic counterparts. These enzymes also play an important role in biotechnological applications such as synthesis of biodegradable polymers, synthesis of optically active drugs and drug intermediates and fine chemicals due to their activity in non-aqueous medium and ability to catalyze chemo-, region and enantioselective reactions. In addition, they consume less energy due to low working temperatures. However, their use in industrial exploitation is limited due to instability at moderate temperatures. Hence there is a need to increase the thermostability through immobilization, directed evolution or protein engineering. Enzymes were discovered usually from culturable microorganisms that account for only 1% of microbial diversity. It is now possible to have access to new enzymes by identifying the gene sequences by metagenome mining through metagenomic based activity-screening protocols. Such approaches can help in searching novel cold active lipases performing transformations of molecules that are difficult or impossible to synthesize through chemical transformations to high value fine chemicals leading to greener technologies. In addition, there are daunting demands for green products to be developed through greener technologies using effective cell factories harboring novel biocatalysts. Such rapidly increasing demands compel us to improve the quality and quantity of lipases to develop greener technologies producing products for human consumption.
Data availability
Not applicable
References
Sheldon, R. A. (2016). Engineering a more sustainable world through catalysis and green chemistry. Journal of the Royal Society, Interface, 13(116), 20160087.
Sheldon, R. A., & Brady, D. (2019). Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem, 12(13), 2859–2881.
Marco, B. A., Rechelo, B. S., Totoli, E. G., Kogawa, A. C., & Salgado, H. R. N. (2019). Evolution of green chemistry and its multidisciplinary impacts: A review. Saudi Pharmaceutical Journal, 27, 1–8.
Kirchman, D. L., Moran, X. A. G., & Ducklow, H. (2009). Microbial growth in the polar ocean: role of temperature and potential impact of climate change. Nature Reviews. Microbiology, 7(6), 451–459.
Gerday, C. (2014). Fundamental of cold active enzymes. In P. Buzzini & R. Margesin (Eds.), Cold-adapted yeasts (pp. 325–350). Berlin: Springer.
Collins, T., & Margesin, R. (2019). Psychrophilic lifestyles: mechanisms of adaptation and biotechnological tools. Applied Microbiology and Biotechnology, 103(7), 2857–2871.
Bar Dolev, M., Braslavsky, I., & Davies, P. L. (2016). Ice-binding proteins and their function. Annual Review of Biochemistry, 85(1), 515–542.
Voets, I. K. (2017). From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter, 13(28), 4808–4823.
Malavenda, R., Rizzo, C., Michaud, L., Gerçe, B., Bruni, V., Syldatk, C., Hausmann, R., & Lo Giudice, A. (2015). Biosurfactant production by Arctic and Antarctic bacteria growing on hydrocarbons. Polar Biology, 38(10), 1565–1574.
Perfumo, A., Banat, I. M., & Marchant, R. (2018). Going green and cold: biosurfactants from low-temperature environments to biotechnology applications. Trends in Biotechnology, 36(3), 277–289.
Shen, L., Liu, Y., Wang, N., Jiao, N., Xu, B., & Liu, X. (2018). Variation with depth of the abundance, diversity and pigmentation of culturable bacteria in a deep ice core from the Yuzhufeng Glacier, Tibetan Plateau. Extremophiles, 22(1), 29–38.
Pandey, N., Jain, R., Pandey, A., & Tamta, S. (2018). Optimisation and characterisation of the orange pigment produced by a cold adapted strain of Penicillium sp. (GBPI_P155) isolated from mountain ecosystem. Mycology, 9(2), 81–92.
Jagannadham, M. V., Chattopadhyay, M. K., Subbalakshmi, C., Vairamani, M., Narayanan, K., Rao, C. M., & Shivaji, S. (2000). Carotenoids of an Antarctic psychrotolerant bacterium, Sphingobacterium antarcticus and a mesophilic bacterium. Sphingobacterium multivorum. Arch. Microbiol., 173(5–6), 418–424.
Casillo, A., Parrilli, E., Sannino, F., Mitchell, D. E., Gibson, M. I., Marino, G., Lanzetta, R., Parrilli, M., Cosconati, S., Novellino, E., Randazzo, A., Tutino, M. L., & Corsaro, M. M. (2017). Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: a strategy for cryoprotection. Carbohydrate Polymers, 156, 364–371.
Caruso, C., Rizzo, C., Mangano, S., Poli, A., Di Donato, P., Finore, I., Nicolaus, B., Di Marco, G., Michaud, L., & Lo Giudice, A. (2018). Production and biotechnological potential of extracellular polymeric substances from sponge-associated Antarctic bacteria. Applied and Environmental Microbiology, 84(4), e01624–e01617.
Joseph, B., Ramteke, P. W., & Thomas, G. (2008). Cold active microbial lipases:some hot issues and recent developments. Biotechnology Advances, 26, 457–470.
Rasol, R., Rashidah, A. R., Sitinurnazuha, R., Smykla, J., Wanmaznah, W. O., & Alias, S. A. (2014). Psychrotrophic lipase producers from Arctic soil and sediment samples. Polish Journal of Microbiology, 63(1), 75–82.
Maiangwa, J., Ali, M. S. M., Salleh, A. B., Rahman, R. N. Z. R. A., Shariff, F. M., & Leow, T. C. (2015). Adaptational properties and applications of cold-active lipases from psychrophilic bacteria. Extremophiles, 19(2), 235–247.
Salwoom, L., Rahman, R. N. Z. R. A., Salleh, A. B., Shariff, F. M., Convey, P., Pearce, D., & Ali, M. S. M. (2019). Isolation, characterization, and lipase production of a cold-adapted bacterial strain Pseudomonas sp. LSK25 isolated from Signy Island, Antarctica. Molecules, 24, 715.
Huang, A. H. C. (1987). Lipases. In P. K. Stumpf & E. E. Cohn (Eds.), The Biochemistry of Lipases (pp. 91–119). New York: Academic Press Inc..
Brady, L., Brzozowski, A. M., Derewenda, Z. S., Dodson, E., Dodson, G., Tolley, S., Turkenburg, J. P., Christiansen, L., Huge-Jensen, B., Norskov, L., Thim, L., & Menge, U. (1990). A serine protease triad forms the catalytic center of a triacylglycerol lipase. Nature, 343(6260), 767–770.
Winkler, F. K., D’Arcy, A., & Hunziker, W. (1990). Structure of human pancreatic lipase. Nature, 343(6260), 771–774.
Moroz, O. V., Blagova, E., Reiser, V., Saikia, R., Dalal, S., Jørgensen, C. I., Bhatia, V. K., Baunsgaard, L. V., Andersen, B., Svendsen, A., & Wilson, K. S. (2019). Novel Inhibitory Function of the Rhizomucor miehei Lipase Propeptide and Three-Dimensional Structures of Its Complexes with the Enzyme. ACS Omega, 4(6), 9964–9975.
Lotti, M., & Alberghina, L. (2007). Lipases: molecular structure and function. In J. Polaina & A. P. MacCabe (Eds.), Industrial enzymes: Structure, function and applications (Vol. 1, pp. 263–300). Dordrecht: Springer.
Bauer, T. L., Buchholz, P. C. F., & Pleiss, J. (2020). The molecular structure of α/β-hydrolases. The FEBS Journal, 287(5), 1035–1053.
Jaeger, K. E., Dijkstra, B. W., & Reetz, M. T. (1999). Bacterial biocatalysts: Molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annual Review of Microbiology, 53(1), 315–351.
Ollis, D. L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S. M., Harel, M., Remington, S. J., Silman, I., Schrag, J., Sussman, J. L., Verschueren, K. H. G., & Goldman, A. (1992). The α/β hydrolase fold. Protein Engineering, 5, 197–211.
Secundo, F., Carrea, G., Tarabiono, C., Gatti-Lafranconi, P., Brocca, S., Lotti, M., Jaeger, K. E., Puls, M., & Eggert, T. (2006). The lid is a structural and functional determinant of lipase activity and selectivity. Journal of Molecular Catalysis B: Enzymatic, 39(1-4), 166–170.
Khan, F. I., Lan, D., Durrani, R., Huan, W., Zhao, Z., & Wang, Y. (2017). The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Frontiers in Bioengineering and Biotechnology, 5, 16.
Skjold-Jorgensen, J., Vind, J., Moroz, O. V., Blagova, E., Bhatia, V. K., & Svendsen, A. (2017). Controlled lid-opening in Thermomyces lanuginosus lipase – an engineered switch for studying lipase function. Biochimica et Biophysica Acta, 1865(1), 20–27.
Yu, X. W., Zhu, S. S., Xiao, R., & Xu, Y. (2014). Conversion of a Rhizopus chinensis lipase into an esterase by lid swapping. Journal of Lipid Research, 55(6), 1044–1051.
Haque, N., & Prabhu, N. P. (2016). Lid dynamics of porcine pancreatic lipase in non-aqueous solvents. Biochimica et Biophysica Acta, 1860(10), 2326–2334.
Singh, Y., Gupta, N., Verma, V. V., Goel, M., & Gupta, R. (2016). Selective disruption of disulphide bonds lowered activation energy and improved catalytic efficiency in TALipB from Trichosporon asahii MSR54: MD simulations revealed flexible lid and extended substrate binding area in the mutant. Biochemical and Biophysical Research Communications, 472(1), 223–230.
Arpigny, J. L., Lamotte, J., & Gerday, C. (1997). Molecular adaptation to cold of an antarctic bacterial lipase. Journal of Molecular Catalysis B: Enzymatic, 3, 29–35.
Wintrode, P. L., Miyazai, & Arnold, F. H. (2000). cold adaptation of a mesophilic subtilism-like protease by laboratory evolution. The Journal of Biological Chemistry, 275(41), 31635–31640.
Feller, G., & Gerday, C. (1997). Psychrophilic enzymes: molecular basis of cold adaptation. Cellular and Molecular Life Sciences, 53(10), 830–841.
Holwerda, K., Verkade, P. E., & de Willigen, A. H. A. (1936). Vergleichende Untersuchungen über die Verseifungsgeschwindigkeit einiger Einsäuriger Triglyceride unter Einfluss von Pankreasextrakt. I. Der Einfluss des Verteilungszustandes der Triglyceride auf die Verseifungsgeschwindigkeit. Recueil des Travaux Chimiques des Pays-Bas, 55, 43–57.
Schonheyder, F., & Volovartz, K. (1945). On the affinity of pig pancreatic lipase for tricaproin in heterogenous solution. Acta Physiologica Scandinavica, 9(1), 57–67.
Reis, P., Miller, R., Krägel, J., Leser, M., Fainerman, V., Watzke, H., & Holmberg, K. (2008). Lipases at interfaces: Unique interfacial properties as globular proteins. Langmuir, 24(13), 6812–6819.
Reis, P., Holmberg, K., Watzke, H., Leser, M. E., & Miller, R. (2009). Lipases at interfaces: A review. Advances in Colloid and Interface Science, 147-148, 237–250.
Yaacob, N., Ahmad Kamarudin, N., Leow, A., Salleh, A., Raja Abd Rahman, R., & Ali, M. M. M. (2017). The Role of Solvent-Accessible Leu-208 of Cold-Active Pseudomonas fluorescens Strain A. M.S8 Lipase in Interfacial Activation, Substrate Accessibility and Low-Molecular Weight Esterification in the Presence of Toluene. Molecules, 22, 1312.
Cheng, C., Jiang, T., Wu, Y., Cui, L., Qin, S., & He, B. (2018). Elucidation of lid open and orientation of lipase activated in interfacial activation by amphiphilic environment, Int J. International Journal of Biological Macromolecules, 119, 1211–1217.
Feller, G., & Gerday, C. (2003). Psychrophilic enzymes: hot topics in cold adaptation. Nature Reviews. Microbiology, 1(3), 200–208.
Duarte, A. W. F., dos Santos, J. A., Vianna, M. V., Viera, J. M. F., Mallagutti, V. H., Inforsato, F. J., Wentzel, L. C. P., Lario, L. D., Rodrigues, A., Pagnocca, F. C., Pessoa Junior, A., & Sette, L. D. (2018). Cold-adapted enzymes produced by fungi from terrestrial and marine environments. Critical Reviews in Biotechnology, 38(4), 600–619.
Ruslan, R., Abd Rahman, R. N., Leow, T. C., Ali, M. S. M., Basri, M., & Salleh, A. B. (2012). Improvement of thermal stability via outer-loop ion pair interaction of mutated T1 lipase from Geobacillus zalihae strain T1. International Journal of Molecular Sciences, 13(1), 943–960.
Siddiqui, K. S. (2015). Some like it hot, some like it cold: Temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnology Advances, 33(8), 1912–1922.
Kuddus, M. (2018). Cold active enzymes in food biotechnology. Journal of Applied Biology and Biotechnology, 63, 58–63.
Bornscheuer, U. T., Huisman, G. W., Kazlauskas, R. J., Lutz, S., Moor, J. C., & Robins, K. (2012). Engineering the third wave of biocatalysts. Nature, 485(7397), 185–194.
Albayati, S. H., Masomian, M., Ishak, S. N. H., Ali, M. S. M., Thean, A. L., Shariff, F. M., Noor, N. D. M., & Rahman, R. N. Z. R. A. (2020). Main Structural Targets for Engineering Lipase Substrate Specificity. Catalysis, 10, 747.
Bassegoda, A., Cesarini, S., & Diaz, P. (2012). Lipase improvement: goals and strategies. Computational and Structural Biotechnology Journal, 2, e209005.
Sharma, P. K., Kumar, R., Mohammad, O., Singh, R., & Kaur, J. (2012). Engineering of a metagenome derived lipase toward thermal tolerance: effect of asparagine to lysine mutation on the protein surface. Gene, 491(2), 264–271.
Gatti-Lafranconi, P., Calderazzo, S. M., Vill, A., Alberghina, L., & Lotti, M. (2008). Unscrambling thermal stability and temperature adaptation in evolved variants of a cold-active lipase. FEBS Letters, 582(15), 2313–2318.
Veno, J., Ahmad Kamarudin, N., Mohamad Ali, M., Masomian, M., & Raja Abd Rahman, R. (2017). Directed evolution of recombinant C-terminal truncated Staphylococcus epidermidis lipase AT2 for the enhancement of thermostability. International Journal of Molecular Sciences, 18(11), 2202.
Veno, J., Raja Abd Rahman, R., Masomian, M., Mahamad Ali, M., & Ahmad Kamarudin, N. (2019). Insight into improved thermostability of cold-adapted Staphylococcal lipase by glycine to cysteine mutation. Molecules, 24(17), 3169.
Yaacob, N., Kamarudin, N. H. A., Leow, A. T. C., Saleh, A. B., Rahman, R. N. Z. R. A., & Ali, M. S. M. (2019). Effects of lid 1 mutagenesis on lid displacement, catalytic performance and thermostability of cold active Pseudomonas AMS8 lipase in toluene. Computational and Structural Biotechnology Journal, 17, 215–228.
Tan, Z., Li, J., Wu, M., & Wang, J. (2014). Enhancing the thermostability of a cold active lipases from Penicillium cyclopium by in silico design of a disulfide bridge. Applied Biochemistry and Biotechnology, 173(7), 1752–1764.
Li, G., Fang, X., Su, F., Chen, Y., Xu, L., & Yan, Y. (2018). Enhancing the thermostability of Rhizomucor miehei lipase with a limited screening library by rational-design point mutations and disulfide bonds. Applied and Environmental Microbiology, 84, e02129–e02117.
Zhang, J. H., Lin, Y., Sun, Y. F., Ye, Y. R., Zheng, A. P., & Han, S. Y. (2012). High throughput screening of B factor saturation mutated Rhizomucor miehei lipase thermostability based on synthetic reaction. Enzyme and Microbial Technology, 50(6-7), 325–330.
Priyanka, P., Tan, Y., Kinsella, G. K., Henehan, G. T., & Ryan, B. J. (2019). Solvent stable microbial lipases: current understanding and biotechnological applications. Biotechnology Letters, 41(2), 203–220.
Ganasen, M., Yaacob, N., Rahman, R. N. Z. R. A., Leow, A. T. C., Basri, M., Salleh, A. B., & Ali, M. S. M. (2016). Cold-adapted organic solvent tolerant alkalophilic family I.3 lipase from an Antarctic Pseudomonas. International Journal of Biological Macromolecules, 92, 1266–1276.
Dachuri, V., Boyineni, J., Choi, S., Chung, H.-S., Jang, S.-H., & Lee, C. (2016). Organic solvent-tolerant, cold-adapted lipases PML and LipS exhibit increased conformational flexibility in polar organic solvents. Journal of Molecular Catalysis B: Enzymatic, 131, 73–78.
Reetz, M. T., Soni, P., Fernandez, L., Gumulya, Y., & Carballeira, J. D. (2010). Increasing the stability of an enzyme toward hostile organic solvents by directed evolution based on iterative saturation mutagenesis using the B-FIT method. Chemical Communications, 46(45), 8657–8658.
Kawata, T., & Ogino, H. (2009). Enhancement of the organic solvent-stability of the LST-03 lipase by directed evolution. Biotechnology Progress, 25(6), 1605–1611.
Patel, P., & Desai, P. (2018). Isolation, identification and production of lipase producing bacteria from oil contaminated soil. BMR Microbiology, 4, 1–7.
Sarmah, N., Revathi, D., Sheelu, G., Yamuna Rani, K., Sridhar, S., Mehtab, V., & Sumana, C. (2018). Recent advances on sources and industrial applications of lipases. Biotechnology Progress, 34(1), 5–28.
Zheng, X., Chu, X., Zhang, W., Wu, N., & Fan, Y. (2011). A novel cold-adapted lipase from Acinetobacter sp. XMZ-26: gene cloning and characterization. Applied Microbiology and Biotechnology, 90(3), 971–980.
Hassan, S. W. M., Abd, E. I., Latif, H. H., & Ali, S. M. (2018). Production of cold-active lipase by free and immobilized marine Bacillus cereus HSS: Application in waste water treatment. Frontiers in Microbiology, 9, 2377.
Kiran, G. S., Shanmughapriya, S., Jayalakshmi, J., Selvin, J., Gandhimathi, R., Sivaramakrishnan, S., Arunkumar, M., Thangavelu, T., & Natarajaseenivasan, K. (2008). Optimization of extracellular psychrophilic alkaline lipase produced by marine Pseudomonas sp. (MSI057). Bioprocess and Biosystems Engineering, 31(5), 483–492.
Tanaka, D., Yoneda, S., Yamashiro, Y., Sakatoku, A., Kayashima, T., Yamakawa, K., & Nakamura, S. (2012). Characterization of a new cold-adapted lipase from Pseudomonas sp. TK-3. Applied Biochemistry and Biotechnology, 168(2), 327–338.
Katiyar, P., Hare, P., & Baghel, V. S. (2017). Isolation, partial purification and characterization of a cold active lipase from Pseudomonas sp. Isolated from Sanopanth glacier of western Himalaya, India. International Journal of Scientific Research and Management, 5, 6106–6112.
Kavitha, M., & Shanthi, C. (2014). Partial purification of cold active lipase from Pseudomonas sp. ITCLP4 isolated from marine samples of Tamilnadu coast. International Journal of Pharma and Bio Sciences, 5, 269–275.
Jain, R., Pandey, N., & Pandey, A. (2020). Aggregation properties of CAL produced by a psychrotolerant strain of Pseudomonas palleroniana (GBPI_508). Biocatalysis and Biotransformation, 38, 263–273.
Kamarudin, N. H., Rahman, R. N., Ali, M. S., Leow, T. C., Basri, M., & Salleh, A. B. (2014). A new cold-adapted, organic solvent stable lipase from mesophilic Staphylococcus epidermidis AT2. The Protein Journal, 33(3), 296–307.
Yang, X., Lin, X., Fan, T., Bian, J., & Huang, X. (2008). Cloning and expression of lipP, a gene encoding a cold-adapted lipase from Moritella sp.2-5-10-1. Current Microbiology, 56(2), 194–198.
Joseph, B., Shrivastava, N., & Ramteke, P. W. (2012). Extracellular cold active lipase of Microbacterium luteolum isolated from Gangotri glacier, western Himalaya: isolation, partial purification and characterization. Journal, Genetic Engineering & Biotechnology, 10(1), 137–144.
Li, M., Yang, L. R., Xu, G., & Wu, J. P. (2016). Cloning and characterization of a novel lipase from Sternotrophomonas maltophila GS11: the first member of a new bacterial lipase family. Journal of Biotechnology, 228, 30–36.
De Pascale, D., Cusano, A. M., Autore, F., Parrilli, E., Di Prisco, G., Marino, G., & Tutino, M. L. (2008). The cold-active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family. Extremophiles, 12(3), 311–323.
Kasana, R. C., Kaur, B., & Yadav, S. K. (2008). Isolation and identification of a psychrotrophic Acinetobacter sp. CR9 and characterization of its alkaline lipase. Journal of Basic Microbiology, 48(3), 207–212.
Zhang, J. W., & Zeng, R. Y. (2008). Molecular cloning and expression of a cold-adapted lipase gene from an antarctic deep sea psychrotrophic bacterium Pseudomonas sp. 7323. Marine Biotechnology, 10(5), 612–621.
Parra, L. P., Reyes, F., Acevedo, J. P., Salazar, O., Andrews, B. A., & Asenjo, J. A. (2008). Cloning and fusion expression of a cold-active lipase from marine Antarctic origin. Enzyme and Microbial Technology, 42(4), 371–377.
Parra, L. P., Espina, G., Devia, J., Salazar, O., Andrews, B., & Asenjo, J. A. (2015). Identification of lipase encoding genes from Antarctic sea water bacteria using degenerate primers: expression of a cold-active lipase with high specific activity. Enzyme and Microbial Technology, 68, 56–61.
Novototskaya-Vlasova, K. A., Petrovskaya, L. E., Rivkina, E. M., Dolgikh, D. A., & Kirpichnikov, M. P. (2013b). Characterization of a cold active lipase from Psychrobacter cryohalolentis K5T and its deletion mutant. Biochemistry (Moscow), 78, 385–394.
Khurana, J., Kumar, R., Kumar, A., Singh, K., Singh, R., & Kaur, J. (2015). New insight into old bacillus lipase: solvent stable mesophilic lipase demonstrating enzyme activity towards cold. Journal of Molecular Microbiology and Biotechnology, 25(5), 340–348.
Jadhav, V. V., Pote, S. S., Yadav, A., Shouche, Y. S., & Bhadekar, R. K. (2013). Extracellular cold active lipase from the psychrotrophic Halomonas sp. BRI 8 isolated from the Antarctic sea water. Songklanakarin Journal of Science and Technology, 35, 623–630.
Baek, K., Lee, Y. M., Shin, S. C., Hwang, K., Hwang, C. Y., Hong, S. G., & Lee, H. K. (2015). Halocynthiibacter arcticus sp. nov., isolated from Arctic marine sediment. International Journal of Systematic and Evolutionary Microbiology, 65(Pt_11), 3861–3865.
Kumar, R., Acharya, V., Singh, D., & Kumar, S. (2018). Strategies for high-altitude adaptation revealed from high-quality draft genome of non-violacein producing Janthinobacterium lividum ERGS5:01. Standards in Genomic Sciences, 13(1), 11.
Kumar, R., Acharya, V., Mukhia, S., Singh, D., & Kumar, S. (2019). Complete genome sequence of Pseudomonas frederiksbergensis ERDD5:01 revealed genetic bases for survivability at high altitude ecosystem and bioprospection potential. Genomics, 111(3), 492–499.
Kumar, A., Muhia, S., Kumar, N., Acharya, V., Kumar, S., & Kumar, R. (2020). A Broad Temperature active lipase purified from a psychrotrophic bacterium of Sikkim Himalaya with potential application in detergent formulation. Frontiers in Bioengineering and Biotechnology, 8, 642.
Ortiz, C., Ferreira, M. L., Barbosa, O., dos Santos, J. C. S., Rodrigues, R. C., Berenguer-Murcia, A., Briand, L. E., & Fernandez-Lafuente, R. (2019). Novozym 435: the “perfect” lipase immobilized biocatalyst? Catalysis Science & Technology, 9(10), 2380–2420.
Peterson, R. A., Bradner, J. R., Roberts, T. H., & Nevalainen, K. M. H. (2009). Fungi from koala (Phascolarctos cinereus) faeces exhibit a broad range of enzyme activities against recalcitrant substrates. Letters in Applied Microbiology, 48, 218–225.
Neang, P. M., Subileau, M., Perrier, V., & Dubreucq, E. (2014). Homologous yeast lipases/acyltransferases exhibit remarkable cold-active properties. Applied Microbiology and Biotechnology, 98(21), 8927–8936.
Cai, Y., Wang, L., Liao, X., Ding, Y., & Sun, J. (2009). Purification and partial characterization of two new cold-adapted lipases from mesophilic Geotrichum sp. SYBC WU-3. Process Biochemistry, 44(7), 786–190.
Lan, D. M., Yang, N., Wang, W. K., Shen, Y. F., Yang, B., & Wang, Y. H. (2011). A novel cold-active lipase from Candida albicans: cloning, expression and characterization of the recombinant enzyme. International Journal of Molecular Sciences, 12(6), 3950–3965.
Canak, I., Berkics, A., Bajcsi, N., Kovacs, M., Belak, A., Teparic, R., Maraz, A., & Mrsa, V. (2015). Cold active lipase from the yeast Candida zeylanoides. Journal of Molecular Microbiology and Biotechnology, 25(6), 403–411.
Demera, L. L., Barahona, P. P., & Carvajal Barriga, E. J. (2019). Production, Extraction and Characterization of Lipases from the Antarctic Yeast Guehomyces pullulans. American Journal of Biochemistry and Biotechnology, 15(2), 75–82.
Kim, H. R., Kim, I. H., Hou, C. T., Kwon, K. I., & Shin, B. S. (2010). Production of a novel cold-active lipase from Pichia lynferdii Y-7723. Journal of Agricultural and Food Chemistry, 58(2), 1322–1326.
Maharana, A. K., & Singh, S. M. (2018a). Cold Active Lipases Produced by Cryptococcus sp. Y-32 and Rhodococcus erythropolis N149 Isolated from Nella Lake, Antarctica. International Journal of Current Microbiology and Applied Sciences, 7(3), 1910–1926.
Maharana, A. K., & Singh, S. M. (2018b). A cold and organic solvent tolerant lipase produced by Antarctic strain Rhodotorula sp. Y-23. Journal of Basic Microbiology, 58(4), 331–342.
Shimizu, Y., & onno, Y., and Tomita, Y. (2020). Wickerhamomyces psychrolipolyticus f.a., sp. nov., a novel yeast species producing two kinds of lipases with activity at different temperatures. International Journal of Systematic and Evolutionary Microbiology, 70(2), 1158–1165.
Mohammed, S., Te’o, J., & Nevalainen, H. (2013). A gene encoding a new cold-active lipase from Antarctic isolate of Penicillium expansum. Current Genetics, 59(3), 129–137.
Ahanger, F. A., Mali, S. M., Wani, N., Sahay, S., & Jain, K. (2018). Screening of Cold-Active Lipase from Psychotropic Fungi. Research & Reviews A Journal of Microbiology & Virology, 8, 1–5.
Pandey, N., Dhaar, K., Jain, R., & Pandey, A. (2016). Temperature dependent lipase production from cold and pH tolerant species of Penicillium. Mycosphere, 7(10), 1533–1545.
Yan, Q., Duan, X., Liu, Y., Jiang, Z., & Yang, S. (2016). Expression and characterization of a novel 1,3-regioselective cold-adapted lipase from Rhizomucor endophyticus suitable for biodiesel synthesis. Biotechnology for Biofuels, 9(1), 86.
Florczaka, T., Daroch, M., Wilkinson, M. C., Białkowska, A., Bates, A. D., Turkiewicz, M., & Iwanejko, L. A. (2013). Purification, characterization and expression in Saccharomyces cerevisiae of LipG7 an enantioselective, cold-adapted lipase from the Antarctic filamentous fungus Geomyces sp. P7 with unusual thermostability characteristics. Enzyme and Microbial Technology, 53, 18–24.
Bjerga, G. E. K., Lale, R., & Williamson, A. K. (2016). Engineering low-temperature expression systems for heterologous production of cold-adapted enzymes. Bioengineered, 7(1), 33–38.
Shuo-shuo, C., Xue-zheng, L., & Ji-hong, S. (2011). Effects of co-expression of molecular chaperones on heterologous soluble expression of the cold active lipase Lip-948. Protein Expression and Purification, 77(2), 166–172.
Cheng, Y. Y., Qian, Y. K., Li, Z. F., Wu, Z. H., Liu, H., & Li, Y. Z. (2011). A novel cold adapted lipase from Sorangium cellulosum strain So0157-2: genecloning, expression, and enzymatic characterization. International Journal of Molecular Sciences, 12(10), 6765–6780.
Chen, R., Guo, L., & Dang, H. (2010). Gene cloning, expression and characterization of a cold-adapted lipase from a psychrophilic deep-sea bacterium Psychrobacter sp. C18. World Journal of Microbiology and Biotechnology, 27, 431–441.
Novototskaya-Vlasova, K. A., Petrovskaya, L., Kryukova, E., Rivkina, E. M., Dolgikh, D. A., & Kirpichnikov, M. P. (2013a). Expression and chaperon-assisted refolding of a new cold-active lipase from Psychrobacter cryohalolenticus K5(T). Protein Expression and Purification, 91(1), 96–103.
Xu, H., Lan, D., Yang, B., & Wang, Y. (2015). Biochemical properties and structure analysis of a DAG-Like lipase from Malassezia globosa. International Journal of Molecular Sciences, 16(3), 4865–4879.
Ji, X., Li, S., Wang, B., Zhang, Q., Lin, L., Dong, Z., & Wei, Y. (2015). Expression, purification and characterization of a functional, recombinant, cold-active lipase (LipA) from psychrotrophic Yersinia enterocolitica. Protein Expression and Purification, 115, 125–131.
Ali, Y., Ahmad, B., Alghamdi, K. M., Kamal, T., Ali, H. S. H. M., Anwar, Y., Hussain, A., & Jogezai, N. U. (2019). Characterization of recombinant cold active lipase from a novel Pseudomonas spp. MG687270. International Journal of Agriculture and Biology, 22, 855–865.
Su, H., Mai, Z., Yang, J., Xiao, Y., Tian, X., & Zhang, S. (2016). Cloning, expression and characterization of a cold active and organic solvent tolerant lipase from Aeromicrobium sp. SCSIO 25071. Journal of Microbiology and Biotechnology, 26(6), 1067–1076.
Santiago, M., Ramirez-Sarmiento, C. A., Zamora, R. A., & Parra, L. P. (2016). Discovery, molecular mechanism, and industrial applications of cold active enzymes. Frontiers in Microbiology, 7, 1408.
Joshi, S., & Satyanarayana, T. (2015). In vitro engineering of microbial enzymes with multifarious applications. Bioresource Technology, 176, 273–283.
Mehta, A., Bodh, U., & Gupta, R. (2017). Fungal lipases: a review. Journal of Biotech Research, 8, 58–77.
Sutton, P. W., Adams, J. P., Archer, I., Auriol, D., Avi, M., Branneby, C., Collis, A. J., Dumas, B., Eckrich, T., Fotheringham, I., ter Halle, R., Hanlon, S., Hansen, M., Holt-Tiffin, K. E., Howard, R. M., Huisman, G. W., Iding, H., Kiewel, K., Kittelmann, M., Kupfer, E., Laumen, K., Lefèvre, F., Luetz, S., Mangan, D. P., Martin, V. A., Meyer, H. P., Moody, T. S., Osorio-Lozada, A., Robins, K., Snajdrova, R., Truppo, M. D., Wells, A., Wirz, B., & Wong, J. W. (2012). Biocatalysis in the fine chemicals and pharmaceutical industries. In J. Whittal & P. W. Sutton (Eds.), Practical methods for biocatalysis and biotransformations (Vol. 2, pp. 2–60). Wiley: New York.
Tutino, M. L., Parrilli, E., De Santi, C., Giuliani, M., Marino, G., & de Pascale, D. (2010). Cold-Adapted esterases and lipases: A biodiversity still under-exploited. Current Chemical Biology, 4, 74–83.
Carvalho, A. C., Fonseca, T., de Mattos, M. C., de Oliveira, M., de Lemos, T. L., Molinari, F., Romano, D., & Serra, I. (2015). Recent advances in lipase-mediated preparation of pharmaceuticals and their intermediates. International Journal of Molecular Sciences, 16(12), 29682–29716.
Kavitha, M. (2016). Cold active lipases – an update. Frontiers in Life Science, 9(3), 226–238.
Kalkote, U. R., Ghorpade, S. R., Joshi, R. R., Ravindranathan, T., Bastawde, K. B., & Gokhale, D. V. (2000). A practical and scalable process for 4-(R)-Hydroxycyclopent-2-en-1-(S)-acetate by desymmetrization of meso-cyclopent-2-en-1,4-diacetate catalyzed by Trichosporon beigelii (NCIM 3326), a cheap biocatalyst. Tetrahedron: Asymmetry, 11(14), 2965–2970.
Bastawde, K.B., Gokhale, D.V., Ravindranathan, T., Ghorpade, S.R., Joshi, R.R., and Kalkote, U.R. (2002). US Patent No. 6, 448,051 B1.
Yadav, K. N., Adsul, M. G., Bastadwe, K. B., Jadhav, D. D., Thulasiram, H. V., & Gokhale, D. V. (2011). Differential induction, purification and characterization of cold active lipase from Yarrowia lipolytica NCIM 3639. Bioresource Technology, 102(22), 10663–10670.
Spelmezan, C. G., Bencze, L. C., Katona, G., Irimie, F. D., Paizs, C., & Tosa, M. I. (2020). Efficient and Stable Magnetic Chitosan-Lipase B from Candida Antarctica Bioconjugates in the Enzymatic Kinetic Resolution of Racemic Heteroarylethanols. Molecules, 25(2), 350.
Tufino, C., Bernal, C., Ottone, C., Romero, O., Illanes, A., & Wilson, L. (2019). Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules, 24(18), 3227.
Gawas, S. D., Lokanath, N., & Rathod, V. K. (2018). Optimization of enzymatic synthesis of ethyl hexanoate in a solvent free system using response surface methodology (RSM). Biocatalysis, 4(1), 14–26.
Musa, N., Latip, W., Abd Rahman, R. N. Z., Salleh, A. B., & Ali, M. S. M. (2018). Immobilization of an Antarctic Pseudomonas AMS8 lipase for low temperature ethyl hexanoate synthesis. Catalysts, 8(6), 234.
Evans, L. (2017). The new law in EU. European Food and Feed Law Review, 12, 49–51.
Shin, M., Seo, J., Bae, Y., Lee, T., Jang, M., & Par, C. (2020). Novel and efficient synthesis of phenethyl formate via enzymatic esterification of formic Acid. Biomolecules, 10(1), 70.
Leemans, L., van Langen, L., Hollmann, F., & Schallmey, A. (2019). Bienzymatic cascade for the synthesis of an optically active-benzoyl cyanohydrins. Catalysts, 9(6), 522.
Kantevari, S., Bantu, R., & Nagarapu, L. (2007). HClO4-SiO2 and PPA-SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent-free conditions: scope and limitations. Journal of Molecular Catalysis A: Chemical, 269, 53–57.
Nandre, K. P., Patil, V. S., & Bhosale, S. V. (2011). CsF mediated rapid condensation of 1,3-cyclohexadione with aromatic aldehydes: comparative study of conventional heating vs ambient temperature. Chinese Chemical Letters, 22(7), 777–780.
Li, J. T., Li, Y. W., Song, Y. L., & Chen, J. F. (2012). Improved synthesis of 2,2’-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound. Ultrasonics Sonochemistry, 19(1), 1–4.
Jiang, L., Wang, B., Li, R. R., Shen, S., Yu, H. W., & Ye, L. D. (2014). Amano lipase DF-catalyzed efficient synthesis of 2, 2’-arylmethylenedicyclohexane-1,3-dione derivatives in anhydrous media. Chinese Chemical Letters, 25(8), 1190–1192.
Cavicchioli, R., Siddiqui, K. S., Andrews, D., & Sowers, K. R. (2002). Low-temperature extremophiles and their applications. Current Opinion in Biotechnology, 13, 253–261.
Ugo, A. K., Amara, A. V., Igwe, C. N., & Kenechuwku, U. (2017). Microbial Lipases: A Prospect for Biotechnological Industrial Catalysis for Green Products: A Review. Fermentation Technology, 6, 144.
Brault, G., Shareck, F., Hurtubise, Y., Lepine, F., & Doucet, N. (2014). Short-chain flavor ester synthesis in organic media by an Escherichia coli whole-cell biocatalyst expressing a newly characterized heterologous lipase. PLoS One, 9, 1–9.
De Souza, M. C. M., dos Santos, P., Freire, R. M., Barreto, A. C. H., Fechine, P. B. A., & Goncales, L. R. B. (2017). Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Brazilian Journal of Chemical Engineering, 34(3), 681–690.
Yu, H., Lee, M. W., Shin, H., Park, K. M., & Chang, P. S. (2019). Lipase-catalyzed solvent-free synthesis of erythorbyl laurate in a gas-solid-liquid multiphase system. Food Chemistry, 271, 445–449.
Cong, S., Tian, K., Zhang, X., Lu, F., Singh, S., Prior, B., & Wang, Z. X. (2019). Synthesis of flavor esters by a novel lipase from Aspergillus niger in a soybean-solvent system. 3 Biotech, 9(6), 244.
Shahidi, F., & Ambigaipalam. (2015). Phenolics and polyphenols in food, beverages and spices: Antioxidant activity and health effects. Journal of Functional Foods, 18, 820–897.
Vosmann, K., Wiege, B., Weitkamp, P., & Weber, N. (2008). Preparation of lipophilic alkyl (hydroxy) benzoates by solvent-free lipase-catalyzed esterification and transesterification. Applied Microbiology and Biotechnology, 80(1), 29–36.
Han, S. Y., Pan, Z. Y., Huang, D. F., Ueda, M., Wang, X. N., & Lin, Y. (2009). Highly efficient synthesis of ethyl hexanoate catalyzed by CALB-displaying Saccharomyces cerevisiae whole-cells in non-aqueous phase. Journal of Molecular Catalysis B: Enzymatic, 59(1-3), 168–172.
Turati, D. F. M., Morais, W. G., Terrasan, C. R. F., Moreno-Perez, S., Pessela, B. C., Fernandez-Lorente, G., Guisan, J. M., & Carmona, E. C. (2017). Immobilization of lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on different hydrophobic supports: modulation of functional properties. Molecules, 22, 339.
Deckelbaum, R. J., & Torrejon, C. (2012). The Omega-3 fatty acid nutritional landscape: Health benefits and sources. The Journal of Nutrition, 142(3), 587S–591S.
Da Boit, M., Sibson, R., Sivasubramaniam, M. S., & Greig, J. R. (2017). Sex differences in the effect of fish oil supplementation on the adaptive response to resistance exercise training in older people: A randomized control trial. The American Journal of Clinical Nutrition, 105(1), 151–158.
Mangiagalli, M., Brocca, S., Orlando, M., & Lotti, M. (2020). The “cold revolution”. Present and future applications of cold active enzymes and ice-binding proteins. New Biotechnology, 55, 5–11.
Al-Ghanayem, A., & Joseph, B. (2020). Current perspective in using cold-active enzymes as eco-friendly detergent additive. Applied Microbiology and Biotechnology, 104(7), 2871–2882.
Valentini, F., Diamanti, A., & Palleschi, G. (2010). New biocleaning strategies on porous building materials affected by biodeterioration event. Applied Surface Science, 256(22), 6550–6563.
Weerasooriya, M. K. B., & Kumarasimghe, A. A. N. (2012). Isolation of alkaline lipase from rubber seed – Partial purification, characterization and its potential applications as a detergent additive. Indian Journal of Chemical Technology, 19, 244–249.
Li, X. L., Zhang, W. H., Wang, Y. D., Dai, Y. J., Zhang, H. T., Wang, Y., Wang, H. K., & Lu, F. P. (2014). A high detergent-performance, cold adapted lipase from Pseudomonas stutzeri PS59 suitable for detergent formulation. Journal of Molecular Catalysis B: Enzymatic, 102, 16–24.
Sahay, S., & Chouhan, D. (2018). Study on the potential of cold-active lipases from psychrotrophic fungi for detergent formulations. Journal, Genetic Engineering & Biotechnology, 16(2), 319–325.
Liu, R., Jiang, X., Mou, H., Guan, H., Hwang, H., & Li, X. (2009). A novel low temperature resistant alkaline lipase from a soda lake fungus strain Fusarium solani N4-2 for detergent application. Biochemical Engineering Journal, 46(3), 265–270.
Hamdan, A. (2018). Psychrophiles: Ecological significance and potential industrial application. South African Journal of Science, 114(5/6), 2017-0254.
Shcherbakova, V., & Troshina, O. (2018). Biotechnological perspectives of microorganisms isolated from the Polar Regions. Microbiology Australia, 39(3), 137–140.
Petropoulos, E., Dolfing, J., Yu, Y., Wade, M. J., Bowen, E. J., Daenport, R. J., & Curtis, T. P. (2018). Lipolysis of domestic water in anaerobic reactors operating at low temperatures. Environmental Science: Water Research & Technology, 4, 1002–1013.
Fan, X., Liang, W., Li, Y., Li, H., & Liu, X. (2017). Identification and immobilization of a novel cold-adapted esterase and its potential for bioremediation of pyrethroid-contaminated vegetables. Microbial Cell Factories, 16, 149.
Hu, J., Cai, W., Wang, C., Du, X., Lin, J., & Cai, J. (2018). Purification and characterization of alkaline lipase production by Pseudomonas aeruginosa HFE733 and application of for biodegradation in food wastewater treatment. Biotechnology and Biotechnological Equipment, 32(3), 583–590.
Le, L. T. H. L., Yoo, W., Jeon, S., Lee, C., Kim, K. K., Lee, H. J., & Kim, T. D. (2020). Biodiesel and flavor compound production using novel promiscuous cold adapted SGNH-type lipase (HaSGNH1) from the psychrophilic bacterium Halocynthiibacter arcticus. Biotechnology for Biofuels, 13(1), 55.
Author information
Authors and Affiliations
Contributions
N. Mhetras and D. Gokhale decided to write the review article. V. Mapare and N. Mhetras performed the literature search and D. Gokhale wrote the review article with the help of N. Mhetras.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable
Consent to Publish
Not applicable
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mhetras, N., Mapare, V. & Gokhale, D. Cold Active Lipases: Biocatalytic Tools for Greener Technology. Appl Biochem Biotechnol 193, 2245–2266 (2021). https://doi.org/10.1007/s12010-021-03516-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03516-w