Abstract
Advanced developments in the field of enzyme technology have increased the use of enzymes in industrial applications, especially in detergents. Enzymes as detergent additives have been extensively studied and the demand is considerably increasing due to its distinct properties and potential applications. Enzymes from microorganisms colonized at various geographical locations ranging from extreme hot to cold are explored for compatibility studies as detergent additives. Especially psychrophiles growing at cold conditions have cold-active enzymes with high catalytic activity and their stability under extreme conditions makes it as an appropriate eco-friendly and cost-effective additive in detergents. Adequate number of reports are available on cold-active enzymes such as proteases, lipases, amylases, and cellulases with high efficiency and exceptional features. These enzymes with increased thermostability and alkaline stability have become the premier choice as detergent additives. Modern approaches in genomics and proteomics paved the way to understand the compatibility of cold-active enzymes as detergent additives in broader dimensions. The molecular techniques such as gene coding, amino acid sequencing, and protein engineering studies helped to solve the mysteries related to alkaline stability of these enzymes and their chemical compatibility with oxidizing agents. The present review provides an overview of cold-active enzymes used as detergent additives and molecular approaches that resulted in development of these enzymes as commercial hit in detergent industries. The scope and challenges in using cold-active enzymes as eco-friendly and sustainable detergent additive are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enzymes are biocatalysts which accelerate the specificity and rate of a reaction by reducing the activation energy, which is extensively used in household and industrial applications from ancient days. In 1833, the first enzyme diastase (now called as amylase) was reported and later in 1913 commercial enzyme preparation, trypsin (Otto Rohm) from animal pancreas was used in detergents (Herbots et al. 2000; Margesin 2009). In 1960s, microbial proteases were produced in bulk amount and used as detergent additives to remove protein stains. Recently, enzymes are used in various biotechnological and industrial applications such as molecular biology, detergent, food and beverages, textiles, medical, pulp, and paper (Wackett 2019). Recent surveys predict that enzyme market is growing tremendously and the global enzyme market may reach 13 to14 billion US dollars in 2025. The demand for enzymes in various industries is increasing due to its specific activity. Additionally, it makes the reaction faster, efficient, selective, and leaving less by-products. In detergent industries, an eco-friendly approach is possible by replacing the harmful chemicals with enzymes (Kumari et al. 2019). The chemicals used in detergents are released directly to the environment which are difficult to be degraded (Cowan-Ellsberry 2014) and pollutes the waterbodies with high alkalinity, biological oxygen demand (BOD), and chemical oxygen demand (COD) (Mahmood et al. 2019). The use of enzymes in detergent increases the value and efficiency and reduces the energy consumption and usage of chemicals, thereby reduces toxic by-products and decreases the environmental pollution. The international market of detergent enzymes might have reached 1.8 million dollars in 2018 with a compound annual growth rate (CAGR) of 11.3% when compared with 2013 (Sarmiento et al. 2015). Around 25–30% of the enzyme market depends on detergent industries and it has become one of the successful applications of enzyme biotechnology (Singh et al. 2016). With advanced developments in biotechnology, industries are looking for greener technology and cost-effective production of detergents. One of such example is the utilization of cold-active enzymes used in detergent, laundry, and dish wash production process. For production of cold-active enzymes, microorganisms living in cold regions are preferred. Research on cold-active enzymes and its application has increased tremendously and reviewed extensively (Joseph et al. 2008; Margesin, 2009; Cavicchioli et al. 2011; Kuddus and Ramteke 2012; Joshi and Satyanarayana 2013; Sarmiento et al. 2015). The applications of cold-active enzymes have attracted worldwide especially in detergent industries. There are numerous reports on purification, characterization, and detergent compatibility studies on cold-active enzymes. According to market survey, detergent industries will be one of the major consumers for hydrolytic enzymes by 2024. The increase in number of research articles published is directly reflecting the interest on searching novel detergent compatible enzymes. Unfortunately, the information on cold-active enzymes in detergent application is disorganized and scattered. In this review, an attempt is made to organize and update the information on current trends and recent developments on cold-active enzymes in detergent applications, especially the aspects enzyme engineering for detergent compatibility.
Cold-active enzymes
Majority of area on Earth’s biosphere is cold, which consists of microbes and other living organisms that are metabolically active even at low temperature. There are reports on microbial community surviving at − 60 °C (Friedmann 1982; Cary et al. 2010). Metabolically active bacteria surviving at − 32 °C (Bakermans and Skidmore 2011) and bacteria that can reproduce at − 15 °C (Planococcus halocryophilus Or1) were isolated from Arctic permafrost (Mykytczuk et al. 2013). These psychrophiles can survive at low temperature and also it can perform various biochemical activities in the environment due to their structural adaptation of cold-active enzymes. In addition to this, their extracellular enzymes also help to degrade the complex materials present in the environment. In mesophilic organisms, the activation energy needed for catalysis becomes low and inadequate, which results in low enzyme activity. The protein gets denatured because of less availability of water molecules (Karan et al. 2012). In these conditions, structural features for specific adaptation makes cold-active enzymes flexible to become active under harsh conditions in psychrophiles with thermoliability when compared with mesophiles and thermophiles (Siddiqui and Cavicchioli 2006). During harsh climate, these microbes face challenges such as low enzyme activity, low reaction rate, alterations in the cellular transport, membrane fluidity, and flexibility protein denaturation (D’Amico et al. 2006). The cellular structure and biomolecules such as enzymes are adapted and flexible to get adjusted to survive at low temperature (De Maayer et al. 2014). The variation in amino acid sequence in protein results in charge difference, structural variance, flexibility, and hydrophobicity; however, it does not retain a clear structure or pattern to show considerable difference for adaptation in extreme conditions (Reed et al. 2013). To overcome these challenges, the membrane fluidity is increased by secretion of unsaturated methyl branched and short acyl-chain fatty acids (Chintalapati et al. 2004). The cold shock protein also aids to increase the cellular process of membrane fluidity and protein folding (Phadtare 2004). All the cell components are structured to function at low temperature and the antifreeze protein helps in preventing ice crystal growth (Munoz et al. 2017). This long-term adaptation also results in the genetic modifications in these microbes to produce enzymes active in low temperature (Tribelli and Lopez 2018). Initially, the enzymes such as protease from Serratia marcescens (McDonald and Chambers 1963) and malic dehydrogenase from Vibrio marinus (Langrige and Morita 1966) were reported from psychrophilic bacteria. Further cold-active enzymes from the cold-adapted organisms were explored. However, the molecular characterization of cold-active enzyme subtilisin, an alkaline protease, was reported in Antarctic Bacillus TA41 (Davail et al., 1994). Currently, there are numerous reports on the enzymes derived from organisms surviving at extreme environmental conditions. Among the extremophilic microbial community, psychrophilic microorganisms are the major group that are explored for industrial and biotechnological applications (Siddiqui et al. 2013). In search of cold-active enzymes cold regions such as Antarctic and Arctic regions, glaciers, deep sea sediments, etc. are explored for isolating such cold-adapted microorganisms (Boetius et al. 2015). Cold-active enzymes have a huge market potential due to its inevitable applications in various industries. The role of cold-active enzymes is expected to witness a substantial growth due to its extensive usage in the coming years which provides a positive outlook for the enzyme market. Selection of wild strains that produces cold-active enzymes and optimizing a maximum production will provide stable enzymes resulting in an increase in commercial yield. Moreover, these organisms are preserved and maintained easily throughout the production process. Psychrophilic enzymes have high catalytic activity and increased affinity towards the substrates at low temperature with reduced energy consumption in various industrial process. Likewise, the diverse adaptations of these enzymes make them to perform well in unfavorable conditions used in industrial process. The key features for adaptation of cold-active enzymes have been reviewed earlier (Feller 2010; Cavicchioli et al. 2011). Briefly, the following modifications on protein make them active under harsh conditions: the structural flexibility because of a reduced amount of hydrophobicity at the core but more on the protein surface. The alteration in amino acid composition such as less arginine/lysine ratio with more glycine residues which increases conformational mobility, fewer proline residues in the loops with more alpha helices and with high number of non-polar residues on the surface of protein molecules also helps in cold adaptation. The lesser inter domain and inter subunit interactions in protein, less disulfide bridges, few hydrogen bonds, reduced electrostatic interactions, fewer metal-binding sites make them active under cold condition. Increased number and size of loops with less secondary structure and oligomerization, along with unfolded protein state showing increased conformational entropy also favours cold adaptation.
Psychrophilic enzymes possess a higher specific activity at low temperature and require very low activation energy when compared with mesophilic and thermophilic enzymes (Gerday 2013). It is widely used in biotechnological and industrial applications because of its low thermal stability and huge energy savings. There are many more possible applications of cold-active enzymes due to their specific activity, easy inactivation, and need of minimal amount for a particular reaction. Especially, in the field of molecular biology, the cold-active enzymes can be deactivated by increasing the temperature to obtain irreversible inactivation without interfering with the main reaction. Even in protein expression, studies done at low temperature can prevent the formation of inclusion bodies which protects the heat liable gene products (Joseph et al. 2008). With all these benefits, psychrophilic enzymes are gaining attention in major fields of biotechnology for preventing the undesirable reactions and to prevent the loss of volatile components. The major disadvantages of these enzymes are low stability and less availability. To overcome these hurdles, development of novel technologies is needed to increase both quality and quantity of cold-active enzymes. However, protein engineering, rDNA technology, and metagenomics approach have been executed to improve the quality of cold-active enzymes. Genetically improved strains may be also used to overcome the bottlenecks of cold-active enzyme production.
Cold-active enzymes and detergents
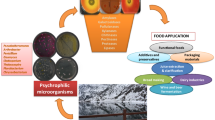
The dirt in clothing includes mainly protein, starch, and lipid components which arises from household and industrial materials. The removal of stains or dirt by heating and beating in turn shortens the life of fabrics and requires more energy. Addition of enzymes in detergents has become common in all the countries that has boomed enzyme market internationally. In cold regions, the water used for washing clothes requires heating, to reach ambient temperature to make the enzymes active for removal of dirt for increasing whiteness and brightness. The introduction of cold-active enzymes, active at alkaline pH, with thermostability has answered the problems in detergent industry. The detergent manufacturers and enzyme producers are seeking for novel enzymes that may fulfill consumer needs, improve efficiency of detergents, and retain the quality of fabrics. A good detergent enzyme should have more shelf life, thermostability, degrade stains effectively, compatible with other components such as perfumes, other enzymes, non-ionic and anionic surfactants, and oxidizing agents (Fig. 1). Hot water is preferred for washing clothes that may cause wear and tear of fabrics. Most of the detergent applications are carried out in harsh conditions such as alkaline pH and low temperature. These conditions may easily denature the standard or mesophilic enzymes used in detergents which have a narrow range of physiochemical activities. To overcome this, replacing mesophilic enzymes with chemicals is the only possibility which may lead to environmental pollution. There is a need to clear such harmful chemical process by replacing with eco-friendly solutions. To achieve this, enzyme capable functioning efficiently at low temperature and alkaline pH can be used as detergent additive because water used for washing has low temperature. Such enzymes used as additives in detergent can improve cold washing thereby reduces energy and improves the life and quality of fabrics. Additionally, it is biodegradable, reduces the use of chemicals, environmental pollution thereby reduces the risk to aquatic organisms. Development of enzyme technology by using enzyme additive in detergents made a tremendous change in reducing amount of chemicals in detergents. A list of cold-active enzymes used in commercially available detergents is given in Table 1. Besides, the recent trend of using suitable enzymes in detergent has created awareness on cold washing and its benefits to protect the fabrics. The usage washing machines using cold water increased from 38 to 53% when compared with 2010 and 2014. Further, the largest detergent technology producers such as Proctor & Gamble and Unilever resolve to make 70% of their machine to load cold washing, for conserving energy and environment by 2020 (Sarmiento et al. 2015).
Cold-active lipase
During winter, the temperature of water drops down which affects the washing efficiency to remove lipid-based stains. During normal wash of fabrics, fatty food stains are not removed effectively. In these circumstances, using hot or warm water to remove the lipid stains is widely practiced. This causes wear and tear to the fabrics that cannot withstand 50–60 °C and consumes lot of energy. Addition of lipase in detergent improves the efficiency of the detergent to remove such stains (Sahay and Chouhan 2018). Lipases are the enzymes that catalyze the hydrolysis of lipids into fatty acids and glycerol or other alcohols (Joseph and Ramteke 2013). However, using normal water with low temperature for washing may reduce the activity of lipases. To overcome this problem, cold-active lipases are used as an additive in the detergents. This decreases the laundering temperature and increases energy savings. It also enhances to remove tough fats and lipid stains from the fabrics in an eco-friendly manner. Cold-active lipases can hydrolyze lipids (fats) thereby removes lipid-based stains such as butter or kitchen used oils and used engine oils at low temperature. The first lipase used in detergent was Lipolase® (Novozymes) produced by Thermomyces lanuginosus (Jurado et al. 2007); later, recombinant lipases were used in detergents. Lipoclean® is a cold-active lipase produced by Novozymes that is used for triglyceride stains. It is active at low temperature (≃ 20 °C), and stable with other enzymes. Cold-active lipase stable at alkaline pH and withstand oxidizing and chelating agents will be suitable for detergent additive. The enzyme should be active in surfactants and effective at low concentration with broader specificity. There is an increased interest to explore cold=active lipase from cold-adapted microorganisms for commercial purposes.
Several cold=active lipases have been isolated from microorganisms and its feasibility to use as detergent additive was studied (Table 2). However, till date only a few fungal cold-active lipases have been reported for detergent compatibility. Penicilium canesense and Pseudogymnoascus roseus producing cold-active metallo-lipase with optimum activity at pH 11 and 9, respectively, stable with detergents (90%) were reported (Sahay and Chouhan 2018). Another cold-active fungal lipase studied for detergent stability was Fusarium solani N4-2 with optimum activity at 30 °C and active at a wide range of temperature (0–30 °C) (Liu et al. 2009). Detergent compatible cold-active lipases were isolated from Pseudomonas stutzeri PS59 (Li et al. 2014); Pseudomonas sp. AKM -L5 (Maharana and Ray 2015); Yersinia enterocolitica strain KM1 (Ji et al. 2015); and Pseudomonas sp. VITCLP4 (Kavitha and Shanthi 2017). Although there are cold-active lipases used in detergents, the increase in demand for a suitable and appropriate lipase is always an urge to search for novel cold-active lipases from microorganisms with unique properties. Cold-active lipase from Acinetobacter sp. XM-26 (Zheng et al. 2011), Bacillus sphaericus MTCC 7526 (Joseph et al. 2013), Microbacterium luteolum (Joseph et al. 2012), and Microbacterium phyllosphaerae MTCC 7530 (Joseph 2006) were also studied for detergent compatibility and stability.
Cold-active protease
Proteases hydrolyze the peptide bonds and breakdown protein (Tavano 2013). Most of the tough stains in fabric are of protein based such as blood, egg stains, cocoa, grass, and human sweat, and addition of protease may serve to fulfill the need of detergency for removing these stains (Kuddus and Ramteke 2012). Cold-active proteases can be used as an additive in detergent along with other components to remove the macromolecular stains. The woolen and silk fabrics are spoiled by processing at high temperature. Treating the silk and wool with cold-active protease can bring good finishing on the surface and new look for the fabrics (Joshi and Satyanarayana 2013). Bio40® (Gebruder Schnyder-Biel) was the first commercially produced protease used in detergent. Later, Novozymes developed proteases (Kannase® or Liquanase®) for cold washing in 1998 and the enzyme was active at low temperature (10–20 °C) and it belongs to serine protease. Another variant of serine protease (Polarzyme®) was released for hand washing detergents in cold water with a broader activity at 5–60 °C. Later, many cold-active proteases were available in markets. Palo Alto (CA, USA) released two cold-active proteases Purafect® and Properase® active at low temperature. Genencor developed Excellase®, a cold-active protease, additive for dishwashing liquids (Sarmiento et al. 2015).
Microorganisms adapted to low temperature are used as the source for cold-active proteases (Kuddus and Ramteke 2012). There are quite a large number of cold-active protease producing bacteria isolated from cold environments. Only few of them have been reported for alkaline stability that can be used as detergent additive (Kuddus and Ramteke 2009; Kuddus and Ramteke 2011). The cold-active protease explored for detergent stability and compatibility is given in Table 3. Bacillus subtilis, isolated from Gangothri glacier, western Himalayas, produced cold-active protease and it was partially purified. The enzyme showed stability in presence of 1% SDS and exhibited enhanced activity in presence of commercially available detergents (Baghel et al. 2005). A closely related species of Chryseobacterium sp. producing cold-active protease which is tolerant to detergents, surfactants, and organic solvents was studied (Mageswari et al. 2017). Cold-active protease isolated from Pseudoalteromonas arctica PAMC 21717 active at pH 9 with broader substrate activity has good compatible with commercially available detergents was reported recently (Park et al. 2018). A cold-active alkophillic protease from Bacillus subtilis WLCP1 active at pH 10 and stable at a wide range of pH 7–11 showed excellent performance in removing blood stains from fabrics (Furhana et al. 2019).
Cold-active amylase
Starch-based stains from fruits, vegetables, sauces, gravy, and cereals are very common in fabrics. For efficient removal of such stains, addition of amylase enzymes is used in detergents (Niyonzima and More 2014). Amylases belong to hydrolases enzymes which randomly break the bonds between glucose units in linear amylose chain of starch by cleaving 1, 4-αD-glycosidic linkages. Amylases are the larger group of enzymes which constitute 25% of the total sale of enzymes. Amylases are consumed by detergent industries both in bulk and contribute total value for the enzyme industries. Among the liquid detergent, 90% of the formulations are made along with amylase (Hmidet et al. 2009). The amylases active at low temperature and high pH are more suitable for using as additive in detergents. Alpha amylase is commonly used for detergency. Stainzyme® was the first amylase active at wide range of temperature introduced by Novozyme. Later, an improved amylase, bleach tolerant and active at low temperature, named Stainzyme plus® was brought into market. An amylase (Preferenz™ S100) active at 16 °C was marketed by DuPont Industrial Biosciences for detergents. Like protease and lipases, isolation and feasibility studies on cold-active amylases as detergent additive are always constantly carried out. However, there are only few reports on cold-active amylase compatible with detergents (Table 4). Cold-active α-amylase from Bacillus cereus GA6 which produced cold-active α-amylase at alkaline conditions (pH 10) was studied for the compatibility with commercially available detergents. It showed 70–92% stable with different detergents at 22 °C (Roohi et al. 2013). This was the first report on cold-active amylase compatible with commercially available detergents. Alpha amylase from a marine bacterium Zunongwangia profunda was isolated and found compatible with detergents. The gene was cloned and expressed in E.coli. The recombinant enzyme was active even at very low (0–5 °C) temperature (Qin et al. 2014). Recently, a cold-active α-amylase was isolated from Bacillus subtilis N8 which produced the enzyme at 15 °C and at a pH of 10. The amylase was maximum active at 25 °C and stable at a pH of 8 and showed 96% stability at a wide range of temperature of 10–40 °C (Arabaci and Arikan 2018). This α-amylase showed high stability in presence of detergents, which supports as an additive in detergents for cold washing. Microorganisms producing amylase, adapted to low temperature and alkaline conditions, are becoming popular for commercially important applications especially in detergent industries.
Cold-active type I pullulanase
Pullulanases are hydrolases that catalyze the hydrolysis of glycosidic linkages (Qoura et al. 2014). Even though there are five groups of pullulanase based on the substrate specificity, cold-active type I pullulanases are tested for detergent stability. During starch hydrolysis, formation of intermolecular cross linking and high viscosity of slurry can be fixed by cold-active pullulanase type I at low temperature (Lu et al. 2018). A gene encoding for cold-active pullulanase from Exiguobacterium sp SH3 was expressed in Bacillus subtilis and E.coli. The product was stable at a wide range of pH (4–11) with maximum activity at pH 8.5 and it was compatible with two commercially available detergents (Rika 7.5% v/v; and Fadisheh 2.5% w/v) for 10 days with 54.5 and 85% activity (Rajaei et al. 2015). In another study, cold-active type I pullulanase from Bacillus pseudofirmus 703 was characterized for detergent stability. The enzyme was stable at pH 8 and active at 45 °C as an optimum temperature. However, the enzyme was active at a wide range of temperature from 25 to 35 °C that helps in cold washing (Lu et al. 2018).
Cold-active cellulase
Bacteria and fungi produce cellulase which can cleave the β 1–4 among glucose molecules in cellulose polymer. Cellulase comprises three groups in which all these groups work synergistically to break or cleave the cellulose polymer (Horn et al. 2012). Along with proteases and lipases, cellulase enzyme is added to detergents to increase the washing efficiency. Cellulase in detergents will care for brightness and color by reducing the fuzz and pills formation in woolen garments. In cotton blend fabrics, the enzyme can increase the color feel and brightness by modifying the cellulose fibrils. It also helps in dirt and stain removal by selectively targeting the cellulose fibrils internally and removing the dirt from inter-fibrils along with other detergent ingredients (Niyonzima 2019). The cellulose fibers present in cotton fabrics get damaged due to repeated usage and washing which can accumulate soil and dirt. Addition of cellulase can digest the damaged cellulose fibers and make the fabrics attractive. Even in textile industries, the cellulase enzyme is used to increase the finishing of fabrics by modifying the protruding fibers from the yarn or fabrics. Thus, treatment with cellulase decreases fabric roughness and increases the smoothness, glossiness, and color brightness (Karmakar and Ray 2011). However, cellulase with pH stability activity at low temperature and its compatibility with detergent components is suitable to use as detergent additive (Kasana and Gulati 2011). Addition of cold-active cellulase in the detergent will increase the washing efficiency of the detergent thereby reduce the consumption of water and lead to huge energy savings (Showell 1999). At present, cellulases active at wide range of temperature (30–60 °C) are added in detergents. Rocksoft™ (Jupiter, FL, USA) and Retrocell, Retrocell ZircoN and Recop (EpyGen Biotech Dubai, UAE) UTA88, 90 (Hunan Youtell Biochemical, Shanghai, China), are some of the mesophilic cellulase active at low temperature are used in detergents (Kasana and Gulati 2011). Studies have been conducted in recombinant cold-active cellulase expressed in Escherichia coli BL21 (DE3) for stability with surfactants (both ionic and non-ionic). The structural stability, chemical unfolding and refolding, has been investigated to explore its stability and activity in detergents (Souza et al. 2016). Novozymes developed a cold-active cellulase (Celluzyme®) active at 15 °C from cold-adapted fungi Humicola insolens and marketed the mix of cellulase as Celluclean®.
Other detergents compatible with cold-active enzymes
Mannanase and pectinase are also having importance in detergent, laundry, and dishwashing industries. Mannans are the thickening agents used in food products such as ice creams and sauces, personal care products such as shampoo, gels, and toothpastes. It is difficult to remove mannan stains which are absorbed into cellulose fibers. Mannanase is the enzyme that can degrade mannan by breaking down the β 1–4 linkage between the mannose into water-soluble carbohydrates (Kim et al. 2018). There are no reports on cold-active mannanase isolated from microorganisms and studied for detergent compatibility. Few mannanase active at low temperature has been reported and used by industries. Mannaway® (Novozymes) active at 20 °C and another enzyme Effectenz™ (DuPont) active at 30 °C are used in detergents. Mannanase is used to formulate products such as healthcare, beauty care, sanitary, hand washes, contact lens cleaners, and surface cleaners (Chauhan et al. 2012).
Pectinases hydrolyze pectin polysaccharides, which are widely found in fruits juices, sauces, and jams. Pectinases are used for bio-scouring process of cotton fabrics, replacing harsh chemical scouring process such as boiling in caustic alkali. This bio-scouring helps to remove pectin that helps the loosened waxes from the fabrics by agitation and surfactants make the fabrics to become high hydrophobicity without fiber deterioration (Wang et al. 2007). Addition of pectinases in the detergent will remove pectin-based stains from fabrics. Till date there are no reports on cold-active pectinases and its compatibility with detergents. However, the detergent industries are targeting cold-active pectinases for using as additive in detergent to improve the efficiency of the product. Novozymes released a pectate lyase (Xpect®) active at 20 °C into market for using in detergents. Cutinase (Degani et al. 2002) and xylanase are used as detergent additives (Csiszar et al. 2001a; Csiszar et al. 2001b). These enzymes active at low temperature with alkaline stability has not been reported so far for detergent compatibility.
Engineering of detergent enzyme
Washing at low temperature had become the trend in society for increasing the life and brightness and retaining the color of the fabrics. Some of the cold-active enzymes show low thermostability and less shelf life. Moreover, the product yield also becomes less compared with mesophilic counterparts (Saeki et al. 2007). The techniques such as NMR spectroscopy, site specific mutagenesis, and X-ray crystallography made to understand the structural and functional aspects and protein sequence of enzymes. Alterations are done by chemio- or regio- or stereo-selectivity to increase the activity and specificity of enzymes (Luetz et al. 2010). Enzymes are modified with increasing activity at wide range of temperature and thermostability by directed evolution and rational design (Vojcic et al. 2015). A schematic representation of enzyme engineering and its advantages is shown in Fig. 2. Subtilisin is non-specific proteases that are widely studied by protein engineering for detergent applications based on the compatibility with detergent matrix, thermostability, active under alkaline pH, and active under wide range of temperatures (20–60 °C). A hybrid enzyme was made from Antarctic Bacillus TA39 protease (S39) by replacing the highly flexible 12 amino acids with mesophilic subtilisin sequence (Topt 55 °C) resulted in increased activity at room temperature, specificity for synthetic substrate, and wider substrate profile (Tindbaek et al. 2004). The enzymes selected for detergent application should be active under high alkaline conditions. Many attempts were made to modify pH-dependent activity of enzymes used in detergent to increase alkaline stability by charge distribution. However, in some cases, the change in amino acid sequence or alteration has resulted in loss of activity rather than increasing the activity at alkaline pH (Tynan-Connolly and Nielsen 2007). The influence of surface charges on pH-dependent enzymes by amino acid substitution was demonstrated earlier (Russell and Fersht 1987). The post translational autocatalytic de-amidation process in Bacillus gibsonii protease (pHopt 11) was done to convert positively charged asparagine and glutamine residues into aspartic acid and glutamic acid resulted in twofold increase pH-dependent activity at pH 8.6 (Jakob et al. 2013). The alteration of surface charge by de-amidation process changes the pH-dependent activity.
The presence of chemical bleaching agents such as hydrogen peroxide in detergent is critical for the fabrics, surface, and enzymes of detergent industry. Enzyme mediate production of oxidative agents such as peroxycarboxylic acid is gaining attraction in detergent industry. The serine hydrolases such as lipases, proteases, and esterase catalyze additional side reaction at different transition states. Enzymes are engineered for perhydrolytic or hydrolytic ratio by site saturation mutagenesis at 12–15 Å at the catalytic triad in earlier reports. An increase of 28-fold perhydrolytic activity of esterase in Pseudomonas fluorescens was observed (Poulouse 1994). A novel esterase from Enterobacter sp. with less thermostability (above 45 °C) was subjected to random mutation and 3.4-fold increase in thermostability was obtained. The homology modeling analysis revealed that hydrophobic Ala was replaced by hydrophilic Asp which increased the solubility and interaction (Ke et al. 2018). Random mutagenesis and recombination of improved strains of Bacillus sphaericus subtilisin (SSII) has shown 6.6-fold increase in turnover number (Kcat 10 °C) and catalytic efficiency by 9.6-fold compared with wild strain (Wintrode et al. 2000). Specific activity and thermostability of Bacillus gibsonii protease active under alkaline conditions was increased by direct evolution by frequent rounds of sequence saturation mutagenesis (SeSaM) (Wong et al. 2004). Cold-active enzymes are potential detergent additive to replace conventional enzymes. Protein engineering for making tailor-made enzymes is to overcome the challenges of low thermostability, stability at alkaline pH, and compatibility with chemical oxidizing agents. There are several reports on improving thermal stability, activity at low temperature, strategies to modulate pH activity, and hypothesis to increase promiscuous activity as bleaching agents by additional catalysis of peroxycarboxylic acid production (Vojcic et al. 2015).
Future outlook
Washing at low temperature is gaining popularity in the coming days. It is clear that enzyme-based detergents have better washing performance when compared with chemical-based or synthetic detergents by retaining the fabric quality. There is a large demand from the industries for the requirement of enzymes which are specifically active for a particular application. Psychrophilic enzymes should accomplish certain characteristics for using in detergent applications. Recent technologies are helpful for researchers to search for novel enzymes compatible with detergents. The recent advancement in protein engineering has developed such enzymes which meet the need or demands by using novel technologies and computational tools. The amino acid mutations have made the cold-active enzymes more stable and increased efficiency at ambient temperature. Thereby amount of energy consumed can be notably reduced by using the cold-active enzymes to carry out the reactions at low temperature. However, further studies are needed to explore the novel sources, compatibility studies on efficacy of detergent enzymes, stability at high pH, and activity at low temperature and perform in presence of chemical agents.
References
Arabaci N, Arikan B (2018) Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep Biochem Biotechnol 48(5):419–426. https://doi.org/10.1080/10826068.2018.1452256
Baghel VS, Tripathi RD, Ramteke PW, Gopal K, Dwivedi S, Rai UN, Singh SN (2005) Psychrotrophic proteolytic bacteria from cold environment of Gangotri glacier, Western Himalaya, India. Enzym Microb Technol 36(5–6):654–659. https://doi.org/10.1016/j.enzmictec.2004.09.005
Bakermans C, Skidmore ML (2011) Microbial metabolism in ice and brine at −5 degrees C. Environ Microbiol 13:2269–2278. https://doi.org/10.1111/j.1462-2920.2011.02485.x
Boetius A, Anesio AM, Deming JW, Mikucki JA, Rapp JZ (2015) Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat Rev Microbiol 13:677. https://doi.org/10.1038/nrmicro3522
Cary SC, McDonald IR, Barrett JE, Cowan DA (2010) On the rocks: the microbiology of Antarctic dry valley soils. Nat Rev Microbiol 8(2):129–138. https://doi.org/10.1038/nrmicro2281
Cavicchioli R, Charlton T, Ertan H, Mohd Omar S, Siddiqui KS, Williams TJ (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4:449–460. https://doi.org/10.1111/j.1751-7915.2011.00258.x
Chauhan PS, Puri N, Sharma P, Gupta KN (2012) Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol 93:1817–1830. https://doi.org/10.1007/s00253-012-3887-5
Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol (Noisy-le-grand) 50:631–642
Cowan-Ellsberry C, Belanger S, Dorn P, Dyer S, McAvoy D, Sanderson H, Versteeg D, Ferrer D, Stanton K (2014) Environmental safety of the use of major surfactant classes in North America. Crit Rev Environ Sci Technol 44:1893–1993. https://doi.org/10.1080/10739149.2013.803777
Csiszar E, Losonczi A, Szakacs G, Rusznak I, Bezur L, Reicher J (2001a) Enzymes and chelating agent in cotton pretreatment. J Biotechnol 89(2):271–279. https://doi.org/10.1016/s0168-1656(01)00315-7
Csiszar E, Urbanszki K, Szakacs G (2001b) Biotreatment of desized cotton fabrics by commercial cellulase and xylanase enzymes. J Mol Catal B Enzym 11(4–6):1065–1072. https://doi.org/10.1016/S1381-1177(00)00149-1
D’Amico S, Collins T, Marx JC, Feller G, Gerday C (2006) Psychrophilic microorganisms: challenges for life. EMBO Rep 7:385–389. https://doi.org/10.1038/sj.embor.7400662
Davail S, Feller G, Narinx E, Gerday C (1994) Cold adaptation of proteins. Purification, characterization, and sequence of the heat-labile subtilisin from the Antarctic psychrophile Bacillus TA41. J Biol Chem 269:17448–17453
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517. https://doi.org/10.1002/embr.201338170
Degani O, Gepstein S, Dosoretz CG (2002) Potential use of cutinase in enzymatic scouring of cotton fiber cuticle. Appl Biochem Biotechnol 103(1):277–290. https://doi.org/10.1385/ABAB:102-103:1-6:277
Feller G (2010) Protein stability and enzyme activity at extreme biological temperatures. J Phys Condens Matter 22:323101. https://doi.org/10.1088/0953-8984/22/32/323101
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215(4536):1045–1053. https://doi.org/10.1126/science.215.4536.1045
Furhana J, Awasthib P, Sharmaa S (2019) Biochemical characterization and homology modelling of cold-active alkophilic protease from Northwestern Himalayas and its application in detergent industry. Biocatal Agric Biotechnol 17:726–735. https://doi.org/10.1016/j.bcab.2019.01.028
Gerday C (2013) Psychrophily and catalysis. Biology 2:719–741. https://doi.org/10.3390/biology2020719
Herbots I, Kottwitz B, Reilly PJ, Antrim RL, Burrows H, Lenting HBM, Viikari L, Suurnakki A, Niku-paavola M, Pere J, Buchert J (2000) Enzymes, non-food application. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, New Jersey
Hmidet N, Ali N, Haddar A, Kanoun S, Alya S, Nasri M (2009) Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: characterization and potential application as detergent additive. Biochem Eng J 47:71–79. https://doi.org/10.1016/j.bej.2009.07.005
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VG (2012) Novel enzymes for the degradation of cellulose. Biotechnol Biofuels 5(1):45. https://doi.org/10.1186/1754-6834-5-45
Jakob F, Martinez R, Mandawe J, Hellmuth H, Siegert P, Maurer KH, Schwaneberg U (2013) Surface charge engineering of a Bacillus gibsonii subtilisin protease. Appl Microbiol Biotechnol 97:6793–6802. https://doi.org/10.1007/s00253-012-4560-8
Ji X, Chen G, Zhang Q, Lin L (2015) Purification and characterization of an extracellular cold-adapted alkaline lipase produced by psychrotrophic bacterium Yersinia enterocolitica strain KM1. J Basic Microbiol 55(6):718–728. https://doi.org/10.1002/jobm.201400730
Joseph B (2006) Isolation, purification and characterization of cold adapted extracellular lipases from psychrotrophic bacteria: feasibility as laundry detergent additive. Ph.D dissertation Allahabad Agricultural Institute-Deemed University, Allahabad
Joseph B, Ramteke PW (2013) Extracellular solvent stable cold active lipase from psychrotrophic Bacillus sphaericus MTCC 7526: partial purification and characterization. Ann Microbiol 63:363–370. https://doi.org/10.1007/s13213-012-0483-y
Joseph B, Ramteke PW, Thomas G (2008) Cold active microbial lipases: some hot issues and recent developments. Biotechnol Adv 26:457–470. https://doi.org/10.1016/j.biotechadv.2008.05.003
Joseph B, Shrivastava N, Ramteke PW (2012) Extracellular cold-active lipase of Microbacterium luteolum isolated from Gangotri glacier, Western Himalaya: isolation, partial purification and characterization. J Genet Eng Biotechnol 10:137–144. https://doi.org/10.1016/j.jgeb.2012.02.001
Joshi S, Satyanarayana T (2013) Biotechnology of cold-active proteases. Biology 2:755–783. https://doi.org/10.3390/biology2020755
Jurado E, Bravo V, Luzon G, Fernandez-Serrano M, Garcia-Roman M, Altmajer-Vaz D, Vicaria JM (2007) Hard-surface cleaning using lipases: enzyme–surfactant interactions and washing tests. J Surfactant Deterg 10:61–70. https://doi.org/10.1007/s11743-006-1009-z
Karan R, Capes MD, DasSarma S (2012) Function and biotechnology of extremophilic enzymes in low water activity. Aquatic Biosystems 8(1):4
Karmakar M, Ray RR (2011) Current trends in research and application of microbial cellulases. Res J Microbiol 6(1):41–53. https://doi.org/10.3923/jm.2011.41.53
Kasana RC, Gulati A (2011) Cellulases from psychrophilic microorganisms: a review. J Basic Microbiol 51:572–579. https://doi.org/10.1002/jobm.201000385
Kavitha M, Shanthi C (2017) Alkaline thermostable cold active lipase from halotolerant Pseudomonas sp. VITCLP4 as detergent additive. Indian J Biotechnol 6:446–455 http://nopr.niscair.res.in/handle/123456789/43324
Ke MM, Ramesh B, Hang YA, Liu ZD (2018) Engineering and characterization of a novel low temperature active and thermo stable esterase from marine. Enterobacter cloacae. Int J Biol Macromol 118:304–310. https://doi.org/10.1016/j.ijbiomac.2018.05.193
Kim S, Lee MH, Lee ES, Nam YD, Seo DH (2018) Characterization of mannanase from Bacillus sp., a novel Codium fragile cell wall-degrading bacterium. Food Sci Biotechnol 27:115–122. https://doi.org/10.1007/s10068-017-0210-3
Kuddus M, Ramteke PW (2009) Cold-active extracellular alkaline protease from an alkaliphilic Stenotrophomonas maltophilia: production of enzyme and its industrial applications. Can J Microbiol 55:1294–1301. https://doi.org/10.1139/w09-089
Kuddus M, Ramteke PW (2011) Production optimization of an extracellular cold-active alkaline protease from Stenotrophomonas maltophilia MTCC 7528 and its application in detergent industry. Afr J Microbiol Res 5(7):809–816. https://doi.org/10.5897/AJMR10.806
Kuddus M, Ramteke PW (2012) Recent developments in production and biotechnological applications of cold-active microbial proteases. Crit Rev Microbiol 38:380–388. https://doi.org/10.3109/1040841X.2012.678477
Kumari U, Singh R, Ray T, Rana S, Saha P, Malhotra K, Daniell H (2019) Validation of leaf enzymes in the detergent and textile industries: launching of a new platform technology. Plant Biotechnol J 17(6):1167–1182. https://doi.org/10.1111/pbi.13122
Langridge P, Morita RY (1966) Thermolability of malic dehydrogenase from the obligate psychrophile, Vibrio marinus. J Bacteriol 92:418–423
Li XL, Zhang WH, Wang YD, Dai YJ, Zhang HT, Wang Y, Wang HK, Lu FP (2014) A high-detergent-performance, cold-adapted lipase from Pseudomonas stutzeri PS59 suitable for detergent formulation. J Mol Catal B Enzym 102:16–24. https://doi.org/10.1016/j.molcatb.2014.01.006
Liu R, Jiang X, Mou H, Guan H, Hwang H, Li X (2009) A novel low-temperature resistant alkaline lipase from a soda lake fungus strain Fusarium solani N4-2 for detergent formulation. Biochem Eng J 46(3):265–270. https://doi.org/10.1016/j.bej.2009.05.016
Lu Z, Hu X, Shen P, Wang Q, Zhou Y, Zhang G, Ma Y (2018) A pH-stable, detergent and chelator resistant type I pullulanase from Bacillus pseudofirmus 703 with high catalytic efficiency. Int J Biol Macromol 109:1302–1310. https://doi.org/10.1016/j.ijbiomac.2017.11.139
Luetz S (2010) Reengineering enzymes. Science. 329:285–287. https://doi.org/10.1126/science.1192224
Mageswari A, Subramanian P, Chandrasekaran S, Karthikeyan S, Gothandam KM (2017) Systematic functional analysis and application of a cold-active serine protease from a novel Chryseobacterium sp. Food Chem 217:18–27. https://doi.org/10.1016/j.foodchem.2016.08.064
Maharana A, Ray P (2015) A novel cold-active lipase from psychrotolerant Pseudomonas sp. AKM-L5 showed organic solvent resistant and suitable for detergent formulation. J Mol Catal B Enzym 120:173–178. https://doi.org/10.1016/j.molcatb.2015.07.005
Mahmood Q, Shaheen S, Bilal M, Tariq M, Zeb BS, Ullah Z, Ali A (2019) Chemical pollutants from an industrial estate in Pakistan: a threat to environmental sustainability. Appl Water Sci 9:47–49. https://doi.org/10.1007/s13201-019-0920-1
Margesin R (2009) Cold active enzymes as new tools in biotechnology. Extremophiles-Vol II. In: Gerday C (ed) Extremophiles. Encyclopedia of life support systems Oxford
McDonald IJ, Chambers AK (1963) Some characteristics of proteinases of an obligately psychrophilic red-pigmented bacterium and of Serratia marcescens. Can J Microbiol 9:871–877. https://doi.org/10.1139/m63-114
Munoz PA, Marquez SL, Gonzalez-Nilo FD, Marquez-Miranda V, Blamey JM (2017) Structure and application of antifreeze proteins from Antarctic bacteria. Microb Cell Factories 16:1–13. https://doi.org/10.1186/s12934-017-0737-2
Mykytczuk NC, Foote SJ, Omelon CR, Southam G, Greer CW, Whyte LG (2013) Bacterial grow that −15 degrees C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or. ISMEJ 7:1211–1226. https://doi.org/10.1038/ismej.2013.8
Niyonzima FN (2019) Detergent-compatible bacterial cellulases. J Basic Microbiol 59(2):134–147. https://doi.org/10.1002/jobm.201800436
Niyonzima FN, More SS (2014) Detergent-compatible bacterial amylases. Appl Biochem Biotechnol 174:1215–1232. https://doi.org/10.1007/s12010-014-1144-3
Park HJ, Han SJ, Yim JH, Kim D (2018) Characterization of an Antarctic alkaline protease, a cold-active enzyme for laundry detergents. Korean J Microbiol 54(1):60–68. https://doi.org/10.7845/kjm.2018.7080
Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 6:125–136
Poulouse AJSB (1994) Selection and method of making enzymes for perhydrolysis system and for altering substrate specificity, specific activity and catalytic efficiency, patent US5352594 a
Qin Y, Huang Z, Liu Z (2014) A novel cold-active and salt-tolerant alpha-amylase from marine bacterium Zunongwangia profunda: molecular cloning, heterologous expression and biochemical characterization. Extremophiles 18:271–281. https://doi.org/10.1007/s00792-013-0614-9
Qoura F, Elleuche S, Brueck T, Antranikian G (2014) Purification and characterization of a cold-adapted pullulanase from a psychrophilic bacterial isolate. Extremophiles 18:1095–1102. https://doi.org/10.1007/s00792-014-0678-1
Rajaei S, Noghabi KA, Sadeghizadeh M, Zahiri HS (2015) Characterization of a pH and detergent-tolerant, cold-adapted type I pullulanase from Exiguobacterium sp. SH3. Extremophiles 19:1145–1155. https://doi.org/10.1007/s00792-015-0786-6
Ranjan K, Lone MA, Sahay S (2016) Detergent compatible cold-active alkaline amylases from Clavispora lusitaniae CB13. J Microbiol Biotechnol Food Sci 5:306–310. https://doi.org/10.15414/jmbfs.2016.5.4.306-310
Reed CJ, Lewis H, Trejo E, Winston V, Evilia C (2013) Protein adaptations in archaeal extremophiles. Archaea 373275. https://doi.org/10.1155/2013/373275
Roohi, Kuddus M, Saima (2013) Cold-active detergent-stable extracellular α-amylase from Bacillus cereus GA6: biochemical characteristics and its perspectives in laundry detergent formulation J Biochem Tech 4(4): 636–644
Russell AJ, Fersht AR (1987) Rational modification of enzyme catalysis by engineering surface-charge. Nature 328:496–500. https://doi.org/10.1038/328496a0
Saeki K, Ozaki K, Kobayashi T, Ito S (2007) Detergent alkaline proteases: enzymatic properties, genes, and crystal structures. J Biosci Bioeng 103:501–508. https://doi.org/10.1263/jbb.103.501
Sahay S, Chouhan D (2018) Study on the potential of cold-active lipases from psychrotrophic fungi for detergent formulation. J Genet Biotechnol 16(2):319–325. https://doi.org/10.1016/j.jgeb.2018.04.006
Sarmiento F, Peralta R, Blamey JM (2015) Cold and hot extremozymes: industrial relevance and current trends. Front Bioeng Biotechnol 3:148. https://doi.org/10.3389/fbioe.2015.00148
Showell MS (1999) Enzymes, detergents. In: Flickinger MC, Drew SW (eds) The encyclopedia of bioprocess technology, 2. Wiley, New York
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433. https://doi.org/10.1146/annurev.biochem.75.103004.142723
Siddiqui KS, Williams TJ, Wilkins D, Yau S, Allen MA, Brown MV, Lauro FM, Cavicchioli R (2013) Psychrophiles. Annu Rev Earth Planet Sci 41:87–115. https://doi.org/10.1146/annurev-earth-040610-133514
Singh D, Thakur S, Thayil SM, Kesavan AK (2019) Characterization of a cold-active, detergent-stable metallopeptidase purified from Bacillus sp. S1DI 10 using Response Surface Methodology. PLoS One 14(5):e0216990. https://doi.org/10.1371/journal.pone.0216990
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3. Biotech 6:174. https://doi.org/10.1007/s13205-016-0485-8
Souza TV, Araujo JN, da Silva VM, Liberato MV, Pimentel AC, Alvarez TM, Squina FM, Garcia W (2016) Chemical stability of a cold-active cellulase with high tolerance toward surfactants and chaotropic agent. Biotechnol Rep 9:1–8. https://doi.org/10.1016/j.btre.2015.11.001
Tavano OL (2013) Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym 90:1–11. https://doi.org/10.1016/j.molcatb.2013.01.011
Tindbaek N, Svendsen A, Oestergaard PR, Draborg H (2004) Engineering a substrate specific cold-adapted subtilisin. PEDS 17:149–156. https://doi.org/10.1093/protein/gzh019
Tribelli PM, Lopez NI (2018) Reporting key features in cold-adapted bacteria. Life 8:E8. https://doi.org/10.3390/life8010008
Tynan-Connolly BM, Nielsen JE (2007) Redesigning protein pKa values. Protein Sci 16:239–249. https://doi.org/10.1110/ps.062538707
Vojcic L, Pitzler C, Korfer G, Jakob F, Martinez R, Maurer KH, Schwaneberg U (2015) Advances in protease engineering for laundry detergents. New Biotechnol 32(6):629–634. https://doi.org/10.1016/j.nbt.2014.12.010
Wackett LP (2019) Microbial industrial enzymes: an annotated selection of World Wide Web sites relevant to the topics in microbial biotechnology. Microb Biotechnol 12(5):1090–1091. https://doi.org/10.1111/1751-7915.13389
Wang Q, Fan X, Hua Z, Gao W, Chen J (2007) Degradation kinetics of pectins by an alkaline pectinase in bioscouring of cotton fabrics. Carbohydr Polym 67:572–575. https://doi.org/10.1016/j.carbpol.2006.06.031
Wang X, Kan G, Ren X, Yu G, Shi C, Xie Q, Wen H, Betenbaugh M (2018) Molecular cloning and characterization of a novel alpha-amylase from Antarctic Sea ice bacterium Pseudoalteromonas sp. M175 and its primary application in detergent. Biomed Res Int 2018:3258383. https://doi.org/10.1155/2018/3258383
Wintrode PL, Miyazaki K, Arnold FH (2000) Cold adaptation of a mesophilic subtilisin like protease by laboratory evolution. J Biol Chem 275:31635–31640. https://doi.org/10.1074/jbc.M004503200
Wong TS, Tee KL, Hauer B, Schwaneberg U (2004) Sequence saturation mutagenesis (SeSaM): a novel method for directed evolution. Nucleic Acids Res 32:e26. https://doi.org/10.1093/nar/gnh028
Zheng X, Chu X, Zhang W, Wu N, Fan Y (2011) A novel cold-adapted lipase from Acinetobacter sp. XMZ-26: gene cloning and characterization. Appl Microbiol Biotechnol 90:971–980. https://doi.org/10.1007/s00253-011-3154-1
Acknowledgments
The authors are thankful to the authorities of Shaqra University, Kingdom of Saudi Arabia, for all the necessary help to perform the related literature survey and research work.
Author information
Authors and Affiliations
Contributions
AAAG collected the data, formatted and wrote the manuscript. BJ formatted the figures, reviewed and corrected the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Ghanayem, A.A., Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl Microbiol Biotechnol 104, 2871–2882 (2020). https://doi.org/10.1007/s00253-020-10429-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10429-x