Abstract
Psychrophilic microorganisms are cold-adapted with distinct properties from other thermal classes thriving in cold conditions in large areas of the earth’s cold environment. Maintenance of functional membranes, evolving cold-adapted enzymes and synthesizing a range of structural features are basic adaptive strategies of psychrophiles. Among the cold-evolved enzymes are the cold-active lipases, a group of microbial lipases with inherent stability–activity–flexibility property that have engaged the interest of researchers over the years. Current knowledge regarding these cold-evolved enzymes in psychrophilic bacteria proves a display of high catalytic efficiency with low thermal stability, which is a differentiating feature with that of their mesophilic and thermophilic counterparts. Improvement strategies of their adaptive structural features have significantly benefited the enzyme industry. Based on their homogeneity and purity, molecular characterizations of these enzymes have been successful and their properties make them unique biocatalysts for various industrial and biotechnological applications. Although, strong association of lipopolysaccharides from Antarctic microorganisms with lipid hydrolases pose a challenge in their purification, heterologous expression of the cold-adapted lipases with affinity tags simplifies purification with higher yield. The review discusses these cold-evolved lipases from bacteria and their peculiar properties, in addition to their potential biotechnological and industrial applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychrophilic microorganisms are cold-adapted with distinct properties from other thermal classes (e.g., thermophiles) (Cavicchioli et al. 2002). Their ubiquity in nature relates to their possession of dynamic cellular processes that ensure their survival, growth and adaptation even to extreme forms of life. Polar environments indeed still represents a dynamic reservoir for habitable psychrophiles normally found in nature colonizing such substantial portion of such extreme terrestrial and aquatic environments as—deep sea/ocean, Antarctic regions, glacial habitats, refrigerated appliances, and on/in plants and animals inhabiting cold regions (Russell 1998; Margesin et al. 2007; Buzzini et al. 2012). More than 90 % of the ocean environments sustain a broad diversity of microbial life and drive the functional capacity of the psychrophilic life. Bio-prospecting of most polar ecosystems was selected for equally diverse indigenous psychrophilic microbial assemblages comprising not only prokaryotes, but also eukaryotes, plants, animals, archaea, eucarya, protists, bacteria, yeasts, unicellular algae and fungi, viruses, flatworms and flagellates (Cavicchioli 2006; Morgan-kiss et al. 2006; Margesin et al. 2007; Parra et al. 2008; Buzzini et al. 2012; Feller 2013) contributing towards carbon and nutrient cycling, bioremediation, production of secondary metabolites, nutrient turnover, biomass production, and litter decomposition in cold ecosystems (Cummings and Black 1999; Trotsenko and Khmelenina 2005; Methé et al. 2005).
With all their cellular processes mediated in the cold environment, it is imperative that components of the cell including metabolism and protein synthesis are suitably adapted against the impact of the cold-shock environment. The most critical metabolic requirement for withstanding the low temperature’s harmful effect is the maintenance of functional membranes, evolving cold-adapted enzymes and synthesizing a range of structural features which endow a high level of flexibility in protein structure enabling biocatalysis, high catalytic efficiency at low temperatures, high degrees of thermolability, lower energy of activation and increased structural flexibility for better substrate access (Thomas and Dieckmann 2002; Siddiqui and Cavicchioli 2006; Siddiqui et al. 2013).
The commercialization of the inherent cellular components of cold-adapted psychrophilic microbes in particular enzymes obtained from them has received great attention. Psychrophilic bacteria have adapted and colonized a variety of cold environments producing cold-adaptive enzymes targeted for their biotechnological potential in detergent and food industries, biotransformation, environmental bioremediation, etc. (Russell 1998) compared to the mesophilic and thermophilic enzymes wherein high activity and interaction between substrates and enzyme are impaired by their high thermostable rigidity (Gerday et al. 2000).
The strategy of adaptation to cold environment is peculiar to microorganisms and their constitutive proteins as well as enzymes. Increased flexibility of molecular structure and high specific activity of cold-adapted enzymes apparently represent their thermosensitivity and a complementary interaction at a reduced energy cost. These and other factors add up to make psychrophilic microorganisms to be considered novel bio-resource for cold-active enzymes and other biological products with a spin-off for biotechnological and catalytic gains (Cavicchioli et al. 2011; Ewert and Deming 2013; Bowman 2013; Siddiqui et al. 2013). Many cold-active enzymes obtained from cold-adapted microorganisms include: protease, lipases, amylases and cellulases which have found wide applications (Aghajari et al. 1996; Alquati et al. 2002; Cieśliński et al. 2005).
Lipases hold huge potential in certain areas of application given their diversity and properties. This review examines cold-adapted lipases from psychrophilic bacteria, their adaptative features, purification strategies and exploitation of their industrial and biotechnological enterprise (Tables 1, 2).
Psychrophilic bacteria
The cold environment is being dominated by an array of aerobic and anaerobic bacteria in great diversity amongst other extremophiles overcoming the adverse effect of the reduced temperature, by developing some molecular shields making them catalytically effective, enabling their survival and maintaining their structural and functional adaptation to such extreme environmental conditions with desired properties (Karner et al. 2001; Deming 2002; Ramteke et al. 2005). Psychrophilic bacteria perform basic functions at frozen environments far below 0 °C and others tolerating such latitude with growth rates at 2–12 °C, with some bacteria becoming more piezophilic at 10 °C (Xu et al. 2003).
Diverse groups of bacteria belonging to the Gram-negative α-, β- and γ-proteobacteria from the forest soil, arctic alpine-tundra soil, stream water, mire sediments, lichen, snow algae and Antarctic lakes have been reported to survive cold-active environments (Gilbert et al. 2004; Männistö and Häggblom 2006). Others include those belonging to the Burkholderia sp., Collimonas sp., Pedobacter sp., Janthinobacter sp., Duganella sp., Dyella sp. and Sphingomonas sp. as well as those of the Gram-positive bacteria Pseudomonas spp., Vibrio spp. The Cytophaga–Flavobacterium–Bacteriodes phylum, Coryneforms, Arthrobacter sp. and Micrococcus sp. (Gilbert et al. 2004; Amico et al. 2006). Psychrophilic and psychrotolerant aerobic methanotrophic bacteria belonging to the genera and species of Methylobacter sp., Methylosphaera sp., Methylocella sp. and Methylocapsa acidiphila, Methylomonas scandinavica also inhabits cold ecosystems (Trotsenko and Khmelenina 2005).
In spite of the dominance of the cold environment by bacteria, both in number and density, they are still found in equivalent proportions in the hydrothermal vents together with Methanogenium and Methanococcus being the most cited genera. In other Antarctic ecosystems, Oscillatoria, Phormidium and Nostoc commune cyanobacteria are said to exercise dominance (Pandey et al. 2004). Heterotrophic bacteria belonging to five major phyla Actinobacteria, Bacteroidetes, Proteobacteria, Firmicutes and Deinococcus–Thermus have been recovered from different continental Antarctica and the Antarctic Peninsula (Peeters et al. 2011).
Many cold-active bacterial communities live and thrive in the low-temperature environments of deep sea hydrothermal vent (Yayanos 1995), Antarctic and Arctic sea ice (Tranter et al. 2004), Antarctic subglacial environment, alpine or glacial transitory ponds and ice-covered lakes (Morgan-kiss et al. 2006), where they represent the most abundant cold-adapted life-forms on earth at the level of species diversity and biomass (Feller and Gerday 2003). Most interesting is the evidence of metabolically active psychrotrophic bacteria in super-cooled, high-altitude cloud droplets (Sattler et al. 2001), surviving subjection to low-temperature, high-pressure, and low-nutrient levels. Considering the potential impact of the biomolecules derived from cold-adapted microorganisms, a great number of microbial biodiversity remains largely unstudied (Kennedy et al. 2008).
Cold-active lipases
Following the discovery of a pancreatic lipase by Claude Bernardin (1856), about 90 % of produced lipases have been obtained from microbial sources (Ellaiah et al. 2004; Kumar and Gupta 2008; Treichel et al. 2010) with many others identified in the environment including, animals and plants (Moussavou Mounguengui et al. 2013). The hunt for new lipases has been on the increase leading to a separate database for true lipases termed lipabase which integrates information about their structural and functional properties including taxonomic and biochemical information (Nagarajan 2012).
Lipases (water-soluble triacylglycerol acylhydrolases, EC 3.1.1.3) are enzymes physiologically catalyzing hydrolysis of insoluble long-chain triacylglycerides to free fatty acids (Treichel et al. 2010; Tran et al. 2013). Besides their natural function, they offer considerable potential in catalyzing bioconversion reactions in non-aqueous media and also catalyze hydrolysis of organic carbonates without need for any cofactors (Pandey et al. 1999; Sharma et al. 2001; Dutra et al. 2008; Rajendran et al. 2009). Lipases by convergent evolution are serine hydrolases (Stergiou et al. 2013) and they demonstrate good chemioselectivity, regioselectivity and enatioselectivity. They also possess unique features of being stable even in organic solvents, low thermostability at elevated temperatures with low costs and enantioselective properties (Thakur 2012; Hosseinpour 2012). Available data regarding several lipases from diverse sources indicate alkaline, acidic and organic solvent tolerant and cold-active lipases as most obviously investigated sources for numerous biotechnological applications given the important role they play in the turnover of various organic materials and biomass into useful products under conditions considered unsuitable to other biomolecules (Ramani et al. 2010; Joseph et al. 2011; Yoo et al. 2011; Mander et al. 2012; Nagarajan 2012; de Abreu et al. 2014).
Conventionally, cold-adapted microorganisms synthesize lipases which have evolved to tolerate the extremely inhospitable conditions of cold habitats with high biocatalytic activity. The need to avoid any causes of structural damage makes them irreversibly adapted to these environments by way of evolving unique mechanism to overcome the adverse influence of low temperatures compared to the lipases from mesophiles or thermophiles. Conserving stability–activity–flexibility relationship is fundamental to their functional and structural adaptive properties (Margesin et al. 2007; Buzzini et al. 2012; Feller 2013). Lowered thermal stability of cold-active lipases allows an equilibrium shift during reactions, because effectively functioning at low temperatures guarantees a promising potential for utilizing lipases in ‘White Biotechnology’ and other industrial applications (Gerday et al. 2000; Hasan et al. 2006; Joseph et al. 2008; Adan Gökbulut and Arslanoğlu 2013).
Cold-adapted lipase are said to offer economic incentives through low energy cost achieved through reduction in heating steps required to function in cold environments. Providing increased reaction yields and minimizing undesirable reactions that can occur at high temperatures by heat-inactivation of the enzymes rather than by use of chemical extraction offers significant advantages to the food industry preventing any modification to the substrates and finished product. Several psychrophlic and psychotrophic bacteria have been exploited for the production of a variety of cold-active lipases across different cold habitats, although current research trends on these classes of enzymes have shown that attention is given to using recombinant and protein engineering strategies to generate strains with promising properties. Few cold-adapted lipase/esterase have been studied. These include the enzymes from Aeromonas sp. LPB4 (Lee et al. 2003), Pseudomonas sp. strain B11-1 (Choo et al. 1998b), Acinetobacter sp. No. 6 (Suzuki et al. 2001), Psychrobacter sp. Ant300 (Kulakova et al. 2004), Photobacterium sp. (Ryu et al. 2006). Gene encoding a cold-active lipase from Antarctic psychrotrophs Penicillium expansum SM3 (Mohammed et al. 2013) Moraxella TA144 (Feller et al. 1991) revealed a novel cold-active lipase. Cold-active lipase from Micrococcus roseus exhibited high activity and stability over a range of temperature regimes in an optimized semisolid state fermentation utilizing agro-industrial substrates (Joseph et al. 2011). Cloned gene (lipA1) of a cold-active lipase has been reported for a Psychrobacter sp. 7195 isolated from a deep-sea sediment (Zhang et al. 2007). Novel cold-active and organic solvent-tolerant lipase displaying remarkable stability have been reported for Stenotrophomonas maltophilia CGMCC 4254 isolated from oil-contaminated soil samples (Li et al. 2013). Soils from Alaskan cold habitat and other cold regions have been exploited as potential sources of novel cold-active lipase (Choo et al. 1998a; Leonov 2010). Characterization of cold-active lipase from cold-adapted bacteria from snow-covered soil, salmon intestine and crab intestine have been predicated upon the thermodynamic shifts in temperatures and reductions in enthalpy energy values (Morita et al. 1997). Culture-independent approach have been applied in constructing a metagenomic library from the unculturable component of microbial communities from various deep-sea sediments, terrestrial environmental niches and other cold habitats towards producing novel cold-active lipase genes from recombinant clones (Kennedy et al. 2008; Jeon et al. 2009).
Structure and cold adaptation in lipases
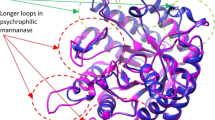
Knowledge of the relationship between extreme environments and their psychrophilic host requires several integrative attempts. Just as studies on cold-active lipases revolves around their isolation, purification characterization so does elucidation of their molecular and functional characteristics provide a better understanding of these enzymes. Strategy towards structural adaptation is a property unique to each enzyme. The thermodynamic stability of psychrophilic lipases is a strategy characterized by the relationship between stability, conformational flexibility or plasticity and their catalytic efficiency conferred upon them by a range of structural systems, useful especially when compared to mesophilic and thermophilic enzymes operating at set conditions of low temperatures. Rapid engineering paradigms involving gene synthesis, cloning and overexpression systems (Emond et al. 2010; Chen et al. 2010; Novototskaya-Vlasova et al. 2013), modular regulation (Palomo et al. 2003; Reetz et al. 2010), X-crystallography (Uppenberg et al. 1995), modification (Juhl et al. 2010; Durmaz et al. 2013), rational modeling (Alquati et al. 2002; Mohamad Ali et al. 2013; Maraite et al. 2013) have proven useful for understanding the structural adaptation of several cloned cold-active lipase.
Array of new methods enabling investigations into the structural and molecular parameters of cold-active lipases has been developed (Parra et al. 2008; Jeon et al. 2009; Do et al. 2013). Cold-active lipolytic genes encoding for two different lipases (Lip-1452 and Lip-948) from an Antarctic Psychrobacter sp. were successfully cloned and expressed with a description of their primary structure given (Xuezheng et al. 2010). A recombinant fusion protein (MBP-lipase) from an Antarctic marine Psychrobacter sp. has been cloned and characterized with significant cold-adapted features and activity (Parra et al. 2008). Comparative studies of the three-dimensional model of a recombinant lipase from Pseudomonas fragi IFO 3458 (PFL) with the structures of mesophilic homologous lipases were shown to account for PFL activity at low temperature (Alquati et al. 2002). This property is conferred by synergistic changes in overall genome content and protein sequence as revealed in the works of Methé et al. (2005) wherein modeling of three-dimensional Colwellia psychrerythraea and genome protein homology suggests changes to proteome composition that may enhance enzyme adaptation at low temperatures. The tendency for obtaining psychrophilic enzymes from a mesophilic and thermophilic counterparts could also be deduced in a homologous substitution of charged residues of Arg and Gluwith Lys and Ala, respectively (Gianese et al. 2001). Reduction in arginine/lysine residues is often less uniformly distributed in psychrophilic than in mesophilic enzymes. A few charged surface residues are involved in stabilizing intramolecular salt bridges and a large proportion of them exposed at the protein surface enable increased solvent interaction and enhance conformational flexibility accompanied by increased thermolability (Alquati et al. 2002; Siddiqui and Cavicchioli 2006; Feller 2013). Another important adaptive response to cold temperature below 0 °C comes from the production of trehalose and exopolysaccharides (EPSs) playing multiple roles in the entrapment, adhesion, retention and survival of microorganisms. It also favors the sequestration and concentration of nutrients, protection against cold shocks damage by modification of the physicochemical environment around the bacterial cell, acting as buffers and cryoprotectant in the prevention of denaturation and precipitation of proteins (Krembs et al. 2002; Ewert and Deming 2013).
Cold-active lipase expression and regulation
Overexpression and secretion of soluble proteins allows for their projected application in finding functional and structural information of large numbers of proteins. The induction of gene expression at sub-optimal growth temperatures also improves the solubility of proteins which is sometimes a major bottleneck hampering heterologous protein production in the periplasm of most microbial host. The synthesis and secretion of lipases by bacteria is influenced by a variety of factors. While rapid protein expression often results in unfolded/misfolded proteins, the reduced environment of the bacterial cytosol and the inability of host cell such as E. coli to perform several eukaryotic post‐translational modifications could result in the insoluble expression of proteins (Francis and Rebecca 2010).
Strategies for recombinant production and expression of cold-adapted lipases and other lipase enzymes, development of alternative promoter and induction strategies, modification of the host cells by engineering strategies and shifting the growth and manipulating the expression conditions with reduction in post-induction temperature (Weickert et al. 1996) has offered the options crucial to abundant yield towards efficient production of a diverse range of soluble heterologous proteins as well as regulating the cytotoxic effect of their host cells. Variety of expression vectors with different affinity tag sequences has been designed for fusion to almost any target protein that can be cloned and expressed in a microbial host. Each of these hosts are also equipped with an N-terminal signal sequence so that they can traverse the cytoplasmic membrane (Schlegel et al. 2013), making them suitable for certain recombinant protein purification procedures. More so, cleavage sites engineered between the affinity tag and the protein of choice enables removal of these tags (Einhauer and Jungbauer 2001). Higher yields of protein obtained with affinity tag makes this alternative economically favorable (Arnau et al. 2006). Thorough consideration is needed especially where the possibility of an adverse effect of the fusion on the applicability to the host system of choice could affect proper protein expression and secretion. A possible strategy is the co-expression of the recombinant protein with molecular chaperone. By employing a combination of functional metagenomic and protein expression technology approach, Jeon et al. (2011) successfully obtained higher yield of purified positive lipase producing clones with cold-activity property. Conversely, they demonstrated that the combination of co-expression of chaperones, removal of signal sequence and induction at low temperature (16 °C) may be effective in regulating soluble expression of lipolytic enzyme-encoding genes. Sometimes expression at low temperature alleviates toxicity, but may often lead to only partial stabilization of the fusion protein (Mujacic et al. 1999). Similar observations were highlighted by Shuo-shuo et al. (2011) wherein a cold-active lipase gene Lip-948, cloned from Antarctic psychrotrophic bacterium (Psychrobacter sp. G) showed enhanced intracellular soluble protein expression when co-expressed with “chaperone consortium” plasmids at a cultivation temperature of 15 °C. Novototskaya-Vlasova et al. (2013) described the influence of multiple parameters as folding enhancers when they developed an efficient protocol for solubilization and subsequent refolding of recombinant Lip2Pc lipase from Psychrobacter cryohalolentis K5T in the presence of a truncated chaperone. Rashid et al. (2001) described the production of low-temperature lipase both intracellularly and extracellularly from a previously reported psychrotrophic bacterium Pseudomonas sp. strain KB700A, which displays sigmoidal growth even at −5 °C. Although the genes were expressed under various conditions, the protein product was consistently produced in an insoluble form as inclusion bodies. However, treatment of insoluble recombinant lipase with urea and refolding by fractional dialysis enhanced purity and homogeneity. Similarly, expression of some lipase have only been achieved in insoluble form necessitating further refolding strategies as reported for a BDLipA cold-adapted lipase produced by Acinetobacter baumannii BD5 cloned and co-expressed in Escherichia coli BL21 (trxB) with a lipase chaperone as an inclusion body (Park et al. 2009). Overproduction, expression and optimization of LipXHis lipase from a psychrophilic deep-sea sediment Psychrobacter sp. strain C18 was achieved by amplifying the lipX lipase encoding gene without a signal peptide (Chen et al. 2010). In Escherichia coli, some proteins and location of secretion signal have been shown to enhance and promote efficient secretion of lipase to the extracellular medium. Thus, some lipases from Pseudomonas fluorescens have been described to secrete lipase through the signal peptide-independent pathway (Duong et al. 1994). Co-expression of rPFL with the foldase of P. aeruginosa as observed by Alquati et al. (2002) did not produce significant improvements in the fraction of soluble lipase, apparently suggesting the hypothesis that rPFL might also be secreted by a signal peptide-independent pathway. A sequence alignment of a novel M37 lipase investigated by Ryu et al. (2006) reveals no signal sequence and the resulting protein when cultivated and induced at 37 and 18 °C was exclusively insoluble and soluble, respectively. However, it was hypothesized that M37 lipase might be another example of extracellular proteins secreted without signal sequence. LipA protein was expressed as a His-tagged fusion protein in E. coli and successfully secreted extracellularly by OmpA secretion signals at the N-terminal regions (Zhang and Zeng 2008).
Mutant host of E. coli (C41(DE3) and C43(DE3) have also been reported to give high saturation cell density, and produced the protein as inclusion bodies at an elevated level without toxic effect. Mutants are frequently used to overcome the toxicity associated with overexpressing recombinant proteins using the bacteriophage T7 RNA polymerase expression system, even when the toxicity of the plasmids is so high that it prevents transformation in the strain (Dumon-Seignovert et al. 2004).
Although several reports have documented efficient secretion and folding of active lipase (Jaeger et al. 1999), over the years efforts have been made towards optimizing protein secretion and expression with several focus on strategies designed to maximize the yields of recombinant proteins and major challenges facing the use of prokaryotic expression system (Hannig and Makrides 1998; Francis and Rebecca 2010).
Purification approaches for cold-active lipases
The Antarctic marine habitats are unique natural laboratories for major research on the evolution of cold-active lipases in extreme environments (Russo et al. 2010). It has become evidently clear that obtaining cold-active lipase from microbial host of these extreme environments does not just stop at isolation, identification and screening/production. However, homogeneity and purity are the currency for achieving their unique purpose as biocatalysts for various industrial and biotechnological applications. Conversely, purity enables the molecular characteristics of cold-active lipases originating from Antarctic bacteria in relation to the adaptation to cold, to acquire high catalytic efficiency at low temperature, enhanced stability and higher flexibility (Nagarajan 2012). A major challenge with cold-active lipases has been obtaining their purified forms, where lipopolysaccharides bonding with lipid hydrolases in the Antarctic microorganisms are difficult to break (Gerday et al. 1997). Several traditional and novel purification and optimization strategies have been developed and employed successfully (Yujun et al. 2008; Basheer and Thenmozhi 2010). Precipitation steps are usually followed by chromatographic steps like gel filtration and affinity chromatography. Following these two is the hydrophobic interaction chromatography (HIC) (Nagarajan 2012). With respect to the desired purity of lipases, different steps from pre- to post-purification periods have involved the use of both physical and molecular technologies, respectively. Ammonium sulfate purification and organic solvent extraction have been used for certain applications just as Iftikhar et al. (2011) have also described a successful purification of lipases of up to 100 % with 710.02-fold. Economically, certain applications of lipases might not be achieved with just the usual procedures of precipitation steps, therefore further purification options are necessary (Gupta et al. 2004).
Industrial potential of cold-adapted lipase
Lipases are adaptable and increasing attention has been drawn to potential applications of psychrophilic lipases from microorganisms populating permanently cold environments (Pfeffer et al. 2007; Długołecka et al. 2008). Some biotechnological and industrial potential of cold-active lipases include the following.
Detergent additives
Lower washing temperature, improved energy conservation and minimization in wear and tear are obvious essential benefits of cold-active lipases in the detergent industries (Gerday et al. 2000). These enzymes have proven useful for cleaning applications that have potential to extend their effectiveness in enzyme-based, low-temperature cleaning formulations for laundry and dishwashers, reducing environmental burden and enabling the biodegradation of undesirable chemicals in detergents, leaving no harmful residues while ensuring environmental sustainability (Joseph et al. 2008). The ability of enzymes to clean effectively in detergents at low temperature has seen a reduction in temperature used for washing procedures in a range of industries. Cold-active lipase have been used as detergent formulations on porous building materials that ordinarily cannot be immersed or moved into cleaning solutions and other mold-infested surfaces reducing the damage normally associated with the use of standard cleaning agents (Valentini et al. 2010). Lipase preparations active at low and ambient temperatures in detergent removes oil stains by decomposing them into more hydrophilic substances while also maintaining the texture and quality of fabrics (Fujii et al. 1986; Weerasoriya and Kumarasinghe 2012).
Textile industry
Successive bio-washing and stone washing of fabric materials in the textile industries often reduce the smoothness of the tissues constituting the main fibers. Pretreatment with cold-adapted lipase reduces the pill-formation and increases the durability and softness of the tissue. They lower the temperature of the process conditions in textile industries. Mechanical resistance of final fabric quality is greatly enhanced as a result of the spontaneous rapid inactivation of cold-adapted enzymes at higher temperature. Enzymatic desizing of materials in fabric has advantages over the traditional process, which uses acid or oxidizing agents. For this purpose, bacterial lipases from Pseudomonas cepacia, Pseudomonas fragi, Pseudomonas fluorescens, Pseudomonas stutzeri has been used (Hasan et al. 2006).
Environmental bioremediation
Microorganisms have been efficiently used in the bioremediation and low molecular wastewater treatment in cold climates and in lowering the amount of toxic compounds previously considered non-degradable (Margesin and Feller 2010). Cold-adapted lipases are potentially applicable as bioremediation agents in wastewater treatment, in situ bioremediation of fat-contaminated cold environment, synthesis of organic compounds and lowering of toxic compounds in the environment such as nitrates, hydrocarbons, aromatic compounds, heavy metals and biopolymers such as cellulase, chitin, lignin, proteins and triacylglycerols (Wakelin and Forster 1997; Margesin and Schinner 1997, 1998; Timmis and Pieper 1999; van Langen et al. 1999; Suzuki et al. 2001; Margesin 2007). Strains preparations with applicable potential in the bioremediation of oil-polluted sites under the conditions of a cold climate have been investigated (Belousova and Shkidchenko 2004; Amara and Salem 2009).
Food industry
Maintaining the ambient temperature of food is a major way of preventing spoilage and deterioration and of minimizing undesirable changes in chemical and physical qualities of food which ordinarily could occur under high-temperature storage conditions. The relevance of cold-active lipase to the food and feed industry most importantly prevents spoilage and undesirable changes in nutritional compositions of most heat-sensitive substrates utilized in food processing (Russell 1998; Gerday et al. 2000; Cavicchioli et al. 2002). Despite their gains to the modern food industry, cold-adapted microorganism have also been implicated in the spoilage of refrigerated meat and raw milk, affecting the quality and shelf-life, thus making these foods unacceptable to consumers (Dieckelmann et al. 1998; Abdou 2003; Samaržija et al. 2012). Psychrotroph-derived lipases have also been applied in the non-aqueous synthesis of a model ester (butyl caprylate). Studies have revealed distinguishing characteristics of cold-active lipases with a strong potential for the organic synthesis of valuable short-chain esters such as flavors used in food and pharmaceuticals (Brault et al. 2012; Li et al. 2013).
Medical and pharmaceutical applications
Chiral intermediates and fine chemicals are in high demand both by pharmaceutical and agrochemical industries, for the preparation of bulk drug substances and agricultural products (Patel 2002). Synthesis and modification of optically pure chiral drugs have been well documented for lipases years ago (Margolin 1993) and recent advances in the synthesis of optically pure compounds have embraced biocatalytic procedures using lipases for the preparation of chiral pharmaceuticals, which offer a clean and ecological way of performing chemical processes in mild reaction conditions and with high degree of selectivity and these are playing an increasingly prominent role (Margolin 1993; Gotor-Fernández et al. 2006a). Simplicity of use, low cost, commercial availability and recycling possibility makes cold-active lipase ideal for the synthesis and resolution of a wide range of nitrogenated compounds in drug synthesis (Gotor-Fernández et al. 2006b).
Low water biocatalysis
Cold temperatures affect the dynamic activity of bulk water as well as the spheres of hydration surrounding the protein surface. The hydration energies of cold-active enzymes are generally less affected by lower temperatures, and their inherent lower surface hydrophobicity is less sensitive than mesophilic proteins, keeping their structures more intact (Fields 2001; Zhong et al. 2011).
Conclusion
Generally, lipase enzymes offer economic benefits in their industrial and biotechnological applications globally. Cold-adapted microorganisms have become potential targets for lipases exploited for numerous biotechnological gains based on their ability to withstand prevailing challenges of permanently cold habitats. Cold-active lipases represent a versatile group of bacterial extracellular enzymes. With adaptive-response strategies, the extreme environments have now become accessible to these cold-adapted psychrophilic enzymes. Researches on the extreme adaptations of cold-adapted microorganisms have stirred up keen interest among several research groups and R&D investment; and several hundreds of researches are ongoing to shed new light on other characteristics of these fascinating organisms that will be of applicable potential benefits.
References
Abdou AM (2003) Purification and partial characterization of psychrotrophic Serratia marcescens lipase. J Dairy Sci 86:127–132. doi:10.3168/jds.S0022-0302(03)73591-7
Adan Gökbulut A, Arslanoğlu A (2013) Purification and biochemical characterization of an extracellular lipase from psychrotolerant Pseudomonas fluorescens KE38. Turkish J Biol 37:538–546. doi:10.3906/biy-1211-10
Aghajari N, Feller G, Gerday C, Haser R (1996) Crystallization and preliminary X-ray diffraction studies of alpha-amylase from the antarctic psychrophile Alteromonas haloplanctis A23. Protein Sci 5:2128–2129. doi:10.1002/pro.5560050921
Alquati C, De Gioia L, Santarossa G et al (2002) The cold-active lipase of Pseudomonas fragi. Eur J Biochem 269:3321–3328. doi:10.1046/j.1432-1033.2002.03012.x
Amara AA, Salem SR (2009) Degradation of Castor Oil and Lipase Production by Pseudomonas aeruginosa. Am J Agric Environ Sci 5:556–563
Amico SD, Collins T, Marx J et al (2006) Psychrophilic microorganisms : challenges for life. EMBO Rep 7:5–9. doi:10.1038/sj.embor.7400662
Arnau J, Lauritzen C, Petersen GE, Pedersen J (2006) Reprint of: current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif 48:1–13. doi:10.1016/j.pep.2011.08.009
Basheer SA, Thenmozhi M (2010) Reverse micellar separation of lipases : a critical review. Int J Chem Sci 8:57–67
Belousova NI, Shkidchenko AN (2004) Low-temperature microbial degradation of oil products differing in the extent of condensation. Appl Biochem Microbiol 40:262–265. doi:10.1023/B:ABIM.0000025949.91999.b4
Berger F, Morellet N, Menu F, Potier P (1996) Cold shock and cold acclimation proteins in the psychrotrophic bacterium Arthrobacter globiformis SI55. J Bacteriol 178:2999–3007
Bowman J (2013) True psychrophiles and part time psychrophiles in the Antarctic
Brault G, Shareck F, Hurtubise Y et al (2012) Isolation and characterization of EstC, a new cold-active esterase from Streptomyces coelicolor A3(2). PLoS One 7:e32041. doi:10.1371/journal.pone.0032041
Bucky AR, Robinsont DS, Hayes PR (1987) Factors Affecting the Heat Stability of Lipase Produced by a Strain of Pseudomonas fluorescens. Food Chem 23:159–173
Buzzini P, Branda E, Goretti M, Turchetti B (2012) Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol Ecol 82:217–241. doi:10.1111/j.1574-6941.2012.01348.x
Cavicchioli R (2006) Cold-adapted archaea. Nat Rev Microbiol 4:331–343. doi:10.1038/nrmicro1390
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13:253–261. doi:10.1016/S0958-1669(02)00317-8
Cavicchioli R, Charlton T, Ertan H et al (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4:449–460. doi:10.1111/j.1751-7915.2011.00258.x
Chen R, Guo L, Dang H (2010) Gene cloning, expression and characterization of a cold-adapted lipase from a psychrophilic deep-sea bacterium Psychrobacter sp. C18. World J Microbiol Biotechnol 27:431–441. doi:10.1007/s11274-010-0475-7
Choo D, Kurihara T, Suzuki T, Soda K (1998a) A Cold-Adapted Lipase of an Alaskan Psychrotroph, Pseudomonas sp. Strain B11–1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol 64:1–7
Choo DW, Kurihara T, Suzuki T et al (1998b) A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol 64:486–491
Cieśliński H, Kur J, Białkowska A et al (2005) Cloning, expression, and purification of a recombinant cold-adapted beta-galactosidase from antarctic bacterium Pseudoalteromonas sp. 22b. Protein Expr Purif 39:27–34. doi:10.1016/j.pep.2004.09.002
Cummings SP, Black GW (1999) Polymer hydrolysis in a cold climate. Extremophiles 3:81–87. doi:10.1007/s007920050102
De Abreu L, Fernandez-Lafuente R, Rodrigues RC et al (2014) Efficient purification-immobilization of an organic solvent-tolerant lipase from Staphylococcus warneri EX17 on porous styrene-divinylbenzene beads. J Mol Catal B Enzym 99:51–55. doi:10.1016/j.molcatb.2013.10.018
De Pascale D, Cusano AM, Autore F et al (2008) The cold-active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family. Extremophiles 12:311–323. doi:10.1007/s00792-008-0163-9
Deming JW (2002) Psychrophiles and polar regions. Curr Opin Microbiol 5:301–309
Dieckelmann M, Johnson La, Beacham IR (1998) The diversity of lipases from psychrotrophic strains of Pseudomonas: a novel lipase from a highly lipolytic strain of Pseudomonas fluorescens. J Appl Microbiol 85:527–536
Długołecka A, Cieśliński H, Turkiewicz M et al (2008) Extracellular secretion of Pseudoalteromonas sp. cold-adapted esterase in Escherichia coli in the presence of Pseudoalteromonas sp. components of ABC transport system. Protein Expr Purif 62:179–184. doi:10.1016/j.pep.2008.07.006
Do H, Lee JH, Kwon MH et al (2013) Purification, characterization and preliminary X-ray diffraction analysis of a cold-active lipase (CpsLip) from the psychrophilic bacterium Colwellia psychrerythraea 34H. Acta Crystallogr, Sect F: Struct Biol Cryst Commun 69:920–924. doi:10.1107/S1744309113019428
Dumon-Seignovert L, Cariot G, Vuillard L (2004) The toxicity of recombinant proteins in Escherichia coli: a comparison of overexpression in BL21(DE3), C41(DE3), and C43(DE3). Protein Expr Purif 37:203–206. doi:10.1016/j.pep.2004.04.025
Duong F, Soscia C, Lazdunski A, Murgier M (1994) The Pseudomonas fluorescens lipase has a C-terminal secretion signal and is secreted by a three-component bacterial ABC-exporter system. Mol Microbiol 11:1117–1126
Durmaz E, Kuyucak S, Sezerman UO (2013) Modifying the catalytic preference of tributyrin in Bacillus thermocatenulatus lipase through in silico modeling of enzyme-substrate complex. Protein Eng Des Sel 26:325–333. doi:10.1093/protein/gzt004
Dutra JCV, da Terzi SC, Bevilaqua JV et al (2008) Lipase production in solid-state fermentation monitoring biomass growth of Aspergillus niger using digital image processing. Appl Biochem Biotechnol 147:63–75. doi:10.1007/s12010-007-8068-0
Einhauer A, Jungbauer A (2001) The FLAG™ peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Biophys Methods 49:455–465. doi:10.1016/S0165-022X(01)00213-5
Ellaiah P, Prabhakar T, Ramakrishna B et al (2004) Production of lipase by immobilized cells of Aspergillus niger. Process Biochem 39:525–528
Emond S, Montanier C, Nicaud J-M et al (2010) New efficient recombinant expression system to engineer Candida Antarctica lipase B. Appl Environ Microbiol 76:2684–2687. doi:10.1128/AEM.03057-09
Ewert M, Deming J (2013) Sea Ice Microorganisms: environmental Constraints and Extracellular Responses. Biology Basel 2:603–628. doi:10.3390/biology2020603
Feller G (2013) Psychrophilic Enzymes: from Folding to Function and Biotechnology. Scientifica (Cairo) 2013:512840. doi:10.1155/2013/512840
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208. doi:10.1038/nrmicro773
Feller G, Thiry M, Arpignya JL, Gerday C (1991) Cloning and expression in Escherichia antarctic strain Moraxellu TA144 c & i of three lipase-encoding genes from the psychrotrophic. Gene 102:111–115
Fields PA (2001) Review: protein function at thermal extremes: balancing stability and flexibility. Comp Biochem Physiol A: Mol Integr Physiol 129:417–431
Francis MD, Rebecca P (2010) Strategies to optimize protein expression in E. coli. Curr. Protoc. Protein Sci 61:5–24. doi:10.1002/ps0524s61
Fujii T, Tatara T, Minagawa M (1986) Studies on applications of lipolytic enzyme in detergency I. Effect of lipase from Candida cylindracea on removal of olive oil from cotton fabric. J Am Oil Chem Soc 63:796–799. doi:10.1007/BF02541967
Gerday C, Aittaleb M, Arpigny JL et al (1997) Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta 1342:119–131
Gerday C, Aittaleb M, Bentahir M et al (2000) Cold-adapted enzymes: from fundamentals to biotechnology. Trends Biotechnol 18:103–107
Gianese G, Argos P, Pascarella S (2001) Structural adaptation of enzymes to low temperatures. Protein Eng 14:141–148
Gilbert JA, Hill PJ, Dodd CER, Laybourn-Parry J (2004) Demonstration of antifreeze protein activity in Antarctic lake bacteria. Microbiology 150:171–180
Gotor-Fernández V, Brieva R, Gotor V (2006a) Lipases: useful biocatalysts for the preparation of pharmaceuticals. J Mol Catal B Enzym 40:111–120
Gotor-Fernández V, Busto E, Gotor V (2006b) Candida antarctica Lipase B: an Ideal Biocatalyst for the Preparation of Nitrogenated Organic Compounds. Adv Synth Catal 348:797–812. doi:10.1002/adsc.200606057
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781. doi:10.1007/s00253-004-1568-8
Hannig G, Makrides SC (1998) Strategies for optimizing heterologous protein expression in Escherichia coli. Focus (Madison) 16:54–60
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzyme Microb Technol 39:235–251. doi:10.1016/j.enzmictec.2005.10.016
Hosseinpour MN (2012) Lipase Production in Solid State Fermentation Using Aspergillus niger: response Surface Methodology. Int J Eng 25:151–160. doi:10.5829/idosi.ije.2012.25.03b.01
Iftikhar T, Niaz M, Jabeen R, Haq IUL (2011) Purification and Characterization of Extracellular Lipases. Pak J Bot 43:1541–1545
Imbert M, Gancel F (2004) Effect of different temperature downshifts on protein synthesis by Aeromonas hydrophila. Curr Microbiol 49:79–83. doi:10.1007/s00284-004-4277-8
Jadhav VV, Pote SS, Yadav A et al (2013) Extracellular cold active lipase from the psychrotrophic Halomonas sp. BRI 8 isolated from the Antarctic sea water. Songklanakarin J Sci Technol 35:623–630
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol 53:315–351. doi:10.1146/annurev.micro.53.1.315
Jeon JH, Kim J-T, Kim YJ et al (2009) Cloning and characterization of a new cold-active lipase from a deep-sea sediment metagenome. Appl Microbiol Biotechnol 81:865–874. doi:10.1007/s00253-008-1656-2
Jeon JH, Kim JT, Lee HS et al (2011) Novel lipolytic enzymes identified from metagenomic library of deep-sea sediment. Evid Based Complement Alternat Med 2011:1–9. doi:10.1155/2011/271419->
Joseph B, Ramteke PW, Kumar PA (2006) Studies on the enhanced production of extracellular lipase by Staphylococcus epidermidis. J Gen Appl Microbiol 52:315–320
Joseph B, Ramteke PW, Thomas G (2008) Cold active microbial lipases: some hot issues and recent developments. Biotechnol Adv 26:457–470
Joseph B, Upadhyaya S, Ramteke P (2011) Production of Cold-Active Bacterial Lipases through Semisolid State Fermentation Using Oil Cakes. Enzyme Res 2011:796407. doi:10.4061/2011/796407
Juhl PB, Doderer K, Hollmann F et al (2010) Engineering of Candida antarctica lipase B for hydrolysis of bulky carboxylic acid esters. J Biotechnol 150:474–480. doi:10.1016/j.jbiotec.2010.09.951
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507–510. doi:10.1038/35054051
Kavitha M, Shanthi C (2013) Isolation and Characterization of Cold active lipase producing Pseudomonas sp. 4 from Marine samples of Tamilnadu Coast. Res J Biotechnol 8:57–62
Kennedy J, Marchesi JR, Dobson AD (2008) Marine metagenomics: strategies for the discovery of novel enzymes with biotechnological applications from marine environments. Microb Cell Fact 7:27. doi:10.1186/1475-2859-7-27
Kim YO, Khosasih V, Nam B-H et al (2012) Gene cloning and catalytic characterization of cold-adapted lipase of Photobacterium sp. MA1-3 isolated from blood clam. J Biosci Bioeng 114:589–595. doi:10.1016/j.jbiosc.2012.06.013
Kim Y, Heo YL, Nam B et al (2013) Molecular Cloning, Purification, and Characterization of a Cold-Adapted Esterase from Photobacterium sp. MA1-3. Fish Aquat Sci 16:311–318
Krembs C, Eicken H, Junge K, Deming J (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res Part I Oceanogr Res Pap 49:2163–2181
Kulakova L, Galkin A, Nakayama T et al (2004) Cold-active esterase from Psychrobacter sp. Ant300: gene cloning, characterization, and the effects of Gly → Pro substitution near the active site on its catalytic activity and stability. Biochim Biophys Acta - Proteins Proteomics 1696:59–65. doi:10.1016/j.bbapap.2003.09.008
Kumar SS, Gupta R (2008) An extracellular lipase from Trichosporon asahii MSR 54: medium optimization and enantioselective deacetylation of phenyl ethyl acetate. Process Biochem 43:1054–1060. doi:10.1016/j.procbio.2008.05.017
Lee H, Ahn M, Kwak S et al (2003) Purification and characterization of cold active lipase from psychrotrophic Aeromonas sp. LPB 4. J Microbiol 41:22–27
Leonov SL (2010) Screening for Novel Cold-Active Lipases from wild type bacteria isolates. Innov Rom Food Biotechnol 6:12–17
Li M, Yang L-R, Xu G, Wu J-P (2013) Screening, purification and characterization of a novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254. Bioresour Technol 148:114–120. doi:10.1016/j.biortech.2013.08.101
Lo Giudice A, Michaud L, de Pascale D et al (2006) Lipolytic activity of Antarctic cold-adapted marine bacteria (Terra Nova Bay, Ross Sea). J Appl Microbiol 101:1039–1048. doi:10.1111/j.1365-2672.2006.03006.x
Mander P, Cho SS, Simkhada JR et al (2012) An organic solvent–tolerant lipase from Streptomyces sp. CS133 for enzymatic transesterification of vegetable oils in organic media. Process Biochem 47:635–642. doi:10.1016/j.procbio.2012.01.003
Männistö MK, Häggblom MM (2006) Characterization of psychrotolerant heterotrophic bacteria from Finnish Lapland. Syst Appl Microbiol 29:229–243. doi:10.1016/j.syapm.2005.09.001
Maraite A, Hoyos P, Carballeira JD et al (2013) Lipase from Pseudomonas stutzeri: purification, homology modelling and rational explanation of the substrate binding mode. J Mol Catal B Enzym 87:88–98. doi:10.1016/j.molcatb.2012.11.005
Margesin R (2007) Alpine microorganisms: useful tools for low-temperature bioremediation. J Microbiol 45:281–285
Margesin R, Feller G (2010) Biotechnological applications of psychrophiles. Environ Technol 31:835–844. doi:10.1080/09593331003663328
Margesin R, Schinner F (1997) Efficiency of indigenous and inoculated cold-adapted soil microorganisms for biodegradation of diesel oil in alpine soils. Appl Environ Microbiol 63:2660–2664
Margesin R, Schinner F (1998) Low-temperature bioremediation of a waste water contaminated with anionic surfactants and fuel oil. Appl Microbiol Biotechnol 49:482–486. doi:10.1007/s002530051202
Margesin R, Neuner G, Storey KB (2007) Cold-loving microbes, plants, and animals–fundamental and applied aspects. Naturwissenschaften 94:77–99. doi:10.1007/s00114-006-0162-6
Margolin AL (1993) Enzymes in the synthesis of chiral drugs. Enzyme Microb Technol 15:266–280
Médigue C, Krin E, Pascal G et al (2005) Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res 15:1325–1335. doi:10.1101/gr.4126905
Methé BA, Nelson KE, Deming JW et al (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci U S A 102:10913–10918. doi:10.1073/pnas.0504766102
Mohamad Ali MS, Mohd FSF, Ganasen M et al (2013) Structural adaptation of cold-active RTX lipase from Pseudomonas sp. strain AMS8 revealed via homology and molecular dynamics simulation approaches. Biomed Res Int 2013:925373. doi:10.1155/2013/925373
Mohammed S, Te’o J, Nevalainen H (2013) A gene encoding a new cold-active lipase from an Antarctic isolate of Penicillium expansum. Curr Genet 59:129–137. doi:10.1007/s00294-013-0394-x
Morgan-kiss RM, Priscu JC, Pocock T et al (2006) Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Mol Biol Rev 70:222–252. doi:10.1128/MMBR.70.1.222
Morita Y, Nakamura T, Hasan Q et al (1997) Cold-active enzymes from cold-adapted bacteria. J Am Oil Chem Soc 74:441–444. doi:10.1007/s11746-997-0103-3
Moussavou Mounguengui RW, Brunschwig C, Baréa B et al (2013) Are plant lipases a promising alternative to catalyze transesterification for biodiesel production? Prog Energy Combust Sci 39:441–456. doi:10.1016/j.pecs.2013.05.003
Mujacic M, Cooper KW, Baneyx F (1999) Cold-inducible cloning vectors for low-temperature protein expression in Escherichia coli: application to the production of a toxic and proteolytically sensitive fusion protein. Gene 238:325–332. doi:10.1016/S0378-1119(99)00328-5
Muryoi N, Sato M, Kaneko S et al (2004) Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. J Bacteriol 186:5661–5671. doi:10.1128/JB.186.17.5661-5671.2004
Nagarajan S (2012) New tools for exploring “old friends-microbial lipases”. Appl Biochem Biotechnol 168:1163–1196. doi:10.1007/s12010-012-9849-7
Nogi Y, Masui N, Kato C (1998) Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles 2:1–7
Novototskaya-Vlasova K, Petrovskaya L, Kryukova E et al (2013) Expression and chaperone-assisted refolding of a new cold-active lipase from Psychrobacter cryohalolentis K5(T). Protein Expr Purif 91:96–103. doi:10.1016/j.pep.2013.07.011
Okuzumi M, Himishi A, Kobayashi T, Fujii T (1994) Photobacterium histaminum sp. nov., a Histamine-Producing Marine Bacterium. Int J Syst Bacteriol 44:631–636
Onarheim AM, Wiik R, Burghardt J, Stackebrandt E (1994) Characterization and identification of two vibrio species indigenous to the intestine of fish in cold sea water. Syst Appl Microbiol 17:370–379
Palomo JM, Muñoz G, Fernández-Lorente G, Mateo C, Fuentes M, Guisan JM, Fernández-Lafuente R (2003) Modulation of Mucor miehei lipase properties via directed immobilization on different hetero-functional epoxy resins: hydrolytic resolution of (R, S)-2-butyroyl-2-phenylacetic acid. J Mol Catal B Enzym 21:201–210
Pandey A, Benjamin S, Soccol CR et al (1999) The realm of microbial lipases in biotechnology. Biotechnol Appl Biochem 29(Pt 2):119–131
Pandey KD, Shukla SP, Shukla PN et al (2004) Cyanobacteria in Antarctica: ecology, physiology and cold adaptation. Cell Mol Biol (Noisy-le-grand) 50:575–584
Park Y-D, Baik KS, Seong CN et al (2006) Photobacterium ganghwense sp. nov., a halophilic bacterium isolated from sea water. Int J Syst Evol Microbiol 56:745–749. doi:10.1099/ijs.0.63811-0
Park I-H, Kim S-H, Lee Y-S et al (2009) Gene cloning, purification, and characterization of a cold-adapted lipase produced by Acinetobacter baumannii BD5. J Microbiol Biotechnol 19:128–135. doi:10.4014/jmb.0802.130
Parra LP, Reyes F, Acevedo JP et al (2008) Cloning and fusion expression of a cold-active lipase from marine Antarctic origin. Enzyme Microb Technol 42:371–377. doi:10.1016/j.enzmictec.2007.11.003
Patel RN (2002) Microbial/enzymatic synthesis of chiral intermediates for pharmaceuticals. Enzyme Microb Technol 31:804–826. doi:10.1016/S0141-0229(02)00186-2
Peeters K, Verleyen E, Hodgson Da et al (2011) Heterotrophic bacterial diversity in aquatic microbial mat communities from Antarctica. Polar Biol 35:543–554. doi:10.1007/s00300-011-1100-4
Pfeffer J, Rusnak M, Hansen C-E et al (2007) Functional expression of lipase A from Candida antarctica in Escherichia coli—a prerequisite for high-throughput screening and directed evolution. J Mol Catal B Enzym 45:62–67. doi:10.1016/j.molcatb.2006.11.006
Rabus R, Ruepp A, Frickey T et al (2004) The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ Microbiol 6:887–902. doi:10.1111/j.1462-2920.2004.00665.x
Rajendran A, Palanisamy A, Thangavelu V (2009) Lipase catalyzed ester synthesis for food processing industries. Brazilian Arch Biol Technol 52:207–219
Ramani K, Chockalingam E, Sekaran G (2010) Production of a novel extracellular acidic lipase from Pseudomonas gessardii using slaughterhouse waste as a substrate. J Ind Microbiol Biotechnol 37:531–535. doi:10.1007/s10295-010-0700-2
Ramteke PW, Joseph B, Kuddus M (2005) Extracellular lipases from anaerobic microorganisms of Antarctic. Indian J Biotechnol 4:293–294
Rashid N, Shimada Y, Ezaki S et al (2001) Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Appl Environ Microbiol 67:4064–4069
Reddy GSN, Matsumoto GI, Schumann P et al (2004) Psychrophilic pseudomonads from Antarctica: pseudomonas Antarctica sp. nov., Pseudomonas meridiana sp. nov. and Pseudomonas proteolytica sp. nov. Int J Syst Evol Microbiol 54:713–719. doi:10.1099/ijs.0.02827-0
Reetz MT, Soni P, Fernández L et al (2010) Increasing the stability of an enzyme toward hostile organic solvents by directed evolution based on iterative saturation mutagenesis using the B-FIT method. Chem Commun (Camb) 46:8657–8658. doi:10.1039/c0cc02657c
Russell NJ (1998) Molecular adaptations in psychrophilic bacteria: Potential for biotechnological applications. 61:1–21. doi:10.1007/BFb0102286
Russo R, Giordano D, Riccio A et al (2010) Cold-adapted bacteria and the globin case study in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Mar Genomics 3:125–131. doi:10.1016/j.margen.2010.09.001
Ryu HS, Kim HK, Choi WC et al (2006) New cold-adapted lipase from Photobacterium lipolyticum sp. nov. that is closely related to filamentous fungal lipases. Appl Microbiol Biotechnol 70:321–326. doi:10.1007/s00253-005-0058-y
Samaržija D, Zamberlin Š, Pogačić T (2012) Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo 62:77–95
Sattler B, Puxbaum H, Psenner R (2001) Bacterial growth in supercooled cloud droplets. Geophys Res Lett 28:239–242. doi:10.1029/2000GL011684
Schlegel S, Rujas E, Ytterberg AJ et al (2013) Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels. Microb Cell Fact 12:1–12
Seo JB, Kim HS, Jung GY et al (2004) Psychrophilicity of Bacillus psychrosaccharolyticus: a proteomic study. Proteomics 4:3654–3659. doi:10.1002/pmic.200401025
Seo HJ, Bae SS, Yang SH et al (2005) Photobacterium aplysiae sp. nov., a lipolytic marine bacterium isolated from eggs of the sea hare Aplysia kurodai. Int J Syst Evol Microbiol 55:2293–2296. doi:10.1099/ijs.0.63765-0
Sharma R, Chisti Y, Banerjee UC (2001) Production, purification, characterization, and applications of lipases. Biotechnol Adv 19:627–662
Shieh WY, Chen Y-W, Chaw S-M, Chiu H-H (2003) Vibrio ruber sp. nov., a red, facultatively anaerobic, marine bacterium isolated from sea water. Int J Syst Evol Microbiol 53:479–484. doi:10.1099/ijs.0.02307-0
Shivaji S, Reddy GSN, Raghavan PUM et al (2004) Psychrobacter salsus sp. nov. and Psychrobacter adeliensis sp. nov. Isolated from Fast Ice from Adelie Land. Antarctica. Syst Appl Microbiol 27:628–635
Shuo-shuo C, Xue-zheng L, Ji-hong S (2011) Effects of co-expression of molecular chaperones on heterologous soluble expression of the cold-active lipase Lip-948. Protein Expr Purif 77:166–172. doi:10.1016/j.pep.2011.01.009
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433. doi:10.1146/annurev.biochem.75.103004.142723
Siddiqui KS, Williams TJ, Wilkins D et al (2013) Psychrophiles. Annu Rev Earth Planet Sci 41:87–115. doi:10.1146/annurev-earth-040610-133514
Sohn JH, Lee J-H, Yi H et al (2004) Kordia algicida gen. nov., sp. nov., an algicidal bacterium isolated from red tide. Int J Syst Evol Microbiol 54:675–680. doi:10.1099/ijs.0.02689-0
Srinivas TNR, Vijaya Bhaskar Y, Bhumika V, Anil Kumar P (2013) Photobacterium marinum sp. nov., a marine bacterium isolated from a sediment sample from Palk Bay. India. Syst Appl Microbiol 36:160–165. doi:10.1016/j.syapm.2012.12.002
Stergiou P-Y, Foukis A, Filippou M et al (2013) Advances in lipase-catalyzed esterification reactions. Biotechnol Adv 31:1846–1859. doi:10.1016/j.biotechadv.2013.08.006
Suzuki T, Nakayama T, Kurihara T et al (2001) Cold-active lipolytic activity of psychrotrophic Acinetobacter sp. strain no. 6. J Biosci Bioeng 92:144–148
Suzuki Y, Haruki M, Takano K et al (2004) Possible involvement of an FKBP family member protein from a psychrotrophic bacterium Shewanella sp. SIB1 in cold-adaptation. Eur J Biochem 271:1372–1381. doi:10.1111/j.1432-1033.2004.04049.x
Thakur S (2012) Lipases, its sources, properties and applications: a Review. Int J Sci Eng Res 3:1–29
Thomas DN, Dieckmann GS (2002) Antarctic sea ice–a habitat for extremophiles. Science 80(295):641–644. doi:10.1126/science.1063391
Timmis KN, Pieper DH (1999) Bacteria designed for bioremediation. Trends Biotechnol 17:201–204. doi:10.1016/S0167-7799(98)01295-5
Tran D-T, Lin Y-J, Chen C-L, Chang J-S (2013) Kinetics of transesterification of olive oil with methanol catalyzed by immobilized lipase derived from an isolated Burkholderia sp. strain. Bioresour Technol 145:193–203. doi:10.1016/j.biortech.2013.01.146
Tranter M, Fountain AG, Fritsen CH et al (2004) Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrol Process 18:379–387. doi:10.1002/hyp.5217
Treichel H, de Oliveira D, Mazutti MA, Di Luccio M, Oliveira Jv (2010) A review on microbial lipases production. Food Bioprocess Technol 3:182–196
Trotsenko YA, Khmelenina VN (2005) Aerobic methanotrophic bacteria of cold ecosystems. FEMS Microbiol Ecol 53:15–26. doi:10.1016/j.femsec.2005.02.010
Uppenberg J, Ohrner N, Norin M et al (1995) Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 34:16838–16851
Valentini F, Diamanti A, Palleschi G (2010) New bio-cleaning strategies on porous building materials affected by biodeterioration event. Appl Surf Sci 256:6550–6563. doi:10.1016/j.apsusc.2010.04.046
Van Langen LM, de Vroom E, van Rantwijk F, Sheldon R (1999) Enzymatic synthesis of beta-lactam antibiotics using penicillin-G acylase in frozen media. FEBS Lett 456:89–92
Wakelin NG, Forster CF (1997) An investigation into microbial removal of fats, oils and greases. Bioresour Technol 59:37–43. doi:10.1016/S0960-8524(96)00134-4
Wang Q, Zhang C, Hou Y et al (2013) Optimization of cold-active lipase production from Psychrophilic Bacterium Moritella sp. 2-5-10-1 by statistical experimental methods. Biosci Biotechnol Biochem 77:17–21. doi:10.1271/bbb.120104
Weerasoriya MKB, Kumarasinghe AAN (2012) Isolation of alkaline lipase from rubber seed—Partial purification, characterization and its potential applications as a detergent additive. Indian J Chem Technol 19:244–249
Wei X, Jiang X, Ye L et al (2013) Cloning, expression and characterization of a new enantioselective esterase from a marine bacterium Pelagibacterium halotolerans B2T. J Mol Catal B Enzym 97:270–277. doi:10.1016/j.molcatb.2013.09.002
Weickert MJ, Doherty DH, Best EA, Olins PO (1996) Optimization of heterologous protein production in Escherichia coli. Curr Opin Biotechnol 7:494–499
Wu G, Wu G, Zhan T et al (2013) Characterization of a cold-adapted and salt-tolerant esterase from a psychrotrophic bacterium Psychrobacter pacificensis. Extremophiles 17:809–819. doi:10.1007/s00792-013-0562-4
Xu Y, Nogi Y, Kato C et al (2003) Moritella profunda sp. nov. and Moritella abyssi sp. nov., two psychropiezophilic organisms isolated from deep Atlantic sediments. Int J Syst Evol Microbiol 53:533–538. doi:10.1099/ijs.0.02228-0
Xuezheng L, Shuoshuo C, Guoying X et al (2010) Cloning and heterologous expression of two cold-active lipases from the Antarctic bacterium Psychrobacter sp. G. Polar Res 29:421–429. doi:10.1111/j.1751-8369.2010.00189.x
Yayanos AA (1995) Microbiology to 10,500 meters in the deep sea. Annu Rev Microbiol 49:777–805. doi:10.1146/annurev.mi.49.100195.004021
Yoo H-Y, Simkhada JR, Cho SS et al (2011) A novel alkaline lipase from Ralstonia with potential application in biodiesel production. Bioresour Technol 102:6104–6111. doi:10.1016/j.biortech.2011.02.046
Yoon J-H, Lee J-K, Kim Y-O, Oh T-K (2005) Photobacterium lipolyticum sp. nov., a bacterium with lipolytic activity isolated from the Yellow Sea in Korea. Int J Syst Evol Microbiol 55:335–339. doi:10.1099/ijs.0.63215-0
Yujun W, Jian X, Guangsheng L, Youyuan D (2008) Immobilization of lipase by ultrafiltration and cross-linking onto the polysulfone membrane surface. Bioresour Technol 99:2299–2303. doi:10.1016/j.biortech.2007.05.014
Yumoto I, Hirota K, Sogabe Y et al (2003) Psychrobacter okhotskensis sp. nov., a lipase-producing facultative psychrophile isolated from the coast of the Okhotsk Sea. Int J Syst Evol Microbiol 53:1985–1989. doi:10.1099/ijs.0.02686-0
Zhang J-W, Zeng R-Y (2008) Molecular cloning and expression of a cold-adapted lipase gene from an Antarctic deep sea psychrotrophic bacterium Pseudomonas sp. 7323. Mar Biotechnol (NY) 10:612–621. doi:10.1007/s10126-008-9099-4
Zhang J, Lin S, Zeng R (2007) Cloning, expression, and characterization of a cold-adapted lipase gene from an antarctic deep-sea psychrotrophic bacterium, Psychrobacter sp 7195. J Microbiol Biotechnol 17:604–610
Zhong D, Kumar S, Zewail AH (2011) Biological water: a critique. Chem Phys Lett 503:1–11. doi:10.1016/j.cplett.2010.12.077
Acknowledgments
This research was supported by the FRGS (03-10-10-965FR) under the Ministry of Higher Education, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Rights and permissions
About this article
Cite this article
Maiangwa, J., Ali, M.S.M., Salleh, A.B. et al. Adaptational properties and applications of cold-active lipases from psychrophilic bacteria. Extremophiles 19, 235–247 (2015). https://doi.org/10.1007/s00792-014-0710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0710-5