Abstract
Flake ice refrigeration is a conventional but commonly employed technique to slow down fish spoilage and deterioration of nutritional values and sensory properties during chilled storage. In the present study, a methanolic extract of red alga Gracilaria verrucosa was characterised, and functional groups, such as alkenes, aldehydes, nitriles, galactans and galactose-4-sulphate were found. Subsequent identification of active compounds revealed the presence of potent preservative agents, such as butylated hydroxytoluene, sulfurous acid, 1,2-propanediol, benzeneacetic acid, cyclononasiloxane and tetracosamethyl-cyclododecasiloxane. The effect of incorporating G. verrucosa at two different concentrations (0.67 and 2.5 g lyophilised alga/L aqueous solution) in the icing medium was tested for the preservation of Indian mackerel (Rastrelliger kanagurta), compared to preservation in traditional ice prepared only from water. Microbial, chemical and sensory qualities were monitored in Indian mackerel chilled in ice with and without G. verrucosa during 15 days storage period. Inhibitory effects (p < 0.05) on the microbial proliferation (mesophilic and psychrophilic bacteria) and chemical markers of fish deterioration (pH, TVB-N, TMA-N and biogenic amines) were evidenced for ice containing both concentrations of G. verrucosa, respectively, relative to the control medium. The sensory score acceptability limit reached 11 days for Indian mackerel stored in traditional ice and 15 days for Indian mackerel stored in ice with G. verrucosa extract. Thus, the icing medium containing G. verrucosa extract improves the quality and safety of Indian mackerel during storage and can be explored by the seafood industry as a biopreservative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indian mackerel (Rastrelliger kanagurta) is a pelagic fish species belonging to the family Scombridae. It is quite abundant on the Indian coast and is a commercially valuable fish due to its high nutritional value and relatively low price. It contributes 7.2% to the total marine fish landing in India and forms the mainstay pelagic fishery along the Indian coast, after sardines (Sofi et al. 2015). Indian mackerel is highly perishable, because of its high susceptibility to lipid oxidation. In dark-fleshed fatty fishes, the onset of oxidation and rancidity occurs rapidly, probably because of the coexisting large amounts of haemoglobin (a well-known activator of lipid oxidation) and lipids (Viji et al. 2015; Viji et al. 2016). Moreover, there are other damage pathways, such as microbiological proliferation, increases in biogenic amines and total volatile base nitrogen content (TVB-N), and decreases in nutritional composition and sensory score.
To prevent this deterioration, flake ice refrigeration is the most commonly employed method to slow down seafood spoilage during short periods of transportation and storage. However, because of the limited shelf life of marine species, icing has been applied in combination with other preservative strategies to improve the shelf life of fish (Miranda et al. 2016a). Among them, several physical, chemical and biological treatments and their combination with packaging have been studied (Senturk and Alpas 2013; Yuan et al. 2016). Additionally, the incorporation of natural preservative compounds such as organic acids (citric or lactic acids) (Garcia-Soto et al. 2014), halophytes (seaweeds Bifurcaria bifurcata and Fucus spiralis) (Miranda et al. 2016a; Miranda et al. 2016b), non-halophytes (mint, pomegranate, grape seed, papaya seed and citrus peel) (Shinde et al. 2015; Sofi et al. 2015; Viji et al. 2015; Viji et al. 2016), herbs (thyme, rosemary, sage, dill, laurel and oregano) (Ozyurt et al. 2012; Ozogul et al. 2017) and ozone (Pastoriza et al. 2008) in the ice medium and chilled storage are considered important preservation methods in order to avoid chemical preservatives.
Macro-algae have been traditionally consumed as part of the diet in Japan, China and Korea, and they constitute a relevant source of nutrients, such as proteins, carbohydrates, fatty acids, vitamins, amino acids, minerals, lipids, dietary fibre and polysaccharides (Sakthivel and Pandima Devi 2015; Chan and Mantanjun 2017). According to European Council Regulation 258/ 1997, marine algae are considered as a food or food ingredient, so their use in the icing medium does not pose a health problem for consumers. However, the use of algae in Western countries has been traditionally focused on the extraction of bioactive compounds of relevance for pharmaceutical, seafood processing and biotechnological industries. Red seaweeds offer the possibility of exploring a wide number of natural bioactive compounds with both antioxidant (Jaswir et al. 2014; Peinado et al. 2014) and antibacterial (Abu et al. 2011; Anantharaman et al. 2011; Jaswir et al. 2014) properties, which could be used to avoid seafood spoilage and enhance shelf life. Moreover, a large number of preservative compounds, such as phenolic and polyphenolic compounds, alkaloids, flavonoids, steroids, terpenes, alkanes and ketones have been isolated from marine algae (Peinado et al. 2014; Maftuch et al. 2016). In this sense, previous researchers have recounted the preservative effect of algal extracts on fish products, such as chilled cod muscle (Wang et al. 2010) and canned Atlantic salmon (Ortiz et al. 2014).
Among the red algae, recent works report Gracilaria verrucosa as having a high content of bioactive compounds, such as flavonoids, quercetin-7-methyl ether (Maftuch et al. 2016), antioxidants, phenols (Gouda et al. 2013; Widowati et al. 2014) and polysaccharides with antibacterial activity (Saraswaty et al. 2015). Additionally, the anticancer, anti-inflammatory and anti-postmenopausal osteoporosis activities of these algae have been described from in vitro and in vivo studies (Lee et al. 2013; Jaswir et al. 2014; Saraswaty et al. 2015; Kim et al. 2017). However, although natural preservative compounds that improve the shelf life of fresh fish have been the focus of intense study in recent years, and the fact that G. verrucosa demonstrates antibacterial activity (Matfuch et al., Maftuch et al. 2016; Widowati et al. 2014), to the best of our knowledge, no data are yet available about G. verrucosa as a fish preservative. Hence, this study was carried out to investigate the effects of iced G. verrucosa extract on biogenic amine formation, biochemical changes and bacterial activity in Indian mackerel muscle during 15 days of chilled storage.
Materials and Methods
Sample Collection
G. verrucosa samples were collected from the Thondi coast and washed with potable water to remove sand and other debris. Seaweeds were identified using the standard manual of the Central Marine Fisheries Research Institute (CMFRI) publication number 62 (Kaliyaperumal et al. 1995). Identified samples were transported to the laboratory and dried at room temperature under shade for 96 h, after which they were powdered. The powdered algae (10 g) was transferred into a 100-ml volumetric flask and 50% ethanol-water (v/v) was added up to the mark. The mixture was stirred for 10 min and afterwards, it was filtered through Whatman paper no 2 under suction. The filtrate was then centrifuged (30 min, 3000g, 4 °C) to obtain a clear supernatant liquid, which was employed for the characterisation of total phenol content and presence of active compounds.
Determination of Total Phenol Content and Antioxidant Properties of the Extract
The total phenol content of lyophilised G. verrucosa was spectrophotometrically assessed (UV-Vis 2450 spectrophotometer; Shimadzu, Japan) using the Folin–Ciocalteu reagent method, as described by Lim et al. (2007). Briefly, 0.5 mL of supernatant was added to a test tube containing 2.25 mL methanol. Afterwards, 0.22 mL Folin–Ciocalteu reagent was added, and the mixture was then stirred for 1 min and allowed to stand for 8 min. Next, 2.0 mL of 7.5% w/v sodium carbonate was added to each tube, and the mixtures were incubated at 25 °C for 120 min. The spectrophotometric absorbance (optical density) of the supernatants and a blank was measured at 756 nm. The concentration of total phenolic compounds in the extract was determined by T = C × V/M, where T = total phenolic content (mg/g) of G. verrucosa in gallic acid equivalents (GAE/g lyophilised alga); C = concentration of gallic acid from the calibration curve (mg/mL); V = volume of the extract (mL); and M = weight of the seaweed extract (g) (Abdelhady et al. 2011).
Antioxidant activity was determined by means of the determination of the 1,1-Diphenyl-2-picryl-hydrazil (DPPH) radical scavenging activity, 2,2-Azino-bis-3 ethylbenzothiozoline-6-sulfonic acid diammonium salt (ABTS) radical scavenging activity, and Evaluation of ferrous ion (Fe2) reducing power, following the procedures described in a previous work (Arulkumar et al. 2018).
Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
An aliquot (2 mg) of the ethanolic supernatant of G. verrucosa and 200 mg KBr (FT-IR grade) were incorporated into solid pellets by applying a hydraulic pressure of 500 kg/m3. The pellet was immediately placed into the sample holder, and the FT-IR spectra were recorded between the range of 7500 and 370 cm−1 (mid-IR) using an FT-IR spectroscopy (Tensor 27; Bruker GmbH, Bremen, Germany).
Characterisation of Active Compounds by Gas Chromatography Mass Spectrometry (GC-MS)
G. verrucosa was analysed by gas chromatography (GC) interfaced with a quadrupole mass spectrometer analyser (QP-2010; Shimadzu), to determine its chemical constituents using a Rtx-PCB capillary column (60 m × 0.25 mm i.d., 0.25 mm film thickness; Restek, Bellefonte, PA, USA). Helium (99.99% purity) was used as the carrier gas at a flow rate of 1 mL/min, and 1 mL of ethanolic supernatant of G. verrucosa was injected in split mode using an autosampler. The injector port, interface and ion source temperatures were set at 250, 270 and 230 °C, respectively. The GC temperature was programmed as follows: 50 °C (1 min), 10 °C/min ramps to 320 °C, 10 min hold. The mass spectrometer (MS) was operated in electron ionisation (EI) mode at 70 eV and at an emission current of 60 mA. Full scan data were obtained in a mass range of m/z 50–500. Interpretation of the mass spectrum analysis was performed by comparison with known compounds stored in the National Institute of Standards and Technology database (NIST, Washington, DC, USA).
Preparation of G. verrucosa Extracts and Icing Systems
Fifteen grams of lyophilised alga was mixed with absolute ethanol (2 × 120 mL), stirred for 30 s and centrifuged at 1900g, 4 °C for 10 min. Next, the supernatant was recovered, diluted to 6 L with distilled water (2.50 g lyophilised alga/L aqueous solution) and then packaged in polyethylene bags and kept frozen at −20 °C, for later use as icing medium (G-2 batch). In the same way, 4 g of lyophilised alga was extracted with ethanol, as described above, to provide a more diluted alga icing medium (0.67 g lyophilised alga/L aqueous solution; G-1 batch). Finally, traditional ice was prepared from distilled water, packaged and kept frozen in the same way as the two other ice media (G-0, control batch). Before addition to individual fish specimens, the different icing systems were ground to obtain ice flakes.
Experimental conditions (content of lyophilised alga extract in the ice) employed in the present study were based on several preliminary tests undertaken at our laboratory in the range 0.2–10.0 g lyophilised alga/L aqueous solution. Thus, 2.50 g/L was the highest concentration that did not modify the sensory descriptors (external odour and colour, as well as flesh odour and flavour) of the fish (data not shown). Consequently, this concentration was considered in the G-2 batch, together with a lower amount (0.67 g/L, G-1 batch), previously employed by Miranda et al. (2016b).
Fish Processing and Sampling
Fresh Indian mackerel fish (R. kanagurta) (n = 100) were obtained from the fish landing centre, Thondi, India (latitude: 9° 44′ 16.83″ N and longitude: 79° 01′ 05.47″ E) and transported in ice to the laboratory, within 10 min. The length and weight of the fish specimens were in the range of 22–25 cm and 340–365 g, respectively. Upon arrival at the laboratory, 10 individual fish specimens were separated and analysed as initial fish (day 0). These fish specimens were divided into three different groups (three individuals per group) that were evaluated independently to achieve the statistical analysis (n = 3). The remaining fish specimens were divided into three batches (30 individuals per batch) that were placed in independent boxes and directly surrounded by different kinds of ice (G-0, G-1 and G-2 batches, respectively), prepared as previously described by Miranda et al. (2016a). Ice was added at a 1:1 (w/w) fish/ ice weight ratio and all batches were placed inside a refrigerated room (4 °C). Boxes that allowed draining of melted ice were used for fish storage. The ice of all batches was renewed when required, to maintain a 1:1 fish/ ice (w/w) weight ratio. Fish samples from all batches were stored for 15 days, with sampling and analysis on days 4, 7, 11 and 15.
Sensory Score
The sensory score was evaluated for G-0, G-1 and G-2 batches of R. kanagurta by a sensory panel consisting of four to six experienced judges. Before sensory evaluation, the judges received specialised training on chilled Indian mackerel, focusing on the evaluation of specimens exhibiting different qualities. The score was based on a nine-point hedonic scale as described by Amerine et al. (1965). Various sensory attributes like appearance, texture, colour, flavour and odour were examined. The score of individual attributes was summed and divided by the number of the attributes examined, to provide an overall acceptability score. A minimum score of 4 was considered as the acceptability threshold.
Microbiological Assessments
Muscle samples (10 g) of Indian mackerel were aseptically taken and homogenised with 90 mL buffered peptone water (HiMedia, Mumbai, India). Serial dilutions were performed in the same diluent. Enumeration of total viable mesophilic count (TVMC) and total viable psychrophilic count (TVPC) were determined by spreading 0.1 mL aliquots on the surface of tryptic soy agar (HiMedia) and incubating at 37 °C for 2 days or at 10 °C for 7 days (Miranda et al. 2016a), respectively. All counts were expressed as log10 CFU/g.
Biochemical Analysis
The pH value was determined using a digital pH meter (Eutech Instruments, Malaysia). The TVB-N content (mg/100 g fish) was measured using the Conway dish method (Cobb et al. 1973). Briefly, 10 g of ground fish muscle samples was extracted with 20 mL of 6% trichloroacetic acid (TCA) and filtered through Whatman No. 2 filter paper. The residue was extracted twice, and the extract was neutralised with 0.2 mL of 2 M NaOH and 0.3 mL saturated sodium bicarbonate, followed by absorption by boric acid and titration with 0.02 N HCl.
Trimethylamine (TMA-N) levels (mg/100 g fish muscle) were determined by the Conway technique, as described above, with the exception that 1 mL of 10% neutralised formalin added before the addition of sodium bicarbonate.
Biogenic Amine Analysis
Detection and quantification of biogenic amines were performed using high-performance liquid chromatography (HPLC). Fish muscle samples (10 g) were homogenised in a waring blender for 3 min. Then, 5 g was transferred to a 50-mL centrifuge tube and homogenised with 20 mL of 6% TCA for 3 min. The homogenates were centrifuged (10,000g/4 °C, 10 min) and filtered through Whatman No. 2 filter paper. Then, TCA was added to the filtrates to give a final volume of 50 mL. A 1 mL aliquot of the extracts was derivatised with dansyl chloride (Sigma-Aldrich, St. Louis, MO, USA), according to the method of Eerola et al. (1993). Tryptamine hydrochloride (83.22 mg), putrescine dihydrochloride (113.32 mg), cadaverine dihydrochloride (107.52 mg), histamine dihydrochloride (104.62 mg), spermine tetrahydrochloride (109.52 mg), spermidine trihydrochloride (87.7 mg), and tyramine hydrochloride (85.22 mg) (Sigma-Aldrich, St. Louis, MI, USA) were used to prepare the solutions and equilibrate column for the identification of BA. Standards were dissolved in 50 mL of a 0.1 M HCl and used as the working solution. The final concentration of each amine was 1.0 mg/mL.
The HPLC system (Hitachi, Tokyo, Japan) consisted of the following: L–7100 pump, Rheodyne 7125 syringe loading sample injector, L-4000 UV-Vis detector (set at 254 nm), D-2500 chromato-integrator, and a LiChrospher 100 RP-18 reversed-phase column (125 × 4.6 mm; 5 μm, Merck, Darmstadt, Germany). The gradient elution program began with 50:50 (v/v) acetonitrile/water at a flow rate of 1.0 mL/min for 19 min, followed by a linear increase to 90:10 acetonitrile/water (1.0 mL/min) during the next 1.0 min; the acetonitrile/water ratio was decreased to 50:50 (1.0 mL/min) for 10 min, then the temperature was set at 22.5 °C.
Statistical Analysis
All determinations were done in triplicate. The results obtained were compared by one-way analysis of variance (ANOVA). The significance of the difference between means was determined by Duncan’s multiple range test. Correlation analysis among the variables (storage time on ice, biochemical indices and microbiological values) was also conducted. The results refer to linear fittings unless indicated. For all kinds of analyses, differences among batches were considered significant for a confidence interval at the 95% level (p < 0.05). All statistical analyses were performed using SPPS version 14 (SPSS, Chicago, IL, USA).
Results and Discussion
Total Phenol Content and Antioxidant Activity
A high polyphenol content (38.8 ± 1.26 GAE/g) was determined in ethanolic extracts of lyophilised G. verrucosa. The results obtained were markedly higher than the 14.58 GAE/g obtained by Widowati et al. (2014) for G. verrucosa, but compatible with those obtained by Miranda et al. (2016a, 2016b) for ethanolic extracts of other algae, such as B. bifurcata and F. spiralis (40.8 ± 8.3 and 53.3 ± 5.0 GAE/g lyophilised alga, respectively).
DHHP radical scavenging activity and ABTS.+ radical scavenging were higher than those reported previously for other Gracilaria species such as G. edulis or G. corticata (Arulkumar et al. 2018). Thus, DHHP radical scavenging activity reached 45.06 ± 1.55%, higher than 23.95% found for G. edulis and 20.32% found for G. corticata. Similar results were found for ABTS.+ radical scavenging activity (54.5 ± 1.22 vs 40.24% reported for G. edulis and 32.65% reported for G. corticata). Contrariwise, ferric ion reducing power (0.126 ± 0.01%) was lower than those previously reported for both G. edulis (0.580%) and G. corticata (0.580%) (Arulkumar et al. 2018).
FT-IR and GC-MS Analysis
Figure 1 depicts the FT-IR spectrum of lyophilised G. verrucosa. FT-IR peak values obtained at 3319.85, 2928.29, 2520.02, 2267.51, 2118.67, 1793.34, 1650.97, 1429.52, 1107.61, 871.84, 855.65, 758.36, 706.45, 601.93, 531.82 and 464.55 cm−1 confirmed the presence of functional groups. The bands at 3319.85 and 2928.29 cm−1 indicated the strong presence of C–H bonds in alkenes, and the band at 2520.02 cm−1 represented C–H stretching in aldehydes. The bands in the region of 2267.51–2118.67 cm−1 are characteristic of nitriles. The band at 1036.00 cm−1 showed the skeleton of galactans. The most important band was that found at 871.84 cm−1, corresponding to agar. The low-density band at 855.65 cm−1 represented galactose-4-sulphate.
The FT-IR spectrum of other Gracilaria species such as Gracilaria edulis showed the characteristic bands (1352.1, 1377.12, 1251.75, 1039.59, 1056.38, 929.65, 927.92, 885.29, 852.5 cm−1) assigned as estersulphate, the skeleton of galactons, 3,6-anhydro-L-galactose, agar specific band, galactose 4-sulphate, galactose 6-sulphate (Sakthivel and Pandima Devi 2015). Similarly, Seedevi et al. (2017) reported the band at 1254.87 which could be attributed to the sulphate group and the region of 1068.49 is equivalent to the skeleton of galactans and agar specific band found in Gracilaria corticata.
In the GC-MS analysis, a total of six different compounds were identified in lyophilised G. verrucosa. Figure 2 and Table 1 provide the target mass ions (m/z) and retention time of all identified compounds. Interestingly, G. verrucosa comprises known potent preservative agents such as butylated hydroxytoluene, sulfurous acid, 1,2-propanediol, benzeneacetic acid, cyclononasiloxane and tetracosamethyl-cyclododecasiloxane. Mohy El-Din and El-Ahwany (2016) identified the main chemical constituents in other red seaweeds, as well as other bioactive compounds, such as ascorbic acid, 2,6-dihexadecanoate, icosapent ethyl, trans-13- octadecenoic acid, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, heptadecane and 1,2-benzenedicarboxylic acid in Jania rubens, and n-hexadecanoic acids, cholesterol, heneicosane, cis-5,8,11,14,17-eicosapentaenoic acid, methyl ester, trans-13-octadecenoic acid, tetradecanoic acid and 1,2-benzenedicarboxylic acid in Pterocladia capillacea. Jassbi et al. (2013) reported that high levels of tetradecanoic acid, 9(Z)-octadecanoic acid, pentadecanoic acid, hexadecanoic acid and octadecanoic acid in red seaweed extract highlight its potential for antioxidant and antibacterial properties. Similarly, Maftuch et al. (2016) noted the most important phenolic compounds in red seaweeds of G. verrucosa, such as quercetin-7-methyl ether, endow antibacterial activity. Coskun et al. (2004) mentioned that quercetin is known to have many beneficial health effects, such as cardiovascular protection, cataract prevention, anticancer, anti-allergic, anti-viral, antibacterial and anti-inflammatory activities.
Sensory Score
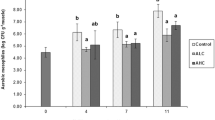
Figure 3 presents the overall acceptability scores for Indian mackerel stored in G-0, G-1 and G-2. The initial quality of Indian mackerel is a characteristic glossy appearance, hard texture, bright convex eyes, reddish gills and fresh seaweed odour; there was a declining trend in quality with storage duration. The shelf life of Indian mackerel was 11 days in the control group G-0 (5.13 ± 0.04), and the sensory score for G-1 and G-2 declined gradually but were always above the minimum acceptability score during the 15 days storage. The shelf life extensions of Indian mackerel with added G. verrucosa extracts were similar to those observed by previous authors that employed other preservative agents. For instance, Viji et al. (2015) treated Indian mackerel with mint and citrus peel extract during ice storage, which improved the sensory score of fish and extended the shelf life by 3–6 days compared with the control samples. In another study, Indian mackerel that was treated with grape seed and papaya seed extracts during ice storage demonstrated an improved sensory score and a 3–5 days shelf life extension compared to the control samples (Sofi et al. 2015).
Microbial Quality of Chilled Indian Mackerel Muscle
The upper limit for bacterial load in the fresh fish, proposed by the International Commission on Microbiological Specifications for Foods (ICMSF, 1986) for human consumption, is 7 log CFU/g. This limit can be applied for both TVMC and TVPC. The TVMC for all three batches (G-0, G-1 and G-2) progressively increased as storage time progressed (r2 = 98–99; Table 2). Surprisingly, TVMC content were significantly different at day 0 in all three batches. No evident reason was found to this initial difference other than possible individual differences in microbiological contamination of fish prior to laboratory arrival. The presence of G. verrucosa extract in the icing medium (G-1 and G-2) resulted in significant (p < 0.05) inhibition of microbial growth as compared with G-0 batches. The greatest inhibition occurred on day 11, and it remained at similar levels as storage advanced. Unlike that in G-0, the mesophilic count in G-1 and G-2 batches was always below 7 log units, even for prolonged storage periods. The results corroborate previous works (Miranda et al. 2016a; Miranda et al. 2016b), in which aerobes indicated the beneficial effects of B. bifurcata and F. spiralis extracts in the icing medium on bacterial inhibition in megrim muscles.
TVPC counts in Indian mackerel batches (Table 2) followed a similar pattern to the TVMC. A progressive increase (p < 0.05) in the microbial enumeration was observed for all batches as storage time progressed (r2 = 94–99). Incorporation of G. verrucosa extract (G-1 and G-2) resulted in significantly (p < 0.05) better control of psychrotroph growth as compared with G-0. It proved that the significant reduction in TVMC and TVPC in mackerel of G-1 and G-2 samples could be attributed to the antibacterial effects of G. verrucosa on aerobic spoilage bacteria, resulting in an extended microbial shelf life relative to that seen for G-0. Although there are no previous works about the preservative of Indian mackerel by G. verrucosa, the results obtained are compatible with prior studies of the inclusion of natural compounds in fish icing systems, e.g. rosemary extract for chilled sardines (Ozyurt et al. 2012), wild thyme hydrosol extract for chilled Transcaucasian barb (Oral et al. 2008), and citric and lactic acid extract for chilled megrim (Garcia-Soto et al. 2014). Other treatments that have been studied are mint and citrus peel extracts (Viji et al. 2015) and grape seed and papaya seed extracts for chilled Indian mackerel (Sofi et al. 2015) or rosemary and basil extracts for chilled Atlantic mackerel (Kaouri and Hassoun 2017).
The presence of natural compounds with antibacterial activity is widespread in red algae, and a wide range of metabolites have been isolated and characterised. G. verrucosa contains alkaloids, flavonoids, tannins, phenols, steroids, saponins and terpenoids, which have been identified as key antimicrobial compounds (Maftuch et al. 2016). The present study has revealed to light different compounds such as butylated hydroxytoluene, sulfurous acid, 1,2-propanediol, benzeneacetic acid, cyclononasiloxane and tetracosamethyl-cyclododecasiloxane which might be responsible for the antibacterial activity against both the Gram-positive and Gram-negative bacteria tested.
Chemical Analysis
Three chemical quality parameters (pH, TVB-N and TMA-N values) closely related to microbial spoilage were investigated. pH progressively increased (p < 0.05) for all batches as storage time progressed (r2 = 0.77–0.99; Table 3). After day 4, G-0 presented higher pH values than those G-1 and G-2, until day 15 (p < 0.05). The increase in pH values throughout storage period may indicate the formation of secondary amines, i.e., ammonia, TMA-N and other alkaline compounds, mainly derived from bacterial actions. The incorporation of G. verrucosa extracts in the icing medium resulted in better control of alkalisation routes, as compared with the control batches. The pH values correlated with the microbial parameters (r2 = 0.75–0.99), particularly TVPC (r2 = 0.70–0.96). Some earlier investigators noticed similar increases in the pH of Indian mackerel during freezing storage (Sofi et al. 2015; Viji et al. 2016). An inhibition of pH increase as a result of using other natural biopreservative compounds in ice during the chilled storage of marine fish has also been documented (Kaouri and Hassoun 2017; Ozogul et al. 2017).
TVB-N is a quality indicator of freshness, which is a measurement of basic volatile compounds recovered by distilling fish muscle, or extracts of fish muscle, under alkaline conditions (Howgate 2010). The concentration of TVB-N in freshly caught fish is typically between 10 and 20 mg/100 g, whereas depending on fish species, levels of 25–35 mg/100 g in fish are considered as the acceptable limit for ice stored fish (EC Commission Regulation 1022/ 2008).
In the present study, marked TVB-N formation (p < 0.05) was observed for all kinds of samples throughout chilled storage (r2 = 0.91–0.99; Table 2). Higher TVB-N was found in G-0 than G-1 and G-2 from day 4 to day 15 (p < 0.05). On days 11 and 15, G-2 had significantly lower TVB-N values relative to G-1 batches. Likewise, Ozyurt et al. (2012) demonstrated that the phenolic compounds in rosemary extracts helped to lower TVB-N formation in sardine muscles, and Viji et al. (2015) suppressed the formation of TVB-N during ice storage of Indian mackerel by the inclusion of mint and citrus peel extracts. Also, Viji et al. (2015) and Sofi et al. (2015) detected reduced TVB-N levels in Indian mackerel during ice storage due to the incorporation of grape-papaya seed and mint extracts, respectively.
The TMA-N value can be used as a quality control index for fish. In this study, the initial TMA-N value for Indian mackerel was 2.4 ± 0.12 mg/100 g, but this rapidly increased during chilled storage (Table 3). Progressive formation of TMA-N ensured in all batches as storage time progressed (r2 = 0.97–0.98). The increase was higher for the G-0 (control) batch than for G-1 and G-2 batches. The values obtained at the end of the storage period (28.43 ± 0.73, 16.86 ± 0.16 and 13.16 ± 0.85 mg/100 g for G-0, G-1 and G-2, respectively) indicated increasing microbial activity in G-0 batch. Consequently, the presence of G. verrucosa extract in the ice led to a remarkable inhibition of TMA-N formation in Indian mackerel muscle. Accordingly, TMA-N values showed good correlation with TVMC (r2 = 95–99), TVPC (r2 = 0.92–0.94) and pH (r2 = 0.87–0.96) throughout storage. In agreement with the present study, previous reports illustrated that the inhibition of TMA-N could be enhanced as a result of incorporating natural preservation agents in ice during chilled storage of marine fish. These studies included the use of rosemary extracts during chilled storage of sardines (Ozyurt et al. 2012), mint extract applied to Indian mackerel (Viji et al. 2015), citric and lactic acid extract employed for megrim (Garcia-Soto et al. 2014), and F. spiralis or B. bifurcata extract applied during the chilled storage of megrim (Miranda et al. 2016a; Miranda et al. 2016b).
Biogenic Amines
The biogenic amine content of fish and fishery products can be used to estimate freshness and degree of spoilage because these amines are found at low levels in fresh fish, and their presence is associated with bacterial spoilage (Ozyurt et al. 2012). The formation of biogenic amines in fish and fishery products depends on many factors including the content of free amino acids, the presence of bacterial amine decarboxylases and the storage conditions of the fishery products. In this sense, high levels of biogenic amines in fish and fishery products are usually derived from inadequate preservation, in which biogenic amines can be largely formed through the microbial decarboxylation of free amino acids by various specific amine-forming enzymes (Park et al. 2010). At the beginning of the study, tryptamine, putrescine, cadaverine, histamine, tyramine, spermidine and spermine were not detected in fresh Indian mackerel (Table 4). In concurrence with these results, Jeya Shakila (1996) did not detect putrescine, cadaverine, histamine and tyramine were detected in fresh Indian mackerel treated with turmeric, pepper, cardamom, cinnamon and clove.
In the present study, the level of histamine, a causative agent for fish poisoning, was significantly higher (p < 0.05) in G-0 than in G-1 and G-2 on day 4 of storage and remained higher until day 15. In comparison, Jeya Shakila et al. (1996) recorded lower values for Indian mackerel (20 mg/100 g) after 4–6 days of storage at 5 °C that might be explained by the differences in fish species and catch area. The legal limits for histamine (<10 mg/100 g) established by the United Nations Food and Agriculture Organisation (FAO, 2012) and Food Safety and Standards Authority of India (FSSAI, 2016) were reached only by G-0 after 15 days of storage. Chong et al. (2014) also reported that histamine reached toxic levels in Indian mackerel after 16 days of ice storage, and the concentration increased to 96.2 mg/100 g when samples were stored at 27 °C for 20 h.
Putrescine and cadaverine levels rose significantly in Indian mackerel during storage under different icing conditions (p < 0.05). The initial putrescine content in Indian mackerel was significantly higher in G-0 than in G-1 and G-2 batches from day 4 to day 15 of chilled storage, and after day 11, G-1 showed significantly higher values than G-2. Jeya Shakila et al. (1996) also found that putrescine and cadaverine levels rose throughout the storage of both Indian mackerel control samples and those treated with spices. Ozyurt et al. (2012) described increasing levels of putrescine (2.35 mg/100 g) and cadaverine (6.92 mg/100 g) during storage periods, reaching 26.11 (control), 22.92 (0.05% rosemary extract) and 18.72 mg/100 g (0.1% rosemary extract) for putrescine and 19.16 (control), 21.25 (0.05% rosemary extract) and 22.07 mg/100 g (0.1% rosemary extract) for cadaverine in sardines stored in ice. Yamanaka et al. (1989) suggested that cadaverine may be used as an indicator of freshness in salmonoids (the acceptable upper limit for cadaverine is 10 mg/100 g). The cadaverine content of mackerel greatly exceeded this value after 11 and 15 days of storage for the G-0 batch and after 15 days for G-1 and G-2 batches and was not well correlated with sensory scores in the present study.
Considerable accumulation of tryptamine, tyramine, spermidine and spermine levels occurred during the storage, irrespective of the icing media. The tryptamine concentration was significantly higher in G-0 than in G-1 and G-2 batches throughout storage (p < 0.05), except on day 0. Similarly, Ozyurt et al. (2012) could not detect tryptamine on day 0 and during storage in control groups, but it was present on days 7 and 12 for sardines stored in ice with 0.05% rosemary extract. The values obtained on day 15 in G-0 exceeded 10 mg/100 g, the upper limit reported by Santos (1996). Kim et al. (2009) recorded tryptamine levels of 10.08 to 22.1 mg/100 g in fresh fish, which are comparable with the findings of the present study.
No spermidine or spermine was detected in the control batch on day 1, but they were evident on day 4 and subsequent sampling days. In both G-1 and G-2 batches, spermidine or spermine was only apparent after day 11 of chilled storage, and always at significantly lower concentrations than in the control batch. Spermidine and spermine are biogenic amines naturally occurring in food, and their formation is not related to bacterial spoilage. Veciana-Nogues et al. (1997) indicated a higher content of spermine than of spermidine in foods of animal origin, but this did not agree with the initial spermidine and spermine levels in Indian mackerel, in the present study. In earlier work, the levels of spermidine (5.97–6.62 mg/100 g) and spermine (10.70–11.27 mg/100 g) were similar to each other during 12–16 h of storage of Indian mackerel at 25 °C (Chong et al. 2014). Consideration of all the results obtained on the biogenic amines content in the present study, it can be concluded that G. verrucosa effectively inhibits the formation of biogenic amines in Indian mackerel samples during chilled ice storage.
Conclusion
In this work, bioactive compounds such as butylated hydroxytoluene, sulfurous acid, 1,2-propanediol, benzeneacetic acid, cyclononasiloxane and tetracosamethyl-cyclododecasiloxane, as well as a high polyphenol content were identified in G. verrucosa extracts. The presence of G. verrucosa extracts in the icing medium during chilled storage of Indian mackerel significantly inhibited microbial growth (TVMC and TVPC), delayed the development of chemical markers of deterioration (increase in pH, TVB-N, and TMA-N), and supressed the formation of all tested biogenic amines. Storage of Indian mackerel in icing medium containing lyophilised G. verrucosa extract can improve the sensory score and reduce biogenic amine formation more than the traditional icing medium, which results in a significant extension of the shelf life of Indian mackerel. Interestingly, the icing medium proposed in this work may constitute a promising strategy that opens the gateway to the application of natural preservatives to enhance the shelf life of seafoods during storage and transportation in the seafood industries.
References
Abdelhady, M. I. S., Motaal, A. A., & Beerhues, L. (2011). Total phenolic content and antioxidant activity of standardized extracts from leaves and cell cultures of three Callistemon species. American Journal of Plant Sciences, 2(06), 847–850.
Abu, O. M., Abdul, A. M., Rowshanul, H. M., & Rezaul, K. M. (2011). Antimicrobial investigation on Manilkara zapota (L.) P. Royen. International Journal of Drug Development and Research, 3, 185–190.
Amerine, M. A., Pongborn, R. H., & Roescler, E. B. (1965). Principles of sensory evaluation of food (p. 602). New York: Academic Press.
Anantharaman, P., Manivannan, K., Karthikai Devi, G., & Balasubramanian, T. (2011). Antimicrobial potential of selected brown seaweed of Vedalai coastal waters, Gulf of Mannar. Asian Pacific Journal of Tropical Biomedicine, 1(2), 114–120.
Arulkumar, A., Rosemary, T., Paramasivam, S., & Babu Rajendran, R. (2018). Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatalysis and Agricultural Biotechnology, 15, 63–71.
Chan, T. P., & Mantanjun, P. (2017). Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chemistry, 221, 302–310.
Chong, C. Y., Abu Bakar, F., Rahman, R. A., Bakar, J., & Zaman, M. Z. (2014). Biogenic amines, amino acids and microflora changes in Indian mackerel (Rastrelliger kanagurta) stored at ambient (25-29°C) and ice temperature (0°C). Journal of Food Science and Technology, 51(6), 1118–1125.
Cobb, B. F., Aoaniz, L., & Thompson, C. A. (1973). Biochemical and microbiology studies on shrimp: Volatile nitrogen and amino nitrogen analysis. Journal of Food Science, 38(3), 431–438.
Commission Regulation (EC) No 1022/2008 of 17 October. (2008). Amending regulation (EC) No 2074/2005 as regards the total volatile basic nitrogen (TVB-N) limits. Official Journal of European Union, L277, 18–20.
Coskun, O., Kanter, M., Armutcu, F., Cetin, K., Kaybolma, B., & Yazgan, O. (2004). Protective effects of quercetin, a flavonoid antioxidant, in absolute ethanol-induced acute gastric ulcer. European Journal of General Medicine, 1(3), 37–42.
Eerola, S., Hinkkanen, R., Lindfors, E., & Hirvi, T. (1993). Liquid chromatographic determination of biogenic amines in dry sausages. Journal of AOAC International, 76(3), 575–577.
European Council Regulation 258/. (1997). Concerning novel foods and novel food ingredients. Official Journal of the European Union, L-43, 1–7.
FAO/WHO. (2012). Expert meeting on the public health risks of histamine and other biogenic amines from fish and fishery products (pp. 8–70). Rome: FAO Headquarters.
Food Safety and Standards Authority of India (FSSAI) (2016). To revise histamine toxin levels in fish, fish products. Available at:http://articles.economictimes.indiatimes.com/2016074/news/79203_1_fish products-fish-species-fssai. Accessed: 03.11.2016.
Garcia-Soto, B., Bohme, K., Velazquez, J. B., & Aubourg, S. P. (2014). Inhibition of quality loss in chilled megrim (Lepidorhombus whiffiagonis) by employing citric and lactic acid icing. International Journal of Food Science and Technology, 49(1), 18–26.
Gouda, S., Moharana, R. R., Das, G., & Patra, J. K. (2013). Free radical scavenging potential of extracts of Gracilaria verrucosa (l) (Harvey): An economically important seaweed from Chilika Lake, India. International Journal of Pharmacy and Pharmaceutical Sciences, 6(1), 707–710.
Howgate, P. (2010). A critical review of total volatile bases and trimethylamine as indices of freshness of fish. Part 1. Determination. Electronic Journal of Environmental Agricultural and Food Chemistry, 9, 29–57.
International Commission on the Microbiological Specifications for Foods (ICMSF). (1986). Microorganism in foods, sampling for microbiological analysis: Principles and specific applications (2nd ed.). Toronto: University of Toronto Press.
Jassbi, A. R., Mohabatia, M., Eslamia, S., Sohrabipourb, J., & Miria, R. (2013). Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iranian Journal of Pharmaceutical Research, 12(3), 339–348.
Jaswir, I., Hammed Tawakalit Tope, A., Raus, R. A., Monsur, H. A., & Ramli, N. (2014). Study on anti-bacterial potentials of some Malaysian brown seaweeds. Food Hydrocolloids, 42, 275–279.
Jeya Shakila, R., Vasundhara, T. S., & Vijaya Rao, D. (1996). Effect of spices on the biogenic amine formation and other quality characteristics of Indian mackerel during refrigerated storage. Asian Fisheries Sciences, 9, 191–199.
Kaliyaperumal, N., Kalimuthu, S., & Ramalingam, J. R. (1995). Economically important seaweeds. CMFRI special publication no. 62, 1–36.
Kaouri, R., & Hassoun, A. (2017). Efficiency of rosemary and basil essential oils on the shelf-life extension of Atlantic mackerel (Scomber scombrus) fillets stored at 2 °C. Journal of AOAC International, 100, 335–344.
Kim, M. K., Mah, J. H., & Hwang, H. J. (2009). Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chemistry, 116, 87–95.
Kim, K. J., Lee, Y. J., Hwang, Y. H., Kang, K. Y., Yee, S. T., & Son, Y. J. (2017). In vitro and in vivo effects of Gracilaria verrucosa extracts on osteoclast differentiation. Journal of Clinical Medicine, 6(3), 32.
Lee, J. C., Hou, M. F., Huang, H. W., Chang, F. R., Yeh, C. C., Tang, J. Y., & Chang, H. W. (2013). Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell International, 13, 1–7.
Lim, Y. Y., Lim, T. T., & Tee, J. J. (2007). Antioxidant properties of several tropical fruits: A comparative study. Food Chemistry, 103(3), 1003–1008.
Maftuch, I. K., Adam, A., & Zamzami, I. (2016). Antibacterial effect of Gracilaria verrucosa bioactive on fish pathogenic bacteria. Egyptian Journal of Aquatic Research, 42, 405–410.
Miranda, J. M., Ortiz, J., Velazquez, J. B., & Aubourg, S. P. (2016a). Quality enhancement of chilled fish by including alga Bifurcaria bifurcate extract in the icing medium. Food & Bioprocess Technology, 9(3), 387–395.
Miranda, J. M., Trigo, M., Velazquez, J. B., & Aubourg, S. P. (2016b). Effect of on icing medium containing the alga Fucus spiralis on the microbiological activity and lipid oxidation in chilled megrim (Lepidorhombus whiffiagonis). Food Control, 59, 290–297.
Mohy El-Din, S. M., & El-Ahwany, A. M. D. (2016). Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). Journal of Taibah University for Science, 10(4), 471–484.
Oral, N., Gulmez, M., Vatansever, L., & Guven, A. (2008). Application of antimicrobial ice for extending shelf life of fish. Journal of Food Protection, 71(1), 218–222.
Ortiz, J., Vivanco, J., & Aubourg, S. P. (2014). Lipid and sensory quality of canned Atlantic salmon (Salmo salar): Effect of the use of different seaweed extracts as covering liquids. European Journal of Lipid Science and Technology, 116(5), 596–605.
Ozogul, Y., Yuvka, I., Ucar, Y., Durmus, M., Kosker, A. R., & Fatih Ozogul, M. (2017). Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LW - Food Science and Technology, 75, 677–684.
Ozyurt, G., Kuley, E., Balikci, E., & Kacar, C. (2012). Effect of the icing with rosemary extract on the oxidative stability and biogenic amine formation in sardine (Sardinella aurita) during chilled storage. Food and Bioprocess Technology, 5(7), 2777–2786.
Park, J. S., Lee, C. H., Kwon, E. U., Lee, H. J., Kim, J. Y., & Kim, S. H. (2010). Monitoring the contents of biogenic amines in fish and fishery products consumed in Korea. Food Control, 21(9), 1219–1226.
Pastoriza, L., Bernárdez, M., Sampedro, G., Cabo, M., & Herrera, J. (2008). The use of water and ice with bactericide to prevent onboard and onshore spoilage of refrigerated megrim (Lepidorhombus whiffiagonis). Food Chemistry, 110, 31–38.
Peinado, I., Giron, J., Koutsidis, G., & Ames, J. M. (2014). Chemical composition and antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Research International, 66, 36–44.
Sakthivel, R., & Pandima Devi, K. (2015). Evaluation of physicochemical properties, proximate and nutritional composition of Gracilaria edulis collected from Palk Bay. Food Chemistry, 174, 68–74.
Santos, M. H. S. (1996). Biogenic amines: Their importance in foods. International Journal of Food Microbiology, 29(2-3), 213–231.
Saraswaty, V., Mozef, T., Risdian, C., & Rasyid, A. (2015). Bioactivity of polysaccharides from Gracilaria verrucosa as α-glucosidase inhibitor. International Symposium on applied Chemistry (ISAC 2015) Procedia Chemistry, 16, 687–693.
Seedevi, P., Moovendhan, M., Vairamani, S., & Shanmugam, A. (2017). Bioactive potential and structural characterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohydrate Polymers, 155, 516–524.
Senturk, T., & Alpas, H. (2013). Effect of high hydrostatic pressure treatment (HHPT) on quality and shelf life of Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 6, 2306–2318.
Shinde, P. A., Reddy, S. V. K., & Patange, S. B. (2015). Quality of Indian mackerel as affected by pomegranate peel and tea leaf extracts during ice storage. SAARC Journal of Agriculture, 13(1), 109–122.
Sofi, F. S., Raja, C. V., Lakshmisha, I. P., & Rattankumar Singh, R. (2015). Antioxidant and antimicrobial properties of grape and papaya seed extract and their application on the preservation of Indian mackerel (Rastrelliger kanagurta) during ice storage. Journal of Food Science and Technology, 53(1), 104–117.
Veciana-Nogues, M. T., Marine-Font, A., & Vidal-Carou, M. C. (1997). Biogenic amines in fresh and canned tuna. Effects of canning on biogenic amine contents. Journal of Agricultural and Food Chemistry, 45, 4324–4328.
Viji, P., Binsi, P. K., Visnuvinayagam, S., Bindu, J., Ravishankar, C. N., & Srinivasa Gopal, T. K. (2015). Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill stored Indian mackerel. Journal of Food Science and Technology, 52(1), 6278–6289.
Viji, P., Panda, S. K., Mohan, C. O., Bindu, J., Ravishankar, C. N., & Srinivasa Gopal, T. K. (2016). Combined effects of vacuum packaging and mint extract treatment on the biochemical, sensory and microbial changes of chill stored Indian mackerel. Journal of Food Science and Technology, 53(12), 4289–4297.
Wang, T., Jonsdottir, R., Kristinsson, H., Thorkelsson, G., Jacobsen, C., Yuca Hamaguchi, P., & Olafsdottir, G. (2010). Inhibition of haemoglobin-mediated lipid oxidation in washed cod muscle and cod protein isolates by Fucus vesiculosus extract and fractions. Food Chemistry, 123, 321–330.
Widowati, I., Lubac, D., Puspita, M., & Bourgougnon, N. (2014). Antibacterial and antioxidant properties of the red alga Gracilaria verrucosa from the north coast of java, Semarang, Indonesia. International Journal of Latest Research in Science and Technology, 3(3), 179–185.
Yamanaka, H., Shiomi, K., & Kikuchi, T. (1989). Cadaverine as a potential index for decomposition of salmonid fishes. Journal of Food Hygienic Society of Japanese, 30(2), 170–174.
Yuan, G., Lv, H., Tang, W., Zhang, X., & Sun, H. (2016). Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control, 59, 818–823.
Acknowledgements
The authors are thankful to the authorities Alagappa University for providing necessary facilities and Financial support by Department of Science and Technology (DST)-Science and Engineering Research Board (SERB), Government of India, New Delhi (Grant No.SR/FT/LS-22/2010; dt. 02.05.2012) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Arulkumar, A., Paramasivam, S. & Miranda, J.M. Combined Effect of Icing Medium and Red Alga Gracilaria verrucosa on Shelf Life Extension of Indian Mackerel (Rastrelliger kanagurta). Food Bioprocess Technol 11, 1911–1922 (2018). https://doi.org/10.1007/s11947-018-2154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2154-x