Abstract
Bifurcaria bifurcata is a widely extended brown macroalga, whose antimicrobial and antioxidant properties have previously been described. In this study, ethanolic extracts of B. bifurcata were included in the icing medium employed for the chilled storage of megrim (Lepidorhombus whiffiagonis). For it, two different concentrations of this brown macroalga extract (0.67 and 2.50 g lyophilized alga L−1 aqueous solution; B-1 and B-2 batches, respectively) were tested for a 14-day storage. The effect of the alga extract was compared with a counterpart batch stored in traditional ice prepared only from water (B-0 batch). Significant (p < 0.05) inhibitions of microbial activity (aerobes, psychrotrophs, lipolytic bacteria, proteolytic bacteria and Enterobacteriaceae) as well as of pH and trimethylamine formation were observed as a result of the incorporation of the alga extract in the icing medium, being this effect especially relevant in the B-2 batch. Concerning lipid damage development, a significantly (p < 0.05) lower formation of free fatty acids (lipid hydrolysis development) and of fluorescent compounds (tertiary lipid oxidation development) in samples corresponding to both alga-including batches could also be observed; this inhibitory effect was more intense in fish belonging to the B-2 batch. The icing medium proposed in this work constitutes a promising strategy in order to apply algae extracts to enhance fish quality retention during the different steps of storage and commercialization of marine species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flake ice refrigeration has been the most commonly employed method to slow down fish damage. However, deterioration of nutritional value and sensory quality during storage has led important decreases in fish shelf lives, thus provoking important economic losses. With the aim of delaying fish damage during chilled storage, a wide number of preservation strategies have been combined with flake ice. Among them, several chemical and physical treatments and their combination with packaging have been evaluated (Ashie et al. 1996; Oms-Oliu et al. 2010; Senturk and Alpas 2013; Campos et al. 2012). Additionally, recent studies accounted for the incorporation of preservative compounds in the icing medium such as natural low-molecular weight organic acids (Sallam 2007; Sanjuás-Rey et al. 2012), vegetable extracts (namely, thyme, rosemary, and oregano) (Oral et al. 2008; Quitral et al. 2009; Özyurt et al. 2012), and ozone (Pastoriza et al. 2008).
Marine algae have traditionally formed part of the Asian diet, especially in countries like Japan, China, and Korea, and constitute a relevant source of beneficial nutrients, such as vitamins, trace minerals, lipids, amino acids, and dietary fibers (Díaz-Rubio et al. 2009; Paiva et al. 2014). The use of alga in Western countries has been traditionally focused on the extraction of compounds of relevance for pharmaceutical, cosmetics, and food industries. Recently, red, green, and brown macroalgae have offered the possibility of exploring a wide variety of natural compounds with potential antioxidant (Wang et al. 2010; Halldorsdóttir et al. 2014), antimicrobial (Sandsdalen et al. 2003; Gupta and Abu-Ghannam 2011), anti-inflammatory, and antitumoral activities (Smit 2004). In this sense, a wide number of preservative metabolites such as polyphenols, terpenes, phlorotannins, steroids, halogenated ketones and alkanes, fucoxanthin, polyphloroglucinol, or bromophenols have been isolated from macroalgae (Fleurence et al. 2012; Peinado et al. 2014).
Among brown macroalgae, Bifurcaria bifurcata is a species found in the Atlantic coast of France, Spain, and Portugal, also extending to the South and West coasts of England and the West Coast of Ireland (Braune 2008; Le Lann et al. 2008). It is usually found in rock pools on the middle and lower shore, particularly on exposed beaches, showing an olive-yellow color and a length up to 50 cm. Previous research on this macroalga accounted for proximate composition analysis (Gómez-Ordóñez et al. 2010) as well as the identification of different kinds of compounds present in it such as diterpenes (Culioli et al. 2001), phenols (Glombitza et al. 1976), sterols (Bouzidi et al. 2008), and polysaccharides (Gómez-Ordóñez and Rupérez 2011). Additionally, the antitumoral and antioxidant activity of this alga has been reported by means of in vitro studies (Zubia et al. 2009).
The present study evaluates the incorporation, to the best of our knowledge for the first time, of B. bifurcata extracts in the icing media and their application to the chilled storage of a fish species of commercial relevance. For it, aqueous solutions including ethanolic extracts of lyophilized B. bifurcata at two different concentrations were tested as icing media. The effects of the alga extracts on microbial activity inhibition and lipid oxidation stability were monitored in megrim (Lepidorhombus whiffiagonis) muscle for up to 14 days of chilled storage. This fish species was chosen due to its abundance in the Northeast Atlantic waters and for its commercial interest in a wide number of European countries such as the UK, France, Ireland, and Spain (FAO 2007).
Materials and Methods
Preparation of B. bifurcata Extracts and Icing Systems
The lyophilized alga B. bifurcata was provided by Porto-Muiños (Cerceda, A Coruña, Spain). Fifteen grams of lyophilized alga were mixed with absolute ethanol (2 × 120 mL), stirred for 30 s, and centrifuged at 3500 rpm for 10 min at 4 °C. Then, the supernatant was recovered and diluted to 6 L with distilled water (2.50 g lyophilized alga L−1 aqueous solution). This solution was packaged in polyethylene bags, kept frozen at −18 °C, and later used as icing medium (B-2 batch). In the same way, 4 g of lyophilized alga were extracted with ethanol as described above in order to provide a more diluted alga-icing medium (0.67 g lyophilized alga L−1 aqueous solution; B-1 batch). Finally, traditional ice was prepared from distilled water that was packaged and kept frozen in the same way as the two other ices (B-0, control batch). Before addition to individual fish specimens, the different icing systems were ground to obtain ice flakes.
Experimental conditions (contents of lyophilized alga extract in the ice) employed in the present study were based on several preliminary tests carried out at our laboratory in the range of 0.10–5.00 g lyophilized alga L−1 aqueous solution. Thus, an increasing presence of alga in the icing medium provided better sensory acceptance (namely, lower putrid odor and taste development). However, if a higher concentration than 2.50 was applied, modification of the external odor and color of the whole fish or the flesh odor and flavor would occur as a result of the alga presence in ice (data not shown). In order to avoid such modifications, this concentration (2.50 g lyophilized alga L−1 aqueous solution) was considered in the B-2 batch. In order to analyze the effect of the alga presence, a lower concentration of the alga was also tested. Thus, the lowest concentration that led to partial sensory performances during the preliminary trials was also chosen (0.67 g L−1, B-1 batch). According to European Council Regulation (1997), algae are considered food or food ingredients, so their use in the icing medium should not constitute any hazard to health.
Fish Material, Processing, and Sampling
Fresh megrim (117 specimens) was caught near the Galician Atlantic coast (North-Western Spain) and transported to the laboratory. Throughout this process (10 h), the fish was maintained in ice. The length and weight of the fish specimens ranged from 21 to 26 cm and from 105 to 132 g, respectively.
Upon arrival to the laboratory, nine individual fish specimens were separated and analyzed as initial fish (day 0). These fish specimens were divided into three different groups (three individuals per group) that were analyzed independently to achieve the statistical analysis (n = 3). The remaining fish specimens were divided into three batches (36 individuals in each batch), that were placed in independent boxes and directly surrounded by different kinds of ice (B-0, B-1, and B-2 batches, respectively), prepared as previously described. Ice was added at a 1:1 fish:ice ratio, and all batches were placed inside a refrigerated room (2 ± 1 °C). Boxes that allowed draining of melted ice were used for fish storage. The ice of all batches was renewed when required to maintain the mentioned fish:ice ratio. Fish samples from all of the batches were stored for a 14-day period, being sampled and analyzed on days 4, 7, 11, and 14. At each sampling time, nine specimens were taken from each batch for analysis and divided into three groups (three individuals in each group) that were studied independently (n = 3).
Microbiological Analyses
Samples of 10 g of fish white muscle were taken aseptically from chilled fish specimens, mixed with 90 mL of 0.1 % peptone water (Merck, Darmstadt, Germany) and homogenized in sterilized stomacher bags (AES, Combourg, France) as previously described (Ben-Gigirey et al. 1998; Ben-Gigirey et al. 1999). Serial dilutions from the microbial extracts were prepared in 0.1 % peptone water.
Total aerobes were investigated by surface inoculation on plate count agar (PCA, Oxoid Ltd., London, UK) after incubation at 30 °C for 48 h. Psychrotrophs were also investigated in PCA, being the incubation carried out at 7–8 °C for 7 days. Enterobacteriaceae were investigated by pour plating using Violet Red Bile Agar (VRBA) (Merck, Darmstadt, Germany) after an incubation period of 24 h at 37 ± 0.5 °C. Microorganisms exhibiting a proteolytic or lipolytic phenotype were determined on casein-agar medium or tributyrin agar, respectively, after incubation at 30 °C for 48 h, as previously described (Ben-Gigirey et al. 2000).
In all cases, microbial counts were transformed into log CFU g−1 muscle before undergoing statistical analysis. All of the analyses were conducted in triplicate.
Chemical Analyses
All solvents and chemical reagents used were of reagent grade (Merck, Darmstadt, Germany). Chemical analyses related to fish quality were carried out on the white muscle of megrim. Total polyphenols content of lyophilized B. bifurcata was assessed by means of the Folin-Ciocalteu colorimetric method (Cary 3E UV–Visible spectrophotometer, Varian; Mulgrave, Victoria, Australia) as described previously (Rodríguez-Bernaldo de Quirós et al. 2010). Measurements were made in triplicate. Gallic acid (GA) was used as standard. Results were expressed as milligram GA per gram lyophilized alga.
The evolution of pH values in megrim muscle along storage time was determined using a 6-mm diameter insertion electrode (Crison, Barcelona, Spain). Trimethylamine-nitrogen (TMA-N) values were determined using the picrate colorimetric (Beckman Coulter, DU 640; London, UK) method, as previously described by Tozawa et al. (1971). This method involved the preparation of a 5 % trichloroacetic acid extract of fish muscle (10 g/25 mL). The results are expressed as mg TMA-N kg−1 muscle.
Lipids were extracted from the fish muscle by the Bligh and Dyer (1959) method, which employs a single-phase solubilization of the lipids using a chloroform–methanol (1:1) mixture. The results were calculated as gram lipid per kilogram muscle. Free fatty acid (FFA) content was determined in the lipid extract of the fish muscle by the Lowry and Tinsley (1976) method based on complex formation with cupric acetate-pyridine followed by spectrophotometric (715 nm) assessment. Results were expressed as gram FFA per kilogram lipids and as milligram FFA kilogram muscle. Peroxide value (PV) was determined spectrophotometrically (Beckman Coulter, DU 640; London, UK) using the lipid extracts via previous peroxide reduction with ferric thiocyanate according to the Chapman and McKay (1949) method. The results were expressed as milliequavalents active oxygen per kilogram lipids. Thiobarbituric acid index (TBA-i) was determined according to Vyncke (1970). This method is based on the reaction between a trichloroacetic acid extract of the fish muscle and thiobarbituric acid. Content of thiobarbituric acid reactive substances (TBARS) was spectrophotometrically measured at 532 nm and calculated from a standard curve using 1,1,3,3-tetraethoxy-propane (TEP). Results were expressed as milligram malondialdehyde per kilogram muscle. Tertiary lipid oxidation compounds resulting from the interaction between oxidized lipids and nucleophilic compounds (namely, protein-like molecules) were measured by fluorescence spectroscopy (Fluorimeter LS 45; Perkin Elmer España; Tres Cantos, Madrid, Spain). In agreement with the previous research (Aubourg et al. 2006), fluorescence measurements were carried out at 393/463 nm and 327/415 nm in the aqueous phase that resulted from the lipid extraction of fish muscle. The relative fluorescence (RF) was calculated as follows: RF = F/F st, where F is the fluorescence measured at each excitation/emission wavelength pair and F st is the fluorescence intensity of a quinine sulfate solution (1 μg mL−1 in 0.05 M H2SO4) at the corresponding wavelength pair. The fluorescence ratio (FR) was calculated as the ratio between the two RF values: FR = RF393/463 nm/RF327/415 nm.
Statistical Analysis
Data obtained from the different microbiological and chemical analyses were subjected to the ANOVA method to explore differences resulting from the effects of both the icing condition and the chilling time; the comparison of means was performed using the least-squares difference (LSD) method. In all cases, analyses were carried out using the PASW Statistics 18 software for Windows (SPSS Inc., Chicago, IL, USA); differences among batches and among chilling times were considered significant for a confidence interval at the 95 % level (p < 0.05) in all cases.
Correlation analysis among parameters (chilling storage time, microbiological values, and chemical scores) was also carried out. The results are referred to linear fittings unless indicated.
Results and Discussion
Microbial Evolution in Megrim Muscle
B. bifurcata ethanolic extracts were preliminarily evaluated in their antimicrobial activity against relevant food-borne spoilage and pathogenic bacteria. Thus, B. bifurcata exhibited antimicrobial activity against Salmonella enterica, Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas fluorescens, Vibrio alginolyticus, and Vibrio parahaemolyticus (data not shown). Additionally, high polyphenol content (40.8 ± 8.3 GA g−1 lyophilized alga) was determined in ethanolic extracts of B. bifurcata.
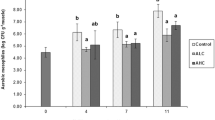
Table 1 shows the evolution of aerobic mesophiles in all three batches. Progressive increases (p < 0.05) were observed in all batches as storage time progressed (r 2 = 0.91–0.92). The presence of B. bifurcata extract in the most concentrated icing medium (B-2 batch) resulted in a significant (p < 0.05) inhibition of microbial growth as compared with B-1 and B-0 (control) batches. The highest inhibition (1.46 log units) was observed on day 7 and remained at similar levels at advanced storage periods. Unlike other batches, aerobes counts in B-2 batch were always below 6 log units, even at advanced storage periods. B-1 batch exhibited better behavior than B-0 (control) batch (lower mean values), although the differences between both batches were not significant (p > 0.05).
The investigation of psychrotrophs in megrim batches is displayed in Table 1. This bacterial group includes bacteria belonging to Flavobacterium, Shewanella, Acinetobacter, Pseudomonas, and Moraxella, among other genera. The evolution of psychrotrophs followed a similar pattern as that observed for aerobes. A progressive increase (p < 0.05) in microbial counts was observed for all batches as storage time progressed (r 2 = 0.86–0.91). The incorporation of alga extract in the B-2 batch resulted in a significantly (p < 0.05) better control of psychrotrophs growth as compared with the other batches. These differences were larger on storage days 7 and 11 and reached a maximum of 1.37 log units. As in the case of aerobes, B-1 batch provided a better protection than B-0 (control) batch (lower mean values), although differences between both batches were only significant (p < 0.05) on day 11. These results are quite in agreement with those observed for aerobes and clearly indicate a beneficial effect of B. bifurcata extract in the icing medium in terms of bacterial inhibition in megrim muscle.
Table 1 shows the evolution of lipolytic bacteria growth in all three batches. As in the case of the other bacterial groups, a progressive increase (p < 0.05) was observed in all batches as storage time progressed (r 2 = 0.93–0.94). Significant (p < 0.05) differences between B-2/B-1 and B-0 (control) batches were observed in the 11–14-day period. These differences reached their maximum (1.11 log units) after 14 days of storage. According to these results, the presence of B. bifurcata extracts in the icing media provided an inhibitory effect on the development of lipolytic bacteria in megrim muscle.
The investigation of Enterobacteriaceae is presented in Table 2. The results were similar for all batches at all sampling times except for day 14, where the batches containing B. bifurcata extract exhibited a significant (p < 0.05) better behavior than the control batch. The low counts determined for this microbial group confirm the good initial microbial quality of megrim specimens.
The study of the evolution of proteolytic microorganisms in all three batches is also presented in Table 2. The active role of proteolytic bacteria in the degradation of fish muscle has been previously informed (Rodríguez et al. 2003). This negative aspect implies that the inhibition of proteolytic bacteria would be of relevance in terms of megrim quality and safety. As for previous bacterial groups, a progressive increase (p < 0.05) was observed in all batches with storage time (r 2 = 0.88–0.89). The results obtained for proteolytic bacteria indicated that the presence of the alga extract in the icing medium provided significant (p < 0.05) inhibition towards this microbial group on days 7 (B-2 batch) and 14 (B-1 batch). The bacterial inhibition reached its maximum (0.78 log units) on day 7.
In spite of disposing of a wide variety of information related to the in vitro antibacterial activity of brown algae, previous research concerning their practical application to seafood can be considered scarce. In the present study, B. bifurcata extracts were included for the first time in the icing media for the chilled storage of a marine fish species. As a result, ethanolic extracts of B. bifurcata were found to exert a remarkable antimicrobial effect for all the five microbial groups investigated in this study: aerobes, psychrothrophs, Enterobacteriaceae, proteolytic, and lipolytic bacteria. This effect was especially relevant at moderate and advanced storage times and would lead to an extended shelf life in the batches treated with the alga, as compared with the control batch. Other authors have reported the inclusion of natural compounds in the icing system, i.e., rosemary extract for chilled sardines (Sardinella aurita) (Özyurt et al. 2012) and wild-thyme hydrosol extract to chilled Transcaucasian barb (Capoeta capoeta capoeta) (Oral et al. 2008).
The presence of natural components with antimicrobial activity is widespread in macroalgae, and a wide range of metabolites has been isolated and characterized. In the Order Fucales (Class Phaeophyceae), natural products of mixed biosynthesis, consisting of terpenes, polyphenols, oligomeric phlorotannins, hydroquinones, and halogenated alkanes and alkenes, have been identified as key antimicrobial components (Smit 2004; Gupta and Abu-Ghannam 2011; Fleurence et al. 2012). Concerning B. bifurcata, Glombitza and Rösener (1974) reported the presence of bifuhalol, a polyhydroxyphenyl ether, on the basis of NMR and IR spectroscopic analysis. In a related Phaeophyceae alga (Fucus vesiculosus), Sandsdalen et al. (2003) identified a polyhydroxylated fucophlorethol as responsible for the antimicrobial activity against both gram-positive and gram-negative bacteria tested. More recently, García-Soto et al. (2015) showed that Fucus spiralis and sorbic acid, when included in a biodegradable film, exerted an inhibitory effect of microbial activity on megrim muscle during chilled storage.
Analysis of the Chemical Changes in Megrim Muscle
Two chemical parameters (pH and TMA-N), closely related to microbial spoilage, were investigated. With respect to pH, a significant (p < 0.05) and progressive increase was observed for all batches as storage time progressed (r 2 = 0.77–0.94, quadratic fitting) (Table 3). The increase of pH in fish muscle indicates the formation of ammonia, TMA, and other alkaline compounds, mainly derived from microbial action. The incorporation of B. bifurcata extracts exerted a better control of the alkalization routes, as compared with the control batch. This effect was significant (p < 0.05) at advanced storage times (11–14-day period), reaching a maximum inhibition value of 0.47 pH units on day 11. pH values showed fair correlation values with the microbial parameters investigated (r 2 = 0.61–0.89), the highest scores being observed with psychrotrophs counts (r 2 = 0.70–0.89).
In agreement with these results, previous studies have reported an inhibition of pH increase as a result of using other natural preservative compounds in ice during the chilled storage of marine species. These studies included the use of oregano and rosemary extracts during the chilled storage of Chilean jack mackerel (Trachurus murphyi) (Quitral et al. 2009), a rosemary extract applied to sardine (Sardinella aurita) (Özyurt et al. 2012) and a wild-thyme hydrosol extract employed for the chilled storage of Transcaucasian barb (Oral et al. 2008). Recently, the presence of Fucus spiralis and sorbic acid in a biodegradable film led to lower pH values during megrim chilled storage (García-Soto et al. 2015).
A marked TMA formation (p < 0.05) was obtained for all kinds of samples throughout chilled storage (r 2 = 0.90–0.93, quadratic fitting) (Table 3), according to the increasing microbial activity. Values obtained at the end of storage period (115–137 mg kg−1) were in agreement with previous research concerning the refrigerated storage of megrim (Aubourg et al. 2006; Sanjuás-Rey et al. 2012). Results obtained showed that the presence of the alga extract in the ice led to a remarkable inhibitory effect on TMA formation in megrim muscle. Thus, fish specimens corresponding to the B-2 batch showed lower TMA mean values than their counterpart control samples for the 7–14-day period; this difference was significant (p < 0.05) at days 7 (B-1 batch) and 14 (B-2 batch).
TMA is one of the most commonly used quality indicators to assess microbial activity in marine species kept under refrigeration conditions. In agreement with the results obtained for the above-mentioned microbial parameters, an inhibitory effect on TMA formation has been obtained as a result of the alga presence in the ice. Accordingly, TMA values showed good correlation values throughout the present study with aerobes (r 2 = 0.73–0.78), psychrotrophs (r 2 = 0.76–0.90), lipolytic bacteria (r 2 = 0.75–0.80), and proteolytic bacteria (r 2 = 0.74–0.79) counts as well as with pH value (r 2 = 0.88–0.92).
Significantly (p < 0.05) progressive FFA formation (g kg−1 lipids; Table 3) was observed in all kinds of samples throughout chilled storage (r 2 = 0.86–0.93, quadratic fitting). An inhibitory effect derived from the presence of the alga in the icing medium could be concluded on the 11–14-day period. At these times, lower mean FFA values were determined in samples corresponding to B-1 and B-2 batches as compared with control batch. Differences were found to be significant (p < 0.05) at day 11 (B-2 batch) and at day 14 (B-1 and B-2 batches). Similar conclusions were obtained when FFA content was considered on muscle basis (mg kg−1 muscle), this being calculated taking into account the lipid content of fish samples (4.4–5.0 g lipid kg−1 muscle, data not shown), On this basis, FFA formation also showed a good correlation value with storage time in all cases (r 2 = 0.85–0.93, quadratic fitting).
FFA formation during chilled storage has been reported to be the result of both endogenous enzyme activity and microbial activity (Ashie et al. 1996; Campos et al. 2012). Before the end of the microbial lag phase (up to 6–9 days, depending on several factors), FFA formation is mostly a result of endogenous enzyme (namely, lipases and phospholipases) activity. Later on, microbial activity gains importance, so that FFA formation is mainly derived from bacterial catabolic processes. The results obtained in the present study can be explained on the basis of these two mechanisms and periods. During the 0–7-day period, little differences were found so that a definite effect of the presence of B. bifurcata in the icing medium could not be concluded. On contrast, later on, when the microbial activity was more intense, the inhibitory effect of the alga was found to be significant. In agreement with this, FFA values determined in the present study showed good correlation values with some indices related to microbial activity, such as psychrotrophs (r 2 = 0.78–0.83), pH value (r 2 = 0.89–0.94), and TMA-N content (r 2 = 0.92–0.93).
Peroxide content (Table 4) showed a marked increasing tendency in the 0–11-day period, which was especially relevant in samples corresponding to the B-2 batch. This was followed by a sharp decrease (p < 0.05) at the end of the chilled storage. Higher mean values were obtained for the B-2 batch when compared with the control batch. Differences were found significant (p < 0.05) for the 4–11-day period.
No significant formation (p > 0.05) of secondary lipid oxidation compounds (i.e., TBARS) could be concluded in all batches for the 0–11-day period (Table 4). At the end of the storage time, and in agreement with the peroxide content decrease, a significant (p < 0.05) formation of TBARS could be depicted in all batches. No significant effect (p > 0.05) of B. bifurcata extract in the icing medium on secondary oxidation compounds could thus be concluded.
A low formation (p > 0.05) of fluorescent compounds was observed in general terms for the 0–7-day period (Table 4). In contrast, a marked increase (p < 0.05) was determined at day 11, this being in agreement with the important peroxide formation also determined at this time. Finally, FR increased (p < 0.05) only in megrim specimens corresponding to B-1 batch. Concerning the effect of B. bifurcata extracts in the icing media, an inhibitory effect on fluorescent compound formation is concluded in the 11–14-day period for the B-2 batch. In addition, samples corresponding to the B-1 batches also exhibited an inhibitory effect at day 11.
Lipid oxidation has been recognized as a complex process where different kinds of molecules are produced, most of them unstable, susceptible to breakdown and to originate low-molecular weight compounds, or to react with other molecules (nucleophilic-type, mostly) present in the fish muscle. As a result of this, determination of each kind of compound cannot always provide an accurate method for the quality loss assessment in fish. In the present study, primary lipid oxidation compounds (peroxides assessment) did not provide a satisfactory correlation with storage time and, accordingly, cannot be considered accurate tools to follow up the lipid oxidation development throughout the whole present experiment. The electrophilic nature of such compounds led them to breakdown or to interact with food constituents possessing nucleophilic functions (Aubourg et al. 2006; Campos et al. 2012). Contrary, TBA-i and FR assessments led to fair correlation values with chilling time (r 2 = 0.75–0.89, quadratic fitting and r 2 = 0.78–0.92, respectively). Additionally, FR values showed fair correlation values with aerobes (r 2 = 0.82–0.85), lipolytic bacteria (r 2 = 0.82–0.89), and proteolytic bacteria (r 2 = 0.81–0.86) counts. In contrast, the best correlation scores for TBARS values were obtained with pH (r 2 = 0.74–0.86) and TMA-N (r 2 = 0.74–0.89).
As photosynthetic organisms, algae are exposed to a combination of light and high oxygen concentration. The lack of structural damage in their organs has led to consider that their protection against oxidation would arise from their natural content on antioxidant substances (Frankel and Meyer 2000). The inhibitory effect of ethanolic extracts of B. bifurcata on the formation of tertiary lipid oxidation compounds (namely, FR value) can be explained on the basis of the high level of polyphenol compounds (40.8 ± 8.3 GA g−1 lyophilized alga) and previous related research. Thus, Glombitza et al. (1976) isolated different polyhydroxyphenyls und phenylethers from B. bifurcata and established their structure by NMR and IR spectroscopy. Later on, other authors reported the antioxidant effect of aqueous methanolic extracts (Connan et al. 2007; Le Lann et al. 2008) and of dichloromethane/methanol extracts (Zubia et al. 2009) of alga B. bifurcata on the basis of different in vitro assays (DPPH, reducing activity, and beta-carotene methods). In such studies, a marked correlation between phenol content (0.96–4.00 % dry weight) and antioxidant activity was proved. In a related alga, F. spiralis, a preliminary identification of active compounds was carried out by means of quadrupole time-of-flight mass spectrometry (Q-TOF-MS) (Tierney et al. 2013). The results supported the assumption that phlorotannins were present and were probably involved in the antioxidant activity. More recently, Ortiz et al. (2014) showed that the inclusion of different kinds of algae (Durvillaea antarctica, Ulva lactuca, Pyropia columbina, Macrocystis piryfera, and Gracilaria chilensis) in the covering medium during salmon canning led to a remarkable rancidity stabilization. Recently, García-Soto et al. (2015) showed that F. spiralis and sorbic acid, when included in a biodegradable film for megrim chilled storage, led to a significant inhibitory effect on lipid oxidation.
Conclusions
The presence of B. bifurcata extracts in the icing media employed for the chilled storage of megrim led to a significant (p < 0.05) inhibition of microbial activity (aerobes, psychrotrophs, lipolytic bacteria, proteolytic bacteria, and Enterobacteriaceae), as well as of pH, trimethylamine formation and lipid damage mechanisms (formation of free fatty acids and tertiary oxidation compounds). These inhibitory effects proved to be more intense in fish specimens corresponding to B-2 batch. According to the strong inhibitory effect on the microbial activity, this effect is considered the main reason that would explain an extended shelf life in the batches treated with the alga, as compared with the control batch.
Most studies on the preservative (namely, antioxidant and antimicrobial) activity of algae have been performed in vitro; however, the practical data derived from studies with commercial foods are still scarce. In this sense, the icing medium proposed in this work may constitute a promising strategy in order to open the way to the application of natural algae extracts for fish storage, to enhance the retention of quality during storage and commercialization.
References
Ashie, I., Smith, J., & Simpson, B. (1996). Spoilage and shelf-life extension of fresh fish and shellfish. Critical Reviews in Food Science and Nutrition, 36, 87–121.
Aubourg, S., Losada, V., Gallardo, J., Miranda, M., & Barros-Velázquez, J. (2006). On-board quality preservation of megrim (Lepidorhombus whiffiagonis) by a novel ozonised-slurry ice system. European Food Research Technology, 223, 232–237.
Ben-Gigirey, B., Baptista, V., de Sousa, J., Villa, T., & Barros-Velázquez, J. (1998). Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. Journal of Food Protection, 61, 608–615.
Ben-Gigirey, B., Baptista, V., de Sousa, J., Villa, T., & Barros-Velázquez, J. (1999). Histamine and cadaverine production by bacteria isolated from fresh and frozen albacore (Thunnus alalunga). Journal of Food Protection, 62, 933–939.
Ben-Gigirey, B., Baptista, V., de Sousa, J., Villa, T., & Barros-Velázquez, J. (2000). Characterization of biogenic amine-producing Stenotrophomonas maltophilia strains isolated from white muscle of fresh and frozen albacore tuna. International Journal of Food Microbiology, 57, 19–31.
Bligh, E., & Dyer, W. (1959). A rapid method of total extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Bouzidi, N., Daghbouche, Y., El Hattab, M., Aliche, Z., Culioli, G., Piovetti, L., Garrigues, S., & De la Guardia, M. (2008). Determination of total sterols in brown algae by Fourier transform infrared spectroscopy. Analytica Chimica Acta, 616, 185–189.
Braune, W. (2008). Meeresalgen. Ein farbbilführer zu den verbreiteten benthischen grün- Braun- und rotalgen der weltmeere (pp. 1–596). Ruggell: Gantner A. R.
Campos, C., Gliemmo, M., Aubourg, S., & Barros-Velázquez, J. (2012). Novel Technologies for the Preservation of Chilled Aquatic Food Products. In A. McElhatton, & P. Amaral Sobral (Eds.), Novel Technologies in Food Science, Chapter 13 (pp. 299–323). New York, USA: Springer.
Chapman, R., & McKay, J. (1949). The estimation of peroxides in fats and oils by the ferric thiocyanate method. Journal of the American Oil Chemists’ Society, 26, 360–363.
Connan, S., Deslandes, E., & Gall, E. (2007). Influence of day-night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. Journal of Experimental Marine Biology and Ecology, 349, 359–369.
Culioli, G., Daoudi, M., Ortalo-Magne, A., Valls, R., & Piovetti, L. (2001). (S)-12-hydroxygeranylgeraniol-derived diterpenes from the brown alga Bifurcaria bifurcata. Phytochemistry, 57, 529–535.
Díaz-Rubio, M. E., Pérez-Jiménez, J., & Saura-Calixto, F. (2009). Dietary fiber and antioxidant capacity in Fucus vesiculosus products. International Journal of Food Science and Nutrition, 60, 23–34.
European Council Regulation (1997). European Community (EC), No 258/97, 27 January 1997 concerning novel foods and novel food ingredients. CELEX-EUR Official Journal L-43, 14/02/1997 (pp. 1–7).
FAO (2007). Fishery statistics. Capture production. Yearbook 2005, 101/1, p. 97. Rome, Italy: Food and Agriculture Organization of the United Nations.
Fleurence, J., Morançais, M., Dumay, J., Decottignies, P., Turpin, V., Munier, M., García-Bueno, N., & Jaouen, P. (2012). What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends in Food Science and Technology, 27, 57–61.
Frankel, E., & Meyer, A. (2000). The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. Journal of the Science of Food and Agriculture, 80, 1925–1941.
García-Soto, B., Miranda, J., Rodríguez-Bernaldo de Quirós, A., Sendón, R., Rodríguez-Martínez, A., Barros-Velázquez, J., & Aubourg, S. (2015). Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). International Journal of Food Science and Technology. doi:10.1111/ijfs.12821.
Glombitza, K., & Rösener, H. (1974). Bifuhalol: Ein diphenyläter aus Bifurcaria bifurcata. Phytochemistry, 13, 1245–1247.
Glombitza, K., Rösener, H., & Koch, M. (1976). Polyhydroxyoligophenyle und phenyläther aus Bifurcaria bifurcata. Phytochemistry, 15, 1279–1281.
Gómez-Ordóñez, E., Jiménez-Escrig, A., & Rupérez, P. (2010). Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Research International, 43, 2289–2294.
Gómez-Ordóñez, E., & Rupérez, P. (2011). FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids, 25, 1514–1520.
Gupta, S., & Abu-Ghannam, N. (2011). Bioactive potential and possible health effects of edible brown seaweeds. Trends in Food Science and Technology, 22, 315–326.
Halldorsdóttir, S., Sveinsdóttir, H., Gudmundsdóttir, A., Thorkelsson, G., & Kristinsson, H. (2014). High quality fish protein hydrolysates prepared from by-product material with Fucus vesiculosus extract. Journal of Functional Foods, 9, 10–17.
Le Lann, K., Jégou, C., & Stiger-Pouvreau, V. (2008). Effect of different conditioning treatments on total phenolic content and antioxidant activities in two sargassacean species: comparison of the frondose Sargassum muticum (yendo) fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycological Research, 56, 238–245.
Lowry, R., & Tinsley, I. (1976). Rapid colorimetric determination of free fatty acids. Journal of the American Oil Chemists’ Society, 53, 470–472.
Oms-Oliu, G., Martín-Belloso, O., & Soliva-Fortuny, R. (2010). Pulsed light treatments for food preservation. A review. Food and Bioprocess Technology, 3, 13–23.
Oral, N., Gülmez, M., Vatansever, L., & Güven, A. (2008). Application of antimicrobial ice for extending shelf life of fish. Journal of Food Protection, 71, 218–222.
Ortiz, J., Vivanco, J., & Aubourg, S. (2014). Lipid and sensory quality of canned Atlantic salmon (Salmo salar): Effect of the use of different seaweed extracts as covering liquids. European Journal of Lipid Science and Technology, 116, 596--605.
Özyurt, G., Kuley, E., Balikçi, E., Kaçar, Ç., Gökdogan, S., Etyemez, M., & Özogul, F. (2012). Effect of the icing with rosemary extract on the oxidative stability and biogenic amine formation in sardine (Sardinella aurita) during chilled storage. Food and Bioprocess Technology, 5, 2777–2786.
Paiva, L., Lima, E., Ferreira Patarra, R., Neto, A., & Baptista, J. (2014). Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chemistry, 164, 128–135.
Pastoriza, L., Bernárdez, M., Sampedro, G., Cabo, M., & Herrera, J. (2008). The use of water and ice with bactericide to prevent onboard and onshore spoilage of refrigerated megrim (Lepidorhombus whiffiagonis). Food Chemistry, 110, 31–38.
Peinado, I., Girón, J., Koutsidis, G., & Ames, J. M. (2014). Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Research International, 66, 36–44.
Quitral, V., Donoso, M. L., Ortiz, J., Herrera, M. V., Araya, H., & Aubourg, S. (2009). Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): effect of a plant extract-icing system. LWT-Food Science and Technology, 42, 1450–1454.
Rodríguez, O., Barros-Velázquez, J., Ojea, A., Piñeiro, C., & Aubourg, S. (2003). Evaluation of sensory and microbiological changes and identification of proteolytic bacteria during the iced storage of farmed turbot (Psetta maxima). Journal of Food Science, 68, 2764–2771.
Rodríguez-Bernaldo de Quirós, A., Frecha-Ferreiro, S., Vidal-Pérez, A., & López-Hernández, J. (2010). Antioxidant compounds in edible brown seaweeds. European Food Research Technology, 231, 495–498.
Sallam, K. (2007). Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control, 18, 566–575.
Sandsdalen, E., Haug, T., Stensvag, K., & Styrvold, O. (2003). The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World Journal of Microbiology and Biotechnology, 19, 777–782.
Sanjuás-Rey, M., García-Soto, B., Fuertes-Gamundi, R., Aubourg, S., & Barros-Velázquez, J. (2012). Effect of a natural organic acid-icing system on the microbiological quality of commercially relevant chilled fish species. LWT-Food Science and Technology, 46, 217–223.
Senturk, T., & Alpas, H. (2013). Effect of high hydrostatic pressure treatment (HHPT) on quality and shelf life of Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 6, 2306–2318.
Smit, A. (2004). Medicinal and pharmaceutical uses of seaweed natural products: a review. Journal of Applied Phycology, 16, 245–262.
Tierney, M., Smyth, T., Rai, D., Soler-Vila, A., Croft, A., & Brunton, N. (2013). Enrichment of phenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chemistry, 139, 753–761.
Tozawa, H., Erokibara, K., & Amano, K. (1971). Proposed modification of dyer’s method for trimethylamine determination in codfish. In R. Kreuzer (Ed.), Fish inspection and quality control (pp. 187–190). London: Fishing News Books Ltd.
Vyncke, W. (1970). Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichmittel, 72, 1084–1087.
Wang, T., Jónsdóttir, R., Kristinsson, H., Thorkelsson, G., Jacobsen, C., Yuca Hamaguchi, P., & Olafsdóttir, G. (2010). Inhibition of haemoglobin-mediated lipid oxidation in washed cod muscle and cod protein isolates by Fucus vesiculosus extract and fractions. Food Chemistry, 123, 321–330.
Zubia, M., Fabre, M. S., Kerjean, V., Le Lann, K., Stiger-Pouvreau, V., Fauchon, M., & Deslandes, E. (2009). Antioxidant and antitumoral activities of some phaeophyta from britanny coasts. Food Chemistry, 116, 693–701.
Acknowledgments
The authors thank Mr. Marcos Trigo and Ms. Monserrat López for their excellent technical assistance. Porto-Muiños (Cerceda, A Coruña, Spain) is greatly acknowledged for kindly providing the lyophilized alga. This work was supported by the Consejo Superior de Investigaciones Científicas (CSIC; Spain) through the project PIE 201370E001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miranda, J.M., Ortiz, J., Barros-Velázquez, J. et al. Quality Enhancement of Chilled Fish by Including Alga Bifurcaria bifurcata Extract in the Icing Medium. Food Bioprocess Technol 9, 387–395 (2016). https://doi.org/10.1007/s11947-015-1626-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1626-5