Abstract
Biogenic amines formation in Indian mackerel of tropical region was investigated during storage at ambient (25–29 °C) and ice temperature (0 °C) in relation with changes of amino acids content and amines forming bacteria. All amines increased significantly during storage at two temperatures except for spermidine and spermine. Histamine concentration of 363.5 ppm was detected after 16 h stored at ambient temperature. Aerobic plate count of fish stored at ambient temperature reached 6.98 log CFU g−1 after 16 h, close to the upper limit (7 log CFU g−1) suggested by International Commission on the Microbiological Specifications for Foods (ICMSF). However, proper icing procedure retarded the formation of histamine effectively, resulting only 8.31 ppm after 16 days of ice storage. Aerobic plate count of 5.99 and 7.72 log CFU g−1 were recorded for fish stored in ice after 16 days and ambient temperature after 20 h, respectively. Histamine exhibited high correlation with histidine (r2 = −0.963, P < 0.01) as well as cadaverine with lysine (r2 = −0.750, P < 0.05). However, tyramine-tyrosine demonstrated a weaker relationship (r2 = −0.138, P > 0.05). As storage time progressed, the amines forming bacteria grew significantly except for that stored in ice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biogenic amines are natural nitrogenous compounds with aliphatic, aromatic or heterocyclic structure (Rabie and Toliba 2011). These compounds can be found in living cells and wide variety of foods and drinks including fish, cheese, wine, meat, vegetable and dairy product. Biogenic amines play important physiological roles in living cells. However, consumption of high amount of biogenic amine may result in food poisoning. The presence of high level of amines in foods is mainly due to bacterial decarboxylation activity toward amino acids (Chong et al. 2011). Histamine is the most toxic biogenic amines responsible for nearly all food amines intoxication (Zaman et al. 2010). The intoxication symptoms are usually mild including reddening on the face, neck and upper chest, vomiting, flushing, nausea and abdominal cramps. Putrescine and cadaverine could enhance the toxicity of histamine by inhibiting diamine oxidase involved in histamine metabolism (Taylor 1986). Whilst tyramine is reported to induce migraine, increase blood pressure and cause hypertension (Shalaby 1996).
Fish is a perishable food and rich in amino acids, which are the precursors of biogenic amines formation. During fish spoilage, bacterial decarboxylases catalyze the conversion of amino acids into biogenic amines. Scombroid poisoning incidents were frequently caused by the consumption of scombroid fish such as mackerel, bonito, tuna, and saury which are rich in histidine (Taylor 1986). However, the available literatures generate inconsistent results on the correlation between the formation of amines and their precursors, amino acids. Wendakoon et al. (1990) found correlation between amino acids and biogenic amines, whereas, Ruiz-Capillas and Moral (2004) only correlated histamine concentration to that of histidine concentration, other amines were not correlated to its corresponding amino acid. Other authors reported the absence of such correlation (Ababouch et al. 1991; Antoine et al. 2002; Ruiz-Capillas and Moral 2002). The profile of biogenic amines in fish is also influenced by the type of microbial flora found in the aquatic environment.
Fresh fish is the most important fishery product with more than one third of world fish production is being traded internationally (FAO 2011). Since biogenic amines might affect the quality and safety of fresh fish, its permissible concentration in the product should be regulated. The United States Food and Drug Administration established the permissible limit of histamine at 50 ppm in edible fish (FDA 1996). Other countries have also set the upper levels of histamine concentrations for edible fish including Europe, 100 ppm, South Africa, 100 ppm and Australia, 200 ppm (Auerswald et al. 2006). The formation of biogenic amines has been studied extensively in various kinds of fish such as tuna, sardine, herring and anchovies. Indian mackerel is common food fish in Southeast Asian countries and can be processed into fish sausage and cracker (keropok). Mackerel is also an important commodity in international trading. However, there is no study available on the profile of biogenic amines and amino acids in Indian mackerel of tropical region. Therefore, this experiment was conducted to examine the formation of biogenic amines in Indian mackerel stored at ambient and ice temperature in relation to free amino acids and microbial changes in the muscle.
Materials and methods
Samples
Indian mackerel was obtained from Beserah, Kuantan, East Coast of Malaysia in the month of February. The fish was transported to laboratory in cooler box under ice condition after 4 h of capture. Whole fresh fish (weight of 90–130 g) were divided into two lots (each lot contained 50 fish). The first lot was stored at ambient temperature (25–29 °C) for 20 h. The second lot was stored in ice-cooler box and kept in cold room. Water in cooler box was drained off and the ice was replaced to maintain the ice temperature. Triplicate muscle samples (each sample consisted of pooled sample of three whole fish) were taken for analysis for every 4 h during ambient temperature storage and every four days during ice storage. Duplicate samples were taken for microbiological analysis.
Preparation of standard amines solution
The concentration of working standard solution of 1.0 mg ml−1 for each amine was prepared according to Hwang et al. (1997). Putrescine dihydrochloride (45.75 mg), cadaverine dihydrochloride (42.85 mg), spermidine trihydrochloride (43.85 mg), spermine tetrahydrochloride (43.0 mg), histamine dihydrochloride (41.4 mg) and tyramine hydrochloride (31.7 mg) were dissolved in 25 ml RO water as a working solution. All the standards were purchased from Sigma Aldrich (St. Louis, MO, USA).
Extraction and derivatization of amines from a fish sample
Five grams of ground fish sample were homogenized with 20 ml 6% trichloroacetic acid (TCA) for 3 min. The homogenate was centrifuged at 8000 g, 4 °C for 10 min and filtered using Whatman No. 2 filter paper. The filtrate was transferred into a volumetric flask and made up to 50 ml. The extract was then derivatized with benzoyl chloride following the procedure proposed by Hwang et al. (1997). One milliliter of extract or mixed amines solution was added with 1 ml of sodium hydroxide (2 M) and 10 μl of benzoyl chloride. The solution was homogenized by vortex mixer and then incubated at 30 °C for 40 min. Two milliliters of saturated NaCl solution was added to stop the benzoylation process and 3 ml of diethyl ether was used to extract the solution. The solution was centrifuged at 10,000 g and 4 °C for 10 min. After centrifugation, the upper layer was transferred into a vial and dried with nitrogen gas. The residue was dissolved in 1 ml of acetonitrile and filtered with 0.2 μm nylon filter. A 20 μl aliquots were injected for HPLC analysis.
Separation of biogenic amines by HPLC
Biogenic amines were determined according to the method developed by Hwang et al. (1997) with modification (Ozogul et al. 2002). The separation of amines was performed using Agilent technologies 1200 series chromatographic system (Waldron, Germany) comprising of vacuum degasser, auto sampler, quaternary pump and diode array detector. The separations were performed on C18 Sunfire™ (150 mm × 4.6 mm, 5.0 μm) column (Waters Corporation, Massachusetts, USA). The samples were detected at 254 nm. The mobile phase was pumped at 0.8 ml min−1 flow rate and consisted of acetonitrile–water solution in a gradient elution program. Initially the acetonitrile–water phase ratio was 45:55 (v/v) and after 1 min the amount of acetonitrile started to increase to achieve 60:40 (v/v), 70:30 (v/v), 80:20 (v/v) and 95:5 (v/v) level in 4th, 8th, 18th and 18.5th min. The composition did not change for the next 3.5 min, and from 22th min to 22.5th min the amount of acetonitrile started to decrease reaching 45:55 (v/v) level. This ratio was maintained for 2.5 min.
Analysis of amino acids
Concentrations of amino acids in samples were analyzed by HPLC. Freeze dried fish powder of 0.06 g was weighed in a test tube. Fifteen milliliters of 6 N HCl was added, the mixture was mixed and flushed with nitrogen for 1 min and was capped immediately. The tube was put in an oven at 110 °C for 24 h. After the hydrolysis, 10 ml AABA (α-amino butyric acid) internal solution was added. The internal standard solution was prepared in 0.1 N aqueous hydrochloric acid containing 0.2578 g of AABA per liter. The sample was poured into volumetric flask, made up to 50 ml and filtered through a syringe filter. For derivatization, 10 μl of the filtered solution was placed into tubes and dried under vacumm for 30 min. A volume of 20 μl of redrying solution (mixture of 200 μl methanol, 200 μl of water and 100 μl triethylamine) was added, mixed and dried again under vacumm for 30 min. A volume of 20 μl of derivatization reagent was added and left at ambient temperature for 20 min, before drying under vacumm for 30 min. The derivatization reagent was comprised of methanol (70%, v/v), triethylamine (10%, v/v), PITC (10%, v/v) and water (10%, v/v). Finally, 100 μl of sample diluents (Buffer A) were added to the dried tube, mixed and ready for injection.
HPLC was conducted using Agilent technologies 1,200 series chromatographic system (Waldron, Germany). The separations were performed on Purospher® STAR RP-18e (250 mm × 4.6 mm, 5.0 μm) column (Merck, Darmstadt, Germany). Buffer A (0.1 M ammonium acetate, pH6.5) and buffer B (0.1 M ammonium acetate containing acetonitrile and methanol, 44:46:10, v/v, pH6.5) were employed as mobile phase in linear gradient program. The flow rate was 1.0 ml min−1 and monitored at 254 nm. The program began with 100% A and 0% B; then a linear gradient was used until 15 min, lowering A to 90% (increasing B to 10%), from 15 min to 30 min 60% A. The amount of buffer A decreased to reach 50% and 0% in 40th and 50th min. Finally, buffer A increased to 100% from 55 min to 57 min. This ratio was maintained until 60th min. Retention times and spectra were compared with pure standards.
Microbiogical analysis
Twenty five grams of fish flesh from each replicate of pooled sample of three fish was transferred aseptically to 225 ml sterile peptone water (0.1% peptone water and 0.85% NaCl) and blended in sterile stomacher bags for 60s. The homogenate was serially diluted with 9 ml peptone water. Dilutions of 0.1 ml was spread on aerobic plate count agar (APC) containing 0.5% NaCl. After the plates were incubated at 37 °C for 48 h, the bacterial colonies were counted. Histamine, cadaverine and putrescine forming bacteria were detected using media supplemented with histidine, lysine or ornithine according to Joosten and Northolt (1989). Bacterial colonies surrounded by purple halo were counted as amines forming bacteria. This incubation temperature was selected for the growth of mesophilic microflora which is reported as a major amines former in literature. Temperature of 30–40 °C was also shown to be optimum for histidine decarboxylase activity of several psychrotrophic microflora (Kanki et al. 2007). As a limitation, this parameter is not favourable to the growth of psychrotrophic bacteria.
Statistical analysis
The effect of storage time on biogenic amines concentration was analyzed using one-way analysis of variance (ANOVA). The means were compared using Duncan test. The significant difference of the results was set at P < 0.05. The software used was SPSS Version 17.0 for windows (SPSS Inc., Chicago, IL, USA). The means were compared in triplicate for biochemical analysis and in duplicate for microbiological analysis. Regression analysis was conducted to determine the relationship between the biogenic amine and amino acid contents of the samples.
Results and discussion
Biogenic amines profile at ambient temperature
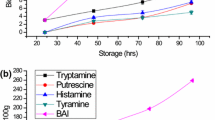
The profile of biogenic amines in Indian mackerel stored at ambient temperature is given in Fig. 1(a). At the beginning of the study, only spermidine and spermine were detected in fresh Indian mackerel. The formation of cadaverine and histamine began before other biogenic amines at 12 h. The considerable amount of biogenic amines was detected after 16 h at ambient temperature. All biogenic amines increased as storage time progressed except for spermidine and spermine. Concentration of biogenic amines increased most rapidly between 16 h to 20 h. Spermidine and spermine showed fluctuation throughout storage period (data not shown). Concentration of spermidine ranged from 5.29 ppm to 6.62 ppm, whereas, spermine ranged from 10.70 ppm to 11.27 ppm over entire storage trial.
Changes in biogenic amines during storage (a) at ambient temperature (25–29 °C) and (b) in ice (0 °C) and (c) in amino acids during storage at ambient temperature (25–29 °C) in Indian mackerel (n = 3). Error bars represent SE. Means with different superscript were significantly different during different storage time. For graph (A), each amine shares the same superscript for the same storage time
In fresh Indian mackerel, spermidine and spermine were the major biogenic amines detected and no other biogenic amines were found. Similar results were reported from other studies (Katikou et al. 2006; Krízek et al. 2004). No histamine was detected in fresh Indian mackerel. Other authors found negligible amount of histamine in fresh sample rich in histidine in anchovy (Chotimarkorn 2011), skipjack tuna (Rossi et al. 2002) and Atlantic mackerel (Prester et al. 2009).
Figure 1(a) shows that cadaverine formation began before putrescine and tyramine and increased steadily at ambient temperature. Cadaverine has been used as potential spoilage indicator (Kumudavally et al. 2011). In the present study, cadaverine was correlated well with storage time (r = 0.827, P < 0.05) at ambient temperature as compared to other biogenic amines. Our results were in agreement with Antoine et al. (2002) and Rossi et al. (2002) where cadaverine and histamine were the major biogenic amines produced and histamine was the most abundant biogenic amines in mahi-mahi and skipjack tuna.
Spermidine and spermine were not significant different throughout storage at ambient temperature. Gokoglu et al. (2004) reported the similar findings for spermidine and spermine in sardine. The concentration of spermine was higher than spermidine during ambient temperature and ice storage and this is typical for foods of animal origin (Kalac and Krausová 2005).
After 20 h storage at ambient temperature, the concentration of tyramine increased progressively to 221 ppm. This value exceeded 100 ppm, the upper limit proposed by Santos (1996). The formation of tyramine in fish has not been frequently reported, however, the concentration of tyramine in fresh fish from retail market was reported ranging from 100.8 ppm to 221.8 ppm (Kim et al. 2009).
The large increase of biogenic amines was observed when aerobic plate count reached 7 log CFU g−1. For storage at ambient temperature, the concentration of histamine was below the level of 10 ppm after 12 h. This level increased to 363.50 ppm after 16 h exceeding 50 ppm the legal level established by FDA for fish species of Scombridae and Clupeidae family. This supports the report of Naila et al. (2011), where considerable amount of histamine was detected after 16 h without chilling. Fish with high histidine content such as Atlantic mackerel and sardines were also reported to exceed 50 ppm for less than 24 h stored at 22 °C (Prester et al. 2009).
In this study, the histamine increased to maximum value of 961.64 ppm after 20 h at 27 °C. This value is higher than that found in Indian mackerel (162 ppm) kept at 32 °C for 24 h (Shakila et al. 2003). There is very few literatures report on concentration of histamine in Indian mackerel. In other fish of scombroid family, more than 500 ppm histamine was detected in snoek and tuna after 1 day exposure at 30 °C (Auerswald et al. 2006). In Atlantic mackerel, 1,090 ppm was detected after 24 h at 22 °C (Prester et al. 2009).
Biogenic amines profile at ice temperature
Changes of biogenic amines in Indian mackerel stored at 0 °C are given in Fig. 1(b). Among major biogenic amines, cadaverine was the first amine developed in Indian mackerel at day 4 of ice storage. There was large increase for putrescine, tyramine and histamine between day 12 to 14 of ice storage. Spermidine was observed to increase from initial value of 6.62 ppm to 12.42 ppm at the end of storage. Spermine did not differed significantly over entire ice storage.
In disagreement with previous studies, it was interesting to notice that spermidine increased significantly after 16 days of ice storage, whereas, spermine was not differed significantly. In the present study, putrescine was found as the most abundant biogenic amines during ice storage. Spermidine is converted to putrescine under action of spermidine synthase (Larqué et al. 2007). The availability of putrescine may contribute to spermidine formation.
Ice storage was found to retard the formation of histamine in Indian mackerel effectively. In the present study, samples stored at ice temperature had highest levels of histamine of 8.31 ppm at the end of storage period. Frank et al. (1985) suggested that mesophilic microflora is more important in producing biogenic amines as compared to psychrophilic microflora. Economou et al. (2007) only detected low level of histamine (<50 ppm) in tuna loin after 12 days at 0–2 °C.
Amino acids
Changes of amino acids in Indian mackerel stored at ambient temperature are given in Fig. 1(c). In general, the concentration of histidine, lysine and tyrosine decreased after 12 h stored at ambient temperature. Similar result was also found in sardine (Ababouch et al. 1991) and fish sauce (Zaman et al. 2011). In contrast, free amino acids were reported to increase during storage in dry-cured grass carp (Zhang et al. 2011). Initially, the concentration of histidine showed fluctuation, after 12 h it reduced significantly from 42.02 mg g−1 DW to 32.59 mg g−1 DW, and then decreased drastically to a minimum value of 19.96 mg g−1 DW at the end of storage. For lysine and tyrosine, the concentrations showed a slight decreasing trend after 12 h. The concentration of lysine and tyrosine decreased from 76.23 mg g−1 DW to 69.26 mg g−1 DW and from 31.28 mg g−1 DW to 27.64 mg g−1 DW, respectively. However, the changes were not significantly different throughout storage period. For ice storage, amino acids did not change significantly over storage period (data not shown). There were no correlations between these amino acids with their respective biogenic amines in sample stored at 0 °C.
Concentration of histidine decreased according to storage time at ambient temperature. There was a strong correlation between histamine and histidine stored at this temperature (r2 = −0.963, p < 0.01). The rate of decrease of histidine was higher than that of lysine and tyrosine reflecting the rate of formation of biogenic amines. After 16 h and 20 h of storage, histamine was produced in high level and the increase was in line with the decrease of histidine. The decrease of histidine was also correlated well with the increase of bacterial count during the same period. This showed the direct relationship between the histamine and its precursor.
Concentration of lysine was higher than that of histidine and tyrosine in muscle of Indian mackerel. There was good correlation between lysine and cadaverine (r2 = −0.750, p < 0.05). Although lysine was found in high concentration, but only low level of cadaverine was observed in this study. This indicates that other factors take part in biogenic amines formation besides the presence of high level of precursor. This result shows that different microflora exhibit different capability in decarboxylation of amino acid. There was a weaker correlation between tyramine and tyrosine (r2 = −0.138), P > 0.05). Concentration of tyrosine increased slightly during the first 12 h. The concentration was reduced to 28.9 and 27.64 mg g−1 DW after 16 and 20 h, respectively. The decomposition rate of tyrosine was lower than the formation rate of tyramine, suggesting that tyrosine was released during the autolytic and bacterial proteolysis of fish muscle. Antoine et al. (2002) found that cadaverine was correlated with lysine (r = −0.61) but the histamine and putrescine were poorly correlated with histidine (r = −0.42) and ornithine (r = −0.27) in mahi-mahi (Coryphae Hippurus).
Season, sex, stage of maturity, age of fish, diet, health and water salinity are the factors influencing free amino acid in muscle (Antoine et al. 1999; Haard 1992). Besides the influence of precursor, proteolysis of fish muscle also releases amino acids, contributing to amine formation (Stratton et al. 1991). The inconsistent results reported for the correlation between amino acids and amines might be explained by the variation of amino acids content, decarboxylation and proteolysis activity affected by microflora species present in fish.
Microbiological analysis
Changes of bacterial count in Indian mackerel stored at ambient and ice temperature are demonstrated in Fig. 2. Initial aerobic plate count of Indian mackerel in this study was 4 log CFU g−1. The bacterial counts increased significantly during storage except for cadaverine and putrescine forming bacteria in fish stored at 0 °C. Samples stored at ambient temperature showed a faster increase rate in bacterial count (Fig. 2a). The aerobic plate count was 6.98 log CFU g−1 after 16 h stored at ambient temperature. This value was almost reached 7 log CFU g−1, the upper limit established by The International Commission on Microbiological Specification for Foods (ICMSF 1986) for rejection of raw fish. For ice storage, the aerobic plate count increased slowly and reached only 5.99 log CFU g−1 after 16 days (Fig. 2b).
Cadaverine and putrescine forming bacterial counts were lower than aerobic plate count by average count of 0.7 log CFU g−1 for the first 8 h in fish stored at ambient temperature. During later stage of storage, the number of cadaverine and putrescine forming bacteria increased and tended to be similar to that found in aerobic mesophilic bacteria at ambient temperature. It seemed likely cadaverine and putrescine forming bacteria were mostly mesophilic bacteria in this study.
For Indian mackerel stored in ice, cadaverine and putrescine forming bacteria remained constant throughout storage period. The results revealed that cadaverine and putrescine forming bacteria were able to survive and decarboxylate amino acids at 0 °C. At the end of storage, 68.3% and 73.5% of aerobic mesophilic bacteria was cadaverine and putrescine forming bacteria. In contrast, Lakshmanan et al. (2002) reported that 96% of the bacterial isolates were cadaverine and putrescine forming bacteria in emperor fish during later stage of ice storage. Therefore, it could be suggested that cadaverine and putrescine forming bacteria in the present study was mostly mesophilic bacteria and was not adapted to grow well at 0 °C.
The proportion of histamine forming bacteria from total microflora count in fish was lower as compared to other bacteria over experimental trial. It was shown that histamine forming bacterial count in fish stored at ambient temperature was lower than aerobic plate count by average count of 2.4 log CFU g−1 throughout storage period. The effect of ice storage was profound in inhibiting the incidence of histamine forming bacteria. The bacteria was only detected after 12 days in ice storage and remained lower than 1.5 log CFU g−1 over storage period.
For ambient temperature storage, concentration of histamine tallied with aerobic plate count achieving acceptable limit after 16 h of storage. Significant amount of histamine was only detected when the sample reached aerobic plate count close to 7 log CFU g−1. This finding was in agreement to that reported by Dalgaard et al. (2006). Aerobic plate count never exceeded 7 log CFU g−1 during ice storage. Correlation was observed between concentration of histamine and aerobic plate count in sample stored at ambient temperature (r2 = 0.678, P < 0.05), but such correlation was not found at 0 °C. The high concentration of histamine in fish stored at ambient temperature could be explained by the proliferation of mesophilic bacteria under optimum growth condition as compared to ice storage.
The use of decarboxylating agar provides a simple and rapid method to identify the strain with amino acid decarboxylation activity. Some authors reported that this method may produce false positive result due to accumulation of alkaline compounds (Kung et al. 2006; Roig-Sagués et al. 1996). However, the previous studies concluded that this method is reliable to determine strain with histidine, tyrosine and ornithine decarboxylation activities confirmed by enzymic or chromatographic method (Roig-Sagués et al. 1997; Moreno-Arribas et al. 2003).
Mietz and Karmas and biogenic amines index
Biogenic amines were suggested by several authors as a quality indicator for fish. Mietz and Karmas (1977) introduced the quality index calculated from the sum of histamine, cadaverine, putrescine over 1 + (spermidine + spermine). Veciana-Nogues et al. (1997) suggested biogenic amines index calculated from the sum of histamine, putrescine, tyramine and cadaverine. Recently, Bakar et al. (2010) reported that Mietz and Karmas index and Biogenic amines index are suitable quality indicators for barramundi. Table 1 shows the different biogenic amine index of Indian mackerel stored at 0 °C and ambient temperature. These two biogenic amines indexes were found in very low value in fish during the first 12 h of storage at ambient temperature. After 16 h of storage at ambient temperature, Mietz and Karmas index and biogenic amines index reached 29.7 ppm and 567.2 ppm respectively, exceeding the rejection value of 10 and 100 ppm which were suggested by Mietz and Karmas (1977) and Muscarella et al. (2005), respectively. This result was in accordance with aerobic plate count and histamine concentration. The bacterial count and histamine achieved 7 log CFU g−1 and 50 ppm respectively after 16 h. After 20 h at ambient temperature, biogenic amines index achieved 1604 ppm. Prester et al. (2009) demonstrated a similar result in Atlantic mackerel (1513 ppm) and sardine (1097 ppm) stored at 22 °C for 24 h. For storage at 0 °C, Mietz and Karmas index never exceeded the value of 10 but biogenic amines index reached 100.3 ppm. This indicated that Mietz and Karmas index and biogenic amines index could be used as a guiding limit value for Indian mackerel. However, more studies should be conducted to establish the guiding limit.
Conclusion
Shelf life of fish in this study was 16 h stored at ambient temperature according to histamine upper level established by US FDA and aerobic plate count set by ICMSF. A direct correlation was found between histidine and histamine produced in fish muscle. The result suggests that proteolysis and decarboxylation influence the balance of amino acids and precursor-amine relationship. The decarboxylation and proteolysis could also be influenced by the strain of microbial group present in fish. Histamine can be produced in high level in Indian mackerel and poses safety hazard if the fish exposed to temperature abuse.
References
Ababouch LH, Afilal ME, Benabdeljelil H, Busta FF (1991) Quantitative changes in bacteria, amino acids and biogenic amines in sardine (Sardina pilchardus) stored at ambient temperature (25–28 °C) and in ice. Int J Food Sci Technol 26:297–306
Antoine FR, Wei CI, Littell RC, Marshall MR (1999) HPLC method for analysis of free amino acids in fish using o- phthaldialdehyde precolumn derivatization. J Agric Food Chem 47:5100–5107
Antoine FR, Wei CI, Otwell WS, Sims CA, Littell RC, Hogle AD, Marshall MR (2002) Analysis of biogenic amines and their precursor free amino acids in mahi-mahi (coryphaena hippurus). J Food Biochem 26:131–152
Auerswald L, Morren C, Lopata AL (2006) Histamine levels in seventeen species of fresh and processed South African seafood. Food Chem 98:231–239
Bakar J, Yassoralipour A, Bakar FA, Rahman RA (2010) Biogenic amine changes in barramundi (Lates calcarifer) slices stored at 0 °C and 4 °C. Food Chem 119:467–470
Chong CY, Abu Bakar F, Russly AR, Jamilah B, Mahyudin NA (2011) The effects of food processing on biogenic amines formation. Int Food Res J 18:837–846
Chotimarkorn C (2011) Quality changes of anchovy (Stolephorus heterolobus) under refrigerated storage of different practical industrial methods in Thailand. J Food Sci Technol 1–9
Dalgaard P, Madsen HL, Samieian N, Emborg J (2006) Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone) - Effect of modified atmosphere packaging and previous frozen storage. J Appl Microbiol 101:80–95
Economou V, Brett MM, Papadopoulou C, Frillingos S, Nichols T (2007) Changes in histamine and microbiological analyses in fresh and frozen tuna muscle during temperature abuse. Food Addit Contam 24:820–832
FAO (2011) Fisheries and Aquaculture topics. Utilization and trade. Topics Fact Sheets. In: FAO Fisheries and Aquaculture Department. http://www.fao.org/fishery/topic/2888/en. Accessed 8 March 2011
FDA (1996) Decomposition and histamine in raw, frozen tuna and mahi-mahi, canned tuna and related species. Compliace Policy Guide. 7108.240.
Frank HA, Baranowski JD, Chongsiriwatana M, Brust PA, Premaratne RJ (1985) Identification and decarboxylase activities of bacteria isolated from decomposed mahimahi (Coryphaena hippurus) after incubation at 0 and 32 °C. Int J Food Microbiol 2:331–340
Gokoglu N, Yerlikaya P, Cengiz E (2004) Changes in biogenic amine contents and sensory quality of sardine (sardina pilchardus) stored at 4 °C and 20 °C. J Food Qual 27:221–231
Haard NF (1992) Control of chemical composition and food quality attributes of cultured fish. Food Res Int 25:289–307
Hwang DF, Chang SH, Shiua CY, Tuu-jyi C (1997) High-performance liquid chromatographic determination of biogenic amines in fish implicated in food poisoning. J Chromatogr B: Biomed Sci Appl 693:23–30
International Commission on the Microbiological Specifications for Foods (ICMSF) (1986) Microorganisms in foods, sampling for microbiological analysis: principles and specific applicationts, 2nd edn. University of Toronto Press, Toronto
Joosten HMLJ, Northolt MD (1989) Detection, growth and amine producing capacity of lactobacilli in cheese. Appl Environ Microbiol 55:2356–2359
Kalac P, Krausová P (2005) A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem 90:219–230
Kanki M, Yoda T, Tsukamoto T, Baba E (2007) Histidine decarboxylases and their role in accumulation of histamine in tuna and dried saury. Appl Environ Microbiol 73(5):1467–1473
Katikou P, Georgantelis D, Paleologos EK, Ambrosiadis I, Kontominas MG (2006) Relation of biogenic amines’ formation with microbiological and sensory attributes in Lactobacillus-inoculated vacuum-packed rainbow trout (Oncorhynchus mykiss) fillets. J Agric Food Chem 54:4277–4283
Kim MK, Mah JH, Hwang HJ (2009) Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem 116:87–95
Krízek M, Vácha F, Vorlová L, Lukásová J, Cupáková S (2004) Biogenic amines in vacuum-packed and non-vacuum-packed flesh of carp (Cyprinus carpio) stored at different temperatures. Food Chem 88:185–191
Kumudavally K, Tabassum A, Radhakrishna K, Bawa A (2011) Effect of ethanolic extract of clove on the keeping quality of fresh mutton during storage at ambient temperature (25 ± 2 °C). J Food Sci Technol 48:466–471
Kung HF, Lee YH, Teng DF, Hsieh PC, Wei CI, Tsai YH (2006) Histamine formation by histamine-forming bacteria and yeast in mustard pickle products in Taiwan. Food Chem 99(3):579–585
Lakshmanan R, Shakila RJ, Jeyasekaran G (2002) Survival of amine-forming bacteria during the ice storage of fish and shrimp. Food Microbiol 19:617–625
Larqué E, Sabater-Molina M, Zamora S (2007) Biological significance of dietary polyamines. Nutr 23:87–95
Mietz JL, Karmas E (1977) Chemical index of canned tuna determined by high pressure liquid chromatography. J Food Sci 42:155–158
Moreno-Arribas MV, Polo MC, Jorganes F, Muñoz R (2003) Screening of biogenic amine production by lactic acid bacteria isolated from grape must and wine. Internat J Food Microbiol 84:117–123
Muscarella M, Iammarino M, Centonze D, Palermo C (2005) Measurement of histamine in seafood by HPLC, CE, and ELISA: comparison of three techniques. Vet Res Commun 29:343–346
Naila A, Flint S, Fletcher GC, Bremer PJ, Meerdink G (2011) Biogenic amines and potential histamine Forming bacteria in Rihaakuru (a cooked fish paste). Food Chem 128(2):479–484
Ozogul, F, Taylor, KDA, Quantick, P, Ozogul, YO (2002) Biogenic amines formation in atlantic herring (Clupea harengus) stored under modified atmosphere packaging using rapid HPLC method. Int J Food Sci Tech 37:515–522
Prester L, Macan J, Varnai VM, Orct T, Vukusic J, Kipcic D (2009) Endotoxin and biogenic amine levels in Atlantic mackerel (Scomber scombrus), sardine (Sardina pilchardus) and Mediterranean hake (Merluccius merluccius) stored at 22 °C. Food Addit Contam-A Chem, Anal, Control, Expo Risk Assess 26:355–362
Rabie M, Toliba A (2011) Effect of irradiation and storage on biogenic amine contents in ripened Egyptian smoked cooked sausage. J Food Sci Technol 1–7. Article in Press. DOI: 10.1007/s13197-011-0444-7
Roig-Sagués AX, Hérnandez-Herrero MM, López-Sabater EI, Rodriguez-Jerez JJ, Mora-Ventura MT (1996) Histidine-decarboxylase activity of bacteria isolated from raw and ripened “Salchichón”, a Spanish cured sausage. J Food Prot 59:516–520
Roig-Sagués AX, Hernández-Herrero MM, López-Sabater EI, Rodríguez-Jerez JJ, Mora-Ventura MT (1997) Evaluation of three decarboxylating agar media to detect histamine and tyramine-producing bacteria in ripened sausages. Lett Appl Microbiol 25:309–312
Rossi S, Lee C, Ellis PC, Pivarnik LF (2002) Biogenic amines formation in Bigeye tuna steaks and whole Skipjack tuna. J Food Sci 67:2056–2060
Ruiz-Capillas C, Moral A (2002) Effect of controlled and modified atmospheres on the production of biogenic amines and free amino acids during storage of hake. Eur Food Res Technol 214:476–481
Ruiz-Capillas C, Moral A (2004) Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmospheres. Amino Acids 26:125–132
Santos MHS (1996) Biogenic amines: their importance in foods. Int J Food Microbiol 29:213–231
Shakila RJ, Vijayalakshmi K, Jeyasekaran G (2003) Changes in histamine and volatile amines in six commercially important species of fish of the Thoothukkudi coast of Tamil Nadu, India stored at ambient temperature. Food Chem 82:347–352
Shalaby AR (1996) Significance of biogenic amines to food safety and human health. Food Res Int 29:675–690
Stratton JE, Hutkins RW, Taylor SL (1991) Biogenic amines in cheese and other fermented foods: A Review. J Food Prot 54:460–470
Taylor SL (1986) Histamine food poisoning: toxicology and clinical aspects. Crit Rev Toxicol 17:91–128
Veciana-Nogues MT, Marine-Font A, Vidal-Carou MC (1997) Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines, and organoleptic changes. J Agric Food Chemi 45:2036–2041
Wendakoon CN, Murata M, Sakaguchi M (1990) Comparison of non-volatile amine formation between the dark and white muscles of mackerel during storage. Nippon Suisan Gakkaishi 56(5):809–818
Zaman MZ, Bakar FA, Selamat J, Bakar J (2010) Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J Food Sci 28:440–449
Zaman MZ, Abu Bakar F, Jinap S, Bakar J (2011) Novel starter cultures to inhibit biogenic amines accumulation during fish sauce fermentation. Int J Food Microbiol 145:84–91
Zhang J, Liu Z, Hu Y, Fang Z, Chen J, Wu D (2011) Effect of sucrose on the generation of free amino acids and biogenic amines in Chinese traditional dry-cured fish during processing and storage. J Food Sci Technol 48:69–75
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chong, C.Y., Abu Bakar, F., Rahman, R.A. et al. Biogenic amines, amino acids and microflora changes in Indian mackerel (Rastrellinger kanagurta) stored at ambient (25–29 °C) and ice temperature (0 °C). J Food Sci Technol 51, 1118–1125 (2014). https://doi.org/10.1007/s13197-012-0621-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0621-3