Abstract
The present study was aimed to investigate the combined effects of vacuum packaging and mint extract treatment on the quality changes of gutted Indian mackerel (Rastrelliger kanagurta) during storage at 0–2 °C for 22 days. Biochemical, total viable count and sensory quality of chill stored mackerel were analysed at periodic intervals. Mint extract treated [dipping in 0.5% (w/v) solution of mint extract for 30 min] and vacuum packed fishes (MEVP) had significantly lower total volatile base nitrogen and trimethyl amine nitrogen compared to those packed under vacuum (CVP) and air (CAP) without mint extract treatment. Nucleotide degradation rate was lower in MEVP followed by CVP and CAP. Vacuum packaging in combination with ME treatment significantly inhibited lipid hydrolysis and lipid oxidation in mackerel as observed from its lower free fatty acid, peroxide value and thiobarbituric acid reactive substances values. Synergistic use of mint extract and vacuum packaging has markedly controlled microbial proliferation in the samples. Based on sensory evaluation, shelf life of Indian mackerel stored at 0–2 °C was determined as 13 days for CAP group, 16 days for CVP group and 21 days for MEVP group, respectively. The present study revealed that combination of vacuum packaging and mint extract treatment can be a promising technology to improve the storage quality of chill stored gutted mackerel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unique biochemical composition of seafood makes it more vulnerable to rapid spoilage, resulting in limited shelf life under normal refrigerated storage conditions. Consumer demand for minimally processed and more natural fish products is continuously increasing. Vacuum packaging is an established technology for minimal food preservation and works by changing the gas proportions in a food environment by removing O2. Vacuum packaging is an ideal technology for preserving oily fishes and fish products under refrigerated or ambient storage conditions (Ozogul et al. 2004; Pezeshk et al. 2011). The positive effects of vacuum packaging can be further enhanced by the treatment with antimicrobials/antioxidants. Addition of synthetic preservatives in food products is strictly regulated due to the toxicological issues of their metabolites (Wilson and Bahna 2005). Hence, recent attention is widely focused on the bioactivities of natural preservatives such as antioxidants present in plant extracts and residual sources. Antioxidants and antimicrobial activity of plant extracts have been investigated for many years and those with potential activities have been applied in several food systems as antioxidant or antibacterial agents (Ahn et al. 2007; Kanatt et al. 2007).
The genus Mentha is an important member of Labitaceae family and is being used as a herbal tea, flavouring agent and as a medicinal plant. Extracts and essential oil of Mentha species have potential antioxidant and antibacterial activities (Moreira et al. 2005; Kanatt et al. 2007). Rosemarinic acid, caffeic acid, eriocitrin and luteolin are the major phenolics reported in Mentha species (Padmini et al. 2010; Kappa et al. 2013) with antibacterial and antioxidant activities (McKay and Blumberg 2006; Singh et al. 2010). Even though there is a vast literature of antioxidant/antibacterial properties of mentha species, the reports on its application in fish and fish product are limited.
Indian mackerel is a medium-fatty fish species which is having a high demand in domestic as well as export markets. It is well established that fatty and semi-fatty species like mackerel are more prone to lipid oxidation as in their muscles, large amounts of haemoglobin (a well-known activator of lipid oxidation) and lipids coexist (Richards and Hultin 2002). Because of this, mackerel is prone to rapid quality deterioration during refrigerated storage, the most prevailing preservation method followed in the domestic market. Therefore, use of various combined preservation techniques and additives is under consideration. Vacuum packaging is commonly employed for preserving fresh fish fillet and lightly preserved products like cold smoked fish fillets. Gutted mackerel is a value added fresh fish product which can fetch better prize at domestic and export marketing. However, the information on the storage quality of vacuum packed gutted Indian mackerel during chilled storage is scarce in literature. Our study was aimed to investigate the effects of combined application of extract from Mentha arvensis (corn mint) and vacuum packaging on the quality changes and shelf life of guttted Indian mackerel during chill storage.
Materials and methods
Materials
Fresh Indian mackerel (weight 200 ± 15 g and length 18 ± 2 cm) was procured from Thoppumpadi fishing harbour, Cochin and brought to the laboratory in iced condition within 15 min. Mint was purchased from the vegetable market located in Cochin.
Mint extract treatment and storage of mackerel
Extract from dried mint leaf was prepared using ethanol as described by Viji et al. (2015). Mackerel was beheaded, eviscerated and washed thoroughly in potable water. Fish was dipped in 2 ppm chlorinated water for 5 min and drained properly. Mackerel was then divided into 3 batches. Batch I was air packed (control air pack-CAP) and batch II was vacuum packed (control vacuum pack-CVP). Batch III was given dip treatment in 0.5% mint extract solution in the ratio 1:1.5 (fish to solution) for 30 min, drained well and vacuum packed (MEVP). Concentration and dipping time in mint extract solution was chosen according to preliminary studies (0.5–1.5 g/100 ml distilled water and 15–45 min); thus, the best combination of concentration and dipping time which improved the keeping quality with less presence of odour and colour of the mint extract in fish was chosen. Pouches made of EVOH multilayer film (300 gauge) were used for packing the fish samples. Two fishes were packed in each pouch. Batch II and batch III were packed under vacuum at −1 bar pressure by a vacuum sealing machine (Vac-star, CH-1786, Switzerland). Batch I was packed under air by using the same machine. Immediately after packing, all the pouches were placed in an insulated box with ice and were kept in a chill room maintained at 0–2 °C. Samples were drawn at regular intervals and were analysed for chemical, microbiological, textural and sensory parameters. All the analyses were done in triplicate and mean values were taken.
Chemical analysis
Proximate composition of the raw fish muscle was determined by AOAC (1998) method. pH of homogenised muscle in distilled water (1:5 w/v) was determined by using a glass electrode digital pH meter (Cyberscan 510, Eutech instruments, Singapore). Total volatile base nitrogen (TVB-N) and Tri methyl amine nitrogen (TMA-N) was estimated by Conway’s micro-diffusion method (Conway, 1950). Lipid oxidation of fish muscle sample was assessed by measuring Thiobarbituricacid (TBARS) value (Tarladgis et al. 1960) as well as Peroxide value (Yildiz et al. 2003). Lipid hydrolysis was assessed by Free Fatty Acid (FFA) value following AOAC (1989) procedure. Nucleotides, namely adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), inosine monophosphate (IMP), inosine and hypoxanthine, were determined from perchloric acid extract of fish meat by the method of Ryder (1985) using the High Performance Liquid Chromatography (K value was calculated as the ratio of sum of inosine and hypoxanthine to the sum of total nucleotides).
Microbiological analysis
Total viable count (TVC) of the samples were analyzed according to the method of Ryser and Schuman (2013). Fifty gram of fish muscle was drawn aseptically and homogenized with 450 ml of phosphate buffer in a filter stomacher bag using a Stomacher® 400 Circulator (Seward Limited, UK) for 2 min. Decimal dilutions were made in phosphate buffer and, 1 ml each of three consecutive dilutions were plated on Plate Count Agar (Difco). Plates were incubated at 35 ± 2 °C for 48 ± 2 h for obtaining total viable count.
Sensory analysis
Sensory evaluation of raw and cooked samples was done by 6 trained panellists. Uniform pieces were cooked in 1% brine for 10 min and assessed. Colour and appearance, texture, odour and flavour of raw and cooked samples were evaluated and scored based on a 9 point hedonic scale as described by Amerine et al. (1965). The overall acceptability score was determined taking into account the total score obtained for raw and cooked samples. Scores of separate attributes were summed up and divided by the total number of attributes to give an overall acceptability score. An overall score of 4 was considered as the border line for acceptability.
Statistical analyses
Results of all analysis were subjected to ANOVA by statistical software, SPSS version 16. Significant differences (at 5% level of significance) between mean values of experimental data of the treatments were found out by Duncan’s multiple range test.
Results and discussion
Proximate composition
Proximate composition of fresh mackerel showed 73.07% moisture, 18.74% protein, 1.60% ash and 5.50% fat. Chemical composition of seafood influences the post harvest handling and storage and also could determine the specific processing techniques and storage conditions suitable for different species. The present result indicated that Indian mackerel is a fatty fish in agreement with Lakshmisha et al. (2012), susceptible to rapid oxidation under storage and hence, vacuum packaging and treatment with antioxidant rich extracts could be useful for inhibiting lipid oxidation during storage.
pH
Variations among pH of the samples are given in Fig. 1a. The initial pH of fresh mackerel sample was measured as 6.2. The low initial pH indicates that the fish has just resolved rigor-mortis stage after death. In CAP group, pH value showed a marginal increase and reached up to 6.47 when it was rejected on 16th day. On the other hand, in CVP and MEVP groups, pH value declined slightly during storage period. pH values of vacuum packed samples were significantly lower than that of the control (p < 0.05) during storage. However, no significant difference (p > 0.05) was found between the CVP and MEVP groups. In a similar study, Houicher et al. (2013) also could not observe any significant difference between the pH values of mint treated and vacuum packed sardine fillets and that of control vacuum pack without any treatment. Slight decrease in pH values observed in vacuum packed samples may be attributed to the production of lactic acid in the same by lactic acid bacteria which grow under microaerophilic conditions. This finding correlated well with the result of lactic acid bacterial counts in the samples under study (results not shown). Decrease or constant levels of pH might be attributed to increasing solubility of CO2 at storage time, effecting on growth of aerobic microflora. These was in agreement with those reported by Erkan et al. (2011) for plant extract treated and vacuum packed smoked rainbow trout during chill storage. Stamatis and Arkoudelos (2007) also observed a similar trend for pH values in vacuum packed chub mackerel during refrigerated storage.
K value
Various measurements of adenine nucleotides and their degradation products in fresh fish are used as chemical indices of freshness because the rates of change of these compounds in many species are parallel with its loss of freshness. As shown in the Fig. 1b, K value of samples increased from an initial value of 7.82, 6.07 and 6.56%, respectively for CAP, CVP, and MEVP samples. The initial K value obtained in our study was slightly lower than the reported value of 12–13% for other fishes (Reddy et al. 1994; Ozogul et al. 2004). A steady increase in K value was noticed in all the samples up to 10th day of storage, followed by a marked rise over the rest of storage period. The rapid increase after 10 days could be due to the sharp drop of IMP and hypoxanthine in fish (Ozogul et al. 2004; Mohan et al. 2009). During the early stages of storage period, endogenous enzymes are primarily responsible for ATP degradation whereas microbial metabolism also contributes to degradation towards later stages of spoilage (Moreira et al. 2005). It has also been proved that conversion of inosine to hypoxanthine is catalyzed by two enzymes: nucleotide phoshorylase (NP) and inosine nucleosidase (IN) and many spoilage bacteria possesses NP and IN which can accelerate Hx generation (Surette et al. 1988). Accordingly, the sharp degradation of IMP and Hx after 10 days storage period could be a result of accelerated breakdown of those compounds by microbial enzymes which was further in agreement with a positive correlation (0.94–0.99) between TVC and K value of fish samples after 10 days storage.
K values were significantly (p < 0.05) higher in CAP samples than MEVP samples. No significant difference was observed between CVP and MEVP samples until the 10th day, but thereafter, mint extract treated samples showed significantly lower K value, which indicated that the presence of mint extract has lowered the rate of degradation of nucleotides. At the limit of sensory acceptability, the levels of K value in samples respectively for CAP, CVP and MEVP were 81.27, 77.57 and 87.54%. The rejection levels of K value observed in the present study were lower to other observations given by Ozogul et al. (2004) for sardines (90%) and Yesudhason et al. (2010) for seer fish steaks (95%), whereas Ozogul et al. (2008) recorded K value as 81% as the limit of acceptability in ice stored grouper. Manju et al. (2007) reported that vacuum packaging is effective in controlling nucleotide degradation in pearl spot stored in ice. The results of our study demonstrated that previous dip treatment in mint extract can further slow down the breakdown of nucleotides by autolytic enzymes. An inhibitory effect of mint extracts on enzymes responsible for biogenic amines development is previously reported by Houicher et al. (2015) in vacuum packed sardine. The present investigation proved that mint extract can also be used for inhibiting the activity of nucleotide degradation enzymes in fishes during storage. This could be due to the active phytochemicals present in mint extract although no previous reports are available for the positive effect of mint extract in controlling nucleotide degradation. It has been reported that phenolic compounds affects enzyme activity possibly through binding with sulfhydril groups of the protein chain or through non specific interactions leading to protein denaturation (Brijesh et al. 2009).
TVB-N
The index TVB-N is commonly used to assess bacterial spoilage in muscle foods. Increase in TVB-N during storage period followed the order CAP > CVP > MEVP. As observed on the Fig. 2a, TVB-N values increased from an initial value of 13.21, 13.21 and 13.9 mg % to a final value of 22.47, 21.36 and 20.86 mg % in CAP, CVP and MEVP groups, respectively on their rejection on 16th, 18th and and 22nd day of storage. After 10 days of storage, MEVP showed significantly lower values (p > 0.05) than CVP samples. The results indicated an apparent preservative effect by mint extract, significantly lowering the TVB-N levels in MEVP samples compared to its counter pack in CVP and CAP samples. In our previous study, mint extract was found to be a potential preservative for controlling TVB-N generation in air packed and chill stored Indian mackerel (Viji et al. 2015). The lower content of TVB-N in treatment groups compared to CVP might be owing to the combined effects of extracts as an antibacterial agent and vacuum on controlling the microbial growth, which is primarily responsible for generating volatile bases during spoilage. Similar findings with rosemary extracts were reported in vacuum packed mackerel burgers (Ucak et al. 2011) and with turmeric extract in vacuum packed rainbow trout steaks (Pezeshk et al. 2011) during chill storage. In a similar study, Houicher et al. (2013) also observed lower TVB-N values in mint extract treated vacuum packed sardine fillet compared to vacuum packed sardine alone. It is well known that the complete removal of oxygen from the pack inhibits the aerobic bacterial growth and the result from present study suggests that combined use of vacuum packaging and mint extract further inhibits the microbial growth; thereby maintains lower TVB-N levels in fish during storage.
TMA-N
Trimethyl amine oxide (TMAO) is used as electron acceptor in anaerobic respiration by most of specific spoilage bacteria. The reduced component formed is TMA which is one of the dominant components of spoiled fish responsible for foul smell once the values reach beyond the acceptable limit. Changes in TMA-N content of samples over the chilled storage are presented in Fig. 2b. A gradual increase in TMA-N formation was noticed in all the samples over the storage period. TMA-N levels in CAP group was significantly higher (p < 0.05) followed by CVP than MEVP samples. Among the vacuum packed samples, significantly lower TMA-N content was observed in MEVP samples after 7th day of storage. All vacuum packed groups contained significantly lower TMA-N than air packed group. Vacuum packaging is demonstrated to have inhibitory effect on growth of microflora responsible for degradation of trimethyl amine oxide which was consistent with the results of various authors (Manju et al. 2007; Pantazi et al. 2008). Presence of mint extract in vacuum further improved the inhibitory effect, leading to lowest TMA-N levels. There are few studies demonstrating significant effects by treatment of Indian mackerel with mint extract (Viji et al. 2015) and pomegranate and grape seed extract (Shinde et al. 2015) on lowering TMA-N levels during refrigerated storage. In this study, all the sample groups didn’t cross the proposed limit of 10–15 mg/100 g set for TMA-N at which the offensive fishy odour occurs. This result was consistent with those of Ishida et al. (1976) and Manju et al. (2007) who reported that, in low temperature storage, such as refrigeration above 0 °C; TMA-N formation slows down noticeably.
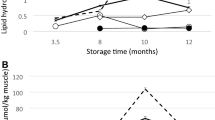
FFA
It is well known that development of FFA is a result of enzymatic hydrolysis of lipids in fish. Triglycerides are cleaved by lipases originating from the digestive tract or microorganisms and the degree of lipid hydrolysis can be evaluated by measuring the amount of free fatty acids. The association between lipolysis and lipid oxidation/rancidity is free (polyunsaturated) fatty acids oxidise more readily than esterified lipid (Ashton 2002). FFA content of all the sample groups presented a gradual increase with slight fluctuations (Fig. 2c). On and after 4 days of storage, significant differences in FFA were noticed among the sample groups. CAP group showed the highest values for FFA during storage followed by CVP group, while the lowest values were with MEVP group. The result revealed that lipid hydrolysis progressed at a slower rate in the samples treated with mint extract. Inhibitory effect on endogenous and microbial lipolytic enzymes in chill stored mackerel by plant extracts (pomegranate peel and green tea leaf extracts) were reported by Shinde et al. (2015). It is well established that vacuum packaging of fish reduces lipid hydrolysis as the growth of aerobic lipolytic bacteria is inhibited under vacuum. In addition, the antimicrobial effects of mint extract have further protected the lipid in mackerel muscle from hydrolysis by bacteria which is evident from the lower FFA values in treated samples compared to that in CVP samples. Similar results were reported by Ucak et al. (2011) for rosemary extract treated and vacuum packed mackerel burgers during storage at 4 °C. Whereas Kenar et al. (2010) and Houicher et al. (2013) obtained insignificant changes in FFA among control and plant extract treated vacuum packed sardine fillets during refrigerated storage.
PV
Quality of fatty fish is rapidly deteriorated due to the oxidation of lipid. The primary product of lipid oxidation is fatty acid hydroperoxide, measured as PV which gives an indication of primary lipid oxidation occurred in fish muscle. This oxidation index showed a marked increase in CAP samples in contrast to samples kept under MEVP and CVP conditions (Fig. 3a). Lower values in MEVP and CVP are attributed to the lack of oxygen in surrounding environment which is the initiator of lipid peroxidation. Moreover, PV values were significantly (p < 0.05) lower in MEVP groups than CVP, owing to the antioxidant activities of the extracts on the fish lipid. The reported antioxidant activity may be due to the presence of phenolic and flavonoid compounds which has the scavenging potential by reducing the free radicals. One of our previous studies demonstrated that ethanolic extract of mint leaf was rich in phenolic content (127 ± 8.2 mg gallic acid eqwt/g) with potential reducing power and radical scavenging activities (Viji et al. 2015). Mentha spp. possesses antioxidant properties due to the presence of active constituents like menthone, menthol, flavonoids, rosmarinic acid and carvone (Padmini et al. 2010). Antioxidant extracts from Mentha spp. has been used to control lipid peroxidation in different food systems including sunflower oil, lamp, pork and chilled fish (Kanatt et al. 2007; Biswas et al. 2012; Houicher et al. 2013). The result of present study revealed that inhibitory effects of vacuum can be further enhanced by mint extract treatment.
TBARS
Hydroperoxides formed during primary oxidation are highly unstable and they break down to aldehydes, ketones and alcohols that are volatile products causing rancid off-flavour in products. The extent of secondary oxidation is determined by measuring the amount of malonaldehyde produced. In this study, a progressive increase in TBARS was observed with the extent of storage days (Fig. 3b). At the beginning of the storage, TBARS values were determined as 0.542, 0.563 and 0.621 mg MDA/kg for the MEVP, CVP and CAP groups, respectively. The values showed a fluctuating trend in all vacuum packed samples with storage time. MEVP samples showed significantly (p < 0.05) lower TBARS than CVP and CAP at any day of storage. In CAP, the TBARS index reached up to 2.642 mg MDA/kg on 16th day of storage. TBARS values, indicating rancidity development remained low and below the limit level (<4 mg MDA/kg fish) at which rancid flavours may become evident in fish in MEVP and CVP groups.
The higher TBARS values in CAP group can be attributed to the higher rate of secondary lipid oxidation in the same mediated by oxygen in the packing environment. Presence of mint extract along with vacuum packaging has markedly retarded the rate of secondary oxidation in mackerel during storage. Similar results with natural antioxidants and vacuum packaging were reported previously (Sarkardei and Howell 2008; Kenar et al. 2010). Combined application of vacuum packaging and plant extract treatment has shown to delay lipid oxidation in rainbow trout fillets stored at 4 ± 1 °C (Pezeshk et al. 2011). Houicher et al. (2013) reported that addition of M. spicata extract @ 1% in vacuum packed sardine has effectively reduced TBARS formation and extended the shelf life during refrigerated storage. The antioxidant activity of mint extract could be related to their free radical chain breaking activity, hydrogen donating ability or metal chelating activity (Kanatt et al. 2007).
TVC
The initial TVC of fresh mackerel was 4.14 log CFU/g, indicating that the mackerel studied was of good quality. TVC values of the three groups increased gradually with storage. As shown in Fig. 4, bacteria grew most quickly in CAP samples followed by CVP and the lowest counts were with MEVP. 10th day onwards, the TVC in control air pack group remained 1 log higher than that of treated groups. Generally, a bacterial load of 7 log10 cfu g−1 is considered as the threshold limit of acceptance as spoilage is detected at this level (ICMSF 2011). The TVC crossed the limit in CAP, CVP and MEVP on 13, 16 and 21st day of storage, respectively. It was interesting to note that the microbiological data did not correlate well with biochemical and sensory results. The result of the present study is agreeing with the statement that higher counts are quite normal in tropical fishes and counts of 8 log10 cfu g−1 may be tolerated for bacterial population rendered “less active” by processing (Aguilera et al. 1992).
Results of present study indicates that vacuum packaging combined with mint extract treatment was effective in inhibiting the microbial spoilage to a great extend compared to air packed and vacuum packed control samples by extending the lag phase and generation time. Similar to our findings, vacuum packaging and pomegranate extract treatment has improved the microbial quality of pacific shrimp during refrigerated storage (Basiri et al. 2014). It has been reported that menthol, the major component of M. arvensis, exhibited excellent antimicrobial activity, even stronger than standard drugs (amoxycillin and fluconazole) against Staphylococcus aureus, Bacillus subtilis and E.coli (Padmini et al. 2010). Viji et al. (2015) also observed noteworthy antibacterial activities in mint extract against food spoilage and pathogenic bacteria tested.
Sensory analysis
Fresh mackerel had characteristic brightness, sea weedy odour, with firm texture and without gaping and discoloration. Sensory analysis of gutted mackerel showed differences in overall acceptability score between samples. Scores of CAP, CVP and MEVP mackerel samples showed a similar pattern of decreasing acceptability. Throughout the storage, MEVP retained the highest sensory scores (Fig. 5). It was observed that presence of mint extract imparted a mild but sensorially acceptable mint flavour in raw fish. The fish samples were considered to be acceptable for human consumption until the sensory score reached 4. Accordingly, the observed shelf life of Indian mackerel, as determined by panellists, was longest in MEVP (21 days), followed by CVP (16 days) and CAP (13 days) at 0–2 °C. So, compared to CAP, CVP extended the shelf life of gutted mackerel by 3 days while MEVP further extended the shelf life by 5 more days. The analysis demonstrated that VP conditions alone did not significantly extend the shelf life of mackerel compared to storage in air, whereas VP along with mint extract treatment did extend the shelf life.
Conclusion
Based on the sensory, biochemical and microbial results evaluated, use of mint extract treatment prior to vacuum packaging might increases the hurdles for microbial growth, thereby delaying quality changes of mackerel more effectively. Vacuum packaging along with mint extract treatment was the most effective for Indian mackerel preservation, achieving a shelf life of 21 days at 0–2 °C. Vacuum packaging of Indian mackerel effectively inhibited the oxidation of lipid as assessed by PV and TBARS value. Treatment of mackerel with ME is recommended prior to vacuum packaging to maintain the quality and to extend the shelf life of mackerel during refrigerated storage.
References
Aguilera JM, Francke A, Figueroa G, Bornhardt C, Cifuentes A (1992) Preservation of minced pelagic fish by combined methods. Int J Food Sci Technol 27:171–177
Ahn J, Grun IU, Mustapha A (2007) Effect of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol 24:7–14
Amerine MA, Pongborn RH, Roescler EB (1965) Principles of sensory evaluation of food. Academic Press, New York, p 602
AOAC (1989) Official methods and recommended practices of American Oil Chemists Society, 5th edn. Association of Official Analytical Chemists, Champaign
AOAC (1998) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Washington
Ashton IP (2002) Understanding lipid oxidation in fish. In: Bremner HA (ed) Safety and quality issues in fish processing. Woodhead Publishing, Sawston, p 507
Basiri S, Shekarforoush SS, Aminlari M, Abhari KH, Berizi E (2014) Influence of combined vacuum packaging and pomegranate peel extracts on shelf life and overall quality of pacific white shrimp (Penaeus vannamei) during refrigerated storage. Iran J Vet Res 15:23–29
Biswas AK, Chatil MK, Sahoo J (2012) Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem 133:467–472
Brijesh K, Tiwari S, Vasilis P, Valdramidis J, Colm POD, Kasiviswanathan M, Paula B, Cullen PJ (2009) Application of natural antimicrobials for food preservation. J Agric Food Chem 57:5987–6000
Conway EJ (1950) Micro-diffusion analysis and volumetric error. Crosby Lockwood and Son Ltd, London
Erkan N, Tosun SY, Ulusoy S, Uretener G (2011) The use of thyme and laurel essential oil treatments to extend the shelf life of bluefish (Pomatomus saltatrix) during storage in ice. J Verbrauch Lebensm 6:39–48
Houicher A, Kuley E, Bendeddouche B, Ozogul F (2013) Effect of Mentha spicata L. and Artemisia campestris extracts on the shelf life and quality of vacuum packed refrigerated sardine (Sarda pilchardus) fillets. J Food Prot 76:1719–1725
Houicher A, Kuley E, Ozogul F, Bendeddouche B (2015) Effects of natural extracts (Mentha spicata L. and Artemisia campestris) on biogenic amine formation of sardine vacuum packed and refrigerated fillets. J Food Process Preserv. doi:10.1111/jfpp12489
ICMSF (International Commission on Microbiological Specifications for Foods) (2011) Microorganisms in foods. 8. Use of data for assessing process control and product acceptance, vol 8. Springer, New York, p 400
Kanatt SR, Chander R, Sharma A (2007) Antioxidant potential of mint (Mentha spicata L.) in radiation processed lamb meat. Food Chem 100:451–458
Kappa K, Hakala E, Orav A, Pohjala A, Vuorela P, Püssa T, Vuorela H, Raal A (2013) Commercial peppermint (Mentha x piperita L.) teas: antichlamydial effects and polyphenolic composition. Food Res Int 53:758–766
Kenar M, Ozogul F, Kuley E (2010) Effects of rosemary and sage tea extracts on the sensory, chemical and microbiological changes of vacuum-packed and refrigerated sardine (Sardina pilchardus) fillets. Int J Food Sci Tech 45:2366–2371
Lakshmisha IP, Sankar TV, Anandan R, Geethalakshmi V, Joseph J (2012) Changes in muscle protein during fat oxidation in Indian mackerel Rastrelliger kanagurta (Cuvier, 1817) and Threadfin Bream Nemipterus japonicus (Bloch, 1791) under accelerated conditions. Fish Technol 49:47–154
Manju S, Jose L, Gopal TKS, Ravisankar CN, Lalitha KV (2007) Effect of sodium acetate dip treatment and vacuum-packaging on chemical, microbiological, textural and sensory changes of pearl spot (Etroplus suratensis) during chilled storage. Food Chem 102:27–35
McKay DL, Blumberg JB (2006) A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phyto Res 20:619–633
Mohan CO, Ravishankar CN, Srinivasa Gopal TK, Ashok Kumar K (2009) Nucleotide breakdown products of seer fish (Scomberomorus commerson) steaks stored in O2 scavenger packs during chilled storage. Innov Food Sci Emerg Technol 10:272–278
Moreira MR, Ponce AG, Del-Valle CE, Roura SI (2005) Inhibitory parameters of essential oils to reduce a foodborne pathogen. Lebensmittel 38:565–570
Ozogul F, Polat A, Ozogul Y (2004) The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sadines (Sardina plichardus). J Food Chem 85:49–57
Ozogul F, Ozogul Y, Kuley E (2008) Nucleotide degradation and biogenic amine formation of wild white grouper (Epinephelus aeneus) stored in ice and at chill temperature (4 °C). Food Chem 108:933–941
Padmini EA, Valaemathi M, Ran U (2010) Comparative analysis of chemical composition and antibacterial activities of Mentha spicata I and Camella sinensis. Asian J Exp Biol Sci 4:772–781
Pantazi D, Papavergou A, Pournis N, Kontominas MG, Savvaidis IN (2008) Shelf-life of chilled fresh Mediterranean swordfish (Xiphias gladius) stored under various packaging conditions: microbiological, biochemical and sensory attributes. Food Microbiol 25:136–143
Pezeshk S, Rezaei M, Hosseini H (2011) Effect of turmeric, shallot extracts and their combinations on quality characteristics of vacuum packaged rainbow trout stored at 4 ± 1 °C. J Food Sci 76:387–391
Reddy NR, Schreiber KS, Bazard KS, Skinner GE, Amstrong DJ (1994) Shelf life of fresh Tilapia fillets packaged in high barrier film with modified atmospheres. J Food Sci 59:260–264
Richards MP, Hultin HO (2002) Contributions of blood and blood components to lipid oxidation in fish muscle. J Agric Food Chem 50:555–564
Ryder JM (1985) Determination of adenine triphosphate and its breakdown products in fish muscle by high performance liquid chromatography. J Agric Food Chem 33:678–680
Ryser ET, Schuman J (2013) Aerobic plate count. In: Doores S, Salfinger Y, Tortorello ML (eds) Compendium of method for the microbiological examination of foods. American Public Health Association, Washington. doi:10.2105/MBEF.0222.013
Sarkardei S, Howell N (2008) Effect of natural antioxidants on stored freeze dried food product formulated using horse mackerel (Triachurus trachurus). J Food Sci Technol 43:309–315
Shinde PA, Reddy VKS, Patange SB (2015) Quality of Indian mackerel as affected by pomegranate peel and tea leaf extracts during ice storage. SAARC J Agric 13:109–122
Singh A, Sharma PK, Garg G (2010) Natural products as preservatives. Int J Pharma Bio Sci 1:601–612
Stamatis N, Arkoudelos J (2007) Quality assessment of Scomber colias japonicus under modified atmosphere and vacuum packaging. Food Cont 18:292–300
Surette ME, Gill TA, LeBlanc PJ (1988) Biochemical basis of post-mortem nucleotide catabolism in cod (Gadus morhua) and its relationship to spoilage. J Agric Food Chem 36:19–22
Tarladgis BG, Watts BM, Younthan MT (1960) A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc 37:44–52
Ucak I, Ozgul Y, Durmus M (2011) The effects of rosemary extract combination with vacuum packing on the quality changes of Atlantic mackerel fish burgers. Int J Food Sci Tech 46:1157–1163
Viji P, Binsi PK, Visnuvinayagam S, Srinivasa Gopal TK (2015) Efficacy of mint (Mentha arvensis) leaf and citrus (Citrus aurantium) peel extracts as natural preservatives for shelf life extension of chill stored Indian mackerel. J Food Sci Technol 52:6278–6289
Wilson BG, Bahna SL (2005) Adverse reactions to food additives. Ann Allergy Asthma Immunol 95:499–507
Yesudhason P, Srinivasa Gopal TK, Ravishankar CN, Lalitha KV, Ashok Kumar K (2010) Effect of potassium sorbate and modified atmosphere packaging on the shelf-life extension of seer fish (Scomberomorus commerson) steaks during iced storage. J Food Biochem 34:339–424
Yildiz G, Wehling R, Cuppett SL (2003) Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils. J Am Oil Chem Soc 80:103–107
Acknowledgements
The assistance offered by the technical staff in Fish Processing division of ICAR-Central Institute of Fisheries Technology, Cochin is deeply acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Rights and permissions
About this article
Cite this article
Viji, P., Panda, S.K., Mohan, C.O. et al. Combined effects of vacuum packaging and mint extract treatment on the biochemical, sensory and microbial changes of chill stored Indian mackerel. J Food Sci Technol 53, 4289–4297 (2016). https://doi.org/10.1007/s13197-016-2425-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2425-3