Abstract
The ability of high hydrostatic pressure treatment (HHPT) to extend the shelf life of Atlantic mackerel (Scomber scombrus) was assessed in this study. For that purpose, fillets were subjected to pressure treatments at 200, 300, 400 MPa at 5, 10, 15 °C for 5 and 15 min. The influence of pressure treatments on the levels of trimethylamine nitrogen (TMA-N) and thiobarbituric acid (TBA) as well as color changes was investigated. The suitable combinations were determined as 200 MPa, 15 °C for 5 min. and 400 MPa, 5 °C for 5 min. In the second stage, the shelf life of samples, which were treated at these conditions, stored at 4 ± 0.5 °C were studied by monitoring pH, color, sensorial features (appearance and odor), TMA-N, TBA, total volatile basic nitrogen, histamine, and total mesophilic aerobic count. The unpressurized mackerel samples were acceptable up to only 7 days compared to 17 and 19 days after 200 and 400 MPa treatments; respectively. The results obtained in this study showed that HHPT in combination with chilled storage can improve the shelf life and quality of fish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atlantic mackerel, which belongs to the family Scombridae, is a pelagic schooling fatty fish which inhabits in various oceans. It demonstrates an exceptional nutritional value in the human diet being rich in minerals, vitamins, and polyunsaturated fatty acids (Ozden 2010). Although it is declared as a wholesome food, it remains underutilized due to its short frozen shelf life (Aubourg et al. 2005).
Fresh form of Atlantic mackerel is the product most marketed, followed by frozen, smoked, and canned form (http://www.fishbase.org, Accessed 30 August 2011). Although freezing is the main commercially used method to slow down enzymatic and bacterial degradation of fish muscle, solidification of water brings about numerous complex physical and physicochemical changes such as gaping, moisture loss, and mechanical damage due to formation of large ice crystals. Occurrence of these degradations has negative economic impact and fish with a lower quality can only be sold as minced or low-priced product, such as canned fish (Jiang and Lee 2006; Love 2006). However, in the canning process, the nutritional and sensorial values of processed fish are reduced (Aubourg 2001).
High hydrostatic pressure treatment (HHPT) is one of the non-thermal technologies that provides safer, high quality, value-added foods which possess higher vitamin retention and higher consumer acceptance than both conventional (e.g., thermally processed foods) and other non-thermal technologies (Doona and Feeherry 2007). According to different factors such as species nature, chemical composition, and size, different effects of HHPT on fish have been reported (Yağız et al. 2007; 2009). Potential detrimental changes, in appearance, texture, and chemical parameter in HHPT products are also dependent on the processing conditions mainly to the applied pressure. Therefore, the undesirable changes may only be minimized by the judicious selection of treatment parameters namely pressure–temperature and the time (Mol et al. 2004; Gómez-Estace et al. 2007; Erkan et al. 2010a,b).
Fish deterioration occurs due to presence of spoilage microflora and endogenous enzymes. During handling and storage, quality deterioration of fresh fish rapidly occurs and limits the shelf life of the product (Tülsner 1994). HHPT can significantly reduce the microbial load by breaking the non-covalent bonds and piercing or changing the permeabilization of the cell membrane, consequently causing enzyme inactivation. The effects on non-covalent bonds together with changes in the permeabilization of the cell membrane cause the microbial reduction enabling a safer product with prolonged shelf life without affecting flavor and nutritional properties (Alpas et al. 1999; 2000; Bozoglu et al. 2004). HHPT is able to achieve these effects via the two causative agents of food spoilage, namely autolysis as well as microbial growth and metabolism (Heinz and Bukow 2010). On the other hand, HHPT induces damage to membranes, denatures proteins and changes the cell morphology; weak energy bonds like hydrogen and hydrophobic bonds can be irreversibly modified, causing substantial effects on the secondary, tertiary and quaternary structures in proteins. It is also reported that HHPT induces oxidative changes in lipids, therefore an important loss of oxidative stability has been underlined (Ohlsson and Bengtsson 2002; Aubourg et al. 2010). Hence, it is important to monitor the physical (color changes) and chemical parameters (trimethylamine nitrogen (TMA-N), thiobarbituric acid (TBA)) as important quality indicators (Erkan et al. 2011a; b).
TMA-N is formed from trimethylamine oxygen (TMA-O) as a result of bacterial enzyme or other endogenous enzymes activities (Erkan and Ozden 2008). Fishy odor takes its source from this compound.
Oxidative rancidity is an important organoleptic characteristic for rejection or approval of fish after prolonged shelf life. Lipid oxidation that can be originated from non-enzymatic and enzymatic reactions is expressed as TBA level. The lipids of fish are more vulnerable to oxidation than other types of meat due to the high concentration of polyunsaturated fats (Amanatidou et al. 2000).
Total volatile basic nitrogen (TVB-N) is interrelated with bacterial growth, during marine fish spoilage along with TMA-N, ammonia and other basic nitrogenous compounds (Baixas-Nogueras et al. 2001; Gómez-Estace et al. 2007). TVB-N analysis is not taken as a criteria to select the optimum conditions in this study as TVB-N level alone is a very unreliable freshness indicator (Castro et al. 2006). Other researchers also underlined that TVB-N was not an index of spoilage; however it could only be evaluated as indicator of advanced spoilage during storage (Ozogul and Ozogul 2000).
The effect of pressure on pH was stated in cod muscle (Angsupanich and Ledward 1998). It was well established that HHPT inactivated microorganisms, some of which could be responsible from formation of volatile substances (amines, bases, acids, etc.). Hence, lower and more stable pH could be expected. Nevertheless, it could not be evaluated alone, it had to be supported by other analyses. Some studies underscored that pH examination was not useful for quality evaluation (Ledward 1995; Erkan and Ozden 2008; Simeonidou et al. 1998).
There is a small amount of pathogenic bacteria in fish. On the other hand, the growth rate of Gram (−) spoilage bacteria are high at low temperatures. Therefore, the fish will be spoiled even before dangerous amounts of pathogens or toxins have developed. The metabolites of spoilage organisms cause off-flavors, odors (fishy, ammoniac) and consequently induce consumer rejection (Huss 1997).
Among biogenic amines, researchers mostly focused on histamine level. Spoiled fish of the Scombridae and Scomberesocidae families commonly cause the food-borne intoxication known as histamine poisoning, in words “scombroid fish poisoning”. Histamine is formed mainly through decarboxylation of histidine by certain bacteria which possess the enzyme histidine decarboxylase (Taylor 1989).
The aspect and color of the food surface is the first quality parameter which is used as a tool by consumers to accept or reject food. Fish fillet color is correlated with heme-based pigment, physical structure of muscle and amount of unbound water due to the affect of the light scattering. Additionally, modifications of protein matrix, enzymatic and non-enzymatic reactions which occur in consequence of myofibrillar protein degradation, and disorganization of myofibrils can cause color changes during storage (Chéret et al. 2005).
Sensory evaluations are an accurate method for assessing freshness in many fish. As the storage time increases, characteristic changes of the appearance, odor, taste and texture of the fish occur (Aubourg, 2001; Olafsdottir et al. 1997). Instrumental methods must be used with sensory evaluation panels to guarantee the accuracy of the results because instrumental methods can not show edibleness of the fish alone (Dokuzlu 2004).
Recent studies with different variety of fish have shown that HHPT may be a useful processing tool. Büyükcan et al. (2009) investigated preservation and shelf life extension of shrimps (250 MPa, 50 °C, 10 min) and clams (220 MPa, 50 °C, 10 min) during storage at 4 °C after HHPT and reported an additional 10 and 16 days of shelflife extension in shrimps and clams; respectively. The effect of HHP treatment on color, TBA and TMA parameters of red mullet was studied by Uretener (2009). The study indicated unchanged TMA-N content for HHPT applied 220 MPa, 15 °C for 10 min, 220 MPa, 25 °C for 5 min, 250 MPa, 7–25 °C for 10 min and 330 MPa, 3 °C for 5 min. Similarly; Erkan and Uretener (2010) determined insignificant TMA-N level than untreated samples in HHPT applied sea bream (220–250–330 MPa, 7 and 15 °C for 5 min; at 330 MPa, 3–7 °C for 10 min). Erkan et al. (2010b) reported 3–4 days of shelf life extension of HHPT red mullet (at 220 MPa for 5 min at 25 °C/at 330 MPa for 5 min at 3 °C) as compared with control samples stored at 4 °C based primarily on microbiological and sensory results. The quality of high-pressure-treated horse mackerel was reported to be best preserved at 250 MPa, 7–15 °C for 5 min, 220 MPa, 15–25 °C for 5 min, 250 MPa, 15 °C for 10 min and 330 MPa, 25 °C for 10 min (Erkan et al. 2011a; b).
Based on these recent literature findings, the effect of different HHPT conditions (200, 300 or 400 MPa at 5, 10, 15 °C for 5 and 15 min) on quality characteristics (color, lipid oxidation, and trimethylamine nitrogen stability) of Atlantic mackerel fillet were investigated to establish the best processing conditions (pressure, temperature, and time) that would be used in the shelf life study. In the second part, the effect of selected HHPT conditions (200 MPa, 15 °C for 5 min and 400 MPa, 5 °C for 5 min) based on chemical, sensory, and microbiological changes, were evaluated during storage of Atlantic mackerel for 21 days at 4 °C. These treatment conditions were selected due to the necessity of determining the minimum HHPT conditions for obtaining desirable levels of microbial destruction while maintaining a maximum degree of sensory and nutritional quality. In case the values of the samples exceeded the specified threshold values two times successively, experiment was terminated.
Materials and Methods
Raw Material and Sample Preparation
Fresh Atlantic mackerel (average weight and length, 200 ± 20 g and 26.5 ± 4.5 cm, respectively) were purchased from a local market in Ankara during the fall of 2010; and until arrival at the laboratory, fish was kept in boxes with ice packs. All fish were headed, eviscerated, washed thoroughly, filleted, and divided in portions of equal weight (15 g). The samples were prepared by wrapping with stretch film, aluminum foil and once more stretch film in order to avoid the contact of sample with the pressure-transmitting medium. They were kept in a freezer at −25 ± 2 °C until they were used. This period did not exceed 1 week. They were thawed in the refrigerator around 4–6 °C overnight and subjected to pressure treatment at 200, 300, or 400 MPa at 5, 10, or 15 °C for 5 or 15 min and TMA-N, TBA, and color analysis were performed to determine the best combination.
For shelf life analysis, samples were prepared as in the first experiment. Two groups of samples were pressurized at 200 MPa at 15 °C for 5 min and at 400 MPa at 5 °C for 5 min and then stored at 4 °C, and the third group samples (control) were stored directly at 4 °C without undergoing high-pressure treatment. After treatment, samples were stored up to 21 days at 4 °C. Samples were placed into oxygen-permeable bags (Seward Medical, England) for the storage study. On each analysis day for each group, samples were taken at random from packaged samples (each packaged sample is approximately 15 g).
HHPT
HHPT was performed with 760.0118-type pressure equipment supplied by SITEC-Sieber Engineering AG, Zurich, Switzerland. The vessel had a volume of 100 ml with ID 24 mm and length 153 mm. A built-in heating–cooling system (Huber Circulation Thermostat, Offenburg, Germany) was used to maintain and control required temperature which is measured by a thermocouple type K in the vessel. The vessel was filled with a pressure-transmitting medium consisting of distilled water. Pressurization rates were 400 MPa/min for 200 MPa, 360 MPa/min for 300 MPa and 340 MPa/min for 400 MPa. Pressure increase and release times were less than 20 s. for each. Pressurization time reported in this study did not include the pressure increase and release times. Control group samples were not pressurized.
Analyses
Unless specified, three independent measurements for all kinds of analyses were performed for each condition. Solely, sensory analyses and color measurements were carried out with six and nine measurements for all conditions, respectively.
Physical Analyses
Measurement of pH
pH was monitored at room temperature in distilled water (1/10 w/v) by using pH meter (Mettler-Toledo MP 220, Schwerzenbach, Switzerland).
Color Analysis
Although there are different color spaces, CIELAB L*, a*, b* color spaces are the most used one due to the uniform distribution of colors. L* is the luminance or lightness component, parameters a* (from green to red) and b* (from blue to yellow) are the two chromatic components. Euclidean distance (ΔE) between two different colors corresponds approximately to the color difference perceived by the human eye. (Leon et al. 2006)
Three different fillet parts were measured in three different locations of each part using CIELAB color system. L* (lightness, range from 0 to 100), a* (from green to red, range from −120 to 120) and b* (from blue to yellow, range from −120 to 120) values were measured with the help of a chromameter (Minolta CR-200, Osaka, Japan). The instrument was standardized before each measurement with a white calibration plate (L = 73.3; a = 8.4; b = 23.0 in CIELAB Color System). Averages and standard deviations of L*, a*, and b* values were calculated as the total color differences.
The total color difference (ΔE), as calculated below, was also used for evaluation where subscript ‘0’ indicates initial color (Lozano and Ibarz 1997).
Microbiological Analyses
For the enumeration of total mesophilic aerobic bacterial count (TMAC), 10 g of fish sample was homogenized in sterile Stomacher bags (Seward Medical, England) with a stomacher (Seward Laboratory Blender Stomacher 400, England) in 90 mL sterile 0.1 % peptone water solution. 0.1 mL samples of serial dilutions (1:10 diluents, 0.1 % peptone water, Merck, Germany) of fish homogenates was spread plated on plate count agar (PCA, Merck, Germany). Total viable counts were determined after incubation for 48 h at 37 °C. Results are expressed as logarithm of colony-forming units per gram of sample ((log CFU)/g fish sample).
Chemical Analyses
Measurement of Trimethylamine Nitrogen Value
TMA-N was determined by the method of Schormüller (1968). Homogenized samples (10 g) were weighed, blended with 90 mL of 10 % trichloroacetic acid (TCA, Merck, Germany) solution and filtrated (Whatman no. 1). 4 ml extract was transferred into test tubes with 1 ml formaldehyde (20 %, Emir Kimya, Turkey), 10 ml toluene (99 %, Emir Kimya, Turkey) and 3 ml potassium hydroxide (KOH) (50 %, Merck, Germany). The tubes were shaken and 5 mL toluene layer was pipetted. Five milliliters of picric acid working solution (0.02 % with H2O-free toluene, Fluka Chemie, Germany) was added. Yellow picrates, which were the end product of interaction of picric acid with primary and secondary amines, were analyzed with maximum absorption at 410 nm against the blank in spectrophotometer (Specord50, Analytic Jena AG, Jena, Germany) in quartz cuvettes (Silica, Sigma). The concentration of TMA-N was calculated from a standard (TMA-HCl) curve. Results of TMA-N were expressed as mg per 100 g of muscle.
Measurement of Thiobarbituric Acid Value
TBA was determined according to Weilmeier and Regenstein (2004) and Khan et al. (2006) as detailed in Erkan and Ozden (2008). Ten grams of fish sample was placed in a tube with 500 μL butylated hydroxytoluene (BHT, Merck, Germany) and1 90 mL of 5 % (w/v) TCA solution (Merck, Germany). The mixture was homogenized with Ultra-Turrax (T-18, IKA-Werke GmbH & Co, Staufen, Germany) at high speed for 2 min and filtrated through a Whatman No.1 filter paper. A portion (5.0 mL) of the mixture that was pipetted into a dry stoppered test tube 1 mL of TBA reagent (0.01 M of the solution of 2-thiobarbituric acid in 90 % acetic acid, Merck, Germany) was added. The test tubes were stoppered, vortexed, and placed in a water bath at 75 °C for 40 min, then cooled. Absorbance of the resultant colored solution was read at 532 nm using spectrophotometer (Specord50, Analytic Jena AG, Jena, Germany). The concentration of malondialdehyde (MDA) was calculated from a standard curve using 1,1,3,3-tetraethoxy-propane (TEP, Merck, Germany) as the standard compound. TBA values were expressed as mg of MDA/kg of sample.
Measurement of Total Volatile Basic Nitrogen Value
TVB-N was determined according to the method of Antonacopoulos and Vyncke (1989). 10 g of fish sample was homogenized with 90 ml of 6.5 % perchloric acid (Merck, Germany) for 1–2 min in an Ultra-Turrax (T-18, IKA-Werke GmbH & Co, Staufen, Germany). The homogenates were filtered through a Whatman No.1 filter paper and 50 mL of filtrate was transferred to a Kjeldahl tube with 150 ml distilled water and subsequently alkalized by 20 % NaOH (Merck, Germany). Tubes were distilled in Kjeldahl apparatus (Simsek Laborteknik Co., Ankara, Turkey) until 50 ml distillate was collected. The distillate was titrated with 0.01 N HCl (Merck, Germany). Results of TVB-N were expressed as milligrams per 100 g of muscle.
Measurement of Histamine Value
Histamine content of fish flesh during storage was determined with commercial rapid test kits (Veratox Quantitative Histamine Test Kits, Neogen Co, USA) by means of the competitive direct enzyme-linked immunosorbent assay (CD-ELISA) method. The tests’ results were read by a micro-well reader (Stat Fax, Neogen Co, USA) with a 650 nm filter. Histamine concentrations were expressed as parts per million (ppm).
Sensory Analyses
Sensory assessment of the control and HHPT samples (uncooked) included appearance and odor parameters. Analysis was conducted by six panelists, who were laboratory trained graduate students in the Department of Food Engineering at METU, according to the hedonic scale proposed by Huss (Huss 1988). In this ten-point descriptive scale, a score of 10–9 indicated “very good quality”, 8.9–8 indicated “good quality”, 7.9–4 represents “acceptable quality”, the score below 3.9 means the sample had strong off-odors’ and was denoted as spoiled.
Statistical Analyses
The results were reported as mean values ± standard deviation. They were analyzed by two- and three-way analysis of variance procedure (according to the number of factors) of SPSS 15 for Windows (SPSS Inc., Chicago, IL, USA) to determine the significant differences (p < 0.05). Tukey test was used as a post-hoc test if a factor had a significant effect (difference between treatments and days of storage) and if the factor had three or more groups.
Results and discussion
Effect of HHPT Parameters on the Quality of Atlantic Mackerel
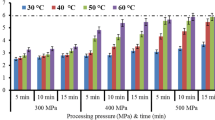
The effects of combined application of pressure, temperature, and holding time on TMA-N and TBA levels of Atlantic mackerel are presented in Table 1. The results coincided well with literature TMA-N data of mackerel species, 1.22 ± 0.06 mg/100 g (Goulas and Kontominas 2005). This value was also consistent with the FAO standards which recommended that good quality fish must contain less than 1.5 mg TMA-N/100 g (Huss 1988). Unpressurized control samples had a TMA-N level of 1.241 ± 0.169 mg/100 g and the scores of some HHPTs were higher than control samples insignificantly (Table 1). In literature, this increase was linked to the damage of the lysosomal membrane by high-pressure treatment (100–200–300–400–500 MPa 10 ± 5 °C for 5 min) and releasing of the enzymes (Chéret et al. 2006). TMA-N content of the samples slightly decreased with increasing pressure (T, t = constant), nevertheless difference was not found as significant in Tukey test for only high pressure as an independent variable. The TMA-N content of horse mackerel samples was not affected also from HHPT application significantly (p ≤ 0.05) (Erkan et al. 2011a; b).
Goulas and Kontominas (2005) studied with unprocessed chub mackerel, TBA level was 0.23 ± 0.05 mg malondialdehyde/kg. The reason of the higher TBA values of control group might be the season of the fish used for experiments. October was the month in which fishing season began in Turkey. TBA number was high due to stocking of fat all summer long. All pressurized samples had higher levels of TBA than the control group. TBA level of the samples increased with increasing pressure. Similarly, Angsupanich and Ledward (1998) indicated that when compared with the fresh sample, 200-MPa-treated sample changed the TBA number a little although 400 MPa or higher pressure treatments increased its value considerably. The reason of this increase was due to the denaturation of heme protein by pressure which released metal ions (Fe and Cu) and catalyzed lipid oxidation (Tanaka et al. 1991; Cheah and Ledward 1997; Angsupanich and Ledward 1998). Likewise, during HHPT, metal ions where released from metal salts (Fe-stearate and Cu-stearate) and catalyzed the oxidation reaction (Cheah and Ledward 1997). The oxidative stability decreased (TBA number increased) with the increasing temperature and decreasing with processing time generally. Despite the lower TBA results of 200 MPa, 10 °C, 5 min- and 300 MPa, 10 °C, 5 min-treated samples, other chemical parameters had to be taken into consideration when determining/selecting the optimum conditions.
The effect of the HHPT on the CIELAB color system (L*, a*, b* and ΔE) of the mackerel was illustrated in Table 2. L*, a*, b* and ΔE values of unpressurized mackerel group were 42.0 ± 0.2, 12.0 ± 1.3, 24.9 ± 2.2, and 31.6 ± 0.4, respectively. Lightness (L*) increased, with increasing pressure and although there are few exceptions, generally increased with holding time. However, it decreased with increasing temperature (p ≤ 0.05); 400 MPa −5 °C, 15 min treatment caused the highest L* value change (56.5 ± 1.0). Angsupanich and Ledward (1998) indicated that translucency loss was measured with the denaturation of myosins with pressure. Similar results were reported for HHPT-treated sea bass (200–250–330 MPa, 3,7,15 and 25 °C, 5 to 10 min) muscle and dolphinfish (200–300–400 MPa at 20 °C for 15 min) where control samples had the lowest L* values among all treatment pressures and lightness increased with increasing pressure and holding time (Gómez-Estace et al. 2007; Erkan et al. 2010a). Redness (a*) of the samples decreased with increasing pressure and temperature and holding time (p ≤ 0.05). Yellowness (b* values) of HHPT-treated Atlantic mackerel samples were not significantly affected (p ≤ 0.05) with pressure. The significant effect on b* values of HHPT-treated sea bass, cold smoked salmon, sliced dolphinfish were also not observed (p ≤ 0.05) (Gómez-Estace et al. 2007; Erkan et al. 2010a; 2010b). Increase in pressure and application time caused a decrease of ΔE scores.
Shelf Life Analyses
The condition which caused lower TMA-N level, comparatively high oxidative stability (low TBA level), and the closest color values to the control samples was 200 MPa, 15 °C, 5 min for Atlantic mackerel. The second combination (400 MPa, 5 °C, 5 min) was also studied for shelf life analyses with respect to the lowest TMA-N level among the treatments as high-pressure treatment can also promote color and oxidation changes that could modify the sensory characteristics (Erkan et al. 2010b) The selection of two different HHPT combinations also enabled the comparison of the effect of relatively low pressure–high temperature (200 MPa, 15 °C) and high-pressure–low-temperature (400 MPa, 5 °C) treatments on the quality parameters studied within the HHPT conditions (200, 300 or 400 MPa at 5, 10, 15 °C for 5 and 15 min) of the study.
The effect of HHPT on TMA-N (in milligrams per 100 g), TBA (in milligrams MDA per kg), TVB-N (in milligrams per 100 g), TMAC (in log cfu per gram) and pH of Atlantic mackerel flesh during refrigerated storage (at 4 ± 0.5 °C) are given in Table 3.
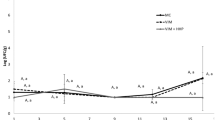
FAO recommended that 10–15 mg TMA-N/100 g must be regarded as the limit of acceptability for human consumption (Connell 1975; Huss 1988). Up to 4 mg TMA/100 g, the fish sample is indicated as “very good quality”, up to 10 mg TMA/100 g it is denoted as marketable, the score of 12 mg TMA/100 g is denoted as spoiled (Kundakçı 1989; Varlık et al. 2000; Mol et al. 2004). Based on these literature information, 10 mg/100 g was used as a threshold TMA-N value in this study. Although the control group has a drastic increase of TMA-N number, 200-MPa-pressurized samples had slower increase; moreover, 400 MPa-treated samples did never exceed the limit of acceptability. Accordingly, control samples were described as spoiled at the 11th day of storage and 200 MPa samples at the 17th day of storage.
TBA value of 1–2 mg MDA/kg was denoted as the limit of acceptability (Connell 1975; Goulas and Kontominas 2005); 1 mg MDA/kg was used as a threshold TBA value in this study. Unpressurized samples of fatty fish showed lower (p < 0.05) oxidation than their counterpart pressurized products at the first day. In literature, it was noted that TBA records might not bring out the actual lipid oxidation rate due to the interaction between malonaldehyde and other fish components such as amines, nucleosides, nucleic acids, proteins, amino acids of phospholipids, or other aldehydes (Aubourg 1993; Simeonidou et al. 1998; Taliadourou et al. 2003). The other limitation of the test was instability and impossibility of determining the organic alcohols and acids which were produced by oxidation of MDA and other short chain carbon products of lipid oxidation for a long period of time (Fernández et al. 1997). Unpressurized mackerel exceeded the threshold values on the 13th day, pressurized samples (both 200- and 400-MPa-treated ones) did not exceed up to the 21st day of analyses.
TVB-N value was denoted as “very good quality” up to 25 mg/100 g. Over 35 mg/100 g was indicated as spoiled (EEC 1995; Goulas and Kontominas 2005; Varlık et al. 2000). Goulas and Kontominas (2005) determined the initial value of unprocessed mackerel as 10.93 ± 0.36 mg N/100 g. Mol et al. (2004) specified this level as 12.34 ± 1.16 mg N/100 g; however, researchers underlined that TVB-N level varied according to the place (such as first depot, second depot, and the retail sellers) where the samples were received. A drastic increase in the TVB-N values of unpressurized samples was detected especially on the ninth day of storage. The threshold value was exceeded at the same day. Slow and regular increase in the TVB-N scores of 200-MPa-treated samples was observed throughout the shelf life analysis.
The limit of acceptability for mesophilic aerobic bacteria was indicated as 106–107 CFU/g (Gobantes et al. 1998; Erkan and Ozden 2008; Gumus et al. 2008). For the present study, 6 log cfu/g was designated as a limit value for consumption. At the beginning of the shelf life analyses, the microbial load of both control and pressurized samples were at undetectable levels (<3.00 log CFU/g). However, as the shelf life lengthened out, the application of pressure slowed down the microbial growth as can be seen from the data given in Table 3. Moreover, the retardation time was extended as the applied pressure increased. Hereunder, unpressurized samples exceeded the acceptability limit at the 11th day; 200 MPa–15 °C–5 min processed ones at 17th day; and 400 MPa–5 °C–5 min processed samples at 19th day of storage. Amanatidou et al. (2000) indicated that commercial shelf life of fish products is usually limited to 1 week of refrigerated (2–8 °C) storage. An inverse relationship between microbiological growth rate and the amount and time of pressure applied was observed in this study as well as the net effect of high pressure on the reduction of microbial growth.
Threshold value for pH was denoted as 6.8–7.0 (Mol et al. 2004). In this study, fish samples which had pH higher than 7.0 were denoted as spoiled. pH of the mackerel samples varied significantly in the literature. Goulas and Kontominas (2005) and Mol et al. (2004) specified this value as 6.12 ± 0.09, although it was indicated as 6.62 ± 0.17 and 6.8 ± 0.1 in other studies (Venugopal and Shahidi 1994; Simeonidou et al. 1998). This difference could take its source from two reasons. The fish were kept in −25 °C until the beginning of the experiments. Freezing made the remaining solution more concentrated due to converting a large proportion of water into ice. The increased concentration changed the acid–base equilibrium (pH). Secondarily, increased concentration consists of slightly soluble salts, phosphate, and other compounds. This phenomenon can result in up to two units of irreversible pH changes (Love 2006; Jiang and Lee 2006). pH of the 400-MPa-treated Atlantic mackerel muscle was higher than 200-MPa-treated samples (except the 7th, 9th, and 11th day of storage), probably due to the denaturation of some protein fractions. Unpressurized samples exceeded the limit on the 7th day, while 400-MPa-treated ones reached this level on the 21st day of storage; 200 MPa treatment did not caused the samples to reach this critic value throughout the storage.
Alkaline compounds (ammonium compounds, TMA, etc.) which are mainly derived from microbial action, cause an increase in pH. In this respect, the results of pH, TMA-N, TVB-N, and TMAC of the samples coincided well with each other. Due to the inhibition of bacterial growth with the help of pressure–temperature and time combination applied; it took more time for the pressurized samples to reach the threshold limit.
Throughout the storage period, L* values of the control group samples increased, although lightness of the 200-MPa-treated samples was quite stable and a trend to decrease was observed for 400-MPa-pressurized samples (p ≤ 0.05) (Table 4). Erkan et al. (2010b) determined an increase for both pressurized (220 MPa/5 min/25 °C and 330 MPa/5 min/3 °C) and unpressurized red mullet muscle which were covered with flexible plastic films during pressurization process and stored at 4 °C during 17 days of storage. However, Gómez-Estace et al. (2007) and Erkan et al. (2011a; b) indicated that lightness of the pressurized (250 MPa/3 °C/5 min, 250 MPa/25 °C/10 min, and 300 MPa/20 °C/15 min) and control group salmon and dolphinfish was quite stable. L* value fluctuations were also reported for sea bream for control and pressurized (250 MPa/3 °C/5 min, 250 MPa/15 °C/5 min) groups in literature (Erkan and Uretener 2010). Redness (a*) of the samples did not significantly changed during storage period (p ≤ 0.05) in agreement with findings of Gómez-Estace et al. (2007). Yellowness (b*) of the control group samples increased from 19.7 ± 0.7 to 34.4 ± 0.7. However, with fluctuations, b* values of the pressurized samples decreased (p ≤ 0.05). Total color difference (ΔE) of untreated samples decreased, a slight increase was determined for the pressurized samples.
Histamine concentrations of foods exceeding 50 ppm (5 mg/100 g fish) are hazardous (FAO 2011). Histamine formation was studied up to 20 days of refrigerated storage of Atlantic mackerel (Table 5). In literature, untreated mackerel samples had a histamine value of 14.40 ± 4.30 (Mol et al. 2004). A great increase in histamine formation of unpressurized samples was observed after the 12th day of storage. Such a large increase was observed on the 20th day for 200-MPa-pressurized samples. The histamine concentration of 400-MPa-pressurized samples did not exceed the critical level until the end of the experiment. The reason of lower histamine concentrations than the control group could be reduced histidine decarboxylase activity. Results were in agreement with literature cited. It had been established that pressures above 300 MPa reduced the enzyme activity to a level of 55 % of the residual (Santibanez 2007).
Decreasing scores in appearance and odor attributes were obtained with increasing time (Table 6). The results corroborated the chemical and microbiological analyses. Pressure caused the fish flesh to have a cooked appearance. These findings were consistent with this literature information indicating a 5 min pressure treatment around 150–200 MPa pressure causes a cooked appearance of mackerel (Matser et al. 2000). According to the panelists of this study, this aspect did not appeal to them, conversely caused them to give lower scores to the pressurized samples at the beginning of the shelf life analysis. Similarly, the reason of lower odor remarks of pressurized samples at the first day was due to the chemical smell after pressurization process. Although the control samples of mackerel had a strong deteriorated fish odor, both of the pressurized samples did not have this kind of intense odor till the end of the shelf life analysis according to panelist evaluations. Instead, a slight off-odor was reported at the 17th day of storage for 200-MPa-pressurized samples. Any fishy smell was not reported for 400-MPa-pressurized samples until the 21st day. Control group samples were evaluated as spoiled on the 9th day; however, pressurized samples exceeded the limits on the 17th and 19th days.
Conclusions
When all the parameters were evaluated; the unpressurized Atlantic mackerel (Scomber scombrus) was acceptable up to only 7 days compared to 17 and 19 days at 200 MPa–15 °C–5 min and 400 MPa–5 °C–5 min treatments; respectively. Accordingly, HHPT in combination with chilled storage can improve the shelf life and quality of fish. For fatty fish, HHPT can be recommended not only for industrial production but also for safe storage even at refrigeration temperatures. With the help of larger and economic high-pressure chambers the industry can manufacture high-quality fish products.
Abbreviations
- HHPT:
-
High hydrostatic pressure treatment
- TMA:
-
Trimethylamine
- TMA-N:
-
Trimethylamine nitrogen
- TMA-O:
-
Trimethylamine oxygen
- TBA:
-
Thiobarbituric acid
- TVB-N:
-
Total volatile basic nitrogen
- MDA:
-
Malondialdehyde
- TMAC:
-
Total mesophilic aerobic count
- TEP:
-
Tetraethoxy-propane
- PCA:
-
Plate count agar
- CFU:
-
Colony-forming unit
- TCA:
-
Trichloroacetic acid
- KOH:
-
Potassium hydroxide
- BHT:
-
Butylated hydroxytoluene
References
Alpas, H., Kalchayanand, N., Bozoglu, F., Sikes, A., Dunne, P., & Ray, B. (1999). Variation in resistance to hydrostatic pressure among strains of foodborne pathogens. Applied and Environmental Microbiology, 65, 4248–4251.
Alpas, H., Kalchayanand, N., Bozoglu, F., & Ray, B. (2000). Interactions of high hydrostatic pressure, pressurization temperature and ph on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. International Journal of Food Microbiology, 60, 33–42.
Amanatidou, A., Schluter, O., Lemkau, K., Gorris, L. G. M., Smid, E. J., & Knorr, D. (2000). Effect of combined application of high pressure treatment and modified atmosphere on the shelf-life of fresh Atlantic salmon. Innovative Food Science and Emerging Technologies, 1, 87–98.
Angsupanich, K., & Ledward, D. A. (1998). High pressure treatment effects on cod (Gadus morhua) muscle. Food Chemistry, 63, 39–50.
Antonacopoulos, N., & Vyncke, W. (1989). Determination of volatile basic nitrogen in fish: a third collaborative study by the West European Fish Technologists Association (WEFTA). Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung, 189, 309–316.
Aubourg, S. P. (1993). Interaction of malondialdehyde with biological molecules-new trends about reactivity and significance. Journal of Food Science and Technology, 28, 323–335.
Aubourg, S.P. (2001). Review: Loss of quality during the manufacture of canned fish products. Food Science and Technology International, 7, 199–215.
Aubourg, S., Rodríguez, A., & Gallardo, J. M. (2005). Rancidity development during frozen storage of mackerel (Scomber scombrus): effect of catching season and commercial presentation. European Journal of Lipid Science and Technology, 107, 316–323.
Aubourg, S., Munizaga, G. T., Reyes, J. E., Rodriguez, A., & Won, M. P. (2010). Effect of high-pressure treatment on microbial activity and lipid oxidation in chilled coho salmon. European Journal of Lipid Science and Technology, 112, 362–372.
Baixas-Nogueras, S., Bover-Cid, S., Vidal-Carou, M. C., Veciana-Nogués, M. T., & Mariné-Font, A. (2001). Trimethylamine and total volatile basic nitrogen determination by flow injection/gas diffusion in Mediterranean hake (Merluccius merluccius). Journal of Agricultural and Food Chemistry, 49(4), 1681–1686.
Bozoglu, F., Alpas, H., & Kaletunc, G. (2004). Injury recovery of foodborne pathogens in high hydrostatic pressure treated milk during storage. FEMS Immunology and Medical Microbiology, 40, 243–247.
Büyükcan, M., Bozoğlu, F., & Alpas, H. (2009). Preservation and shelf-life extension of shrimps and clams by high hydrostatic pressure. International Journal of Food Science and Technology, 44, 1495–1502.
Castro, P., Padron, J. C. P., Cansino, M. J. C., Velazguez, E. S., & Rafael, M. D. L. (2006). Total volatile base nitrogen and its use to assess freshness in European sea bass stored in ice. Food Control, 17, 245–248.
Cheah, P. B., & Ledward, D. A. (1997). Catalytic mechanism of lipid oxidation following high pressure treatment in pork fat and meat. Journal of Food Science, 62, 1135.
Chéret, R., Chapleau, N., Ladrat, C. D., Bagnis, V., & Lamballerie, M. (2005). Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. Journal of Food Science, 70, 477–483.
Chéret, R., Aránzazu, H. A., Delbarre-Ladrat, C., de Lambarrie, M., & Verrez-Bagnis, V. (2006). Proteins and proteolytic activity changes during refrigerated storage in sea bass (Dicentrarchus labrax L.) muscle after high-pressure treatment. European Food Research and Technology, 222, 527–535.
Connell, J. J. (1975). Control of fish quality. Fishing News (Books) Ltd. Farnham, Surrey, UK.
Dokuzlu, C. (2004). Gıda Analizleri (2nd ed.). Bursa, Turkey: Marmara Kitapevi Yayınları.
Doona, C. J., & Feeherry, F. E. (Eds.). (2007). High pressure processing of foods. Iowa: IFT.
EEC. (1995). Total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Commission Decision 95/149/EEC of 8 March 1995. Journal of European Communities, L97, 84–87.
Erkan, N., & Ozden, O. (2008). Quality assessment of whole and gutted sardine (Sardine pilchardus) stored in ice. International Journal of Food Science and Technology, 43(9), 1549–1559.
Erkan, N., & Uretener, G. (2010). The effect of high hydrostatic pressure on the microbiological, chemical and sensory quality of fresh gilthead sea bream (Sparus aurata). European Food Research and Technology, 230, 533–542.
Erkan, N., Uretener, G., & Alpas, H. (2010a). Effects of high pressure treatment on physicochemical characteristics of fresh sea bass (Dicentrarchus labrax). Journal of Consumer Protection and Food Safety, 5, 83–89.
Erkan, N., Uretener, G., & Alpas, H. (2010b). Effect of high pressure (HP) on the quality and shelf life of red mullet (Mullus surmelutus). Innovative Food Science and Emerging Technologies, 11, 259–264.
Erkan, N., Uretener, G., & Alpas, H. (2011a). The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innovative Food Science and Emerging Technologies, 12, 104–110.
Erkan, N., Uretener, G., Alpas, H., Selcuk, A., Ozden, O., & Buzrul, S. (2011b). Effect of high hydrostatic pressure (HHP) treatment on physicochemical properties of horse mackerel (Trachurus trachurus). Food and Bioprocess Technology, 4, 1322–1329.
FAO. (2011). The state of world fisheries and aquaculture 2010. Italy: Rome.
Fernández, J., Perez-Alvarez, J. A., & Fernández-Lopez, J. A. (1997). Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chemistry, 59, 345–353.
Gobantes, I., Choubert, G., & Gomez, R. (1998). Quality of pigmented (astaxanthin and canthaxanthin) rainbow trout (Oncorhynchus mykiss) fillets stored under vacuum packaging during chilled storage. Journal of Agricaltural Food Chemisty, 46, 4358–4362.
Gómez-Estace, J., Gómez-Guillén, P., & Montero, P. (2007). High pressure effects on the quality and preservation of cold-smoked dolphinfish (Coryphaena hippurus) fillets. Food Chemistry, 102, 1250–1259.
Goulas, A. E., & Kontominas, M. G. (2005). Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chemistry, 93, 511–520.
Gumus, B., Ikiz, R., Unlusayin, M., & Gulyavuz, H. (2008). Quality changes of salted red mullet (Mullus barbatus L., 1758) during vacuum packaged stored at +4°C. E.U. Journal of Fisheries and Aquatic Sciences, 25, 101–104.
Heinz, V., & Bukow, R. (2010). Food preservation by high pressure. Journal für Verbraucherschutz und Lebensmittelsicherheit, 5, 73–81.
Huss, H. H. (1988). Fresh fish quality and quality changes. FAO Fisheries Series, No 29. Rome, Italy: FAO.
Huss, H. H. (1997). Control of indigenous pathogenic bacteria in seafood. Food Control, 8, 91–98.
Jiang, S. T., & Lee, T. C. (2006). Freezing seafood and seafood products: Priciples and applications. In Y. H. Hui (Ed.), Handbook of food science, technology, and engineering (pp. 39(1)–39(22)). New York: CRC.
Khan, M. A., Parrish, C. C., & Shahidi, F. (2006). Effects of mechanical handling, storage on ice and ascorbic acid treatment on lipid oxidation in cultured Newfoundland blue mussel (Mytilus edulis). Food Chemistry, 99, 605–614.
Kundakçı, A. (1989). Kefal ve lüferin ön bekleme kosullarının kaliteye etkileri. E.Ü. Su Ürünleri Fak. Dergisi, 6, 23–24.
Ledward, D. A. (1995). High pressure processing—the potential. In: High Pressure Processing of Food (pp. 1-5). Nottingham, UK: Nottingham University Pres.
Leon, K., Mery, D., Pedreschi, F., & Leon, J. (2006). Color measurement in L*a*b* units from RGB digital images. Food Research International, 39, 1084–1091.
Love, R. M. (2006). Fish biology and food science. In Y. H. Hui (Ed.), Handbook of food science, technology, and engineering (pp. 35(1)–35(20)). New York: CRC.
Lozano, J. E., & Ibarz, A. (1997). Color changes in concentrated fruit pulp during heating at high temperatures. Journal of Food Engineering, 31, 365–373.
Matser, A. M., Stegeman, D., Kals, J., & Bartels, P. V. (2000). Effects of high pressure on colour and texture of fish. High Pressure Research, 19, 109–115.
Mol, S., Ozden, O., Erkan, N., & Baygar, T. (2004). İthal uskumruların değisik çözülme koşullarındaki kalite parametrelerinin belirlenmesi. Turkish Journal of Veterinary and Animal Sciences, 28, 1071–1077.
Ohlsson, T., & Bengtsson, N. (Eds.). (2002). Minimal processing technologies in the food industry. Florida, USA: CRC.
Olafsdottir, G., Martinsdottir, E., Oehlenschlager, J., Dalgaard, P., Jensen, B., Undeland, I., Mackie, I., Henehan, G., Nielsen, J., & Nilsen, H. (1997). Methods to evaluate fish freshness in research and industry. Trends in Food Science and Technology, 8, 258–265.
Ozden, O. (2010). Micro, macro mineral and proximate composition of Atlantic bonito and horse mackerel: a monthly differentiation. International Journal of Food Science, 45(3), 578–586.
Ozogul, F., & Ozogul, Y. (2000). Comparison of methods used for determination of total volatile basic nitrogen (TVB-N) in rainbow trout (Oncorhynchus mykiss). Turk Journal of Zoology, 24, 113–120.
Santibanez, R. (2007). The effect of high hydrostatic pressure on histidine decarboxylase and histamine forming bacteria. Masters Abstracts International, 46, no.03, 89 CSA, Illumina.
Schormüller, J. (1968). Handbuch der Lebensmittel Chemie;Band III/2 Teil.Tiersiche Lebensmittel Eier, Fleisch, Buttermilch (pp. 1561–1578-1584). Berlin: Springer.
Simeonidou, S., Govaris, A., & Vareltzis, K. (1998). Quality assessment of seven Mediterranean fish species during storage on ice. Food Research International, 30, 479–484.
Taliadourou, D., Papadopoulos, V., Domvridou, E., Savvaidis, I. N., & Kontominas, M. G. (2003). Microbiological, chemical and sensory changes of whole and filleted Mediterranean aquacultured sea bass (Dicentrarchus labrax) stored in ice. Journal of the Science of Food and Agriculture, 83, 1373–1379.
Tanaka, M., Xueyi, Z., Nagashima, Y., & Taguchi, T. (1991). Effect of high pressure on the lipid oxidation in sardine meat. Nippon Suisan Gakkaishi, 57, 957–963.
Taylor, S. L. (1989). Histamine poisoning (scombroid fish poisoning): an allergylike intoxication. Clinical Toxicology, 27, 225–240.
Tülsner, M. (1994). Fischverarbeitung. Bd.1 – Rohstoffeigenschaften von Fisch und Grundlagen der Verarbeitungsprozesse. Hamburg: Behr’s Verlag. ISBN: 3-86022-196-5.
Uretener, G. (2009). The effect of high hydrostatic pressure (HHP) on quality and shelf life of fish. M.Sc. Thesis. Istanbul University, Institute of Science, Department of Fisheries and Seafood Processing, Istanbul, Turkey.
Varlık, C., Baygar, T., Ozden, O., Erkan, N., & Metin, S. (2000). Soğukta depolanan karideslerin (Parapenaeus longirostris, LUCAS 1846) bazı duyusal, fiziksel ve kimyasal parametrelerinin belirlenmesi. Turkish Journal of Veterinary and Animal Sciences, 24, 181–185.
Venugopal, V., & Shahidi, F. (1994). Thermostable water dispersion from myofibrillar proteins of atlantic mackerel (Scomber scombrus). Journal of Food Science, 59, 265–268.
Weilmeier, D. M., & Regenstein, J. M. (2004). Cooking enhances the antioxidant properties of polyphosphates. Journal of Food Sciences, 69, FCT16–FCT23.
Yağız, Y., Kristinsson, H. G., Balaban, M. O., & Marshall, M. R. (2007). Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). Journal of Food Science, 72(9), C509–C515.
Yağız, Y., Kristinsson, H. G., Balaban, M. O., Bruce, A. W., Ralat, M., & Marshall, M. R. (2009). Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chemistry, 116, 828–835.
Acknowledgments
The authors would like to thank Dr. K. Bayramova for help in statistical analyses. This work was supported by TUBİTAK-TOVAG project No: 108O668 and METU BAP-project (BAP-03-14-2011-003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senturk, T., Alpas, H. Effect of High Hydrostatic Pressure Treatment (HHPT) on Quality and Shelf Life of Atlantic Mackerel (Scomber scombrus). Food Bioprocess Technol 6, 2306–2318 (2013). https://doi.org/10.1007/s11947-012-0943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0943-1