Abstract
Salt stress usually results in severe physiological damage to plants. Melatonin (N-acetyl-5-methoxytryptamine) is an important growth regulator that adapts plants to abiotic stress. The present study evaluated the role of melatonin application on inducing salt tolerance in sugar beet (Beta vulgaris L.). The protective role of melatonin (0, 30, 60, and 90 µM) was examined by measuring leaf photosynthetic characteristics, antioxidant system, and osmotic adjustment substances of sugar beet seedlings under salt (300 mM Na+) and non-salt stresses. The results showed that salt stress resulted in significantly reduced biomass, reduced photochemical activity of photosystem II (PSII), and evoked the production of reactive oxygen species (ROS). In contrast, the application of melatonin significantly increased antioxidant enzyme activities (SOD, POD, and CAT) under salt stress, reduced ROS accumulation (MDA and O2·−), and enhanced photosynthesis in seedlings. There was no significant difference in the above indicators of melatonin pretreatment under control condition (non-salinized). On day 1 of stress application, the concentration of sucrose decreased significantly, and the concentration of proline and H2O2 increased significantly under melatonin treatment. On day 7, soluble sugar and betaine concentrations increased significantly. Current research speculates that melatonin enhances cellular energy metabolism and may be involved in activating the antioxidant system to eliminate ROS. In conclusion, these results indicated that the application of 60 µM melatonin could act as a feasible way to alleviate the salt stress in sugar beet production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity accounts for a main abiotic stress inhibiting plant growth in the world (Zhu 2001). Currently, approximately 900 million hectares of land is salinized globally, including around 20% of cultivated land and 50% of irrigated land (Cristiano et al. 2016; FAO 2009). Furthermore, the annual world farmland has been reduced by 2 million hectares owing to secondary salinization, resulting in reduced plant productivity (Ke et al. 2016).
Salinity is a complex abiotic stress, including physiological water deficiency, ion toxicity, oxidative damage, nutrient disturbance, metabolic disturbance, photoinhibition and altered main cell enzymatic activities (Chen et al. 2007; Cuin and Shabala 2010; Farouk and Al-Amri 2019c; Farouk and Arafa 2018; Helaly et al. 2018; Munns and Tester 2008). Plants can establish the antioxidant systems and accumulate osmotic adjustment substances in the presence of abiotic stresses (Farouk and Al-Amri 2019c). However, the in-build system of plants to adapt to salinity is insufficient to prevent damage to crops. Therefore, it is urgent to find effective ways to improve the salt tolerance of crops.
Melatonin is an endogenous growth regulator synthesized by animals and plants under stress (Kolár and Machácková 2010; Reiter et al. 2014). As melatonin was first discovered in plants in 1993, considerable progress has been made in this field (Farouk and Al-Amri 2019a, b; Van Tassel et al. 2010). Until date, several studies have demonstrated that plant melatonin could be an essential regulator of redox homeostasis (Arnao and Hernández-Ruiz 2018). It is associated with several physiological functions such as growth (Farouk and Al-Amri 2019b), rooting (Arnao and Hernández-Ruiz 2017), seed germination (Zhang et al. 2017b), and photosynthesis (Li et al. 2017a), as well as protection against abiotic stress (Arnao and Hernández-Ruiz 2015, 2017; Farouk and Al-Amri 2019b; Wang et al. 2018). Melatonin plays a critical role in plants to scavenge reactive oxygen species (ROS), which is the first-line defense to resist environmental and endogenous oxidative stresses (OS) (Arnao and Hernández-Ruiz 2018; Farouk and Arafa 2018). Wang et al. (2016) investigated the potential role of melatonin in salt tolerance and found that 50 to 150 µM exogenous melatonin promoted cucumber seedling growth and photosynthesis under salt stress (200 mM NaCl). Similarly, Li et al. (2017a) found that melatonin treatments at diverse concentrations (50, 150, and 500 µM) mitigated OS and the reduced photosynthetic rate of watermelon leaf samples under 300 mM NaCl stress. According to the results from Siddiqui et al. (2019), applying melatonin promoted tomato plant development in the meantime of reducing ROS contents through increasing the non-enzymatic antioxidant and antioxidant (including catalase and superoxide dismutase). However, information on how melatonin is involved in the salt tolerance of crops is still scarce (Chao et al. 2012; Wei et al. 2015).
Sugar beet (Beta vulgaris L.) represents the main sugar crop in the world, which shows high resistance to abiotic stresses (Bor et al. 2003). However, there is at present no literature related to melatonin involvement in regulating sugar beet response to abiotic stress is available. This work determined the roles of exogenous melatonin in sugar beet growth, antioxidant system, osmotic regulation and photosynthesis in the presence of salt stress; analyzed the regulatory mode; and laid the foundation for further elucidating the physiological and molecular mechanisms of its regulation of salt stress.
Materials and methods
Plant materials and growth conditions
Our experiments were carried out in the Northeast Agricultural University (126°63′ E, 45°44′ N, Harbin, P.R. China). We grew the sugar beet seeds (KWS0143, KWS, Germany) into pots that contained vermiculite within a plant growth room under the following conditions, 23 °C at day, 18 °C at night and 14 h/10 h light/dark cycle.
In our experiments, the relative humidity (RH) and light intensity were set at 60 ± 5% and 450 µmol m–2 s–1 PAR, respectively. Following seedling emergence, they were irrigated once a day with a 1/2 concentration of Hoagland’s solution for 10 days. The uniform seedlings were later transferred to the above nutrient solution. The pH was maintained at 7.0 during the entire plant development period, while nutrient solution was replaced at intervals of 3 days.
Experimental design
Melatonin pretreatment (a culture with 1/2 Hoagland’s solution containing 0, 30, 60 or 90 µM melatonin for 3 days) was performed upon the unfolding of the initial true leaf pair. Salt treatment (S; NaCl and Na2SO4 in a 2:1 molar ratio) was started after the pretreatment (after 3 days), following which the Na+ concentration elevated to 100, 200 and 300 mM in sequence within 3 days (Hossain et al. 2017). A randomized block design comprised 8 treatments: (1) 1/2 Hoagland’s solution (M0); (2) 1/2 Hoagland’s solution + 30 µM melatonin (M30); (3) 1/2 Hoagland’s solution + 60 µM melatonin (M60); (4) 1/2 Hoagland’s solution + 90 µM melatonin (M90); (5) 1/2 Hoagland’s solution + 300 mM Na+ (M0 + S); (6) 1/2 Hoagland’s solution + 30 µM melatonin + 300 mM Na+ (M30 + S); (7) 1/2 Hoagland’s solution + 60 µM melatonin + 300 mM Na+ (M60 + S); (8) 1/2 Hoagland’s solution + 90 µM melatonin + 300 mM Na+ (M90 + S). Sugar beet seedlings were sampled at 1 day and 7 days after salt (300 mM Na+) treatment, and physiological parameters were measured. Each collected sample was subjected to liquid nitrogen freezing at once, followed by preservation under − 80 °C for subsequent biochemical analysis. All experiments were performed thrice and each treatment included three biological replicates.
Endogenous melatonin, Na+, and K+, concentration measurements
Melatonin was extracted from the leaves of sugar beet in line with Pape and Lüning’s description (2006). Afterwards, we used the melatonin ELISA kit (EK-DSM; Buhlmann Laboratories AG, Schonenbuch, Switzerland) to quantify endogenous melatonin in line with specific protocols. Melatonin concentrations were expressed as pg g–1 (fresh mass, FM).
The concentrations (mg g−1 DW) of Na+ and K+ were determined using the method described by Chen et al. (2018). Leaf samples were subjected to 48 h of oven-drying under 75 °C. Later, HNO3:HClO4 (5:1 v/v) was added to digest 0.1 g dry sample till the solution became clear. Thereafter, we measured K+ and Na+ contents through inductively coupled plasma mass spectrometry (Optimal 2100DV; PerkinElmer Instruments, Waltham, MA).
Leaf relative water content and photosynthetic pigments’ concentration
We determined relative water content (RWC) by the formula as follows: RWC = (fresh weight − dry weight)/(turgid weight − dry weight) × 100% (Smart and Bingham 1974).
We measured photosynthetic pigment contents by the acetone approach (Lichtenthaler and Wellburn 1983). To be specific, we isolated photosynthetic pigments from the freshly prepared samples contained within the 80% acetone. Then, the UV-754 spectrophotometer (Zealquest Scientific; Shanghai, China) was employed to measure supernatant absorbance (OD) values at 470, 645, and 663 nm. Later, the corrected extinction coefficients were utilized to calculate the Car, chlorophyll (Chl) a and Chl (a + b) contents, which were presented in the manner of mg g–1 FW.
Gas exchange and photosynthetic activity measurements
We adopted the photosynthesis system (GFS-3000; WALZ, Germany) to measure gas exchange in the leaf samples attached using the light source of 3040-L LED. Typically, we set the photosynthetic photon flux density (PPFD) and cuvette air flow rate as 400 µmol m–2 s–1 and 750 mL min–1, respectively. At the same time, we determined the stomatal conductance (gs), net photosynthetic rate (PN), transpiration rate (E) and intercellular CO2 contents (Ci). Each experiment was carried out for thrice, with 5 leaf samples from diverse plants being used under every replicate. In this experiment, we used the initial 2 completely folding leaf samples on the top (Zou et al. 2019).
Chlorophyll fluorescence measurements
After leaf samples were adapted to dark for 25 min, we used the Portable Chlorophyll Fluorometer (PAM-2500; WALZ, Germany) to measure Chl fluorescence parameters within leaf samples. In the meantime, we measured the maximal (Fv/Fm) and actual ([Y(II)]) photochemical efficiency of PSII, as well as the non-photochemical [Y(NPQ)] and photochemical (qP) quenching, respectively (Pfündel et al. 2008).
Determination of RuBPcase activity
RuBPcase (ribulose-1, 5-bisphosphatecarboxylase) activity was determined using an ELISA kit according to the manufacturer’s (CHUNDUBIO Ltd., China). 5 mL PBS (pH = 7.4) was used to extract 0.5 g freshly prepared leaf samples. Supernatant OD value was detected at 450 nm, while the eventual enzymatic activity was presented in the manner of µmol CO2 g–1 FM−1 min–1.
Determination of malondialdehyde (MDA), superoxide radical, and hydrogen peroxide (H2O2)
Thiobarbituric acid (TBA) was used to extract MDA, and later the supernatant OD values were measured at 450, 532 and 600 nm according to Shi et al.’s method (2013).
We applied Liu and Pang’s approach (2010) to determine superoxide (O2·−) contents. O2·− was extracted from plant materials using the potassium phosphate buffer (pH 7.8) supplemented with 7 mM α-naphthylamine and 17 mM sulfonamide, and incubated for 20 min under 25 °C. Finally, we determined the OD value at 530 nm and presented O2·− content in the manner of nmol g−1 FM min−1.
H2O2 contents were measured according to Velikova et al.’s description (2000). First, root tissues were extracted with 0.1% (w/v) trichloroacetic acid, followed by the addition of PBS and the 1 M KI solution to react for 1 h in dark. Then, we determined the OD value at 390 nm. A standard curve was produced using solutions of known H2O2 concentration, with H2O2 content expressed in mM g FM−1.
Analysis of antioxidant enzymes
Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured using the NBT method of Stewart and Bewley (Stewart and Bewley 1980). Enzymatic activities were presented in the manner of unit g–1 (FM). To be specific, we monitored the guaiacol oxidation rate by Fu et al.’s method (2014) to determine peroxidase (POD, EC 1.11.1.7) activity at 470 nm, then expressed in mmol (guaiacol) min−1 g−1 (FM). In addition, we determined catalase (CAT, EC 1.11.1.6) activity by Aebi’s method (1984).
Flavonoids’ concentration measurements
Contents of flavonoids were determined by Pekal and Pyrzynska’s method (2014). Briefly, 0.3 mL NaNO2 (5%, w/v) was blended with 1 mL of the 50% ethanolic extract, followed by the addition of 0.5 mL AlCl3 (2%, w/v), as well as 0.5 mL NaOH solution (1 M) for neutralization. At 10 min later, we detected the OD value at 510 nm. Meanwhile, the standard flavonoid solution was utilized to prepare the standard curve.
Determination of soluble sugar and sucrose
We measured the content of soluble sugar through the anthrone approach according to Spiro’s description (1966). In brief, we blended 100 μL root extract into the solution supplemented with 2.1 mM anthrone, 1.09 mM thiourea and 1.08 M H2SO4 till 3 ml. Thereafter, we heated the mixed solution for 10 min under 100 °C, and measured the OD value at 620 nm. In addition, we also constructed the calibration curve based on D-glucose for reference.
The sucrose content was determined by a spectrophotometric method following Alcázar et al. description (2005).
Determination of proline and betaine concentration
Proline concentration was determined as described by Bates et al. (1973). 3% (w/v) sulfosalicylic acid was utilized to extract 0.5 g samples. Thereafter, acid ninhydrin along with glacial acetic acid was added, and the obtained mixed solution was maintained for 1 h under 100 °C within the water bath, while the ice bath was used to terminate the reaction. We used toluene to extract proline and measured its OD value at 520 nm. Meanwhile, the proline level was determined based on the standard curve, which was presented in the manner of standard µg g–1 FM.
This study determined the glycine betaine (betaine) level according to Nishimura et al. description (2001). In brief, 0.1 g dried leaf powder was blended sufficiently with 5 ml distilled water. Then, we extracted the supernatants and filtered them using the 0.2-µm filter membrane. For the subsequent esterification, Gorham’s approach (1984) after modification was applied. To be specific, 100 µL standard betaine solution or plant extract was added into the microtube and blended with 50 µL buffer solution consisting of 100 mM KH2PO4,100 mM KHCO3 and acetonitrile at 1:1:4 (v/v), followed by the addition of 300 µL of the 20 mg/mL p-bromophenacyl bromide solution contained within acetonitrile. Later, we capped the tube and then heated it for 90 min under 80 °C. Afterwards, we adopted the centrifugal evaporator to evaporate the reaction mixture till dryness under 80 °C. Later, the electrolyte solution was run, and each sample was injected in hydrostatic mode (10 cm, 10 s), with the potential applied and peak being 15 kV and 254 nm, respectively. At last, we used the standard betaine solution calibration curve to obtain betaine levels in plant extracts.
Statistical analysis
Values were presented in the manner of mean ± SD. All experiments were totally randomized and three replicates were set for each experiment. GraphPad Prism 8.3 (San Diego, CA, USA) and Visio 2013 were used to create figures. SPSS22.0 (IBM, Chicago, IL) was employed for all statistical analyses, while Duncan’s multiple range test was conducted to compare means.
Results
Effects of exogenous application of melatonin on the growth in sugar beet under salt stress
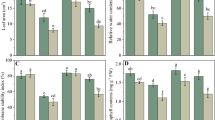
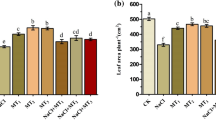
Relative water content and plant biomass of the leaves were measured to study how exogenous melatonin affected sugar beet development. As shown in Fig. 1a, the LRWC was significantly reduced under salt stress, whereas melatonin pretreatment significantly attenuated leaf water loss. On day 7, comparison with the non-salt control, the LRWC of M0 + S was significantly reduced by 43.66%, whereas the change of M60 + S treatment was insignificant. No significant was observed in the biomass of sugar beet seedlings between treatments under non-salt conditions (Fig. 1b). The application of salt stress inhibited plant development under salt stress in the presence or absence of melatonin (Fig. 2). For salt-exposed sugar beet seedlings subjected to melatonin pretreatment, their biomass elevated. Compared with M0 + S, the biomass of M30 + S, M60 + S, M90 + S leaves and petiole was increased significantly (P < 0.05), of which M60 increased 51.00% of leaf biomass and 43.79% of whole plant biomass.
Effects of different concentrations of melatonin on the growth of sugar beet seedlings. a Leaf relative water content of sugar beet seedlings under 0, 30, 60 and 90 µM melatonin and exposed to salt stress for 1 day. b Plant biomass in sugar beet seedlings with 0, 30, 60, and 90 µM melatonin treatments and exposure to salt stress for 7 days. c Melatonin concentration in sugar beet leaves following different treatments for 7 days. d Na+ content e K+ content, and f Na + /K + ratio in sugar beet leaves following different treatments for 7 days. M represents no salt stress after melatonin pretreatment, while M + S stands for melatonin treatment combined with salt treatment. In the figures, the diverse uppercase letters on the top of columns stand for statistically significant differences (P < 0.05; Duncan’s range test) in salt treatment compared with control. The diverse lowercase letters on the top of columns stand for statistically significant differences (P < 0.05; Duncan’s range test) among melatonin treatments. The same as below.
Effects of exogenous application of melatonin on endogenous melatonin, Na+ and K+ concentration measurements under salt stress
As shown in Fig. 1c, the endogenous melatonin concentration of the leaves increased remarkably with exogenous treatment, and especially under salt stress. As shown in Fig. 1d, e, the application of exogenous melatonin significantly (P < 0.05) reduced the Na+ concentration of the beet seedlings and increased the K+ concentration, while reducing the Na+/K+ ratio. Compared with M0 + S, the Na+/K+ ratio of M30 + S, M60 + S, and M90 + S was decreased significantly (P < 0.05), of which 60 mM melatonin effect was the most obvious.
Effects of exogenous application of melatonin on photosynthesis and chlorophyll fluorescence in sugar beet under salt stress
Photosynthesis shows tight correlation with plant development. Therefore, we analyzed the gas exchange chlorophyll concentration, carotenoids, together with Chl fluorescence parameters. As a result, melatonin made no obvious difference to the above parameters under non-salt stress conditions (Figs. 3 and 4). However, carotenoids and Chl markedly reduced after salt treatment (P < 0.05). On day 7, the concentration of chlorophyll and carotenoids of melatonin treatment M60 + S and M90 + S were higher compared with M0 + S. The chlorophyll content of M60 was increased by 51.08%, whereas carotenoid concentration was increased by 47.70%. As shown in Fig. 3d, RuBPcase activity showed no significant change between treatments on the first day of stress. On day 7, salt stress significantly decreased RuBPcase activity, which then increased and finally decreased with increasing melatonin concentration. Among them, M60 + S-treated treatment showed the greatest enzymatic activity, evidently increased compared with control.
Effects of different concentrations of melatonin on photosynthetic pigments and the RuBPcase activity. a Chlorophyll a concentration, b chlorophyll concentration, c carotenoid concentration and d RuBPcase activity of sugar beet seedlings with 0, 30, 60, and 90 µM melatonin treatments and exposure to salt stress for 1 and 7 days
Leaf Fv/Fm and qP significantly decreased on the day 1 of salt stress, and were efficiently kept under melatonin exposure (Fig. 4). On day 7 of salt stress, the difference of Fv/Fm and qP between all treatments was insignificant, whereas the Y(II) for M60 + S treatment was still significantly increased. Despite after a decrease on day 7, leaf Y(NPQ) increased substantially after salt treatment. Melatonin efficiently suppressed the elevation in Y(NPQ), whereas M60 treatment produced the lowest effect.
Salt exposure remarkably decreased photosynthesis (Fig. 5). On day 1 of stress, PN, E and Gs of M0 + S decreased sharply, until these recovered on day 7. The melatonin treatment significantly alleviated the salt stress, and M60 + S-treated PN, E and gs higher than M0 + S by 818.25%, 605.95% and 705.11%, respectively.
Effects of different concentrations of melatonin on gas exchange parameters. a Net photosynthetic rate (PN), b transpiration rate (E), c stomatal conductance (gs), and d intercellular CO2 concentration (Ci) of sugar beet seedlings with 0, 30, 60 and 90 µM melatonin treatments and exposure to salt stress for 1 and 7 days
Exogenous melatonin mitigated ROS damage while enhancing the antioxidant enzymatic activities within salt-exposed sugar beet
As shown in Fig. 6a–c, on day 1 and 7 day after salt stress, compared with M0, M0 + S resulted in increased in MDA concentrations of sugar beet leaves by 174.13% and 203.61% and O2·− by 55.54% and 72.90%, respectively. However, melatonin treatment significantly reduced MDA and O2·− under salt stress. Compared with M0 + S, M60 + S produced lower MDA (− 46.30% and − 12.89%) and O2·− (− 31.24% and − 23.19%) on days 1 and 7 (P < 0.05). On day 1 of salt stress, with the increase in melatonin concentration, the concentration of H2O2 in leaves first increased first and then decreased. The trend on day 7 of stress was the opposite of that on day 1, in which M60 + S and M90 + S was significantly reduced.
Effects of different concentrations of melatonin on reactive oxygen species and the antioxidant system. a Malondialdehyde (MDA), b superoxide anion (O2·−), c catalase (H2O2), d superoxide dismutase (SOD), e peroxidase (POD) and f catalase (CAT) of sugar beet seedlings with 0, 30, 60, and 90 µM melatonin treatments and exposure to salt stress for 1 and 7 days
The results showed that under non-salt stress conditions, melatonin did not change the activity of SOD, POD and CAT (Fig. 6). Under salt stress, the activity of SOD, POD, and CAT in melatonin-treated sugar beet seedlings was significantly higher than that under M0 + S. On day 1 of stress, the SOD, POD, and CAT activities of M60 + S treatment were 68.46%, 210.56%, and 79.13% higher than that under M0 + S, respectively. On day 7 of stress, SOD and CAT activities were maximal, and the CAT activity was approximately twice that on day 1. However, compared day 7, the POD activity was stronger on day 1 under salt stress.
Exogenous application of melatonin altered accumulation of osmolytes in sugar beet under salt stress
The accumulation of proline, betaine, and flavonoids in sugar beet seedlings was evaluated in this study. Figure 7 demonstrates that salt stress significantly enhanced the accumulation of compatible solutes in sugar beet seedlings. Salt stress reduced the sucrose concentration of leaves; however, it increased the soluble sugar concentration. In the absence of salt exposure, melatonin treatment did not significantly affect sucrose or reduce sugar level. Upon salt exposure, melatonin treatment remarkably decreased sucrose level within leaves of sugar beet while markedly increasing soluble sugar content (P < 0.05). Compared with day 1 of salt stress, the trend on day 7 was more pronounced.
Effects of different concentrations of melatonin on osmotic adjustment substances. a Soluble sugar concentration, b sucrose concentration, c proline concentration, d flavonoid concentration, and e betaine concentration of sugar beet seedlings with 0, 30, 60, and 90 µM melatonin treatments and exposure to salt stress for 1 and 7 days
Relative to controls, betaine and proline contents markedly elevated upon salt exposure (P < 0.05), whereas the concentration of flavonoids was significantly reduced considerably. On day 1 of salt stress, melatonin treatment significantly increased the concentration of proline and betaine in sugar beet leaves (P < 0.05) and inhibited the reduction in flavonoid concentration. The increase in proline concentration was the most pronounced, M30 + S, M60 + S and M90 + S increased by 91.22%, 216.95%, and 28.21%, respectively, compared with M0 + S (Fig. 7). Compared with day 1 day, proline concentration was significantly decreased on day 7 of salt stress, whereas the concentration of betaine increased significantly by 4.86%, 34.78%, and 29.89%, respectively.
Discussion
Biomass is a reliable indicator of plant growth, whereas salt stress significantly suppresses plant growth (Egea et al. 2018; Sui et al. 2018). Melatonin exerts a vital part in the increase of biomass through elevating PN in the exposure to salt (Li et al. 2017b). Overcoming osmotic stress accounts for a crucial strategy for the adaptation to salt exposure in plants. In this work, salt exposure markedly suppressed sugar beet seedling growth, reduced PN, and promoted plant leaf dehydration, resulting in reduced plant biomass (Fig. 1). However, melatonin effectively alleviated the water loss of beet seedlings and significantly improved PN, which in turn increased the biomass of the plants.
Different concentrations of exogenous melatonin could increase the endogenous melatonin concentration only to a certain extent, with no absolute effect. The leaf endogenous melatonin content was dose-dependent when sugar beet roots were pre-treated with exogenous melatonin (Fig. 1c). On the contrary, certain studies have suggested that relatively high exogenous melatonin concentration has a more effective promoting effect on tomato (Siddiqui et al. 2019) and corn seedlings (Chen et al. 2018). Compared with the results of these studies, multiple concentrations may be more representative. Consistent with the observations from Li et al. on watermelon seedlings (Li et al. 2017a), exogenous melatonin content had first increased and then weakened impacts on sugar beet seedling growth.
Salt exposure has certain impact on plant photosynthesis via non- and stomatal limitations (Zhou et al. 2016). The stomatal limitation is attributed to the partial closure of the stomata, resulting in a reduced concentration of CO2 entering the mesophyll cells of the plant, which in turn blocks photosynthesis (Sharkey et al. 2007). In this work, the decrease in E and gs and the high level of Ci indicated that reduced PN could be a result of physiological dryness which caused by the high osmotic stress of salinity, leading to almost complete closure of stomata (Fig. 4). The carbon dioxide generated simultaneously during plant respiration increases Ci. In the late stage of melatonin application, PN, gs, and Ci increased indicating melatonin maintenance of stomatal opening under salt stress as the primary reason for elevated PN in the leaves of sugar beet seedlings.
The relationship between abiotic stress and RuBPcase activity is highly controversial. Studies have shown that leaves do not alter RuBPcase activity when they are within the acceptable RWC range (Bota et al. 2004; Lawlor 1995). However, there exist studies that hold the opposite view and believe that increasing the RuBPcase activity contributes to crop growth and can also increase the crop yield under adverse conditions (Ren et al. 2018). RuBPcase activity was not markedly changed during the initial salt treatment stage, which might be caused by the physiological drought of sugar beet (Fig. 3). Under long-term salt stress, the RuBPcase activity of sugar beet leaves was significantly reduced that improved with melatonin pretreatment.
A series of photoreaction processes caused by photosynthetic pigments (chlorophyll, carotenoids, etc.) in plants to absorb photosynthetically active radiation forms the basis of photosynthesis (Fig. 3). Studies have shown that salt stress reduces the photosynthetic pigment concentration of plant leaves. Melatonin maintains a high pigment concentration and enhances photosynthesis. Results from studies on corn and watermelon concluded the same (Chen et al. 2018; Li et al. 2017b).
Fv/Fm and Y(II) are indicative of the real and maximum light energy conversion efficiency of Photosystem II, respectively (Genty et al. 1989; Kitajima and Butler 1975). In the current study, the Fv/Fm and Y(II) of the leaves significantly increased compared with the non-melatonin treatment, and PN, E, and gs increased in the later stage of stress (Fig. 3), which indicated that melatonin exerted a positive regulatory effect on plant photosynthetic systems under salt stress. Chen et al. (2018) showed that melatonin treatment could alleviate oxidative damage in photosynthetic organs. Under salt stress, melatonin increased E or gs; however, it did not decrease LRWC, indicating that melatonin could positively affect water conservation and photosynthesis protection (Ahmad et al. 2019; Chen et al. 2018).
Until date, only a few studies are available on the protective effect of melatonin on the oxidative defense system of sugar beet in the presence of salt exposure. Salt exposure frequently leads to the disturbance in ROS generation and scavenging, thereby increasing ROS accumulation and aggravating oxidative damage to proteins, lipids, and nucleic acids (Tahjib-Ul-Arif et al. 2019; Zhang et al. 2019). Excessive amounts of ROS within plant cells will induce lipid peroxidation while increasing H2O2, MDA and O2·− levels (Farouk and Al-Amri 2019b, c). In the present study, melatonin-treated MDA and O2·− accumulation was lower, indicating that melatonin attenuated the membrane lipid peroxidation under salt stress and reduced the oxidative damage of sugar beet seedlings under salt conditions (Fig. 6). In salt-stressed oat (Gao et al. 2019), corn (Chen et al. 2018), and soybean (Wei et al. 2015), melatonin was found to be involved in reducing the concentration of MDA, thus keeping the membrane permeability and integrity (Li et al. 2019). Critical antioxidant enzymes, including POD, CAT and SOD, usually exert important parts in preventing damage to plant cell membrane systems by ROS accumulation (Jaleel et al. 2009; Tahjib-Ul-Arif et al. 2019; You and Chan 2015). As shown in Fig. 6, the activity of SOD, POD, and CAT in sugar beet leaves was significantly increased in the current study, with POD playing the most crucial role, indicating that melatonin effectively decreased ROS accumulation and avoided membrane structural or functional degradation in sugar beet cells in the presence of salt exposure. These results are strengthened by those of the study that reports that melatonin effectively participates in physiologically regulating plants upon salt exposure (Li et al. 2019). In the present study, the flavonoid concentration following melatonin treatment under salt stress was opposite to that of Y(NPQ). Flavonoids were inhibited by salt stress, and melatonin pretreatment protected the leaves by decreasing the reduction of flavonoids.
Recently, numerous studies have demonstrated that H2O2 acts as the signal transduction element in plants in the presence of biotic or abiotic stress (Li et al. 2018; Neto et al. 2005; Smirnoff and Arnaud 2019). In the present study, melatonin pretreatment significantly reduced MDA and O2·− levels in the early stage of stress; however, it increased H2O2 concentration in seedlings on day 1, which could be attributed to the fact that H2O2 as a signaling substance involved in plant responses to stress (Neto et al. 2005). On day 7, H2O2 contents in M60 + S and M90 + S were lower than that of M0 + S, suggesting that it was cleared by the antioxidant system induced by melatonin (Chen et al. 2018).
The cellular energy status has been suggested to be a vital modulator for plant growth as well as stress decrease (Jamsheer and Laxmi 2015). In our study, salt stress significantly reduced sucrose concentration; however, it increased the concentration of soluble sugar (Fig. 7). Melatonin treatment effectively promoted this process. According to certain studies, plants are required to consume energy in response to stress (Crawford et al. 2018; De Block et al. 2005). In the early stages of salt stress, melatonin reduced the concentration of soluble sugar. We speculate that melatonin could cope with stress by enhancing energy metabolism in cells (Zhang et al. 2017a; Zhao et al. 2020). Cells can produce large amounts of ATP through sucrose metabolism and TCA cycling accompanied by the accumulation of several primary and secondary metabolites, such as proline, betaine, and flavonoids. The accumulation of soluble sugars, proline, and betaine ensures plant photosynthesis (Ashraf and Foolad 2007; Rady et al. 2018; Wei et al. 2015). In general, an increase in soluble sugar and proline concentration helps reduce damage under abiotic stress (Ben Ahmed et al. 2010; Yang et al. 2007).
Proline and betaine account for 2 main organic permeants accumulating within multiple plant varieties upon environmental stress (Farouk and Al-Amri 2019c). Generally, plants respond to abiotic stress on the basis of certain permeates production and accumulation. These permeations not only positively affect membrane and enzyme integrity but also exert vital parts in plant osmotic adjustment that mediates growth under stress conditions (Liu et al. 2018; Vicente et al. 2016). Our data suggest that melatonin could first act through responsive regulation of proline (early stage) and betaine (late stage) and subsequently activate an antioxidant defense system to combat cellular damage caused by salt stress (Fig. 8).
Conclusion
Our study demonstrated that exogenous application of melatonin could alleviate inhibition of the growth of sugar beet seedlings which exposed to salt stress. Melatonin may rapidly enhance the production of H2O2 as a signaling substance in sugar beet leaves, induce high levels of proline synthesis, and perform the osmotic adjustment. Simultaneously, melatonin could enhance the activity of protective enzymes to remove ROS.
Author contribution statement
LL contributed to the experimental design, data analyses and manuscript writing; CFL and ZJG conceived the study, contributed to the experimental design, and revised the manuscript; DL, BW, ZYW, PFZ, XYL, JTC, SYZ, CLZ and YBW helped with the sample preparation and data analyses; and all the authors approved the manuscript.
Abbreviations
- Car:
-
Carotenoids
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- Ci:
-
Intercellular CO2 concentration
- E :
-
Transpiration rate
- F v/F m :
-
Maximum quantum yield of PSII
- LRWC:
-
Leave relative water content
- MDA:
-
Malondialdehyde content
- g s :
-
Stomatal conductance
- Pn:
-
Net photosynthetic rate
- q L :
-
Estimates the fraction of open PSII centers
- POD:
-
Peroxidase
- q P :
-
Photochemical quenching
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Y(II):
-
Effective quantum yield of PSII
- Y(NO):
-
Quantum yield of nonregulated non-photochemical energy dissipation
- Y(NPQ):
-
Quantum yield of regulated non-photochemical energy dissipation
References
Aebi H (1984) Catalase in vitro methods. Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad S et al (2019) Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 7:e7793. https://doi.org/10.7717/peerj.7793
Alcázar Á, Jurado JM, Martín MJ, Pablos F, González AG (2005) Enzymatic-spectrophotometric determination of sucrose in coffee beans. Talanta 67:760–766. https://doi.org/10.1016/j.talanta.2005.04.005
Arnao MB, Hernández-Ruiz J (2015) Functions of melatonin in plants: a review. J Pineal Res 59:133–150. https://doi.org/10.1111/jpi.12253
Arnao MB, Hernández-Ruiz J (2017) Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta Physiol Plant 39:127. https://doi.org/10.1007/s11738-017-2428-3
Arnao MB, Hernández-Ruiz J (2018) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci. https://doi.org/10.1016/j.tplants.2018.10.010
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Ben Abdullah F (2010) Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agric Food Chem 58:4216–4222. https://doi.org/10.1021/jf9041479
Bor M, Ozdemir F, Turkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84. https://doi.org/10.1016/s0168-9452(02)00338-2
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681. https://doi.org/10.1111/j.1469-8137.2004.01056.x
Chao L et al (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53:298–306. https://doi.org/10.1111/j.1600-079X.2012.00999.x
Chen Z et al (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145:1714–1725. https://doi.org/10.1104/pp.107.110262
Chen YE et al (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 164:349–363. https://doi.org/10.1111/ppl.12737
Crawford T, Lehotai N, Strand A (2018) The role of retrograde signals during plant stress responses. J Exp Bot 69:2783–2795. https://doi.org/10.1093/jxb/erx481
Cristiano G, Camposeo S, Fracchiolla M, Vivaldi G, De Lucia B, Cazzato E (2016) Salinity differentially affects growth and ecophysiology of two mastic tree (Pistacia lentiscus L.) accessions. Forests 7:156. https://doi.org/10.3390/f7080156
Cuin TA, Shabala S (2010) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885. https://doi.org/10.1111/j.1365-3040.2007.01674.x
De Block M, Verduyn C, De Brouwer D, Cornelissen M (2005) Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J 41:95–106. https://doi.org/10.1111/j.1365-313X.2004.02277.x
Egea I et al (2018) The SlCBL10 calcineurin B-like protein ensures plant growth under salt stress by regulating Na+ and Ca2+ homeostasis. Plant Physiol 176:1676–1693. https://doi.org/10.1104/pp.17.01605
FAO (2009) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush. Accessed 10 Oct 2019
Farouk S, Al-Amri S (2019a) Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind Crops Prod 142:111823. https://doi.org/10.1016/j.indcrop.2019.111823
Farouk S, Al-Amri SM (2019b) Exogenous melatonin-mediated modulation of arsenic tolerance with improved accretion of secondary metabolite production, activating antioxidant capacity and improved chloroplast ultrastructure in rosemary herb. Ecotoxicol Environ Saf 180:333–347. https://doi.org/10.1016/j.ecoenv.2019.05.021
Farouk S, Al-Amri SM (2019c) Exogenous zinc forms counteract NaCl-induced damage by regulating the antioxidant system, osmotic adjustment substances, and ions in canola (Brassica napus L. cv. Pactol) plants. J Soil Sci Plant Nutr 19:887–899. https://doi.org/10.1007/s42729-019-00087-y
Farouk S, Arafa SA (2018) Mitigation of salinity stress in canola plants by sodium nitroprusside application Span. J Agric Res 16:0802. https://doi.org/10.5424/sjar/2018163-13252
Fu Y et al (2014) Bioaccumulation, subcellular, and molecular localization and damage to physiology and ultrastructure in Nymphoides peltata (Gmel.) O. Kuntze exposed to yttrium. Environ Sci Pollut Res 21:2935–2942. https://doi.org/10.1007/s11356-013-2246-0
Gao WY, Feng Z, Bai QQ, He JJ, Wang YJ (2019) Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int J Mol Sci. https://doi.org/10.3390/ijms20051176
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta (BBA)-Gen Subj 990:87–92. https://doi.org/10.3390/ijms20051176
Gorham J (1984) Separation of plant betaines and their sulphur analogues by cation-exchange high-performance liquid chromatography. J Chromatogr 287:345–351. https://doi.org/10.1016/S0021-9673(01)87710-4
Helaly M, Farouk S, Arafa SA, Amhimmid NB (2018) Inducing salinity tolerance of rosemary (Rosmarinus officinalis L.) plants by chitosan or zeolite application. Asian J Adv Agric Res 5:1–20. https://doi.org/10.9734/AJAAR/2018/40051
Hossain MS, Elsayed AI, Moore M, Dietz KJ (2017) Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J Exp Bot 68:1283–1298. https://doi.org/10.1093/jxb/erx019
Jaleel CA et al (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436. https://doi.org/10.1007/s11738-009-0275-6
Jamsheer KM, Laxmi A (2015) Expression of Arabidopsis FCS-Like Zinc finger genes is differentially regulated by sugars, cellular energy level, and abiotic stress. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00746
Ke Q et al (2016) Transgenic poplar expressing codA exhibits enhanced growth and abiotic stress tolerance. Plant Physiol Biochem 100:75–84. https://doi.org/10.1016/j.plaphy.2016.01.004
Kitajima M, Butler W (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta (BBA)-Bioenerg 376:105–115. https://doi.org/10.1016/0005-2728(75)90209-1
Kolár J, Machácková I (2010) Melatonin in higher plants: occurrence and possible functions. J Pineal Res 39:333–341. https://doi.org/10.1111/j.1600-079X.2005.00276.x
Lawlor D (1995) The effects of water deficit on photosynthesis. Environ Plant Metab 5:129–160
Li H et al (2017a) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci 8:295. https://doi.org/10.1111/j.1600-079X.2005.00276.x
Li H et al (2017b) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00295
Li Q, Lv LR, Teng YJ, Si LB, Ma T, Yang YL (2018) Apoplastic hydrogen peroxide and superoxide anion exhibited different regulatory functions in salt-induced oxidative stress in wheat leaves. Biol Plant 62:750–762. https://doi.org/10.1007/s10535-018-0808-1
Li JP, Liu J, Zhu TT, Zhao C, Li LY, Chen M (2019) The role of melatonin in salt stress responses. Int J Mol Sci. https://doi.org/10.3390/ijms20071735
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents analysis (Peach). Biochem Soc Transc 11:591–592
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Biol Ecol 382:82–87. https://doi.org/10.1016/j.jembe.2009.11.005
Liu D, Liu M, Liu X-L, Cheng X-G, Liang Z-W (2018) Silicon priming created an enhanced tolerance in alfalfa (Medicago sativa L.) seedlings in response to high alkaline stress. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00716
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Neto ADDA, Prisco JT, Enéas-Filho J, Medeiros JVR, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces saltstress acclimation in maize plants. J Plant Physiol 162:1114–1122. https://doi.org/10.1016/j.jplph.2005.01.007
Nishimura N, Zhang J, Abo M, Okubo A, Yamazaki S (2001) Application of capillary electrophoresis to the simultaneous determination of betaines in plants. Anal Sci 17:103–106. https://doi.org/10.2116/analsci.17.103
Pape C, Lüning K (2006) Quantification of melatonin in phototrophic organisms. J Pineal Res 41:157–165. https://doi.org/10.1111/j.1600-079X.2006.00348.x
Pekal A, Pyrzynska K (2014) Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods 7:1776–1782. https://doi.org/10.1007/s12161-014-9814-x
Pfündel E, Klughammer C, Schreiber U (2008) Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl Notes 1:21–24
Rady MO, Semida WM, El-Mageed TAA, Hemida KA, Rady MM (2018) Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci Hortic 240:614–622. https://doi.org/10.1016/j.scienta.2018.06.069
Reiter RJ, Tan DX, Galano A (2014) Melatonin: exceeding expectations. Physiology 29:325–333. https://doi.org/10.1152/physiol.00011.2014
Ren B, Zhang J, Dong S, Liu P, Zhao B (2018) Exogenous 6-benzyladenine improves antioxidative system and carbon metabolism of summer maize waterlogged in the field. J Agro Crop Sci 204:175–184. https://doi.org/10.1111/jac.12253
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040. https://doi.org/10.1111/j.1365-3040.2007.01710.x
Shi H, Ye T, Chan Z (2013) Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 71:226–234. https://doi.org/10.1016/j.plaphy.2013.07.021
Siddiqui MH et al (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci. https://doi.org/10.3390/ijms20020353
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260. https://doi.org/10.1104/pp.53.2.258
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214. https://doi.org/10.1111/nph.15488
Spiro RG (1966) Analysis of sugars found in glycoproteins. Methods Enzymol 8:3–26. https://doi.org/10.1016/0076-6879(66)08005-4
Stewart RR, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248. https://doi.org/10.1104/pp.65.2.245
Sui N, Wang Y, Liu SS, Yang Z, Wang F, Wan SB (2018) Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00007
Tahjib-Ul-Arif M, Afrin S, Polash MAS, Akter T, Ray SR, Hossain MT, Hossain MA (2019) Role of exogenous signaling molecules in alleviating salt-induced oxidative stress in rice (Oryza sativa L.): a comparative study. Acta Physiol Plant. https://doi.org/10.1007/s11738-019-2861-6
Van Tassel DL, Roberts N, Lewy A, O’Neill SD (2010) Melatonin in plant organs. J Pineal Res 31:8–15. https://doi.org/10.1034/j.1600-079X.2001.310102.x
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(59):66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vicente O, Al Hassan M, Boscaiu M (2016) Contribution of osmolyte accumulation to abiotic stress tolerance in wild plants adapted to different stressful environments. Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, pp 13–25. https://doi.org/10.1007/978-81-322-2616-1_2
Wang L, Liu J, Wang W, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54:19–27. https://doi.org/10.1007/s11099-015-0140-3
Wang Y, Reiter RJ, Chan Z (2018) Phytomelatonin: a universal abiotic stress regulator. J Exp Bot 69:963. https://doi.org/10.1093/jxb/erx473
Wei W et al (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707. https://doi.org/10.1093/jxb/eru392
Yang C, Chong J, Li C, Kim C, Shi D, Wang D (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276. https://doi.org/10.1007/s11104-007-9251-3
You J, Chan ZL (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci. https://doi.org/10.3389/fpls.2015.01092
Zhang JR et al (2017a) Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct Plant Biol 44:961–968. https://doi.org/10.1071/fp17003
Zhang N et al (2017b) Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci Rep 7:503. https://doi.org/10.1038/s41598-017-00566-1
Zhang W, Liu SH, Li CC, Zhang PY, Zhang PY (2019) Transcriptome sequencing of Antarctic moss under salt stress emphasizes the important roles of the ROS-scavenging system. Gene 696:122–134. https://doi.org/10.1016/j.gene.2019.02.037
Zhao Y, Yu H, Zhou J-M, Smith SM, Li J (2020) Malate circulation: linking chloroplast metabolism to mitochondrial ROS. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2020.01.010
Zhou XT, Zhao HL, Cao K, Hu LP, Du TH, Baluska F, Zou ZR (2016) Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01823
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71. https://doi.org/10.1016/S1360-1385(00)01838-0
Zou CL et al (2019) Photosynthetic capacity, osmotic adjustment and antioxidant system in sugar beet (Beta vulgaris L.) in response to alkaline stress. Photosynthetica 57:350–360. https://doi.org/10.32615/ps.2019.010
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32071973 and 31671622). Special thanks to Fuxin Zhao for his help and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Communicated by Z.-L. Zhang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Wang, Z., Gai, Z. et al. Exogenous application of melatonin improves salt tolerance of sugar beet (Beta vulgaris L.) seedlings. Acta Physiol Plant 44, 57 (2022). https://doi.org/10.1007/s11738-022-03389-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03389-4