Abstract
Kochia sieversiana (Pall.) C. A. M., a naturally alkali-resistant halophyte, was chosen as the test organism for our research. The seedlings of K. sieversiana were treated with varying (0–400 mM) salt stress (1:1 molar ratio of NaCl to Na2SO4) and alkali stress (1:1 molar ratio of NaHCO3 to Na2CO3). The concentrations of various solutes in fresh shoots, including Na+, K+, Ca2+, Mg2+, Cl−, SO 2−4 , NO −3 , H2PO −3 , betaine, proline, soluble sugar (SS), and organic acid (OA), were determined. The water content (WC) of the shoots was calculated and the OA components were analyzed. Finally, the osmotic adjustment and ion balance traits in the shoots of K. sieversiana were explored. The results showed that the WC of K. sieversiana remained higher than 6 [g g−1 Dry weight (DW)] even under the highest salt or alkali stress. At salinity levels >240 mM, proline concentrations increased dramatically, with rising salinity. We proposed that this was not a simple response to osmotic stress. The concentrations of Na+ and K+ all increased with increasing salinity, which implies that there was no competitive inhibition for absorption of either in K. sieversiana. Based on our results, the osmotic adjustment feature of salt stress was similar to that of alkali stress in the shoots of K. sieversiana. The shared essential features were that the shoots maintained a state of high WC, OA, Na+, K+ and other inorganic ions, accumulated largely in the vacuoles, and betaine, accumulated in cytoplasm. On the other hand, the ionic balance mechanisms under both stresses were different. Under salt stress, K. sieversiana accumulated OA and inorganic ions to maintain the intracellular ionic equilibrium, with close to equal contributions of OA and inorganic ions to anion. However, under alkali stress, OA was the dominant factor in maintaining ionic equilibrium. The contribution of OA to anion was as high as 84.2%, and the contribution of inorganic anions to anion was only 15.8%. We found that the physiological responses of K. sieversiana to salt and alkali stresses were unique, and that mechanisms existed in it that were different from other naturally alkali-resistant gramineous plants, such as Aneurolepidium chinense, Puccinellia tenuiflora.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The salinization of soil is a widespread environmental problem and an important factor in limiting agricultural productivity. Although the world’s land surface occupies about 13.2 × 109 ha, no more than 7 × 109 ha are potentially arable and only 1.5 × 109 ha are currently cultivated. Of the cultivated lands, about 0.34 × 109 ha (23%) are saline and another 0.56 × 109 ha (37%) are sodic (Läuchli and Lüttge 2002). In saline and sodic soils, Na+, Ca2+, Mg2+, and K+ are the main cations of dissoluble mineral salts, and Cl−, SO 2−4 , HCO −3 , CO 2−3 , and NO −3 are the corresponding main anions (Läuchli and Lüttge 2002). These ions all come from neutral salts or alkaline salts. We can further classify the natural salt stress, in terms of the salt characteristics, into neutral salt stress, alkaline salt stress, and mixed salt stress. Some reports have clearly demonstrated that alkaline salt stress and neutral salt stress are two distinct kinds of stresses for plants and should be called alkali stress and salt stress, respectively (Shi and Yin 1993). In fact, alkaline salts (NaHCO3 and Na2CO3) have been shown to cause much stronger destructive effects on plants than neutral salts (NaCl and Na2SO4) (Shi and Yin 1993). When a salinized soil contains HCO3 − and/or CO3 2−, which consequentially elevates the soil pH, plants undergo the damaging effects of both salt and alkali stress. However, relatively little attention has been given to alkali stress. Even so, there have been some reports about high-pH calcareous soils (Brand et al. 2002; Nuttall et al. 2003), alkaline soil (Hartung et al. 2002), alkaline salt stress (Campbell and Nishio 2000; El-Samad and Shaddad 1996; Shi and Yin 1992; Yan et al. 2005), and mixed salt stress (Shi and Wang 2005; Shi and Sheng 2005) in the literature. These reports not only clearly demonstrate the actual existence of alkali stress, but also show that the effects of alkali stress are more severe than salt stress (Shi and Yin 1993; Tang and Turner 1999).
Halophytes have been studied as the principal species for exploiting and recovering salt-alkalinized soil in order to address the growing problem of utilizing salinized and alkalized lands (Zheng and Li 1999). An increasing number of reports on distribution (Zhao et al. 2002), exploitation (Zhao et al. 2002), and physiological mechanisms of salt resistance (Short and Colmer 1999; Khan et al. 2000a; Peng et al. 2004) of halophytes have been published. However, few studies have been done on the alkali resistance aspect of halophytes. In fact, soil salinization and alkalization frequently co-occur, and this has caused severe problems in some areas. For example, in northeast China, alkalinized grassland covers more than 70% of land area, and is expanding (Kawanabe and Zhu 1991).
Salt stress in the soil generally involves osmotic stress and ion injury (De-Lacerda et al. 2003; Ghoulam et al. 2002; Soussi et al. 1998; Khan et al. 2000b). Moreover, high-salt environments can break the ion homeostasis of plant cells, destroy the ionic balance, and affect the distributions of K+ and Ca2+ in cells (Niu et al. 1995). It is necessary to osmotically adjust and re-establish the ion balance in cells for plants living under salt stress (Li et al. 2003). Comparing alkali stress with salt stress, there is an added high-pH effect. The high-pH caused by alkali stress directly affects the absorption of mineral elements and interferes with the re-establishment of ionic balance. To resist alkali stress, plants not only have to regulate the intracellular pH to keep ionic balance, but also have to spend materials and energy to regulate pH in their root environment. Osmotic adjustment and intracellular ion balance play key roles in plant salt resistance and alkali resistance. Therefore, our objective was to demonstrate the differences between osmotic adjustment and ion balance traits and differences between adapting to salt and alkali conditions, using an alkali resistant halophyte Kochia sieversiana (Pall.) C. A. M.. K. sieversiana is not only a herbage used in traditional Chinese medicine (Zhai et al. 1996), but is also a forage plant with high salt and alkali resistance (Zhao et al. 2002; Zheng and Li 1999). It can survive and grow well even at a pH above ten (Zheng and Li 1999). It often invades alkali soils as a pioneer plant and forms one dominant halophyte community. However, there have been no reports on the salt and alkali resistant characteristics of K. sieversiana.
Two neutral salts, NaCl and Na2SO4, and two alkaline salts, NaHCO3 and Na2CO3, are the main salt components in the alkalized grassland of northeast China where K. sieversiana is commonly found. In this paper, these two neutral salts and two alkaline salts were mixed, respectively, at molar ratios of 1:1. The resulting mixtures, with varying concentrations in the range of 0–400 mM, were used to simulate different salinity and alkalinity stress conditions and to treat the seedlings of K. sieversiana, in order to probe its physiological adaptive traits to salt stress and alkali stress.
Materials and methods
Plant materials
Seeds of K. sieversiana were collected from the native grassland located in Changling county, Jilin province, northeast China, and were sown in 17 cm diameter plastic pots containing 2.5 kg of washed sand. Each pot contained 25 seedlings and seedlings were sufficiently watered with Hoagland nutrient solution every 2 days. Evaporated water was replenished with distilled water at other times. All pots were placed outdoors and were kept out of rain. Temperatures during the experiment were in the range of 22–26°C during the day and 19–22°C at night.
Design of simulated saline and alkaline conditions
Salt mixtures of 80, 160, 240, 320, and 400 mM were prepared using combinations of either two neutral salts, NaCl and Na2SO4, mixed at a 1:1 molar ratio, for the salt stress treatment, or two alkaline salts, NaHCO3 and Na2CO3, also mixed at a 1:1 molar ratio, for the alkali stress treatment. These treatment concentrations referred to the total salt concentrations of NaCl + Na2SO4 or NaHCO3 + Na2CO3. Therefore, in the 400 mM solution, a mixture of 200 mM NaCl and 200 mM Na2SO4 would result in total ion concentrations of 600 mM Na + 200 mM Cl + 200 mM SO4. The five concentration treatments for each salt pair were applied to the plants as described below. The treatment groups were designated S1–S5 for the salt stress group and A1–A5 for the alkali stress group. The pH values of treatment solutions ranged from 6.60 to 6.95 in the salt stress group and from 9.77 to 10.14 in the alkali stress group.
Stress treatment
The seedlings were subjected to stress treatment when they were 6 weeks old. Thirty-six pots of uniformly growing seedlings were randomly divided into 12 sets, three pots per set. Each pot was considered a single replicate. Each set contained three replicates. One set was used as an untreated control. A second set was used for determining dry (DW)and fresh weights (FW) at the beginning of treatment and the remaining ten sets were treated with the various stress treatments. Stress treatments were performed daily at around 5–6 p.m. by thoroughly watering treated plants with 500 ml of treatment solution per pot, in three portions. Control plants were maintained by watering with nutrient solution. On the first day, all pots were treated using the 80 mM treatment solutions. Concentrations of treatment solutions were increased daily at 80 mM increments, as appropriate for sets with higher designated concentrations. As each set reached the designated concentration, that concentration was maintained until the end of the experiment. After the concentrations of the two sets with the highest concentration, 400 mM, were reached, treatment continued for another 7 days. The total treatment duration was 12 days.
Physiological indices measurements
All plants were harvested the morning after the final treatment. The plants were first washed with tap water, then distilled water. Roots and shoots were separated and the FWs were determined for each plant. A portion of the fresh samples were taken to measure the physiological indices. The remainder of the samples were oven-dried at 80°C for 15 min, then vacuum-dried at 40°C to constant weight and the DWs were recorded. The water content (WC) was calculated using the formula (FW-DW)/DW, and expressed as g g−1 DW.
Pressurized liquid chromatography was used for the determination and analysis of organic acids (OA). Standard samples of malic acid, citric acid, oxalic acid (OXA), succinic acid, α-ketoglutaric acid, and tartaric acid were chromatographically pure, and ultrapure water was prepared by using a Millipore system. A Hypersil C18 column (4.6 × 250 mm2, 5 μm) (Shimadzu Corp., Kyoto, Japan) and a Shimadzu RID-10A ultraviolet detector (214 nm) were used. The mobile phase contained 0.5% (in mass fraction) KH2PO4 and 0.5 mM tetra-n-butyl ammonium hydrogen sulfate, pH = 2.0, and treated with an ultrasonic generator for 20 min to degas. The column temperature was set at 40°C, the column pressure was set at 20 MPa, and the injection volume was 5 μl. The flow rate was 0.5 ml/min. All sample solutions were filtered through a Millipore system (0.45 μm) before use.
Dry samples of plant material (100 mg) were treated with 20 ml deionized water at 100°C for 20 min and the extract was taken to determine free inorganic ion contents. The contents of NO3 −, Cl−, SO4 2−, and H2PO4 − were determined by ion chromatography (DX-300 ion chromatographic system, AS4A-SC chromatographic column, CDM-II electrical conductivity detector, mobile phase: Na2CO3/NaHCO3 = 1.7/1.8 mM, DIONEX, Sunnyvale, CA, USA). A flame photometer was used for the determination of K+ and Na+ concentrations (Wang and Zhao 1995) and complexometric titration was used for the determination of free Ca2+ and free Mg2+ concentrations (Bao 1981).
Fresh samples were taken to determine the contents of proline, total soluble sugar (SS) and betaine. The contents of proline and total SS were measured, respectively, using ninhydrin and anthrone (Zhu et al. 1983). The betaine content was determined using the method of Grieve and Grattan (1983). The concentrations of solutes were expressed in mM based on shoot WC. The results were equal to the molar concentration of each solute in the living plants.
In order to determine the pH of the plant tissue sap, fresh plant shoots were thoroughly washed three times with neutral deionized water and surface water dried using filter paper. The shoots were then crushed and the tissue sap was extruded. The pH was measured with a digital pH meter.
Dissociation extent of organic acid computation
The dissociation extents of each OA in plant tissue were calculated using a special program, GEOCHEM-PC 2.0 (Parker et al. 1987), based on the plant tissue pH.
Statistical data analysis
Statistical analysis of the data, which involved data processing and variance analysis (ANOVA), was performed using the statistical program SPSS 14.0. All the acquired data were represented by an average of the three replicate measurements and standard errors (SE). Significance was tested at the 5% level.
Results
Water content
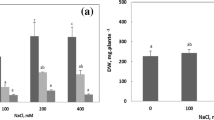
With increasing salt stress and alkali stress, WC in shoots decreased slightly (P < 0.01). Reductions under alkali stress (P < 0.01) were greater than those under salt stress (Fig. 1). The WC remained as high as 6 g g−1 DW even under the alkali stress of 400 mM (treatment A5). The relatively high WC of K. sieversiana was apparently not caused by becoming succulent, but was caused by a special pathway of salinity tolerance, which should be further investigated.
Effects of salt and alkali stresses on the water contents of K. sieversiana shoots. The 6-week-old K. sieversiana seedlings were treated with salt stress (NaCl : Na2SO4 = 1:1; pH 6.28–6.95) and alkali stress (NaHCO3 : Na2CO3 = 1:1; pH 9.77–10.14) for 12 days. The values are means (±SE) of triplicate samples
Inorganic cations
The Na+ concentrations of shoots increased with increasing salinity under both salt stress (P < 0.0001) and alkali stress (P < 0.0001). The extents of the increases were similar under both types of stress at the same salinity, except at 400 mM, where Na+ concentrations were much higher under alkali stress than under salt stress (Fig. 2a). The K+ concentration of the control was relatively high and K+ concentrations also increased with increasing salinity under both stresses (salt stress: P < 0.001; alkali stress: P < 0.0001) with the increment being greater under alkali stress than under salt stress (Fig. 2b). The above results indicated that the absorption and transportation of Na+ and K+ are unique for K. sieversiana. The free Ca2+ concentrations increased slightly with increasing salinity under salt stress (P < 0.0001) and the increments were much larger under alkali stress (P < 0.0001, Fig. 2c). Under salt stress, the change in free Mg2+ concentrations (Fig. 2d) with increasing salinity was insignificant, and a steady increase of free Mg2+ concentrations with increasing salinity was observed under alkali stress (P < 0.0001). As indicated in Fig. 2, the responses of K+, free Ca2+ and free Mg2+ to salt and alkali stresses were similar. The incremental changes of these three cations under alkali stress were all greater than under salt stress.
Effects of salt and alkali stresses on concentrations of Na+ (a), K+ (b), free Ca2+ (c), and free Mg2+ (d) in the fresh shoots of K. sieversiana. The 6-week-old K. sieversiana seedlings were treated with salt stress (NaCl : Na2SO4 = 1:1; pH 6.28–6.95) and alkali stress (NaHCO3 : Na2CO3 = 1:1; pH 9.77–10.14) for 12 days. The values are means (±SE) of triplicate samples
Inorganic anions
With increasing salinity, Cl− concentrations remained relatively unchanged under alkali stress, but increased quickly under salt stress (P < 0.0001) reaching a maximum value of 5.85 times that of the control (Fig. 3a). The SO4 2− concentration increased only slightly under alkali stress (P < 0.0001) with increasing salinity, but increased significantly under salt stress (P < 0.0001) and reached 6.71 times that of the control (Fig. 3b). Under salt stress, the concentration of H2PO3 − increased with increasing salinity (P < 0.01). In comparison, H2PO3 − concentrations only increased slightly under alkali stress (P < 0.01, Fig. 3d). Furthermore, H2PO3 − concentrations under alkali stress were always much lower than those of the control, which might be closely related to the deposition of phosphate caused by the high pH of alkali stress. Data in Fig. 3 suggests that the responses of Cl−, SO4 2−, and H2PO3 − to salt stress and alkali stress were very similar. Their concentrations under salt stress were all much higher than those under alkali stress at the same salinity. The NO3 − concentration under salt stress was higher than in the control (P < 0.05) and appeared to have no significant dependence on salinity (Fig. 3c). The concentration of NO3 − under alkali stress was the same as that of salt stress at 80 mM salinity and decreased with increasing salinity (P < 0.0001).
Effects of salt and alkali stresses on concentrations of Cl− (a), SO4 2− (b), NO3 − (c), and H2PO3 − (d) in the fresh shoots of K. sieversiana. The 6-week-old K. sieversiana seedlings were treated with salt stress (NaCl : Na2SO4 = 1:1; pH 6.28–6.95) and alkali stress (NaHCO3 : Na2CO3 = 1:1; pH 9.77–10.14) for 12 days. The values are means (±SE) of triplicate samples
Organic solutes
Effects of salt and alkali stresses on proline content
When salinity was below 240 mM, no significant change in proline concentration was observed under either salt or alkali stress. When salinity was raised above 240 mM, the proline concentration increased with rising salinity (salt stress: P < 0.0001; alkali stress: P < 0.0001). The maximum proline concentration was 12.87 times that of the control under salt stress and 17 times that of the control under alkali stress. The responses of proline concentrations to salinity were similar under both stresses before 400 mM (Fig. 4a). However, at 400 mM salinity, the proline concentration under alkali stress was higher than that under salt stress (Fig. 4a).
Effects of salt and alkali stresses on concentrations of proline (a), betaine (b), soluble sugar (c), and organic acid (d) in the fresh shoots of K. sieversiana. The 6-week-old K. sieversiana seedlings were treated with salt stress (NaCl : Na2SO4 = 1:1; pH 6.28–6.95) and alkali stress (NaHCO3 : Na2CO3 = 1:1; pH 9.77–10.14) for 12 days. The values are means (±SE) of triplicate samples. SS soluble sugar, OA organic acid, OXA oxalic acid
Effects of salt and alkali stresses on betaine content
Betaine is one of the major secondary metabolites of K. sieversiana, and it reaches very high levels even under non-stress conditions. In this experiment, the betaine content of the control had already reached up to 1.67% of DW and was expected to further increase under stress conditions. With increasing salinity, the betaine concentration in shoots increased rapidly and to similar extents under salt stress (P < 0.0001) and alkali stress (P < 0.0001) (Fig. 4b). However, at the highest salinity, 400 mM, the betaine concentration under alkali stress was higher than under salt stress (Fig. 4b).
Effects of salt and alkali stresses on soluble sugar content
The stress response of SS concentrations was observed to be different than the responses of proline and betaine concentrations. With increasing stress intensity, the increase in SS concentration was greater under alkali stress (P < 0.0001) than under salt stress (P < 0.001, Fig. 4c).
Effects of salt and alkali stresses on organic acid content, components, and extent of dissociation
The OA contents in K. sieversiana shoots are shown in Fig. 4d. Malic acid, succinic acid, and OXA were detected in the control sample. Succinic acid and OXA were detected in the salt stress group and four OA species, malic acid, succinic acid, citric acid, and OXA, were detected in the alkali stress group. OXA was clearly the dominant component in all cases, with the content of OXA being 83.8, 88.7–88.9, and 82.2–84.5% of the total of OA in the control, salt stress groups, and alkali stress groups, respectively. It appears that OA is another major metabolite, in addition to betaine, and accounts for 12–17.8% of DW in the shoots of K. sieversiana (Fig. 4d). The overall trend of changes in total OA (salt stress: P < 0.0001; alkali stress: P < 0.00001) and OXA (salt stress: P < 0.0001; alkali stress: P < 0.00001) concentrations under salt and alkali stresses were basically identical. Under salt stress, the OAs did not accumulate at low salinity and the OA concentrations began to slowly increase after salinity was higher than 240 mM (P < 0.001). In contrast, under alkali stress, both the total OA concentrations and OXA concentrations increased greatly with increasing salinity (P < 0.0001). At the same salinity, the concentrations of total OA and OXA were both much higher under alkali stress than under salt stress. Furthermore, the calculated results using GEOCHEM (Parker et al. 1987) showed that the dissociation extent of OAs was over 99% for control and over 98.6% for both stress treatments.
Tissue pH
The tissue pH value in K. sieversiana shoots under both stresses was in close agreement with the control. The tissue pH value was 6.64 in the control and the mean tissue pH value at different stress intensities was 6.56 under salt stress and 6.62 under alkali stress (Table 1). The stress intensity did not affect the tissue pH value.
Discussion
Solute accumulation and osmotic adjustment
The deleterious effects of salt stress result primarily from osmotic stress and ion toxicities (De-Lacerda et al. 2003; Ghoulam et al. 2002; Soussi et al. 1998; Khan et al. 2000b). Under alkali stress, in addition to the above factors, plants also have to deal with stress of elevated pH. The high pH in soil surrounding the roots is not only detrimental to the supplying capacity of mineral nutrients (Shi and Zhao 1997), but also directly destroys the structure and function (such as membrane selectivity) of root cells (Shi and Yin 1993; Shi and Wang 2005; Shi and Sheng 2005). Despite the high-pH caused by alkali stress, accumulation of solutes and osmotic adjustment are the main physiological responses to both salt and alkali stress. As a dicotyledon and an alkali-resistant halophyte species, K. sieversiana has a distinctive physiological response to salt and alkali stresses.
Generally, plants can reduce WC as a quick and economical approach to osmotic adjustment in response to osmotic stress (Lissner et al. 1999). This is also true for K. sieversiana. The WC of K. sieversiana decreased with increased salt and alkali stress, and the extent of reductions under alkali stress was greater than under salt stress (Fig. 1). However the WC of K. sieversiana remained higher than is generally found in other stressed plants (Sheng et al. 1999; Yin et al. 2003; Lissner et al. 1999; Song et al. 2006). The WC of K. sieversiana remained high at 6 (g g−1 DW) even under alkali stress of 400 mM. Despite not being a succulent plant, K. sieversiana was able to maintain a high WC under severe osmotic stress. This might be one of the main physiological characteristics of K. sieversiana that allow it to have strong resistance to salt and alkali stresses. The high WC of cells results from vacuoles enlarging, which cause the cell surface to increase and make the layer of protoplasm thinner. Cell surface area and the distance between plasmalemma and vacuole membranes are key factors in deciding the speed and power of consumption of inorganic ions and organic small molecules going in and out of each cell. Therefore, maintaining a high WC might be a key characteristic of K. sieversiana that allow it to accumulate osmolytes with minimum energy consumption.
Halophytes are reported to accumulate large amounts of Na+ under salt and alkali stresses (Shi and Wang 2005; Shi and Sheng 2005; Moghaieb et al. 2004; Khan et al. 2000a; Short and Colmer 1999). Plants generally compartmentalize Na+ into vacuoles to avoid Na+ toxicity in the cytosol (Serrano and Rodriguez-Navarro 2001; Zhu 2003). At the same time, plants will also synthesize compatible low-molecular weight organic solutes, such as betaine, proline, free sugar, and polyalcohol, in the cytoplasm to prevent dehydration. Furthermore, some of these organic solutes also can protect biomacromolecules in cytoplasm (Munns 2002; Moghaieb et al. 2004; Parida and Das 2005). Plants under salt-alkaline stress usually absorb Na+ and simultaneously inhibit K+ absorption (Khan et al. 2000b; Short and Colmer 1999; Shi and Wang 2005; Shi and Sheng 2005). However, in the case of K. sieversiana, both Na+ and K+ contents in the shoots increased with increasing salinity. This implied that there was no competitive inhibition between the absorption of Na+ and K+. The absorption mechanism of K. sieversiana for Na+ and K+ appears to be unique and deserves further investigation. In reports about other plants, such as Aneurolepidium chinense (Shi and Yin 1992; Shi and Wang 2005) and Helianthus annuus (Shi and Sheng 2005), Na+ content was observed to increase with increasing alkalinity at the same salinity, which suggests that alkali stress might have a stronger destructive effect on the control of Na+ absorption in roots than salt stress. However, in the case of K. sieversiana, there is no significant difference between the effects of salt stress and alkali stress on the Na+ content in shoots except for treatments at 400 mM. That is, the effect of alkali stress on root functions is similar to that of salt stress at salinity below 400 mM. This implies that the adapting mechanism of K. sieversiana roots, to the high-pH alkali stress, may be different than in other plants, such as A. chinense and H. annuus and also deserves further investigation.
Results shown in Table 2 indicated that the K+ concentration was higher than other ions in the control. However, Na+ concentrations were the highest in the salt and alkali stress groups, and the proportion of Na+ to total ions increased with increasing stress intensity. Thus, Na+ is the main inorganic osmolyte under either salt or alkali stress. Mg2+ is the key component of chlorophyll and Ca2+ can maintain membrane stability, help to form cell walls and take part in signal transduction. Some researchers have shown that exogenous Ca2+ could relieve salt stress toxicity, which is presumed due to exogenous Ca2+ being able to enhance selective absorption of K+ (Parida and Das 2005). The Ca2+ and Mg2+ accumulations in many plants are inhibited by salt stress (Khan et al. 1999; Khan 2001). However, our observations of K. sieversiana were to the contrary. The free Ca2+ and Mg2+ in K. sieversiana shoots increased with increasing salinity under both salt and alkali stress, and the extent of increases under alkali stress were higher than under salt stress. Although free Ca2+ and Mg2+ increased under both stresses, their contributions to osmotic adjustment were small because the ratio of Ca2+ and Mg2+ to the total solute was very low (Table 2).
From Fig. 4a, it is obvious that proline accumulation in K. sieversiana shoots is unique compared with other plants under salt and alkali stresses (Ghoulam et al. 2002; Shi and Wang 2005; Shi and Sheng 2005). For K. sieversiana, proline did not accumulate at low salinity and only began to accumulate when stress intensity was higher than 240 mM. In general, the accumulation of proline, a main organic osmolyte, relates closely with osmotic stress intensity (Shi 1995). But for K. sieversiana, proline only accumulated sharply after the stress intensity had passed a certain threshold. For example, at 320 mM salinity, the proline concentration under salt and alkali stresses increased to 13 and 17 times that of the control, respectively (Fig. 4a). However, proline concentration was very low compared to betaine concentration. At just 0.034–0.154% of the total solute (Table 2), the contribution of proline to osmotic adjustment was insignificant. These phenomena perhaps meant that the change of proline concentration in K. sieversiana might not result from the response to osmotic stress, but resulted from metabolism being interrupted by high-stress intensity or from an adaptive response with special physiological function.
Betaine is the main secondary nitrogen-containing metabolite of K. sieversiana, and its content is very high even under non-stress conditions (1.67% of DW). With a maximum value of 4.14% of DW, the betaine concentration was significantly higher than the concentrations of SS and proline under salt and alkali stresses (Table 2). Furthermore, with rising stress intensity, not only did betaine concentrations increase significantly (Fig. 4b), but the proportion relative to the total solute concentration also increased (Table 2). The above results indicated that betaine was the main organic osmolyte in K. sieversiana cytoplasm. Although betaine was the dominant component of organic solutes that are not OA, the betaine concentration was low compared to common inorganic solutes such as Na+ and K+ (Table 2). This probably resulted from K. sieversiana having a high WC, enormous vacuoles and little cytoplasm.
Under alkali stress, the OA accumulated in plants usually distributes in vacuoles to neutralize the cations (Shi et al. 2002). Table 2 clearly shows that Na+, K+, and OA are all important osmolytes in vacuoles for both stresses. The contribution of Cl- under salt stress was up to 10.7%, which is much greater than under alkali stress. Under salt stress, the sum of the concentrations of Na+, K+, Cl−, and OA was 78.2% of all solutes. Under alkali stress, the concentrations of Na+, K+, and OA added up to 88.3% of the total concentration of all solutes.
Betaine, proline, and free sugar are three organic osmolytes distributed principally in protoplasm. Table 2 clearly shows that betaine is the main osmolyte of the three under both stresses. The proportion contributed by betaine to osmotic adjustment in protoplasm was 82 and 88% of the total of all three solutes under salt stress and alkali stress, respectively.
In conclusion, the osmotic adjustment mechanism in K. sieversiana shoots appears essentially the same between salt and alkali stresses. For both stresses, the key feature of K. sieversiana pertaining to osmotic adjustment was the ability to accumulate Na+, K+, and OA in vacuoles and to accumulate a large amount of betaine in the protoplasm. The difference between the two stresses was that the contribution of Cl− under salt stress was much greater than under alkali stress, but the contribution of OA under alkali stress was much greater than under salt stress (Table 2).
Ion balance
A stable tissue pH, as a result of intracellular ion balance, is necessary for plants to maintain normal metabolism. In a living plant, as long as the plant can adapt to the environment, the pH value in its tissue should be stable regardless of how the environmental pH value changes. This was also verified by our experimental results. The tissue pH in K. sieversiana shoots under both stresses was in basic agreement with the control (Table 1). The data in Table 3 suggests that there was a different mechanism of ion balance, between salt stress and alkali stress, to maintain intracellular pH stability in the shoots of K. sieversiana.
Under salt stress, plants accumulate cations such as Na+ and K+ (Khan et al. 2000a; Parida and Das 2005), and simultaneously accumulate inorganic anions such as Cl− (Santa-Cruz et al. 2002; Ghoulam et al. 2002), NO3 −, and SO4 2− or synthesized organic anions (Sagi et al. 1997) to keep ion balance. Under alkali stress, plants not only need to maintain intracellular ion balance, but also need to maintain pH stability. Our previous reports showed that OA accumulation may be an important means for some plants such as A. chinense (Shi and Wang 2005), Puccinellia tenuiflora (Shi et al. 2002) and H. annuus (Shi and Sheng 2005) to adapt to alkali stress, maintain intracellular ion balance and perform pH adjustments. However, in K. sieversiana, the mechanism of ion balance in shoots appears to differ from those plants.
As shown in Table 3, there is no significant difference in cation components between salt stress and alkali stress in K. sieversiana. The dominant intracellular cations in all cases were Na+ and K+, and these two cations contributed to ∼90% of the total positive charge, even under non-stress (90.4%). The contribution of the free Ca2+ and Mg2+ to the total positive charge was minimal. The total anion component in K. sieversiana shoots was almost unchanged under both stresses. However, different anions differed in their contributions to the overall negative charge and how the contribution changed with stress intensity. Under salt stress, the percentages of various anions contributing to the total negative charge ranged from high to low as follows: OA, Cl−, SO4 2−, NO3 −, H2PO3 −; and under alkali stress, the order was OA, NO3 −, SO4 2−, Cl−, H2PO3 −. Although OA was the main anion for both stresses, the percentage of its contribution to total negative charge under alkali stress was significantly higher than under salt stress. In addition, the effects of stress intensity on anion concentrations varied for different anions and under different type of stresses. With rising salinity, salt stress enhanced the contribution of Cl− and weakened the contribution of OA. However, the trends were reversed for alkali stress. For both stresses, the contribution of SO4 2− increased and the contribution of NO3 − deceased with increasing salinity. In summary, under salt and alkali stresses, K. sieversiana accumulated OA, Cl−, SO4 2−, H2PO3 −, and other inorganic anions to balance the massive influx of cations. Under salt stress, OA and inorganic anions each contributed about half of the total negative charge. However, under alkali stress, OA became the dominant component and contributed in average 84.2% of total negative charge, while the total contribution of the inorganic anions was only 15.8%.
H2PO3 − and NO3 − concentrations of K. sieversiana under alkali stress were significantly lower than under salt stress of same intensity (Fig. 3), which suggested that high pH may inhibit anion uptake. Although the energy consumption from absorbing inorganic ions is far less than from synthesizing organic compounds (Munns 2002), K. sieversiana has to enhance OA synthesis to remedy the shortage of inorganic anions under alkali stress (Fig. 3). Therefore, the energy consumption for K. sieversiana to adapt to alkali stress is greater than for salt stress, which may be one of the reasons that its relative growth rate under alkali stress was lower than that under salt stress (Yang et al. manuscript in preparation).
The accumulation and secretion of OAs are a physiological response of plants to various stresses such as drought (Timpa et al. 1986), Al3+ toxicity (Li et al. 2000), P deficiency (Koyama et al. 2000), Fe deficiency (López-Millán et al. 2000; Li et al. 2001), and alkali stress (Shi et al. 2002). There is a difference in the accumulation of OAs when some plants respond to salt and alkali stress (Shi et al. 2002; Yan et al. 2005). We found that OA was the dominant factor in maintaining ionic equilibrium, in K. sieversiana under alkali stress, and was different from factors for adapting to salt stress.
The result of the component analysis of OA showed that OXA was the dominant component in K. sieversiana and was also the main species of OA accumulated under salt and alkali conditions. Some alkali resistant gramineous species, such as A. chinense (Yan et al. 2000) and P. tenuiflora (Shi et al. 2002), were found to accumulate predominantly citric acid, specifically under alkali stress. K. sieversiana accumulated OXA under both salt and alkali stresses, however, the extent of accumulation under alkali stress was much higher than under salt stress. Nevertheless, A. chinense (Yan et al. 2000) and P. tenuiflora (Shi et al. 2002) did not accumulate any OA under salt stress. In conclusion, the OA metabolic regulation was closely related to the plant alkali resistance. In the process of adapting to alkali stress, plants with different adaptive pathways will have different OA metabolic regulating characteristics. These data implied that the natural alkali-resistant plants might have different alkali-resistant physiological pathways, which should be further investigated.
Conclusion
Despite what we know about alkali resistant plants similar to K. sieversiana, little is known about the physiological mechanism of plants resisting alkali stress. Understanding the mechanism of native halophytes resisting alkali stress is important for the ecological recovery and exploitation of the salt-alkalinized soil, discovering natural alkali resistant genes, and developing alkali-resistance biotechnology. K. sieversiana grows perennially in highly salt-alkalinized habitats, forcing evolution to form special mechanisms for osmoregulation and ion balance in order to survive and fulfill its life cycle. The traits of osmoregulation are similar for both stresses. These include maintenance of a high WC, and accumulation of inorganic ions dominated by Na+ and K+, and small organic molecules dominated by betaine. However, ionic equilibrium mechanisms under the two different stresses are different. Under salt stress, K. sieversiana accumulates OA and inorganic anions to maintain the intracellular ionic balance. The contributions of OA and inorganic anions to total negative charge were approximately equal. However, under alkali stress, the OA, which is dominated by OXA, is the dominant contributor to the negative charge. The contribution of inorganic anions to the negative charge was just 15.8%.
Plant alkali resistance may involve two aspects, intracellular pH adjustment and extracellular pH adjustment (pH adjustment in the root-external microenvironment). Within certain stress intensities, the intracellular main physiological indexes of K. sieversiana were not significantly different under salt and alkali stresses. This phenomenon implies that the high pH of the root environment under alkali stress might be limited to the outside of the cell and did not affect the inside of cell. In other words, extracellular pH adjustment may be the key feature allowing K. sieversiana to adapt to alkali stress. The physiological responses of K. sieversiana to salt stress and alkali stress are different from other plants, such as A. chinense (Shi and Wang 2005), P. tenuiflora (Shi and Yin 1993), H. annuus (Shi and Sheng 2005). These results suggest there may be different characteristics or types of physiological pathways, in nature, for plant resistance to alkali stress.
Abbreviations
- OA:
-
Organic acid
- OXA:
-
Oxalic acid
- SS:
-
Soluble sugar
- WC:
-
Water content
- DW:
-
Dry weight
- FW:
-
Fresh weight
References
Bao SD (1981) Determine of cation. In: Bao SD (ed) Analysis methods for soil and agriculture chemistry. China Agriculture Press, Beijing, China, pp 100–200
Brand JD, Tang C, Rathjen AJ (2002) Screening rough-seeded lupins (Lupinus pilosus Murr. and Lupinus atlanticus Glads.) for tolerance to calcareous soils. Plant Soil 245:261–275
Campbell SA, Nishio JN (2000) Iron deficiency studies of sugar beet using an improved sodium bicarbonate-buffered hydroponics growth system. J Plant Nutr 23:741–757
De-Lacerda CF, Cambraia J, Oliva MA, Ruiz HA, Prisco JT (2003) Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ Exp Bot 49:107–120
El-Samad HMA, Shaddad MAK (1996) Comparative effect of sodium carbonate, sodium sulphate, and sodium chloride on the growth and related metabolic activities of pea plants. J Plant Nutr 19:717–728
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Grieve CM, Grattan SR (1983) Rapid assay for determination of water-soluble quaternary-amino compounds. Plant Soil 70:303–307
Hartung W, Leport L, Ratcliffe RG, Sauter A, Duda R, Turner NC (2002) Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinum L.) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 240:191–199
Kawanabe S, Zhu TC (1991) Degeneration and conservation of Aneurolepidium chinense grassland in Northern China. J Jpn Grassland Sci 37:91–99
Khan MA (2001) Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aquat Bot 70:259–268
Khan MA, Ungar IA, Showalter AM (1999) Effects of salinity on growth, ion content, and osmotic relations in Halopyrum mocoronatum (L.) Stapf. J Plant Nutr 22:191–204
Khan MA, Ungar IA, Showalter AM (2000a) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45:73–84
Khan MA, Ungar IA, Showalter AM (2000b) Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann Bot 85:225–232
Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D (2000) Overexpression of mitochondrial citrate synthase in arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant Cell Physiol 41:1030–1037
Läuchli A, Lüttge U (2002) Salinity in the soil environment. In: Tanji KK (ed) Salinity: environment-plants-molecules. Boston Kluwer Academic Publishers, Boston, USA, pp 21–23
Li PH, Zhang H, Wang BS (2003) Ionic homeostasis of plant under salt stress. Acta Bot Boreal-Occident Sin 23:1810–1817
Li DH, He LY, Liu WD (2001) Correlation between abiotic-stress in soil and organic-acid secretion by root. J Wuhan Bot Res 19:497–507
Lissner J, Schierup HH, Comın FA, Astorga V (1999) Effect of climate on the salt tolerance of two Phragmites australis populations. I. Growth, inorganic solutes, nitrogen relations and osmoregulation. Aquat Bot 64:317–333
Li XF, Ma JF, Matsumoto H (2000) Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol 123:1537–1544
López-Millán AF, Morales F, Andaluz S, Gogorcena Y, Abadía A, Rivas JDL, Abadía J (2000) Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol 124:885–898
Moghaieb REA, Saneoka H, Fujita K (2004) Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritime. Plant Sci 166:1345–1349
Munns R (2002) Comparative physiology of salt and water stress. Plant cell Environ 25:239–250
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Nuttall G, Armstrong RD, Connor DJ (2003) Evaluating physicochemical constraints of calcarosols on wheat yield in the Victorian southern Mallee. Aust J Agric Res 54:487–497
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Peng YH, Zhu YF, Mao YQ, Wang SM, Su WA, Tang ZC (2004) Alkali grass resists salt stress through high [K+] and an endodermis barrier to Na+. J Exp Bot 55:939–949
Parker DR, Zelazny LW, Kinraide TB (1987) Improvements to the program geochem. Soil Sci Soc Am J 51:488–491
Sagi M, Dovrat A, Kipnis T, Lips H (1997) Ionic balance, biomass production, and organic nitrogen as affected by salinity and nitrogen source in annual ryegrass. J Plant Nutr 20:1291–1316
Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin MC (2002) The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci 162:825–831
Serrano R, Rodriguez-Navarro A (2001) Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 13:399–404
Sheng YM, Shi DC, Xiao HX, Xu Y (1999) Effect of mixed salts with various neutral and alkaline on the growth of sunflower. J Chin Northeast Norm Univ 4:65–69
Shi DC, Yin LJ (1992) Strain responses in Na2CO3−stressed Leymus chinensis seedlings and their mathematical analysis. Acta Bot Sin 34:386–393
Shi DC, Yin LJ (1993) Difference between salt (NaCl) and alkaline (Na2CO3) stresses on Puccinellia tenuiflora (Griseb.) Scribn et Merr. plants. Acta Bot Sin 35:144–149
Shi DC, Yin SJ, Yang GH, Zhao KF (2002) Citric acid accumulation in an alkali-tolerant plant Puccinellia tenuiflora under alkaline stress. Acta Bot Sin 44:537–540
Shi DC, Wang D (2005) Effects of various salt-alkali mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil 271:15–26
Shi DC, Sheng Y (2005) Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot 54:8–21
Shi DC (1995) Relaxation of Na2CO3 stress on Puccinellia tenuiflora (Griseb.) Scribn. et Merr. Plants by neutralizing with H3PO4. Acta Prataculturae Sin 4:34–38
Shi DC, Zhao KF (1997) Effects of sodium chlorideand carbonateon growth of Puccinellia tenuiflora and on present state of mineral elements in nutrient solution. Acta Prataculturae Sin 6:51–61
Song J, Feng G, Tian CY, Zhang FS (2006) Osmotic adjustment traits of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum in field or controlled conditions. Plant Sci 170:113–119
Short DC, Colmer TD (1999) Salt tolerance in the halophyte Halosarcia pergranulata subsp. Pergranulata. Ann Bot 83:207–213
Soussi M, Ocana A, Lluch C (1998) Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicerarietinum L.). J Exp Bot 49:1329–1337
Tang C, Turner NC (1999) The influence of alkalinity and water stress on the stomatal conductance, photosynthetic rate and growth of Lupinus angustifolius L. and Lupinus pilosus Murr. Aust J Exp Agric 39:457–464
Timpa JD, Burke JJ, Quiseberry JE, Wendt CW (1986) Effect of water stress on organic acid and carbohydrate composition of cotton plant. Plant Physiol 82:724–730
Wang BS, Zhao KF (1995) Comparison of extractive methods of Na+ and K+ in wheat leaves. Plant Physiol Commun 3:50–52
Yan H, Shi DC, Yin SJ, Zhao W (2000) Effects of saline-alkaline stress on the contents of nitrogen and several organisms of Aneurolepidium chinense. J Chin Northeast Norm Univ 32:47–52
Yan H, Zhao W, Sheng YM, Shi DC, Zhou DW (2005) Effects of alkali-stress on Aneurolepidium chinense and Helianthus annuus. Chin J Appl Ecol 16:1497–1501
Yin SJ, Shi DC, Yan H (2003) Main strain responses in the plants of Puccinellia tenuiflora (Griseb.).scribn et merr. to alkaline (Na2CO3) stress. Acta Prataculturae Sin 12:51–57
Zhao KF, Fan H, Ungar IA (2002) Survey of halophyte species in China. Plant Sci 163:491–498
Zhai YJ, Feng XH, Kang YG (1996) Pharmacognostlcal idntification of fructus Kochia sieversiana. J Chin Med Mater 6:283–285
Zheng HY, Li JD (1999) Form and dynamic trait of halophyte community. In: Zheng HY, Li JD (eds) Saline plants in songnen plain and restoration of alkaline-saline grass. Science Press, Beijing, China, pp 137–138
Zhu GL, Deng XW, Zuo WN (1983) Determination of free proline in plants. Plant Physiol Commun 1:35–37
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30671491 and No. 30571318) and the Program for Changjiang Scholars and Innovative Research Team in University from the Ministry of Education of China (IRT0519), and partly from the National Key Basic Research Program of China (2007CB106800).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John McPherson Cheeseman.
Rights and permissions
About this article
Cite this article
Yang, C., Chong, J., Li, C. et al. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294, 263–276 (2007). https://doi.org/10.1007/s11104-007-9251-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9251-3