Abstract
Intensified salt stress is an acute hindrance to crop cultivation, whereas plant signaling molecules can efficiently prompt salinity tolerance. Therefore, this study was accomplished to explore the potential salinity stress-mitigating effect of different signaling molecules in rice. The rice (cv. BRRI dhan29) seeds were immersed in 20 mM KNO3, 0.15 mM H2O2, 0.8 mM AsA (ascorbic acid) and 10 mM CaCl2 solutions for 24 h. Eventually, primed seeds were exposed to 75 mM NaCl in Petri dishes during germination. Moreover, 14-day-old rice seedlings were pretreated with different agents, viz., KNO3, H2O2, AsA and CaCl2 (concentrations were same as previous), for 2 days. Primed and non-primed seedlings were grown for 4 days under 75 mM NaCl stress condition. The result revealed that salt stress caused reduced germination indices and pre-seedling and seedling growth inhibition and impaired photosynthetic capacity, whereas catalase (CAT), ascorbate peroxidase (APX) and peroxidase (POX) activities were decreased in salt-treated plants. However, application of the four signaling molecules promoted the germination indices and growth and resisted chlorosis. Pretreatment with CaCl2 and AsA was observed to be relatively more efficient in conferring salinity tolerance of rice as reflected from the significant enhanced germination and growth in the saline medium by increasing reactive oxygen species (ROS) scavenging capacity, both at germination and seedling stage. All the selected signaling molecules significantly detoxified excess ROS, i.e., H2O2 and \({\text{O}}_{2}^{ \cdot - }\) and reduced lipid peroxidation by up-regulating the enzymes, CAT, APX and POX. Moreover, H2O2 and KNO3 pretreatment also mitigated salt-imposed oxidative stress and enhanced growth performance of rice seedlings. Overall, the study confirms that CaCl2 and AsA pretreatment were more effective than H2O2 and KNO3 priming to improve salt tolerance in rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity has imposed an enormous challenge to developing countries in the path of ensuring food security. As a result of climate changes, new arable croplands are likely to be stressed by salts and the increasing salinity has a significant impact on grain crop production (Rengasamy 2006). Nearly 397 million ha lands are exposed to soil salinity worldwide (FAO 2010). It is also considered as a very crucial environmental stressor that hinders rice cultivation in Bangladesh, where about 0.223 million ha (26.7%) new land is affected by various degrees of salinity during about the last four decades (Soil Resource Development Institute (SRDI) 2010).
More or less all growth and developmental stages of rice plants are injured by salt stress. However, among those stages, particularly germination of seeds and establishment of seedling stages are mostly vulnerable to salt-induced stress (Kochak-zadeh et al. 2013). Numerous physio-biochemical changes occur in plants when subjected to excess salt concentrations including reduced absorption of water (Park et al. 2016), ionic imbalance (Jaleel et al. 2008), reduction of enzyme activities (Hütsch et al. 2016), disruption of nitrogen metabolism (Lorenzo et al. 2001), reduction of photosynthetic pigment content (Pessarakli 2016) and accretion of compatible biological solutes, for instance some soluble carbohydrates and amino acid proline (Yancey 2005). Salinity induces imbalance in cellular redox resulting in accelerated accumulation of reactive oxygen species (ROS) and the most common ROS are hydrogen peroxide (H2O2), superoxide radical (\({\text{O}}_{2}^{ \cdot - }\)) and hydroxyl radical (OH·) (Apel and Hirt 2004). These changes ultimately trigger the deterioration of many vital processes within the plants cell and eventually inhibit or postpone the germination, increase the plant mortality rate, arrest growth and finally reduce crop productivity (Ambede et al. 2012).
Rice is considered as a staple food and widely grown cereal crop in Bangladesh. It occupies approximately 80% of the country’s arable lands with an annual production of 33.98 million metric tons (BBS 2014). It is known to be a salt-susceptible crop, and as a result when the soil salt concentration level increases above 4 dS m−1 EC its productivity is substantially diminished (Munns et al. 2006). So, it is not possible to cultivate rice in salinity-affected areas and thus these become fallow areas. So, salinity management is necessary to improve productivity under saline conditions.
Until now, several endeavors have been taken to upgrade the salinity tolerance in rice through conventional breeding program; however, progress has been quite slow with inadequate commercial success (Fita et al. 2015). Exogenous protective chemical application modulates endogenous defensive mechanisms and alleviates salt-induced damages in the vegetative tissues of rice. The protective role of such chemicals on the reduction of salinity-induced damages is linked with the stimulated action of oxidant scavenging enzymes and compatible osmolyte accumulation (Wahid and Shabbir 2005; Afzal et al. 2012).
Priming with exogenous chemicals is an easy and efficient method that is regarded as a practical approach to overcome the salinity-induced growth hindrance of plants (Wahid and Shabbir 2005; Paul and Roychoudhury 2016). It enhances seed performance by conferring positive physiological amendment leading to faster and more harmonized germination of seeds (Patade et al. 2009) apart from inducing early resistance. There are adequate reports showing that priming causes change of different cellular, subcellular and molecular processes in seeds, consequently boosting germination and growth of various plants under diverse environmental stress conditions (Beckers and Conrath 2007).
The positive effects of various signaling molecule priming have been reported in some crop plants such as Triticum aestivum (wheat) (Iqbal and Ashraf 2007), Saccharum officinarum (sugarcane) (Patade et al. 2009) and Helianthus annuus (sunflower) (Moeinzadeh et al. 2010). Previously, priming with KNO3 showed salinity and drought stress mitigation in sunflower (Kaya et al. 2006). Exogenous H2O2 application enhanced salinity resistance in rice seedlings (Roy et al. 2016), and pretreatment with H2O2 also prevented the oxidative damage of wheat seedlings (Wahid et al. 2007).
As an antioxidant molecule, ascorbic acid (AsA) plays a prominent function against oxidative stress and also protects the photosynthetic process under stressful conditions (Ashraf et al. 2008). It performs a wide range of physiological and biochemical functions in plants which eventually regulate the growth, differentiation and metabolism of plants under adverse conditions (Khan et al. 2011). Exogenous AsA application alleviates salt-induced negative effects in plants, as it increases endogenous AsA content, improves chlorophyll contents and enhances proline accumulation (Roy et al. 2016). The salt tolerance in crop plants also can be improved by using salt solutions such as CaCl2 for priming (Afzal et al. 2008). Afzal et al. 2012 described the positive effect of CaCl2 on salinity stress mitigation of aromatic rice. Moreover, seeds presoaked in these chemicals might be utilized to ensure superior growth, mostly in stressful environments (Yazdanpanah et al. 2011).
The effects of pretreatment with different signaling molecules and growth regulators on different plant species have been reported from time to time; however, few reports exist on the comparative effects of priming with KNO3, H2O2, AsA and CaCl2 on germinated pre-seedlings and seedlings of rice under salinity stress. Considering the above facts, the experiment was undertaken to explore the potential roles of pretreatment with KNO3, H2O2, AsA and CaCl2 to ameliorate the oxidative stress-induced impairments in the germinated pre-seedlings and seedlings of rice subjected to salt stress.
Materials and methods
Experimental growth conditions and treatments at the germination stage
The present investigation was accomplished at the Department of Biochemistry and Molecular Biology, Bangladesh Agricultural University, Mymensingh, under growth chamber condition (temperature and relative air humidity were 25.6 ± 1.0 °C and 73.2 ± 3.0%, respectively) using BRRI dhan29, a high yielding salt-susceptible rice cultivar. Initially, uniform size rice seeds were treated with 0.10% mercuric chloride for 3 min, followed by washing several times with double-distilled water to sterilize the seeds. After that, those seeds were carefully de-husked.
Firstly, for priming, the de-husked seeds were soaked in KNO3, H2O2, AsA and CaCl2 in separate screw-capped bottles and the seeds were immersed in double-distilled water in case of control treatment. After that, the treated rice seeds were kept in the dark for 24 h. Subsequently, after washing seeds several times with double-distilled water, the seeds were positioned on 9 cm Petri dishes having three layers of Whatman filter papers and incubated for 7 days for germination study. Each Petri dish contained 50 rice seeds. 20 ml of 75 mM NaCl solution was poured into each Petri dish for salt treatments. A Petri dish containing 20 ml distilled water acted as a control in the experiment (non-saline conditions). After every 2 days, the solutions were renewed. The following treatments were maintained: C, control; S, 75 mM NaCl (salted control); K, distilled water + 20 mM KNO3 primed seed; S + K, 75 mM NaCl + 20 mM KNO3 primed seed; H, distilled water + 0.15 mM H2O2 primed seed; S + H, 75 mM NaCl + 0.15 mM H2O2 primed seed; A, distilled water + 0.8 mM AsA primed seed; S + A, 75 mM NaCl + 0.8 mM AsA primed seed; Ca, distilled water + 10 mM CaCl2 primed seed; S + Ca, 75 mM NaCl + 10 mM CaCl2 primed seed.
At 12 h interval, the number of germinated seeds was recorded up to the 7th day after sowing (DAS). Seeds were counted as germinated when the radicle length was 1 mm or more. Shootlet samples were collected for further biochemical analysis. The experiment was performed in completely randomized block design with three independent replicates.
Germination indices and pre-seedling’s growth measurements
The morphological parameters (rootlet length, shootlet height) at the germination stage were calculated on the 7th DAS. Germination percentage (GP) (Afrin et al. 2019), germination indices (GI) (Tiquia 2010), vigor index (VI) (Hangarter 1997) and mean germination time (MGT) (Matthews and Khajeh Hosseini 2006) were computed according to the given equations:
where,
Here, n indicates the number of germinated seeds on Dth day, D indicates the number of DAS.
Seedling stage experiment
Uniformly germinated rice seedlings were grown in hydroponic condition using Cooper’s nutrient solution (Cooper 1988) with some modifications in 250 ml plastic pots. The nutrient solution had been prepared by adding the following nutrients to 10 l distilled water: KH2PO4 2.63 g, KNO3 5.83 g, Ca(NO3)2·4H2O 10.03 g, FeSO4·5H2O 2 g, MgSO4 0.79 g, MnSO4 0.061 g, H3BO3 0.017 g, CuSO4·5H2O 0.004 g, Na2MoO4 0.003 g, ZnSO4 0.0044 g. After every 3 days, the nutrient solutions were renewed. Around 150 rice seedlings were placed in each plastic pot. After 14 days, seedlings were pretreated with different signaling molecules, viz,. KNO3, H2O2, AsA and CaCl2 (concentrations are same as germination stage) for 2 days. Subsequently, the rice seedlings were exposed to salt stress (75 mM NaCl) and grown for 4 days. Thus, the treatments of the present experiment were: control (C), 75 mM NaCl (S), 75 mM NaCl + 20 mM KNO3 (S + K), 75 mM NaCl + 0.15 mM H2O2 (S + H), 75 mM NaCl + 0.8 mM AsA (S + A), 75 mM NaCl + 10 mM CaCl2 (S + Ca). All treatments were replicated three times in an identical growth environment. After 4 days of salt exposure, morphological data were obtained and the study of biochemical change the second leaves of seedlings were collected.
Salinity-induced growth impairment measurements of seedlings
Through the precise examination and assessing dry weight (DW), the salt-induced growth impairment of rice seedlings was measured. Seedlings were collected and dried at 60 °C for 4 days to determine DW and stated as mg seedling−1. To determine plant height, the distance of the shoot base to the tip of the longest leaf of ten seedlings was measured and averaged.
Assessment of leaf chlorophyll contents of rice pre-seedlings
The total photosynthetic pigment, viz., total Chl content of rice pre-seedlings was measured using spectrophotometer (Shimadzu, UV-1201, Kyoto, Japan) following the protocol as described by Metzner et al. (1965) with some minor modifications. The 0.5 g sample was taken in a screw-capped tube and 10 ml 80% (v/v) aqueous acetone was added and kept for 7 days for extraction of pigments. The plant acetone extract was centrifuged for 10 min at 4000×g and the supernatant was used for spectrophotometric absorbance reading at 644 and 663 nm wavelengths. The following equations were applied to compute the chlorophyll contents and the total Chl content was determined as the sum of chlorophyll a and b content and expressed as mg g−1 fresh weight (FW):
Evaluation of lipid peroxidation and hydrogen peroxide of pre-seedlings and seedlings
Lipid peroxidation of rice leaves was quantified by means of assessing malondialdehyde (MDA) according to the protocol as described by Heath and Packer (1968) with some modifications. The rice leaves (0.1 g leaves) were ground in 5.0% (w/v) trichloroacetic acid (TCA) and subsequently centrifuged at 11,500×g for 10 min at 4 °C. The supernatant was collected in a screw-capped tube, followed by the addition of 4 ml 20% TCA having 0.5% of thiobarbituric acid. Afterward, screw-capped tubes were incubated in a hot water bath and heated at 90 °C for 15 min. The screw-capped tubes were immediately transferred in an ice bath and centrifuged again at 11,500×g for 12 min. The absorbance of the chromophore was measured in a spectrophotometer (Shimadzu, UV-1201, Kyoto, Japan) at 532 nm. MDA content was estimated using the extinction coefficient of 155 mM−1 cm−1 and denoted as nmol of MDA g−1 FW.
Leaf tissue H2O2 content was estimated following the method of Velikova et al. (2000) with some modifications. Rice leaves (0.1 g) were ground with 1.0 ml of 0.10% TCA and centrifuged at 11,500×g for 12 min at 4 °C temperature. The 0.5 ml supernatant was taken in a test tube, 10 mM potassium phosphate buffer (pH 7.0) (0.5 ml) and 1.0 M potassium iodate (1.0 ml) were added and the mixture was incubated under dark condition for 60 min. The absorbance was quantified at 390 nm wavelength (Shimadzu, UV-1201, Kyoto, Japan) and H2O2 content was stated as nmol g−1 FW.
Histochemical detection of superoxide (\({\text{O}}_{2}^{ \cdot - }\))
Localization of \({\text{O}}_{2}^{ \cdot - }\) rice seedling leaves was spotted by means of nitroblue tetrazolium chloride (NBT). The collected leaves of rice seedlings were cleaned thoroughly with dH2O to eliminate the extraneous material associated with the tissues. The selected leaves of each treatment were dipped into 0.10% NBT solution, prepared by dissolving 0.05 g NBT with 50 mM potassium phosphate buffer (pH 7.5), in a Petri dish. Afterward, the Petri dish was wrapped completely with aluminum foil and incubated at room temperature for 24 h. The leaves were decolorized by immersing in boiling ethanol (90%) for 10 min. The photograph was taken in 60% glycerol.
Assessment of ROS scavenging enzymes activity
To assess the ROS scavenging capacity, different antioxidant enzymes’ activity were determined. The catalase activity (CAT, EC 1.11.1.6) was estimated following the method as described by Tahjib-Ul-Arif et al. (2018a). The absorbance of the reaction mixture was noted at 240 nm wavelength for 120 s and the activity of CAT was calculated employing the extinction coefficient 40 M−1 cm−1. Another antioxidant enzyme, namely ascorbate peroxidase activity (APX, EC 1.11.1.11), was quantified using the method developed by Nakano and Asada (1981), with the extinction coefficient 2.8 mM−1 cm−1. Lastly, the peroxidase (POX, EC 1.11.1.7) activity was also assessed using guaiacol as a substrate corresponding to the method of Nakano and Asada (1981) using 26.6 mM−1 cm−1 as extinction coefficient.
Evaluation of osmolyte proline accumulation
The method of Bates et al. (1973) was employed to determine the proline content of rice leaves with some modifications. The collected fresh leaves (0.5 g) were ground with a mortar and pestle in 10 ml of 3.0% sulfosalicylic acid solution. After that, the extract was centrifuged at 4000×g for 10 min to separate the plant debris. The supernatant (2 ml) was taken in a screw-capped tube, 2 ml acid ninhydrin solution and 2 ml of glacial acetic acid were added, and the mixture was kept at 100 °C for 1 h to start the reaction. Immediately after heating, the tubes were cooled in an ice bath and the chromophore was extracted in toluene. Finally, the absorbance was computed at 520 nm (Shimadzu, UV-1201, Kyoto, Japan).
Statistical analysis
Analysis of variance was done by applying the Dunnett’s method for multiple comparisons with Minitab 17.0 statistical software. Means of different treatments were compared with only salt-stressed treatment (S) and statistical differences were expressed either at the 5% or 1% level of probability.
Results
Seed priming improves germination and growth characters of pre-seedlings under salt stress
The impacts of different signaling molecules on germination and growth parameters of rice pre-seedlings under 75 mM NaCl stress are presented in Table 1. GP, GI and VI varied considerably among the treatments. Salinity reduced GP by 11% compared with that of the control condition. In both saline and non-saline environments, priming with KNO3 did not show any significant effect on GP, whereas priming with H2O2, AsA and CaCl2 showed a significant increment of GP compared to control and salted rice seedlings. Again, the lowest GI was recorded under the saline condition and priming with different agents increased GI. Under normal conditions, pretreatment with H2O2, AsA and CaCl2 significantly increased GI, but only AsA and CaCl2 priming increased GI by 51 and 58%, respectively, under saline condition compared to the salt-treated condition. Salt stress significantly decreased VI (45%), whereas under salt stress priming with AsA and CaCl2 increased VI (by 99 and 125%, respectively) significantly. On the other hand, KNO3 and H2O2 priming increased VI non-significantly compared to salt-stressed condition. Under salt-stressed condition, only H2O2 priming significantly reduced MGT and no significant change of MGT was evident on employing other signaling molecules.
To evaluate the consequences of salt stress and stress-alleviating functions of different signaling molecules on the growth parameters of pre-seedlings, we monitored the shootlet and rootlet length (SL and RL, respectively) change (Table 1; Fig. 1a). Salt stress significantly reduced shootlet and rootlet length by 34 and 44%, respectively, in rice pre-seedlings compared with the control condition. The priming with AsA and CaCl2 significantly increased the shooltet length in salt-stressed pre-seedlings by 40 and 48%, respectively, in comparison with salt-treated only plants. Moreover, under NaCl stress conditions, seed priming with KNO3 and H2O2 enhanced shootlet length non-significantly. Likewise, KNO3, AsA and CaCl2 priming increased rootlet length significantly by 50, 45 and 62%, respectively, compared to saline condition but H2O2 priming did not show any significant effect on root length elongation. Also, under non-stress conditions, AsA, CaCl2, and H2O2 enhanced SL. The highest shootlet length and rootlet length under salt-stressed condition were recorded from the seedlings receiving CaCl2 priming, followed by AsA priming (Table 1).
Phenotypic appearance of rice a pre-seedlings and b seedlings under different treatments. Control (C), 75 mM NaCl (S), 20 mM KNO3 (K), 75 mM NaCl + 20 mM KNO3 (S + K), 0.15 mM H2O2 (H), 75 mM NaCl + 0.15 mM H2O2 (S + H), 0.8 mM ascorbic acid (A), 75 mM NaCl + 0.8 mM ascorbic acid (S + A), 10 mM CaCl2 (Ca), 75 mM NaCl + 10 mM CaCl2 (S + Ca)
Priming enhances the growth of rice seedlings under salt stress
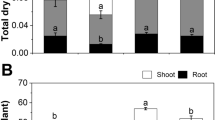
As shown in Fig. 1b, NaCl stress in rice seedlings for 4 days displayed toxicity symptoms, including chlorosis, yellowing and death of leaf tips. Salinity also caused arrested growth, as witnessed by a lower plant height under salt-stressed condition (Fig. 2a). Salt-induced stress decreased plant height and biomass production significantly by 33 and 44%, respectively, compared to that of control. Priming with KNO3, H2O2, AsA and CaCl2 to the salt-stressed rice plants noticeably reinstated plant growth and dry weight compared to the only salt-stressed seedlings. However, among these agents, the maximum plant length enhancement under salinity stress condition was observed as a result of pretreatment with AsA followed by CaCl2 (Fig. 2).
Effects of different signaling molecules (KNO3, H2O2, ascorbic acid (AsA) and CaCl2) on a plant height and b plant dry weight in BRRI dhan29 rice cultivar under 75 mM NaCl stress. Data are the mean value (n =3) ± standard deviation (SD). The SD in each case is represented by the vertical bar in each graph. Here, ‘asterisk’ indicates statistical differences at P < 0.05 and ‘double asterisks’ indicates P < 0.01 and ‘ns’ indicates non-significant differences and ‘c’ indicates the salt-stressed control to which all other treatments are compared based on Dunnett’s multiple comparison method. Control (C), 75 mM NaCl (S), 75 mM NaCl + 20 mM KNO3 (S + K), 75 mM NaCl + 0.15 mM H2O2 (S + H), 75 mM NaCl + 0.8 mM AsA (S + A), 75 mM NaCl + 10 mM CaCl2 (S + Ca)
Priming adjusts ROS accumulation and lipid peroxidation
The NaCl-induced oxidative stress triggered over-accumulation of ROS in the rice seedlings both at germination and seedling stage, leading to increased lipid peroxidation. The tissue H2O2 content significantly increased by 23.6 and 33%, correspondingly, in the salt-treated rice seedlings at germination and seedling stage, relative to the stress-free control seedlings. However, treating salt-stressed rice seedlings with KNO3, H2O2, AsA and CaCl2 contributed to the significant reduction of H2O2 by 44.8, 40, 40 and 38.2%, respectively, at germination stage, whereas at seedling stage only AsA and CaCl2 significantly decreased endogenous H2O2 content by 46 and 54%, compared with salt-stressed only plants (Fig. 3b). The salinity stress imposed oxidative stress which caused severe damage of cellular membrane that can be measured in terms of lipid peroxidation product MDA content in the leaves of rice seedlings. Similarly, salt stress enhanced MDA content both at germination and seedling stage by 110 and 18.7%, respectively, relative to control plants (Fig. 3a). All the signaling molecules reduced the level of MDA compared with salt-treated plants, both at germination and seedling stage. However, a significant reduction of MDA content was recorded due to the priming with KNO3 (31.9%), AsA (73%) and CaCl2 (68.6%) at the germination stage, and with H2O2 (25%) and AsA (36%) at seedling stage in comparison with only NaCl-stressed treatment. At the germination stage, under normal conditions, priming with all four agents did not affect endogenous H2O2 and MDA content significantly (Fig. 3).
Effects of different signaling molecules (KNO3, H2O2, ascorbic acid (AsA) and CaCl2) on a MDA content and b H2O2 content in BRRI dhan29 rice cultivar under 75 mM NaCl stress. Data are the mean value (n =3) ± standard deviation (SD). The SD in each case is represented by the vertical bar in each graph. Here, ‘asterisk’ indicates statistical differences at P < 0.05 and ‘double asterisks’ indicates P < 0.01 and ‘ns’ indicates non-significant differences and ‘c’ indicates the salt-stressed control to which all other treatments are compared based on Dunnett’s multiple comparison method. Control (C), 20 mM KNO3 (K), 0.15 mM H2O2 (H), 0.8 mM AsA (A), 10 mM CaCl2 (Ca), 75 mM NaCl (S), 75 mM NaCl + 20 mM KNO3 (S + K), 75 mM NaCl + 0.15 mM H2O2 (S + H), 75 mM NaCl + 0.8 mM AsA (S + A), 75 mM NaCl + 10 mM CaCl2 (S + Ca)
We also carried out the histochemical detection of \({\text{O}}_{2}^{ \cdot - }\) in fresh rice leaves at the seedling stage under salt-stressed and non-stressed conditions as shown in Fig. 4. Frequent dark blue spots appeared in the salt-treated rice leaves when stained with NBT. Priming with KNO3, H2O2, AsA and CaCl2 remarkably diminished the NBT-stained spots in salt-stressed rice seedlings relative to only salt-stressed seedlings. Moreover, almost no spots were visible in the leaf lamina of rice seedlings pretreated with AsA.
Priming modulates antioxidant enzyme activities
We also examined the governing role of these selected signaling molecules on some selected oxidant scavenging enzymes such as CAT, APX and POX, both at germination and seedling stages (Fig. 5). The results of the present experiment uncovered that salt stress caused a decrease of CAT activity both at germination and seedling stage by 13.8 and 80.7%, respectively, relative to salt-free control conditions. In contrast, KNO3, H2O2, AsA and CaCl2 pretreatment prior to salt stress rescued CAT activity by 77.4, 77, 42 and 77.4%, respectively, at the germination stage and by 82, 82.1, 63 and 56.5% at the seedling stage (Fig. 5a).
Effects of different signaling molecules [KNO3, H2O2, ascorbic acid (AsA) and CaCl2] on a catalase (CAT) activity (mmol min−1 g−1 FW), b ascorbate peroxidase (APX) activity (μmol min−1 g−1 FW) and peroxidase (POX) activity (μmol min−1 g−1 FW) in BRRI dhan29 rice cultivar under 75 mM NaCl stress. Data are the mean value (n =3) ± standard deviation (SD). The SD in each case is represented by the vertical bar in each graph. Here, ‘asterisk’ indicates statistical differences at P < 0.05 and ‘double asterisks’ indicates P < 0.01 and ‘ns’ indicates non-significant differences and ‘c’ indicates the salt-stressed control to which all other treatments are compared based on Dunnett’s multiple comparison method. Control (C), 20 mM KNO3 (K), 0.15 mM H2O2 (H), 0.8 mM AsA (A), 10 mM CaCl2 (Ca), 75 mM NaCl (S), 75 mM NaCl + 20 mM KNO3 (S + K), 75 mM NaCl + 0.15 mM H2O2 (S + H), 75 mM NaCl + 0.8 mM AsA (S + A), 75 mM NaCl + 10 mM CaCl2 (S + Ca)
The rice pre-seedlings and seedlings exposed to NaCl stress had decreased APX activity by 14.8 and 28.5%, respectively, compared with control (Fig. 5b). Among the signaling molecules, the only pretreatment with AsA at the germination stage significantly decreased and KNO3 at the seedling stage significantly increased APX activity in NaCl-treated seedlings in comparison with only salt-treated plants.
Salt stress did not alter POX activity at the germination stage, but significantly decreased (40.3%) at the seedling stage compared with the control. Priming with all four agents significantly decreased POX activity in salt-stressed pre-seedlings, but enhanced in salt-stressed seedlings in comparison with NaCl-treated only plants (Fig. 5c). Under salinity stress-free conditions, only H2O2 priming extensively decreased CAT activity and increased APX activity, whereas KNO3, AsA and CaCl2 priming significantly enhanced CAT activity and did not display any noteworthy effect on the activity of APX, relative to control. In contrast, POX activity significantly reduced because of pretreatment with all the signaling molecules compared with control (Fig. 5).
Priming of signaling molecules amplifies photosynthetic pigment in rice seedlings under salt stress
A considerable variation of chlorophyll pigment content was observed as a result of salt stress (Fig. 6a). A sharp decrease in total chlorophyll content (60.5%) in rice leaves was noticed in salt-stressed seedlings compared to the non-stressed control seedlings. Prior to salt-stress treatment, increased leaf total chlorophyll pigment content was recorded due to priming with KNO3, H2O2, AsA and CaCl2. The maximum total chlorophyll content under salinity stress condition was observed with CaCl2 priming treatment. These agents increased total chlorophyll contents to more than that in the control condition. Under non-saline conditions, KNO3, AsA and CaCl2 pretreatment improved total chlorophyll content significantly compared with stress-free control plants.
Effects of different signaling molecules (KNO3, H2O2, ascorbic acid (AsA) and CaCl2) on a total chlorophyll content and b proline content in BRRI dhan29 rice cultivar under 75 mM NaCl stress. Data are the mean value (n =3) ± standard deviation (SD). The SD in each case is represented by the vertical bar in each graph. Here, ‘asterisk’ indicates statistical differences at P < 0.05 and ‘double asterisks’ indicates P < 0.01 and ‘ns’ indicates non-significant differences and ‘c’ indicates the salt-stressed control to which all other treatments are compared based on Dunnett’s multiple comparison method. Control (C), 75 mM NaCl (S), 75 mM NaCl + 20 mM KNO3 (S + K), 75 mM NaCl + 0.15 mM H2O2 (S + H), 75 mM NaCl + 0.8 mM AsA (S + A), 75 mM NaCl + 10 mM CaCl2 (S + Ca)
Priming maintains osmoprotection
Salinity stress triggered a considerable accumulation of proline in the rice leaves compared with control (Fig. 6b). Under salt stress, KNO3 and CaCl2 reduced proline content by 52.7 and 15.6%, respectively, compared to NaCl-treated only seedlings. Conversely, AsA priming increased and H2O2 priming did not cause a significant change of proline content in comparison with only salt-treated plants. Under normal conditions, priming with all those agents increased proline accumulation in the leaves of rice seedlings in comparison to control conditions.
Discussion
Application of various exogenous protectants to minimize the unfavorable consequences of environmental stressors has enormous inferences equally from theoretic and functional viewpoints (Uchida 2003; Sivritepe et al. 2005). The usefulness of different pretreatment application techniques depends on the emergence and establishment of seedlings. Therefore, in the present experiment, the effects of KNO3, H2O2, AsA and CaCl2 on inducing salinity tolerance of rice were examined at the germination and seedling stage. Various parameters such as germination, growth, physiological attributes, ROS accumulation and antioxidant activity in primed seeds and seedlings were studied under NaCl-induced salinity stress.
It is an established fact that seed germination as well as plant growth severely hindered by salt-induced stress and seed priming could be used to improve the salt-stress tolerance of rice seedling (Ashraf and Harris 2004; Afzal et al. 2006). A similar phenomenon was also noticed in our experiment, where salinity showed deleterious effect on germination and growth performances of rice pre-seedlings (Table 1). Seed priming with KNO3, H2O2, AsA and CaCl2 has previously been testified in different crops to recover crop growth under various stressed conditions (Gondim et al. 2010; Ramzan et al. 2010; Azooz et al. 2013; Farooq et al. 2008). In our current experiment, seed priming with these signaling molecules efficiently mitigated the harmful consequences of salt-induced stress on germination of rice seeds as apparent from notably shortened MGT by H2O2, increased germination percentage by CaCl2, AsA and H2O2, increased VI by CaCl2 and AsA and increased GI by CaCl2 priming. In addition, all these signaling molecules increased germination percentage, VI and GI than in the salted condition (Table 1).
In this study, priming with CaCl2 proved to be superior to other treatments in alleviating salinity-induced inhibition of germination percentage, VI and GI (Table 1). This finding is in concordance with earlier reports, which demonstrated that CaCl2 priming effectively altered the detrimental consequences of salt stress on wheat (Farooq et al. 2008), Zea mays (maize) (Ashraf and Rauf 2001) and rice (Afzal et al. 2012) seed germination-related parameters. This positive stimulus of CaCl2 priming on germination-related parameters of rice might be as a result of the enhancement of expression, stability and activity of α-amylase, a key germination enzyme (Afzal et al. 2012). Moreover, under NaCl stress conditions, plants were exposed to excess Na+ ions, whereas Ca2+ can counteract the uptake of Na+ and eventually mitigate the deadly influence of excess Na+ ions on germination of seeds (Anil et al. 2005). Alternatively, the priming with H2O2 and AsA significantly alleviated salt stress-induced growth inhibition by increasing the germination indices (Azooz et al. 2013; Kilic and Kahraman 2016). It might be due to either by reestablishing osmotic equilibrium or intensifying the uptake and transfer of essential minerals nutrients (Kaya et al. 2010).
At germination stage, priming with all four agents increased growth, i.e., shootlet and rootlet length of rice pre-seedlings in saline condition. Shootlet length increased more pronouncedly due to CaCl2 and AsA, whereas rootlet length increased due to CaCl2, AsA and KNO3 (Table 1; Fig. 1a). The growth-enhancing effect of KNO3, CaCl2 and AsA priming were in agreement with previous studies on Capsicum annuum (hot pepper) (Amjad et al. 2007), Solanum lycopersicum (Farooq et al. 2005), Cuminum cyminum (Shahi-Gharahlar et al. 2010) and sunflower (Bajehbaj 2010) under saline condition. Moreover, salt stress at rice seedling stage also led to a decline of the growth and biomass production (Figs. 1b, 2a, b), perhaps by disturbing the enzymatic functions and cellular metabolic processes that suggested salt-induced toxicity in plants. However, pretreatment with KNO3, H2O2, AsA and CaCl2 boosted the rice seedling’s overall growth performances under NaCl-stress conditions, indicating the protective role of these signaling molecules in alleviating salt-induced toxicity, where AsA and CaCl2 priming showed relatively better results (Fig. 2). Parallel findings have also been reported in several crop species under drought and salinity stresses (Shalata and Neumann 2001; Alhasnawi et al. 2015; Rahman et al. 2016). These signaling molecules might fuel cell proliferation in root apical meristematic tissues, amending plant growth (Farooq et al. 2006). The increased seedling growth due to the CaCl2 and KNO3 pretreatment might be because of the amplified nuclear copying in dividing cells (Mavi et al. 2006). Calcium ions are interconnected with numerous vital mitotic incidents, mainly in the metaphase–anaphase transition and breakdown of nuclear membranes (Hepler 1994). The Ca2+ level is intimately linked to the inception of cytoplasmic division (Chang and Meng 1995). It also activates calmodulins and increases cytokinin production, which can augment cell division (Snedden and Fromm 2001). Excess Na+ has mitodepressive effect and lowers the cell number entering mitotic division (Teerarak et al. 2009). Exogenous K+ counteracts excess Na+ uptake (Abbasi et al. 2014) and K+ channel is crucially in favor of normal progression of the cell division cycle. K+ is also involved in alkalization of cytoplasm and regulation of intracellular osmotic pressure which increases the cell division rate (Sano et al. 2007). Seed priming considerably upgrades rootlet growth (Hassanpouraghdam et al. 2009). Priming with AsA alleviates the growth-inhibitory effects of salinity stress successfully and enhances the protein translation process (Garg and Kapoor 1972), by stabilizing the cell membranes (Rodriguez-Aguilera et al. 1995) and modulating oxidant scavenging capacity (Ejaz et al. 2012).
A common symptom of salt stress is annihilation of chlorophyll pigments that leads to impaired photosynthesis (Mishra et al. 2013), as also observed in our study (Fig. 6a). More importantly, it was observed previously that the total chlorophyll content of NaCl-stressed rice seedlings increased significantly as a result of pretreatment with CaCl2 (Farooq et al. 2008; Afzal et al. 2012), AsA (Azooz et al. 2013), H2O2 (Wahid et al. 2007) and KNO3 (Nawaz et al. 2017) priming, where reduction of over-accumulation of ROS protects chlorophyll from degradation during stress (Sevengor et al. 2011; Hayat et al. 2012; Wani et al. 2016). A similar result was also found in our present experiment (Fig. 6a). The increment of chlorophyll pigments is expected to permit higher rates of photosynthetic activity to meet plant nutritional requirement under salinity (Afzal et al. 2012).
Under various environmental stresses, accumulation of ROS in plants is a well-known phenomenon (Miller et al. 2010; Gill and Tuteja 2010; Anjum et al. 2011). Our present study demonstrated that increased levels of \({\text{O}}_{2}^{ \cdot - }\) and H2O2 (Figs. 3b, 4) were definitely linked to the NaCl-induced oxidative surge in rice seedlings. The salinity-induced excess ROS generation interrelated with the peroxidation of lipids as reflected by considerably higher MDA content in salt-stressed rice seedlings (Fig. 3a), which also indicated the extent of membrane damage and also a similar outcome was reported in maize plant grown in saline medium (Tahjib-Ul-Arif et al. 2018b). When salt-stressed rice seedlings were pretreated with KNO3, H2O2, AsA and CaCl2, a lower ROS production and MDA content were observed both at germination and seedling stage, which reflects the potentiality of those signaling molecules on direct ROS scavenging (Fig. 3) and adjustment of oxidants scavenging mechanisms in abolishing ROS (Fig. 5). Among the exogenous protectants, AsA most effectively eliminated ROS followed by CaCl2, both at the germination and seedling stage (Fig. 3). Our experimental findings are also in line with several previous studies (Shalata and Neumann 2001; Ashraf et al. 2015; Rahman et al. 2016). AsA is a front-line oxidant scavenger that promptly extinguishes excess ROS produced within the cells (Gill and Tuteja 2010) and CaCl2, KNO3 and H2O2 stimulate antioxidant enzymes (Saed-Moocheshi et al. 2014; Ashraf et al. 2015; Rahman et al. 2016).
Plants possess a distinct antioxidant defense system: for instance, CAT, POX and APX that scavenges toxic ROS and protects plants from oxidative damage (Anjum 2015). It is clear that salt tolerance in plants is closely associated with elevated levels of antioxidant capacity (Tahi et al. 2008). Excess H2O2 is toxic for plants and CAT converted it to non-toxic H2O and O2 which has the highest turnover number of all enzymes (Sanchez-Casas and Klessig 1994). In our current experiment, the NaCl-stressed rice seedlings exhibited a significantly decreased CAT activity (Fig. 5a). Several previous reports showed similar results in rice under salt stress (Mishra et al. 2013; Wutipraditkul et al. 2015). The decreased CAT action in salt-stressed seedlings might be as a result of over-accumulation of H2O2 (Fig. 3b) that was elevated when plants were subjected to NaCl-imposed oxidative stress and parallel findings were documented in earlier reports (Mishra et al. 2013; Hasanuzzaman et al. 2014). Exogenous application of KNO3, H2O2, AsA and CaCl2 stimulated CAT activity in salt-treated rice pre-seedlings and seedlings along with lowered the accumulation of H2O2 (Figs. 3b, 5a). Analogous outcomes were also narrated by Saed-Moocheshi et al. (2014), Ashraf et al. (2015) and Rahman et al. (2016) in maize and rice crops. Moreover, the APX activity also decreased both at the germination and seedling stages under salinity-induced stress conditions, whereas the POX activity only decreased at the seedling stage (Fig. 5b), which is in harmony with earlier results in rice (Mishra et al. 2013; Hasanuzzaman et al. 2014). However, when the NaCl-stressed rice seedlings were treated with exogenous CaCl2 and H2O2 it further accelerated POX activity (Fig. 5c) along with other antioxidant enzymes activity which might detoxify H2O2, leading to lower APX activity (Fig. 5b). The current results are also in concordance with preceding findings (Mohammad 2013; Rahman et al. 2016), where they reported that treatment with Ca2+ and H2O2 promoted the activity of several antioxidant enzymes under environmentally stressful condition. On the contrary, KNO3 and AsA pretreatment increased APX and POX activity at the seedling stage, which was supported by Saed-Moocheshi et al. (2014) and Alhasnawi et al. (2016). AsA mitigates the toxic ROS generated due to NaCl-induced salinity stress and oxidizes to dehydroascorbate in this process of ROS detoxification (Noctor and Foyer 1998; Razaji et al. 2012). This also resulted in lower antioxidant enzymatic activity (Fig. 5).
Proline, an osmolyte, plays a crucial function in plant osmotic adjustment and is often considered as an important marker of salt-stress tolerance (Elham et al. 2011; Uddin et al. 2012). Salinity stress increased the proline content in rice plants (Fig. 6b), which is substantiated by some earlier experiments (Ain-Lhout et al. 2001; Azooz et al. 2013). Only AsA priming increased proline content under salt stress and improved plant growth. A parallel result was also found in durum wheat (Triticum turgidum) by Azzedine et al. (2011). On the contrary, several previous reports suggest a negative relation between proline accumulation and salt tolerance and considered proline accumulation as a symptom to salinity stress (Kanawapee et al. 2012). On the other hand, seed priming with KNO3 reduced proline content (Fig. 6b), which is in agreement with Anosheh et al. (2011) who reported that higher proline accumulation negatively affects the growth of maize plants. Surprisingly, H2O2 and CaCl2 priming induced no change in proline content in salt-stressed rice plants. A completely different result was found by Gondim et al. (2010) and Tamimi (2016). Further study is required to clarify this issue.
Therefore, among the signaling molecules, CaCl2 and AsA were found to be promising candidates in augmenting salinity tolerance of rice plants owing to their functions in supporting plant growth and the protection of photosynthetic pigment loss. Activation of antioxidant enzymes and detoxification of ROS due to pretreatment with CaCl2 helped the plants to overcome adverse effect of salinity, and thus plants maintain lower proline content. On the other hand, AsA pretreatment also maintained enhanced plant growth maybe by stimulating POX, maintaining higher proline content and acting as ROS scavenging antioxidant. That is why plant may maintain relatively lower CAT and APX content. Moreover, H2O2 and KNO3 pretreatment alleviates salinity-induced adverse effects by modulating the oxidant scavenging mechanisms, but is less effective in maintaining plant growth compared to CaCl2 and AsA pretreatment.
Author contribution statement
MT-U-A and SA designed the study. MT-U-A, SA, SRR and TA conducted the experiment as well as collected different data during the study period of the germination stage. MASP and MT-U-A conducted the experiment again and also conducted the seedling stage experiment. MTH and MAH supervised the whole study. MT-U-A and SA wrote the first draft. Finally, MTH revised the manuscript.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CaCl2 :
-
Calcium chloride
- CAT:
-
Catalase
- EC:
-
Electrical conductivity
- GI:
-
Germination indices
- H2O2 :
-
Hydrogen peroxide
- KNO3 :
-
Potassium nitrate
- MDA:
-
Malondialdehyde
- MGT:
-
Mean germination time
- \({\text{O}}_{2}^{ \cdot - }\) :
-
Superoxide
- POX:
-
Peroxidase
- VI:
-
Vigor index
References
Abbasi GH, Akhtar J, Anwar-ul-Haq M et al (2014) Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak J Bot 46:135–146
Afrin S, Tahjib-Ul-Arif M, Sakil MA, Sohag AAM, Polash MAS, Hossain MA (2019) Hydrogen peroxide priming alleviates chilling stress in rice (Oryza sativa L.) by enhancing oxidant scavenging capacity. Fundam Appl Agric 4:713–722
Afzal I, Basra SMA, Farooq M, Nawaz A (2006) Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int J Agric Biol 8:23–28
Afzal I, Rauf S, Basra SMA, Murtaza G (2008) Halopriming improves vigor, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ 54:382–388. https://doi.org/10.17221/408-PSE
Afzal I, Butt A, Rehman HU et al (2012) Alleviation of salt stress in fine aromatic rice by seed priming. Aust J Crop Sci 6:1401–1407
Ain-Lhout F, Zunzunegui M, Barradas MCD et al (2001) Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 230:175–183. https://doi.org/10.1023/A:1010387610098
Alhasnawi AN, Kadhimi AA, Isahak A et al (2015) Exogenous application of ascorbic acid ameliorates detrimental effects of salt stress in rice (MRQ74 and MR269) seedlings. Asian J Crop Sci 7:186–196. https://doi.org/10.3923/ajcs.2015.186.196
Alhasnawi AN, Che Radziah CMZ, Kadhimi AA et al (2016) Enhancement of antioxidant enzyme activities in rice callus by ascorbic acid under salinity stress. Biol Plant 60:783–787. https://doi.org/10.1007/s10535-016-0603-9
Ambede JG, Netondo GW, Mwai GN, Musyimi DM (2012) NaCl salinity affects germination, growth, physiology, and biochemistry of bambara groundnut. Braz J Plant Physiol 24:151–160. https://doi.org/10.1590/S1677-04202012000300002
Amjad M, Ziaf K, Iqbal Q et al (2007) Effect of seed priming on seed vigour and salt tolerance in hot pepper. Pak J Agric Sci 44:408–414
Anil VS, Krishnamurthy P, Kuruvilla S et al (2005) Regulation of the uptake and distribution of Na + in shoots of rice (Oryza sativa) variety Pokkali: role of Ca2+ in salt tolerance response. Physiol Plant 124:451–464. https://doi.org/10.1111/j.1399-3054.2005.00529.x
Anjum NA (2015) Book review: oxidative damage to plants-antioxidant networks and signaling. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00452
Anjum SA, Xie X, Wang L et al (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6:2026–2032. https://doi.org/10.5897/AJAR10.027
Anosheh HP, Sadeghi H, Emam Y (2011) Chemical priming with urea and KNO3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress. J Crop Sci Biotechnol 14:289–295. https://doi.org/10.1007/s12892-011-0039-x
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16. https://doi.org/10.1016/j.plantsci.2003.10.024
Ashraf M, Rauf H (2001) Inducing salt tolerance in maize (Zea mays L.) through seed priming with chloride salts: growth and ion transport at early growth stages. Acta Physiol Plant 23:407–414. https://doi.org/10.1007/s11738-001-0050-9
Ashraf M, Athar HR, Harris PJC, Kwon TR (2008) Some prospective strategies for improving crop salt tolerance. Adv Agron 97:45–110. https://doi.org/10.1016/S0065-2113(07)00002-8
Ashraf MA, Rasheed R, Hussain I et al (2015) Hydrogen peroxide modulates antioxidant system and nutrient relation in maize (Zea mays L.) under water-deficit conditions. Arch Agron Soil Sci 61:507–523. https://doi.org/10.1080/03650340.2014.938644
Azooz MM, Alzahrani AM, Youssef MM (2013) The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (‘Vicia faba’ L.). Aust J Crop Sci 7:2091
Azzedine F, Gherroucha H, Baka M (2011) Improvement of salt tolerance in durum wheat by ascorbic acid application. J Stress Physiol Biochem 7:27–37
Bajehbaj AA (2010) The effects of NaCl priming on salt tolerance in sunflower germination and seedling grown under salinity conditions. Afr J Biotechnol 9:1764–1770
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
BBS (2014) Bangladesh bureau of statistics, statistical year book of Bangladesh. Ministry of Planning, Government of the People’s Republic of Bangladesh, pp 33–36
Beckers GJM, Conrath U (2007) Priming for stress resistance: from the lab to the field. Curr Opin Plant Biol 10:425–431. https://doi.org/10.1016/j.pbi.2007.06.002
Chang DC, Meng C (1995) A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J Cell Biol 131:1539–1545
Cooper A (1988) The ABC of NFT. Nutrient film technique. Grower Books, London, p 181
Ejaz B, Sajid ZA, Aftab F (2012) Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp. hybrid cv. HSF-240 under salt stress. Turk J Biol 36:630–640. https://doi.org/10.3906/biy-1201-37
Elham R, Mehdi GS, Hasan ANB (2011) The effect of salinity on the growth, morphology and physiology of Echium amoenum Fisch. Mey. Afr J Biotechnol 10:8765–8773. https://doi.org/10.5897/AJB10.2301
FAO (2010) Food and Agricultural Organization of the United Nations, TerraSTAT database
Farooq M, Basra SMA, Saleem BA et al (2005) Enhancement of tomato seed germination and seedling vigor by osmopriming. Pak J Agric Sci 42:3–4
Farooq M, Barsa SMA, Wahid A (2006) Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regul 49:285–294. https://doi.org/10.1007/s10725-006-9138-y
Farooq M, Basra SMA, Rehman H, Saleem BA (2008) Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J Agron Crop Sci 194:55–60. https://doi.org/10.1111/j.1439-037X.2007.00287.x
Fita A, Rodríguez-Burruezo A, Boscaiu M et al (2015) Breeding and domesticating crops adapted to drought and salinity: a new paradigm for increasing food production. Front Plant Sci 6:686–690. https://doi.org/10.3389/fpls.2015.00978
Garg OP, Kapoor V (1972) Retardation of leaf senescence by ascorbic acid. J Exp Bot 23:699–703. https://doi.org/10.1093/jxb/23.3.699
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gondim FA, Gomes-Filho E, Lacerda CF et al (2010) Pretreatment with H2O2 in maize seeds: effects on germination and seedling acclimation to salt stress. Braz J Plant Physiol 22:103–112. https://doi.org/10.1590/S1677-04202010000200004
Hangarter RP (1997) Gravity, light and plant form. Plant Cell Environ 20:796–800
Hasanuzzaman M, Alam MM, Rahman A et al (2014) Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res Int 2014:1–17. https://doi.org/10.1155/2014/757219
Hassanpouraghdam MB, Pardaz JE, Akhtar NF (2009) The effect of osmo-priming on germination and seedling growth of Brassica napus L. under salinity conditions. J Food Agric Environ 7:620–622
Hayat S, Hayat Q, Alyemeni MN et al (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hepler PK (1994) The role of calcium in cell division. Cell Calcium 16:322–330
Hütsch BW, Osthushenrich T, Faust F et al (2016) Reduced sink activity in growing shoot tissues of maize under salt stress of the first phase may be compensated by increased PEP-carboxylase activity. J Agron Crop Sci 202:384–393. https://doi.org/10.1111/jac.12162
Iqbal M, Ashraf M (2007) Seed treatment with auxins modulates growth and ion partitioning in salt-stressed wheat plants. J Integr Plant Biol 49:1003–1015. https://doi.org/10.1111/j.1672-9072.2007.00488.x
Jaleel CA, Sankar B, Sridharan R, Panneerselvam R (2008) Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk J Biol 32:79–83
Kanawapee N, Sanitchon J, Lontom W, Threerakulpisut P (2012) Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil 358:235–249. https://doi.org/10.1007/s11104-012-1179-6
Kaya MD, Okçu G, Atak M et al (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron 24:291–295. https://doi.org/10.1016/j.eja.2005.08.001
Kaya C, Tuna AL, Okant AM (2010) Effect of foliar applied kinetin and indole acetic acid on maize plants grown under saline conditions. Turk J Agric For 34:529–538. https://doi.org/10.3906/tar-0906-173
Khan AL, Hamayun M, Kim Y-H et al (2011) Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol Biochem 49:852–861
Kilic S, Kahraman A (2016) The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley. Pol J Environ Stud 25:1053–1059. https://doi.org/10.15244/pjoes/61852
Kochak-zadeh A, Mousavi S, Eshraghi-nejad M (2013) The effect of salinity stress on germination and seedling growth of native and breeded varieties of wheat. J Nov Appl Sci 2:703–709
Lorenzo H, Siverio JM, Caballero M (2001) Salinity and nitrogen fertilization and nitrogen metabolism in rose plants. J Agric Sci 137:77–84. https://doi.org/10.1017/S0021859601001150
Matthews S, Khajeh Hosseini M (2006) Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci Technol 34:339–347. https://doi.org/10.15258/sst.2006.34.2.09
Mavi K, Ermis S, Demir I (2006) The effect of priming on tomato rootstock seeds in relation to seedling growth. Asian J Plant Sci 5:940–947. https://doi.org/10.3923/ajps.2006.940.947
Metzner H, Rau H, Senger H (1965) Investigations on the synchronizability of single pigment-deficient mutants of chlorella. Planta 65:186–194. https://doi.org/10.1007/BF00384998
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19. https://doi.org/10.1007/s00709-011-0365-3
Moeinzadeh A, Sharif-Zadeh F, Ahmadzadeh M, Tajabadi Fh (2010) Biopriming of Sunflower (“Helianthus annuus” L.) seed with ‘Pseudomonas fluorescens’ for improvement of seed invigoration and seedling growth. Aust J Crop Sci 4:564
Mohammad H (2013) Hydrogen peroxide priming stimulates drought tolerance in mustard (Brassica juncea L.) seedlings. Plant Gene Trait 4:109–123. https://doi.org/10.5376/pgt.2013.04.0020
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043. https://doi.org/10.1093/jxb/erj100
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nawaz F, Naeem M, Akram A et al (2017) Seed priming with KNO3 mediates biochemical processes to inhibit lead toxicity in maize (Zea mays L.). J Sci Food Agric 97:4780–4789. https://doi.org/10.1002/jsfa.8347
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279. https://doi.org/10.1146/annurev.arplant.49.1.249
Park HJ, Kim W-Y, Yun D-J (2016) A new insight of salt stress signaling in plant. Mol Cells 39:447. https://doi.org/10.14348/molcells.2016.0083
Patade VY, Bhargava S, Suprasanna P (2009) Halopriming imparts tolerance to salt and PEG induced drought stress in sugarcane. Agric Ecosyst Environ 134:24–28. https://doi.org/10.1016/j.agee.2009.07.003
Paul S, Roychoudhury A (2016) Seed priming with spermine ameliorates salinity stress in the germinated seedlings of two rice cultivars differing in their level of salt tolerance. Trop Plant Res 3:616–633. https://doi.org/10.22271/tpr.2016.v3.i3.082
Pessarakli M (2016) Handbook of photosynthesis. CRC Press, Boca Raton
Rahman A, Nahar K, Hasanuzzaman M, Fujita M (2016) Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00609
Ramzan A, Hafiz IA, Ahmad T, Abbasi NA (2010) Effect Of priming with potassium nitrate and dehusking on seed germination of gladiolus (Gladiolus alatus). Pak J Bot 42:247–258
Roy P, Tahjib-Ul-Arif MM, Akter T et al (2016) Advances in Environmental Biology Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv Environ Biol 10:148–154
Razaji A, Asli DE, Farzanian M (2012) The effects of seed priming with ascorbic acid on drought tolerance and some morphological and physiological characteristics of safflower (Carthamus tinctorius L.). Ann Biol Res 3:3984–3989
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023. https://doi.org/10.1093/jxb/erj108
Rodriguez-Aguilera JC, Navarro F, Arroyo A et al (1995) Vitamin C stabilization as a consequence of the plasma membrane redox system. Protoplasma 184:229–232
Saed-Moocheshi A, Shekoofa A, Sadeghi H, Pessarakli M (2014) Drought and salt stress mitigation by seed priming with KNO3 and urea in various maize hybrids: an experimental approach based on enhancing antioxidant responses. J Plant Nutr 37:674–689. https://doi.org/10.1080/01904167.2013.868477
Sanchez-Casas P, Klessig DF (1994) A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant Physiol 106:1675–1679
Sano T, Ivashikina N, Hedrich R, Soriichirou S (2007) The role of potassium ion in cell division and cell cycle progression of tobacco BY-2 cells. Plant Cell Physiol 48:294. https://doi.org/10.14841/jspp.2007.0.294.0
Sevengor S, Yasar F, Kusvuran S, Ellialtioglu S (2011) The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. Afr J Agric Res 6:4920–4924
Shahi-Gharahlar A, Khademi O, Farhoudi R, Mirahmadi SF (2010) Influence of salt (NaCl, CaCl2, KNO3) stress on germination and early seedling growth traits of cumin (Cuminum cyminum L.) seed. Seed Sci Biotechnol 4:37–40
Shalata A, Neumann PM (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J Exp Bot 52:2207–2211. https://doi.org/10.1093/jexbot/52.364.2207
Sivritepe HÖ, Sivritepe N, Eriş A, Turhan E (2005) The effects of NaCl pre-treatments on salt tolerance of melons grown under long-term salinity. Sci Hortic (Amsterdam) 106:568–581. https://doi.org/10.1016/j.scienta.2005.05.011
Snedden WA, Fromm H (2001) Calmodulin as a versatile calcium signal transducer in plants. New Phytol 151:35–66. https://doi.org/10.1046/j.1469-8137.2001.00154.x
Soil Resource Development Institute (SRDI) (2010) Saline soils of Bangladesh, SRMAF Project, Ministry of Agriculture, Government of the People’s Republic of Bangladesh, pp 1–55
Tahi H, Wahbi S, El Modafar C et al (2008) Changes in antioxidant activities and phenol content in tomato plants subjected to partial root drying and regulated deficit irrigation. Plant Biosyst Int J Deal Asp Plant Biol 142:550–562. https://doi.org/10.1080/11263500802410900
Tahjib-Ul-Arif M, Roy PR, Sohag AAM, Afrin S, Rady MM, Hossain MA (2018a) Exogenous calcium supplementation improves salinity tolerance in BRRI dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. J Crop Sci Biotechnol 21:383–394
Tahjib-Ul-Arif M, Siddiqui MN, Sohag AAM, Sakil MA, Rahman MM, Polash MAS, Mostofa MG, Tran LSP (2018b) Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9867-y
Tamimi SM (2016) Effect of seed priming on growth and physiological traits of five Jordanian wheat (Triticum aestivum L.) landraces under salt stress. J Biosci Agric Res 11:906–922. https://doi.org/10.18801/jbar
Teerarak M, Bhinija K, Thitavasanta S, Laosinwattana C (2009) The impact of sodium chloride on root growth, cell division, and interphase silver-stained nucleolar organizer regions (AgNORs) in root tip cells of Allium cepa L. Sci Hortic (Amsterdam) 121:228–232. https://doi.org/10.1016/j.scienta.2009.01.040
Tiquia SM (2010) Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 79:506–512. https://doi.org/10.1016/j.chemosphere.2010.02.040
Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42:318–343
Uddin MK, Juraimi AS, Ismail MR et al (2012) Physiological and growth responses of six turfgrass species relative to salinity tolerance. Sci World J. https://doi.org/10.1100/2012/905468
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wahid A, Shabbir A (2005) Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul 46:133–141. https://doi.org/10.1007/s10725-005-8379-5
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294. https://doi.org/10.1016/j.jplph.2006.01.005
Wani AS, Ahmad A, Hayat S, Tahir I (2016) Is foliar spray of proline sufficient for mitigation of salt stress in Brassica juncea cultivars? Environ Sci Pollut Res 23:13413–13423. https://doi.org/10.1007/s11356-016-6533-4
Wutipraditkul N, Wongwean P, Buaboocha T (2015) Alleviation of salt-induced oxidative stress in rice seedlings by proline and/or glycinebetaine. Biol Plant 59:547–553. https://doi.org/10.1007/s10535-015-0523-0
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. https://doi.org/10.1242/jeb.01730
Yazdanpanah S, Baghizadeh A, Abbassi F (2011) The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. Afr J Agric Res 6:798–807. https://doi.org/10.5897/AJAR10.405
Acknowledgements
The authors have gratefully acknowledged the technical support provided by the Central Laboratory, Bangladesh Agricultural University, Mymensingh, during this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. K. Nagar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tahjib-Ul-Arif, M., Afrin, S., Polash, M.A.S. et al. Role of exogenous signaling molecules in alleviating salt-induced oxidative stress in rice (Oryza sativa L.): a comparative study. Acta Physiol Plant 41, 69 (2019). https://doi.org/10.1007/s11738-019-2861-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2861-6