Abstract
Bioaccumulation, subcellular distribution, and acute toxicity of yttrium (Y) were evaluated in Nymphoides peltata. The effects of Y concentrations of 1–5 mg L−1 applied for 4 days were assessed by measuring changes in photosynthetic pigments, nutrient contents, enzymatic and non-enzymatic antioxidants, and ultrastructure. The accumulation of Y in subcellular fractions decreased in the order of cell wall > organelle > soluble fraction. Much more Y was located in cellulose and pectin than in other biomacromolecules. The content of some mineral elements (Mg, Ca, Fe, Mn, and Mo) increased in N. peltata, but there was an opposite effect for P and K. Meanwhile, ascorbate, and catalase activity decreased significantly for all Y concentrations. In contrast, peroxidase activity was induced, while initial rises in superoxide dismutase activity and glutathione content were followed by subsequent declines. Morphological symptoms of senescence, such as chlorosis and damage to chloroplasts and mitochondria, were observed even at the lowest Y concentration. Pigment content decreased as the Y concentration rose and the calculated EC50 and MPC of Y for N. peltata were 2 and 0.2 mg L−1 after 4 days of exposure, respectively. The results showed that exogenous Y was highly available in water and that its high concentration in water bodies might produce harmful effects on aquatic organisms. N. peltata is proposed as a biomonitor for the assessment of metal pollution in aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) are a collection of 15 chemical elements between lanthanum and lutetium in the periodic table, and also include scandium and yttrium. They are usually classed into light REEs (LREEs, lanthanum–europium) and heavy REEs (HREEs, yttrium, and gadolinium–lutetium). In China, REEs have been widely used in industry, forestry, animal husbandry, aquaculture, and agriculture. As a result, large amounts of dissolved REEs have been discharged into the aquatic environment. However, REEs have not yet been characterized as essential plant nutrients or environmentally hazardous metals (Shtangeeva and Ayrault 2007).
Several studies have reported the bioavailability and environmental and ecological effects of REEs on freshwater organisms (Chua 1998; Yang et al. 1999; Barry and Meehan 2000; Wang et al. 2007; Ippolito et al. 2010; Xu et al. 2012). Environmental residues of REEs were found to enter into aquatic flora (Eichhornia crassipes) (Chua 1998). In a static laboratory aquatic microcosm, the accumulative levels of REEs in duckweed were far higher than those in Daphnia, shellfish, and fish, indicated that phytoplankton was more likely influenced by exogenous REEs release into the aquatic environment (Yang et al. 1999). Our previous reports indicated that REEs resulted in nutrient imbalance, oxidative stress, and damage to organelles in Hydrilla verticillata (Wang et al. 2007) and Hydrocharis dubia (Xu et al. 2012). It is well known that the physical and chemical characteristics of HREEs differ from those of LREEs, e.g., HREEs normally possess relatively small ionic radii and have relatively high stability constants for naturally relevant complexes in comparison with LREEs (Wei et al. 2001). However, the subcellular distribution and molecular interaction between REEs and biological molecules in plants, especially HREEs, remains unclear.
Nymphoides peltata, a macrophyte, is frequently used as a food source for fish, pigs, and fowls, and hence, enters the human food chain. Some studies have investigated the mechanism of cadmium (Cd) accumulation in this aquatic plant and found it was sensitive to Cd levels (Lavid et al. 2001). However, no information is available on the REEs accumulation capacity of N. peltata and the accompanying metabolic changes therein. In the present study, the responses of N. peltata to yttrium (Y), a commonly used HREE, with regard to subcellular distribution, Y-binding biological molecules and its influence on nutrient elements, antioxidative systems, and ultrastructure were studied in detail. The aim of this study was to provide valuable information towards understanding the effects of REEs on plants and determining the environmental toxicology and food safety associated with the wide use of REEs in China.
Materials and methods
Plant material and treatments
N. peltata plants were collected from Lake Pipa, Nanjing, China, washed with distilled water, and maintained in hydroponic tubes under the laboratory conditions (115 μmol · m−2 s−1 light irradiance, 14 h photoperiod and 25 °C/18 °C day/night temperature) in 1/10 Hoagland solution. After a week, well-developed, mature plants were transferred to glass beakers filled with 2-L nutrient solution as mentioned above while PO4 3− ions had not been added to avoid REEs precipitation (Hu et al. 2002; Wang et al. 2007; Xu et al. 2012). Y was added at 0, 1, 2, 4, and 5 mg L−1 as YCl3 · 6H2O for 4 days. The levels of YCl3 · 6H2O selected in the present experiment were based on pre-experimental studies and Y concentration higher than 5 mg L−1 was found to be lethal to the plants. All solutions were refreshed every period of 2 days and 0.1 mM KH2PO4 was applied through foliage at the end of each photoperiod (Hu et al. 2002). All experiments were performed in triplicate.
Analysis of leaf damage
To obtain digital images, leaf discs were scanned individually with an Epson Perfection V700 Photo (J221A, Japan). Software settings were color photo, grey scale (48 bits) and resolution 600 dpi. No post-scanning editing (e.g., picture brightness, picture contrast, intensity of picture, etc.) was performed. The digital images were analyzed using WinFolia PRO 2011 software (Regent Instruments Inc., Canada). The proportion of leaf area damaged (PLAD) is defined as a fraction of the number of pixels in the damaged area to the total number of pixels of the entire leaf image.
Leaf tissue fractionation and Y analysis
The subcellular distribution of Y within the leaves was determined according to the procedure of Xiong et al. (2009). Plants (2.0 g FW) were homogenized with 20 mL pre-cold extraction buffer (containing 50 mM Tris–HCl (pH 7.5), 250 mM sucrose, and 1.0 mM dithiothritol). The homogenate was then centrifuged at 500×g for 5 min at 4 °C. The pellet was considered as cell wall fraction. The supernatant was further centrifuged at 20,000×g for 45 min at 4 °C. This pellet was taken as organelle fraction. The resultant supernatant solution was referred as soluble fraction. All homogenizations and subsequent fractionations were performed at 4 °C. Y concentrations were determined by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES, Leeman labs, Prodigy, USA) after decomposition by HNO3 and HClO4.
Chemical forms extraction
In order to study Y contents in different biological molecules, a chemical sequence extraction method was applied to the leaves of N. peltata using various extraction media (Lai et al. 2006): (a) 2.0 g dry plants were ground to powder and extracted with ether for 8 h (soxhlet extraction) to get crude lipids (F1); (b) the resulted residue was extracted with 30 mL boiling water for 3.5 h and centrifuge at 800×g for 10 min, repeat two times, and combine all the supernatant followed by concentrating with rotary evaporator under reduced pressure to get crude polysaccharides (F2); and (c) the residue obtained from step 2 was extracted with 20 mL, 0.1 mol L−1 NaOH at 80 °C for 2 h (repeat 2 times), combine all the solutions, and centrifuge at 10,000×g for 10 min. The resulted supernatant is crude proteins (F3), and the residue is cellulose and pectin (F4). Y concentrations in the biomacromolecules were determined by ICP-AES.
Nutrient elements measurement
Leaves were washed thoroughly with 10 mM EDTA at 4 °C for 30 min under stirring followed by double distilled water to remove metals adsorbed to the surface. They were digested with HNO3/HClO4 (10:1) at 160 °C. The solution samples were analyzed for nutrient elements by ICP-AES.
Electron microscopy
Samples from leaves of control and Y-treated plants of N. peltata were initially fixed for 2 h at 4 °C in 2.5 % glutaraldehyde, followed by postfixation with 1 % osmium tetroxide. The samples were subsequently dehydrated in a graded series of acetone and embedded in Epon 812 resin. The embedded samples were sectioned (70 nm) with a diamond knife (LKB Products). The ultrathin section was stained with uranyl acetate and lead citrate before it was examined by Hitachi H-7650 transmission electron microscope (TEM).
Photosynthetic pigment assay
Chlorophylls (Chl) and carotenoids (Car) content (∼0.4 g fresh samples) was extracted with 80 % acetone and absorbances (A) at 470, 647, and 663 nm recorded on a spectrophotometer (Thermo GENESYS 10). The contents of Chl a, Chl b, and Car were determined according to Lichtenthaler (1987).
Analysis of antioxidants
Plant material (0.5 g) was put in a pre-cooled mortar, in which 0.05 M phosphate-buffered saline (PBS) buffer was added. After grinding in an ice bath, the homogenate was centrifuged at 10,000×g for 20 min at 4 °C. Supernatant was use to measure the activities of enzymes. Superoxide dismutase (SOD) activity was determined according to Stewert and Bewley (1980). One unit of SOD activity is defined as the amount of enzyme that inhibits by 50 % nitrite formation. Peroxidase (POD) activity was assayed using the guaiacol method (Maehly 1955) and calculated by the increment in the absorbance at 470 nm min−1 · g−1 fresh weight. Catalase (CAT) activity was measured by assaying hydrogen peroxide forming stable complex with ammonium molybdate (De Gara et al. 2003). One unit of CAT activity was defined as the decomposition of 1 μmol of hydrogen peroxide per minute.
Determination of reduced glutathione (GSH) and ascorbate (AsA)
The content of GSH was tested following the method of Anderson (1985). AsA content was assayed using the method described by Law et al. (1983).
Statistical analysis
Data presented in this article are the mean values ± S.D. from at least three individual experiments. The coefficients of correlation (r value) were calculated by using Excel 2003. To confirm the variability of data and validity of results, all the data were subjected to the analysis of variance (ANOVA).
Results
Leaf damage
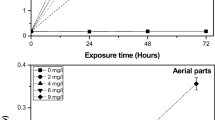
Exposure of N. peltata to Y for 4 days caused significant leaf area damage, starting with typical dotted chlorosis and developing into necrosis as the Y concentration of treatment increased (Fig. 1b–e), in comparison to control plants (Fig. 1a). The estimated leaf damage level expressed as PLAD increased in a dose-dependent manner (p < 0.01, Fig. 2). The maximal PLAD was 84 % in leaves exposed to 5 mg L–1 Y for 4 days.
Subcellular distribution of Y
Y was not detected in unpolluted N. peltata. However, Y exposure induced a significant increase in Y concentration in the cell wall, organelle, and the soluble fraction (Table 1). The highest concentration was in the cell wall (73–86 %), followed by organelle (8–19 %) and then the soluble fraction (4–8 %).
Y-binding forms
The sequential extraction analysis results for Y-binding forms in the leaves of N. peltata showed that 59–67 % of the total cellular Y content was located in the cellulose and pectin components. The Y content in other subcellular components was relatively lower and followed a gradient with the sequence: cellulose and pectin (59–67 %) > crude protein (24–29 %) > crude polysaccharides (4–15 %) > crude lipid (1–3 %; Table 2).
Nutrient elements
Compared with controls, the Y treatment caused a significant decrease in P and K and an increase in Ca, Mg, Mn, and Mo in the leaves of N. peltata at all Y concentrations. The coefficients of correlation showed that the relativity between the above six elements and Y concentration was found highly significant (r P = −0.99, p < 0.01; r K = −0.98, p < 0.01; r Ca = 0.99, p < 0.01; r Mg = 0.99, p < 0.01; r Mn = 0.99, p < 0.01; r Mo = 0.99, p < 0.01), respectively. Fe levels reached a peak value at 4 mg L−1, which was 2.4 times that of controls; however, despite its decline at the highest concentration, Fe remained higher than in controls (Table 3).
Ultrastructural change
The normal chloroplast in leaves of N. peltata appeared to be oval with an orderly arrangement of grana and stroma thylakoids (Fig. 3a). Swelling of thylakoid grana was observed (Fig. 3b) after treatment with 1 mg L−1 Y. In the leaves treated with 4 mg L−1 Y, the inner structure of the chloroplast was disorganized and breakage occurred on the outer membrane of some chloroplasts (Fig. 3c); The highest dose of Y damaged most outer membranes of the chloroplasts, and the swollen grana and stroma were dispersed into the ground substance (Fig. 3d).
Effects of yttrium (Y; 1, 2, 4, and 5 mg L−1) after 4 days exposure on ultrastructure of chloroplasts. a Chloroplasts in control leaf cells. b Leaf cells treated with 1 mg L−1 Y, showing swelling of thylakoid grana. c Leaf cells treated with 4 mg L−1 Y, showing breakage occurred in membrane of chloroplast. d Disintegrated chloroplasts in 5 mg L−1 Y-treated fronds
Photosynthetic pigment
As is seen from Fig. 4, compared with control, a sharp reduction of Chl a, Chl b, and Car was registered in the leaves of N. peltata treated with 5 mg L−1 Y (81 %, 53 %, and 72 %, respectively).
Yttrium (Y) influence on the chlorophyll, Chl a and Chl b and carotenoid (Car) contents in leaves of N. peltata after 4 days of exposure to 1, 2, 4, and 5 mg L−1 Y. Data are means ± S.D. (n = 3); a, b, c, and d letters denote significant differences between treatments for each parameter (p < 0.05, one-way ANOVA)
The antioxidant system
Antioxidant enzyme analysis showed considerable changes in SOD, POD, and CAT activities after treatment with Y (Table 4). There was a significant increase in POD (p < 0.01) and a decrease in CAT (p < 0.05) activities in the leaves of Y-treated N. peltata at all concentrations, compared to controls. In contrast to POD and CAT, SOD activity reached a peak when the leaves were treated with 1 mg L−1, increasing by 22 %; however, as Y concentration rose, SOD activity declined and the maximum reduction was recorded as 23 % in leaves exposed to 5 mg L−1.
Only at concentration of 1 mg L−1 Y, a statistically significant increase of GSH was observed. In contrast AsA (Table 4) decreased sharply (p < 0.01) with increased Y concentration. The maximum loss (40 %) was detected at 5 mg L−1 Y.
Discussion
The main factors which determine the ecological hazards associated with the use of REEs are their concentrations in the environment and their bioavailability to aquatic organisms (Barry and Meehan 2000). Under the studied circumstances, N. peltata leaves accumulated Y in a concentration-dependent manner. When the Y concentrations reached 5 mg L−1 for 4 days, the estimated ratios of accumulated Y content in dry weight to initial metal concentration in the culture medium were 495–648. In comparison to terrestrial plants, the epidermis of water plants does not have a marked protective function but plays a role in the uptake of nutrients from the water and in gas exchange (Fahn 1989). Hydropotes (water drinkers) in the epidermis of the immersed surface of leaves of N. peltata are thought to be structures that facilitate water and salt transport into and out of the plant (Lavid et al. 2001). Xu et al. (2012) also observed significant absorption of REEs in leaves of H. dubia. These all indicate that REEs are highly available to floating aquatic plants through leaf surfaces. Consequently, the bioaccumulation of Y in N. peltata leaves was also accompanied by an induction of varieties of morphogenetic, ultrastructural, and physiological damage. In the present experiment, <0.02‰ of chloride was added to the water solution—the biological effects of such a small amount of chloride could be ignored (Chu et al. 2003). Therefore, the influence of YCl3 on N. peltata should be mainly due to the action of Y.
There are several potential mechanisms by which REEs may exert toxicity on aquatic organisms; however, the exact mode of action remains unknown (Barry and Meehan 2000). To understand the physiological and biochemical bases of the environmental biological effect of REEs on plants, it is necessary to evaluate the chemical behavior of REEs in the plant cell, such as whether REEs are deposited in extracellular or intracellular locations. In the present study, Y analysis at the subcellular level demonstrated that a large proportion of Y (73–85 %) was stored in the cell wall (Table 1). Our findings were consistent with those of Lai et al. (2006), who reported that about 68 % of REEs were located in the cell wall of Pronephrium simplex; and also of Wei et al. (2005), who reported that 56 % of Y was found in the cell wall of Dicranopteris dichotoma leaves. The deposition in cell wall was believed to be passive and to some extent uncontrollable (Lai et al. 2006), as the many carboxylate groups in cell wall can effectively bind with REE ions (Kelley et al. 1999). Furthermore, the present study clearly demonstrated that 15–27 % of Y could enter N. peltata leaf cells; likely with the help of carriers such as protein and hormones (Ni 1995). Trace amounts of REEs disturbed a number of metabolic processes, evident not only in the present study but also in other investigations (Wang et al. 2010), wherein terbium (Tb) changed the contents of the cytosolic nutrient elements in horseradish.
The distribution patterns of Y in the subcellular components of N. peltata showed that 59–67 % of Y was bound to cellulose and pectin (Table 2), indicating a relatively high accumulation of Y in these molecules. Cellulose and pectin are chief components of cell wall, and therefore, the high accumulation of Y in cellulose and pectin was consistent with leaf Y being predominantly in the cell wall (Table 1). It is well known that pectic acid is a polygalacturonic acid and that most of its carboxyl groups are not esterified, and so it can provide many binding sites for metal ions (Lai et al. 2006). Crude polysaccharides and crude lipids have much lower Y content, and therefore, cannot be the main reason for Y bioaccumulation. However, about 24–29 % of Y can bind with proteins. In contrast, the amount of LREEs (e.g., La, Ce, Pr, and Nd) account for 84.9 % of total REEs in the protein fraction in P. simplex, indicating that LREEs may have much stronger capacity to bind with the proteins than HREEs (Lai et al. 2006).
REEs have important effects on membrane stability, therefore, they strongly affect ionic interactions with the cell (Hu et al. 2002; Hu et al. 2004; Shtangeeva and Ayrault 2007; Wang et al. 2010; Xu et al. 2012). In the present study, Y had a negative effect on uptake of P and K, a finding consistent with those of Wang et al. (2010), who reported a decrease in P and K contents in Armoracia rusticana sprayed with Tb. However, in contrast to the present observations, no P and K deficiency was induced by La application in H. dubia (Xu et al. 2012). REEs exposure resulted in significant increases in Ca concentration in test plants (Wang et al. 2010; Shtangeeva and Ayrault 2007)—similar results to the present study concerning Ca content. It was shown that REEs can stimulate Ca influx in various animal cells (Cheng et al. 1999). However, the mechanism of the stimulation in plant cells is still unclear and indicates an active Ca–REE exchange (Shtangeeva and Ayrault 2007). In contrast to present observations, Hu et al. (2002) demonstrated decreases in Ca, Mg and Mn contents of Triticum aestivum seedlings exposed to La and Ce; and our earlier study (Xu et al. 2012) showed a reduction in Ca and Mg contents of leaves of H. dubia treated with La compared to controls. These conflicting data may be due to the different plant species and distinct REEs involved and also because complex phenomena are involved in the influences of REEs on nutrient uptake (Hu et al. 2004).
In the present study, Chl a, Chl b, and Car contents decreased significantly as the concentration of Y increased from 1 to 5 mg L−1. Reduction in chlorophyll and carotenoid content was also reported for aquatic plants Hydrilla verticullata (Wang et al. 2007) and H. dubia (Xu et al. 2012). In naturally grown fern D. dichotoma, Y could be bound to the porphyrin ring of Chl a (Wei et al. 2005). Low chlorophyll content after REEs treatment may be due to (a) impairment in the supply of Mg and Zn, which are required for chlorophyll synthesis (Xu et al. 2012); (b) chlorophyll degradation caused by lipid peroxidation (Wang et al. 2007); and (c) damage to the chloroplast ultrastructure (Xu et al. 2012). In the present study, the total chlorophyll content in plants at different Y concentrations at 4 days was measured in order to determine the EC50 value. The EC50 is the concentration of a toxicant that produces 50 % reduction of chlorophyll content in experimental plants compared to controls. The maximum permissible concentration (MPC) was calculated by considering 10 % of EC50 as a conventional safety factor for relevant studies (Mohan and Hosetti 2006). The EC50 of Y for N. peltata and MPC in the present study were 2 and 0.2 mg L−1 for 4 days of exposure, respectively.
It is well known that the oxidative stress induced by REEs can be an indirect effect of REEs causing the increased production of reactive oxygen species (ROS) and lipid peroxides (Wang et al. 2007; Ippolito et al. 2010; Xu et al. 2012). To avoid oxidative damage by ROS, antioxidants, including key enzymes (i.e., SOD, POD, and CAT) and redox metabolites (i.e., GSH, AsA, and Car), are involved in ROS detoxification (Mittler 2002). In the present study, however, the level of SOD, CAT, AsA, and Car declined gradually with increasing Y concentrations (>1 mg L−1), which may be an important reason for ROS accumulation (Wang et al. 2007). In contrast, POD activity and GSH content at all Y concentrations was higher than those in controls (Table 4). This increment in the antioxidants is suggested as a stress marker without any positive physiological role in the stress responses (Ippolito et al. 2010; Xu et al. 2012). In fact, Y treatment caused significant oxidative damage in N. peltata as evidenced by the breakdown of the outer membranes of the chloroplast (Fig. 3b–d), indicating that Y accumulation could cause damage to plant cells.
Conclusions
In the present study, N. peltata accumulated significant amounts of Y. The Y concentrations followed a gradient sequence: cell wall > organelle > soluble fraction. Cellulose, pectin, and proteins were the main storage of Y in N. peltata. The bioaccumulation of Y in N. peltata leaves could induce severe physiological and cellular alterations, such as nutrient imbalance, oxidative stress, and cell damage, demonstrating its potential toxicity to aquatic plants. As evident by its positive and negative impacts on certain metabolic parameters, we propose its use as an in situ biomonitor for metal pollution and water quality. The maximum permissible concentration of Y for aquatic plants relative to environmental safety was suggested to be 0.2 mg L−1. Further work is necessary to evaluate its implications for higher trophic levels that depend upon N. peltata as an animal feedstock.
References
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Barry MJ, Meehan BJ (2000) The acute and chronic toxicity of lanthanum to Daphnia carinata. Chemosphere 41:1669–1674
Cheng Y, Yao H, Lin H, Lu J, Li R, Wang K (1999) The events relating to lanthanide ions enhanced permeability of human erythrocyte membrane: binding, conformational change, phase transition, perforation and ion transport. Chem.- Biol. Interact 121:267–289
Chu HY, Zhu JG, Xie ZB, Zeng Q, Li ZG, Cao ZH (2003) Availability and toxicity of exogenous lanthanum in a haplic acrisols. Geoderma 115:121–128
Chua H (1998) Bioaccumulation of environmental residues of rare earth elements in aquatic flora Eichhornia crassipes (Mart.) Solms in Guangdong Province of China. Sci Total Environ 214:79–85
De Gara L, de Pinto MC, Moliterni VM, D’Egidio MG (2003) Redox regulation and storage processes during maturation in kernels of Triticum durum. J Exp Bot 54:249–258
Fahn A (1989) Plant anatomy. Pergamon Press, Oxford
Hu X, Ding ZH, Chen YJ, Wang XR, Dai LM (2002) Bioaccumulation of lanthanum and cerium and their effects on the growth of wheat (Triticum aestivum L.) seedlings. Chemosphere 48: 621–629
Hu ZY, Richter H, Sparovek G, Schnug E (2004) Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: A review. J Plant Nutr 27:183–220
Ippolito MP, Fasciano C, Aquin LD, Morgana M, Tommasi F (2010) Responses of antioxidant systems after exposition to rare earths and their role in chilling stress in common duckweed (Lemna minor L): a defensive weapon or a boomerang? Arch Environ Contam Toxicol 58:42–52
Kelley C, Mielke RE, Dimaquibo D (1999) Adsorption of Eu (III) onto roots of water hyacinth. Environ Sci Technol 33:1439–1443
Lai Y, Wang QQ, Yang LM, Huang BL (2006) Subcellular distribution of rare earth elements and characterization of their binding species in a newly discovered hyperaccumulator Pronephrium simplex. Talanta 70:26–31
Lavid N, Schwartz A, Lewinsohn E, Tel-Or E (2001) Phenols and phenol oxidases are involved in cadmium accumulation in the water plants Nymphoides peltata (Menyanthaceae) and Nymphaeae (Nymphaeaceae). Planta 214:189–195
Law MY, Charles SA, Halliwell B (1983) Gluathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol 148:350–382
Maehly AC (1955) Plantper oxidase. Methods Enzymol 2:801–813
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohan BS, Hosetti BB (2006) Phytotoxicity of cadmium on the physiological dynamics of Salvinia natans L. grown in macrphyte ponds. J Environ Biol 27:701–704
Ni JZ (1995) Bioinorganic Chemistry of Rare Earth Elements. Science Press, Beijing
Shtangeeva I, Ayrault S (2007) Effects of Eu and Ca on yield and mineral nutrition of wheat (Triticum aestivum) seedlings. Environ Exp Bot 59:49–58
Stewert RC, Bewley JD (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–248
Wang X, Shi GX, Xu QS, Xu BJ, Zhao J, van Genuchten MT (2007) Lanthanum-and cerium-induced oxidative stress in submerged Hydrilla verticillata plants. Russ J Plant Physiol l54:693–697
Wang LH, Zhou Q, Huang XH (2010) Effects of heavy metal terbium on contents of cytosolic nutrient elements in horseradish cell. Ecotoxicol Environ Saf 73:1012–1017
Wei ZG, Yin M, Zhang X, Hong FS, Li B, Tao Y, Zhao GW, Yan CH (2001) Rare earth elements in naturally grown fern Dicranopteris linearis in relation to their variation in soils in South-Jiangxi region (Southern China). Environ Pollut 114:345–355
Wei ZG, Hong FS, Yin M, Li HX, Hu F, Zhao GW, Wongchung Wong J (2005) Subcellular and molecular localization of rare earth elements and structural characterization of yttrium bound chlorophyll a in naturally grown fern Dicranopteris dichotoma. Microchem 80:1–8
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xu QS, Fu YY, Min HL, Cai SJ, Sha S, Cheng GY (2012) Laboratory assessment of uptake and toxicity of lanthanum (La) in the leaves of Hydrocharis dubia (Bl.) Backer. Environ Sci Pollut Res 19:3950–3958
Yang XY, Yin DQ, Sun H, Wang XR, Dai LM, Chen YJ, Cao M (1999) Distribution and bioavailability of rare earth elements in aquatic microcosm. Chemosphere 39:2443–2450
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No.31170162) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).The authors wish to thank Kaihe Du for his technical assistance in the TEM, Yttrium samples analyzed by Nanjing Normal University Center for Analysis and Testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Fu, Y., Li, F., Xu, T. et al. Bioaccumulation, subcellular, and molecular localization and damage to physiology and ultrastructure in Nymphoides peltata (Gmel.) O. Kuntze exposed to yttrium. Environ Sci Pollut Res 21, 2935–2942 (2014). https://doi.org/10.1007/s11356-013-2246-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2246-0