Abstract
Background
This study evaluated early and medium-term changes in bone turnover markers, and their associations with weight loss, total bone mineral density (BMD), and hormonal changes after biliopancreatic diversion (BPD).
Methods
Ancillary study from a one-year prospective cohort of 16 individuals assessed before, 3 days, 3 and 12 months after BPD. Bone turnover markers (C-terminal telopeptide (CTX), intact osteocalcin (OC), sclerostin, and osteoprotegerin (OPG)) and several hormones were measured at each visit. Total BMD by DXA was assessed at baseline, 3 and 12 months after BPD. Three participants were lost to follow-up.
Results
CTX increased significantly at 3 days (+ 66%), 3 months (+ 219%), and 12 months (+ 295%). OC decreased at 3 days (− 19%) then increased at 3 months (+ 69%) and 12 months (+ 164%). Change in sclerostin was only significant between 3 days and 3 months (+ 13%), while change in OPG was significant between baseline and 3 days (+ 48%) and baseline and 12 months (+ 45%). CTX increase correlated negatively with weight loss at 3 (r = − 0.63, p = 0.009) and 12 months (r = − 0.58, p = 0.039), and total BMD decrease (r = − 0.67, p = 0.033) at 12 months. Change in insulin and adiponectin correlated with changes in bone turnover markers independently of weight loss.
Conclusion
BPD causes an earlier and greater increase in bone resorption over bone formation markers and a decrease in total BMD. Sclerostin did not increase as expected following extensive weight loss. Changes in insulin and adiponectin seem to play a role in the activation of bone remodeling after BPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is gaining in popularity as a treatment of severe obesity [1]. Biliopancreatic diversion with duodenal switch (BPD) is a weight loss procedure that includes a restrictive component (sleeve gastrectomy) and an important modification in caloric and nutrient absorption. It is one of the most effective surgery to induce weight loss and resolve comorbidities associated with obesity [2, 3]. Despite clear benefits on several health outcomes, mounting evidence suggests that especially bariatric procedures that induce malabsorption, including BPD, can adversely affect bone health [4]. Indeed, they have been shown to increase biochemical markers of bone turnover, reduce bone mineral density (BMD), impair bone microarchitecture and strength, and increase fracture risk [5,6,7,8].
Numerous studies have described changes in bone turnover markers and BMD after all types of bariatric procedures [4, 9], but assessments were performed after at least 6 months postoperatively. Early marked increase in the bone resorption marker C-terminal telopeptide (CTX) (i.e., at 10 days) and an increase in sclerostin were reported after Roux-en-Y gastric bypass (RYGB) [10, 11], but no study evaluated these outcomes after BPD. This knowledge is important as it may confirm that changes in bone remodeling also occur rapidly after BPD, justifying early intervention to prevent future bone loss and deterioration of bone microarchitecture.
Mechanisms by which bariatric surgery affects bone remodeling are probably multifactorial but remain incompletely understood. Among those factors, weight loss per se and alterations in the secretion of pancreatic, gut- and adipose tissue-derived hormones induced by weight loss and by anatomical modifications resulting from intestinal bypass are likely involved [12,13,14]. No previous study has reported factors associated with bone remodeling after BPD.
This study aim was to evaluate the early- and medium-term effects of BPD on bone turnover markers (CTX and intact osteocalcin (OC)) and on regulators of bone turnover (sclerostin and osteoprotegerin (OPG)). A secondary exploratory aim was to assess correlations between changes in bone remodeling markers and change in total BMD, weight loss and changes in several hormones that may potentially affect bone remodeling. We hypothesized that BPD will lead to marked early increases in bone turnover markers, sclerostin and OPG, and that bone resorption markers will increase to a greater extent and earlier than bone formation markers. We also hypothesized that changes in bone remodeling markers would correlate with weight loss, change in total BMD and hormones such as insulin, adipokines, and gut-derived hormones.
Materials and Methods
Study Design and Population
This is an ancillary study from a one-year prospective observational cohort study that aimed primarily at assessing fatty acid metabolism during type 2 diabetes remission after BPD [15]. Patients undergoing BPD at a tertiary bariatric care center (Institut de cardiologie et de pneumologie de Québec-Université Laval (IUCPQ) located in Quebec City, Canada) were offered consecutively to participate in the study from July 2012 to July 2014. Briefly, 16 men and women aged 18–65 years, with a BMI ≥ 35 kg/m2, and with or without type 2 diabetes according to the American Diabetes Association’s criteria, were evaluated before and after BPD. Exclusion criteria included but were not limited to: pharmacological treatment with fibrate, insulin, thiazolidenedione, beta-blocker, corticosteroids, hormone replacement therapy, treatment for osteoporosis, or any other medication known to affect bone or lipid metabolism, uncontrolled thyroid disease, decompensated or severe renal or hepatic disease other than non-alcoholic fatty liver disease, or other serious medical condition. Participants suffering from major medical or surgical complications following surgery were also excluded. The protocol was approved by the IUCPQ Ethics Committee. All subjects signed the informed consent prior to study inclusion.
Calcium and Vitamin D Supplementation
Participants were prescribed variable doses of vitamin D3 before BPD to ensure normal preoperative 25-hydroxyvitamin D (25OHD) levels. A standard prescription including a multivitamin pill per day (Centrum Forte®), calcium carbonate 500 mg twice daily, vitamin D (vitamin D2 50,000 IU per day), vitamin A, and iron was given to all participants after surgery, and doses were adjusted based on blood results at 3 months and 12 months.
Outcome Measures
Outcome measures were assessed before BPD (a mean of 45 ± 8 days before surgery) as well as at 3 days, 3 months, and 12 months after BPD. Blood was sampled in the morning after a 12-h fast and plasma was frozen at − 80 °C. Fasting frozen plasma was used to measure CTX (intra-assay CV 1.8–4.5% and inter-assay CV 2.5–6.5%) and intact OC (intra-assay CV 0.7–2.4% and inter-assay CV 2.0–4.1%) with an automated electrochemiluminescence assay (Elecsys, Roche Diagnostics). OPG (Biomedica Immunoassays, Vienna, AT; intra-assay CV 2–3% and inter-assay CV 3–5%) and sclerostin (TECOmedical Group, Sissach, CH; intra-assay CV 3.4–4.0% and inter-assay CV 4.3–4.8%) were also quantified with an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. Glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), leptin, adiponectin, and insulin were assessed, as previously described [16]. Parathyroid hormone (PTH) (Elecsys, Roche Diagnostics) and 25OHD (Cobas 8000, Roche; DEQAS certification obtained) were also measured by an ECLIA method. Bodyweight was measured before, 3 months, and 12 months after BPD in light clothing to the nearest 0.1 kg using a bioimpedance scale (InBody520, body composition analyzer, Biospace, LA, California) calibrated for subjects with severe obesity, and height with a wall-mounted stadiometer to the nearest 0.1 cm. BMI was calculated and reported in kilogram per square meter. Assessment of total BMD and body composition by dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy, GE) was also performed before surgery and at 3 months and 12 months after BPD.
Statistical Analyses
Statistical analyses were performed using JMP Pro version 12.1.0 and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Concentrations of bone turnover markers, regulatory proteins influencing bone metabolism, hormonal factors, and BMD results, as well as their changes at each time point, were assessed using a general linear mixed model with a variance component covariance structure for repeated measures. The chosen matrix was based on minimal Akaike information criterion (AICc). The subject-specific intercept was considered a random effect, and time was considered a fixed effect. If required, variables were log-transformed prior to analysis to ensure normality of distribution. The Tukey-Kramer adjustment was used for multiple comparison tests, comparing all timepoints versus all other timepoints. Spearman correlations were used to assess changes from baseline value in bone turnover markers and total BMD. Partial Spearman correlations adjusted for weight loss were used to assess correlations between changes from baseline in bone turnover markers and hormonal factors. No subgroup analysis is presented since all results were similar between participants with or without type 2 diabetes at baseline. Data are presented as mean ± SD, unless stated otherwise. A two-tailed p value < 0.05 was considered statistically significant for all analyses.
Results
Baseline Characteristics of the Participants
Baseline characteristics of the participants are shown in Table 1. The study sample included five men and 11 women with a mean age of 41.6 ± 8.8 years and a mean BMI of 49.4 ± 5.6 kg/m2. At baseline, 11 participants had type 2 diabetes and two women were postmenopausal. Two participants did not complete the 12-month visit due to pregnancy (n = 1) and a diagnosis of low-grade breast cancer (n = 1) during follow-up. Moreover, one participant’s 12-month frozen sample was lost.
Weight Loss and Changes in Body Composition and Total BMD After BPD
Three months after surgery, mean total weight decreased by 19% (p < 0.0001), with a reduction of 10% (p < 0.0001) in fat mass and 9% (p < 0.0001) in fat-free mass (FFM). Mean total weight loss reached 43% (p < 0.0001) 12 months after BPD, which was mainly explained by a decline in fat mass (− 34% at 12 months, p < 0.0001) as FFM remained stable during this period (− 9%, p > 0.05 between 3 and 12 months). Total BMD decreased by 4.7% (p = 0.0007) at 3 months and by 9.9% (p < 0.0001) at 12 months.
Changes in Bone Turnover Markers After BPD
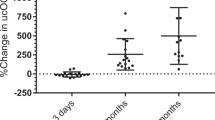
Descriptive data of bone turnover markers at each timepoint are presented in Table 2. Despite non-significant weight loss, CTX started to rise significantly by 66% (p < 0.0001) at 3 days after BPD, followed by further increase that reached 219% (p < 0.0001) at 3 months and 295% (p < 0.0001) at 12 months. Intact OC first decreased significantly by 19% (p = 0.01) at 3 days after BPD, but then increased significantly by 69% (p < 0.0001) and 164% (p < 0.0001) at 3 and 12 months, respectively (Fig. 1). Although we found statistically significant changes in sclerostin between 3 days and 3 months (+ 13%, p = 0.007), changes were modest overall. Changes in OPG were only significant between baseline and 3 days (+ 48%, p = 0.02) and baseline and 12 months (+ 45%, p = 0.03) after BPD.
Change in percentage from baseline in CTX and OC at 3 days, 3 months, and 12 months after BPD. CTX increased as early as 3 days after BPD and continued to increase at 3 months and 1 year, while OC first decreased at 3 days and then increased 3 months and 1 year after BPD. BPD, biliopancreatic diversion with duodenal switch; CTX, C-terminal telopeptide; OC, intact osteocalcin
Changes in Hormonal Factors After BPD
Descriptive data of all hormones at each timepoint are shown in Table 3. Fasting insulin decreased rapidly at 3 days postoperatively (− 53%, p < 0.0001) and continued to decline up to 12 months after BPD (− 71% at 3 months and − 83% at 12 months, p < 0.0001 for both timepoints). Fasting levels of GLP-1 first increased at 3 days after BPD (+ 46%, p < 0.002), returned to the baseline level at 3 months and then did not change significantly 12 months after BPD. Leptin levels decreased significantly throughout follow-up (− 40% at 3 days,
− 62% at 3 months and − 90% at 12 months, p < 0.0001 for all timepoints) whereas adiponectin levels were unchanged at 3 days after surgery, but then increased at 3 months (+ 80%, p < 0.0001) and 12 months (+ 241%, p < 0.0001). PTH initially increased at 3 days after BPD (+ 87%, p < 0.0001) but then remained within normal limits at 3 and 12 months. Besides, 25OHD levels remained unchanged at 3 days and 3 months after surgery but increased significantly at 12 months after BPD (+ 61%, p < 0.0001). Fasting GIP did not change at any timepoint after BPD.
Correlations Between Changes in Bone Turnover Markers, Changes in BMD, Weight Loss and Changes in Hormonal Factors After BPD
Change in CTX was the only bone turnover marker associated with weight loss at 3 months (r = − 0.63, p = 0.009) and 12 months (r = − 0.58, p = 0.039) after BPD. Besides, a negative correlation between changes in sclerostin and fat mass was found at 3 months after surgery (r = − 0.59, p = 0.015), which did not remain significant at 12 months after BPD (r = − 0.23, p = 0.451). Furthermore, change in CTX correlated with change in total BMD (r = − 0.67, p = 0.033) at 12 months after BPD. Correlations adjusted for weight loss were also found between changes in bone turnover markers and changes in insulin and adiponectin. At 3 days after BPD, change in sclerostin correlated negatively with change in insulin (r = − 0.60, p = 0.018). At 3 months, a negative correlation was found between change in CTX and change in insulin (r = − 0.59, p = 0.019) as well as between change in sclerostin and change in adiponectin (r = − 0.60, p = 0.018). At 12 months after BPD, change in OC correlated negatively with change in adiponectin (r = − 0.66, p = 0.021). There were no significant correlations between changes in GLP-1, GIP, leptin, PTH, and 25OHD, with changes in any bone turnover marker.
Discussion
In this study, we examined for the first time the early changes in bone turnover markers and their association with weight loss and changes in BMD, fasting insulin, 25OHD, PTH, several gastrointestinal hormones, and adipokines in patients with severe obesity who had undergone BPD. As early as 3 days postoperatively despite likely non-significant weight loss, BPD resulted in a striking increase in the bone resorption marker CTX and a significant decrease in the bone formation marker intact OC. While CTX continued to rise up to 1 year after surgery, the increase in intact OC was delayed and smaller, resulting in a twofold higher augmentation of bone resorption over bone formation markers at 1 year. Moreover, contrary to our hypothesis, sclerostin did not increase substantially after BPD despite extensive weight loss. Besides, we found significant negative correlations between change in CTX and total weight loss and change in sclerostin and fat mass reduction, as well as between change in CTX and change in total BMD. Significant negative correlations were also observed between changes in several bone turnover markers and changes in insulin and adiponectin. Moreover, correlations between bone turnover markers and hormones were independent of weight loss, suggesting an implication of glucose-insulin metabolism and adiponectin in the activation of bone remodeling following BPD.
Our results extend current knowledge on the effects of malabsorptive bariatric procedures on bone metabolism by adding to the limited data on changes in bone turnover markers after BPD, and by providing novel information on changes occurring very early after this bariatric procedure. Indeed, we could identify only a handful of studies that assessed changes in bone turnover markers after BPD [17,18,19,20]. Of these, only two presented prospective changes in bone turnover markers relatively late after BPD [17, 20]. Similar to our results, the increase in CTX was more than two times larger than the increase in OC at 3 months [20] and 1 year [17, 20] after surgery. In the study by Marceau et al., significant increases in OC and in bone alkaline phosphatase were shown at 4 years after BPD, but bone resorption markers were not measured [21]. As opposed to the paucity of data after BPD, numerous studies addressed changes in bone turnover markers after RYGB [10, 11, 13, 18, 20, 22,23,24,25]. Although no study compared directly both procedures in similar populations, the increments in bone resorption markers reported after RYGB resembled what we found after BPD, with increases in CTX varying between 80–200% at 3 months and 144–220% at 12 months after RYGB, vs. 219% and 295% after BPD in our study. Despite the abundant literature on changes in bone turnover markers > 3 months after RYGB, only the study by Yu et al. measured bone turnover markers as early as 10 days after surgery [10]. In this study, CTX increased by 69% at 10 days, matching the 66% increase in CTX that we observed at only 3 days after BPD.
A novel finding of our study is the significant decrease in the bone formation marker intact OC at 3 days after surgery, which was followed by a significant augmentation at 3 and 12 months. While several studies demonstrated that after RYGB, the increase in bone formation was smaller and delayed compared with the increase in bone resorption markers [10, 11, 23], an early reduction in bone formation following any bariatric procedure has not been documented previously. Moreover, in the only study that assessed bone formation markers early after RYGB, P1NP remained unchanged at 10 days after surgery while it increased by roughly 95% at 1 year [10]. The later increase in bone formation could be explained by the coupling of bone formation with bone resorption [26]. Possible explanations for the initial decrease and then the delayed increase in bone formation include the acute caloric restriction following BPD. Indeed, human and animal studies have shown that caloric restriction results in a decrease of bone formation [27,28,29]. Oxidative stress increasing after surgery [30] may also contribute to lower bone formation since it has been associated with decreased osteoblast differentiation and activity, and increased apoptosis [31].
In line with the greater increase in bone resorption markers over bone formation markers, we found a decrease in total BMD of nearly 10% at 1 year that was associated with the increase in the bone resorption marker CTX. This magnitude of BMD loss is well over the expected decrease of 0.5–1% per year in a population composed mainly of premenopausal women and young men [32]. Our results are also in agreement with existing literature suggesting an adverse effect of mixed restrictive and malabsorptive bariatric surgery on BMD [33].
Another interesting and unexpected finding of our study is the lack of significant increment of sclerostin following extensive weight loss. Indeed, reduced bone strain caused by weight loss is predicted to stimulate sclerostin production by the bone osteocytes [11, 24, 34, 35]. In concordance with this, Muschitz et al. reported a two to threefold increase in sclerostin levels as early as 1 month after RYGB, which was sustained over 2 years [11]. Nevertheless, another study found a time course change in sclerostin after RYGB that was similar to our study with an initial increase of about 60% over the first 3 months (vs. a smaller increment of 13% at 3 months in our study) followed by a return to baseline levels at 12 months [23]. One possible explanation for the divergent results obtained between studies is the use of different assays to measure sclerostin. Still, we used the ELISA kit that was reported as the most accurate and specific assay [36]. Lastly, we found that OPG levels were elevated at 3 days and 12 months after BPD, but not at 3 months. The significance of higher OPG levels at certain timepoints after BPD remains uncertain, especially in light of opposite results obtained from the only other study that measured OPG levels after bariatric surgery, which found reduced OPG levels at 3, 12 and 24 months after RYGB [23].
Several mechanisms have been put forward to explain the increase in bone remodeling after BPD. First, it may be explained by the large amount of weight loss experienced after this procedure [37, 38]. Indeed, the significant correlations that we found between the increase in CTX and weight loss at 3 and 12 months after surgery and between change in sclerostin and fat mass at 3 months, which was also reported in the study by Biagioni et al. [23], lends support to this hypothesis. However, the increase in CTX as early as 3 days after surgery while weight was presumably still unchanged suggests that weight loss is not the primary driver of the early increase in bone resorption following BPD, and that other mechanisms are involved. For instance, inflammation, oxidative stress and increased cortisol levels resulting from postoperative stress may stimulate bone resorption early after surgery [39, 40], but data to support their implication after bariatric surgery is lacking.
Hormonal changes resulting from anatomical modifications induced by surgery may also contribute to postoperative stimulation of bone remodeling [18, 19, 25, 35, 41]. Indeed, the fact that we found strong correlations that were independent of weight loss between changes in bone resorption marker CTX and changes in insulin at 3 months after BPD suggests that insulin-glucose homeostasis may be implicated in the activation of bone remodeling after this surgery. Consistent with our results, two studies found negative associations between change in CTX and change in insulin [42, 43], while another study could not find any association [44]. These inconsistent findings underscore the need for additional studies to confirm that the relationship between bone metabolism and insulin described in animal studies also exists in humans [45,46,47,48]. Furthermore, although changes in sclerostin were minor overall, we found a strong negative correlation between change in sclerostin and change in insulin at 3 days after BPD. This correlation is supported by the results of a large cohort study where negative associations between sclerostin and fasting insulin were found [49], and another study that showed a link between hyperglycemia and higher sclerostin levels [50]. Lastly, our findings, along with these data, suggest a crosstalk between bone and glucose-insulin metabolism.
In addition, we observed significant negative correlations between both changes in OC and sclerostin, with changes in adiponectin, suggesting that adipokines may also be involved in the activation of bone remodeling following BPD. Although no study reported an association between change in sclerostin and change in adiponectin, association between OC and adiponectin are documented in in vitro and animal studies. Indeed, adiponectin and OC may act in an endocrine loop as adiponectin stimulate OC expression in osteoblasts [51, 52], and OC enhances adiponectin expression in adipose tissue [53, 54]. Thus, changes in adiponectin may affect bone remodeling after BPD but larger studies that confirm the correlations that we observed in this small cohort are needed.
Gut-derived hormones including GIP and GLP-1 as well as PTH have also been implicated in the regulation of bone metabolism [55,56,57,58]. However, in line with the study by Yu et al., we did not find that changes in GLP-1 and GIP after bariatric surgery were associated with changes in bone turnover markers [10]. Moreover, no correlations were found between PTH and any of the bone turnover markers, which may be explained by generally normal levels of PTH maintained throughout the study. Nevertheless, an elevation in PTH level was observed at 3 days after BPD, which may be explained by transient hypocalcemia. Unfortunately, calcium levels were not measured.
Our study has notable strengths. Indeed, this is the first study to evaluate early changes in bone turnover markers, OPG, and sclerostin after BPD. Moreover, we measured a large number of hormonal factors potentially contributing to changes in bone turnover after bariatric surgery. However, our study also has limitations including small sample size, which limited our power to find significant associations between changes in bone turnover markers and hormonal changes as well as to assess significant differences between individuals with or without type 2 diabetes, men and women and across age groups. Furthermore, assessment of bone microarchitecture and other bone remodeling markers (including P1NP, RANK, and RANKL) could have provided valuable information to improve our understanding of the changes in bone metabolism after BPD. Moreover, hormones were only measured in the fasting state, and postprandial assessments would have been more appropriate for gut-derived hormones such as GLP-1 and GIP. Also, we did not measure serum calcium and calcium carbonate was prescribed to our patients instead of calcium citrate, which is typically recommended after malabsorptive bariatric surgery. Moreover, given the limitations stated above, our analyses remain exploratory. Finally, BPD only represents about 1% of all bariatric surgeries performed worldwide.
In conclusion, BPD leads to an earlier and greater increase in bone resorption over bone formation markers, potentially contributing to bone loss, as determined by a significant reduction in total BMD. Moreover, sclerostin did not increase as expected following massive weight loss, suggesting that factors other than mechanical unloading could be involved in the regulation of sclerostin levels by osteocytes. Finally, changes in insulin and adiponectin may play a role in the activation of bone remodeling after BPD. Associations between gut- and adipose tissue-derived hormones and bone turnover markers should be further investigated in a larger cohort of patients post bariatric surgery. In addition, studies exploring potential mechanisms to explain the early reduction in bone formation and the impaired sclerostin response after BPD should be conducted. These studies may guide the development of strategies to mitigate the acute activation of bone remodeling and future bone loss after BPD.
References
Ozsoy Z, Demir E. Which bariatric procedure is the most popular in the world? A bibliometric comparison. Obes Surg. 2018.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Claudia G, Schafer AL. Bone health after bariatric surgery. JBMR Plus. 2018;2(3):121–33.
Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore). 2015;94(48):e2087.
Yu EW, Bouxsein ML, Putman MS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9.
Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794.
Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25(1):151–8.
Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29(7):1507–18.
Yu EW, Wewalka M, Ding SA, et al. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):714–22.
Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891–901.
Hosseinzadeh-Attar MJ, Golpaie A, Janani L, et al. Effect of weight reduction following bariatric surgery on serum visfatin and adiponectin levels in morbidly obese subjects. Obes Facts. 2013;6(2):193–202.
Bruno C, Fulford AD, Potts JR, et al. Serum markers of bone turnover are increased at six and 18 months after Roux-en-Y bariatric surgery: correlation with the reduction in leptin. J Clin Endocrinol Metab. 2010;95(1):159–66.
Kotidis EV, Koliakos GG, Baltzopoulos VG, et al. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16(11):1425–32.
Grenier-Larouche T, Carreau AM, Geloen A, et al. Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes. 2017;66(11):2743–55.
Plourde CE, Grenier-Larouche T, Caron-Dorval D, et al. Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity (Silver Spring). 2014;22(8):1838–46.
Tsiftsis DD, Mylonas P, Mead N, et al. Bone mass decreases in morbidly obese women after long limb-biliopancreatic diversion and marked weight loss without secondary hyperparathyroidism. A physiological adaptation to weight loss? Obes Surg. 2009;19(11):1497–503.
Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity (Silver Spring). 2011;19(12):2388–93.
Balsa JA, Botella-Carretero JI, Peromingo R, et al. Chronic increase of bone turnover markers after biliopancreatic diversion is related to secondary hyperparathyroidism and weight loss. Relation with bone mineral density. Obes Surg. 2010;20(4):468–73.
Granado-Lorencio F, Simal-Anton A, Salazar-Mosteiro J, et al. Time-course changes in bone turnover markers and fat-soluble vitamins after obesity surgery. Obes Surg. 2010;20(11):1524–9.
Marceau P, Biron S, Lebel S, et al. Does bone change after biliopancreatic diversion? J Gastrointest Surg. 2002;6(5):690–8.
Ivaska KK, Heliovaara MK, Ebeling P, et al. The effects of acute hyperinsulinemia on bone metabolism. Endocr Connect. 2015;4(3):155–62.
Biagioni MFG, Mendes AL, Nogueira CR, et al. Bariatric Roux-En-Y gastric bypass surgery: adipocyte proteins involved in increased bone remodeling in humans. Obes Surg. 2017;27(7):1789–96.
Hofso D, Bollerslev J, Sandbu R, et al. Bone resorption following weight loss surgery is associated with treatment procedure and changes in secreted Wnt antagonists. Endocrine. 2016;53(1):313–21.
Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98(2):541–9.
Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481.
Villareal DT, Fontana L, Das SK, et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res. 2016;31(1):40–51.
Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–88.
Ahn H, Seo DH, Kim HS, et al. Calorie restriction aggravated cortical and trabecular bone architecture in ovariectomy-induced estrogen-deficient rats. Nutr Res. 2014;34(8):707–13.
Murri M, Garcia-Fuentes E, Garcia-Almeida JM, et al. Changes in oxidative stress and insulin resistance in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(3):363–8.
Wauquier F, Leotoing L, Coxam V, et al. Oxidative stress in bone remodelling and disease. Trends Mol Med. 2009;15(10):468–77.
O'Flaherty EJ. Modeling normal aging bone loss, with consideration of bone loss in osteoporosis. Toxicol Sci. 2000;55(1):171–88.
Rodriguez-Carmona Y, Lopez-Alavez FJ, Gonzalez-Garay AG, et al. Bone mineral density after bariatric surgery. A systematic review. Int J Surg. 2014;12(9):976–82.
Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27(5):1215–21.
Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165–74.
Piec I, Washbourne C, Tang J, et al. How accurate is your sclerostin measurement? Comparison between three commercially available sclerostin ELISA kits. Calcif Tissue Int. 2016;98(6):546–55.
Topart P, Becouarn G, Ritz P. Weight loss is more sustained after biliopancreatic diversion with duodenal switch than Roux-en-Y gastric bypass in superobese patients. Surg Obes Relat Dis. 2013;9(4):526–30.
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:Cd003641.
Neumann E, Muller-Ladner U, Frommer KW. Inflammation and bone metabolism. Z Rheumatol. 2014;73(4):342–8.
Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81.
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37.
Valderas JP, Padilla O, Solari S, et al. Feeding and bone turnover in gastric bypass. J Clin Endocrinol Metab. 2014;99(2):491–7.
Tonks KT, White CP, Center JR, et al. Bone turnover is suppressed in insulin resistance, independent of adiposity. J Clin Endocrinol Metab. 2017;102(4):1112–21.
Frost M, Balkau B, Hatunic M, et al. The relationship between bone turnover and insulin sensitivity and secretion: cross-sectional and prospective data from the RISC cohort study. Bone. 2018;108:98–105.
Wei J, Ferron M, Clarke CJ, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014;124(4):1–13.
Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–19.
Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26(4):677–80.
Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308.
Yu OH, Richards B, Berger C, et al. The association between sclerostin and incident type 2 diabetes risk: a cohort study. Clin Endocrinol. 2017;86(4):520–5.
Kang J, Boonanantanasarn K, Baek K, et al. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J Periodontal Implant Sci. 2015;45(3):101–10.
Luo XH, Guo LJ, Yuan LQ, et al. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309(1):99–109.
Kanazawa I, Yamaguchi T, Yano S, et al. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007;8:51.
Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–41.
Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014;15(2):149–56.
Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19(7):905–12.
Luo XH, Guo LJ, Xie H, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21(10):1648–56.
Nissen A, Christensen M, Knop FK, et al. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99(11):E2325–9.
Zhao C, Liang J, Yang Y, et al. The impact of glucagon-like Peptide-1 on bone metabolism and its possible mechanisms. Front Endocrinol (Lausanne). 2017;8:98.
Funding
The Canadian Institutes of Health Research (MOP 97947), Diabetes Canada and CHU de Québec Foundation provided funding for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
AMC is the recipient of Fonds de recherche du Québec-Santé (FRQ-S) and Diabetes Canada scholarships. ACC is the recipient of the GSK Chair in Diabetes of Université de Sherbrooke. LM reports non-financial support from Roche Diagnostics Canada, personal fees from Amgen, personal fees from Eli Lilly, personal fees from Abbvie, personal fees from Bristol-Myers Squibb, personal fees from Novartis, outside the submitted work. AT and LB receive funding from Johnson Johnson Medical Companies and Medtronic for research studies on bariatric surgery. CG is a clinical research scholar of the FRQ-S and the recipient of a Diabetes Canada New Investigator Award. She received research funding from Technologies Khlôros, and speaker honoraria from Amgen, Eli Lilly, and Janssen. All other authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Turcotte, AF., Grenier-Larouche, T., Ung, RV. et al. Effects of Biliopancreatic Diversion on Bone Turnover Markers and Association with Hormonal Factors in Patients with Severe Obesity. OBES SURG 29, 990–998 (2019). https://doi.org/10.1007/s11695-018-3617-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3617-x