Abstract
Dynamic viscoelasticity measurements were performed for concentrated solutions of konjac glucomannan in an ionic liquid. The entanglement coupling appeared in the rheological data for each solution was characterized in terms of the molecular weight between entanglements (M e) as an average size of the transient entanglement network. The value of M e for konjac glucomannan in the molten state was estimated to be 1.8 × 103 (in g mol−1), being significantly smaller than that for cellulose, although the molecular weight and linkage of the repeating units were the same between these polysaccharides. This result suggested that the configuration of the repeating monosaccharide unit affected the entanglement network of these polysaccharides reflecting the single chain characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Konjac glucomannan is an important food material as a dietary fiber. It is recognized that konjac glucomannan contains small amount of acetyl groups and deacetylation in the alkaline solutions induces crosslinking between glucomannan chains which leads to gelation. So far extensive studies on the intermolecular aggregation of glucomannan chains in aqueous systems have been made using various experimental techniques including rheological measurement; current researches have ranged to the modification of the chemical structure of glucomannan as well as to the mixing of other polysaccharides such as carrageenan and agarose into the pure glucomannan aqueous systems to alter the interaction between glucomannan chains, thereby producing food materials having new physicochemical properties [1–4]. In comparison with the extensive studies on the intermolecular aggregation of glucomannan described above, it seems that few studies have been performed on the single chain properties such as the chain stiffness of glucomannan [5–7]. Further studies on the single chain properties are crucial for advanced application of konjac glucomannan, because the physicochemical properties of glucomannan hydrogels are determined not only by the intermolecular behavior but also by the single chain properties.
It is well known that polymers of high molecular weight like konjac glucomannan in solution tend to show the so-called entanglement coupling above certain concentrations, where there is a transient network of interpenetrating polymer chains and the global motion of each chain is restricted topologically [8, 9]. The entanglement coupling can be characterized by the molecular weight between entanglements (M e), which corresponds to an average size of the entanglement network. Although M e for a polymer in solution is dependent on the concentration, M e for a polymer in the molten state without a solvent (M e,melt) becomes a material constant for the polymer; namely, M e,melt is one of the single chain properties of the polymer. So far there has been no information on M e nor on M e,melt for konjac glucomannan, because it is actually impossible to obtain aqueous solutions of konjac glucomannan above the concentration of entanglement coupling and, moreover, the molten state of konjac glucomannan cannot be attained due to the thermal degradation before melting.
In this study, M e values for konjac glucomannan in concentrated solutions have been estimated from dynamic viscoelasticity obtained by rheological measurement. The concentrated solutions of konjac glucomannan have been prepared with an ionic liquid [10]. The dynamic viscoelasticity data have indicated the existence of entanglement coupling in the solutions and have given M e values. Then M e,melt for konjac glucomannan has been determined from the concentration dependence of M e. Comparison has been made with cellulose with regard to the relationship between M e,melt and the molecular structure.
Materials and methods
Materials
Konjac glucomannan (RHEOLEX® RS, kindly gifted by Shimizu Chemical, Japan) was dried in a vacuum oven at 80 °C overnight just before use. According to the manufacturer, RHEOLEX® is a heteropolysaccharide consisting of β-(1,4)-linked d-glucose and d-mannose in the ratio of ca. 1:1.6 [11] and the molecular weight (in g mol−1) determined by viscometry using Ubbelohde-type viscometers is 1.03 × 106. The ionic liquid solvent used in this study was 1-butyl-3-methylimidazolium acetate (BmimAc) (BASF, Germany) [12]. Concentrated solutions of konjac glucomannan were prepared in the following way. The powdery polysaccharide was added to BmimAc in a dry glass vessel, and the mixture was stirred at ca. 100 rpm on a hot plate at 80 °C for 2 h, and then the mixture was put in a vacuum oven set at 80 °C to avoid moisture and then kept for around 22 h to dissolve glucomannan completely. The concentration of glucomannan (c) ranged from 21 to 63 kg m−3 (3–6 wt%). The values of c prepared were rather low, depending on the solubility of glucomannan in BmimAc, but entanglement coupling appeared in all the solutions due to the high molecular weight of the sample (1.03 × 106), as shown below. In the calculation of c, 1.0 × 103 kg m−3 was assumed for the density of konjac glucomannan in the molten state, and the reported value of 1.055 × 103 kg m−3 was used for the density of BmimAc [13].
Methods
Rheological measurement was carried out with a rheometer (ARES, now TA Instruments, USA). The glucomannan solutions were sandwiched between a cone-plate geometry with the diameter of 25 mm and the cone angle of 0.1 rad under nitrogen atmosphere. The dynamic viscoelasticity measurement was performed as the angular frequency (ω) dependence of the storage modulus (G′) and the loss modulus (G″) from 0.01 to 100 s−1 with a strain amplitude (γ) of 0.1. Linear viscoelastic region was confirmed up to γ = 0.3. The dynamic viscoelasticity data were obtained in the temperature (T) range from 0 to 100 °C at every 20 °C. Steady shear flow measurement was also conducted for the glucomannan solutions except for the solution of c = 63 kg m−3 over the shear rate (\(\dot{\gamma }\)) from 0.01 to 10 s−1 at 80 °C with the same cone-plate geometry.
Results and discussion
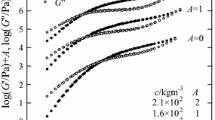
Figure 1 shows an example of the results of the dynamic viscoelasticity measurement; (a) G′ and (b) G″ for the solution of c = 63 kg m−3 at each temperature are plotted against ω. The values of G′ and G″ at a given ω generally decrease as the temperature increases, although the order of G″ cannot be clearly observed in comparison with that of G′. Each curve can be superposed to one another only by an appropriate horizontal shift; for instance, the G′ curve at 100 °C is overlapped with that at 80 °C by multiplying ω by 0.31 and form a single smooth curve, and furthermore overlapping of the G″ curves can be also made with the same factor. This method is the so-called time–temperature superposition. The superposed curves finally obtained are drawn in Fig. 2.
Figure 2 shows the master curves of G′ and G″ for the glucomannan solutions; the curves for the solutions of different c are shifted vertically by a constant A to avoid overlapping. Each pair of curves of G′ and G″ at a given c has been obtained by superposing the data of G′ and G″ at different temperatures according to the time–temperature superposition principle. The reference temperature (T r) is 80 °C and the horizontal shift factor is a T. The figure illustrates that the time–temperature superposition principle holds for all the glucomannan solutions. The values of a T used for drawing the master curves are plotted against 1/T in Fig. 3; the straight line in the figure is best fitted to the data points. It is shown that a T at a given T are almost the same regardless of c and all data points fall on the single line. This indicates that the T-dependence of a T can be represented by an Arrhenius-type equation, as expected for polymer solutions without gelation. The master curves for each c can be divided into two regions based on the values of G′ and G″. In the first region, where G′ < G″, the relation G″ ∝ ω is observed at the end of low ωa T; exactly speaking, G″ curve for the solution of c = 63 kg m−3 does not reach the terminal behavior within the ωa T region observed, although the behavior is similar to the rest of the samples. Obviously, this region corresponds the flow region known for polymer solutions; namely, konjac glucomannan is considered to be dissolved completely in BmimAc without any aggregations at each c examined. The second region, where G′ > G″, can be identified as the rubbery plateau, where the G′ curve reaches a plateau, although each G′ curve in the figure still has a slight tilt in this region. The tilt probably originates from the polydispersity of the glucomannan sample [14]. The existence of rubbery plateau means that there is the entanglement coupling between glucomannan chains in the solutions [9, 15, 16]. Actually, the plateau region broadens with increasing c, as expected from the fact that the entanglement coupling becomes more important with increasing c.
T-dependence of a T for obtaining the master curves in Fig. 2. Data points are well fitted by a single line
It is well known that the zero-shear viscosity (η 0) can be obtained from the terminal behavior of the flow region according to the definition [9, 17]
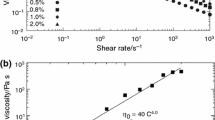
Figure 4 shows the double logarithmic plot of η 0 against c. The values of η 0 have been estimated from Fig. 2 for the solutions exhibiting the terminal relation, G″ ∝ ω; namely, the solution of c = 63 kg m−3 is eliminated, as mentioned above. The figure also includes η 0 obtained from the steady shear flow measurement; η 0 has been determined as the constant value of the viscosity plotted against \(\dot{\gamma }\) observed in the low \(\dot{\gamma }\) region. It is observed that the values of η 0 from the dynamic and the steady flow methods coincide with each other at each c, which ensures the accuracy of the measurements, and that the slope of the plot is estimated to be 5.3.
There are several experimental procedures for determining the plateau modulus (\(G_{N}^{0}\)) from a tilted rubbery plateau [9]. In this study, \(G_{N}^{0}\) at each c has been obtained as the value of G′ at ω where the loss tangent (tanδ; tanδ = G″/G′) attains the minimum in Fig. 2 [13]. With \(G_{N}^{0}\), M e for a polymer in a concentrated solution of c can be calculated from the analogy with rubber elasticity as [9]
where R (=8.31 J mol−1 K−1) is the gas constant. As an example, the master curve of tanδ for the solution of c = 63 kg m−3 in the vicinity of the minimum is plotted in Fig. 5 together with the original data of G′ and G″, which is a part of the curves shown in Fig. 2. In the figure, \(G_{N}^{0}\) can be determined to be 7.1 × 103 Pa at ωa T = 7.6 × 102 s−1, as indicated by a broken line, which gives M e = 2.6 × 104 (in g mol−1). The values of M e for the other solutions were determined in a similar manner.
Master curve of tanδ for the solution of c = 63 kg m−3 in the vicinity of the minimum. The data of G′ and G″ are parts of the curves shown in Fig. 2. The broken line indicates ω at which tanδ attains the minimum
As for \(G_{N}^{0}\) of polymer solutions with entanglement coupling, Colby has reported that the solvent quality for the solute can be evaluated from the concentration dependence [18]. Figure 6 shows the double-logarithmic plot of \(G_{N}^{0}\) against c for glucomannan solutions. The data points can be well fitted by a straight line with a slope of 2.3, but it is still impossible to distinguish the solvent quality of BmimAc for konjac glucomannan using this exponent, as expected from the close exponents for good solvent and θ-solvent.
Figure 7 shows log M e versus log c plot. In addition to the data points for the four solutions treated in Figs. 2 and 3, M e for the solution of c = 32 kg m−3 separately prepared is plotted in the figure. As seen, reproducibility is confirmed for M e obtained in this study. A straight line with a slope of −1 is also drawn in the figure to fit the data points. The slope of the line is derived from the relation M e ∝ c −1, which is well known for concentrated polymer solutions [8, 19, 20]. Since the line represents the c-dependence of M e well, as seen in the figure, and the density of konjac glucomannan in the molten state is assumed to be 1.0 × 103 kg m−3, M e,melt for konjac glucomannan can be estimated as M e at c = 1.0 × 103 kg m−3 of the line to be 1.8 × 103. It should be noted that konjac glucomannan in the molten state is not available actually [21]. However, it is possible to assume an imaginary “molten state” of this polysaccharide. Based on the fact that M e,melt is originally considered for amorphous polymers in the molten state [22], we define the “molten state” as an assembly of flexible polymer chains without solvent molecules. There are no other experimental data on the conformation of glucomannan chains in the BmimAc solutions, but the fact that the dynamic viscoelasticity shown in Fig. 2 is very similar to those of solutions of flexible polymers suggests random coil conformations [23]. Hence, M e,melt obtained in this study by extrapolating M e for the solutions to the density of glucomannan can be comparable with M e,melt of amorphous polymers. Using the molecular weight of the monosaccharide unit for glucomannan (M unit), commonly 162 for glucose and mannose units, this value of M e,melt can be converted to the number of monosaccharide units between entanglements (N unit) of 11. Here, it should be mentioned that even though M unit is calculated taking acetyl groups existing in konjac glucomannan at 1 group per 19 repeating units into consideration, the value of M unit is unchanged [24]. We would also emphasize that M e,melt and N unit for the imaginary “molten state” are basically intrinsic properties for glucomannan chain and are independent of solvent, although no comparison of M e,melt and N unit values can be made at present due to lack of available data using other solvents.
The relationship between M e,melt and the molecular structure has been investigated for a large variety of polymers by several research groups but are still unsettled [25–28]. In our previous study, M e,melt (or N unit) for linear d-glucans such as cellulose and amylose have been compared in terms of the effects of the linkage between the repeating monosaccharide units [26]. It has been found that the linkage is influential on M e,melt within the polysaccharides consisting of the same monosaccharide unit of d-glucose. Regarding the molecular structure, konjac glucomannan can be considered as a linear polysaccharide consisting of β-(1,4)-linked d-glucose and d-mannose in the ratio of ca. 1:1.6. Compared with cellulose, which is exclusively composed of β-(1,4)-linked d-glucose, the repeating units of glucomannan are partly substituted with d-mannose having the same M unit and linkage. The values of M e,melt = 1.8 × 103 and N unit = 11 for konjac glucomannan estimated in this study are significantly smaller than those for cellulose (M e,melt: 3.2 × 103–3.5 × 103; N unit: 20–22) [13, 26]. This difference can be attributed to the configuration of the repeating monosaccharide units. It appears that the configuration of the monosaccharide unit is also influential on M e,melt (or N unit) for linear polysaccharides. As far as we know, the effect of configuration with regard to the relationship between M e,melt and the molecular structure of polysaccharides is first mentioned in the present study. Finally, it is emphasized that comparison in terms of configuration under the same M unit and linkage made in this study is characteristic of polysaccharides, as well as our previous study for the effect of the linkage between the same repeating units, and that the information obtained in this study can make a unique contribution to understanding the relationship between M e,melt and the molecular structure.
Conclusion
Dynamic viscoelasticity as well as steady flow behavior was examined for concentrated solutions of konjac glucomannan in BmimAc. The rheological data indicates the existence of entanglement coupling between konjac glucomannan without gelation in all the solutions examined. The value of M e for konjac glucomannan in solution was firstly determined from the rubbery plateau at each c and then M e,melt for konjac glucomannan in the molten state was estimated to be 1.8 × 103 as one of the chain properties. It could be concluded that configuration of the repeating monosaccharide unit is an important factor in determining M e,melt for linear polysaccharides in comparison with cellulose. Combination with other techniques for examining chain properties such as chain stiffness might enhance understanding of the relationship between M e,melt and the molecular structure of polysaccharides. In this study, konjac glucomannan was dried at 80 °C before measurement, as mentioned above; it might be interesting to investigate the effects of drying temperature on the rheological properties in the future.
References
S. Kobayashi, S. Tsujihata, T. Hibi, Y. Tsukamoto, Preparation and rheological characterization of carboxymethyl konjac glucomannan. Food Hydrocoll. 16, 289–294 (2002)
C.F. Mao, W. Klinthong, Y.C. Zeng, C.H. Chen, On the interaction between konjac glucomannan and xanthan in mixed gels: an analysis based on the cascade model. Carbohydr. Polym. 89, 98–103 (2012)
K. Nishinari, E. Miyoshi, T. Takaya, P.A. Williams, Rheological an DSC studies on the interaction between gellan gum and konjac glucomannan. Carbohydr. Polym. 30, 193–207 (1996)
H. Zhang, M. Yoshimura, K. Nishinari, M.A.K. Williams, T.J. Foster, I.T. Norton, Gelation behavior of konjac glucomannan with different molecular weights. Biopolymers 59, 38–50 (2001)
M.S. Koek, A.S. Abdelhameed, S. Ang, G.A. Morris, S.E. Harding, A novel global hydrodynamic analysis of the molecular flexibility of the dietary fibre polysaccharide konjac glucomannan. Food Hydrocoll. 23, 1910–1917 (2009)
B. Li, B.J. Xie, Single molecular chain geometry of konjac glucomannan as a high quality dietary fiber in East Asia. Food Res. Int. 39, 127–132 (2006)
Y. Lu, L. Zhang, X. Zhang, Y. Zhou, Effects of secondary structure on miscibility and properties of semi-IPN from polyurethane and benzyl konjac glucomannan. Polymer 44, 6689–6696 (2003)
M. Doi, S.F. Edwards, The Theory of Polymer Dynamics (Clarendon, Oxford, 1986)
J.D. Ferry, Viscoelastic Properties of Polymers (Wiley, New York, 1980)
X. Chen, Y. Zhang, F. Ke, J. Zhou, H. Wang, D. Liang, Solubility of neutral and charged polymers in ionic liquids studies by laser light scattering. Polymer 52, 481–488 (2011)
K. Kato, K. Matsuda, Studies of the chemical structure of konjac glucomannan: I. Isolation and characterization of oligosaccharides from the partial hydroyzate of the mannan. Agric. Biol. Chem. 33, 1446–1453 (1969)
J. Horinaka, Y. Urabayashi, T. Takigawa, M. Ohmae, Entanglement network of chitin and chitosan in ionic liquid solutions. J. Appl. Polym. Sci. 130, 2439–2443 (2013)
J. Horinaka, R. Yasuda, T. Takigawa, Entanglement properties of cellulose and amylose in an ionic liquid. J. Polym. Sci. B Polym. Phys. 49, 961–965 (2011)
S. Onogi, T. Masuda, K. Kitagawa, Rheological properties of anionic polystyrenes. I. Dynamic viscoelasticity of narrow-distribution polystyrenes. Macromolecules 3, 109–116 (1970)
S.J. Dalsin, M.A. Hillmyer, F.S. Bates, Linear rheology of polyolefin-based bottlebrush polymers. Macromolecules 48, 4680–4691 (2015)
Q. Chen, H. Masser, H.S. Shiau, S. Liang, J. Runt, P.C. Painter, R.H. Colby, Linear viscoelasticity and Fourier Transform infrared spectroscopy of polyether-ester-sulfonated copolymer ionomers. Macromolecules 47, 3635–3644 (2014)
F.B. Khorasani, R. Poling-Skutvik, R. Krishnamoorti, J.C. Conrad, Mobility of nanoparticles in semidilute polyelectrolyte solutions. Macromolecules 47, 5328–5333 (2014)
R.H. Colby, Structure and linear viscoelasticity of flexible polymer solutions: comparison of polyelectrolyte and neutral polymer solutions. Rheol. Acta 49, 425–442 (2010)
T. Masuda, N. Toda, Y. Aoto, S. Onogi, Viscoelastic properties of concentrated solutions of poly(methyl methacrylate) in diethyl phthalate. Polym. J. 3, 315–321 (1972)
N. Nemoto, T. Ogawa, H. Odani, M. Kurata, Shear creep studies of narrow-distribution poly(cis-isoprene). III. Concentrated solutions. Macromolecules 5, 641–644 (1972)
K. Werner, L. Pommer, M. Broström, Thermal decomposition of hemicelluloses. J. Anal. Appl. Pyrolysis 110, 130–137 (2014)
S.J. Park, P.S. Desai, X. Chen, R.G. Larson, Universal relaxation behavior of entangled 1,4-polybutadiene melts in the transition frequency region. Macromolecules 48, 4122–4131 (2015)
A. Shabbir, H. Goldansaz, O. Hassager, E. van Ruymbeke, N.J. Alvarez, Effect of hydrogen bonding on linear and nonlinear rheology of entangled polymer melts. Macromolecules 48, 5988–5996 (2015)
K. Maekaji, Relationship between stress relaxation and syneresis of konjac glucomannan gel. Agric. Biol. Chem. 42, 177–178 (1978)
W.W. Graessley, S.F. Edwards, Entanglement interactions in polymers and the chain contour concentration. Polymer 22, 1329–1334 (1981)
J. Horinaka, A. Okuda, R. Yasuda, T. Takigawa, Molecular weight between entanglements for linear d-glucans. Colloid Polym. Sci. 290, 1793–1797 (2012)
S. Wu, Chain structure and entanglement. J. Polym. Sci. B Polym. Phys. 27, 723–741 (1989)
L.J. Fetters, D.J. Lohse, D. Richter, T.A. Witten, A. Zirkel, Connection between polymer molecular weight, density, chain dimensions, and melt viscoelastic properties. Macromolecules 27, 4639–4647 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horinaka, Ji., Okamoto, A. & Takigawa, T. Rheological characterization of konjac glucomannan in concentrated solutions. Food Measure 10, 220–225 (2016). https://doi.org/10.1007/s11694-015-9296-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-015-9296-6