Abstract
Entanglement network of carboxymethyl cellulose (CMC) was characterized based on the dynamic viscoelasticity of the concentrated solutions in an ionic liquid. According to the concentration dependence of the molecular weight between entanglements (M e), M e for the molten state (M e,melt) for CMC was estimated to be 3.9 × 103 as a chain variable reflecting the chemical structure of the polysaccharide. Furthermore, relations between M e,melt and other chain variables were examined to elucidate the specificity in the entanglement properties of CMC and related polysaccharides. It was shown that the number of entanglement strands (P e), the ratio of the cube of the tube diameter, and the volume occupied by the entanglement strand, for CMC was 72 being significantly larger than the universal value of ca. 20 recognized for flexible polymers. Anomalous values of P e > 20 were also obtained for related polysaccharides such as cellulose and amylose.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer chains in melts or in concentrated solutions tend to interpenetrate so that their chain dynamics are topologically constrained. This so-called entanglement coupling of polymer chains, which is influential in the rheological behavior of the polymer systems at long times, can be characterized by the molecular weight between entanglements (M e) (Masuda et al. 1972; Ferry 1980; Doi and Edwards 1986). In fact, M e is often referred to as an essential quantity for discussing the rheological behavior of polymer systems with the entanglement coupling. In addition, M e for a polymer melt (M e,melt) is recognized as a material constant reflecting the chemical structure of the polymer. This means that M e,melt is not only a rheological parameter representing the whole polymer system but also one of fundamental variables describing the nature of the individual polymer chain. Since there are various fundamental chain variables besides M e,melt describing the chain dimension as well as the entanglement nature, the establishment of interrelations among the chain variables should be helpful in understanding the nature of the entanglement coupling. So far, many experimental and theoretical studies have been conducted for flexible polymers and several interrelations have been obtained empirically (Lin 1987; Kavassalis and Noolandi 1989; Wu 1989; Graessley and Edwards 1981; Colby et al. 1992; Fetters et al. 1994, 2007). Above all, there is an interesting suggestion known as the Lin Noolandi conjecture (Lin 1987; Kavassalis and Noolandi 1989; Colby et al. 1992; Fetters et al. 2007). With the characteristic ratio (C ∞), the unperturbed mean-square end-to-end distance of a real polymer chain which consists of n backbone bonds of length l is given by 〈R 2〉0 = C ∞ nl 2 = Nb 2. This equation means that a real chain can be expressed by the equivalent freely jointed chain with N Kuhn monomers of length b. The occupied volumes of a chain V c and a Kuhn monomer V 0, whose molar masses are M and M 0 (M 0 = M/N), respectively, are given by V c = M/ρN Aand V 0 = M 0/ρN A, where ρ is the density and N A is the Avogadro’s number. Hence, the packing length p, which is defined as the ratio of V c and 〈R 2〉0, is given by:
In a similar manner, the so-called tube diameter a, which is the unperturbed root-mean-square end-to-end distance of an entanglement strand of the molar mass M e, is obtained as a 2 = N e b 2, where N e is the number of Kuhn monomers in this strand. The occupied volume of the entanglement strand V e is given by V e = M e/ρN A = V 0 N e. Then, the Lin Noolandi conjecture suggests that P e, the number of entanglement strands of the volume V e within an entanglement strand volume a 3, becomes a fixed quantity. Here, P e is defined as:
Since P e for a polymer can be calculated using M e and C ∞, the above criterion has been tested about a wide variety of flexible polymers whose chain variables have been reported in the literature (Fetters et al. 2007). However, comparison among the chain variables for polysaccharides is far from satisfactory partly due to the experimental difficulties (Brown et al. 1963; Nakanishi et al. 1993; Horinaka et al. 2012a).
Carboxymethyl cellulose (CMC) is a polysaccharide whose hydrogens on the hydroxyl groups are partly substituted by carboxymethyl groups. This polysaccharide is soluble in water and therefore has been widely used as thickening and stabilizing agents applicable to, for example, foods, cosmetics, and dyes. The variety of practical applications of CMC emphasizes the importance of the rheological behavior of CMC systems. Actually, extensive studies on the rheological behavior of the aqueous systems have been performed at a broad range of experimental conditions (Kuroiwa et al. 1967; Abdelrahim and Ramaswamy 1995; Werner-Michael et al. 1996; Broniarz-Press et al. 2003; Lopez et al. 2017), but the concentration of CMC has been limited to the dilute or semi-dilute region (Wu et al. 2009), where the entanglement coupling is of no importance. Accordingly, to our knowledge, the entanglement coupling of CMC has hardly been examined at the present stage. Regarding aqueous solutions of CMC, concentration dependence of viscosity has been studied and a dynamic crossover has been related to entanglement (Lopez et al. 2017). Furthermore, the fact that CMC in the molten state is not available due to thermal degradation before melting, as is often the case with polysaccharides, makes estimation of M e,melt for CMC difficult. Regarding the chemical structure of CMC, the repeating units of glucopyranose are remarkably bulky compared to those of conventional flexible polymers (Eliezer and Hayman 1957; Brown et al. 1963). Taking this specificity in the chemical structure into consideration, it seems interesting to examine interrelations among the chain variables for CMC, that is, interrelations differing from those about the flexible polymers can be expected.
It has been demonstrated in our previous studies that M e,melt for polysaccharides can be estimated from the rheological behavior of their concentrated solutions prepared by using ionic liquids as the solvents (Horinaka et al. 2009, 2011, 2012a, b, 2013, 2015). In this study, it is shown that the same method holds good in the case of CMC: Rheological measurements have been carried out for ionic liquid solutions of CMC in the concentrated region to characterize the entanglement coupling by M e, and then, M e,melt for CMC has been obtained from the concentration dependence of M e. One reason for choosing CMC is that there are enough reported values of the chain variables available (Eliezer and Hayman 1957; Brown et al. 1963). The relations between M e,melt and other chain variables are examined for CMC as well as for other polysaccharides.
Experimental
Materials
A commercial grade of CMC sodium salt (Wako, Japan) was used as received. The sodium ion (Na+) content was reported as 0.065–0.085 in weight by the manufacturer, and therefore, the degree of substitution by carboxymethyl groups of this sample was estimated to be around 0.7 assuming every carboxymethyl group has the counter ion of Na+. The solvent 1-butyl-3-methylimidazolium acetate (BmimAc) (BASF, Germany) was used without further purification. The melting point and density of this solvent were assumed identical to those of BmimAc in our earlier study (Horinaka et al. 2013), namely – 20 °C and 1.055 × 103 kg−3, respectively, although the manufacturer’s data sheet was not available at the time of this study. Concentrated solutions of CMC were prepared in the following manner. Powdery sample of CMC sodium salt was added into BmimAc in a dry glass vessel, and the mixture was stirred with a stainless spatula on a hot plate at 70 °C until obvious inhomogeneity was disappeared. Then, the mixture was left on a hot plate at 70 °C for about 8 h to dissolve the powdery sample completely. The concentration of CMC (c) was ranged from 9.8 × 101 to 2.9 × 102 kg−3, i.e., from 10 to 30 wt%. In the calculation of c, the density of CMC sodium salt was assumed to be 1.0 × 103 kg−3 and the weight fraction of Na+ was eliminated. The samples were immediately used for rheological measurements after preparation.
Measurements
Rheological measurements were carried out with an ARES rheometer (now TA Instruments, USA) under a nitrogen atmosphere. A cone-plate geometry with a diameter of 25 mm and a cone angle of 0.1 rad. was used. Dynamic viscoelasticity, or the frequency (ω) dependence of the storage modulus (G’) and the loss modulus (G”), for the CMC solutions was examined with a strain amplitude (γ) of 0.1. The value of γ was determined so that the measurement could be performed in the linear viscoelasticity region. The measurement temperature (T) ranged from 0 to 80 °C.
Results and discussion
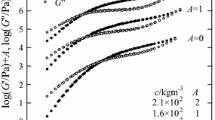
Figure 1 shows the master curves of G' and G'' for all the CMC solutions examined in this study. Here, the reference temperature (T r) has been chosen to be 80 °C, and a T stands for the horizontal shift factor. The master curves indicate that the time-temperature superposition principle holds very well for all the CMC solutions. In each pair of the master curves, two characteristic domains are seen: the flow zone and the rubbery plateau region. The flow zone in the low ω region is typical of polymer solutions and at c ≤ 1.9 × 102 kg−3 even the terminal flow behavior characterized by the relation G'' ∝ ω appears in the curves. The rubbery plateau, the higher ω region where G’ > G'', implies the existence of the entanglement coupling between CMC chains in the solutions; the plateau region becomes wider as c increases, as expected (Onogi et al. 1970; Masuda et al. 1972). It is also seen that the plateau of G’ is actually tilted to some extent, which is probably due to the polydispersity of the CMC employed (Horinaka et al. 2011).
Figure 2 shows a T determined for obtaining the master curves in Fig. 1 plotted against 1/T; there are no data points at the lowest temperature for the solutions of c ≥ 2.4 × 102 kg−3. Although a T at a given T are almost independent of c, weak dependence on c could be observed at low temperatures; that is, a T at a given T becomes larger with c. It is impossible for us to explain why such a c-dependence appears, but similar trend has been obtained in our previous studies on polysaccharide solutions. Assuming all data points fall on a single line, as drawn in the figure, the best fit line gives the activation energy of flow of 86 kJ mol−1.
Concerning the CMC solutions at c ≤ 1.9 × 102 kg−3, the zero-shear viscosity (η 0) can be determined from the terminal flow behavior appearing in the master curves shown in Fig. 1. The estimated values of η 0 are plotted double-logarithmically as a function of c in Fig. 3. Scaling of the viscosity has been obtained to be η 0 ∝ c 3.3; interestingly, the exponent is close to those (3.4–3.8) reported for the viscosity of aqueous solution of CMC at high concentrations (Kastner et al. 1997; Truzzolillo et al. 2009; Wu et al. 2009; Lopez et al. 2015).
Using the master curves of G' and G'' with a tilted rubbery plateau, the plateau modulus (\( {G}_N^0 \)) can be determined as the G' value at ωa T where the loss tangent (tanδ = G''/G') curve attains a minimum. For example, \( {G}_N^0 \) for the CMC solution of c = 2.4 × 102 kg−3 has been determined to be 4.2 × 104 Pa, which gives M e (in gmol−1) of 1.7 × 104 for CMC at this concentration according to the following equation (Onogi et al. 1970; Ferry 1980; Doi and Edwards 1986):
where R is the gas constant. The values of M e for other CMC solutions were determined in the similar way. Figure 4 shows the double-logarithmic plot of the obtained M e against c. A straight line with a slope of − 1 is also drawn in the figure to fit the plot best taking the well-known relation M e ∝ c −1 for polymer solutions into consideration (Bhattacharjee et al. 2002; Huang et al. 2013); it can be confirmed that the c dependence of M e also holds well for the CMC solutions. Small deviations from the straight line are probably due to the uncertainty in the determination of M e. Another scaling M e ∝ c −4/3, which is also known for polymer solutions (Struglinski and Graessley 1985; Lee et al. 2006), has been tested against the data points in Fig. 4. It is obvious that the scaling with − 1 is better than that with − 4/3 for the data points. If the density of the amorphous CMC is assumed to be 1.0 × 103 kg−3, M e,melt for CMC can be estimated as a value of M e at c = 1.0 × 103 kg−3 in Fig. 4, namely 3.9 × 103. The extrapolation to the molten state has been validated for several synthetic polymers (Struglinski and Graessley 1985; Bhattacharjee et al. 2002; Lee et al. 2006; Huang et al. 2013).
As described in the introduction part, M e,melt is a material constant characterizing the polymer chain, and there might be interesting interrelations between M e,melt and other chain variables. For flexible polymers, it has been concluded that P e apparently becomes constant regardless of the polymer species with the average value of 20.6 (Fetters et al. 2007). Now, similar analysis is possible for CMC; using M e,melt = 3.9 × 103 obtained in this study and C ∞ = 11.7 provided in the literature (Brown et al. 1963), P e is evaluated to be 72. It should be noted that P e for CMC is significantly larger than the universal value for flexible polymers. Although it is impossible at the present stage to mention exactly which factors bring about this difference, we suppose that the bulkiness of the repeating units of CMC is attributable; actually, a glucopyranose ring of CMC has the length of ca. 5Å (Brown et al. 1963), while most flexible polymers have the main-chain bond length of less than 2Å (Fetters et al. 2007). In an earlier study on the solution properties of CMC in cadoxen, it has been reported that the chains of CMC have displayed hydrodynamics of random-coil polymers (Brown et al. 1963). Hence, we think that chains of CMC in BmimAc, which has a great ionic strength, are also in the random-coil conformation in spite of the difference in the solvent, suggesting that the anomalous P e for CMC does not correlate with the chain conformation. To support the anomaly of P e for CMC, values of P e for other polysaccharides have been estimated based on our previous results on M e,melt as well as reported C ∞ in the literature. It is noted that all the C ∞ values quoted here are experimental data although the measurement conditions are not necessarily the same and that C ∞ given by model calculations are not employed because of the large researcher-dependence. Table 1 shows the obtained chain variables for the polysaccharides including their Kuhn length (b; = C ∞ l 0; l 0 being the average main-chain bond length) in ascending order of M e,melt. In the lower part of the same table, the corresponding values for several flexible polymers have been extracted from the literature for comparison taking the variation in M e,melt into account (Fetters et al. 2007). It is noteworthy that all the values of P e for polysaccharides are surely larger than the universal value for flexible polymers, although the values depend on the polysaccharide species without any obvious trends.
Since the universal value of P e is originally supposed to hold for flexible polymers, i.e., for chains where a >> b, P e has been plotted against a/b in Fig. 5 using the values given in Table 1. The dotted line in Fig. 5 indicates the average value of P e = 20.6, and four data points for the flexible polymers in Table 1 are almost on this line. Compared to the coincidence for the flexible polymer, P e values for polysaccharides are significantly larger than the universal value. Here, the value of a/b for the flexible polymers ranges from 1.9 to 8.3, and those for three polysaccharides pullulan, curdlan, and amylose are within this range. This fact implies that the anomaly of P e for polysaccharides cannot be attributed to merely the chain inflexibility of polysaccharides—at least for the three polysaccharides. The figure also shows that P e values for polysaccharides with a/b < 1, namely cellulose and xanthan, are extremely high, which might be due to the inflexibility of the chain. Although the limit of applicability of the universal P e in terms of a/b cannot be determined quantitatively, the anomalous values of P e for CMC and guar gum with the intermediate a/b could be explained by their semi-flexible chain characteristics.
Plot of P e against a/b from the values in Table 1. The dotted line indicates the average value of P e = 20.6
Conclusions
The dynamic viscoelasticity for the concentrated solutions of CMC was examined to characterize the entanglement network of CMC in terms of M e. M e,melt = 3.9 × 103 together with C ∞ in the literature gave P e = 72 for CMC which is significantly larger than the universal value for flexible polymers. Taking a flexibility parameter a/b into consideration, this anomalous value of P e might be due to the semi-flexible chain character. On the other hand, it was found that other polysaccharides pullulan, curdlan, and amylose, whose values of a/b are comparable with flexible polymers of P e ≈ 20, also have significantly large P e, as shown in Fig. 5. The anomaly of P e might be the specific characteristics of polysaccharide chains.
References
Abdelrahim KA, Ramaswamy HS (1995) High temperature/pressure rheology of carboxymethyl cellulose. Food Res Int 28:285–290
Bhattacharjee PK, Oberhauser JP, McKinley GH, Leal LG, Sridhar T (2002) Extensional rheometry of entangled solutions. Macromolecules 35:10131–10148
Broniarz-Press L, Rozanski J, Dryjer S, Bednarz J (2003) Effect of strong electrolyte addition on rheological properties of aqueous solutions of sodium carboxymethyl cellulose. Int J Appl Mech Eng 8:207–212
Brown W, Henley D, Öhman J (1963) Studies on cellulose derivatives. Part I. The dimensions and configuration of sodium carboxymethyl cellulose in cadoxen and the influence of the degree of substitution. Makromol Chem 62:164–182
Buliga GS, Brant DA (1987) Temperature and molecular weight dependence of the unperturbed dimensions of aqueous pullulan. Int J Biol Macromol 9:71–76
Cai J, Liu Y, Zhang L (2006) Dilute solution properties of cellulose in LiOH/urea aqueous system. J Polym Sci B 44:3093–3101
Colby RH, Rubinstein M, Viovy JL (1992) Chain entanglement in polymer melts and solutions. Macromolecules 25:996–998
Doi M, Edwards SF (1986) The theory of polymer dynamics. Clarendon, Oxford
Eliezer I, Hayman HJG (1957) Mean square length and mean square radius of gyration of 1,4′-polysaccharides and of polybutadienes. J Polym Sci 23:387–402
Ferry JD (1980) Viscoelastic properties of polymers. John Wiley & Sons, New York
Fetters LJ, Lohse DJ, Richter D, Witten TA, Zirkel A (1994) Connection between polymer molecular weight, density, chain dimensions, and melt viscoelastic properties. Macromolecules 27:4639–4647
Fetters LJ, Lohse DJ, Colby RH (2007) Chain dimensions and entanglement spacings. In: Mark JE (ed) Physical properties of polymers handbook, 2nd edn. Springer, Berlin, pp 447–454
Goebel KD, Brant DA (1970) The configuration of amylose and its derivatives in aqueous solution. Experimental results. Macromolecules 3:634–643
Graessley WW, Edwards SF (1981) Entanglement interactions in polymers and the chain contour concentration. Polymer 22:1329–1334
Horinaka J, Honda S, Takigawa T (2009) Rheological properties of concentrated solutions of gellan in an ionic liquid. Carbohydr Polym 78:576–580
Horinaka J, Yasuda R, Takigawa T (2011) Entanglement properties of cellulose and amylose in an ionic liquid. J Polym Sci B Polym Phys 49:961–965
Horinaka J, Okuda A, Yasuda R, Takigawa T (2012a) Molecular weight between entanglements for linear D-glucans. Colloid Polym Sci 290:1793–1797
Horinaka J, Yasuda R, Takigawa T (2012b) Rheological properties of concentrated solutions of galactomannans in an ionic liquid. Carbohydr Polym 89:1018–1021
Horinaka J, Urabayashi Y, Takigawa T, Ohmae M (2013) Entanglement network of chitin and chitosan in ionic liquid solutions. J Appl Polym Sci 130:2439–2443
Horinaka J, Tanaka M, Takigawa T (2015) Rheological properties of ionic liquid solutions of xanthan. Colloid Polym Sci 293:2709–2712
Huang Q, Mednova O, Rasmussen HK, Alvarez NJ, Skov AL, Almdal K, Hassager O (2013) Concentrated polymer solutions are different from melts: role of entanglement molecular weight. Macromolecules 46:5026–5035
Kastner U, Hoffmann H, Donges R, Hilbig J (1997) Structure and solution properties of sodium carboxymethyl cellulose. Colloids Surf A Physicochem Eng Asp 123:307–328
Kavassalis TA, Noolandi J (1989) Entanglement scaling in polymer melts and solutions. Macromolecules 22:2709–2720
Kuroiwa S, Ogawa M, Nakamura M (1967) Superposition of viscoelastic data for solutions of carboxymethyl cellulose. Bull Chem Soc Jpn 40:2195–2197
Lee JH, Goldberg JM, Fetters LJ, Archer LA (2006) Linear viscoelastic behavior of symmetric and asymmetric star polymer solutions. Macromolecules 39:6677–6685
Lin YH (1987) Number of entanglement strands per cubed tube diameter, a fundamental aspect of topological universality in polymer viscoelasticity. Macromolecules 20:3080–3083
Lopez CG, Rogers SE, Colby RH, Graham P, Cabral JT (2015) Structure of sodium carboxymethyl cellulose aqueous solutions: a SANS and rheology study. J Polym Sci B Polym Phys 53:492–501
Lopez CG, Colby RH, Graham P, Cabral JT (2017) Viscosity and scaling of semiflexible polyelectrolyte NaCMC in aqueous salt solutions. Macromolecules 50:332–338
Masuda T, Toda N, Aoto Y, Onogi S (1972) Viscoelastic properties of concentrated solutions of poly(methyl methacrylate) in diethyl phthalate. Polym J 3:315–321
Nakanishi Y, Norisuye T, Teramoto A, Kitamura S (1993) Conformation of amylose in dimethyl sulfoxide. Macromolecules 26:4220–4225
Nakata M, Kawaguchi T, Kodama Y, Konno A (1998) Characterization of curdlan in aqueous sodium hydroxide. Polymer 39:1475–1481
Onogi S, Masuda T, Kitagawa K (1970) Rheological properties of anionic polystyrenes. I. Dynamic viscoelasticity of narrow-distribution polystyrenes. Macromolecules 3:109–116
Picout DR, Ross-Murphy SB, Errington N, Harding SE (2001) Pressure cell assisted solution characterization of polysaccharides. 1. Guar gum. Biomacromolecules 2:1301–1309
Sato T, Norisuye T, Fujita H (1984) Double-stranded helix of xanthan in dilute solutions: evidence from light scattering. Polym J 16:341–350
Struglinski MJ, Graessley WW (1985) Effects of polydispersity on the linear viscoelastic properties of entangled polymers. 1. Experimental observations for binary mixtures of linear polybutadiene. Macromolecules 18:2630–2643
Truzzolillo D, Bordi F, Cametti C, Sennato S (2009) Counterion condensation of differently flexible polyelectrolytes in aqueous solutions in the dilute and semidilute regime. Phys Rev E 79:011804
Werner-Michael K, Arne Henning K, Wiebke K, Heiko T, Juergen E, Joern-Bernd P (1996) Characterization of aqueous carboxymethyl cellulose solutions in terms of their molecular structure and its influence on rheological behavior. Polymer 37:2723–2731
Wu S (1989) Chain structure and entanglement. J Polym Sci B Polym Phys 27:723–741
Wu Q, Shangguan Y, Du M, Zhou J, Song Y, Zheng Q (2009) Steady and dynamic rheological behaviors of sodium carboxymethyl cellulose entangled semi-dilute solution with opposite charged surfactant dodecyl-trimethylammonium bromide. J Colloid Interface Sci 339:236–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horinaka, Ji., Chen, K. & Takigawa, T. Entanglement properties of carboxymethyl cellulose and related polysaccharides. Rheol Acta 57, 51–56 (2018). https://doi.org/10.1007/s00397-017-1053-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-017-1053-9