Abstract
Purpose
The composition of the fish parasite community depends on several factors related to the environment, the host and its biology. This study aimed to evaluate the influence of environmental factors in anthropized and conserved areas on the endoparasite community structure in fish at different trophic levels, in addition to verifying that some species of Digenea are indicators of conserved environments.
Methods
The study was carried out in the Upper Juruá River region, Western Amazon, Brazil. Six sampling sites were selected in this region and grouped in conserved and degraded environments. Fish were caught from periods of drought and flood, using passive and active sampling methods. Fish collected were measured, weighed, necropsied and the parasites found were counted, fixed, and subjected to morphological analysis. Physical and chemical variables and environmental characteristics were measured in all sites.
Results
The present study demonstrated that environmental variables in a floodplain system can influence the richness, diversity, composition and abundance of endoparasites in hosts at different trophic levels. In addition, anthropized environments may favor the abundance of some generalist parasites and present a more homogeneous biota between seasonal periods compared to conserved environments.
Conclusion
Study contributed with information supporting the importance of conservation of aquatic environments, and demonstrated that fish parasites can be excellent indicators of environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endoparasites are organisms transmitted through a food web involving intermediate, paratenic and/or definitive hosts [1]. Among fish endoparasites, helminths have a direct life cycle and complex life cycle and require multiple hosts at different trophic levels, and thus transmission is dependent on prey-predator relationships [2, 3]. Interactions involving hosts and parasites can provide essential ecosystem functions and services, contributing to biomass flow, food web connectivity and population control, as well as driving the evolution of other species [4,5,6,7]. Furthermore, obligatory dependence of parasites on their hosts can make these organisms vulnerable to environmental changes, even before their hosts are at risk of extinction [8, 9]. This is because the composition of the fish endoparasites community depends on several factors related to the environment (low water quality, changes in pH, oxygen dissolved level, variations in temperature, water level and seasonality effects) and their hosts (feeding behavior, physiology, age, sex and biology) [10].
Furthermore, seasonality is also an important factor in structuring the parasite community, in the case of the Amazon, periodic floods and droughts are major forces coordinating the lowland systems [11]. Biological and biochemical exchanges occur between aquatic and terrestrial environments determining productivity, reproduction and population dynamics of aquatic organisms, as well as consumer-resource interactions [12]. Thus, understanding how seasonal and environmental variation influences the dynamics of parasite infection is necessary to better understand, for example, the impacts caused by human actions [13, 14].
Anthropic changes are transforming seasonal cycles and environmental characteristics, which can impact host physiology and phenology, on the one hand, and temporal peaks in the epidemiological dynamics of the parasite, on the other [15]. Furthermore, these impacts can be particularly pronounced in aquatic ecosystems [16,17,18,19]. Fish parasites can face a dual threat and be directly vulnerable to extinction due to climate change or invasive species and indirectly vulnerable through host co-extinction [20,21,22]. These organisms also react to different specific environmental conditions, such water quality variation, environmental stress and pollution [23,24,25].
The choice of hosts to assess the environmental, seasonal and human influence is fundamental to understand how the endoparasite fauna responds to these factors. Thus, selecting hosts with different feeding habits can be important, as the diet of these organisms influences and reflects the presence of endoparasites in environments [26]. For example, detritivorous species consume organic matter, algae, detritus and microorganisms [27] and thus may ingest intermediate hosts of endoparasites [28]. Omnivorous hosts are opportunistic, feeding on a wide variety of items including fish, detritus, crustaceans, seeds, fruits, leaves, insects and mollusks [28, 29], which make them suitable hosts for endoparasites in different environments [30, 31]. However, piscivorous hosts are dominant consumers of intermediate fish within the food web, so they can present a high load of parasites due to their high trophic level [3, 32].
In this context, the present study aimed to evaluate the influence of environmental and seasonal factors in anthropized and conserved areas on the endoparasite community structure in fish at different trophic levels. The following hypotheses were tested: (i) endoparasites show greater species richness and diversity in conserved environments, and greater abundance and dominance in anthropized areas, regardless of trophic characteristics of the hosts. The high diversity of parasites is found in conserved sites, while anthropized environments present a greater abundance of more opportunistic organisms [19, 33, 34]. (ii) Endoparasites found in fish, mainly trematode species (Digenea), are indicators of conserved environments. The disturbance of aquatic environments can negatively influence the intermediate hosts of certain parasites, in addition to inhibiting the reproductive physiology [35] and the encysting process of some helminth species [36], altering the behavior of individuals with free-living stages, such as digeneans, impairing locomotion and the ability to find hosts [37]. (iii) and, drought and flooding periods and environmental characteristics are responsible for influencing the endoparasite community structure in conserved environments. This may occur because the natural floodplain system presents a dynamic structure mainly maintained by fluctuations in the water level, affecting the dynamics of definitive and intermediate hosts, and consequently, the structure and composition of their parasites [11, 38]. However, in anthropized environments, as in the present study, it is expected to find similar temporal distribution patterns in the endoparasite community, because human activities can reduce allochthonous food sources, increase silting, alter the flow and cause eutrophication of aquatic systems [39], negatively affecting rare and more sensitive species and reducing species variability in the community of endoparasites [40].
Materials and Methods
Study Area
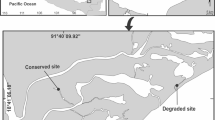
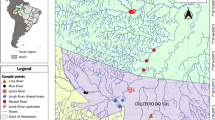
The study was carried out in the Upper Juruá River region, Western Amazon, near municipalities of Cruzeiro do Sul, state of Acre, and Guajará, state of Amazonas, Brazil (07° 37′ 52″ S and 72° 40′ 12″ W). Six sampling sites were selected and grouped in conserved environments, that is, places with dense vegetation, but used by man for extraction or use of natural resources, and anthropized environments, which present urban areas, roads, rural areas and small forest fragments. To categorize the environments, the Rapid Habitat Diversity Assessment was used according to Callisto et al. [41] for each sampling site. This rapid habitat diversity assessment protocol assesses the characteristics of stream sections and the level of environmental impacts from human activity, based on the protocol proposed by the Ohio Environmental Protection Agency (U.S. EPA, 1987). This document is represented by 10 (ten) parameters: 1–type of occupation of watercourse banks (main activity); 2–erosion near and/or on the banks of the river, silting in its bed; 3–anthropogenic changes; 4–vegetation cover on the bed; 5–odor in the water; 6–oiliness of the water; 7–water transparency; 8–sediment odor (bottom); 9–oiliness of the bottom; 10–type of bottom. Each parameter has 3 criteria for assigning the score, which can be 4, 2 or 0 points, depending on environmental conditions and assignment of the evaluator. The studied environments with anthropized characteristics were: (i) downstream and (ii) the Juruá River (7°40′34.1″S 72°39′39.5″W), under a high degree of degradation, located in the urban center, highways, rural areas and preserved fragments; and (iii) Môa River (7°37′18″S 72°47′47″W) presented deforested areas with roads, urban part and the presence of pastures, suffers from the effect of removal of sand from its remnants, but presented fragments of conserved forests. The conserved environments were: (i) Crôa River (7°71′48.30″S 72°53′34.98″W), which presented rural stretches and logging; the conserved stretches were used by the community for ecotourism activities; (ii) Paranã River (7°17′13″S 72°36′49″W) has areas subjected to logging, but with stretches of preserved vegetation where a riverside population lives; and (iii) Gama River (7°37′13″S 72°16′49″W), an area subjected to logging and farm implantation, but has stretches with a high degree of conservation (Fig. 1).
Sampling
Fish were caught (SISBIO—Authorizations for activities with a scientific purpose 59,642-2/2019) from March 2019 to April 2021, during the periods of drought (May, June, August and September) and flooding (February, March, November and December). In each region of the sub-basins, three conserved and three anthropized sites were selected, the total sampled area was 14 km2, including the main river, lakes and streams surrounding these areas.
Passive fish collections were conducted using 12 gill nets with 80 m in length and 3.0 m in height, with mesh sizes of 1.5 cm, 2.5 cm, 3.5 cm, 5.5 cm between opposite knots, in areas of rivers, lakes and streams. Nets were set in the early afternoon, remaining exposed for 24 h. Inspections were carried out every 4 h, in which samples were obtained for the morning, afternoon and night periods. Active collections were performed with a trawl net of 25 m in length and 2.5 m in height; nets were trawled along the banks of lakes, rivers and streams. A 12 m in length and 1.8 m in height cast net was also used for sampling, for 24 h; at every 4 h, six casts were carried out on the bank, six in the water flow and six in backwater areas.
Fish caught were identified according to literature [27, 42, 43], length (cm), Weight (mg) and necropsied in situ. Some individuals, after biometry evaluation, were fixed in 10% formalin and taken to the laboratory, where they were deposited in the Núcleo de ictiologia do vale do Juruá (NIVAJ), Universidade Federal do Acre.
Twelve species of host fish were selected according to their trophic characteristics found in the literature including [3, 27, 29, 32], three detritivorous, three omnivorous, three piscivorous and three invertivorous (Table 1).
Collection and Analysis of Parasite
Fish were fresh necropsied for endoparasite collection. Internal organs of fish were removed and individually separated in Petri dishes containing 0.65% sodium chloride solution. Endoparasites were placed in Petri dishes and observed under a stereomicroscope. The Cestoda, Nematoda, Acanthocephala, and Pentastomida found were fixed in 5% formaldehyde and preserved in 70% alcohol at 65 °C. Digenea were fixed by slight compression between the slide and the coverslip in heated 70% alcohol. Digenea, Cestoda and Acanthocephala were stained in Langeron's carmine, dehydrated by an increasing alcohol series, from 70 to 100% alcohol, cleared in phenol and beech creosote, and then mounted between a slide and coverslip in Canada balm. Nematodes and pentastomids were cleared and mounted on semi-permanent slides in phenol. Helminths were identified according to Travassos et al. [44], Thatcher [45], Moravec [46], Martins and Yoshitoshi [47], Jones et al. [48], Giesen et al. [49], and Miller and Cribb [50].
Environmental Variables
The environmental variables (supplementary material 1) pH, electrical conductivity (µS.cm), water temperature (˚C), dissolved oxygen (mg.L), turbidity (NTU), total dissolved solids (TDS) and chlorophyll α were measured during the 24 h of collection in the margin, middle and bottom regions using a multiparameter probe. A Secchi disk was used to measure the transparency (cm) and depth profiles (m) of aquatic environments. Water samples for physical-chemical analysis were taken using a Van Dorn bottle and stored for analysis. Analyses of physical and chemical variables were carried out using a spectrophotometer, according to the methods proposed by Apha, 2012 [51] for analysis of zinc (zinc method); nitrite (N 202 (1-naphthyl)-ethylenediamine (NTD) method), nitrate (N-(1-naphthyl)-ethylenediamine (NTD) method), total nitrogen (persulfate method), ammonia nitrogen (indophenol method), total fhosphate (ascorbic acid and molybdenum blue method) and soluble orthophosphate (ascorbic acid and molybdenum blue method).
The water level and river flow were measured using rulers from the stations of the Agência Nacional das águas (ANA), upstream of the sampling sites. Rainfall, temperature and humidity data for the region were obtained from INMET (Instituto Nacional de Meteorologia) data for the years 2019 to early 2021.
Data Analysis
Prevalence, intensity and mean abundance of endoparasite populations were determined according to Bush et al. [52]. The following descriptors, based on the structure of infracommunities, were calculated: abundance, richness, Shannon–Wiener diversity and Berger–Parker dominance. Parametric analysis of variance (ANOVA) was applied to test for significant differences in abundance, richness, diversity, dominance of endoparasites and environmental variables between anthropized and conserved environments in different hydrological periods, the Tukey’s post-hoc test was applied to evaluate the difference between the sites. Assumptions of normality and homoscedasticity were met.
Principal Coordinate Analysis (PCoA) was summarized to assess the dissimilarity of endoparasites found in piscivorous, omnivorous, detritivorous and invertivorous host fish, between environments and seasonal periods [53]. A multivariate permutational analysis of variance (PERMANOVA) was performed to assess changes in endoparasite species composition between sampling sites. A total of 999 permutations were run to assess significance, paired PERMANOVA was used to assess for significant differences between sites.
In order to determine which species were indicators of environmental conditions between anthropized and conserved environments, the Indicator Value Index (IndVal) was applied [54]. The indicator value of a species can range from 0 to 100, reaching its maximum when all individuals of a species occur at all sites within a single group, the significance value of the indicator was tested for each species with a test of Monte Carlo with 4999 permutations.
Pearson correlation coefficient “r” was estimated to determine possible correlations between physical and chemical variables and the richness, diversity and abundance of endoparasites between the anthropized and conserved sites. To check for differences in physical and chemical variables between environments, during the periods of flooding and drought, and influence on the distribution of endoparasite species, a Canonical Correspondence Analysis (CCA) was performed. Matrices were log-transformed to homogenize the values of the variables, except for pH, and the effect of rare species was not removed, since for parasites, rare species can provide site-specific information. Subsequently, a Monte Carlo test with 999 permutations was run to test the significance of CCA axes [53]. Statistical analyses were performed in software R 3.2.4 (R Development Core Team 2018), using the vegan [55] and permute [56] packages for PCoA and according to the "ADONIS" function of the vegan package [55] for PERMANOVA. The level of statistical significance adopted was p ≤ 0.05.
Results
Fish Endoparasite Fauna
In total, 5832 endoparasites were found, belonging to 61 species, being 26 Digenea, four Cestoda, 20 Nematoda, nine Acanthocephala, and two Pentastomida,
In conserved environments, during the flooding, a total of 1240 endoparasites belonging to 39 species were found, 11 Digenea, 1 Cestoda, 18 Nematoda, seven Acanthocephala, and two Pentastomida. The highest prevalence was observed for Dadaytrema oxycephalum Diesing, 1836, while the highest mean abundance and mean intensity was for Cosmoxynema vianai Travassos, 1949 and Cosmoxynemoides aguirrei Travassos, 1948, in detritivorous fish. As for omnivorous fish, Sharpilosentis peruviensis Lisitsyna, Scholz and Kuchta, 2015 was the parasite with the highest prevalence, mean abundance and mean intensity. Among piscivorous, the highest prevalence, abundance and mean intensity were registered for Prosthenhystera obesa Diesing, 1850 and Bellumcorpus majus Kohn, 1962. Among the invertivorous, P. obesa and Crassicutis cichlasomae Manter, 1936 were the most prevalent, with greater mean abundance and mean intensity. In conserved environments, during the drought period, 1319 endoparasites belonging to 53 species were observed, including 23 Digenea, four Cestoda, 17 Nematoda, seven Acanthocephala, and two Pentastomida. Among detritivorous hosts, Paramphistomidae gen. sp. and Cucullanus pinnai pinnai Travassos, Artigas and Pereira, 1928 were the parasites with the highest prevalence and C. aguirei, with the highest prevalence and mean abundance. Among omnivorous hosts, the highest prevalence was observed for Dadaytremoides parauchenipteri Lunaschi, 1989, S. peruviensis and D. oxycephalum. Among piscivorous fish, Posthodiplostomum sp. and Procamallanus inopinatus Travassos, Artigas and Pereira, 1928 were the most prevalent, and Austrodiplostomum sp. showed the highest mean intensity. For the invertivorous species, C. cichlasomae and Clinostomum sp. were the most prevalent, and Ithyoclinostomum dimorphum Diesing, 1850 had the highest mean intensity (Table 2).
In anthropized areas, during the flooding, 1358 endoparasites were collected and during the drought, 1575 were collected. During these two periods, the richness was 27 species, including 11 Digenea, 12 Nematoda and five Acanthocephala. During the flooding, among detritivorous fish, Gorytocephalus elongorchis Thatcher, 1979 and Contracaecum sp. showed the highest prevalence, intensity and mean abundance. During the drought, Neoechinorhynchus curemai Noronha, 1973 was the parasite with the highest prevalence and mean abundance, and Contracaecum sp. had the highest mean intensity. Among omnivorous hosts, D. parauchenipteri and C. viana were the most prevalent species with the highest mean abundance, and Rondonia rondoni Travassos, 1920 presented the highest mean intensity during the flooding. In the drought, Austrodiplostomum sp. and S. peruviensis were the parasites with the highest prevalence, abundance and mean intensity. P. inopinatus, Contracaecum sp., B. majus and Austrodiplostomum sp. were the most prevalent endoparasite species during the flooding and drought among piscivorous fish. In both periods, Austrodiplostomum sp. showed the highest mean intensity. Among the invertivorous, in both periods, Procamallanus peraccuratus Pinto, Fabio, Noronha and Rolas, 1976 and I. dimorphum were the parasites with the highest prevalence and mean abundance (Table 2).
For the endoparasite fauna of detritivorous hosts, species richness was significantly higher in conserved environments (ANOVA p = 0.003). The difference occurred between the environments in the drought (Tukey-p = 0.002) and in the flooding (Tukey-p = 0.003) season. The same was observed in omnivorous fish (ANOVA p = 0.004) between environments in different seasonal periods (Drought: Tukey-p = 0.02; Flooding: Tukey-p = 0.001). For piscivorous and invertivorous, the difference in richness (ANOVA p = 0.01) occurred during the drought between anthropized and conserved environments (Tukey-p = 0.01) (Table 3). Differences in the number of individuals of endoparasites were found between anthropized and conserved environments in the fauna of detritivorous (ANOVA p = 0.02), omnivorous (ANOVA-p = 0.002) and piscivorous (ANOVA p = 0.02) fish. For detritivorous, the difference occurred between environments during the flooding season (Tukey-p = 0.001) and between anthropized areas in the drought and flooding season (Tukey-p = 0.02). For omnivorous and piscivorous, the difference in abundance of endoparasites was observed between conserved and anthropized environments in the flooding season (Tukey-p < 0.05) and between conserved environments, in the flooding, and the anthropized environments, in the drought (Tukey-p < 0.05) (Table 3). The lowest diversity of endoparasites in detritivorous fish was verified in the drought in anthropized environments and showed a significant difference (ANOVA p = 0.001) from conserved environments in both seasonal periods (Tukey-p < 0.05), there was also a difference in diversity between anthropized environments in the drought and flooding season (Tukey-p < 0.05). For piscivorous hosts, the difference was seasonal (ANOVA p = 0.002), between the drought and flooding environments (Tukey-p = 0.001). As for invertivorous fish, the diversity of endoparasites was different between anthropized and conserved environments during flooding (Tukey-p = 0.001) and drought (Tukey-p = 0.004) (Table 3). For endoparasite dominance, the difference was detected in piscivorous (ANOVA p = 0.001) and invertivorous (ANOVA p = 0.02) hosts. In piscivorous hosts, the difference was observed between anthropized and conserved environments during the flooding, whereas for invertivorous, the dominance was higher in anthropized environments compared to conserved environments in both periods (Tukey-p < 0.05) (Table 3).
The species composition of endoparasites in omnivorous, piscivorous (Fig. 2), detritivorous and invertivorous fish (Fig. 3) showed variability between environments in different sampling season. In endoparasites of piscivorous (PCoA: p = 0.001) fish, differences were detected between conserved and anthropized environments during the flooding (PCoA: p = 0.01); conserved environments in the flooding and anthropized in the drought (PCoA: p = 0.002). Gorytocephalus elongorchis (IndVal = 0.682; p = 0.02), G. genarchella (IndVal = 0.612; p = 0.02) and A. compactum (IndVal = 0.732; p = 0.03) were the indicator species of conserved environments, which influenced the variations.
Principal coordinate analysis (PCoA) showing the variability in species composition of endoparasites of omnivorous and piscivorous fish between conserved and anthropized sampling sites in the periods of flooding and drought. A Piscivorous; B omnivorous. 1—Conserved environment (Flooding); 2—conserved environment (Drought); 3—anthropized environment (Flooding); 4—anthropized environment (Drought)
Principal coordinate analysis (PCoA) showing the variability in species composition of endoparasites of detritivorous and invertivorous fish between conserved and anthropized sampling sites in the periods of flooding and drought. A Invertivorous; B detritivorous. 1—Conserved environment (Flooding); 2—conserved environment (Drought); 3—anthropized environment (Flooding); 4—anthropized environment (Drought)
For omnivorous hosts, there was also a difference in endoparasite composition (PCoA: p = 0.001), the difference occurred between environments in the flooding (PCoA:p = 0.01), the conserved environments in the flooding and anthropized environments in the drought (PCoA:p = 0.002). The indicator species influencing the variability between environments were P. inopinatus (IndVal = 0.698; p = 0.02), A. compactum (IndVal = 0.567; p = 0.02), in anthropized environments, C. pinnai (IndVal = 0.582; p = 0.02) and Contracaecum sp. (IndVal = 0.657; p = 0.01) in conserved environments.
For detritivorous hosts, the difference occurred (PCoA:p = 0.001) between anthropized and conserved environments in flooding (PCoA:p = 0.02) and drought (PCoA: p = 0.01) periods. The species that indicated this variability were P. inopinatus (IndVal = 0.763; p = 0.03) and Monticellia sp. (IndVal = 0.687; p = 0.02), in anthropized areas and N. travassossi (IndVal = 0.568; p = 0.01) and C. pinnai (IndVal = 0.654; p = 0.01) in conserved environments.
As for endoparasites in invertivorous fish, the differences occurred (PCoA: p = 0.001) between environments during the flooding (PCoA: p = 0.03), conserved environments during the flooding and anthropized environments during the drought (PCoA: p = 0.02) and between environments during the drought (PCoA: p = 0.01). Endoparasite species influencing this variation were P. peraccuratus (IndVal = 0.622; p = 0.01), C. manteri (IndVal = 0.622; p = 0.02) and C. cihlasomae (IndVal = 0.672; p = 0.03) in conserved environments, I. dimorphum (IndVal = 0.592; p = 0.03) and Contracaecum sp. (IndVal = 0.692; p = 0.03) in anthropized environments.
Species Composition and Environmental Variables
Variables of electrical conductivity, pH, TDS, nitrite, nitrate, orthophosphate, zinc, phosphate, phosphorus, chlorophyll α and nitrogen variables were higher in anthropized environments. And dissolved oxygen presented higher content in conserved environments. As for the level and flow of water, values were high during the flooding in all environments (supplementary material 1).
During the flooding season, richness and diversity of endoparasites in piscivorous fish showed a positive correlation with chlorophyll α and the level of dissolved oxygen and a negative correlation with river flow in conserved environments. In the same seasonal period, in anthropized environments, the oxygen level showed a correlation with richness, diversity and abundance of endoparasites. During the drought, in anthropized environments, pH and TDS showed a positive correlation with the endoparasite abundance in piscivorous hosts. For the invertivorous fauna, in conserved environments, during the flooding, richness and diversity of species indicated a correlation with oxygen and river water level, in addition, the species diversity showed a positive relationship with Chlorophyll α, and the richness, a negative correlation with water flow. The endoparasite abundance was also negatively correlated with zinc levels in these environments during the flooding period, and with chlorophyll α in the drought.
In anthropized areas, during the drought, the levels of dissolved oxygen were correlated with the richness and abundance of endoparasites in invertivorous fish (Table 4). Total nitrogen showed a positive correlation with the richness and diversity of endoparasites of omnivorous hosts, in anthropic environments in the drought, and a negative correlation in conserved environments during the flooding (Table 4). Total phosphorus showed a positive correlation with richness and abundance of endoparasites in omnivorous hosts in the drought, and a negative correlation with diversity in conserved environments during the flooding (Table 4). This environment during the flooding also showed a correlation between the abundance of endoparasites and the levels of dissolved oxygen. For detritivorous, richness, diversity and abundance of endoparasites showed negative relationships with chlorophyll α, conductivity, river water level, water flow, zinc and nitrogen during drought in anthropized environments (Table 4). In conserved environments, during the flooding, the diversity of endoparasites in detritivorous fish was negatively related to total phosphorus and during the drought, to zinc concentration (Table 4).
The ordination indicated that between environments, the first two axes explained 76.7% distribution of the endoparasite fauna in invertivorous fish. The main environmental variables indicating the correlation between sampling sites and the parasite endofauna were conductivity, oxygen, pH, TDS and zinc. Dissolved oxygen and pH influenced species composition in anthropized and conserved environments, where the related species were S. peruvensis and C. manteri in conserved environments, whereas I. laterifilamenta and C. aguirre, in anthropized environments. Conductivity, TDS and zinc negatively influenced the anthropized sites between the two periods, in which the correlated parasite was I. dimorphum (Fig. 4; Table 5).
Ordination of environments and species of endoparasites in invertivorous hosts by Canonical Correspondence Analysis (CCA). A Ordination of environments between seasonal periods and limnological characteristics. B Ordination of species and limnological characteristics. Chlo chlorophyll α; Temp temperature; Cond conductivity; Ox oxygen; TDS total dissolved solids; TP total phosphorus; Z zinc
The two ordination axes explained 71.1% distribution of endoparasites found in omnivorous hosts, influenced by conductivity, temperature, TDS and zinc, in anthropized areas, mainly during the drought period. The related species were D. oxycephalum and P. peraccuratus. Species distribution was also positively influenced by total phosphorus and negatively influenced by oxygen level, water flow and river water level in conserved areas in both periods the main correlated species were R. rondoni, P. obesa and Contracaecum sp. (Fig. 5; Table 5).
Ordination of environments and species of endoparasites in omnivorous hosts by Canonical Correspondence Analysis (CCA). A Ordination of environments between seasonal periods and limnological characteristics. B Ordination of species and limnological characteristics.; Chlo chlorophyll α; Cond conductivity; Ox oxygen; TDS total dissolved solids; Temp temperature; Lev river level; Flo flow; NT total nitrogen; PT total phosphorus; Z zinc
The ordination axes explained 61.4% distribution of the endoparasite fauna in detritivorous hosts. The chlorophyll α content, conductivity, zinc, TDS and temperature were the environmental variables that influenced the distribution in anthropized areas during the periods of flooding and drought, in which P. inopinatus and Monticellia sp. were the species that influenced this correlation (Fig. 6; Table 5).
Ordination of environments and species of endoparasites in detritivorous hosts by Canonical Correspondence Analysis (CCA). A Ordination of environments between seasonal periods and limnological characteristics. B Ordination of species and limnological characteristics. Chlo chlorophyll α; Cond conductivity; Ox oxygen; TDS total dissolved solids; Temp temperature; Lev river level; Flo flow; NT total nitrogen; PT total phosphorus; Z zinc
For the distribution of endoparasites in piscivorous hosts, the axes explained 72.0% variation, influenced by the environmental variables chlorophyll α, conductivity, TDS and temperature. Posthodiplostomum sp. was the main correlated species in anthropized areas (Fig. 7; Table 5).
Ordination of environments and species of endoparasites in piscivorous hosts by Canonical Correspondence Analysis (CCA). A Ordination of environments between seasonal periods and limnological characteristics. B Ordination of species and limnological characteristics. Chlo chlorophyll α; Cond conductivity; Ox oxygen; TDS total dissolved solids; Temp temperature; Flo flow; NT total nitrogen; PT total phosphorus; Z zinc
The Monte Carlo test applied to ordination axes showed that the correlation between environmental variables and the species involved was significant for the set of CCA axes (p < 0.001).
Discussion
Richness, Diversity, Abundance and Composition of Endoparasites
The present study showed a greater richness and diversity of endoparasites in conserved environments, whereas anthropized environments presented a greater abundance and lower richness of endoparasites in hosts at different trophic levels. In response to human activities, fish parasite communities may increase or decrease in prevalence, abundance and diversity [57]. According to Marcogliese [58], diversity and richness of endoparasite species can reduce in response to environmental degradation. These reductions in parasite richness are believed to parallel the loss of species diversity with free-living stages, such as Digenea, and part of the populations of intermediate hosts that are impacted by environmental changes [19, 25]. Lafferty [57], predicted that some Digenea species may be sensitive to anthropic disturbances, which may explain the greater richness of these endoparasites in conserved environments when compared to the anthropized environments of the present study.
The results also supported the hypothesis that conserved areas present a variation in the richness of taxa between the seasonal periods. The study indicated that the greatest richness of Digenea was found during the drought period and decreased during the flooding, when Nematoda was the taxon with the highest species richness. In anthropized areas, the richness of endoparasites remained constant between the two seasonal periods. This is probably related to the fact that in conserved natural environments, the flood regime directly or indirectly influences the distribution of species, influencing the increase or decrease in richness of certain aquatic organisms [11], such as fish parasites. According to Yamada et al. [59], it is possible that the flooding conditions imposed on ecosystems may lead to differences in the levels of parasite infections, depending on the taxonomic group and the availability of intermediate and/or definitive hosts. The flooding period may favor the life cycle of some parasites, such as nematodes [60], as infections by some nematode species during this period may be associated with the seasonal dietary composition of their hosts [46, 61]. The fact that drought favors some species of digeneans may be related to the transportation of small, mobile, parasite-free stages out of the aquatic ecosystem by large floods, due to increased water flow, and thus reducing the richness of these parasites [62, 63]. In addition, during the dry season, the reduction in river water level increases the density of invertebrate and fish communities [64]. This can induce the overlap of intermediate and definitive hosts in a shrunken environment [65], facilitating the transmission of parasites with complex life cycles, such as digeneans [60]. However, in anthropized environments, environmental conditions may not be favorable for the occurrence of certain species of parasites. Because, in addition to environmental degradation reducing the host fauna, it can negatively influence the biotic characteristics of ecosystems, allowing only the presence of opportunistic, generalist species with low host specificity, which manage to complete their life cycle in both seasonal periods [66, 67], which may explain the low variation in species richness in these environments.
The present study showed a contrast in the endoparasite fauna of detritivorous fish between conserved and anthropized environments, where there was a higher prevalence of Digenea in conserved environments, mainly species of the family Cladorchiidae. As this group of hosts ingest large amounts of organic matter from the sediment [68], they may have ingested some of these organisms in the free-living stage [6]. These endoparasites of the family Cladorchiidae encyst in vegetation until they are predated upon by potential definitive hosts, for example, herbivorous, detritivorous and omnivorous fish [45]. Nevertheless, in anthropized environments during periods of flooding and drought, detritivorous hosts showed higher prevalence, abundance and intensity of infection by G. elongorchis and N. curemai, in addition to Contracaecum sp.. This does not mean that these endoparasite species occur in these hosts only in anthropized environments, as these parasites are commonly found in Prochilodontidae and Curimatidae fish in conserved areas, as observed here and in other studies [69,70,71]. In fact, the present study demonstrated that anthropic activities can induce an imbalance in the infection by certain species of endoparasites. This is because, in anthropized systems, the high input of nutrients can lead to a disproportionate growth of intermediate hosts, such as ostracods, and increase parasitic infection by acanthocephalans [72,73,74,75]. Furthermore, it can result in an increase in Contracaecum populations in wild fish populations [76, 77].
Endoparasites of omnivorous hosts indicated similar prevalence in conserved and anthropized environments, during the flooding period. However, there was greater abundance and intensity of infection of R. rondoni nematodes in anthropized areas. This parasite is known to occur in different fish species and river systems at high intensities [78,79,80]. They are viviparous parasites and their direct life cycle can allow the dissemination of numerous eggs with several filaments and larvae in the marginal vegetation of water bodies [81]. As anthropized aquatic environments can favor the dissemination of some species of aquatic plants [82], these micro-habitats become environments favorable to the reproduction of these nematodes, which can colonize omnivorous fish that forage in these environments [83].
On the other hand, endoparasites in piscivorous and invertivorous hosts showed high richness and diversity in the drought season in conserved environments. During the drought, invertebrate and fish communities may present higher diversity due to the reduction of river levels and hydrological disconnection of some environments in floodplain areas [64]. This can induce an increase in density, overlapping of intermediate and definitive hosts in a reduced environment [65], facilitating the transmission of parasites with a complex life cycle [60]. In anthropized environments, especially during the drought season, there was an increase in the dominance of endoparasites in piscivorous and invertivorous hosts. An expected pattern in these areas, as environmental degradation induces a change in community structure towards dominance of tolerant species [84, 85]. Thus, richness decreases as a result of the disappearance of taxa as the level of environmental degradation increases and the number of sensitive species is reduced, while the number of tolerant species may increase [19, 57].
The piscivorous and invertivorous host fish showed high prevalence and mean abundance of Posthodiplostomum sp. and Clinostomum sp. in conserved environments and Austrodiplostomum sp. and I. dimorphum in anthropized areas. Although studies indicate that water quality is an important factor for the infection of parasite species of the family Diplostomidae [86] Austrodiplostomum sp. stood out in the present study for being present in both conserved and anthropized environments. Other factors may be influencing these metacercariae in these environments, first is the generalist characteristic of these species, as the ability to infect different hosts can facilitate the permanence and proliferation of these parasites under adverse environmental conditions [31, 87, 88]. The second factor may be related to the increase in parasite load of metacercariae in eutrophic environments, as the concentration of nutrients in this region can influence the increase of some species of tolerant invertebrates that serve as food for intermediate hosts of these species [89].
Adult digeneans, such as P. obesa, C. cichlasomae, D. parauchenipteri and B. majus, found in piscivorous, omnivorous and invertivorous fish occurred, mainly in conserved environments. This suggests that these environments present autogenic endoparasite species and these fish may be playing an important role as definitive hosts. However, in all anthropized areas of the present study, P. obesa, C. cichlasomae were not observed, which may suggest that some autogenic species may be more susceptible to local extinction [90]. For example, a study showed that P. obesa disappeared after anthropic actions in the Paraná River [59], that is, the increase in anthropization can destabilize the parasite community, mainly some autogenic species. Because these organisms complete their entire life cycle within the limits of an aquatic ecosystem, and may not be able to colonize other environments in time, as in the case of allogenic species [91].
The composition of endoparasites in piscivorous, omnivorous, invertivorous and detritivorous hosts were dissimilar between anthropized and conserved areas in different seasonal periods. Thus, it was evidenced that the seasonality influenced the endoparasite community, as suggested in other fish parasite studies [92,93,94]. It is well established that the hydrological regime and the degree of environmental conservation are important factors in controlling environmental heterogeneity, and consequently in organizing communities in floodplain systems [95, 96].
The indicator endoparasite species influencing the variation in the infracommunity of omnivorous and detritivorous hosts were P. inopinatus in anthropized environments and C. pinnai pinnai, in conserved environments during the flooding period. These nematode species were also found in fish species from the Amazon region, mainly during the flooding period [97,98,99]. This may occur because during flooding and flood in the Amazon, environmental conditions are more favorable for some aquatic organisms, so there are a large number of individuals influencing the occurrence of infective larval forms in their hosts [100]. The endoparasite P. inopinatus is a generalist species found in different families of fish at different trophic levels, including detritivorous and omnivorous species, which ingest a wide variety of food items [101, 102]. This species has already been found infecting Astyanax paranae Eigenmann, 1914 only in highly polluted areas, indicating that this nematode can be used as a bioindicator of anthropized areas [103]. The nematode C. pinnai pinnai may also have low host specificity, and can parasitize several fish species [79, 80, 104]. It can be found in omnivorous or invertebrate-predator fish that feed mainly on aquatic insects [105]. As the diversity and richness of aquatic insects are greater in conserved environments [106], this may justify the presence of this Nematoda in these places.
Gorytocephalus elongorchis and G. genarchella were the indicator species contributing to the variation of endoparasite fauna in piscivorous hosts in conserved rivers during flooding. The endoparasite C. maintaini contributed to the dissimilarity of the parasite endofauna of invertivorous, also in conserved environments. This may indicate that these species found environmental conditions, as well as intermediate and definitive hosts, to complete their life cycles. The transmission of endoparasites with a complex life cycle and free-living stage can be considered a good environmental indicator for these environments [18, 58, 107, 108].
Environmental Variables and Endoparasites
The present study indicated the variation in environmental factors during the hydrological cycle periods influenced the richness, diversity, composition and abundance of the endoparasite fauna of fish between environments with different degrees of conservation.
The variation in chlorophyll α in environments of the present study, influenced the diversity, richness and abundance of endoparasites of piscivorous and invertivorous hosts in conserved environments. This environmental factor also determined the variation in the composition of endoparasites in piscivorous hosts. The presence of chlorophyll α in floodplains indicates a good source of phytoplankton contributing to the diet of diverse organisms, such as zooplankton, containing abundant species of diatoms and green algae [109,110,111]. Aquatic insects feed on plankton and attract intermediate consumer fish, which serve as food for piscivorous fish. Birds consume piscivorous fish, and so endoparasites can complete their life cycle. This means that environmental factors, such as chlorophyll α, model host assemblages which in turn contribute to the maintenance of parasite assemblages [112]. Nevertheless, in anthropized environments of the present study, chlorophyll α showed high concentration and negative correlation with the richness and diversity of endoparasites in detritivorous hosts, as well as the high concentration of nitrogen, total phosphorus and zinc. The diversity and richness of endoparasites in omnivorous fish also responded negatively to the concentration of phosphorus and nitrogen in the environments of this study. In fact, it has been suggested that unfavorable environmental conditions affect some species of parasites in anthropized environments with excess chlorophyll α, nitrogen, phosphorus, among other nutrients [33, 113].
The composition of some species of detritivorous, piscivorous, invertivorous and omnivorous parasites in the present study were influenced by the high concentration of total solids (TDS), temperature and electrical conductivity in anthropized environments. High conductivity occur in environments with high TDS concentration and temperature, and indicate disturbed environments [114]. According to [115, 116], waters with high conductivity are more productive and, therefore, harbor some invertebrates that are intermediate hosts for endoparasites and allow some species to succeed. In addition, the higher temperature during drought in anthropized environments may favor the development of certain metacercariae species [117, 118] as observed in the present study, where the metacercariae I. dymorfum and Posthodiplostomum sp. were related to these environments and environmental factors.
The present study indicated that pH was more alkaline in anthropized areas during the drought period, and influenced the abundance of parasites in piscivorous hosts and the composition of endoparasites in invertivorous species. Where the nematodes C. aguirre and P. inopinatus were the most correlated endoparasite that influenced this correlation. In floodplain regions, studies on anthropized aquatic environments indicated that pH increases during algal blooms in dry season due to photosynthesis, which may result in increased nutrient release [119, 120]. This may favor the presence of some species of copepods, which are intermediate hosts of endoparasites such as C. aguirre and P. inopinatus [102, 106, 121, 122], which may explain this relationship. The present study also showed that zinc found in anthropized areas influenced the composition of endoparasites in omnivorous, invertivorous and detritivorous hosts. Some studies have shown that zinc generate a direct negative effect, especially in parasite-free life stages [123, 124].
The increase in the level of dissolved oxygen positively influenced the diversity and richness of endoparasites in piscivorous and insectivorous hosts, and also the abundance of parasites of omnivorous fish in anthropized environments during the flooding season. The flood pulse influences the abiotic environment, mainly oxygen levels [125], which is one of the environmental parameters exerting a direct effect on fish growth and production and an indirect effect on nutrient [126]. This may justify its positive correlation with the richness and diversity of endoparasites in several studies [127, 128]. This variable also explained the species composition of endoparasites of omnivorous and invertivorous in conserved environments in periods of drought and flooding, in which C. manteri, D. oxycephalum and P. obesa were the affected parasites. Dissolved oxygen can contribute to the life cycle of Digenea species by aiding the energy metabolism of these organisms [129,130,131].
The rise in river water level and flow negatively influenced the richness and diversity of endoparasites in piscivorous hosts, and positively in invertivorous fish. The diversity of zooplankton and other invertebrates is greater during the flooding, and provides fish with better feeding conditions [60, 68]. This may have influenced the fauna of invertivorous in the present study. These hosts belong to the family Cichlidae, according to Tavares-Dias et al. [60], some species of this family had higher helminth infections during the flooding due to increased availability of food resources. This influenced the increased ingestion of infectious stages of these trophically transmitted endoparasites. Regarding endoparasites of piscivorous hosts, the present study suggests that the reduction in richness and diversity in these hosts should be associated with a reduction in the consumption of some species of parasitized fish. According to Luz-Agostinho et al. [132], during the flooding, the dispersion of aquatic biota occurs by increasing the water level reducing the concentration of prey, such as fish at lower trophic levels, and thus reducing food consumption for these piscivorous fish. As a result, the hydrological cycle should affect interspecific relationships, particularly predation. Thus, flooding increases the number of shelters and reduces the density of prey, which can influence the fauna of parasites trophically transmitted to piscivorous hosts.
Conclusions
In conclusion, endoparasites showed higher species richness and diversity in conserved environments and greater abundance and dominance in anthropized areas. The periods of drought and flooding were responsible for influencing the endoparasite community structure in conserved environments. In anthropized areas, the distribution patterns of the endoparasite community between seasonal periods were similar. In addition, Digenea species were indicators of conserved environments, and the more generalist metacercariae were indicators of anthropized environments. Environmental and host variables in a floodplain system can influence the richness, diversity, composition and abundance of endoparasites in hosts at different trophic levels.
Data availability
Not applicable.
References
Beevi MR, Radhakrishnan S (2012) Community ecology of the metazoan parasites of freshwater fishes of Kerala. J Parasit Dis 36:184–196. https://doi.org/10.1007/s12639-012-0101-8
Luque JL, Poulin R (2008) Linking ecology with parasite diversity in Neotropical fishes. J Fish Biol 72:189–204. https://doi.org/10.1111/j.1095-8649.2007.01695.x
Valtonen ET, Marcogliese DJ, Julkunen M (2010) Vertebrate diets derived from trophically transmitted fish parasites in the Bothnian Bay. Oecologia 162:139–152
Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W (2008) Homage to linnaeus: how many parasites? How many hosts? Proc Natl Acad Sci USA 105:11482–11489. https://doi.org/10.1073/pnas.0803232105
Dunne JA, Lafferty KD, Dobson AP, Zander CD (2013) Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biol 11:e1001579. https://doi.org/10.1371/journal.pbio.1001579
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol 21:381–385. https://doi.org/10.1016/j.tree.2006.04.007
Sato T, Watanabe K, Kanaiwa M, Niizuma Y, Harada Y, Lafferty KD (2011) Nematomorph parasites drive energy flow through a riparian ecosystem. Ecology 92:201–207
Dunn AM (2009) Parasites and biological invasions. J adv parasitol 68:161–184. https://doi.org/10.1016/S0065-308X(08)00607-6
MacKenzie K, Pert C (2018) Evidence for the decline and possible extinction of a marine parasite species caused by intensive fishing. Fish Sci Res 198:63–65. https://doi.org/10.1016/j.fishres.2017.10.014
Takemoto R, Pavanelli G, Lizama M, Bellay S (2009) Diversity of parasites of fish from the upper Paraná River floodplain. Brazil Braz J Biol 69:691–705. https://doi.org/10.1590/S1519-69842009000300023
Junk W, Bayley PB, Sparks RE (1989) The flood pulse concept in river- foodplain systems. Can J Fish Aquat 1:110–127
Winemiller KO, Andrade MC, Arantes CC, Bokhutlo T, Bower LM, Cunha ER, Robertson CR (2023) Can spatial food web subsidies associated with river hydrology and lateral connectivity be detected using stable isotopes? Food Webs 34:e00264
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7:369–374
Poulin R (2020) Meta-analysis of seasonal dynamics of parasite infections in aquatic ecosystems. Parasitol Int 50:6–7. https://doi.org/10.1016/j.ijpara.2020.03.006
Lafferty KD (2009) The ecology of climate change and infectious diseases. Ecology. https://doi.org/10.1890/08-0079.1
Barber I (2007) Parasites, behaviour and welfare in fish. Appl Anim Behav Sci 104(3–4):251–264
Malhi Y, Roberts JT, Betts RA, Killeen TJ, Li W, Nobre CA (2008) Climate change, deforestation, and the fate of the Amazon. Science 319:169–172. https://doi.org/10.1126/science.1146961
Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Can J Zool 79:1331–1352
Sures B, Nachev M, Selbach C, Marcogliese DJ (2017) Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology.’ Parasit Vectors 10:1–19. https://doi.org/10.1186/s13071-017-2001-3
Carlson CJ, Burgio KR, Dougherty ER, Getz WM (2017) Parasite biodiversity faces extinction and redistribution in a changing climate. Sci Adv 3:e1602422. https://doi.org/10.1126/sciadv.1602422
Lafferty KD (2012) Biodiversity loss decreases parasite diversity: theory and patterns. Philos Trans R Soc Lond B Biol Sci 367:2814–2827
Strona G, Lafferty KD (2016) Environmental change makes robust ecological networks fragile. Nat Commun. https://doi.org/10.1038/ncomms12462
Khan RA, Thulin J (1991) Influence of pollution on parasites of aquatic animals. J Adv Parasitol 30:201–238. https://doi.org/10.1016/S0065-308X(08)60309-7
Landsberg JH, Blakesley BA, Reese RO, Mcrae G, Forstchen PR (1998) Parasites of fish as indicators of environmental stress. Environ Monit Assess 51:211–232. https://doi.org/10.1023/A:1005991420265
Mackenzie K (1999) Parasites as pollution indicators in marine ecosystems: a proposed early warning system. Mar Pollut Bull 38:955–959. https://doi.org/10.1016/S0025-326X(99)00100-9
Baia RRJ, Florentino AC, Silva LMA, Tavares-Dias M (2018) Patterns of the parasite communities in a fish assemblage of a river in the Brazilian Amazon region. Acta Parasitol 63:304–316. https://doi.org/10.1515/ap-2018-0035
Silvano RAM (2020) Fish and fisheries in the Brazilian Amazon. Springer International, Rio grande do Sul. https://doi.org/10.1007/978-3-030-49146-8
Almeida VLL, Hahn NS, Vazzoler AEAM (1997) Feeding patterns in five predatory fishes of the high Parana River floodplain (PR, Brazil). Ecol Freshw Fish 6:123–133. https://doi.org/10.1111/j.1600-0633.1997.tb00154.x
Garcia L, Pinya S, Colomar V, Mayol J (2018) The first recorded occurrences of the invasive crab Callinectes sapidus Rathbun, 1896 (Crustacea: Decapoda: Portunidae) in coastal lagoons of the Balearic Islands (Spain). Bioinvasions Rec 7:191–196
Pantoja C, Scholz T, Luque JL, Jones A (2018) New genera and species of paramphistomes (Digenea: Paramphistomoidea: Cladorchiidae) parasitic in fishes from the Amazon basin in Peru. Syst Parasitol 95:611–624. https://doi.org/10.1007/s11230-018-9808-y
Ramos IP, Brandão H, Zanatta AS, Carvalho ED (2013) Interference of cage fish farm on diet, condition factor and numeric abundance on wild fish in a Neotropical reservoir. Aquaculture 414–415:56–62
Poulin R, Leung TLF (2011) Body size, trophic level, and the use of fish as transmission routes by parasites. Oecologia 166:731–738
Falkenberg JM, Golzio JESA, Pessanha A, Patrício J, Vendel AL, Lacerda ACF (2019) Gill parasites of fish and their relation to host and environmental factors in two estuaries in northeastern Brazil. Aquat Ecol 53:109–118. https://doi.org/10.1007/s10452-019-09676-6
Nachev M, Sures B (2009) The endohelminth fauna of barbel ( Barbus barbus ) correlates with water quality of the Danube River in Bulgaria. Parasitology 136:545–552. https://doi.org/10.1017/S003118200900571X
Gheorghiu C, Cable J, Marcogliese DJ, Scott ME (2007) Effects of waterborne zinc on reproduction, survival and morphometrics of Gyrodactylus turnbulli (Monogenea) on guppies (Poecilia reticulata). Parasitol Int 37:375–381. https://doi.org/10.1016/j.ijpara.2006.09.004
Morley NJ, Crane M, Lewis JW (2003) Toxicity of cadmium and zinc to the decaudised cercarial life-span of Diplostomum spathaceum (Trematoda: Diplostomidae). Parasitology 127:497–506. https://doi.org/10.1017/s0031182003003949
Pietrock M, Marcogliese DJ (2003) Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol 19:293–299. https://doi.org/10.1016/S1471-4922(03)00117-X
Eiras JC, Takemoto RM, Pavanelli GC (2010) Diversidade dos parasitas de peixes de água doce do Brasil. Clichetec, Maringá
Castello L, McGrath DG, Hess LL, Arantes CC (2013) The vulnerability of Amazon freshwater ecosystems. Conserv Lett 6:217–229. https://doi.org/10.1111/conl.12008
Marcogliese DJ, Pietrock M (2011) Combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol 27:123–130
Callisto M, Ferreira WR, Moreno P, Goulart M, Petrucio M (2002) Aplicação de um protocolo de avaliação rápida da diversidade de habitats em atividade de ensino e pesquisa (MG-RJ). Acta Limnol Bras 14:91–98
Silvano RAM (2001) Peixes do Alto Rio Juruá (Amazonas, Brasil). EdUSP, São Paulo
Torrente-Vilara G, Queiroz LD, Ohara WM (2013). Um breve histórico sobre o conhecimento da fauna de peixes do Rio Madeira. São Paulo.
Travassos L, Freitas JF, Kohn A (1969) Trematódeos do Brazil. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro
Thatcher VE (2006) Amazon fish parasites, 2°. Pensoft Publishers, Moscow
Moravec F (1998). Nematoides de peixes de água doce da Região Neotropical. República Tcheca, Academia de Ciencias da República Tcheca.
Martins ML, Yoshitoshi ER (2003) A new nematode species Goezia leporini n. sp. (Anisakidae) from cultured freshwater fish Leporinus macrocephalus (Anostomidae) in Brazil. Braz J Biol 63:497–505. https://doi.org/10.1590/S1519-69842003000300016
Jones A, Jones A, Bray RA, Gibson DI (2005) Keys to the Trematoda, vol 2. CABI Publishing and The Natural History Museum, London
Giesen SC, Takemoto RM, Calitz F, Lizama MDLAP, Junker K (2013) Infective pentastomid larvae from Pygocentrus nattereri Kner (Pisces, Characidae) from the Miranda River, Pantanal, Mato Grosso do Sul State, Brazil, with notes on their taxonomy and epidemiology. Folia Parasitol 60:457–468. https://doi.org/10.14411/fp.2013.049
Miller TL, Cribb TH (2008) Family Cryptogonimidae Ward, 1917, In Keys to the Trematoda, vol 3. CABI, Wallingford
Gutwiński P, Cema G, Ziembińska-Buczyńska A, Wyszyńska K, Surmacz-Gorska J (2021) Long-term effect of heavy metals Cr(III), Zn(II), Cd(II), Cu(II), Ni(II), Pb(II) on the anammox process performance. J Water Process Eng 39:101668. https://doi.org/10.1016/j.jwpe.2020.101668
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol Res 83:575–583
Legendre P, Legendre L (2012) Numerical ecology. Elsevier, Oxford
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Oksanen J (2016). Design decisions and implementation details in vegan. Vignette of the Package Vegan. R Package Version, 2-4.
Simpson, G. L. (2018). Permute: functions for generating restricted permutations of data. R Package.
Lafferty KD (1997) Environmental parasitology: what can parasites tell us about human impacts on the environment? Parasitol Today 13:251–255. https://doi.org/10.1016/S0169-4758(97)01072-7
Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Parasitol Int 35:705–7016. https://doi.org/10.1016/j.ijpara.2005.01.015
Yamada POF, Yamada FH, da Silva RJ, Anjos LA (2017) Ecological implications of floods on the parasite communities of two freshwater catfishes in a Neotropical floodplain. Acta Parasitol. https://doi.org/10.1515/ap-2017-0039
Tavares-Dias M, Oliveira MSB, Gonçalves RA, Silva LMA (2014) Ecology and seasonal variation of parasites in wild Aequidens tetramerus, a Cichlidae from the Amazon. Acta Parasitol 59:158–164. https://doi.org/10.2478/s11686-014-0225-3
Soylu E (2013) Metazoan parasites of perch Perca fluviatilis L. from Lake Sığırcı, Ipsala. Turkey Pak J Zool 45:47–52
Pietrock M, Hursky O (2011) Fish and ecosystem health as determined by parasite communities of lake whitefish (Coregonus clupeaformis) from Saskatchewan boreal lakes. Water Qual Res J Canada 46:219–229. https://doi.org/10.2166/wqrjc.2011.004
Marcogliese DJ (2016) The distribution and abundance of parasites in aquatic ecosystems in a changing climate: more than just temperature. Integr Comp Biol 56:611–619. https://doi.org/10.1093/icb/icw036
Nhiwatiwa T, De Bie T, Vervaeke B, Brendonck L (2009) Invertebrate communities in dry-season pools of a large subtropical river: patterns and processes. Hydrobiologia 630:169–186. https://doi.org/10.1007/s10750-009-9790-0
Choudhury A, Dick TA (2000) Richness and diversity of helminth communities in tropical freshwater fishes: empirical evidence. J Biogeogr 27:935–956. https://doi.org/10.1046/j.1365-2699.2000.00450.x
Guardone L, Ricci E, Susini F, Polsinelli E, Guglielmone G, Armani A (2021) First detection of Eustrongylides excisus (Nematoda: Dioctophymatidae) in big-scale sand smelt (Atherina boyeri) from the lake Massaciuccoli (Northwest Tuscany, Italy): implications for public health and seafood quality. Food Control 120:107517
Rautenberg KA, da Silveira EL, Vaz-dos-Santos AM (2021) Feeding trends of Psalidodon paranae in an impacted Neotropical basin: a multifactor and integrative approach. Environ Biol Fishes 104:89–105
Cardone IB, Lima-Junior SE, Goitein R (2006) Diet and capture of Hypostomus strigaticeps (Siluriformes, Loricariidae) in a small brazilian stream: relationship with limnological aspects. Braz J Biol 66:25–33. https://doi.org/10.1590/S1519-69842006000100005
Arévalo EG, Morey GMA, Malta JCO (2018) Fauna parasitária de Prochilodus nigricans (Prochilodontidae) de lagos de várzea da Amazônia brasileira Parasitic fauna of Prochilodus nigricans (Prochilodontidae) from Brazilian Amazon floodplain lakes. Biota Amazôn 1:19–21. https://doi.org/10.18561/2179-5746
Leite LAR, Pelegrini LS, Agostinho BN, de Azevedo RK, Abdallah VD (2018) Biodiversity of the metazoan parasites of Prochilodus lineatus (Valenciennes, 1837) (Characiformes: Prochilodontidae) in anthropized environments from the Batalha River, São Paulo State Brazil. Biota Neotrop 18:e20170422. https://doi.org/10.1590/1676-0611-bn-2017-0422
Moreira LHA, Yamada FH, Ceschini TL, Takemoto RM, Pavanelli GC (2010) The influence of parasitism on the relative condition factor (Kn) of Metynnis lippincottianus (Characidae) from two aquatic environments: the upper Parana river floodplain and Corvo and Guairacá rivers. Brazil Acta Sci 32:83–86. https://doi.org/10.4025/actascibiolsci.v32i1.3668
DeMont DJ, Corkum KC (1982) The life cycle of Octospiniferoides chandleri Bullock, 1957 (Acanthocephala: Neoechinorhynchidae) with some observations on parasite-induced, photophilic behavior in ostracods. J Parasitol 68:125–130. https://doi.org/10.2307/3281334
Merritt SV, Pratt I (1964) The life history of Neoechinorhynchus rutili and Its Development in the Intermediate Host (Acanthocephala: Neoechinorhynchidae). J Parasitol 50:394–400. https://doi.org/10.2307/3275843
Mikhailova EI, Kusenko KV (2018) Advanced development of the cystacanths of Neoechinorhynchus beringianus (Eoacanthocephala: Neoechinorhynchidae) living in intermediate hosts. Invert Zool 15:92–102. https://doi.org/10.15298/invertzool.15.1.07
de Sousa LF, Morey GAM, de Oliveira Malta JC (2018) The development of Neoechinorhynchus buttnerae (Eoacanthocephala: Neoechinorhynchidae) in its intermediate host Cypridopsis vidua in Brazil. Acta Parasitol 63:354–359. https://doi.org/10.1515/ap-2018-0040
Martins ML, Onaka EM, Moraes FR, Fujimoto RY (2001) Mebendazole treatment against Anacanthorus penilabiatus (Monogenea, Dactylogyridae) gill parasite of cultivated Piaractus mesopotamicus (Osteichthyes, Characidae) in Brazil. Efficacy Hematol Acta Parasitol 46:332–336
Shamsi S (2019) Parasite loss or parasite gain? Story of Contracaecum nematodes in antipodean waters. Parasite Epidemiol Control 4:e00087. https://doi.org/10.1016/j.parepi.2019.e00087
Kohn A, Fernandes BMM, Macedo B, Abramson B (1985) Helminths parasites of freshwater fishes from Pirassununga, SP Brazil. Mem Inst Oswaldo Cruz 80:327–336. https://doi.org/10.1590/S0074-02761985000300009
Reis MS, Santos CP, Nunes JLS, Mugnai R (2021) Lista de verificação de nematoides parasitando peixes na Amazônia brasileira. J Helmintol 95:e75. https://doi.org/10.1017/S0022149X21000729
Virgilio LR, Martins WMO, Lima FS, Takemoto RM, Camargo LMA, Meneguetti DUO (2022) Endoparasite fauna of freshwater fish from the upper Juruá River in the Western Amazon Brazil. J Helminttol 96:e55. https://doi.org/10.1017/S0022149X2200027X
Dias PG, Furuya WM, Pavanelli GC, Machado MH, Takemoto RM (2004) Carga parasitária de Rondonia rondoni, Travassos, 1920 (Nematoda, Atrictidae) e fator de condição do armado, Pterodoras granulosus, Valenciennes, 1833 (Pisces, Doradidae). Acta Scient 26:151–156. https://doi.org/10.4025/actascibiolsci.v26i2.1613
Fares ALB, Nonato FAS, Michelan TS (2020) New records of the invasive macrophyte, Urochloa arrecta extend its range to eastern Brazilian Amazon altered freshwater ecosystems. Acta Amazon 50:133–137. https://doi.org/10.1590/1809-4392201903831
Fernando AME, Súarez YR (2021) Resource use by omnivorous fish: effects of biotic and abiotic factors on key ecological aspects of individuals. Ecol Freshw Fish 30:222–233. https://doi.org/10.1111/eff.12578
Holt EA, Miller SW (2011). Bioindicators: Using Organisms to Measure Environmental Impacts. Nature Education Knowledge.
Parmar TK, Rawtani D, Agrawal YK (2016) Bioindicators: the natural indicator of environmental pollution. Front Life Sci 9:110–118. https://doi.org/10.1080/21553769.2016.1162753
Flores-Lopes F (2014) The occurence of black spot disease in Astyanax aff. fasciatus(characiformes: characidae) in the Guaíba Lake basin, RS. Brazil B J Biolog 74:127–134. https://doi.org/10.1590/1519-6984.08312
Lymbery AJ, Lymbery SJ, Beatty SJ (2020) Fish out of water: aquatic parasites in a drying world. Int J Parasitol Parasites Wildl 12:300–307. https://doi.org/10.1016/j.ijppaw.2020.05.003
Salgado-Maldonado G (2006) Checklist of helminth parasites of freshwater fishes from Mexico. Zootaxa 1324:351–357. https://doi.org/10.11646/zootaxa.1324.1.1
Hirshfield MF, Morin RP, Hepner DJ (1983) Increased prevalence of larval Eustronglylides (Nematoda) in the mummichog, Fundulus heteroclitus (L.), from the discharge canal of a power plant in the Chesapeake Bay. J Fish Biol 23:135–142
Fellis KJ, Esch GW (2005) Variation in life cycle affects the distance decay of similarity among bluegill sunfish parasite communities. J Parasitol 91:1484–1486. https://doi.org/10.1645/GE-578R.1
Esch GW, Kennedy CR, Bush AO, Aho JM (1988) Patterns in helminth communities in freshwater fish in Great Britain: alternative strategies for colonization. Parasitology 96:519–532. https://doi.org/10.1017/S003118200008015X
Chagas De Souza D, Lima Correa L, Tavares-Dias M (2018) Ithyoclinostomum dimorphum Diesing, 1850 (Digenea, Clinostomidae) in Hoplias malabaricus (Erythrinidae) with the fi rst report of infection of the eyes. Helminthologia 55:343–349. https://doi.org/10.2478/helm-2018-0028
Lizama MLAP, Takemoto RM, Pavanelli G (2006) Parasitism influence on the hepato, splenosomatic and weight/length relation and relative condition factor of Prochilodus lineatus (Valenciennes, 1836) (Prochilodontidae) of the Upper Paraná River floodplain Brazil. Rev Bras Parasitol Vet 15:116–122
Lizama MAP, Takemoto RM, Pavanelli GC (2006) Influence of the seasonal and environmental patterns and host reproduction on the metazoan parasites of Prochilodus lineatus. Braz Arch Biol Technol 49:611–622. https://doi.org/10.1590/S1516-89132006000500011
Chaparro G, O’Farrell I, Hein T (2019) Multi-scale analysis of functional plankton diversity in floodplain wetlands: effects of river regulation. Sci Total Environ 667:338–347. https://doi.org/10.1016/j.scitotenv.2019.02.147
Petsch DK, Pinha GD, Takeda AM (2017) Dispersal mode and flooding regime as drivers of benthic metacommunity structure in a neotropical floodplain. Hydrobiologia 788:131–141. https://doi.org/10.1007/s10750-016-2993-2
Cavalcante PHO, da Silva MT, Pereira ANS, Gentile R, Santos CP (2020) Helminth diversity in Pimelodus blochii Valenciennes, 1840 (Osteichthyes: Pimelodidae) in two Amazon Rivers. Parasitol Res 119:4005–4015. https://doi.org/10.1007/s00436-020-06906-x
Gonçalves RA, Oliveira MSB, Neves LR, Tavares-Dias M (2016) Seasonal pattern in parasite infracommunities of Hoplerythrinus unitaeniatus and Hoplias malabaricus (Actinopterygii: Erythrinidae) from the Brazilian Amazon. Acta Parasitol 61:119–129. https://doi.org/10.1515/ap-2016-0016
Negreiros LP, Pereira FB, Tavares-Dias M, Tavares LER (2018) Community structure of metazoan parasites from Pimelodus blochii in two rivers of the Western Brazilian Amazon: same seasonal traits, but different anthropogenic impacts. Parasitol Res 117:3791–3798. https://doi.org/10.1007/s00436-018-6082-5
Fujimoto RY, Couto MVS, Sousa NC, Madi RR, Eiras JC, Martins ML (2018) Seasonality of Procamallanus (Spirocamallanus) inopinatus (Nematoda: Camallanidae) Infection in Bryconops melanurus (Characiformes: Iguanodectidae). Bol Inst Pesca 44:e334. https://doi.org/10.20950/1678-2305.2018.44.4.334
Bonato KO, Burress ED, Fialho CB (2017) Dietary differentiation in relation to mouth and tooth morphology of a neotropical characid fish community. Zool Anz 267:31–40. https://doi.org/10.1016/j.jcz.2017.01.003
Neves LR, Silva LMA, Florentino AC, Tavares-Dias M (2020) Distribution patterns of Procamallanus (Spirocamallanus) inopinatus (Nematoda: Camallanidae) and its interactions with freshwater fish in Brazil. Rev Bras Parasitol Vet 29:1–15. https://doi.org/10.1590/s1984-29612020092
Ribeiro CAO, Katsumiti A, França P, Neto FF (2013) Biomarkers responses in fish (Atherinella brasiliensis) of paranaguá bay, southern Brazil, for assessment of pollutant effects. Braz J Oceanogr 61:1–11
Brandão ML, Moreira J, Luque JL (2014) Checklist of platyhelminthes, acanthocephala, nematoda and arthropoda parasitizing penguins of the world. Check List 10:562–573. https://doi.org/10.15560/10.3.562
Hoshino MDFG, Hoshino ÉM, Tavares-Dias M (2014) First study on parasites of Hemibrycon surinamensis (Characidae), a host from the eastern Amazon region. Braz J Vet Parasitol 23:344–347. https://doi.org/10.1590/S1984-29612014069
Brasil LS, Luiza-Andrade A, Calvão LB, Juen L (2020) Aquatic insects and their environmental predictors: a scientometric study focused on environmental monitoring in lotic environmental. Environ Monit Assess 192:194e. https://doi.org/10.1007/s10661-020-8147-z
Blanar CA, Munkittrick KR, Houlaha J, MacLatchy DL, Marcogliese DJ (2009) Pollution and parasitism in aquatic animals: a meta-analysis of effect size. Aquat Toxicol 93:18–28. https://doi.org/10.1016/j.aquatox.2009.03.002
Marcogliese DJ (2004) Parasites: small players with crucial roles in the ecological theater. EcoHealth 1:151–164. https://doi.org/10.1007/s10393-004-0028-3
Keckeis S (2003) The significance of zooplankton grazing in a floodplain system of the River Danube. J Plankton Res 25:243–253. https://doi.org/10.1093/plankt/25.3.243
Kiss KT (1987) Phytoplankton studies in the Szigetköz section of the Danube during 1981-1982. Arch Hydrobiol 78:247–273
Lewis WM, Hamilton SK, Rodríguez MA, Saunders JF, Lasi MA (2001) Foodweb analysis of the Orinoco floodplain based on production estimates and stable isotope data. J North Am Benthol Soc 20:241–254. https://doi.org/10.2307/1468319
Berkhout BW, Borregaard MK, Brandl R, Thieltges DW (2020) Host assemblage and environment shape β-diversity of freshwater parasites across diverse taxa at a continental scale. Glob Ecol Biogeogr 29:39–49. https://doi.org/10.1111/geb.13005
Madi RR, Ueta MT (2009) O papel de Ancyrocephalinae (Monogenea: Dactylogyridae), parasito de Geophagus brasiliensis (Pisces: Cichlidae), como indicador ambiental. Rev Bras Parasitol Vet 18:38–41. https://doi.org/10.4322/rbpv.01802008
Valendolf NJ, Barbosa SMW, Henrique CG, Cervelin FI, Aparecida VV, Almeida J, Xavier C (2022) Bacterial diversity in aerated facultative lagoon treating kraft cellulose effluent with bioaugmentation. BioResources 17:6556–6568. https://doi.org/10.15376/biores.17.4.6556-6568
Bhatnagar A, Devi P (2013) Water quality guidelines for the management of pond fish culture. Int J Environ Sci 3:1980–2009. https://doi.org/10.6088/ijes.2013030600019
Sreenivasan A (1976) Limnological studies of and primary production in temple pond ecosystems. Hydrobiologia 48:117–123. https://doi.org/10.1007/BF00040163
Morley NJ, Lewis JW (2015) Thermodynamics of trematode infectivity. Parasitology 142:585–597. https://doi.org/10.1017/S0031182014001632
Morley NJ, Lewis JW (2017) Thermodynamics of egg production, development and hatching in trematodes. J Helminthol 91:284–294. https://doi.org/10.1017/S0022149X16000249
Benassi RF, de Jesus TA, Coelho LHG, Mitsch WJ (2021) Eutrophication effects on CH4 and CO2 fluxes in a highly urbanized tropical reservoir (Southeast, Brazil). Environ Sci Pollut Res Int 28:42261–42274. https://doi.org/10.1007/s11356-021-13573-7
Qu JB, Chu LY, Yang M, Xie R, Hu L, Chen WM (2006) A pH-responsive gating membrane system with pumping effects for improved controlled release. Adv Funct Mater 16:1865–1872. https://doi.org/10.1002/adfm.200500897
Junk WJ, Robertson BA (1997) The central amazon floodplain. Ecological studies (Analysis and Synthesis). Springer, Berlin
Takemoto RM, Lizama MA, Guidelli GM, Pavanelli GC (2004) Parasitos de peixes de águas continentais. sanidade de organismos aquáticos. Editora Varela São Paulo, Sao Paulo
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EAH (2003) Analysis of transgenic indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Cross MA, Irwin SWB, Fitzpatrick SM (2001) Effects of heavy metal pollution on swimming and longevity in cercariae of Cryptocotyle lingua (Digenea: Heterophyidae). Parasitology 143:499–507. https://doi.org/10.1017/S0031182001008708
Thomaz SM (2021) Propagule pressure and environmental filters related to non-native species success in river-floodplain ecosystems. Hydrobiologia 849:3679–3704. https://doi.org/10.1007/s10750-021-04624-8
Onada OA, Akinwole AO, Ajani EK (2015) Study of interrelationship among water quality parameters in earthen pond and concrete tank. PeerJPrePrints 3:845v1. https://doi.org/10.7287/peerj.preprints.845v1
Santos RDS, Marchiori N, Santarem VA, Takahashi HK, Mourino JLP, Martins ML (2012) Austrodiplostomum compactum (Lutz, 1928) (Digenea, Diplostomidae) in the eyes of fishes from Paraná river. Brazil Acta Scient 34:225–231. https://doi.org/10.4025/actascibiolsci.v34i2.9337
Ventura AS, Pádua SBD, Ishikawa MM, Martins ML, Takemoto RM, Jeronimo GT (2018) Endoparasites of Gymnotus sp. (Gymnotiformes: Gymnotidae) from commercial baitfish farming in Pantanal basin Central Brazil. Bol Inst Pesca 44:e322. https://doi.org/10.20950/1678-2305.2018.322
Olivier L, Stirewalt MA (1952) An efficient method for exposure of mice to cercariae of Schistosoma mansoni. J Parasitol 38:19–23
Puinyabati H, Shomorendra M, Kar D (2013) Correlation of water’s physico-chemical characteristics and trematode parasites of Channa punctata (Bloch) in Awangsoi lake, Manipur. India J appl nat sci 5:190–193. https://doi.org/10.31018/jans.v5i1.304
Wanja DW, Mbuthia PG, Waruiru RM, Bebora LC, Ngowi HA (2020) Natural concurrent infections with black spot disease and multiple bacteriosis in farmed Nile tilapia in Central Kenya. Vet Med Int 2020:1–8. https://doi.org/10.1155/2020/8821324
Luz-Agostinho K, Agostinho A, Gomes L, Júlio-Jr H, Fugi R (2009) Effects of flooding regime on the feeding activity and body condition of piscivorous fish in the upper Paraná River floodplain. B J Biol 69:481–490. https://doi.org/10.1590/S1519-69842009000300004
Acknowledgements
We thank the Laboratory of Animal Biology and Limnology of the Federal University of Acre for helping with the data analysis and with the necessary equipment to carry out the study.
Funding
Not avaliable.
Author information
Authors and Affiliations
Contributions
All authors collected the data and provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Ethical Approval
We certify that Fish species reported in the study is not threatened, and all procedures were approved by the ethics committee of the institution where the study was conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11686_2023_685_MOESM1_ESM.tif
Supplementary file1 Mean, standard deviation and ANOVA values, for physical and chemical variables in environments A - anthropized and C - Conserved, in the periods of D - Drought and F - Flooding (TIF 186 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Virgilio, L.R., da Silva Lima, F., keppeler, E.C. et al. Endoparasite Communities of Fish at Different Trophic Levels in the Western Brazilian Amazon: Human, Environmental and Seasonal Influence. Acta Parasit. 68, 612–636 (2023). https://doi.org/10.1007/s11686-023-00685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00685-y