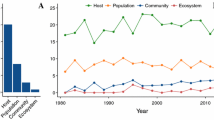
Abstract
Effective management of our natural resources requires an understanding of ecosystem structure and function; effectively, an ecosystem-based approach to management. Parasites occur, albeit cryptically, in almost all ecosystems, yet they are usually neglected in studies on populations and communties of organisms. Parasites can have pronounced or subtle effects on hosts affecting host behavior, growth, fecundity, and mortality. Furthermore, parasites may regulate host population dynamics and influence community structure. Many parasites have complex life cycles and depend for transmission on the presence of a variety of invertebrate and vertebrate intermediate hosts. Often transmission involves predator–prey interactions. Thus, parasites reflect the host’s position in the food web and are indicative of changes in ecosystem structure and function. Parasites can provide information on population structure, evolutionary hypotheses, environmental stressors, trophic interactions, biodiversity, and climatic conditions. I use examples from diverse freshwater and marine systems to demonstrate that parasites should be incorporated into research and monitoring programs to maximize information gathered in ecosystem-based studies and resource management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Parasitic organisms are often neglected in the management and conservation of biological resources and ecosystems. They are analagous to “extras” in a theatrical production who do not have speaking parts, yet are crucial to a deeper comprehension of the ongoing scene. They are most often small, short-lived, and rarely observed in the external environment, or more commonly hidden within organisms during their parasitic phase. Their effects on their hosts may be obvious and profound, or more subtle, reflecting principal characters or supporting players on the ecosystem stage. Typically, they attract attention only when they cause pathology and disease, or somehow degrade biological products, thus reducing production yields and economic benefits. This is a role where they are only temporarily prominent on the scene and always panned by their critics. Yet, virtually all species are host to at least one parasite species, with the remitting probability that parasitic organisms outnumber free-living species (Price, 1980). Thus parasites comprise an important component of the cast of organisms in ecological theaters throughout the world, including freshwater and marine, whose effects can be manifested in the evolutionary play of life (with apologies to Hutchinson, 1965).
Research on parasites can provide a great deal of information about their host organisms and habitats. A population or ecosystem-scale approach to parasitology can be applied not only to the control of disease, but to the proper management and conservation of aquatic resources, be they species targeted for harvest or areas designated as protected. In this article, I summarize basic parasitic life-styles and transmission patterns and review the various applications of this information to species management in aquatic ecosystems. I outline a holistic approach whereby knowledge of parasites can be applied to conservation of multispecies systems to aid in conservation management. Examples are given from freshwater and marine ecosystems including plants, invertebrates, and vertebrates, for the implications of parasitism extend beyond commercially exploited species to include all trophic levels and organisms within habitats. The goal is to foster an appreciation for the role played by parasites as the drama of life unfolds on the global ecosystem stage.
LIFE-STYLES AND TRANSMISSION PATTERNS
For epidemiological purposes, parasites are traditionally divided into microparasites and macroparasites (Anderson and May, 1979; May and Anderson, 1979). Microparasites consist of small organisms that are primarily unicellular, including viruses, bacteria, and protozoans, but also multicellular organisms of small size (< 50 μm) such as myxozoans. These organisms typically multiply in or on the host and are often associated with disease. Transmission is usually direct but may be indirect, involving alternate hosts (e.g., myxozoans), or vectors (e.g., many protozoans). Macroparasites are larger, multicellular organisms such as monogeneans, digeneans, cestodes, nematodes, acanthocephalans, arthropods, leeches, and others. These typically undergo sexual reproduction in or on a host, but do not normally proliferate there (the production of cercariae by digeneans in molluscan intermediate hosts is an exception). They often possess complex life cycles, with one or more intermediate hosts required for development or growth (Figs. 1 and 2). These parasites are sometimes clearly detrimental to their hosts, but in many cases their effects are more subtle and difficult to measure.
Life cycle of a typical digenean (Euhaplorchis californiensis) in a salt marsh. Piscivorous birds such as egrets or herons serve as definitive hosts. Eggs are passed with feces, which are ingested by the first intermediate host (horn snail, Cerithidea californica). Cercariae are asexually produced in the snail and released into the water, infecting a suitable second intermediate fish host (Pacific killifish, Fundulus parvipinnus) upon contact. The parasite then encysts as a metacercaria in the brain. The life cycle is completed when the bird eats the infected fish. The parasite impacts upon the ecosystem at multiple trophic levels. Infected snails are castrated, possibly affecting snail population levels. Infected fish display behavioral alterations such that they become 30 times more susceptible to predation. Conceivably, the presence of the parasite permits the persistence of piscivorous waterfowl by facilitating predation (Lafferty and Morris, 1996).
Life cycle of a typical nematode (Eustrongylides ignotus) in the aquatic environment. Piscivorous birds, including great blue herons (Ardea herodius), great egrets (Casmeroidius albus), and snowy egrets (Egretta thula), are definitive hosts. Eggs are passed into the water with the feces and are ingested by oligochaetes, the first intermediate hosts. Numerous fish species, including mosquitofish (Gambusia holbrooki) may function as second intermediate hosts, acquiring the parasite by eating infected oligochaetes. The avian definitive hosts become infected when they consume the fish, thus completing the life cycle. This parasite causes pathology in piscivorous fish hosts and renders smaller forage fish more susceptible to predation (Coyner et al., 2001). In addition, the parasite causes significant mortality among nestling birds. Furthermore, the parasites’ abundance is amplified in eutrophic conditions, probably via effects on oligochaete populations. Thus, this parasite has impacts at different trophic levels, and these impacts are substantially amplified by anthropogenic nutrient enrichment.
For those parasites with complex life cycles, a variety of transmission modes has evolved. Transmission may involve one or more free-living infective stages, where the infective stage is passively ingested by the next host in the parasite’s life cycle, for example, certain larvae of digeneans (metacercariae) that encyst in the external environment; free-swimming cestode larvae (coracidia) that are preyed upon by crustacean intermediate hosts; or parasite eggs of many groups. Free-living infective stages also may be transmitted actively by penetrating the next host in the life cycle, as with the larval cercariae of many digeneans in fish and other organisms, and nematode larvae in moist soil. Parasite life cycles may involve a parasitic infective stage in one precursor host that must be ingested for transmission to occur. Examples can be found among all the principal helminth groups (except monogeneans) including many digeneans, and all species of cestodes, nematodes, and acanthocepahalans. In these cases, occurrence of a parasite in a host reflects predator–prey interactions between the host and its prey and predators. The diversity of parasites within a host reflects the presence of diverse intermediate and definitive hosts in the ecosystem participating in the parasites’ life cycles. Thus, by the nature of their different life cycles, the parasites in a host population provide information on that organism’s role in the food web (Marcogliese and Cone, 1997a; Marcogliese, 2001b, 2002, 2003).
IMPACTS ON HOSTS AND COMMUNITIES
By their very nature, parasites have a variety of impacts on their hosts. They impose energetic demands, alter behavior, affect morphology and appearance, reduce fecundity and growth, and cause mortality. These effects are well documented in numerous host–parasite systems in both freshwater and marine habitats. Behavioral alterations may lead to increased vulnerability to predation. In some cases, this is a pathological side effect, but in others it may enhance transmission to the next host in the life cycle. Thus, parasites can affect the diet of predators, influencing predator–prey dynamics (Fig. 1) and competitive interactions between that host and other organisms (Price et al., 1986; Lafferty et al., 2000). Parasites can affect sex ratio and mate choice (Minchella and Scott, 1991). Taken together with the effects listed above, it seems likely that parasites can have an impact on host fitness, and thus, play a role in natural selection of host characteristics. A list of parasites that affect commercial fish stocks through parasite-induced host mortality, reduction in fecundity, or reduction in market value or weight appears in Dobson and May (1986). Pathology caused by parasitism is widespread in all organisms (Kinne, 1980–1990; Woo, 1995; Bondad-Reantaso et al., 2001), and virtually all organs and tissues can be damaged by a plethora of parasitic organisms. Examples of parasites of a range of invertebrate and vertebrate aquatic organisms that have different negative impacts on their hosts are listed in Table 1. Infection with many types of parasites causes sublethal effects in virtually all types of organisms, including ctenophores, cnidarians, molluscs, crustaceans, insects, echinoderms, chaetognaths, fish, and plants (see supplementary Table 3, available for viewing by subscribers only at http://www.springerlink.com), as well as amphibians, waterfowl, and aquatic mammals Though most parasites do not normally kill their hosts, death can result from infection. Parasites of numerous different types of organisms, from algae through vertebrates, have been shown to cause parasite-induced host mortality (see supplementary Table 4, available for viewing by subscribers only at http://www.springerlink.com). Clearly, the effects of parasites are manifest throughout the food webs of aquatic systems, and not confined to well-studied commercial species. These lists of host–parasite associations represent a variety of invertebrate and vertebrate taxa at different trophic levels, along with different types of impacts. Virtually all types of parasites manifest some sort of effect and all types of organisms are impacted in some way, no matter where they occur within a food web.
Parasites also function as ecosystem engineers by directly or indirectly modifying the environment of other organisms (i.e., the host phenotype). A parasite may alter host biology such that new habitat for other species is formed (Thomas et al., 1999; Lafferty et al., 2000).
If prevalence and abundance are high, parasites can have a significant impact on the host population, and regulate its numbers (Anderson and May, 1979; May and Anderson, 1979). If that particular host population is a dominant species in an ecosystem, then the presence of the parasite may have consequences for the entire food web and ecosystem structure (Dobson and Hudson, 1986; Minchella and Scott, 1991; McCallum and Dobson, 1995). Such parasites are termed “keystone parasites” by Minchella and Scott (1991). For example, two different parasites are known to control local populations of the green sea urchin (Strongylocentrotus droebachiensis) which can overgraze kelp beds and completely alter coastal ecosystems. In Norwegian waters, populations of sea urchins are limited by the nematode Echinomermella matsi, while in Nova Scotia by the protozoan Paramoeba invadens (reviewed in Hagen, 1996). Outbreaks of the microsporidian Cougourdella sp. reduce populations of the dominant grazer in the system, the caddisfly Glossosoma nigrior, thus permeating increases in the production of periphyton and the abundance of other invertebrate grazers in Michigan streams (Kohler and Wiley, 1997). The parasitic plant Cuscuta salina preferentially infects the dominant competitor Salicornia virginiana over three other subordinate salt marsh plants, thus affecting community composition and dynamics (Pennings and Callaway, 2002). Parasites may actually drive plant succession, with very small effects on competitive ability being translated into community-wide consequences (Dobson and Crawley, 1994). These few examples illustrate the broad range of communities that can be influenced by parasitic infections in key species.
Depending on the location within the food web of a species infected by parasites, impacts may permeate through a bottom-up or a top-down cascade on the rest of the web. It should be stressed that organisms found throughout the food web are subjected to the impacts of parasitism. These impacts strongly suggest that it is imperative to consider parasites in management and conservation of their hosts.
Parasites may exert more subtle effects on host communities. For example, epizootics of plerocercoids of the cestode Ligula intestinalis in their intermediate host, the roach (Rutilus rutilus), clearly determine not only the population structure of the roach via parasite-induced host mortality and sterility but of another sympatric cyprinid fish, the rudd (Scardinius erythrophthalmus) in Slapton Ley via roach–rudd interactions (Fig. 3; Kennedy et al., 2001). Such interactions only became apparent after collection of long-term data and may generally be more common than previously assumed in other host–parasite systems.
Schematic displaying role of the cestode Ligula intestinalis in population cycles of the roach, Rutilus rutilus (direct interactions) and rudd, Scardinius erythrophthalmus (indirect interactions) in Slapton Ley, United Kingdom. In the first phase of the epizootic cycle, roach population dynamics are controlled by the parasite population. In the second phase, roach population dynamics determine infection levels of L. intestinalis. Initiation of a new cycle (dashed line) may depend on local conditions and is not obligatory (after Kennedy et al., 2001). PIHM, parasite-induced host mortality.
In terms of conservation, the introduction or elimination of a parasite may affect interactions between a variety of species within a community (Dobson and Hudson, 1986; Kennedy et al., 2001). Furthermore, parasites and disease are a major threat to endangered species (McCallum and Dobson, 1995), especially if those species are maintained at high densities in small, fragmented areas that promote transmission and parasite exchange across species (Scott, 1988; McCallum and Dobson, 1995; Holmes, 1996). In addition, introductions and other emerging infectious diseases, including parasites, are a potentially serious threat to endangered species and biodiversity at large (Cunningham et al., 2003; Daszak and Cunningham, 2003).
PARASITES AS INDICATORS OF HOST BIOLOGY
Numerous studies in aquatic systems have effectively used parasites as indicators of host stocks and their ontogenetic or seasonal migrations. For some recent reviews, consult in Williams et al. (1992), Arthur (1997), and MacKenzie and Abaunza (1998) for rationale, examples, and guidelines. Most of the studies to date involve marine species of fish, but some work in fresh waters has successfully used parasites to discriminate among stocks (Marcogliese et al., 2001, and references therein). Because many parasites are transmitted via predator–prey interactions, and parasites possess a variety of complex life cycles with different intermediate hosts, parasites are excellent indicators of host diet (Williams et al., 1992; Marcogliese and Cone, 1997a). In fact, for numerous reasons, parasites provide complementary information to that obtained through stomach content analysis. Parasites reflect trophic interactions over weeks or months, whereas gut contents provide details of the animal’s diet only over the last 24 hours or less (Williams et al., 1992; Curtis, 1995). They can indicate ontogenetic shifts in diet, whether hosts feed on more than one trophic level, niche shifts due to competition or other factors, individual feeding specializations within a population, seasonal changes in diet, and temporary links in a food web such as periodic migrants into a system (reviewed in Williams et al., 1992; Marcogliese and Cone, 1997a; Marcogliese, 2003). When combined with other techniques used in fisheries science such as meristics and population genetics, research managers have at their disposal a very powerful array of tools to study the biology of virtually any organism. Moreover, and not insignificantly, parasites are used as indicators of historical biogeography and phylogenetics of their hosts (Brooks and Hoberg, 2000).
PARASITES AS INDICATORS OF ECOSYSTEM STRESS, FOOD WEBS, AND BIODIVERSITY
A number of reviews summarize existing knowledge of the relationship between parasites and pollution, and parasites as indicators of stress (Overstreet and Howse, 1977; Overstreet, 1988, 1993, 1997; Khan and Thulin, 1991; Poulin, 1992; MacKenzie 1993, 1999; MacKenzie et al. 1995; Lafferty, 1997; Lafferty and Kuris, 1999). Basically, parasites can be used as indicators of environmental stress in a way analogous to their employment to differentiate among host populations or stocks. Many parasites possess complex life cycles and depend on the presence of one or more intermediate or paratenic hosts for transmission. Should the abundance of any of these hosts decline, for example, by exposure to chemical contaminants, then transmission of the parasite may be impaired. Similar results occur if infected hosts are more sensitive to effects of contaminants and are selectively removed (MacKenzie et al., 1995). Furthermore, free-living stages of parasites or those inhabiting the external surface or gastrointestinal tract are directly exposed to toxicants, and those parasites may be directly susceptible to pollution (Poulin, 1992; Overstreet, 1997; MacKenzie, 1999). See Table 1 in Pietrock and Marcogliese (2003) for a list of various endohelminths where survival and infectivity of their free-living stages are susceptible to toxicological effects caused by environmental contaminants. Parasites demonstrate different types of sensitivity to contaminants and environmental stress in aquatic hosts and ecosystems (see supplementary Table 5, available for viewing by subscribers only at http://www.springerlink.com). In terms of other relationships with pollutants, intestinal parasites appear to be more sensitive bioaccumulators of heavy metals than their fish hosts, and may serve as excellent indicators of heavy metal pollution (Sures et al., 1999; Sures, 2001, 2003). Alternatively, if a host’s immune response is compromised by toxin exposure, its parasite burden may increase. Such a situation is often observed for monogeneans and protozoans that proliferate in hosts that inhabit polluted habitats (Overstreet, 1997). Commonly parasites that increase in abundance in contaminated habitats often possess direct life cycles (see Table 1 in MacKenzie et al., 1995, for numerous examples). Interpreted another way, abundance of infections with endoparasitic helminths tends to decrease, while those of ectoparasites tend to increase with pollution (MacKenzie, 1999). Moreover, many facultative parasites such as pathogenic viruses and bacteria also proliferate under these circumstances. Guidelines for selecting the most appropriate host–parasite combinations and the most vulnerable stages as indicators are provided in MacKenzie (1993, 1999) and MacKenzie et al. (1995).
Just as entire communities of free-living organisms are affected by environmental stress, so are the communities of parasites that infect any particular host species. Diversity and species richness may increase under stressful conditions, but more often a decrease occurs, at least for endoparasites with indirect life cycles. Reductions in parasite species richness have been observed following acidification, eutrophication, and chemical contamination (Table 2; see also supplementary Table 6 for a more comprehensive list, available for viewing by subscribers only at http://www.springerlink.com). These reductions in parasite diversity are believed to parallel diversity loss in free-living species, because populations of intermediate hosts are impacted by environmental changes. Furthermore, parasite communities may recover concomitantly with free-living communities when conditions improve (Cone et al., 1993).
However, resource managers must be aware that parasite taxa respond differently to environmental perturbations (Curtis, 1995; Lafferty, 1997; Marcogliese and Cone, 1997b; Lafferty and Kuris, 1999; MacKenzie, 1999), depending on the life cycle of the parasite, the concentration and type of contaminant, and the exposure time (Overstreet and Howse, 1977; Khan and Thulin, 1991; Poulin, 1992). Thus, generalizations about the relationship between parasitism and pollution cannot be made without taking into account the biology of individual species. Kennedy (1997) further highlights the intrinsic difficulties to using parasites as indicators of pollution, but concludes that they can be excellent, nonspecific, early-warning indicators of environmental change.
Because many parasites depend on predator-prey relationships for transmission, parasites are sentinels for food web interactions. One species of parasite may depend on the presence of only one or a few intermediate and paratenic hosts for transmission, however, the total parasite diversity within a host represents a diversity of life cycles that utilize numerous different organisms as hosts at some point in their life cycles (Figs. 4 and 5). Not only do parasites provide information on a host’s diet, but this information is complementary, and in many ways superior, to gut content analysis (see above). Furthermore, information on a host’s predators can also be derived from a host’s parasites. Thus, the parasite fauna within a host population provides information about the role of the host in the food web, and the variety of predator–prey relationships in which it participates (Marcogliese and Cone, 1997a, b; Marcogliese, 2002, 2003).
Potential transmission pathways involving predator–prey interactions for helminth parasites in freshwater environments. In this figure and in Figure 5, other types of parasites are not shown for simplicity’s sake, nor are interactions involving free-living infective stages depicted (e.g., cestode coracidia and digenean miracidia and cercariae). For both this figure and Figure 5, specificity for the intermediate and definitive hosts within any one life cycle (and any one compartment in the diagrams) will vary with individual parasite species. Routes of trophic pathways will also vary with parasite species, with some being obligate and others facultative, depending on the nature of the host–parasite interaction. In addition, within each life cycle, parasites may follow more than one path to reach the definitive host, again depending on the specificity of the host–parasite interaction. Reprinted in adapted form from Marcogliese and Cone (1997a), copyright 1997, with permission from Elsevier Science.
Potential transmission pathways involving predator–prey interactions for helminth parasites in marine environments (see Fig. 4 for details about the food web diagram). Note that the marine food web appears more complex than the freshwater web. This is a result of the presence of an additional trophic level in marine systems, that of large invertebrate predators, which play a relatively greater role in marine food chains than in freshwater food chains. Reprinted in adapted form from Marcogliese and Cone (1997a), copyright 1997, with permission from Elsevier Science.
Parasite life cycles evolve in tandem with the evolution of their hosts’ life history traits, and have adapted to long-standing predator–prey interactions. Thus, the presence of particular parasites in a host may also tell us something about the stability of the ecosystem (George-Nascimento, 1987; Marcogliese and Cone, 1997a; Marcogliese, 2003). Moreover, parasites may actually maintain the stability and integrity of ecosystems (Brooks and Hoberg, 2001). Environmental changes resulting from global warming, for example, may disrupt synchronous population cycles of predators and their prey in aquatic habitats, interfering with parasite transmission between those organisms (Marcogliese, 2001a). The climate change-related environmental perturbations that can affect parasitism include alterations in temperature regimes, precipitation, host range, water levels and flow rates, eutrophication, stratification, extent of ice cover, acidification, oceanic circulation patterns, and UV radiation (Marcogliese, 2001a).
Parasites may be excellent, economical, early-warning indicators of changes to environmental conditions and ecosytem health (MacKenzie, 1993, 1999; Overstreet, 1993, 1997; Lafferty, 1997; Marcogliese and Cone, 1997b; Marcogliese, 2003). This concept can be expanded to changes in biodiversity by taking into account the nature of parasites, their life cycles, and transmission. Biodiversity and its measurement have been an increasing concern for research managers and conservationists. By definition, biodiversity includes not only species diversity, but the ecological complexes of which they are a part (Glowka et al., 1994). Developing appropriate indicators for biodiversity has been a difficult task. The idea that certain taxa can be used as surrogates is popular, but few indicators have proven reliable, especially across different ecosystems. Because parasites respond to environmental stressors and track food webs via their transmission processes, it is logical to extend their use to indicators of biodiversity. They have the further advantage that they belong to many distinct and unrelated taxa. Thus, there are no phylogenetic constraints such as those imposed when specific taxa are used as indicators. In addition, because they infect hosts on different trophic levels, their usage is not trophically constrained (Marcogliese and Cone, 1997b; Marcoglies, 2003).
Those organisms in the middle of the food web such as small fish may be best suited as biodiversity indicators. They tend to be more heavily infected than top piscivores, because they prey upon intermediate and paratenic hosts and thus acquire parasites that may or may not mature in them (George-Nascimento, 1987). They, in turn, are preyed upon by larger piscivores and pass on larval parasites to these organisms, where they may or may not mature. In addition, predators on small fish include not only larger fish, but other vertebrates such as birds and mammals. Thus, it is possible to obtain information on food web pathways linking the aquatic and terrestrial milieux.
In summary, parasites are ubiquitous in the aquatic environment. They have impacts ranging from the subtle, to the sublethal, to the lethal. Their impacts on hosts are propagated up and down food webs and thus are manifested throughout entire communities. Like free-living organisms, they are affected by biotic and abiotic changes to the environment. Parasites are effective indicators of many aspects of host biology and thus extremely useful as management and conservation tools. Moreover, they are uniquely situated within food webs, and their transmission processes may permit their usage as indicators of environmental stress, food-web structure, and biodiversity. Indeed, far from being mere extras without speaking parts in the ecological theater, parasites may be bit players but with incredibly important roles who should step forward and take a bow as the curtain goes down on the ecosystem stage. Critics must acknowledge the significance of their wonderfully complex roles that are intricately woven into the scripts of virtually all the principal players in the theater of life.
References
RM Anderson RM May (1979) ArticleTitlePopulation biology of infectious diseases: part I Nature 280 361–367
JR Arthur (1997) Recent advances in the use of parasites as biological tags for marine fish TW Flegel IH MacRae (Eds) Diseases in Asian Aquaculture III Fish Health Section, Asian Fisheries Society Manila 141–154
TCM Bakker D Mazzi S Zala (1997) ArticleTitleParasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation Ecology 78 1098–1104
JA Balbuena E Karlsbakk AM Kvenseth M Saksvik A Nylund (2000) ArticleTitleGrowth and emigration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring (Clupea harengus) Journal of Parasitology 86 1271–1275
I Barber FA Huntingford (1995) ArticleTitleThe effects of Schistocephalus solidus (Cestoda: Pseudophyllidea) on the foraging and shoaling behaviour of three-spined sticklebacks, Gasterosteus aculeatus Behaviour 132 1223–1240
Bondad-Reantaso MG, McGladdery SE, East I, Subasinghe RP (2001) Asian diagnostic guide to aquatic animal diseases. FAO Fisheries Technical Paper 402/2, FAO, Rome, Italy
K Broeg S Zander A Diamant W Korting G Kruner I Paperna et al. (1999) ArticleTitleThe use of fish metabolic, pathological and parasitological indices in pollution monitoring. I. North Sea Helgoländer Marine Research 53 171–194
DR Brooks EP Hoberg (2000) ArticleTitleTriage for the biosphere: the need and rationale for taxonomic inventoried and phylogenetic studies of parasites Comparative Parasitology 67 1–25
DR Brooks EP Hoberg (2001) ArticleTitleParasite systematics in the 21st century: opportunities and obstacles Trends in Parasitology 17 273–275
AF Brown D Pascoe (1989) ArticleTitleParasitism and host sensitivity to cadmium: an acanthocephalan infection of the freshwater amphipod Gammarus pulex Journal of Applied Ecology 26 473–487
JA Butler RE Millemann (1971) ArticleTitleEffect of the “salmon poisoning” trematode, Nanophyetus salmincola, on the swimming ability of juvenile salmonid fishes Journal of Parasitology 57 860–865
DK Cone DJ Marcogliese WD Watt (1993) ArticleTitleMetazoan parasite communities of yellow eels (Anguilla rostrata) in acidic and limed rivers of Nova Scotia Canadian Journal of Zoology 71 177–184
WW Cort KL Hussey DJ Ameel (1960) ArticleTitleSeasonal fluctuations in larval trematode infections in Stagnicola emarginata angulata from Phragmites Flats on Douglas Lake Journal of the Helminthological Society of Washington 27 11–13
DF Coyner SR Schaack MG Spalding DJ Forrester (2001) ArticleTitleAltered predation susceptibility of mosquitofish infected with Eustrongylides ignotus Journal of Wildlife Diseases 37 556–560
DF Coyner MG Spalding DJ Forrester (2003) ArticleTitleInfluence of treated sewage on infections of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in eastern mosquitofish (Gambusia holbrooki) in an urban watershed Comparative Parasitology 70 205–210
AA Cunningham P Daszak JP Rodriguez (2003) ArticleTitlePathogen pollution: defining a parasitological threat to biodiversity conservation Journal of Parasitology 89 (Suppl) S78–S83
LA Curtis (2002) ArticleTitleEcology of larval trematodes in three marine gastropods Parasitology 124 (Suppl) S43–S56
M Curtis (1995) ArticleTitleThe ecological parasitology of charrs: relationships between parasites and food web structure in northern lakes Nordic Journal of Freshwater Research 71 92–101
P Daszak AA Cunningham (2003) ArticleTitleAnthropogenic change, biodiversity loss, and a new agenda for emerging diseases Journal of Parasitology 89 (Suppl) S37–S41
CL Densmore VS Blazer TB Waldrop PS Pooler (2001) ArticleTitleEffects of whirling disease on selected hematological parameters in rainbow trout Journal of Wildlife Diseases 37 375–378
A Dobson M Crawley (1994) ArticleTitlePathogens and the structure of plant communities Trends in Ecology & Evolution 9 393–398
A Dobson PJ Hudson (1986) ArticleTitleParasites, disease and the structure of ecological communities Trends in Ecology & Evolution 1 11–15
A Dobson RM May (1986) The effects of parasites on fish populations—theoretical aspects MJ Howell (Eds) Parasitology—quo vadit? Proceedings of ICOPA VI Australian Academy of Science Canberra, Australia 363–370
L Dusek M Gelnar S Sebelová (1998) ArticleTitleBiodiversity of parasites in a freshwater environment with respect to pollution: metazoan parasites of chub (Leuciscus cephalus L.) as a model for statistical evaluation International Journal for Parasitology 28 1555–1571
D Ebert (1994) ArticleTitleGenetic differences in the interactions of a microsporidian parasite and four clones of its cyclically parthenogenetic host Parasitology 108 11–16
D Ebert M Lipsitch KL Mangin (2000) ArticleTitleThe effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites American Naturalist 156 459–477
SA Fischer WE Kelso (1988) ArticleTitlePotential parasite-induced mortality in age-0 bluegills in a floodplain pond of the lower Mississippi River Transactions of the American Fisheries Society 117 565–573
P Galli L Mariniello G Crosa M Ortis AQ Ambrogi S D’Amelio (1998) ArticleTitlePopulations of Acanthocephalus anguillae and Pomphorhynchus laevis in rivers with different pollution levels Journal of Helminthology 72 331–335
P Galli G Crosa L Mariniello M Ortis S D’Amelio (2001) ArticleTitleWater quality as a determinant of the composition of fish parasite communities Hydrobiologia 452 173–179
M Gelnar S Sebelová L Dusek B Koubková P Jurajda S Zahrádková (1997) ArticleTitleBiodiversity of parasites in freshwater environment in relation to pollution Parassitologia 39 189–199
MA George-Nascimento (1987) ArticleTitleEcological helminthology of wildlife animal hosts from South America: a literature review and a search for patterns in marine food webs Revista Chilena de Historia Natural 60 181–202
L Glowka F Burhanne-Guilmin H Synge JA McNeeley L Gundling (1994) A guide to the Convention on Biological Diversity IUCN—The World Conservation Union Gland, Switzerland
NT Hagen (1996) Sea urchin outbreaks and epizootic disease as regulating mechanisms in coastal ecosystems A Eleftheriou AD Ansell CJ Smith (Eds) Biology and Ecology of Shallow Coastal Waters Olsen & Olsen Fredensborg, Denmark 303–308
A Halmetoja ET Valtonen E Koskenniemi (2000) ArticleTitlePerch (Perca fluviatilis L.) parasites reflect ecosystem conditions: a comparison of a natural lake and two acidic reservoirs in Finland International Journal for Parasitology 30 1437–1444
M Heath N Nicoll (1991) ArticleTitleInfection of larval herring by helminth parasites in the North Sea and the effect on feeding incidence Continental Shelf Research 11 1477–1489
JC Holmes (1996) ArticleTitleParasites as threats to biodiversity in shrinking ecosystems Biodiversity and Conservation 5 975–983
GE Hutchinson (1965) The Ecological Theatre and the Evolutionary Play Yale University Press New Haven, CT
KC Jacobson MR Arkoosh AN Kagley ER Clemons TK Collier E Casillas (2003) ArticleTitleCumulative effects of natural and anthropogenic stress on immune function and disease resistance in juvenile Chinook salmon Journal of Aquatic Animal Health 15 1–12
KT Jensen KN Mouritsen (1992) ArticleTitleMass mortality in two common soft-bottom invertebrates, Hydrobia ulvae and Corophium volutator—the possible role of trematodes Helgoländer Meeresuntersuchungen 46 329–339
BE Keas HD Blankespoor (1997) ArticleTitleThe prevalence of cercariae from Stagnicola emarginata (Lymnaeidae) over 50 years in northern Michigan Journal of Parasitology 83 536–540
CR Kennedy (1997) ArticleTitleFreshwater fish parasites and environmental quality: an overview and caution Parassitologia 39 249–254
CR Kennedy PC Shears JA Shears (2001) ArticleTitleLong-term dynamics of Ligula intestinalis and roach Rutilus rutilus: a study of three epizootic cycles over thirty-one years Parasitology 123 257–269
RA Khan J Thulin (1991) ArticleTitleInfluence of pollution on parasites of aquatic animals Advances in Parasitology 30 201–238
O Kinne (1980–1990) Diseases of Marine Animals, Vol 1–14 John Wiley and Biologische Anstalt Helgoland Chichester, UK and Hamburg, Germamny
WD Klein OW Olsen DC Bowden (1969) ArticleTitleEffects of intestinal fluke, Crepidostomum farionis, on rainbow trout, Salmo gairdneri Transactions of the American Fisheries Society 98 1–6
SL Kohler MJ Wiley (1997) ArticleTitlePathogen outbreaks reveal large-scale effects of competition in stream communities Ecology 78 2164–2176
AK Kumaraguru FWH Beamish PTK Woo (1995) ArticleTitleImpact of a pathogenic haemoflagellate, Cryptobia salmositica Katz, on the metabolism and swimming performance of rainbow trout, Oncorhynchus mykiss (Walbaum) Journal of Fish Disease 18 297–305
KD Lafferty (1997) ArticleTitleEnvironmental parasitology: what can parasites tell us about human impacts on the environment? Parasitology Today 13 251–255
KD Lafferty AM Kuris (1999) ArticleTitleHow environmental stress affects the impacts of parasites Limnology and Oceanography 44 925–931
KD Lafferty AK Morris (1996) ArticleTitleAltered behavior of parasitized killifish increases susceptibility to predation by bird final hosts Ecology 77 1390–1397
KD Lafferty F Thomas R Poulin (2000) Evolution of host phenotype manipulation by parasites and its consequences R Poulin S Morand A Skorping (Eds) Evolutionary Biology of Host-parasite Relationships: Theory Meets Reality Elsevier Amsterdam 117–127
JH Landsberg BA Blakesley RO Reese G McRae PR Forstchen (1998) ArticleTitleParasites of fish as indicators of environmental stress Environmental Monitoring and Assessment 51 211–232
D Lemly GW Esch (1984) ArticleTitleEffects of the trematode Uvulifer ambloplitis on juvenile bluegill sunfish, Lepomis macrochirus: ecological implications Journal of Parasitology 70 475–492
K MacKenzie (1993) ArticleTitleParasites as biological indicators Bulletin of the Scandinavian Society for Parasitology 1 1–10
K MacKenzie (1999) ArticleTitleParasites as pollution indicators in marine ecosystems: a proposed early warning system Marine Pollution Bulletin 38 955–959
K MacKenzie P Abaunza (1998) ArticleTitleParasites as biological tags for stock discrimination of marine fish: a guide to procedures and methods Fisheries Research 38 45–56
K MacKenzie HH Williams B Williams AH McVicar R Siddall (1995) ArticleTitleParasites as indicators of water quality and the potential use of helminth transmission in marine pollution studies Advances in Parasitology 35 85–144
DJ Marcogliese (2001a) ArticleTitleImplications of climate change for parasitism of animals in the aquatic environment Canadian Journal of Zoology 79 1331–1352
DJ Marcogliese (2001b) ArticleTitlePursuing parasites up the food chain: implications of food web structure and function on parasite communities in aquatic systems Acta Parasitologica 46 82–93
DJ Marcogliese (2002) ArticleTitleFood webs and the transmission of parasites to marine fish Parasitology 124 (Suppl) S83–S99
DJ Marcogliese (2003) ArticleTitleFood webs and biodiversity: are parasites the missing link? Journal of Parasitology 89 (Suppl) S106–S113
DJ Marcogliese DK Cone (1996) ArticleTitleOn the distribution and abundance of eel parasites in Nova Scotia: influence of pH Journal of Parasitology 82 389–399
DJ Marcogliese DK Cone (1997a) ArticleTitleFood webs: a plea for parasites Trends in Ecology & Evolution 12 320–325
DJ Marcogliese DK Cone (1997b) ArticleTitleParasite communities as indicators of ecosystem stress Parassitologia 39 227–232
DJ Marcogliese DK Cone (2001) ArticleTitleMyxozoan communities parasitizing Notropis hudsonius (Cyprinidae) at selected localities on the St. Lawrence River, Quebec: possible effects of urban effluents Journal of Parasitology 87 951–956
DJ Marcogliese P Dumont A Gendron Y Mailhot E Bergeron JD McLaughlin (2001) ArticleTitleSpatial and temporal variation in abundance of Diplostomum spp. in walleye (Stizostedion vitreum) and white suckers (Catostomus commersoni) from the St. Lawrence River Canadian Journal of Zoology 79 355–369
RM May RM Anderson (1979) ArticleTitlePopulation biology of infectious diseases: part II Nature 280 455–461
CP McCahon MJ Poulton (1991) ArticleTitleLethal and sub-lethal effects of acid, aluminum and lime on Gammarus pulex during repeated simulated episodes in a Welsh stream Freshwater Biology 25 169–178
CP McCahon SJ Maund MJ Poulton (1991) ArticleTitleThe effect of the acanthocephalan parasite (Pomphorhynchus laevis) on the drift of its intermediate host (Gammarus pulex) Freshwater Biology 25 507–513
H McCallum A Dobson (1995) ArticleTitleDetecting disease and parasite threats to endangered species and ecosystems Trends in Ecology & Evolution 10 190–194
DJ Minchella ME Scott (1991) ArticleTitleParasitism: a cryptic determinant of animal community structure Trends in Ecology & Evolution 6 250–254
RM Overstreet (1988) ArticleTitleAquatic pollution problems, southeastern U.S. coasts: histopathological indicators Aquatic Toxicology 11 213–239
RM Overstreet (1993) Parasitic diseases of fishes and their relationship with toxicants and other environmental factors JA Couch JW Fournie (Eds) Pathobiology of Marine and Estuarine Organisms CRC Press Boca Raton, FL 111–156
RM Overstreet (1997) ArticleTitleParasitological data as monitors of environmental health Parassitologia 39 169–175
RM Overstreet HD Howse (1977) ArticleTitleSome parasites and diseases of estuarine fishes in polluted habitats of Mississippi Annals of the New York Acadamy of Science 298 427–462
D Pascoe P Cram (1977) ArticleTitleThe effect of parasitism on the toxicity of cadmium to the three-spined stickleback, Gasterosteus aculeatus L Journal of Fish Biology 10 467–472
AF Pasternak K Pulkkinen VN Mikheev T Hasu ET Valtonen (1999) ArticleTitleFactors affecting abundance of Triaenophorus infection in Cyclops strenuus, and parasite-induced changes in host fitness International Journal for Parasitology 29 1793–1801
KR Patterson (1996) ArticleTitleModelling the impact of disease-induced mortality in an exploited population: the outbreak of the fungal parasite Ichthyophonus hoferi in the North Sea herring (Clupea harengus) Canadian Journal of Fisheries and Aquatic Sciences 53 2870–2887
SC Pennings RM Callaway (2002) ArticleTitleParasitic plants: parallels and contrasts with herbivores Oecologia 131 479–489
L Pennycuick (1971) ArticleTitleQuantitative effects of three species of parasites on a population of three-spined sticklebacks, Gasterosteus aculeatus Journal of Zoology, London 165 143–162
M Pietrock DJ Marcogliese (2003) ArticleTitleFree-living endohelminth stages: at the mercy of environmental conditions Trends in Parasitology 19 293–299
SJ Plaistow J-P Troussard F Cézilly (2001) ArticleTitleThe effect of the acanthocephalan parasite Pomphorhynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex International Journal for Parasitology 31 346–351
R Poulin (1992) ArticleTitleToxic pollution and parasitism in freshwater fish Parasitology Today 8 58–61
PW Price (1980) Evolutionary Biology of Parasites Princeton University Press Princeton, NJ
PW Price M Westoby B Rice PR Atsatt RS Fritz JN Thompson et al. (1986) ArticleTitleParasite mediation in ecological interactions Annual Reviews in Ecology and Systematics 17 487–505
K Pulkkinen AF Pasternak T Hasu ET Valtonen (2000) ArticleTitleEffect of Triaenophorus crassus (Cestoda) infection on behavior and susceptibility to predation of the first intermediate host Cyclops strenuus (Copepoda) Journal of Parasitology 86 664–667
H Rosenthal (1967) ArticleTitleParasites in larvae of the herring (Clupea harengus L.) fed with wild plankton Marine Biology 1 10–15
AE Rumpus CR Kennedy (1974) ArticleTitleThe effect of the acanthocephalan Pomphorhynchus laevis upon the respiration of its intermediate host, Gammarus pulex Parasitology 68 271–284
P Salathé D Ebert (2003) ArticleTitleThe effects of parasitism and inbreeding on the competitive ability in Daphnia magna: evidence for synergistic epistasis Journal of Evolutionary Biology 16 976–985
ME Scott (1988) ArticleTitleThe impact of infection and disease on animal populations: implications for conservation biology Conservation Biology 2 40–56
R Siddall AW Pike AH McVicar (1993) ArticleTitleParasites of Buccinum undatum (Mollusca: Prosobranchia) as biological indicators of sewage-sludge dispersal Journal of the Marine Biological Association of the United Kingdom 73 931–948
R Siddall AW Pike AH McVicar (1994) ArticleTitleParasites of flatfish in relation to sewage sludge dumping Journal of Fish Biology 45 193–209
K Stoltze K Buchmann (2001) ArticleTitleEffect of Gyrodactylus derjavini infections on cortisol production in rainbow trout fry Journal of Helminthology 75 291–294
T Sulgostowska G Banaczyk B Grabda-Kazubska (1987) ArticleTitleHelminth fauna of flatfish (Pleuronectiformes) from Gdansk Bay and adjacent areas (south-east Baltic) Acta Parasitologica Polonica 31 231–240
B Sures (2001) ArticleTitleThe use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review Aquatic Ecology 35 245–255
B Sures (2003) ArticleTitleAccumulation of heavy metals by intestinal helminths in fish: an overview and perspective Parasitology 126 (Suppl) S53–S60
B Sures R Siddall H Taraschewski (1999) ArticleTitleParasites as accumulation indicators of heavy metal pollution Parasitology Today 15 16–21
F Thomas R Poulin T Meeüs Particlede J-F Guégan F Renaud (1999) ArticleTitleParasites and ecosystem engineering: what roles could they play? Oikos 84 167–171
ET Valtonen JC Holmes M Koskivaara (1997) ArticleTitleEutrophication, pollution, and fragmentation: effects on parasite communities in roach (Rutilus ruitlus) and perch (Perca fluviatilis) in four lakes in central Finland Canadian Journal of Fisheries and Aquatic Sciences 54 572–585
HH Williams K MacKenzie AM McCarthy (1992) ArticleTitleParasites as biological indicators of the population biology, migrations, diet, and phylogenetics of fish Reviews in Fish Biology and Fisheries 2 144–176
PTK Woo (1995) Fish Diseases and Disorders, Vol 1. Protozoan and Metazoan Infections CABI Publishing Wallingford, CT
CD Zander (1998) ArticleTitleEcology of host parasite relationships in the Baltic Sea Naturwissenschaften 85 426–436
Supplementary References (Cited in Supplementary Tables 3–6)
AT Abd Allah MQ Wanas SN Thompson (1996) ArticleTitleThe effects of lead, cadmium, and mercury on the mortality and infectivity of Schistosoma mansoni cercariae Journal of Parasitology 82 1024–1026
RD Adlard RJG Lester (1994) ArticleTitleDynamics of the interaction between the parasitic isopod, Anilocra pomacentri, and the coral reef fish, Chromis nitida Parasitology 109 311–324
GS Aeby (1991) ArticleTitleBehavioral and ecological relationships of a parasite and its hosts within a coral reef system Pacific Science 45 263–269
F Alvarez AH Hines ML Reaka-Kudla (1995) ArticleTitleThe effects of parasitism by the barnacle Loxothylacus panopaei (Gissler) (Cirripeda: Rhizocephala) on growth and survival of the host crab Rhithropanopeus harrisii (Gould) (Brachyura: Xanthidae) Journal of Experimental Marine Biology and Ecology 192 221–232
RL Baker BP Smith (1997) ArticleTitleConflict between antipredator and antiparasite behaviour in larval damselflies Oecologia 109 622–628
TCM Bakker D Mazzi S Zala (1997) ArticleTitleParasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation Ecology 78 1098–1104
JA Balbuena E Karlsbakk AM Kvenseth M Saksvik A Nylund (2000) ArticleTitleGrowth and emigration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring (Clupea harengus) Journal of Parasitology 86 1271–1275
I Barber FA Huntingford (1995) ArticleTitleThe effects of Schistocephalus solidus (Cestoda: Pseudophyllidea) on the foraging and shoaling behaviour of three-spined sticklebacks, Gasterosteus aculeatus Behaviour 132 1223–1240
CW Bean IJ Winfield (1989) ArticleTitleBiological and ecological effects of a Ligula intestinalis (L.) infestation of the gudgeon, Gobio gobio (L.) in Lough Neagh, Northern Ireland Journal of Fish Biology 34 135–147
WM Bethel JC Holmes (1977) ArticleTitleIncreased vulnerability of amphiods to predation owing to altered behavior induced by larval acanthocephalans Canadian Journal of Zoology 55 110–115
A Bonn M Gasse J Rolff A Martens (1996) ArticleTitleIncreased fluctuating asymmetry in the damselfly Coenagrion puella is correlated with ectoparasitic water mites: implications for fluctuating asymmetry theory Oecologia 108 596–598
NP Boyce (1979) ArticleTitleEffects of Eubothrium salvelini (Cestoda: Pseudophyllidea) on the growth and vitality of sockeye salmon, Oncorhynchus nerka Canadian Journal of Zoology 57 597–602
NP Boyce WC Clarke (1983) ArticleTitle Eubothrium salvelini (Cestoda: Pseudophyllidea) impairs seawater adaptation of migrant sockeye salmon yearlings (Oncorhynchus nerka) from Babine Lake, British Columbia Canadian Journal of Fisheries and Aquatic Sciences 40 821–824
NP Boyce SB Yamada (1977) ArticleTitleEffects of a parasite, Eubothrium salvelini (Cestoda: Pseudophyllidea), on the resistance of juvenile sockeye salmon, Oncorhynchus nerka, to zinc Journal of the Fisheries Research Board of Canada 34 706–709
A Braband TA Bakke BA Faafeng (1994) ArticleTitleThe ectoparasite Ichthyophthirius multifiliis and the abundance of roach (Rutilus rutilus): larval fish epidemics in relation to host behaviour Fisheries Research 20 49–61
P Brassard ME Rau MA Curtis (1982a) ArticleTitleInfection dynamics of Diplostomum spathaceum cercariae and parasite-induced mortality of fish hosts Parasitology 85 489–493
P Brassard ME Rau MA Curtis (1982b) ArticleTitleParasite-induced susceptibility to predation in diplostomiasis Parasitology 85 495–501
J Brattey (1983) ArticleTitleThe effects of larval Acanthocephalus lucii on the pigmentation, reproduction, and susceptibility to predation of the isopod Asellus aquaticus Journal of Parasitology 69 1172–1173
K Broeg S Zander A Diamant W Korting G Kruner I Paperna et al. (1999) ArticleTitleThe use of fish metabolic, pathological and parasitological indices in pollution monitoring. I. North Sea Helgoländer Marine Research 53 171–194
AF Brown D Pascoe (1989) ArticleTitleParasitism and host sensitivity to cadmium: an acanthocephalan infection of the freshwater amphipod Gammarus pulex Journal of Applied Ecology 26 473–487
SA Bullard S Frasca SuffixJr GW Benz (2001) ArticleTitleGill lesions associated with Erpocotyle tiburonis (Monogenea: Hexabothriidae) on wild and aquarium-held bonnethead sharks (Sphyrna tiburo) Journal of Parasitology 87 972–977
H-P Bulnheim (1978) ArticleTitleInteraction between genetic, external and parasitic factors in sex determination of the crustacean amphipod Gammarus duebeni Helgoländer Meeresuntersuchungen 31 1–33
D Bumann G Puls (1996) ArticleTitleInfestation with larvae of the sea anemone Edwardsia lineata affects nutrition and growth of the ctenophore Mnemiopsis leidyi Parasitology 113 123–128
CW Burns (1989) ArticleTitleParasitic regulation in a population of Boekella hamata Brehm (Copepoda: Calanoida) Freshwater Biology 21 421–426
JA Butler RE Millemann (1971) ArticleTitleEffect of the “salmon poisoning” trematode, Nanophyetus salmincola, on the swimming ability of juvenile salmonid fishes Journal of Parasitology 57 860–865
M Byrne A Cerra T Nishigaki M Hoshi (1997) ArticleTitleInfestation of the testes of the Japanese sea star Asterias amurensis by the ciliate Orchitophyra stellarum: a caution against the use of this ciliate for biological control Diseases of Aquatic Organisms 28 235–239
AM Christensen B Kanneworff (1965) ArticleTitleLife history and biology of Kronborgia amphipodicola Christensen & Kanneworff (Turbellaria, Neorhabdocoela) Ophelia 2 237–252
DK Cone DJ Marcogliese WD Watt (1993) ArticleTitleMetazoan parasite communities of yellow eels (Anguilla rostrata) in acidic and limed rivers of Nova Scotia Canadian Journal of Zoology 71 177–184
WW Cort KL Hussey DJ Ameel (1960) ArticleTitleSeasonal fluctuations in larval trematode infections in Stagnicola emarginata angulata from Phragmites Flats on Douglas Lake Journal of the Helminthological Society of Washington 27 11–13
DF Coyner SR Schaack MG Spalding DJ Forrester (2001) ArticleTitleAltered predation susceptibility of mosquitofish infected with Eustrongylides ignotus Journal of Wildlife Diseases 37 556–560
DF Coyner MG Spalding DJ Forrester (2003) ArticleTitleInfluence of treated sewage on infections of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in eastern mosquitofish (Gambusia holbrooki) in an urban watershed Comparative Parasitology 70 205–210
AE Crews GW Esch (1987) ArticleTitleHistopathology of larval trematode infections in the freshwater pulmonate snail, Helisoma anceps Journal of Invertebrate Pathology 49 76–82
KW Cummins MA Wilzbach (1988) ArticleTitleDo pathogens regulate stream invertebrate populations? Proceedings—International Association of Theoretical and Applied Limnology 23 1232–1243
LA Curtis (2002) ArticleTitleEcology of larval trematodes in three marine gastropods Parasitology 124 (Suppl) S43–S56
S D’Amelio L Gerasi (1997) ArticleTitleEvaluation of environmental deterioration by analysing fish parasite biodiversity and community structure Parassitologia 39 237–241
DJ DeMont KC Corkum (1982) ArticleTitleThe life cycle of Octospiniferoides chandleri (Bullock, 1957) (Acanthocephala: Neoechinorhynchidae) with some observations on parasite-induced, photophilic behavior in ostracods Journal of Parasitology 68 125–130
CL Densmore VS Blazer TB Waldrop PS Pooler (2001) ArticleTitleEffects of whirling disease on selected hematological parameters in rainbow trout Journal of Wildlife Diseases 37 375–378
A Diamant A Banet I Paperna HV Westernhagen K Broog G Kruener et al. (1999) ArticleTitleThe use of fish metabolic, pathological and parasitological indices in pollution monitoring. II. The Red Sea and Mediterranean Helgoländer Marine Research 53 195–208
L Dusek M Gelnar S Sebelová (1998) ArticleTitleBiodiversity of parasites in a freshwater environment with respect to pollution: metazoan parasites of chub (Leuciscus cephalus L.) as a model for statistical evaluation International Journal for Parasitology 28 1555–1571
D Ebert (1994) ArticleTitleGenetic differences in the interactions of a microsporidian parasite and four clones of its cyclically parthenogenetic host Parasitology 108 11–16
D Ebert M Lipsitch KL Mangin (2000) ArticleTitleThe effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites American Naturalist 156 459–477
J Escos CL Alados JM Emlen S Alderstein (1995) ArticleTitleDevelopmental instability in the Pacific hake parasitized by myxosporeans Kudoa spp. Transactions of the American Fisheries Society 124 943–945
NA Evans (1982a) ArticleTitleEffect of copper and zinc on the life cycle of Notocotylus attenuatus (Digenea: Notocotylidae) International Journal for Parasitology 12 363–369
NA Evans (1982b) ArticleTitleEffect of copper and zinc upon the survival and infectivity of Echinoparyphium recurvatum cercariae Parasitology 85 295–303
SA Fischer WE Kelso (1988) ArticleTitlePotential parasite-induced mortality in age-0 bluegills in a floodplain pond of the lower Mississippi River Transactions of the American Fisheries Society 117 565–573
P Galli L Mariniello G Crosa M Ortis AO Ambrogi S D’Amelio (1998) ArticleTitlePopulations of Acanthocephalus anguillae and Pomphorhynchus laevis in rivers with different pollution levels Journal of Helminthology 72 331–335
P Galli G Crosa L Mariniello M Ortis S D’Amelio (2001) ArticleTitleWater quality as a determinant of the composition of fish parasite communities Hydrobiologia 452 173–179
Q Gao P Nie (2000) ArticleTitleLead content in the monogenean Ancyrocephalus mogurndae and in different organs of its host, the mandarin fish, Siniperca chuatsi China Environmental Science 20 233–236
M Gelnar B Koubková K Plànková P Jurajda (1994) ArticleTitleReport on metazoan parasites of fishes of the river Moravia with remarks on the effects of water pollution Helminthologia 31 47–56
M Gelnar S Sebelová L Dusek B Koubková P Jurajda S Zahrádková (1997) ArticleTitleBiodiversity of parasites in freshwater environment in relation to pollution Parassitologia 39 189–199
DJ Guth HD Blankespoor J Cairns SuffixJr (1977) ArticleTitlePotentiation of zinc stress caused by parasitic infection of snails Hydrobiologia 55 225–229
JF Guthrie RL Kroger (1974) ArticleTitleSchooling habits of injured and parasitized menhaden Ecology 55 208–210
A Guttowa B Boniecka (1975) ArticleTitleThe effect of Foschlor and MCPA upon the embryo development of Fasciola hepatica (Trematoda) and Triaenophorus nodulosus (Cestoda) Bulletin de l’Academie Polonaise des Sciences 23 391–397
NT Hagen (1996) Sea urchin outbreaks and epizootic disease as regulating mechanisms in coastal ecosystems A Eleftheriou AD Ansell CJ Smith (Eds) Biology and Ecology of Shallow Coastal Waters Olsen & Olsen Fredensborg, Denmark 303–308
A Halmetoja ET Valtonen E Koskenniemi (2000) ArticleTitlePerch (Perca fluviatilis L.) parasites reflect ecosystem conditions: a comparison of a natural lake and two acidic reservoirs in Finland International Journal for Parasitology 30 1437–1444
M Heath N Nicoll (1991) ArticleTitleInfection of larval herring by helminth parasites in the North Sea and the effect on feeding incidence Continental Shelf Research 11 1477–1489
J Heinonen JVK Kukkonen IJ Holopainen (1999) ArticleTitleThe effects of parasites and temperature on the accumulation of xenobiotics in a freshwater clam Ecological Applications 9 475–481
S Helluy (1984) ArticleTitleRelations hôtes-parasites du trématode Microphallus papillorobustus (Rankin, 1940). III—Facteurs impliqués dans les modifications du comportement des Gammarus hôtes intermédiaires et tests de prédation Annales de Parasitologie Humaine et Comparée 59 41–56
DD Hoggarth (1990) ArticleTitleThe effects of parasitism by the rhizocephalan, Briarosaccus callosus Boschma on the lithodid crab, Paralomis granulosa (Jacquinot) in the Falkland Islands Crustaceana 59 156–170
RB Holliman LP Esham (1977) ArticleTitleToxicity of cadmium to Schistosoma mansoni cercariae: effects on vitality and developmental ability in white mice Hydrobiologia 56 81–88
DK Howe PM Nollen (1992) ArticleTitleThe effects of pH and salinity on the behavior of Philophthalmus gralli (Trematoda) miracidia Transactions of the Illinois State Academy of Science 85 73–77
KC Jacobson MR Arkoosh AN Kagley ER Clemons TK Collier E Casillas (2003) ArticleTitleCumulative effects of natural and anthropogenic stress on immune function and disease resistance in juvenile Chinook salmon Journal of Aquatic Animal Health 15 1–12
PA Jansen TA Bakke (1993) ArticleTitleRegulatory processes in the monogenean Gyrodactylus salaris Malmberg—Atlantic salmon (Salmo salar L.) association. II. Experimental studies Fisheries Research 17 103–114
KT Jensen KN Mouritsen (1992) ArticleTitleMass mortality in two common soft-bottom invertebrates, Hydrobia ulvae and Corophium volutator—the possible role of trematodes Helgoländer Meeresuntersuchungen 46 329–339
MW Johnson TA Dick (2001) ArticleTitleParasite effects on the survival, growth, and reproductive potential of yellow perch (Perca flavescens Mitchill) in Canadian Shield lakes Canadian Journal of Zoology 79 1980–1992
SC Johnson RB Blaylock J Elphick KD Hyatt (1996) ArticleTitleDisease induced by the sea louse (Lepeophtheirus salmonis) (Copepoda: Caligidae) in wild sockeye salmon (Oncorhynchus nerka) stocks of Alberni Inlet, British Columbia Canadian Journal of Fisheries and Aquatic Sciences 53 2888–2897
BE Keas HD Blankespoor (1997) ArticleTitleThe prevalence of cercariae from Stagnicola emarginata (Lymnaeidae) over 50 years in northern Michigan Journal of Parasitology 83 536–540
A Kelly MJ Hatcher AM Dunn (2003) ArticleTitleThe impact of a vertically transmitted microsporidian, Nosema granulosis on the fitness of its Gammarus duebeni host under stressful environmental conditions Parasitology 126 119–124
RA Khan (1987) ArticleTitleEffects of chronic exposure to petroleum hydrocarbons on two species of marine fish infected with a hemoprotozoan, Trypanosoma murmanensis Canadian Journal of Zoology . 2703–2709
RA Khan J Kiceniuk (1983) ArticleTitleEffects of crude oils on the gastrointestinal parasites of two species of marine fish Journal of Wildlife Diseases 19 253–258
RA Khan EM Lee D Barker (1990) ArticleTitle Lernaeocera branchialis: a potential pathogen to cod ranching Journal of Parasitology 76 913–917
WD Klein OW Olsen DC Bowden (1969) ArticleTitleEffects of intestinal fluke, Crepidostomum farionis, on rainbow trout, Salmo gairdneri Transactions of the American Fisheries Society 98 1–6
RZ Klekowski A Guttowa (1968) ArticleTitleRespiration of Eudiaptomus gracilis infected with Diphyllobothrium latum Experimental Parasitology 22 279–287
SL Kohler WK Hoiland (2001) ArticleTitlePopulation regulation in an aquatic insect: the role of disease Ecology 82 2294–2305
SL Kohler MJ Wiley (1997) ArticleTitlePathogen outbreaks reveal large-scale effects of competition in stream communities Ecology 78 2164–2176
AK Kumaraguru FWH Beamish PTK Woo (1995) ArticleTitleImpact of a pathogenic haemoflagellate, Cryptobia salmositica Katz, on the metabolism and swimming performance of rainbow trout, Oncorhynchus mykiss (Walbaum) Journal of Fish Diseases 18 297–305
RH Kussat (1969) ArticleTitleA comparison of aquatic communities in the Bow River above and below sources of domestic and industrial wastes from the city of Calgary Canadian Fish Culturist 40 3–31
KD Lafferty (1993) ArticleTitleEffects of parasitic castration on growth, reproduction and population dynamics of the marine snail Cerithidea californica Marine Ecology Progress Series 96 229–237
KD Lafferty AK Morris (1996) ArticleTitleAltered behavior of parasitized killifish increases susceptibility to predation by bird final hosts Ecology 77 1390–1397
JH Landsberg BA Blakesley RO Reese G McRae PR Forstchen (1998) ArticleTitleParasites of fish as indicators of environmental stress Environmental Monitoring and Assessment 51 211–232
D Lemly GW Esch (1984) ArticleTitleEffects of the trematode Uvulifer ambloplitis on juvenile bluegill sunfish, Lepomis macrochirus: ecological implications Journal of Parasitology 70 475–492
H Liao L Shih (1956) ArticleTitleContribution to the biology and control of Bothriocephalus gowkongensis Yeh, a tapeworm parasitic in the young grass carp (Ctenopharyngodon idellus C .and V.) Acta Hydrobiologica Sinica 2 129–185
G Loot S Lek SP Brown J-F Guégan (2001) ArticleTitlePhenotypic modification of roach (Rutilus rutilus L.) infected with Ligula intestinalis L. (Cestoda: Pseudophyllidea) Journal of Parasitology 87 1002–1010
AR Lyndon (1996) The role of acanthocephalan parasites in the predation of freshwater isopods by fish SPR Greenstreet ML Tasker (Eds) Aquatic Predators and Their Prey Fishing News Books Oxford, UK 26–32
K MacKenzie HH Williams B Williams AH McVicar R Siddall (1995) ArticleTitleParasites as indicators of water quality and the potential use of helminth transmission in marine pollution studies Advances in Parasitology 35 85–144
DJ Marcogliese DK Cone (1996) ArticleTitleOn the distribution and abundance of eel parasites in Nova Scotia: influence of pH Journal of Parasitology 82 389–399
DJ Marcogliese DK Cone (1997) ArticleTitleParasite communities as indicators of ecosystem stress Parassitologia 39 227–232
DJ Marcogliese DK Cone (2001) ArticleTitleMyxozoan communities parasitizing Notropis hudsonius (Cyprinidae) at selected localities on the St. Lawrence River, Quebec: possible effects of urban effluents Journal of Parasitology 87 951–956
DJ Marcogliese JJ Nagler DG Cyr (1998) ArticleTitleEffects of exposure to contaminated sediments on the parasite fauna of American plaice (Hippoglossoides platessoides) Bulletin of Environmental Contamination and Toxicology 61 88–95
CP McCahon MJ Poulton (1991) ArticleTitleLethal and sub-lethal effects of acid, aluminum and lime on Gammarus pulex during repeated simulated episodes in a Welsh stream Freshwater Biology 25 169–178
CP McCahon SJ Maund MJ Poulton (1991) ArticleTitleThe effect of the acanthocephalan parasite (Pomphorhynchus laevis) on the drift of its intermediate host (Gammarus pulex) Freshwater Biology 25 507–513
DG McCurdy MR Forbes JS Boates (1999a) ArticleTitleEvidence that the parasitic nematode Skrjabinoclava manipulates host Corophium behaviour to increase transmission to the sandpiper, Calidris pusilla Behavioral Ecology 10 351–357
DG McCurdy MR Forbes JS Boates (1999b) ArticleTitleTesting alternative hypotheses for variation in amphipod behaviour and life history in relation to parasitism International Journal for Parasitology 29 1001–1009
GA Messick JD Shields (2000) ArticleTitleEpizootiology of the parasitic dinoflagellate Haematodinium sp. in the American blue crab Callinectes sapidus Diseases of Aquatic Organisms 43 139–152
A Moles (1983) ArticleTitleEffect of parasitism by mussel glochidia on growth of coho salmon Transactions of the American Fisheries Society 112 201–204
A Moles N Hale (2003) ArticleTitleUse of physiological responses in Mytilus trossulus as integrative bioindicators of sewage pollution Marine Pollution Bulletin 46 954–958
JF Morado AK Sparks (1990) Preliminary report on the diseases and parasites of juvenile walleye pollock, Theragra chalcogramma, from the Gulf of Alaska FO Perkins TC Cheng (Eds) Pathology in Marine Science Academic Press San Diego, CA 201–213
F Moravec M Gelnar R Ergens T Scholz (1997) ArticleTitleMetazoan parasites of fishes from the section of the Vltava River supposed to be affected by the operation of the Temelín nuclear electric power-station, Czech Republic Acta Societatis Zoologicae Bohemicae 61 65–76
NJ Morley M Crane JW Lewis (2001a) ArticleTitleToxicity of cadmium and zinc to Diplostomum spathaceum (Trematoda: Dilpostomidae) cercarial survival International Journal for Parasitology 31 1211–1217
NJ Morley M Crane JW Lewis (2001b) ArticleTitleToxicity of cadmium and zinc to encystment and in vitro excystment of Parorchis acanthus (Digenea: Philophthalmidae) Parasitology 122 75–79
NJ Morley M Crane JW Lewis (2001c) ArticleTitleToxicity of cadmium and zinc to miracidia of Schistosoma mansoni Parasitology 122 81–85
NJ Morley M Crane JW Lewis (2002) ArticleTitleToxic effects of cadmium and zinc on the transmission of Echinoparyphium recurvatum cercariae Journal of Helminthology 76 157–163
NJ Morley M Crane JW Lewis (2003a) ArticleTitleEffects of cadmium and zinc on orientation behaviour of Echinoparyphium recurvatum (Digenea: Echinostomatidae) cercariae Diseases of Aquatic Organisms 56 89–92
NJ Morley M Crane JW Lewis (2003b) ArticleTitleToxicity of cadmium and zinc to the cercarial activity of Diplostomum spathaceum (Trematoda: Diplostomidae) Folia Parastiologica 50 57–60
NJ Morley M Crane JW Lewis (2003c) ArticleTitleToxicity of cadmium and zinc to the decaudised cercarial life-span of Diplostomum spathaceum (Trematoda: Diplostomidae) Parasitology 127 497–506
DT Nolan P Reilly SE Wendelaar Bonga (1999) ArticleTitleInfection with low numbers of the sea louse Lepeophtheirus salmonis induces stress-related effects in postsmolt Atlantic salmon (Salmo salar) Canadian Journal of Fisheries and Aquatic Sciences 56 947–959
Overstreet, RM (1985) Some parasitological aspects of shrimp culture in the United States, In: Parasitology and Pathology of Marine Organisms of the World Ocean, Hargis, WJ, Jr (editor), National Oceanic and Atmospheric Administration (NOAA) Technical Report NMFS25, NOAA, Seattle, WA, pp 117–122
RM Overstreet (1997) ArticleTitleParasitological data as monitors of environmental health Parassitologia 39 169–175
RM Overstreet HD Howse (1977) ArticleTitleSome parasites and diseases of estuarine fishes in polluted habitats of Mississippi Annals of the New York Academy of Science 298 427–462
EP Papapanagiotou JP Trilles (2001) ArticleTitleCymothoid parasite Ceratothoa parallela inflicts great losses on cultured gilthead sea bream Sparus aurata in Greece Diseases of Aquatic Organisms 45 237–239
I Paperna A Diamant RM Overstreet (1984) ArticleTitleMonogenean infestations and mortality in wild and cultured Red Sea fishes Helgoländer Meeresuntersuchungen 37 445–462
D Pascoe P Cram (1977) ArticleTitleThe effect of parasitism on the toxicity of cadmium to the three-spined stickleback, Gasterosteus aculeatus L Journal of Fish Biology 10 467–472
AF Pasternak K Pulkkinen VN Mikheev T Hasu ET Valtonen (1999) ArticleTitleFactors affecting abundance of Triaenophorus infection in Cyclops strenuus, and parasite-induced changes in host fitness International Journal for Parasitology 29 1793–1801
KR Patterson (1996) ArticleTitleModelling the impact of disease-induced mortality in an exploited population: the outbreak of the fungal parasite Ichthyophonus hoferi in the North Sea herring (Clupea harengus) Canadian Journal of Fisheries and Aquatic Sciences 53 2870–2887
S Pearre SuffixJr (1979) ArticleTitleNiche modification in Chaetognatha infected with larval trematodes (Digenea) Internationale Revue der gesamten Hydrobiologie 64 193–206
SC Pennings RM Callaway (2002) ArticleTitleParasitic plants: parallels and contrasts with herbivores Oecologia 131 479–489
L Pennycuick (1971) ArticleTitleQuantitative effects of three species of parasites on a population of three-spined sticklebacks, Gasterosteus aculeatus Journal of Zoology, London 165 143–162
II Perevozchenko ON Davydov (1974) ArticleTitleDDT in some fish cestodes Hydrobiological Journal 10 72–75
M Pietrock DJ Marcogliese (2003) ArticleTitleFree-living endohelminth stages: at the mercy of environmental conditions Trends in Parasitology 19 293–299
M Pietrock T Meinelt DJ Marcogliese CEW Steinberg (2001) ArticleTitleInfluence of aqueous sediment extracts from the Oder River (Germany/Poland) on survival of Diplostomum sp. (Trematoda: Dipostomidae) cercariae Archives of Environmental Contamination and Toxicology 40 327–332
M Pietrock DJ Marcogliese JD McLaughlin (2002a) ArticleTitleEffects of cadmium upon longevity of Diplostomum sp. (Trematoda: Diplostomidae) cercariae Chemosphere 47 29–33
M Pietrock DJ Marcogliese T Meinelt JD McLaughlin (2002b) ArticleTitleEffects of mercury and chromium upon longevity of Diplostomum sp. (Trematoda: Diplostomidae) cercariae Parasitology Research 88 225–229
SJ Plaistow J-P Troussard F Cézilly (2001) ArticleTitleThe effect of the acanthocephalan parasite Pomphorhynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex International Journal for Parasitology 31 346–351
MD Powell D Fisk BF Nowak (2000) ArticleTitleEffects of graded hypoxia on Atlantic salmon infected with amoebic gill disease Journal of Fish Biology 57 1047–1057
R Poulin (1993) ArticleTitleAge-dependent effects of parasites on anti-predator responses in two New Zealand freshwater fish Oecologia 96 431–438
R Poulin (1994) ArticleTitleMate choice decisions by parasitized female upland bullies, Gobiomorphus breviceps Proceedings of the Royal Society of London, Series B 256 183–187
K Pulkkinen AF Pasternak T Hasu ET Valtonen (2000) ArticleTitleEffect of Triaenophorus crassus (Cestoda) infection on behavior and susceptibility to predation of the first intermediate host Cyclops strenuus (Copepoda) Journal of Parasitology 86 664–667
DC Radabaugh (1980) ArticleTitleChanges in minnow, Pimephales promelas Rafinesque, schooling behaviour associated with infections of brain-encysted larvae of the fluke, Ornithodiplostomum ptychocheilus Journal of Fish Biology 16 621–628
R Rahkonen J Aalto P Koski J Särkkä K Juntunen (1996) ArticleTitleCestode larvae Diphylobothrium dendriticum as a cause of heart disease leading to mortality in hatchery-reared sea trout and brown trout Diseases of Aquatic Organisms 25 15–22
LW Reimer (1995) ArticleTitleParasites especially of piscean hosts as indicators of the eutrophication in the Baltic Sea Applied Parasitology 36 124–135
MR Riggs D Lemly GW Esch (1987) ArticleTitleThe growth, biomass, and fecundity of Bothriocephalus acheilognathi in a North Carolina cooling reservoir Journal of Parasitology 73 893–900
R Rosen TA Dick (1983) ArticleTitleDevelopment and infectivity of the procercoid of Triaenophorus crassus Forel and mortality of the first intermediate host Canadian Journal of Zoology 61 2120–2128
H Rosenthal (1967) ArticleTitleParasites in larvae of the herring (Clupea harengus L.) fed with wild plankton Marine Biology 1 10–15
AE Rumpus CR Kennedy (1974) ArticleTitleThe effect of the acanthocephalan Pomphorhynchus laevis upon the respiration of its intermediate host, Gammarus pulex Parasitology 68 271–284
EA Rumyantsev (1997) ArticleTitleFish parasites as ecological indicators of lake eutrophication Russian Journal of Ecology 28 347–350
JA Sakanari M Moser CA Reilly TP Yoshino (1984) ArticleTitleEffects of sublethal concentrations of zinc and benzene on striped bass, Morone saxatilis (Walbaum) infected with larval Anisakis nematodes Journal of Fish Biology 24 553–563
P Salathé D Ebert (2003) ArticleTitleThe effects of parasitism and inbreeding on the competitive ability in Daphnia magna: evidence for synergistic epistasis Journal of Evolutionary Biology 16 976–985
GJ Sandland CP Goater (2001) ArticleTitleParasite-induced variation in host morphology: brain-encysting trematodes in fathead minnows Journal of Parasitology 87 267–272
P Sasal C Pampoulie (2000) ArticleTitleAsymmetry, reproductive success and parasitism of Pomatoschistus microps in a French lagoon Journal of Fish Biology 57 382–390
C Schludermann R Konecny S Laimgruber JW Lewis F Schiemer A Chovanec et al. (2003) ArticleTitleFish macroparasites as indicators of heavy metal pollution in river sites in Austria Parasitology 126 (Suppl) S61–S69
ME Scott (1987) ArticleTitleTemporal changes in aggregation: a laboratory study Parasitology 94 583–595
JD Shields RK Okazaki AM Kuris (1990) ArticleTitleBrood mortality and egg predation by the nemertean, Carcinonemertes epialti, on the yellow rock crab, Cancer anthonyi, in southern California Canadian Journal of Fisheries and Aquatic Sciences 47 1275–1281
R Siddall S Clers Particledes (1994) ArticleTitleEffect of sewage sludge on the miracidium and cercariae of Zoogonoides viviparus (Trematoda: Digenea) Helminthologia 31 143–153
R Siddall AW Pike AH McVicar (1993) ArticleTitleParasites of Buccinum undatum (Mollusca: Prosobranchia) as biological indicators of sewage-sludge dispersal Journal of the Marine Biological Association of the United Kingdom 73 931–948
R Siddall AW Pike AH McVicar (1994) ArticleTitleParasites of flatfish in relation to sewage sludge dumping Journal of Fish Biology 45 193–209
P Sirois JJ Dodson (2000) ArticleTitleInfluence of turbidity, food density and parasites on the ingestion and growth of larval rainbow smelt Osmerus mordax in an estuarine turbidity maximum Marine Ecology Progress Series 193 167–179
MA Solangi RM Overstreet (1980) ArticleTitleBiology and pathogenesis of the coccidium Eimeria funduli infecting killifishes Journal of Parasitology 66 513–526
A Soleng ABS Poléo NEW Alstad TA Bakke (1999) ArticleTitleAqueous aluminium eliminates Gyrodactylus salaris (Platyhelminthes, Monogenea) infections in Atlantic salmon Parasitology 119 19–25
G Sprengel H Luchtenberg (1991) ArticleTitleInfection by endoparasites reduces maximum swimming speed of European smelt Osmerus eperlanus and European eel Anguilla anguilla Diseases of Aquatic Organisms 11 31–35
K Stoltze Buchmann (2001) ArticleTitleEffect of Gyrodactylus derjavini infections on cortisol production in rainbow trout fry Journal of Helminthology 75 291–294
T Sulgostowska G Banaczyk B Grabda-Kazubska (1987) ArticleTitleHelminth fauna of flatfish (Pleuronectiformes) from Gdansk Bay and adjacent areas (south-east Baltic) Acta Parasitologica Polonica 31 231–240
T Sulgostowska B Jerzewska J Wicikowski (1990) ArticleTitleParasite fauna of Myoxocephalus scorpius (L.) and Zoarces viviparus (L.) from environs of Hel (south-east Baltic) and seasonal occurrence of parasites Acta Parasitologica Polonica 35 143–148
B Sures N Riemann (2003) ArticleTitleAnalysis of trace metals in the Antarctic host-parasite system Notothenia coriiceps and Aspersentis megarhynchus (Acanthocephala) caught at King George Island, South Shetland Islands Polar Biology 26 680–686
B Sures R Siddall H Taraschewski (1999) ArticleTitleParasites as accumulation indicators of heavy metal pollution Parasitology Today 15 16–21
B Sures H Taraschewski J Rokicki (1997) ArticleTitleLead and cadmium content of two cestodes, Monobothrium wageneri and Bothriocephalus scorpii, and their fish hosts Parasitology Research 83 618–623
B Sures S Zimmerman C Sonntag D Stüben H Taraschewski (2002) ArticleTitleThe acanthocephalan Paratenuisentis ambiguous as a sensitive indicator of the precious metals Pt and Rh from automobile catalytic converters Environmental Pollution 122 401–405
AJ Szalai TA Dick (1991) ArticleTitleRole of predation and parasitism in growth and mortality of yellow perch in Dauphin Lake, Manitoba Transactions of the American Fisheries Society 120 739–751
J Taskinen (1998) ArticleTitleInfluence of trematode parasitism on the growth of a bivalve host in the field International Journal for Parasitology 28 599–602
A Théron H Moné C Gérard (1992) ArticleTitleSpatial and energy compromise between host and parasite: the Biomphalaria glabrata-Schistosoma mansoni system International Journal of Parasitology 22 91–94
F Thomas R Poulin (1998) ArticleTitleManipulation of a mollusc by a trophically transmitted parasite: convergent evolution or phylogenetic inheritance? Parasitology 116 431–436
U Tillman K-J Hesse A Tillman (1999) ArticleTitleLarge-scale parasitic infection of diatoms in the Northfrisian Wadden Sea Journal of Sea Research 42 255–261
JW Treasurer T Turnbull (2000) ArticleTitleThe pathology and seawater performance of farmed Atlantic salmon infected with glochidia of Margaritifera margaritifera Journal of Fish Biology 57 858–866
ET Valtonen JC Holmes M Koskivaara (1997) ArticleTitleEutrophication, pollution, and fragmentation: effects on parasite communities in roach (Rutilus ruitlus) and perch (Perca fluviatilis) in four lakes in central Finland Canadian Journal of Fisheries and Aquatic Sciences 54 572–585
P van Banning OLM Haenen (1990) Effects of the swimbladder nematode Anguillicola crassus in wild and farmed eel, Anguilla anguilla FO Perkins TC Cheng (Eds) Pathology in Marine Science Academic Press San Diego, CA 317–330
SA Vance (1996) ArticleTitleThe effect of the mermithid parasite Gasteromermis sp. (Nematoda: Mermithidae) on the drift behaviour of its mayfly host, Baetis bicaudatus (Ephemeroptera: Baetidae): a trade-off between avoiding predators and locating food Canadian Journal of Zoology 74 1907–1913
S Vance BL Peckarsky (1997) ArticleTitleThe effect of mermithid parasitism on predation of nymphal Baetis bicaudatus (Ephemeroptera) by invertebrates Oecologia 110 147–152
I Varo AC Taylor JC Navarro F Amat (2000) ArticleTitleEffect of parasitism on respiration rates of adults of different Artemia strains from Spain Parasitology Research 86 772–774
GE Vaughan DW Coble (1975) ArticleTitleSublethal effects of three ectoparasites on fish Journal of Fish Biology 7 283–294
JO Washburn DR Mercer JR Anderson (1991) ArticleTitleRegulatory role of parasites: impact on host population shifts with resource availability Science 253 185–188
AM Wegeberg KT Jensen (1999) ArticleTitleReduced survivorship of Himasthla (Trematoda, Digenea)-infected cockles (Cerastoderma edule) exposed to oxygen deficit Journal of Sea Research 42 325–331
ME White EN Powell CL Kitting (1984) ArticleTitleThe ectoparasitic gastropod Boonea(= Odostomia) impressa: population ecology and the influence of parasitism on oyster growth rates Marine Ecology 5 283–299
RL Willey PA Cantrell ST Threlkeld (1990) ArticleTitleEpibiotic euglenoid flagellates increase the susceptibility of some zooplankton to fish predation Limnology and Oceanography 35 952–959
HH Williams KJ MacKenzie (2003) ArticleTitleMarine parasites as pollution indicators: an update Parasitology 126 (Suppl) S27–S41
MA Williams MC Penlington JA King D Hoole CT Arme (1998) ArticleTitle Ligula intestinalis (Cestoda) infection of roach (Rutilus rutilus) (Cyprinidae): immunocytochemical investigations into the salmon- and chicken-II type gonadotrophin-releasing hormone (GnRH) systems in host brains Acta Parasitologica 43 232–235
CT Wolmarans E Yssel VL Hamilton-Attwell (1988) ArticleTitleToxic effects of chromium on Schistosoma haematobiutm miracidia Bulletin of Environmental Contamination and Toxicology 41 928–935
CD Zander (1998) ArticleTitleEcology of host parasite relationships in the Baltic Sea Naturwissenschaften 85 426–436
DA Zelmer GW Esch (1998) ArticleTitleInteractions between Halipegus occidualis and its ostracod second intermediate host: evidence for castration? Journal of Parasitology 84 778–782
S Zimmermann B Sures H Taraschewski (1999) ArticleTitleExperimental studies on lead accumulation in the eel-specific endoparasites Anguillicola crassus (Nematoda) and Paratenuisentis ambiguus (Acanthocephala) as compared with their host, Anguilla anguilla Archives of Environmental Contamination and Toxicology 37 190–195
Acknowledgments
I thank Drs. Jenny Cook, Peter Daszak, Michael Pietrock, and two reviewers for providing helpful comments on the manuscript. I am grateful to Dr. Frank Morado for the invitation to present the opening address at a special symposium of the American Fisheries Society in Phoenix, Arizona in August 2001, upon which this work is based.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Tables 3, 4, 5, 6 and additional references for this article are available for viewing by subscribers only at http://www.springerlink.com
Rights and permissions
About this article
Cite this article
Marcogliese, D. Parasites: Small Players with Crucial Roles in the Ecological Theater. EcoHealth 1, 151–164 (2004). https://doi.org/10.1007/s10393-004-0028-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-004-0028-3