Abstract
Psalidodon paranae is an endemic small Characidae from the Upper Paraná River ecoregion (Neotropical region, Brazil), where many watercourses are affected by anthropic activities. The Verde River (VR) was chosen as a model to study the feeding trends of P. paranae. Based on empirical observations, hypotheses were set concerning its occurrence, the environmental representativeness of its diet and the multifactors modulating ontogenetic, spatial, and temporal diet variations. To evaluate these hypotheses for the first time, an integrative approach was applied (modelling and multivariate techniques). Standardized samplings were performed monthly during one year at four sites. Influence of environmental variables on fish distribution was evaluated by means a general linear model. Stomach content analysis of P. paranae allowed the calculation of gravimetric frequency of consumed food categories. Ontogenetic, spatial, and temporal differences on diet were evaluated by means a permutational multivariate analysis of variance, and the influence of environmental variables on them with a canonical correspondence analysis. From the total of 301 specimens caught (4.6–13.4 cm total length range), 216 individuals of P. paranae presented stomach with contents. The species consumed 32 different food categories in VR, highlighting aquatic and terrestrial angiosperms, and beetles. The methods applied identified significative spatial and temporal differences in P. paranae diet as result of multifactors (palaeogeomorphology, abiotic, biotic, and anthropic) operating in the VR. Heterogeneity and complexity of VR and P. paranae occurrence and feeding trends evidenced that the opportunistic and generalist behavior lead to intrinsic patterns of each fish population at each watercourse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population dynamics of freshwater fishes are controlled by selective pressures from stochastic and deterministic multifactors operating in the watersheds, related to palaeogeomorphology, abiotic and biotic factors (Jackson et al. 2001). There are four broad categories concerning resources acquired to sustain fish population dynamics: maintenance, production of somatic tissues, reproduction, and energy reserves (Souza et al. 2015). Trophic ecology is the basis for these processes, operating in a strong integrative way that affects fish distribution, and influences intra- (i.e., ontogeny) and interspecific interactions (i.e., competition, resources partition and predation) (Matthews 1998; Wootton 1998). Because of this, it is one of the most important aspects about species bionomy, influencing the structure and composition of fish populations (Windell and Bowen 1978; Krebs 2014), and supporting other important approaches at community and ecosystem levels (Gelwick and McIntyre 2017).
The Neotropical region presents a high diversity of freshwater habitats (Lowe-McConnell 1987) and, as consequence it has the most diverse freshwater ichthyofauna of the world, around to 5100 species (Reis et al. 2016). In the Southeastern Neotropical region, the Upper Paraná River ecoregion (UPRE, Abell et al. 2008) comprises more than 300 fish species, many of them endemic (Langeani et al. 2007; Albert et al. 2011), and with diverse feeding strategies associated with high trophic plasticity (Abelha et al. 2001). Notwithstanding, UPRE is highly affected by anthropic activities (Agostinho et al. 2007). Among the rivers that compose the UPRE, the Verde River (VR) was a third order tributary chosen as a model to investigate community (results already available in Silveira et al. 2018) and population dynamics responses to multifactors (i.e., palaeogeomorphology, abiotic and biotic) operating along the river. But why VR?
During the second half of twentieth century, VR fishes used to be exploited by the local community for subsistence (i.e., feeding and commercialization) and recreationally. In the last 20–30 years, an empirical reduction of fish populations was perceived. At the same time, VR and its surrounding landscapes has been growly exploited for agriculture, livestock, and urban zoning, receiving treated and untreated effluents (Rocha and Weirich Neto 2010; Silveira et al. 2018). Ecologically, it presents many of the features found in watercourses from the Neotropical region and UPRE, with high diversity of habitats along its course that includes canyon formations and sets of riffles, pools and cascades, with several lotic and lentic habitats. For conservation purposes, VR is partially into two important protected areas (PAs), the Campos Gerais National Park and the Devonian Escarpment Environmental Protection Area. This set of elements becomes the VR an excellent model to represent Neotropical watercourses that mix natural, rural and urban zones, comprising “the problem identification and consideration of the possible causes of the problem” (Zale et al. 2012).
The lambari, Psalidodon paranae Eigenmann, 1914, is endemic from streams and rivers of UPRE (Garutti and Britski 2000). Formerly named Astyanax paranae, a taxonomic revision reintroduced the genus Psalidodon (Terán et al. 2020, also accepted by Fricke et al. 2020). In this way, this is the one of the first studies using the name P. paranae. It is an opportunistic and generalist fish species (Ferreira 2007; Carvalho et al. 2015; Leite and Silva 2018). In streams, its diet is modulated according to the environmental conditions (Ferreira 2007; Ferreira et al. 2012), an ability also recorded in reservoirs (Abelha et al. 2006). Due to its ontogenetic, spatial, and temporal trophic plasticity (Esteves 1996; Vilella et al. 2002; Bennemann et al. 2005), allied to its high frequency and abundance at VR (Silveira et al. 2018), P. paranae was selected to be studied in terms of trophic ecology. It was hypothesized that: (i) Psalidodon paranae, as an opportunistic and generalist fish species, would be found along the entire watercourse; (ii) its trophic ecology will represent the heterogeneity of VR, in terms of diversity of aquatic habitats and distinct degrees of environmental conditions (i.e., water quality and habitat complexity); and (iii) multifactors will modulate ontogenetic, spatial and temporal fluctuations on its diet.
To evaluate these hypotheses, traditional and more recent methods were combined and applied in an integrative approach. Datasets were frequency of P. paranae and stomach content analyses based on gravimetric frequency (Garvey and Chipps 2012; Gelwick and McIntyre 2017). Gravimetric frequency was individually calculated, ensuring data variability (i.e., each individual stomach as a sampling unit) (Chipps and Garvey 2007; Silveira et al. 2020). The integrative approach included additive General Linear Model (GLM) (Venables and Dichmont 2004; Zuur et al. 2009) and multivariate techniques (ter Braak 1986; Anderson 2001, 2014; Legendre and Legendre 2012) to analyze fluctuations in datasets and their relationships with environmental variables.
Materials and methods
Study area
This study was performed in Verde River (Fig. 1) in the context of the project “Structure of assemblages and population dynamics of the Neotropical ichthyofauna from a Paranaense microbasin, Southern Brazil”, developed to attend a research call for environmental evaluation and conservation purposes. Systematic samplings were taken monthly from May/2016 to April/2017 in four sampling sites, considering the project objectives and outline (Zale et al. 2012).
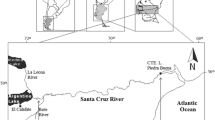
Verde River basin and sampling sites: a) South America and the Upper Paraná River Ecoregion (UPRE, in black); b) Tibagi River Basin (TRB, in black) in UPRE; c) Pitangui River Basin (PRB, in black) in TRB; d) Verde River (in black) in the PRB; e) Hydrography; f) Devonian Escarpment Environmental Protection Area; g) Campos Gerais National Park; h) Water flow direction. (Modified from Silveira et al. 2018)
Sampling sites were characterized by means of a rapid bioassessment protocol [RBP, Supplementary Material, adapted from Barbour et al. (1999) and Casatti et al. (2006)]. Headwater (site 1, 25°06′19.67″S 50°01′23.21″W, Fig. 2a) is composed by young and shallow soils (Humic Litholic Neosols, less than 0.1 m depth; Bhering et al. 2007; Sá 2007; Guimarães et al. 2014) that supporting steppe-type vegetation (Moro and Carmo 2010). Headwater stretch is lentic and presents sandy riverbed, well-preserved riverbanks and relatively preserved riparian vegetation. Riverbanks have submerged roots and branches, in addition to branches and leaves touching the water surface. Downstream this sampling site there are a series of riffles and cascades followed by a small dam (2 m height) and a largest cascade (10 m height) that constitute ecological barriers for fish upstream displacements (Silveira et al. 2018).
Middle and river mouth stretches are composed by relatively young soils (Haplic Cambiosol, less than 1.0 m deep; Bhering et al. 2007; Sá 2007; Guimarães et al. 2014), supporting more complex vegetations. Upper middle stretch (site 2, 25°04′46.29″S 50°04′56.53″W, Fig. 2b) is composed by riffle-pool sequences and series of small cascades. Riverbed is complex, presenting riffles associated with rocky substrates, and pools associated with plant debris (e.g., branches, roots) and sandy substrates. Riparian vegetation is relatively preserved, presenting steppe-type vegetation and Mixed Ombrophilous Forest (Moro and Carmo 2007). Riverbanks are formed by rocky outcrops and are relatively well-preserved. Headwater and upper middle stretches are surrounded by intensive and semi-intensive agricultural crops in which inadequate handling soils and indiscriminate use of agrochemicals are common (Rocha and Weirich Neto 2010).
Between sites 2 and 3 there are agricultural areas close to the river and sloping areas occupied by urban zoning, where the original vegetation was replaced by grass. At the lower middle stretch (site 3, 25°03′26.11″S 50°07′25.06″W, Fig. 2c), river structure also is composed by riffle-pool sequences and small cascades. Nonetheless, riffle stretches are short, and pools are in advanced siltation process. Riparian vegetation is composed by relatively few preserved Mixed Ombrophilous Forest, with few individuals in contact with water. Riverbanks are eroded and accumulate tree debris (e.g., trunks and branches) and garbage. Between sites 3 and 4, the Pilão de Pedra and Lajeado Grande Streams flow to the RV. These VR tributaries cross extensive urban areas, being contaminated by garbage and untreated sewage (Sequinel et al. 2011).
Immediately upstream the site 4, VR receives treated sewage effluents from the municipal sewage treatment plant (Sanitation Company of Paraná - SANEPAR). At the river mouth (site 4, 25°02′28.85″S 50°07′35.59″W, Fig. 2d), riverbed is silted, presenting great amounts of sand and sludge. Riverbanks are eroded and present garbage and some tree debris in contact with water. Riparian vegetation is formed by degraded Mixed Ombrophilous Forest.
Data collection
Standardized monthly samplings of fishes and environmental variables were conducted in a synoptic design at the four sites above described. For fish sampling, a pilot survey in VR revealed the impossibility to use electrofishing due to low conductivity values (median of 15 μS/cm, average ± standard deviation of 44.95 ± 62.82 μS/cm), then requiring the use of passive and active fishing gears (Lagler 1978; Zale et al. 2012). Passive gears included gillnets (1.2-, 1.5-, 2-, 3-, 4-, 5- and 6 cm) with 24 h of sampling effort (between Saturday 17:00 and Sunday 17:00). Fish removals occurred at each eight hours. Active gears included cast net (1.2 cm mesh) and scoop net (38 cm opening, 40 cm deep and 0.4 cm mesh) with one hour of sampling effort (30 min day and 30 min night). Fishes were anesthetized with benzocaine (250 mg/L) and sacrificed in field (license of the Chico Mendes Institute for Biodiversity conservation no. 40132–2 and no. 51797–1; Certificate of the Ethics Committee in the Use of Animals UFPR no. 38/2015). At laboratory, specimens were measured (total length, TL, 0.01 cm) and eviscerated. Stomachs were fixed in formalin 4% for ulterior analyses.
In every fish sampling, environmental variables were measured/collected (Table 1) in the same hour and in the same stretch of each sampling site (standardized procedures). Dissolved oxygen and pH were obtained using an oximeter LT Lutron DO-5519. Water samples were collected using one-liter bottles, previously sterilized (nitric acid 10% v/v during 24 h followed by rinse using distilled water), in triplicates to determine the concentrations of dissolved chlorides, total ammonia nitrogen and total suspended solids. Bottles were opened and closed underwater (~10 cm depth). Each bottle was packaged in an individual plastic bag, placed in an ice box, and transported to laboratory. Physical parameters were measured according to standard methodology (Baird et al. 2017). River depth and width, and water velocity were measured as well as habitat structure and complexity (sensu Barbour et al. 1999; Casatti et al. 2006), these last based on riverbed complexity, riverbank stability and riparian vegetation integrity (all of them described in RBP, Supplementary Material).
Spatial and temporal fish occurrence
Selectivity of different fishing gears can restrict the data pooling for analyses, which must be tested a priori (Lagler 1978; Fauconnet et al. 2015). In this sense, there were no differences among abundances (Kruskal-Wallis test, H = 1.904, p value = 0.386) and variances (Levene test, L = 0.31, p value = 0.738) of P. paranae by length classes and fishing gears. Spatial and temporal differences in the lambari occurrence were analyzed using length frequency distributions (length classes from 4 to 13 cm) by sites (1, 2, 3 and 4) and quarters (winter: May/July; spring: August/October; summer: November/January; autumn: February/April), adopted to attain numerical sufficiency. This data was subjected to a Scheirer-Ray-Hare test followed by a post-hoc Mann-Whitney test (Sokal and Rohlf 2012).
The influence of environmental variables (transformed log x + 1) in P. paranae distribution was analyzed using a generalized linear model with negative binomial distribution (GLM-nb). The GLM-nb avoids overdispersion and allows the analysis of a dependent variable (i.e., P. paranae occurrence by site by sampling, totalizing 48 lines) with high frequencies of small numbers and zeros (Venables and Dichmont 2004; Zuur et al. 2009). Variance Inflation Factor (VIF) was applied to remove multicollinear variables (VIF > 3). A stepwise Akaike-based selection procedure using forward and backward elimination was applied to determine the best subset of terms for the model.
Feeding study
Empty stomachs were discarded and stomachs with food were analyzed under optical stereomicroscope and microscope, both coupled to a camera and an image analyzer system. Prey items were identified to the lowest possible taxonomic level (Gelwick and McIntyre 2017; Silveira et al. 2020) by using specific references for Neotropical region (Bicudo and Bicudo 1970; Domínguez and Fernández 2009; Hamada et al. 2014). The water excess trapped around prey items was removed with filter paper and then they were weighted in an analytical balance (Wi, 0.0001 g), and grouped in food categories.
Psalidodon paranae diet description was based on gravimetric frequency (%Wi) of consumed food categories (calculated according Chipps and Garvey 2007 and Silveira et al. 2020). Frequency of occurrence (%Oi) was additionally calculated and presented as a comparative referential to other studies in the Supplementary Material. Ontogenetic (i.e., length classes), spatial (i.e., sites) and temporal (i.e., quarters) diet differences were tested by using Wi values (transformed log x + 1) (Sokal and Rohlf 2012) in a three-way permutational multivariate analysis of variance (PERMANOVA; Euclidian distance; 9999 permutations) (Anderson 2001, 2014). Influences of environmental variables in P. paranae diet were analyzed by means of a canonical correspondence analysis (CCA; ter Braak 1986; Legendre and Legendre 2012) using environmental variables (32 columns; score-Z transformed data) as exploratory matrix, and Wi values (216 lines; transformed log x + 1 data) as response matrix.
All analyses were done with R 3.6.3 version (R Core Team 2020). Functions krustal.test (stats package, R Core Team 2020) and leveneTest (car package; Fox and Weisberg 2019) were used to test data from different fishing gears. Functions ScheirerRayHare (rcompanion; Mangiafico 2019) and wilcox.test (stats; R Core Team 2020) were used for population distribution analyses. Functions glm.nb (MASS; Venables and Ripley 2002), vif (car), and simulatedResiduals and testResiduals (DHARma; Hartig 2020) were used to perform GLM-nb, remove multicollinear variables and model validation, respectively. Functions adonis and cca (vegan; Oksanen et al. 2019) were used to perform PERMANOVA and CCA, respectively.
Results
Spatial and temporal fish occurrence
A total of 301 P. paranae specimens with total length ranging from 4.6 to 13.4 cm were caught in the Verde River (headwater: 135; upper middle stretch: 162; lower middle stretch: 4) (Fig. 3). No specimens were recorded at river mouth. There were remarkable differences in the spatial distribution of P. paranae (H = 28.1867, p value <0.0001) (Table 2) due to lower number of individuals at lower middle stretch in relation to the other sampling sites (site 1 vs. 3: U = 5.0311; p value <0.0001; site 2 vs. 3: U = 5.9662; p value <0.0001).
The distribution of P. paranae was significantly affected by environmental variables regarding river structure and water quality (Table 3). It was positively correlated with river depth (p value <0.0001) and negatively correlated with river width (p value = 0.0337) and ammoniacal nitrogen concentration (p value = 0.0491). Statistically, dissolved oxygen concentration did not affect P. paranae distribution (p value = 0.0519). Other VR environmental variables (see Table 1) were removed from the model due to their multicollinearity (identified via VIF analyses) and low explicative power (analyzed via stepwise Akaike-based selection). The analysis of standardized residuals indicated model adequacy (details in Supplementary Material).
Feeding study
A total of 216 individuals of P. paranae presented stomach with contents, 94 at headwater and 122 at upper middle stretch (Table 4). No individuals with stomach contents were recorded at the lower middle stretch, and this site was not considered in the following results. Psalidodon paranae consumed 32 different food categories in the VR (Table 4). The most important food categories concerning weights were Aquatic Angiosperms (%Wi = 17.2%), Coleoptera (%Wi = 15.8%), Terrestrial Angiosperms (%Wi = 14.6%), Hymenoptera (%Wi = 9.4%) and Insect fragments (%Wi = 8.7%). Plastic debris occurred in only one stomach at headwater. Bacillariophyta, Bryophyta, Bivalvia, Isopoda, Blattodea, Isoptera and Neuroptera were exclusively consumed at the upper middle stretch. Five individuals from headwater and twelve from upper middle stretch showed nematode parasite infestation in their stomachs. Frequency of occurrence of consumed food categories is presented in Supplementary Material.
Ontogenetic dietary shifts were not significant (PERMANOVA, F = 1.0677, p = 0.2930), but they were between sites (F = 4.2363, p = 0.0005) and among quarters (F = 2.0454, p = 0.0207) (Table 5). Despite of this, some ontogenetic tendencies can be highlighted. Lambaris in the length class of 4 cm mainly consumed terrestrial invertebrates (Muscidae: %Wi = 94.7), while those from length class of 5 cm mainly ingested Coleoptera (%Wi = 17.8), Insect fragments (%Wi = 17.1), Hymenoptera (%Wi = 15.4) and Simuliidae (%Wi = 13.4). From the length class of 6 cm, P. paranae gradually increased the consumption of plants, highlighting Aquatic (Podostemaceae) and Terrestrial Angiosperms (fruits/seeds and leaves). Notwithstanding, animal resources were still important in the diet of the bigger length classes, highlighting terrestrial (Coleoptera and Hymenoptera) and aquatic insects (Chironomidae larvae).
Spatial and temporal trends in P. paranae diet together to influence of environmental variables were revealed by CCA (Fig. 4). Like in the descriptive analysis, the same diet tendencies were summarized by the four first CCA axes (73.0% of the constrained inertia), allowing a detailed diagnosis.
Results of CCA revealing relationship between environmental variables and consumed food categories by Psalidodon paranae in the Verde River. Spatial tendencies regarding (a) axes 1 and 2 and (b) axes 3 and 4. Temporal tendencies regarding (c) axes 1 and 2 and (d) axes 3 and 4. Cyano = Cyanophyta; Bacyl = Bacillariophyta; Bryoph = Bryophyta; AquaAng = Aquatic Angiosperm; TerAng = Terrestrial Angiosperm; PlanfFrag = Plant fragments; Oligo = Oligochaeta; Bival = Bivalvia; Isopd = Isopoda; Arane = Araneae; Blatt = Blattodea; Coleop = Coleoptera; Cerato = Ceratopogonidae; Chiro = Chironomidae; Musc = Muscidae; Psycho = Psychodidae; Simul = Simuliidae; Ephem = Ephemeroptera; Hemip = Hemiptera; Hymeno = Hymenoptera; Isopt = Isoptera; Lepidop = Lepidoptera; Neurop = Neuroptera; Odon = Odonata; Trichop = Trichoptera; InsctFrag = Insect fragments; InsctUnidtf = Unidentified insects; InvEgg = Invertebrate eggs; Teleo = Teleostei; Plast = Plastic debris; Det/Sed = Detritus/Sediments; Unidtf = Unidentified matters; O2 = dissolved oxygen; Cl = dissolved chlorides; AmnNit = ammoniacal nitrogen; TotSol = total suspended solids; Depth = river depth; Width = river width; WatVel = water velocity; SbstCmpx = substrate complexity; RipIntg = riparian vegetation integrity
Spatial tendencies showed that although P. paranae consumed similar food categories both in headwater and upper middle stretches, its diet was more diverse at site 2. Negative axis 1 (25.4%; Fig. 4a, see also Table 4) retained several food resources exclusively consumed at the upper middle stretch, including Isoptera (%Wi = <0.1%), Bryophyta (%Wi = <0.1%), Neuroptera (%Wi = 0.1%), Isopoda (%Wi = 0.4%) and Blattodea (%Wi = 0.5%). On the other hand, positive axis 1 highlighted food resources higher exploited at the headwater than upper middle stretch, including Teleostei (%Wi = 3.5% vs. %Wi = 0.5%), Coleoptera (%Wi = 23.4% vs. %Wi = 10.0%) and Lepidoptera (%Wi = 2.9% vs. %Wi = 1.0%). Higher values of substrate complexity and dissolved chlorides were associated with the upper middle stretch (negative axis 1) while higher values of dissolved oxygen and riparian integrity were associated with the headwater (positive axis 1). The same trends were evidenced in axis 2 (17.9%) that highlighted food resources higher exploited at the headwater than upper middle stretch, including Plant fragments (%Wi = 4.4% vs. %Wi = 2.4%), Oligochaeta (%Wi = 4.6% vs. %Wi = 0.8%), Simuliidae (%Wi = 1.8% vs. %Wi = 0.2%), Invertebrate eggs (%Wi = 0.9% vs. %Wi = <0.1%) and Teleostei. Indeed, the ratio plant-animal consumption (%Wplant / %Wanimal) was lower at the headwater (0.37) than upper middle stretch (1.04), indicating high consumption of animal resources at the headwater and a balanced exploitation of animal and plant resources at the upper middle stretch.
Axis 3 (15.4%; Fig. 4b) highlighted food categories exclusively consumed at the upper middle stretch, including Bivalvia (%Wi = 0.2%), Bryophyta and Isoptera. This axis also highlighted foods categories consumed at the upper middle stretch in higher proportions than headwater, including Psychodidae (%Wi = 0.2% vs. %Wi = 0.1%), Ceratopogonidae (%Wi = 0.2% vs. %Wi = 0.1%), Chironomidae (%Wi = 2.7% vs. %Wi = 1.2%), Hymenoptera (%Wi = 10.5% vs. %Wi = 8.1%), Aquatic Angiosperms (%Wi = 16.2% vs. %Wi = 12.5%) and Detritus/Sediments (%Wi = 6.5% vs. %Wi = 5.0%). Axis 4 (14.2%) showed the same trends described for axes 1 and 3 concerning food categories consumed at the upper middle stretch. It is noteworthy that its positive side highlighted the exclusive consumption of Bacillariophyta at the upper middle stretch (%Wi = 1.3%), and a higher consumption of Muscidae (%Wi = 1.23% vs. %Wi = 0.54%) and Simuliidae at the upper middle stretch than headwater. Water velocity, substrate complexity and suspended solids were associated with the consumption of aquatic food resources at the upper middle stretch, whilst riparian integrity was related to consumption of terrestrial resources, mainly at the headwater.
Temporal tendencies were revealed by subspace projections concerning the different quarters of axes 1 and 2 (Fig. 4c, see also Table 4). They indicated that P. paranae diet was structured by some food categories consumed in similar proportions along different quarters. Nonetheless, the position occupied by these different subspaces along the projections of axes 1 and 2 indicated fluctuations in the global composition of diet composition in each quarter. In general, ratio between plant-animal consumption decreased from the winter (0.83) through spring (0.65) to summer (0.44), indicating increases in consumption of animal resources. Nevertheless, this trend was reverted in autumn (0.73).
During the winter, P. paranae diet was focused on Terrestrial Angiosperms (%Wi = 21.8%), followed by Hymenoptera (%Wi = 13.9%), Aquatic Angiosperms (%Wi = 10.9%), Insect fragments (%Wi = 10.4%) and Detritus/Sediments (%Wi = 11.9%). In the spring, it mainly consumed Aquatic Angiosperms (%Wi = 25.5%) and Coleoptera (%Wi = 25.0%), in addition to Terrestrial Angiosperms (%Wi = 7.8%), Hymenoptera (%Wi = 7.7%) and Insect Fragments (%Wi = 7.3%). In the summer, Terrestrial Angiosperms were again the most exploited food category (%Wi = 17.2%), followed by Coleoptera (%Wi = 15.4%), Insect Fragments (%Wi = 12.1%), Hymenoptera (%Wi = 11.7%) and Aquatic Angiosperms (%Wi = 7.6%). In this period, decreases in Coleoptera consumption were balanced by substantial increases in the consumption of Lepidoptera (%Wi = 5.2%), Chironomidae (%Wi = 5.1%) and Teleostei (%Wi = 4.8%). Finally, during the autumn, P. paranae diet was mainly focused on Terrestrial Angiosperms (%Wi = 21.2%) followed by Aquatic Angiosperms (%Wi = 9.6%), Insect Fragments (%Wi = 8.6%) and Hymenoptera (%Wi = 7.8%).
Axes 3 and 4 (Fig. 4d) reinforced diet trends identified from winter to summer. Nevertheless, it is noteworthy that differential position of autumn subspace in relation to others was related to exclusive consumption of Isopoda and Neuroptera in this season, as well as to the higher consumption of Bacillariophyta, Oligochaeta, Araneae, Muscidae, Blattodea and Insect Unidentified in this season when compared with global diet of the others.
Discussion
Recalling the hypotheses, despite its opportunistic and generalist behavior, Psalidodon paranae occurred mainly (i.e., with constant frequency and abundance) at headwater and upper middle stretch (refusing hypothesis i). Due to this, its trophic ecology was able to represent the heterogeneity of VR (i.e., diversity of aquatic habitats and distinct degrees of environmental quality) only at those sites (partially confirming hypothesis ii), in which multifactors (i.e., palaeogeomorphologic, abiotic and biotic) modulated ontogenetic, spatial, and temporal fluctuations on P. paranae diet (confirming hypothesis iii). These findings were supported by the integrative approach chosen, following discussed.
The requirement of different fishing gears to assure diversity completeness and sample sufficiency (Ishyama et al. 2016; Silveira et al. 2018) is a reality in the Neotropical region. Although the use of multiple fishing gears could insert biases due to differential selectivity of each gear, a priori sampling planning and a posteriori statistical tests are peremptory to assure data suitability (Lagler 1978; Fauconnet et al. 2015), a care observed in the present study. The absence of significance among abundances and variances of individuals by length classes by fishing gears allowed the use of combined data for population dynamic assessments (Fauconnet et al. 2015; Silveira et al. 2018). The gravimetric frequency (%Wi) was used as a quantitative measurement to estimate the nutritional importance of each consumed food category (Garvey and Chipps 2012). Following Ahlbeck et al. (2012) and Silveira et al. (2020), the choice of %Wi considered P. paranae behaviour and its prey features, being the best method to an opportunist and generalist species that consumes a wide range of prey sizes and types (i.e., animal, plant) from aquatic and terrestrial origin. The presentation of frequency of occurrence (%Oi) as a referential to allow comparisons with other studies followed Baker et al. (2014), Buckland et al. (2017) and Silveira et al. (2020). These authors do not recommend the use of composite indices in diet assessments, reinforcing Ahlbeck et al. (2012) that modelled approaches to test the capacity of single and composite indices to describe fish diets. The occurrence and feeding data of P. paranae supported the integrative approach herein used, including GLM, PERMANOVA and CCA. These methodologies are already consolidated and were applied with straightness and care concerning premises and control quality, avoiding any misinterpretations of multifactors as confounding factors (Gotelli and Ellison 2013). Then, what biological and environmental data from VR are telling?
Several authors empirically observed that P. paranae prefers upstream sites of watercourses, arguing in favor of biological and environmental factors (Araujo-Lima and Oliveira 1998; Garutti and Britski 2000; Vilella et al. 2002; Gealh and Silveira 2014; Silveira et al. 2018). Herein, this was tested and demonstrated: P. paranae mainly occurred at the upstream sites of VR (i.e., headwater and upper middle stretch) that were deeper and narrower than downstream sites (i.e., lower middle stretch and river mouth), which are affected by siltation processes. In VR, downstream sites presented the worst environmental conditions related to water quality and habitat complexity. The combination of high values of ammoniacal nitrogen, near to reference of chronic toxicity for aquatic life (1.9 mg/L, USEPA 2013), and values of dissolved oxygen lower than the international limits set (6.5 mg/L; CCME 1999) explained the rare occurrence (at the lower middle stretch) and absence (at river mouth) of lambari at downstream sites. In addition, total suspended solids concentrations in the downstream sites were higher than the background levels recorded in upstream sites, indicating ecological risk for the aquatic biota (CCME 2002). In this way, VR sampling sites followed a gradient from “more preserved” (headwater) to “highly affected” areas (mouth river). This was empirically realized prior to this study, stimulating this research project. The results obtained confirmed the historical and continuous impacts of anthropic activities affecting VR, avoiding any negligence/misperception related to the “shifting baseline syndrome” (Pauly 1995; Soga and Gaston 2018).
Diet of P. paranae presented varied tendencies, following well-posited theories about fish feeding strategies (Gerking 1994; Zavala-Camin 1996; Wootton 1998), and spatial and temporal influence of multifactors operating along watercourses (Jackson et al. 2001; Thorp et al. 2006; McCain 2013). In VR, these multifactors (i.e., paleogeomorphology, abiotic and biotic) were clearly identified, assessed and analyzed following a systematic and standardized project design, with well-defined objectives and hypotheses (Zale et al. 2012; Gotelli and Ellison 2013). More than this, they revealed the complexity of P. paranae occurrence and diet in relation to the current state of VR, a scenario common to UPRE and Neotropical region.
Remarkable differences of lambari diet were related to headwater and upper middle stretch, despite significant temporal variations too. Ontogenetic dietary shifts followed expected patterns explained by fish development, concerning features not evaluated here, such as mouth size and related feeding apparatus, energetic demands, swimming ability, learning processes, among others (Sabino and Castro 1990; Gerking 1994; Zavala-Camin 1996; Wootton 1998). Despite of this, ontogenetic variations also explained part of spatial and temporal feeding tendencies observed in P. paranae from VR.
In the spatial perspective, individuals at VR headwater mainly depended on terrestrial resources, highlighting Coleoptera and angiosperm seeds. This area is inside two overlapped Protected Areas (PAs), the Devonian Escarpment Protection Area (DEEPA) and the Campos Gerais National Park (CGNP). Not surprisingly, the best condition of riparian vegetation integrity of VR was recorded at the headwater. These finds match with P. paranae diet from other riverine systems that presented preserved riparian vegetation (Ferreira et al. 2012; Gealh and Silveira 2014), thus confirming the importance of this vegetation as an energetic source for aquatic systems (Vannote et al. 1980; Pusey and Arthington 2003). But this perception is not so simple at VR.
The headwater of VR is naturally poorly shaded (see Fig. 2a) due to the riparian steppe-type vegetation (Moro and Carmo 2010), a plant supported by the young and shallow soil (less than 0.1 m) that predominates in the area (Bhering et al. 2007; Sá 2007; Guimarães et al. 2014). Consequently, this river stretch is opened and exposed to high amounts of light. In this way, headwater should present higher primary production (Burrell et al. 2014; Bleich et al. 2015) allied to higher diversity of consumers and complex trophic interactions (Ceneviva-Bastos and Casatti 2014). This partially occurs, but due to the water enrichment caused by fertilizers used in agriculture crops in the surrounding landscapes, increasing natural concentrations of dissolved phosphorus and chlorophyll alpha (Silveira et al. 2018). This increase in the primary production was not reflected in the consumers productivity once that P. paranae consumed lower amounts of resources from aquatic origin at the headwater. These contradictory finds were related to the riverbed features of VR headwater as follow.
Riverbed of VR plays an important role in the feeding pattern of lambari at headwater. The predominance of sandy substrate in this area is related to the VR geomorphology, pressed on the Furnas formation rocks and presenting high susceptibility to erosion, magnified by the inadequate practices of soils handling and suppression of native cover in the surrounding landscapes (Bhering et al. 2007; Sá 2007; Melo et al. 2010; Rocha and Weirich Neto 2010; Guimarães et al. 2014). Both PAs that partially includes VR ensure the conservation of native riparian vegetation only up to 30 m from the riverbed. Beyond this legal strip, there are intensive agricultural and livestock areas. As consequence, headwater riverbed is silted, homogenizing the watercourse (Zeni and Casatti 2014). This exerted a negative pressure on the diversity and abundance of potential prey such as invertebrates (Parkhill and Gulliver 2002; Pusey and Arthington 2003; Jones et al. 2012), leading to a few consume of these resources by P. paranae at the headwater.
In a different way, the diet of P. paranae at the upper middle stretch was more balanced in relation to the headwater. In this site, lambari decreased its consumption of terrestrial insects and increased the exploitation of resources from aquatic origin, highlighting Podostemaceae, an angiosperm found attached to rocky substrates (Mello et al. 2011). Two explanations can justify this tendency. The lower degree of riparian vegetation conservation observed in this site could compromise the availability and abundance of terrestrial resources (Pusey and Arthington 2003). At the same time, the presence of macrophytes enhanced the heterogeneity of this stretch, and allied to riffles, pools and plant debris, they provided conditions for a high diversity and abundance of periphyton and macroinvertebrates (Ceneviva-Bastos and Casatti 2014). These factors modulated the diet of P. paranae at different levels in VR, and the substrate complexity implied in the presence of macrophytes, more pronounced and structural at the upper middle stretch (cf. CCA results).
Diet differences between sites were also related to the prevalence of smaller individuals at headwater (< 7 cm of body length) and bigger ones at upper middle stretch (> 10 cm). Sabino and Castro (1990) postulated that small fishes (juveniles) have high protein demands due to a rapid growth, while in big fishes (adults) this demand reduces, enable them to diversify the diet. Studies with Psalidodon indicated substantial increases in the consumption of plants along its ontogeny associated with increases in intestine length and the attainment of sexual maturity (Wolff et al. 2009; Mazzoni et al. 2005, 2010). In the Pitangui River, from which VR is a tributary, P. paranae attains sexual maturity between 7 and 9 cm of body length (Moraes et al. 2010). If this pattern is the same at VR, juveniles and adults are segregated between headwater and upper middle stretch, reinforcing the tendencies observed in this study, even without statistical differences on ontogenetic dietary shifts. These questions remain opened for further investigations.
Temporal variations on P. paranae diet were lesser significative than spatial ones. Integrative effects were arduous extracted from the analyses performed, based on the empirical knowledge about the lambari (and other Neotropical species) and field experience at VR, two valuable factors that avoided misinterpretations (Gotelli and Ellison 2013). Dry and rainy seasons are remarkable and well-defined at the Neotropical region, influencing the diversity, abundance, and availability of food supplies from aquatic and terrestrial origin for the ichthyofauna (Lowe-McConnell 1987; Vazzoler et al. 1997; Winemiller and Jepsen 1998). This applies to VR. During dry seasons (i.e., winter and autumn), P. paranae consumed high amounts of food resources from terrestrial origin (an overall ratio of ~0.46), enhancing this consumption (an overall ratio of ~0.56) during rainfall seasons (i.e., spring and summer). This is because during rainy periods there was an increase in the abundance of food resources from terrestrial origin, which were carried to the river by the rain (Lowe-McConnell 1987). At the same time, the increase of the river flow carried potential aquatic food resources downstream, reducing their availability as food supply in upstream stretches (Esteves 1996; Wolff et al. 2006).
It is notable that P. paranae occurrence and trophic ecology at VR are a complex issue, modulated by multifactors that results in its trophic plasticity (Esteves 1996; Vilella et al. 2002; Bennemann et al. 2005). This is a consequence of genetic diversity and ecomorphological features of Psalidodon, playing an important role in its evolutive and ecological success in the Neotropical region (Pazza and Kavalco 2007; Mise et al. 2013; Portella et al. 2017; Terán et al. 2020). Its large eyes imply in high visual sensitivity and acuity orienting, and the set of high aspect ratio of caudal fin, tall body and terminal mouth allows active and continuous swimming with fast movements up- and downward (Ferreira 2007; Mise et al. 2013; Portella et al. 2017). These characteristics must be considered with the current results, which demonstrated that P. paranae exploits a wide range of prey items from different origins as a response to ontogeny, spatial and temporal factors, and anthropic activities.
Conclusion
Previous studies pointed out that the opportunistic and generalist behavior of Psalidodon paranae is modulated by multifactors that affect the availability of food supplies. Apparently, there is no novelty in the results presented here, but it is exactly opposite. A set of hypotheses were tested, and based on results and discussion it was possible to posit:
-
(i)
Omnivory and trophic opportunism do not ensure an ability to inhabit every environment of a heterogenous watercourse.
-
(ii)
The effects exerted by the multifactors analyzed (i.e., palaeogeomorphologic, abiotic and biotic) were tested and explained, demonstrating their degree of importance.
-
(iii)
Empirical assumptions regarding P. paranae occurrence, and the possibility to use its diet as a record of the conditions of VR were result of a systematic and scientific research. In this case, both occurrence and feeding trends were related to multifactors.
-
(iv)
The usual generalization finds in the literature about P. paranae population dynamics, and in the specific case about its trophic ecology, must be viewed as a scientific provocation. Actually, these “general patterns” refer to extremely intrinsic patterns of each population at each watercourse.
In this way, although P. paranae tends to feed on the most abundant food resources available, it is necessary to investigate those items few exploited carefully. Some responses to the palaeogeomorphologic, abiotic and biotic factors are found only in these items lesser consumed.
The investigations of heterogeneous watercourses like VR, a model for the Neotropical region, demands the inclusion of multifactors and the integrative approach. Despite of the efficiency and robustness of the analyses performed, some questions remain open for further investigation, including (i) prey items availability in the environment, (ii) the influence of reproductive biology, age, growth and mortality of P. paranae, (iii) population dynamics, including trophic ecology, of other fishes of VR.
Independent of these questions, monitoring of aquatic environments are an alarming lack concerning Brazilian policies (i.e. environmental, educational, economic, social, and cultural). Studies like this must be a routine, but they remain scanty, a limitation for long-term evaluations, compromising an adequate management.
Code availability
Not applicable.
Data availability
Not applicable.
References
Abelha FCM, Agostinho AA, Goulart E (2001) Plasticidade trófica de peixes de água doce. Acta Sci 23:425–434. https://doi.org/10.4025/actascibiolsci.v23i0.2696
Abelha FCM, Goulart E, Kashiwaqui EAL, Silva MR (2006) Astyanax paranae Eigenmann, 1914 (Characiformes: Characidae) in the Alagados reservoir, Paraná, Brazil: diet composition and variation. Neotrop Ichthyol 4:349–356. https://doi.org/10.1590/S1679-62252006000300006
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Balderas SC, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Pérez MHS, Petry P (2008) Freshwater Ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 5:403–414. https://doi.org/10.1641/B580507
Agostinho AA, Pelicice FM, Petry AC, Gomes LC (2007) Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health 10:174–186. https://doi.org/10.1080/14634980701341719
Ahlbeck I, Hansson S, Hjerne O (2012) Evaluating fish diet analysis methods by individual-based modelling. Can J Fish Aquat Sci 69:1184–1201. https://doi.org/10.1139/f2012-051
Albert JS, Petry P, Reis RE (2011) Major biogeographical and phylogenetic patterns. In: Albert JS, Reis RE (eds) Historical biogeography of Neotropical freshwater fishes. University of California Press, Berkeley, pp 21–58
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ (2014) Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef:1–15. https://doi.org/10.1002/9781118445112.stat07841
Araujo-Lima CARM, Oliveira EC (1998) Transport of larval fish in the Amazon. J Fish Biol 53:297–306. https://doi.org/10.1111/j.1095-8649.1998.tb01033.x
Baird RB, Eaton AD, Rice EW (2017) Standard methods for the examination of water and wastewater. 23rd. ed. American Public Health Association, Washington
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: Periphyton, benthic macroinvertebrates and fish. US. Environmental Protection Agency, Washington
Bennemann ST, Gealh AM, Orsi ML, Souza LM (2005) Ocorrência e ecologia trófica de quatro espécies de Astyanax (Characidae) em diferentes rios da bacia do rio Tibagi, Paraná, Brasil. Iheringia Ser Zool 95:247–254. https://doi.org/10.1590/S0073-47212005000300004
Bhering SB, Santos HG, Manzatto CV, Bognola IA, Fasolo PJ, Carvalho AP, Potter RO, Aglio ML, Silva JS, Chaffin CE, Carvalho Junior W (2007) Mapa de solos do Estado do Paraná. Embrapa Solos, Rio de Janeiro
Bicudo CE, Bicudo RMT (1970) Algas de águas continentais brasileiras. Fundação Brasileira para o Desenvolvimento do Ensino de Ciências, São Paulo
Bleich ME, Piedade MTF, Mortati AF, André T (2015) Autochthonous primary production in southern Amazon headwater streams: novel indicators of altered environmental integrity. Ecol Indic 53:154–161. https://doi.org/10.1016/j.ecolind.2015.01.040
Burrell TK, O'Brien JM, Graham SE, Simon KS, Harding JS, McIntosh AR (2014) Riparian shading mitigates stream eutrophication in agricultural catchments. Freshw Sci 33:73–84. https://doi.org/10.1086/674180
Carvalho DR, Castro D, Callisto M, Moreira MZ, Pompeu PS (2015) Isotopic variation in five species of stream fishes under the influence of different land uses. J Fish Biol 87:559–578. https://doi.org/10.1111/jfb.12734
Casatti L, Langeani F, Silva AM, Castro RMC (2006) Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz J Biol 66:681–696. https://doi.org/10.1590/S1519-69842006000400012
CCME (1999) Canadian water quality guidelines for the protection of aquatic life: dissolved oxygen. Canadian Environmental Quality Guidelines, Winnipeg, Canada
CCME (2002) Canadian water quality guidelines for the protection of aquatic life: Total particulate matter. Canadian Environmental Quality Guidelines, Winnipeg, Canada
Ceneviva-Bastos M, Casatti L (2014) Shading effects on community composition and food web structure of a deforested pasture stream: evidences from a field experiment in Brazil. Limnologica 46:9–21. https://doi.org/10.1016/j.limno.2013.11.005
Chipps SR, Garvey JE (2007) Assessment of diets and feeding patterns. In: Guy CS, Brown ML (eds) Analysis and interpretation of freshwater fish data. American Fisheries Society, Bethesda, pp 473–514
Domínguez E, Fernández HR (2009) Macroinvertebrados bentónicos sudamericanos: sistemática y biología. Fundación Miguel Lillo, Tucumán
Esteves KE (1996) Feeding ecology of three Astyanax species (Characidae, Tetragonopterinae) from a floodplain lake of Mogi-Guagu River, Parana River basin, Brazil. Environ Biol Fish 46:83–101. https://doi.org/10.1007/BF00001701
Fauconnet L, Trenkel VM, Morandeau G, Caill-Milly N, Rochet M-J (2015) Characterizing catches taken by different gears as a step towards evaluating fishing pressure on fish communities. Fish Res 164:238–248. https://doi.org/10.1016/j.fishres.2014.11.019
Ferreira KM (2007) Biology and ecomorphology of stream fishes from the rio Mogi-Guaçu basin, southeastern Brazil. Neotrop Ichthyol 5:311–326. https://doi.org/10.1590/S1679-62252007000300012
Ferreira A, Gerhard P, Cyrino JEP (2012) Diet of Astyanax paranae (Characidae) in streams with different riparian land covers in the Passa-Cinco River basin, southeastern Brazil. Iheringia Ser Zool 102:80–87. https://doi.org/10.1590/S0073-47212012000100011
Fox J, Weisberg S (2019) An R companion to applied regression. 3rd ed. Sage, Thousand Oaks. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 06 December 2019
Fricke R, Eschmeyer WN, Van der Laan R (2020). Eschmeyer's catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Accessed 29 December 2020
Garvey JE, Chipps SR (2012) Diets and energy flow. In: Zale AV, Parrish DL, Sutton TM (eds) Fisheries techniques, 3rd edn. American Fisheries Society, Bethesda, pp 733–779
Garutti V, Britski HA (2000) Descrição de uma nova espécie de Astyanax (Teleostei: Characidae) da bacia do Alto Paraná e considerações sobre as demais espécies do gênero na bacia. Comun Mus Ciênc Tecnol PUCRS Ser Zool 13:65–88
Gealh AM, Silveira EL (2014) Conhecendo os peixes do rio. In: Gealh AM, Melo MS (eds) Rio São João, Carambeí-Pr: Fonte de vida, cuidados devidos. Editora UEPG, Ponta Grossa, pp 181–203
Gelwick FP, McIntyre PB (2017) Trophic relations of stream fishes. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology. Volume 1: Ecossystem structure. 3rd ed. Academic Press, London
Gerking SD (1994) Feeding ecology of fish. Academic Press, San Diego
Gotelli NJ, Ellison AM (2013) A primer of ecological statistics. 2nd edn. Sinauer Associates Inc, Sunderland
Guimarães GB, Godoy LC, Melo MS, Flügel Filho JC (2014) Geodioversidade. In: Gealh AM, Melo MS (eds) . Pr: fonte de vida, cuidados devidos. Editora UEPG, Ponta Grossa, Rio São João, pp 15–37
Hamada N, Nessimian JL, Querino RB (2014) Insetos aquáticos na Amazônia Brasileira: taxonomia, biologia e ecologia. INPA, Manaus
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.3.2.0. https://CRAN.R-project.org/package=DHARMa. Accessed 06 December 2019
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities — the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. https://doi.org/10.1139/cjfas-58-1-157
Jones JI, Murphy JF, Collins AL, Sear DA, Nadend PS, Armitagee PD (2012) The impact of fine sediment on macro-invertebrates. River Res App 28:1055–1071. https://doi.org/10.1002/rra.1516
Krebs C (2014) Ecological methodology, 3rd edn. Updated 14 march 2014. https://www.zoology.ubc.ca/~krebs/books.html. Accessed March 2014.
Lagler KL (1978) Capture, samplings and examination of fishes. In: Bagenal TB (ed) Methods for assessment of fish production in fresh waters, 3th edn. Blackwell Scientific Publications, Oxford, pp 7–47
Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L (2007) Ichthyofauna diversity of the upper rio Paraná: present composition and future perspectives. Biota Neotrop 7:181–197. https://doi.org/10.1590/S1676-06032007000300020
Legendre P, Legendre L (2012) Numerical ecology. 3nd ed. Elsevier, Amsterdam
Leite GFM, Silva FTC (2018) Effects of temporal changes on resource availability in the diet of Astyanax paranae (Pisces, Characidae) in tropical headwater streams. Limnetica 37:117–128. https://doi.org/10.23818/limn.37.10
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. University Press, Cambridge
Mangiafico S (2019) rcompanion: functions to support extension education program evaluation. R package version 2.3.21. https://cran.r-project.org/web/packages/rcompanion/index.html. Accessed 06 December 2019
Matthews WJ (1998) Patterns in freshwater fish ecology. Springer-Science+Business Media, Dordretch
Mazzoni R, Mendonça RS, Caramaschi EP (2005) Reproductive biology of Astyanax janeiroensis (Osteichthyes, Characidae) from the Ubatiba River, Maricá, RJ, Brazil. Braz J Biol 65:643–649. https://doi.org/10.1590/S1519-69842005000400012
Mazzoni R, Nery LL, Iglesias-Rio R (2010) Ecologia e ontogenia da alimentação de Astyanax janeiroensis (Osteichthyes, Characidae) de um riacho costeiro do Sudeste do Brasil. Biota Neotrop 10:53–60. https://doi.org/10.1590/S1676-06032010000300005
McCain KN (2013) Moving large river ecology from past theories to future actions: a review. Rev Fish Sci 21:39–48. https://doi.org/10.1080/10641262.2012.753867
Melo MS, Guimarães GB, Santana ÁC (2010) Fisiografia da bacia do rio Pitangui. In: Gealh AM, Melo MS, Moro RS (eds) Pitangui, rio de contrastes: seus lugares, seus peixes, sua gente. Editora UEPG, Ponta Grossa, pp 11–21
Mello AS, Tavares AS, Trevisan R (2011) Podostemaceae in southern Brazil. Rodriguésia 62:867–855. https://doi.org/10.1590/S2175-78602011000400013
Mise FT, Fugi R, Pagotto JPA, Goulart E (2013) The coexistence of endemic species of Astyanax (Teleostei: Characidae) is propitiated by ecomorphological and trophic variations. Biota Neotrop 13:21–28. https://doi.org/10.1590/S1676-06032013000300001
Moraes MFPG, Cornélio D, Barbola IF (2010) Aspectos da biologia reprodutiva dos peixes do rio Pitangui. In: Gealh AM, Melo MS, Moro RS (eds) Pitangui, rio de contrastes: seus lugares, seus peixes, sua gente. Editora UEPG, Ponta Grossa, pp 127–139
Moro RS, Carmo MRB (2007) A vegetação campestre nos Campos Gerais. In: Melo MS, Moro RS, Guimarães GB (eds) Patrimônio Natural dos Campos Gerais do Paraná. Editora UEPG, Ponta Grossa, pp 93–98
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2019) vegan: community ecology package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan. Accessed 06 December 2019
Parkhill KL, Gulliver JS (2002) Effect of inorganic sediment on whole-stream productivity. Hydrobiologia 472:5–17. https://doi.org/10.1023/A:101636322
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10:430
Pazza R, Kavalco KF (2007) Chromosomal evolution in the neotropical characin Astyanax (Teleostei, Characidae). Nucleus 50:519–543
Portella T, Lobón-Cerviá J, Manna LR, Bergallo HG, Mazzoni R (2017) Eco-morphological attributes and feeding habits in coexisting characins. J Fish Biol 90:129–146. https://doi.org/10.1111/jfb.13162
Pusey BJ, Arthington AH (2003) Importance of the riparian zone to the conservation and management of freshwater fish: a review. Mar Freshw Res 54:1–16. https://doi.org/10.1071/MF02041
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed 20 July 2020
Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA (2016) Fish biodiversity and conservation in South America. J Fish Biol 89:12–47. https://doi.org/10.1111/JFB.13016
Rocha CH, Weirich Neto PH (2010) Padrões de uso das terras e implicações ambientais. In: Gealh AM, Melo MS, Moro RS (eds) Pitangui, rio de contrastes: seus lugares, seus peixes, sua gente. Editora UEPG, Ponta Grossa, pp 23–41
Sá MFM (2007) Os solos dos Campos Gerais. In: Melo MS, Moro RS, Guimarães GB (eds) Patrimônio Natural dos Campos Gerais do Paraná. Editora UEPG, Ponta Grossa, pp 59–72
Sabino J, Castro RMC (1990) Alimentação, período de atividade e distribuição espacial dos peixes de um riacho da Floresta Atlântica (Sudeste do Brasil). Rev Bras Biol 50:23–36
Sequinel R, Arrúa MEP, Costa W (2011) Um levantamento das concentrações dos íons NO3−,PO43−, K+, Ca2+ and Mg2+ presentes nas águas do rio Verde e sua correlação com as atividades humanas existentes na área. Publicatio UEPG-Ciências Exatas e da Terra, Agrárias e Engenharias 17:29–37. https://doi.org/10.5212/Publ.Exatas.v.17i1.0003
Silveira EL, Ballester ELC, Costa KA, Scheffer EWO, Vaz-dos-Santos AM (2018) Fish community response to environmental variations in an impacted Neotropical basin. Ecol Freshw Fish 27:1126–1139. https://doi.org/10.1111/EFF.12420
Silveira EL, Semmar N, Cartes JE, Tuset VM, Lombarte A, Ballester ELC, Vaz-dos-Santos AM (2020) Methods for trophic ecology assessment in fishes: a critical review of stomach analyses. Rev Fish Sci Aquac 28:71–106. https://doi.org/10.1080/23308249.2019.1678013
Soga M, Gaston KJ (2018) Shifting baseline syndrome: causes, consequences, and implications. Front Ecol Environ 16:222–230. https://doi.org/10.1002/fee.1794
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research, 4th edn. W. H. Freeman, New York
Souza UP, Ferreira FC, Braga FMS, Winemiller KO (2015) Feeding, body condition and reproductive investment of Astyanax intermedius (Characiformes, Characidae) in relation to rainfall and temperature in a Brazilian Atlantic Forest stream. Ecol Freshw Fish 24:123–132. https://doi.org/10.1111/eff.12131
ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179. https://doi.org/10.2307/1938672
Terán GE, Benitez MF, Mirande JM (2020) Opening the Trojan horse: phylogeny of Astyanax, two new genera and resurrection of Psalidodon (Teleostei: Characidae). Zool J Linn Soc-Lond: zlaa019. https://doi.org/10.1093/zoolinnean/zlaa019
Thorp JH, Thoms MC, Delong MD (2006) The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res App 22:123–147. https://doi.org/10.1002/rra.901
USEPA (2013) Aquatic life ambient water quality criteria for ammonia – freshwater. Environmental Protection Agency, Washington
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137. https://doi.org/10.1139/f80-017
Vazzoler AEAM, Agostinho AA, Hahn NS (1997) A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. EDUEM, Maringá
Venables WN, Ripley BD (2002) Modern applied statistics with S. Fourth Edition. Springer, New York
Venables WN, Dichmont CM (2004) GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fish Res 70:319–337. https://doi.org/10.1016/j.fishres.2004.08.011
Vilella FS, Becker FG, Hartz SM (2002) Diet of Astyanax species (Teleostei, Characidae) in an Atlantic Forest river in southern Brazil. Braz Arch Biol Technol 45:223–232. https://doi.org/10.1590/S1516-89132002000200015
Windell JT, Bowen SH (1978) Methods for study of fish diets based on analysis of stomach contents. In: Bagenal T (ed) Methods for assessment of fish production in fresh waters. Blackwell Scientific Publications Ltd, Oxford, pp 219–226
Winemiller KO, Jepsen DB (1998) Effects of seasonality and fish movement on tropical river food webs. J Fish Biol 53:267–296. https://doi.org/10.1111/j.1095-8649.1998.tb01032.x
Wolff LL, Abilhoa V, Rios FS, Donatti L (2009) Spatial, seasonal and ontogenetic variation in the diet of Astyanax aff. fasciatus (Ostariophysi: Characidae) in an Atlantic Forest river, southern Brazil. Neotrop Ichthyol 7:257–266. https://doi.org/10.1590/S1679-62252009000200018
Wootton RJ (1998) Ecology of teleost fishes. 2nd Edn. Kluwer Academic Publishers, Dordrecht
Zale AV, Sutton TM, Parrish DL (2012) Conducting fisheries investigations. In: Murphy BR, Willis DW (eds) Fisheries Techniques. 3nd ed. American Fish Society, Bethesda, pp 1–14
Zavala-Camin LA (1996) Introdução aos estudos sobre alimentação natural em peixes. EDUEM, Maringá
Zeni JO, Casatti L (2014) The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 726:259–270. https://doi.org/10.1007/s10750-013-1772-6
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science+Business Media, New York
Acknowledgements
Data come from the project “Structure of assemblages and population dynamics of the Neotropical ichthyofauna from a Paranaense microbasin, Southern Brazil”, granted by Araucária Foundation (State of Paraná Research Foundation) and Boticário Group Foundation for Nature Protection. The first author thanks Araucária Foundation for the scholarship. The second author thanks CAPES (Finance Code 001) for the Doctorate scholarship. The last author thanks CNPq for the research grant (no. 310451/2018-3). Sampling was licensed by the Chico Mendes Institute for Biodiversity conservation (Authorizations no. 40132-2 and no. 51797-1), and by Certificate of the Ethics Committee in the Use of Animals UFPR (Authorization no. 38/2015). Editors (mentioning Dr. David Noakes, deceased) and referees presented hard comments for the initial version of this manuscript, but all of them were constructive, allowing an enrichment of this paper and a personal and professional growth for all authors.
Funding
Araucária Foundation (State of Paraná Research Foundation) and Boticário Group Foundation for Nature Protection. CAPES (Finance Code 001). CNPq (research grant no. 310451/2018–3).
Author information
Authors and Affiliations
Contributions
André Martins Vaz-dos-Santos and Estevan Luiz da Silveira were responsible by the study conception, sampling design, data collection and analyses. The second author and Kathleen Angélica Rautenberg contributed preparing materials, lab and data analysis, and producing graphical plots. All authors contributed to the textual production process and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethics approval
Sampling was licensed by the Chico Mendes Institute for Biodiversity conservation (Authorizations no. 40132–2 and no. 51797–1), and by Certificate of the Ethics Committee in the Use of Animals UFPR (Authorization no. 38/2015).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/competing interests
The authors declare that they have no conflict of interests concerning its manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 714 kb)
Rights and permissions
About this article
Cite this article
Rautenberg, K.A., da Silveira, E.L. & Vaz-dos-Santos, A.M. Feeding trends of Psalidodon paranae in an impacted Neotropical basin: a multifactor and integrative approach. Environ Biol Fish 104, 89–105 (2021). https://doi.org/10.1007/s10641-021-01058-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01058-y