Abstract
The flood pulse is the main driving force influencing river floodplain ecosystems. The dominant role of the flood pulse on the success of non-native species (NNSs) is what differentiates floodplains from other ecosystems, in terms of invasion. In this review, I discuss some patterns related to the performance of NNSs in response to the flood pulse. First, floods connect floodplain habitats and spread propagules of NNSs, causing ‘propagule pulses’ in these ecosystems. After the establishment of NNSs, floodplains may function as steppingstones for future invasions, because propagule pulses enhance invasions in nearby landscapes. Second, the flood pulse changes environmental filters, with consequences for invasion success and for the coexistence of native and NNSs. Flooding represents a disturbance that enhances the success of some NNSs by reducing biotic resistance and changing resource availability, but diminishes the success of others. Drought enhances the invasion success mainly of NNSs that colonize the aquatic-terrestrial transition zone. Third, impacts caused by river regulation and global changes alter the flood pulse, which in turn affects invasion success. There is a great degree of idiosyncrasy in these patterns, but they pose a broad perspective that helps to understand and manage NNSs in floodplains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floodplain ecosystems are widespread throughout the world and have received especial attention from ecologists and limnologists in recent last decades. These ecosystems are defined as “areas that are periodically inundated by the lateral overflow of rivers or lakes, and/or by direct precipitation or groundwater; the resulting physicochemical environment causes the biota to respond by morphological, anatomical, physiological, phenological, and/or ethological adaptations, and produce characteristic community structures” (Junk et al., 1989). Floodplains have been severely threatened by human activities, mainly in temperate regions (Hudon et al., 2005; Grizzetti et al., 2017; EEA, 2018). In contrast, South America, Africa and Asia still have relatively well preserved and even pristine river-floodplain ecosystems (RFEs), like the Orinoco, Amazon, Paraguay (Pantanal) and Paraná RFEs in South America, the Nile, Zambezi, Niger, Senegal and Congo RFEs in Africa, and the Mekong RFE in Asia.
The flood pulse (Box 1) is the main driving force of RFEs and encompasses a predictable hydrological cycle with extreme environmental conditions during high water (flooding) and low water (drought) periods (Junk et al., 1989; Neiff, 1990). Enhanced connectivity among habitats during flooding occurs with various components, including water, sediments and organisms (Junk et al., 1989; Fuller & Death, 2018). This hydrological connectivity related to the flood pulse is a key factor for maintaining the structure, functioning and biodiversity in RFEs (Bunn & Arthington, 2002; Agostinho et al., 2005; Thomaz et al., 2007; Lasnea et al., 2008; Fernandes et al., 2009; Johnson et al., 2016; Oliveira et al., 2020). The flood pulse has ecological features that include the magnitude, frequency, seasonal timing, predictability, duration and rate of change of flow conditions, to which species are evolutionarily selected (Lytle & Poff, 2004).
RFEs are highly valuable for conservation of biodiversity and the multiple ecosystem services they provide (Ward et al., 1999; Agostinho et al., 2004; Fernandes et al., 2009). RFEs are among the most complex and dynamic ecosystems in the world and are the most biodiverse among aquatic ecosystems, but they are highly subject to multiple stressors, which compromise their conservation, including high numbers of non-native, invasive species (Box 1) (Tockner et al., 2010). There is evidence that RFEs and riparian corridors are considered ‘hotspots of non-native species’ (NNSs) because they have been invaded by a myriad of non-native animals and plants (e.g., Molina & Paggi, 2008; Sousa, 2011; Petrášová et al., 2013; Hejda et al., 2015; Pelicice et al., 2017; Tonella et al., 2018; Williams et al., 2018; Ruaro et al., 2020).
Non-native species are a cause of concern because they impact biodiversity, ecosystem functioning and human economy (Simberloff et al., 2013) and their efficient management is difficult (Simberloff, 2021). Owing to the broad impacts caused by NNSs that become invasive in numerous ecosystems, there is evidence that we are approaching the ‘Exocene’, which is a “new functioning era” with prominent roles of invasive species in changing the quality and functions of ecosystems throughout the planet (e.g., Bolpagni, 2021). Thus, knowing the patterns and causes of invasion by NNSs in RFEs is key to improving the conservation status of these important ecosystems and preserving the ecological services they provide. The study of NNSs in RFEs also provides a unique opportunity to understand the costs and benefits of flow-regime adaptations exhibited by species subject to a gradient of flow regimes (e.g., Lytle & Poff, 2004). In this sense, NNSs may be taken as experimental models that would not be obtained by other means because of ethical reasons.
In this review, I use concepts from the field of invasion biology merged with the flood pulse concept to explore some peculiarities of invasions of RFEs by NNSs. Although I prioritize RFEs with predictable water level oscillations and floods that overflow the riverbank (the classical RFEs with a flood pulse described by Junk et al., 1989), I also use examples of RFEs where floods are less predictable or where multiple floods occur during the rainy season, and thus the ideas I develop here apply to these ecosystems as well. I first state the rationale to explain why the invasion dynamic in RFEs differs from the invasion dynamic in other aquatic ecosystems. Then, more specifically, I seek to answer the following questions: (i) How are propagule pressure (an important determinant of NNS success) and the flood pulse related? (ii) How do environmental filters of invasion relate to the flood pulse? (iii) Do changes promoted by the flood pulse enhance the chances of coexistence of native and NNSs? And (iv) what can be expected of NNSs in current and future scenarios of increasing impacts in RFEs? These four questions were explored with empirical evidence obtained by observational investigations found by a systematic survey of the literature (see details in Sup. Mat. 1). Because RFEs include species inhabiting the aquatic-terrestrial transitional zone (ATTZ, sensu Junk et al., 1989) (Box 1), I use various examples of organisms which are not obligatory aquatic, along with some examples of riparian corridors that also experience seasonal flooding. Last but not least, I emphasize that this paper is not an extensive review about NNSs in RFEs and, thus, papers which do not relate invasive success with the flood pulse are outside its scope.
Linking invasion biology and the flood-pulse concept
The success of any NNS in an ecosystem depends on propagule pressure (Williamson & Fitter, 1996; Colautti et al., 2006; Duncan, 2011), species invasiveness (Rejmánek, 2011; Grotkopp et al., 2002) and community and ecosystem invasibility (Moyle & Light, 1996; Fridley, 2011) (Box 1). Using factors related to propagule pressure (see Box 1) and invasibility helps to explain why ecosystems differ in their susceptibility to invasion. Taking into consideration that the flood pulse is the principal driving force responsible for the structure and ecological dynamics of RFEs (Junk et al., 1989; Neiff, 1990), it seems intuitive that it is also one of the most important driving forces that affect the performance (Box 1) and success of NNSs in RFEs. The influence of the flood pulse on NNSs success is indeed shown in a variety of case studies (see Tables 1 and 2; Sup. Mat. 2, 4 and 5), although the lack of effects of the flood pulse on NNS success has also been recorded (e.g., Warren et al., 2015; Kettenring et al., 2016). The predictable seasonal changes of habitats and communities in response to the flood pulse and the enhanced connectivity of habitats during seasonal flooding distinguishes these ecosystems from aquatic ecosystems where the water level changes irregularly (e.g., streams, small rivers, most reservoirs and lakes) and from aquatic ecosystems with small water level oscillations [e.g., large lakes, some types of wetlands with nearly constant water levels, such as ombrotrophic bogs (Mitsch & Grosselink, 2015), and reservoirs where inflow is similar to outflow]. Thus, the interference that seasonal and predictable flood pulses cause to NNSs performance (Box 1) makes RFEs unique ecosystems in terms of invasion dynamics.
Box 1: Definition of terms used in this review
Aquatic-terrestrial transition zone (ATTZ) | This is the floodplain area that alternates between the aquatic and terrestrial environments, also known as the ‘moving littoral’ (Junk et al., 1989) |
Biotic acceptance hypothesis | This hypothesis predicts that “natural ecosystems tend to accommodate the establishment and coexistence of introduced species despite the presence and abundance of native species” (Stohlgren et al., 2006; Fridley et al., 2007; Richardson et al., 2011). This hypothesis is synonymous of “the rich gets richer” concept and it is usually corroborated at larger spatial scales, differently from the biotic resistance hypothesis, usually corroborated at small spatial scales (plots with few m2). |
Biotic resistance hypothesis | This hypothesis was first formulated to Elton (1958) and it is “the ability of native species to prevent an invader from becoming established by their interactions with propagules of the invader” (Gurevitch, 2011). The resistance may extend to the post – establishment survival, proliferation and spread of the NNSs (Richardson et al., 2011). |
Disturbance | It is “any event that produces a change in ecosystem structure and resource availability” (Hobbs, 2011). According to Connell (1978), disturbances are events (e.g., storms, waves, floods etc.) that kill or damage organisms. |
Flood pulse | It is the “pulsing of the river discharge” (Junk et al., 1989), and it encompasses the periodical (seasonal) change in water level (and river flow), with its typical phases of low water, rising water, high water and falling water. |
Invasibility | This term “describes the susceptibility of biological communities to colonization and dominance by introduced organisms” (Fridley, 2011). Invasibility usually refers to abiotic and biotic features that are related to invasion success. |
Invasiveness | This term is related with the species innate traits and is defined as “the degree to which a species is able to reproduce, spread from its place of introduction, and establish in new locations” (Rejmánek, 2011). |
Invasive species | “A naturalized species that produces reproductive offspring, often in very large numbers, and that spreads over large areas. This definition is usually used by ecologists.” (Simberloff & Rejmánek, 2011) |
NNS success, invasion success | A NNS reaches success when there are evidences that its population colonizes and establishes in an ecosystem, where the species maintains its population overtime. |
NNS performance | Performance is related with any measurement of the population, like abundance, biomass, recruitment, cover (for plants) or dominance. |
Non-native species (NNS) | Any species introduced outside its native range. Also known as ‘exotic’ or ‘alien’. |
Propagule | I use the word “propagule” when I refer to any structure that can generate a new organism (e.g., seeds, eggs, plant fragments, rhizomes etc.) or even an entire organism (young or adult) arriving in a habitat. |
Propagule pulse | Spread of propagules caused by seasonal flooding in river floodplain ecosystems, which helps NNSs to succeed in these ecosystems. |
Propagule pressure | The propagule pressure relates to the number and sizes of propagules, and how they arrive considering the spatial and temporal scales (Simberloff, 2009; Duncan, 2011). This term usually has a connotation related to transport from native to the invasive range (e.g., Richardson et al., 2011). However, I use its broader meaning which refers also to release of propagules that helps subsequent spread of NNSs throughout the invaded ecosystem (see Duncan, 2011). Thus, any release of propagules of a NNS occurring in the RFE will be accounted for as an increase in the propagule pressure. |
Traits | The functional trait of an organism can be defined as “The characteristics of an organism that are considered relevant to its response to the environment and/or its effects on ecosystem functioning” (Díaz & Cabido, 2001). |
Merging the main hypotheses that explain the invasibility of communities and ecosystems with the flood-pulse concept allows a glimpse of three general characteristics of RFEs with regard to invasion dynamics. The first characteristic is that propagule pressure plays a disproportionate role in explaining NNSs success in RFEs, because flooding connects habitats and spreads propagules within these ecosystems very rapidly and in seasonal pulses. The significant role that flooding plays in the dispersal of organisms has been widely recognized for native species (e.g., Padial et al., 2014) and may also be important for NNSs success (Diez et al., 2012; Fig. 1). Peaks of propagule dispersal during seasonal flooding in RFEs can be considered a ‘propagule pulse’. A single, large and predictable propagule pulse occurs in large RFEs that experience bank overflow once a year (like the ones defined by Junk et al., 1989) and thus, these predictable propagule pulses that repeat annually can be considered a unique characteristic of this type of RFE. Moreover, propagule pulses can occur many times per year in RFEs associated with rivers having multiple peaks in water level per year, such as the Upper Paraná RFE (Brazil), for example, where there are from one to three floods during the rainy season (Agostinho et al., 2004). In addition to the dispersal of propagules by flooding, which is typical of RFEs, there are also other natural and human-driven mechanisms, including the transport of propagules originating outside the RFE and from internal sources, that contribute to spreading propagules throughout the year in these ecosystems. Thus, hereafter, I will use the term ‘propagule pressure’ when I discuss mechanisms that help the dispersal of non-native species in RFEs that are not related to seasonal flooding. I will use ‘propagule pulse’ exclusively to refer to dispersal occurring during seasonal flooding.
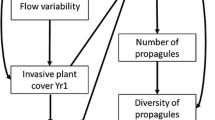
Conceptual model of the relationship between flood dynamic (flood pulse—upper panels) and invasion success in RFEs. Flooding promotes rapid dispersal of invasive propagules throughout floodplain habitats (‘propagule pulses’), leading to enhanced propagule pressure, which is initially associated with propagules arriving in the main channel of the river (a). Right after arriving during high water, some NNSs fail to colonize certain habitats (b). Environmental filters also influence invasion success during low water (e.g., drought) (c). Once the species is stablished in a given RFE, it again disperses during flooding and the propagules are dispersed back to the river, enhancing even more the role of propagule pressure for the success of the species (d). After the establishment of a NNS in the RFE, this ecosystem may work as steppingstone for invasions in nearby landscapes (see propagules going back to the river channel in d). The figures of the upper panel were taken from Castello et al. (2015). Dark blue is the main channel of the river, light blue circles are floodplain lakes and red circles the invasive organisms. X represents the habitats where the propagules arrived but did not colonize or establish because of biotic and/or abiotic filters. A, B and P inside the circles indicate abiotic filters, biotic filters and propagule pressures, respectively, and their sizes are proportional to their hypothetical relative roles in non-native species success (see Fig. 2 for additional details). Arrows in the top figure show the corresponding water phase shown in the figures below
The second characteristic is that the biotic and abiotic filters that affect NNS success in RFEs change seasonally and also in a predictable way in response to the flood pulse. Seasonal changes associated with the flood pulse influence the abiotic medium (e.g., water flow, nutrient availability, oxygen levels, pH etc.) of the local habitats of RFEs and as well as interspecific relationships (e.g., competition, predation and facilitation). Because invasibility depends on biotic and abiotic filters, these seasonal changes also affect NNS performance in a unique way, which varies seasonally in accordance with the hydrodynamic determined by the flood pulse (Figs. 1b, c and 2).
Merging concepts from the field of invasion biology with the flood pulse concept indicates that there exist some peculiarities to the process of invasion of RFEs. The success of invasive species is related to propagule pressure (P) and biotic (B) and abiotic (A) filters, along with their interactions (I; hatched). Propagule pressure has a disproportionate role on RFEs because flooding disperses propagules to local habitats within the floodplain (‘propagule pulses’). Human influences (H) may enhance propagule pressure and change abiotic and biotic filters. Different from other ecosystems, environmental filters related to the abiotic and biotic medium change according to water level oscillations, which, in turn, also affects invasion success. The figure is inspired in Catford et al. (2009)
The third characteristic is related to the second, but has to do with the fact that populations of native and NNSs change (in terms of abundance, population size structure, reproduction, feeding etc.) in response to the flood pulse (e.g., Junk et al., 1989; Tockner et al., 2000; Bunn & Arthington, 2002; Oliveira et al., 2015; Quirino et al., 2015, 2017). If native and NNSs have dissimilar seasonal populational dynamics and/or use different RFE habitats, both types of species may coexist, even if they have strong negative interactions (e.g., competition or predation). I will show numerous examples that this indeed occurs for different groups of organisms in RFEs.
Finally, both propagule pressure (and propagule pulse) and the environment in RFEs are affected by humans, bringing consequences for invasion success (Fig. 2). Among various impacts, two might be currently important and may become enhanced in the future: (i) the flow regulation caused by reservoirs and other structures, which alters the flood pulse and water quality and enhances propagule pressure; and (ii) global changes, which will alter hydrodynamics. I will briefly discuss their potential effects on invasive success in the last section of this review.
Propagule pressure and propagule pulse in river-floodplain ecosystems
Propagule pressure is an important mechanism that explains invasion success (Colautti et al., 2006; Simberloff, 2009; Duncan, 2011). The concept of propagule pressure in invasion biology is analogous to the concept of the “mass effect” model in a meta-community (Leibold et al., 2004; Tonkin et al., 2018). Mass effect is based in the net flow of individuals and/or propagules associated with populations whose abundances differ in different patches (Shmida & Wilson, 1985). Mass effect depends on the existence of local patches with different environmental characteristics that are sufficiently connected to allow dispersal from sources to sink populations (Leibold et al., 2004). RFEs fulfill these conditions because they have different patches (floodplain lakes, canals, marshes and a variety of habitats in the ATTZ) that are colonized to different degrees by populations of native and NNSs. During flooding, these different habitats become connected to a maximum degree (Thomaz et al., 2007), leading to propagule pulses (Fig. 1a). For plants that colonize the ATTZ and for macrophytes that colonize all types of permanent aquatic habitats in the RFE, hydrochory is the main pathway of arriving and dispersal in riparian corridors and in other RFE habitats (Burkart, 2001; Wang et al., 2011; Catford & Jansson, 2014; Chundi et al., 2017; Mao et al., 2019; Jones et al., 2020; West et al., 2020), although there are other mechanisms involved (see below).
Because propagule pressure is a key mechanism that contributes to invasion success, it has to be taken into account when explaining the reasons why ecosystems differ in their susceptibility to invasion (Colautti et al., 2006; Simberloff, 2009). For example, local habitats that receive a great number of propagules may be successfully invaded even if their abiotic characteristics are unsuitable (Leibold et al., 2004; Sepulveda, 2018; Tonkin et al., 2018; Amo et al., 2021) or if biotic resistance (Box 1) is maintained constant (Louback-Franco et al., 2020). In addition, when propagules are released repeatedly (as occurs over the years with seasonal propagule pulses during flooding in RFEs), there is an increased chance that the population finds favourable conditions to establish (Duncan, 2011). However, it is difficult to accurately predict the performance of NNSs using only models applied to their spread because RFEs are highly heterogeneous and the success of any invader will also depend on habitat characteristics (Merrin et al., 2005; see Fig. 1b, c and “Abiotic and biotic filters of invasion in river-floodplain ecosystems” below).
A NNS usually arrives at an ecosystem by a propagule pulse originating from propagules carried by the main river during flooding (Fig. 1a). Once this NNS establishes in the RFE, propagule pulses also originate from local sources, and the spread of NNSs no longer depends only on propagules carried from outside the ecosystem by the main river channel (Fig. 1d). Thus, after a NNSs is successfully established in a RFE, propagule pulses enhance its maintenance in the ecosystem. In addition, after establishment in a RFE, propagules of NNSs can go back to the river channel during flood recession (e.g., Pearce & Smith, 2001; Thomas et al., 2005, 2006; Watterson & Jones, 2006; Catford & Jansson, 2014; Bennett et al., 2015; Kropf et al., 2017; Lewerentza et al., 2019 for plants; Pegg et al., 2002; Pollux & Korosi, 2006; Stuart & Jones, 2006; Díez-del-Molino et al., 2016; Reshetnikova et al., 2017; Koehn et al., 2018 for fish; Collas et al.,2017 for snails), enhancing propagule pressure downstream and upstream, in the case of some animals (see Jones & Stuart, 2009; Macdonald & Crook, 2014; Norman & Whitledge, 2015 for fish). Taking into consideration this enhancement of propagules generated in RFEs and the possibility of their spread by flooding (evidenced by the above references), it can be said that RFEs facilitate regional invasions. Within this context, spread by river flow after flooding constitutes an “unaided pathway” because it enhances the natural spread of NNSs in new regions (Hulme, 2009). Because RFEs may facilitate regional invasions, they can be considered ‘steppingstones’ for dispersal of NNSs in the surrounding landscapes (Fig. 1d), in the same fashion as reservoirs (Havel et al., 2005; Johnson et al., 2008; Gois et al., 2015). One example of the steppingstone role of RFEs is the rapid increase observed in the frequency of Hydrilla verticillata L.f. Royle in the Itaipu Reservoir (Brazil/Paraguay) after this macrophyte invaded a RFE upstream from the reservoir (Thomaz et al., 2009).
The importance of propagule pulses for the success of NNSs, however, differs among different groups of organisms, because the regulation of dispersal in river networks (including RFEs) depends on the life-history and dispersal traits of species (Box 1) (Padial et al., 2014; Tonkin et al., 2018). In terms of life history, for example, some NNSs that are more adapted to terrestrial habitats may not benefit from dispersal by flooding (Catford et al., 2011). In regard to dispersal traits, a study employing metacommunity theory showed that larger organisms (e.g., macrophytes and fish) are more dependent on dispersal to succeed, while smaller organisms (e.g., microalgae and zooplankton) are more dependent on environmental characteristics, because the former have lower dispersal ability than the latter (Padial et al., 2014). Using a general perspective, and disregarding other important details that influence establishment (e.g., reproduction form), NNSs may follow this same logic, and thus I expect that large-sized NNSs would depend more on propagule pulses (in terms of number of propagules) to succeed in RFEs than small-sized organisms. In contrast, the success of small-sized organisms would depend more on abiotic and biotic filters (discussed in the next section).
Propagule pulses also depend on flood characteristics. Water level dynamics change between years, especially in RFEs whose rivers are regulated by dams (Gehrke & Harris, 2001; Agostinho et al., 2004). In the Upper Paraná River floodplain, for example, less intense flooding does not overflow the riverbank, while other more intense flooding caused by the El Niño phenomenon does and connects all habitats to a greater extent (Fernandes et al., 2009; Souza-Filho, 2009; Simões et al., 2013). Another example occurs in RFEs in Wisconsin, where bank overflow helps NNSs of plants to succeed (Johnson et al., 2016). It follows from these examples that propagule pulses are probably more important for the success of NNSs during inundations that overflow the riverbank than during less intense flooding. Nevertheless, in addition to flooding that overflows the riverbank, one cannot disregard the important role of other less intense inundations for the dispersal of NNSs in RFEs, as is the case of flow pulses (inundations without bank overflow; Tockner et al., 2000). Despite lacking the high degree of connectivity with the main river as occurs during bank overflow, flooding during flow pulses also connects different habitats within the RFE, helping the spread of NNSs inside the ecosystem.
Empirical evidence about the important role of propagule pulses in RFEs abound in the literature (Table 1; Sup. Mat. 2). Effective dispersal of NNSs by flooding occurs in a variety of RFEs distributed in all continents, and for different groups of organisms, from increases in seed banks and plants (e.g., Andrew et al., 2012) to the efficient spread of fish (e.g., DeGrandchamp et al., 2008; Wu et al., 2013). The importance of propagules for NNS success in RFEs is also demonstrated by some examples where NNS success correlates positively with the degree of connectivity of the local habitats. For example, the number of NNSs of invertebrates increases with the degree of hydrological connectivity in the Rhone RFE in France (Paillex et al., 2017), and in the Upper Paraná RFE in Brazil (Amo et al., 2021), indicating that connection by flooding is an important mechanism for NNS success in these RFEs.
Although I emphasize the role of flooding on propagule pulses, one cannot disregard other natural and human mediated dispersal pathways of NNSs in RFEs. Examples of natural dispersers include fish (ichthyochory; Cantanhêde et al., 2008; Catford & Jansson, 2014; Jones et al., 2020) and wild terrestrial animals (Oliveira et al., 2006; Mworia et al., 2011). For example, propagule pulses may be enhanced if NNSs use other species as hosts (e.g., non-native parasites) or if they are used as prey. This is the case for the invasive bivalves Limnoperna fortunei Dunker, 1856 and Corbicula fluminea O. F. Müller, 1774, which are eaten by the fish Pterodoras granulosus Valenciennes, 1821 (Cantanhêde et al., 2008). The movement of these fish during high water increases propagule pressure in RFE habitats.
Dispersal of NNSs also occurs during low water phases for aquatic and terrestrial species. The spread of aquatic NNSs (propagule pressure) continues to occur during low water, but this pathway predominates in habitats that remain connected. For example, propagules of the macrophyte Hydrilla verticillata abound in lakes connected to the Paraná RFE (Brazil) during low water periods (personal observation). Finally, there are RFEs where flooding (an indication of seed vector) is not related to the presence of NNSs in specific habitats. This occurs, for example, for long-established NNSs (decades to centuries), which are no longer dispersal limited (Warren et al., 2015).
Humans also enhance disturbances (Box 1) and the spread of aquatic species in RFEs (e.g., Hudon et al., 2005; Chipps et al., 2006; Oliveira et al., 2006; Díez-del-Molino et al., 2016; Johnson et al., 2016; Reshetnikova et al., 2017). Cattle, for example, help to spread NNSs of plants in a RFE in Kenya (Mworia et al., 2011). In an RFE in the USA, the practice of rafting increases the success of NNSs of plants (Pearce & Smith, 2003) and in another RFE, modification of aquatic habitats and public access are associated with the invasion success of the bullfrog Lithobates catesbeianus Shaw, 1802 (Sepulveda et al., 2015; Sepulveda, 2018). In the Elbe RFE (Czech Republic), expansion of the Prussian carp Carassius auratus gibelio (Bloch) is explained by escapes from aquaculture activities (Slavík & Bartoš, 2004). In Australian RFEs, the spread of invasive plants is enhanced by human interference during low water (Catford et al., 2011). These dispersal mechanisms, which are unrelated to propagule pulses, enhance even more the spread and success of NNSs in RFEs.
In summary, the propagule pulses and other dispersal mechanisms that enhance propagule pressure should be taken into account when explaining the success of NNSs in RFEs. The spread of NNSs can be reduced with flow manipulations (see “Human impacts on hydrodynamics and their effects on non-native species in river-floodplain ecosystems” later in this review) and other methods of management that reduce disturbances and the spread of NNSs during low water periods.
Abiotic and biotic filters of invasion in river-floodplain ecosystems
Life history and pre-adaptation to the flood pulse
Invasion success depends on the interaction between propagule pressure and other driving forces (Fig. 2). The flood pulse seasonally changes the physical and chemical environment, and the abundance of native populations in RFEs, which in turn may stimulate those NNSs that have a life history associated with RFEs (or with similar ecosystems) in their native ranges and which have pre-adaptations to the flood pulse. For example, the life history of Cyprinus carpio Linnaeus, 1758, is associated with spring flooding, which partially explains its success in unstable habitats subjected to variations in water level, including RFEs (King et al., 2003; Jones & Stuart, 2009; Bajer & Sorensen, 2010; Maiztegui et al. 2019). Another example occurs in the Upper Paraná River floodplain (Brazil), where 10 NNSs of fish successfully became established (Espínola et al., 2015; Agostinho et al., 2015; Gois et al., 2015; Tonella et al., 2018). The majority of these NNSs of fish are pre-adapted to the flood pulse because they are native to the RFEs of the Pantanal and Amazon, which may have helped their success in the Upper Paraná RFE. Similarly, the fish Misgurnus anguillicaudatus Cantor, 1842, invades seasonally inundated Mediterranean rice fields (Spain), where the flood regime is similar to the one reported in the native ranges of this species in Japan (Clavero et al., 2015).
There are species that did not evolved in RFEs but succeed in these ecosystems anyway. For plants, traits responsible for resilience to water level fluctuations are found in species adapted to episodic disturbances other than the flood pulse, making these disturbance-adapted plants potential colonizers of RFEs and riparian corridors (Catford & Jansson, 2014). According to these authors, few NNSs that invade riparian corridors are specifically adapted to the abiotic conditions of these ecosystems. Other examples showing that pre-adaptation to the flood pulse is not a prerequisite to invade RFEs are the macrophyte Hydrilla verticillata and the mussels Limnoperna fortunei and Corbicula fluminea, which colonize RFEs on many continents (e.g., Drago et al., 2004; Oliveira et al., 2006, 2010a, b; Sardina et al., 2011; Sousa, 2011; Paillex et al., 2013, 2017; Zilli, 2013; Besacier-Monbertrand et al., 2014; Pander et al., 2016; Souza et al., 2017). These three species are found in ecosystems other than RFEs in their native ranges (Wei et al., 2013; Zhang et al., 2014; Li et al., 2017; Williams et al., 2018), indicating that their evolutionary histories were not exclusively associated with environments that experience flood pulses. High reproduction and growth rates associated with broad tolerance to environmental conditions, high plasticity, opportunistic traits and generalist habits (e.g., Barko et al., 2006; Simões et al., 2009; Budde et al., 2011; Sousa, 2011; Fobert et al., 2013; Ho et al., 2013; Paolucci et al., 2014; Agostinho et al., 2015; Crespo et al., 2015; Flanagan et al., 2015; Díez-del-Molino et al., 2016; Pander et al., 2016; Cuda et al., 2017; Tonella et al., 2018; Lewerentza et al., 2019), pre-dispose these three species and other NNSs to tolerance of the water level oscillations and associated environmental changes in RFEs.
Species with life histories associated with RFEs in their native ranges do not necessarily succeed in RFEs in introduced ranges. The peacock bass Cichla ocellaris Bloch & Schneider, 1801, for example, is native to Amazonian RFEs, but this species took c. 10 years to become a successful invader in the Upper Paraná River floodplain in Brazil (Agostinho et al., 2007; Espínola et al., 2015; Ortega et al., 2020). According to these authors, the species (which is a visual predator) only succeeded after water transparency was enhanced following dam construction upstream from the RFE.
It is important to highlight that numerous species that successfully colonize and establish in RFEs with natural flood pulses may be even more successful (in terms of invasion) when hydrodynamics are changed by human interference. For example, despite having traits which are favoured to some degree by flooding, C. carpio (and other species) (see Table 2) attains higher invasion success and impacts in RFEs where flow is regulated, i.e., where natural flooding has been reduced or prevented (Bunn & Arthington, 2002). Similarly, the Amazonian fish Cichla kelbery Kullander & Ferreira, 2006, invades other RFEs outside its native range (e.g., Agostinho et al., 2007), but is found at much higher abundances in reservoirs (Ortega et al., 2015; Pelicice et al., 2015). These examples point out that alterations to river flow may be key to explaining invasions in RFEs (see “Human impacts on hydrodynamics and their effects on non-native species in river-floodplain ecosystems”).
The role of disturbance for invasion success in RFEs
According to Tockner et al. (2010), RFEs are disturbance-dominated ecosystems. Flood pulses characterized by seasonal flooding and drought may be considered natural disturbances since they change communities, ecosystem structure and resources availability. A variety of native species colonizing RFEs were selected by seasonal disturbance determined by hydrodynamics (Luz-Agostinho et al., 2009; Davis et al., 2018), and their abundances change seasonally in response to the flood pulses (Junk et al., 1989; Tonella et al., 2019). In the context of invasion biology, disturbance is considered a key factor for explaining invasion success because disturbances create ‘invasion windows’ by reducing densities of native species temporarily and/or by altering the supply of resources (Davis et al., 2000; Hershner & Havens, 2008; Hobbs, 2011). Reduced populations of native species facilitates the success of NNSs because it diminishes biotic resistance (Levine 2000), while increased resources directly enhances the chances of invasion because invasive species can take advantage of these ‘invasion windows’ (Davis et al., 2000; Hobbs, 2011). For example, flooding causes disturbances in riparian communities, enhances nutrients and makes habitats less shaded than adjacent uplands, thus resulting in greater invasion than in adjacent areas (Fridley, 2011; Table 2; Sup. Mat. 4). Another example comes from a large survey conducted in European and Japanese RFEs, which found that annual and perennial NNSs of plants (which colonize the ATTZ) are the most species rich, followed by invasive woody plants and macrophytes (Müller & Okuda, 1998). The same pattern is observed in riparian zones, which experience high degrees of invasion by NNSs (Catford & Jansson, 2014). However, the effects of flooding on NNSs are not always positive, and there are examples where flooding disturbance prevents invasions (Table 2; Sup. Mat. 4). Within this context, the change of the natural disturbance regime, instead of the natural disturbance itself, can be considered the main driver of NNSs success (Catford et al., 2011; 2014; see “Human impacts on hydrodynamics and their effects on non-native species in river-floodplain ecosystems” below). In addition, drought also opens ‘invasion windows’ for NNSs, mainly those that do well in the ATTZ (Table 2; Sup. Mat. 4).
Reductions in the abundances of native species caused by disturbances also increases the success of NNSs indirectly through enhanced resource availability (i.e., part of the resources that were used by native species becomes available for NNSs). This positive feedback may enhance even more the success of NNSs, because reduced abundances of native species (implying less biotic resistance) enhance resources surplus. However, these opportunities only contribute to the success of NNSs if the disturbances are followed by enhanced propagule pressure or, in RFEs, by propagule pulses (see “Propagule pressure and propagule pulse in river-floodplain ecosystems”).
The influence of different flood-pulse phases on the success of non-native species
The flood pulse represents a temporal gradient of conditions that change from low to high water levels, and vice versa, to which environmental features and organisms respond continuously. However, researchers working with floodplain ecology usually identify responses of environmental characteristics and organisms to extreme water levels represented by flooding (high water) and drought (low water) conditions. The emphasis on responses of the environment and organisms to extreme conditions is probably because these responses are easier to identify. Taking this into account, below I use empirical evidence to discuss four general possibilities about the driving forces that determine the performance and success of NNSs during different flood-pulse phases (see Table 2 and details about them in Sup. Mat. 4). In general, at least for aquatic organisms, the abiotic medium (e.g., depth, oxygen, underwater light) probably has a major role during the high-water period, when its features may become unfavourable for many species, while biotic features (e.g., biotic resistance) may play a major role during low water periods because biotic interactions act more intensively in aquatic environments during this phase (Thomaz et al., 2007; Quirino et al., 2015; see Fig. 1b, c).
It is important to highlight, however, that the driving factors identified in any investigation are not static, since one species may have some traits enhanced during flooding and other traits enhanced during drought. For example, the NNS Tamarix chinensis Lour. is out-competed by native plants when the hydrological regime is restored, indicating that its competitive ability is negatively affected by flooding (Bhattacharjee et al., 2009); however, recruitment for the species is triggered by large annual flooding (Birken & Cooper, 2006), indicating a positive effect of flooding for the ‘reproduction trait’. Another example occurs with plant communities in the Moolayember Creek floodplain (Australia), where the seed bank of NNSs decreases but their abundance increases in response to flooding (Osunkoya et al., 2014). These examples were able to identify opposite effects of flooding for different traits of NNSs.
Another complication to separating the effects of the flood pulse using only extremes (flooding versus drought) is that both extremes might negatively impact specific traits of a species, as is the case of the bullfrog Lithobates catesbeianus in the Yellowstone RFE in the USA (Sepulveda, 2018). In this case, the species probably recovers in the floodplain during periods of intermediate water level (e.g., rising or decreasing water) or has other (not measured) traits affected positively during these extremes.
Despite these difficulties in analysing the effects of the flood pulse on NNS success, I use here a systematic survey (see Sup. Mat. 1) to search for how the performance of NNSs is related to flooding (high water conditions) and drought (low water conditions) in RFEs. This means that an investigation may identify factors related to species success in a specific water level phase but not in another. In this case, it does not mean that the other phase does not have a role for the studied NNS, but just that the effect was not clearly stated by the authors. More detailed and specific statements about the rationale behind the choice of separating the flood pulse into extremes (low versus high water) are provided in Supplementary Material 3.
There are examples showing enhanced performance for numerous NNSs with flooding, which contributes to their colonization, establishment, spread and/or impacts in RFEs. These examples fit classical theory predicting that invasion success is facilitated by disturbances (see “Propagule pressure and propagule pulse in river-floodplain ecosystems”). For example, the disturbance associated with flooding can determine the dominance of graminoids (including NNSs) by increasing germination after removing herbaceous litter (Andrew et al., 2012) and can determine the dominance of grasses that invade stream sides where floods scour native vegetation (Barden, 1987). The success of NNSs of plants following high-amplitude flood-pulses may be explained by increases in resources and relaxed competition with native species after flooding (Toth, 2015). Invasion windows that benefit NNSs of plants can also open during flooding by litter removal, the scouring of native vegetation (which reduces biotic resistance) and the formation of gaps (which enhances resources availability) (Cunha & Junk, 2004; De Jager et al., 2013). In general, plant species with fast life cycles are favoured by flooding. This is evidenced in riparian corridors, where the number of NNSs of macrophytes and herbs with annual life cycles is large (Chundi et al., 2017) and the number of NNSs with annual cycles can be even higher than the number of native species (Catford et al., 2011; Catford & Jansson, 2014). An aquatic animal example is the fish C. carpio, whose successful invasion of RFEs is at least in part related to flooding, which increases resource availability (food), decreases biotic resistance and changes abiotic filters (Barko et al., 2006; Jones & Stuart, 2009; Beesley et al., 2012) (Table 2; Sup. Mat. 4).
In contrast to the above examples, there are investigations showing that NNSs are hampered or have their performance negatively impacted by flooding (Table 2; Sup. Mat. 4). These species are supposedly those whose life-history is not associated with the flood pulse. Plant species that do not grow and complete their life cycle rapidly (e.g., k-strategy plants) are also usually negatively affected by flooding (e.g., Johnson et al., 2016; Boever et al., 2017). There is also evidence that sometimes the negative effects of flooding, those that make habitats less suitable, are compensated by positive effects that promote propagule pulses (see Sousa et al., 2010 for an example with macrophytes and Chapman & Warburton, 2006 for an example with fish). Finally, it is worth noting that despite the negative effects that flooding has on some NNSs, the high heterogeneity of RFEs enhances the number and types of refugia (e.g., lateral channels, abandoned meanders and floodplain lakes), from which recolonization occurs after inundations (DeGrandchamp et al., 2008; Díez-del-Molino et al., 2016; Fuller & Death, 2018).
There are NNSs whose performance is enhanced during drought (Table 2; Sup. Mat. 4). This occurs mainly with species that are more adapted to terrestrial habitats, species that develop in the ATTZ and in riparian zones, and some aquatic macrophytes that prefer nearly constant water levels (Catford et al., 2011, 2014). A combination of shade and drought stress may also enhance the biomass of NNSs of plants (González-Muñoz et al., 2014). In these cases, invasive success was associated with a reduction in biotic resistance by native species and changes in environmental filters caused by terrestrialization. Although terrestrial and ATTZ NNSs of plants are the ones that are more benefited by droughts, aquatic NNSs may also take some advantage of this condition. In the Loire River floodplain (France), the number of NNSs of fish increased with isolation, indicating that drought enhances invasion success (Lasnea et al., 2008). Similarly, floating-leaved macrophytes may invade water bodies where floods have been reduced by river regulation (Rajakaruna et al., 2017).
Finally, there are studies showing that the performance of NNSs is negatively related to droughts (Table 2; Sup. Mat. 4), but I found far less cases showing this possibility. One example is found in the Phongolo RFE (South Africa – Mozambique border), where none of the Mozambique tilapia Oreochromis massambicus Peters, 1852, collected during the drought were parasitized by the invasive Lernaea cyprinaceae Linnaeus, 1758, while fish collected during the flood period were infected (Welicky et al., 2017). This decline in infection during drought occurred because the water became hypersaline during this period, which represents an abiotic filter for this invasive freshwater parasite.
In summary, there are plenty of examples showing that NNSs may be benefited by flooding while others are hampered. Similarly, there are NNSs that benefit from drought, and a few others that are hampered. The most important conclusion, nevertheless, is that this collection of empirical evidence clearly shows that the success of NNSs depends on the flood pulse and on its consequences for the abiotic and biotic filters in RFEs. However, these examples also show that the responses of NNSs to the flood pulse are highly idiosyncratic.
When the flood pulse allows the coexistence of native and non-native species
Coexistence of individual native and non-native species
A general theory about species interactions, which applies to invasion biology, predicts that the possibility of coexistence between two species is enhanced with reduced niche overlap and increased species fitness (Chesson & Kuang, 2008; Rejmánek, 2011). The seasonal alterations to chemical and physical characteristics, community composition and resource availability caused by the flood pulse interfere with niche overlap and species fitness, with consequences for the coexistence of native and NNSs. For example, if native and NNSs change their feeding habits during the flood period, as observed for a variety of groups (e.g., Abujanra et al., 2009; Quirino et al., 2015; 2017), their niche overlap would be reduced during this period, enhancing the possibility of coexistence over longer periods. Similarly, native species that enhance recruitment following seasonal floods (e.g., Oliveira et al., 2020) may have an advantage over NNSs that are not adapted to the flood pulse, which may also enhance the possibility of coexistence.
Coexistence of native and NNSs has been documented in RFEs, but in the majority of cases the explanations are not related to the flood pulse. For example, the invasive macrophyte H. verticillata co-occurs with native submerged macrophytes in the Paraná RFE in Brazil, but coexistence can be explained by habitat segregation independently of the flood pulse (Sousa et al., 2010). In this same ecosystem, trophic and habitat segregation and different traits explain the coexistence of native and NNSs of fish (Pereira et al., 2007; Zaia Alves et al., 2017; Rodrigues et al., 2018). However, what is of interest in the context of the present review is whether (and how) the coexistence of native and NNSs is mediated by alterations associated with the flood pulse.
There are indirect examples that suggest that the coexistence of native and NNSs can indeed be related to environmental changes caused by the flood pulse. Although the authors of some of these investigations do not explicitly associate their findings with the coexistence of native and NNSs, one can infer from them that the possibility of coexistence is at least enhanced by inter-specific differences mediated by the flood pulse. For example, in an Australian RFE the dominance of one native and one NNS of plant shifts in response to the flood pulse (Price et al., 2010; 2011a), which probably allows the coexistence of these species throughout the hydrological cycle.
For fish, different movements of native and NNSs in response to the flood pulse (Williams & Gregory, 2018; Williams et al., 2018) may help coexistence. Coexistence between native and non-native fish may also be enhanced if they colonize different sites during the flood pulse (Ho et al., 2013), which can be associated with changes in recruitment rates and resource use of both types of species. Changes in feeding habits may also be involved in co-existence, as suggested for juveniles of C. carpio and of the native fish Hypseleotris sp., whose diet and trophic levels become more different during high water (Mazumder et al., 2012). In this case, one can speculate that such differences that occur, at least during high water, may alleviate competition and enhance the chances of coexistence throughout the year. There is also evidence that native and NNSs of fish do not reproduce at the same time, and that native species use different habitats than NNSs, which give the native species an additional competitive edge over the NNSs (Sommer et al., 2014).
In addition to these examples of empirical evidence is a modelling study that suggests that the flood pulse may also mediate the coexistence of amphibians. Doubledee et al. (2003) found that the occurrence of winter floods increases the mortality of the non-native bullfrog Rana catesbeiana Shaw, 1802, but does not affect the native Rana aurora draytoni Baird & Girard, 1852. These different effects of flooding diminish the probability of extinction of native species, thus enhancing the chances for coexistence.
Coexistence of native and non-native species at the assemblage level
There is indirect evidence suggesting that a high number of species (assemblages) of native and of NNSs can coexist, at least in some RFEs. This evidence comes from studies conducted with different groups of organisms showing that there are positive relationships between native and NNSs richness in RFEs. For example, positive correlations between the richness of native and NNSs of plants have been found in a variety of riparian corridors and RFEs (Tabacchi & Planty-Tabacchi, 2005; Tabacchi et al., 2005; Uowolo et al., 2005; Stokes et al., 2010; Petrášová et al., 2013). In the Paraná RFE in Brazil, the richness of native and NNSs of fish correlate positively independent of the spatial scale analyzed (Santos et al., 2018). Another example of coexistence is with invertebrates in the Rhône River (France), where the highest functional diversity of native and NNSs occurs at sites with intermediate connection with the river channel (Paillex et al., 2013). According to these authors, niche filtering and competitive exclusion are low at these sites. Thus, in this case, one can conclude that the flood pulse (which allows connection among sites) helps coexistence.
Positive relationships between native and NNSs richness usually occur at larger spatial scales and opposes the idea of biotic resistance found at smaller scales (see Fridley et al., 2007). These positive correlations are in accordance with the ‘biotic acceptance hypothesis’ (Box 1), which predicts that the “rich get richer” or good sites for native species are also good for NNSs (e.g., Stohlgren et al., 2006; Fridley et al., 2007; Richardson et al., 2011). Thus, these results obtained for RFEs indicate that where a positive correlation occurs between native and NNSs, habitats that support more native species are also prone to be more invaded and that more species can coexist in these sites. The factors explaining this pattern in RFEs are not well understood, but disturbances, habitat heterogeneity and resource availability (Uowolo et al., 2005; Santos et al., 2018), which are typical features of RFEs, might be involved.
Other factors allowing positive correlations between native and NNSs are environmental suitability and propagule supply (Stokes et al., 2010). Because these features are related to the flood pulse in RFEs, one can suppose that the flood pulse has at least some role in explaining the support of the acceptance hypothesis in RFEs with positive correlations between native and NNSs richness.
Human impacts on hydrodynamics and their effects on non-native species in river-floodplain ecosystems
The natural hydrodynamics of some RFEs (along with their physical and chemical features), including the natural flood pulse, have changed in response to numerous human activities, such as irrigation, water supply and hydroelectricity production (Gehrke & Harris, 2001; Lytle & Poff, 2004; Fernandes et al., 2009; Agostinho et al., 2013; Johnson et al., 2016; Winemiller et al., 2016). Alterations in hydrodynamics (i.e., the disturbance regime), along with physical and chemical water features, in turn, may help NNSs to thrive in RFEs (Poff et al., 1997; Müller & Okuda, 1998; Bunn & Arthington, 2002; Lytle & Poff, 2004; Simões et al., 2009; McShane et al., 2015). These alterations are related to a variety of aspects of the natural flood pulse, such as flooding and drought frequencies, amplitudes and seasonality. It is important to state that changes to disturbance dynamics related to the flood pulse (rather than the disturbance per se) enhance invasion by (a) reducing the abundance of native species (less biotic resistance) and (b) providing hydrological conditions that favor certain NNSs that are adapted to them (Catford et al. 2011, 2014).
Despite the examples showing that NNSs succeed with flooding or drought events, there are numerous other examples showing that the success of NNSs of macrophytes, terrestrial plants and fish increases even more in response to alterations to natural hydrodynamics (natural food pulse) (Table 3; Sup. Mat. 5). Flow regulation enhances the terrestrialization of RFEs (see below) and when it diminishes flooding intensity it also decreases connectivity in RFEs (Thomaz et al., 2007), with consequences for native and NNSs that depend on flooding for dispersal (see “Propagule pressure and propagule pulse in river-floodplain ecosystems”). Flow supplementation that causes permanence in floodplain lake water may enhance the invasion of NNSs of macrophytes, including the water hyacinth Eichhornia crassipes Mart. (Solms) (Tibby et al., 2019). Increases in temporal variability is another type of flow alteration that may increase the number of NNSs, which is in line with the fact that NNSs are usually more adapted to disturbances (Brummer et al., 2016).
In view of these examples showing that flow alteration affects the success of NNSs in RFEs, management that re-stablishes natural flood pulses in regulated rivers is a possibility for reducing the success of NNSs (e.g., Catford et al. 2011, 2014). ‘Designed disturbance’, which drives particular processes and stimulates particular native species assemblages, is a technique that helps conservation and restoration (Hobbs, 2011). For example, simulating historic flood pulses has the potential to reduce the success of NNSs of plants (Hobbs, 2011; Dawson et al., 2017a, b; Dong et al., 2019) and benefit native plants (Glenn & Nagler, 2005; Nagler et al., 2005; Bhattacharjee et al., 2009; Stokes et al., 2010; Dixon et al., 2015; Price et al., 2011a) and native fish (Barko et al., 2006). Even if NNSs of plants are ruderal with high growth rates, their increment during droughts is hampered by regular floods, which reduces their dominance (Stokes et al., 2010; Lunt et al., 2012).
Despite massive evidence that flow rehabilitation may enhance native relative to NNSs in RFEs and riparian corridors, there are cases where flow regulation does not affect the success of NNSs and where the use of flow rehabilitation does (and will probably) not control NNSs in river corridors and RFEs (Birken & Cooper, 2006; Mortenson et al., 2012). In addition, the restoration of environmental flow may benefit NNSs in some RFEs (Howell & Benson, 2000; Taylor & Ganf, 2005; Toth, 2010; Paillex et al., 2013; 2015; Stoffels et al., 2014). These examples show that management aiming at reducing NNS success and restoring native diversity in RFEs is not an easy task. It is possible that managing the flow regime to reduce the abundances of NNSs would not necessarily benefit native species because these two groups respond differently to flow regimes (Brummer et al., 2016). Owing to the idiosyncrasies found in RFEs, the rehabilitation of river flow with the aim of NNSs control has to be evaluated for each individual case.
Global changes may also impact the success of NNSs in RFEs. A first, direct effect is related to temperature itself (e.g., Paillex et al., 2017). However, the greatest long-term impacts on NNS success may be related to extreme climatic events (ECE) associated with global changes (Diez et al., 2012). ECEs include changes in natural hydrodynamics, which will be altered because rainfall is predicted to increase in some river basins and decrease in others (Aldous et al., 2011; Marengo et al., 2012; Hoegh-Guldberg et al., 2018), and thus change the natural flood pulse. An increase in the number of large flooding events will probably occur in some river basins, with consequences for the geomorphology and biota of RFEs (Death et al., 2014 and references therein). The fragmentation, spread, colonization and growth of NNSs of plants may be facilitated by large and more frequent floods (Merrin et al., 2005; Truscott et al., 2006; Fobert et al., 2013; Gibson-Reinemer et al., 2017). Increases of NNSs in response to these ECEs occur, in part, because these disturbances open ‘invasion windows’ by killing and/or stressing resident organisms, and by enhancing resource availability (Davis et al., 2000; Diez et al., 2012). In contrast, changes in the natural flood pulse in regions where rainfall will decrease may cause more frequent and intense droughts (i.e., ‘terrestrialization’ of RFEs), which will probably facilitate the success of NNSs that colonize the ATTZ and riparian corridors, as exemplified by a variety of examples where this has already happened (see also Kopéc et al., 2014; McShane et al., 2015; Table 2 and Sup. Mat. 4).
Finally, there are other anthropogenic disturbances (e.g., raising cattle and agriculture) which contribute to enhancing invasion success in RFEs. For example, livestock grazing impacts native more than non-native annual plants in the Barmah-Millewa RFE in Australia (Lunt et al., 2012). Land use together with reservoir flooding were found to enhance the presence of NNSs of plants in riparian corridors in China (Chundi et al., 2017) and disturbances associated with recreational use enhance the number and frequency of NNSs of plants in the Potamic RFE in the USA (Pyle, 1995). Human disturbances in RFEs can be even more important to the enhancement of invasion by herbaceous plants than the natural flood disturbance (Andrew et al., 2012). In addition, human disturbances may extend impacts over time. For example, land use legacies have been shown to be important in this regard (Dawson et al., 2017a, b), evidencing that past disturbances may influence NNSs success long after they have cessed.
Conclusions
This survey showed that despite restrictions imposed by the flood pulse to NNSs whose life history is not related to seasonal fluctuations in water level in their native ranges, numerous species successfully invade RFEs all over the world. Their ruderal characteristics, high plasticity and fast spread, facilitated by the flood pulse, may be some of the factors that predispose these NNSs to invade RFEs. Studying species traits related to these characteristics may enhance our ability to predict which NNSs will succeed in RFEs.
This review also shows that alterations of the flood pulse impact RFEs and affect the success of NNSs, and that this is currently accepted as fact by scientists and managers. However, this literature survey also evidenced that it is very difficult to predict and quantify responses of NNSs to altered flow regimes (see also Bunn & Arthington, 2002).
Despite idiosyncrasies, invasions of RFEs are mainly related to factors associated with the disturbance regime, represented by the flood pulse in these ecosystems. Within this context, and according to the examples I found in this review, at least two cases can be identified within the context of “invasion syndrome”, a theory that unifies pathways, species traits and characteristics of the recipient ecosystem to enhance predictability of invasion success and impacts (Novoa et al., 2020). One possibility includes aquatic species with traits adapted to the flood pulse, which are usually introduced through aquaculture or ballast water (pathway) and are dispersed by water through hydrochory during annual flooding (a unique feature of RFEs). Examples of this possibility are the grass carp and golden mussel. A second possibility also considers transport of propagules by water (hydrochory), but includes NNSs with traits adapted to drier sites (riparian corridors and the AATZ) and whose means of introduction are associated with feedstock and horticulture. These last species are predicted to invade more successfully RFEs that suffer terrestrialization, and numerous species of grasses (see Novoa et al., 2020) are good examples. Thus, knowing which type of effect a given alteration will cause to the flood pulse, and how NNSs respond to it, is key to understanding, predicting and proposing management strategies aimed at avoiding the spread and impacts of NNSs in RFEs.
References
Abujanra, F., A. A. Agostinho & N. S. Hahn, 2009. Effects of the flood regime on the body condition of fish of different trophic guilds in the Upper Paraná River floodplain, Brazil. Brazilian Journal of Biology 69: 469–479.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2004. Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Ecohydrology & Hydrobiology 4: 255–268.
Agostinho, A. A., H. I. Suzuki, R. Fugi & D. C. Alves, 2015. Ecological and life history traits of Hemiodus orthonops in the invasion process: looking for clues at home. Hydrobiologia 746: 415–430.
Agostinho, A. A., F. M. Pelicice, A. C. Petry, L. C. Gomes & H. F. Júlio Jr., 2007. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquatic Ecosystem Health & Management 10: 174–186.
Agostinho, A. A., S. M. Thomaz & L. C. Gomes, 2005. Conservation of the Biodiversity of Brazil’s Inland Waters. Conservation Biology 19: 646–652.
Agostinho, A. A., L. C. Gomes, C. C. Bonecker & S. M. Thomaz, 2013. Padrões e variação de longo prazo na Planície de Inundação do Alto Rio Paraná. In: Tabarelli, M., C. F. D. Rocha, H. P. Romanowski, O. Rocha & L. C. Lacerda (eds) PELD – CNPq: dez anos do Programa de Pesquisas Ecológicas de longa duração do Brasil: achados, lições e perspectivas. Recife-PE., Ed. Universitária da UFPE. p. 165-196 (Série de publicações PELD).
Aldous, A., J. Fitzsimons, B. Richter & L. Bach, 2011. Droughts, floods and freshwater ecosystems: evaluating climate change impacts and developing adaptation strategies. Marine and Freshwater Research 62: 223–231.
Amo, V. E., J. Ernandes-Silva, D. A. Moi & R. P. Mormul, 2021. Hydrological connectivity drives propagule pressure of Limnoperna fortunei (Dunker, 1857) in a tropical river-floodplain system. Hydrobiologia (in press).
Andrew, A. M., S. R. Moe, Ø. Totland & K. T. Munishi, 2012. Species composition and functional structure of herbaceous vegetation in a tropical wetland system. Biodiversity and Conservation 21: 2865–2885.
Baart, I. S., I. Zsuffa Hohensinner & T. Hein, 2013. Supporting analysis of floodplain restoration options by historical analysis. Environmental Science & Policy 34: 92–102.
Bajer, P. G. & P. W. Sorensen, 2010. Recruitment and abundance of an invasive fish, the common carp, is driven by its propensity to invade and reproduce in basins that experience winter-time hypoxia in interconnected lakes. Biological Invasions 12: 1101–1112.
Barden, L. S., 1987. Invasion of Microstegium vimineum (Poaceae), an exotic, annual, shade-tolerant, C4 grass, into a North Carolina floodplain. The American Midland Naturalist 118: 40–45.
Barko, V. A., D. P. Herzog & M. T. O’Connell, 2006. Response of fishes to floodplain connectivity during and following a 500-year flood event in the unimpounded upper Mississippi River. Wetlands 26: 244–257.
Barrat-Segretain, M. H., 2001. Invasive species in the Rhône River floodplain (France): replacement of Elodea canadensis Michaux by E. nuttallii St. John in two former river channels. Archif für Hydrobiologie 152: 237–251.
Beesley, L., A. J. King, F. Amtstaetter, J. D. Koehn, B. Gawne, A. Price, D. L. Nielsen, L. Vilizzi & S. N. Meredith, 2012. Does flooding affect spatiotemporal variation of fish assemblages in temperate floodplain wetlands? Freshwater Biology 57: 2230–2246.
Bennett, A. J., W. C. Conway, C. E. Comer, H. M. Williams & S. B. Bosworth, 2015. Seedbank potential of chinese tallow tree (Triadica sebifera) in a Texas bottomland hardwood forest. Natural Areas Journal 35: 581–584.
Besacier-Monbertrand, A. L., A. Paillex & E. Castella, 2014. Hort-term impacts of lateral hydrological connectivity restoration on aquatic macroinvertebrates. River Research and Applications 30: 557–570.
Bhattacharjee, J., J. P. Taylor Jr., L. M. Smith & D. A. Haukos, 2009. Seedling competition between native cottonwood and exotic saltcedar: implications for restoration. Biological Invasions 11: 1777–1787.
Bino, G., S. Wassens, R. T. Kingsford, R. F. Thomas & J. Spencer, 2018. Floodplain ecosystem dynamics under extreme dry and wet phases in semi-arid Australia. Freshwater Biology 63: 224–241.
Birken, A. S. & D. J. Cooper, 2006. Processes of Tamarix invasion and floodplain development along the lower Green River, Utah. Ecological Applications 16: 1103–1120.
Boever, C. J., M. D. Dixon, W. C. Johnson, M. L. Scott & T. P. Malloy Jr., 2017. Effects of a large flood on woody vegetation along the regulated Missouri River, USA. Ecohydrology 12:
Bolpagni, R. 2021. Invasive alien plants in freshwater ecosystems: towards global dominance? Hydrobiologia (in press).
Boyd, L., R. M. Nally & J. Read, 2005. Does fallen timber on floodplains influence distributions of nutrients, plants and seeds? Plant Ecology 177: 165–176.
Brummer, T. J., A. E. Byrom, J. J. Sullivan & P. E. Hulme, 2016. Alien and native plant richness and abundance respond to different environmental drivers across multiple gravel floodplain ecosystems. Diversity and Distributions 22: 823–835.
Budde, K. B., L. Gallo, P. Marchelli, E. Mosner, S. Liepelt, B. Ziegenhagen & I. Leyer, 2011. Wide spread invasion without sexual reproduction? A case study on European willows in Patagonia, Argentina. Biological Invasions 13: 45–54.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Burkart, M., 2001. River corridor plants (Stromtalpflanzen) in Central European lowland: A review of a poorly understood plant distribution pattern. Global Ecology & Biogeography 10: 449–468.
Cantanhêde, G., N. S. Hahn, E. A. Gubiani & R. Fugi, 2008. Invasive molluscs in the diet of Pterodoras granulosus (Valenciennes, 1821) (Pisces, Doradidae) in the Upper Parana´ River floodplain, Brazil. Ecology of Freshwater Fish 17: 47–53.
Caruso, B. S., L. Edmondson & C. Pithie, 2013a. Braided River Flow and invasive vegetation dynamics in the Southern Alps, New Zealand. Environmental Management 52: 1–18.
Caruso, B. S., C. Pithie & L. Edmondson, 2013b. Invasive riparian vegetation response to flow regimes and flood pulses in a braided river floodplain. Journal of Environmental Management 125: 156–168.
Castello, L., V. J. Isaac & R. Thapa, 2015. Flood pulse effects on multispecies fishery yields in the Lower Amazon. Royal Society Open Science 2: 150299. https://doi.org/10.1098/rsos.150299.
Castro-Díez, P., G. Valle, N. G. Muñoz & A. Alonso, 2014. Can the life-history strategy explain the success of the exotic trees Ailanthus altissima and Robinia pseudoacacia in Iberian floodplain forests? Plos One 6:
Catford, J. A., R. Jansson & C. Nilsson, 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions 15: 22–40.
Catford, J. A., B. J. Downes, C. J. Gippel & P. A. Vesk, 2011. Flow regulation reduces native plant cover and facilitates exotic invasion in riparian wetlands. Journal of Applied Ecology 48: 432–442.
Catford, J. A. & R. Jansson, 2014. Drowned, buried and carried away: effects of plant traits on the distribution of native and alien species in riparian ecosystems. New Phytologist 204: 19–36.
Catford, J. A., W. K. Morris, P. A. Vesk, C. J. Gippel & B. J. Downes, 2014. Species and environmental characteristics point to flow regulation and drought as drivers of riparian plant invasion. Diversity and Distributions 20: 1084–1096.
Chapman, P. & K. Warburton, 2006. Postflood movements and population connectivity in gambusia (Gambusia holbrooki). Ecology of Freshwater Fish 15: 357–365.
Chundi, C., W. Shengjun, M. C. Douglas, M. Maohua, Z. Janjuan, L. Mingquan & T. Xiaoxia, 2017. Effects of local and landscape factors on exotic vegetation in the riparian zone of a regulated river: Implications for reservoir conservation. Landscape and Urban Planning 157: 45–55.
Chesson, P. & J. J. Kuang, 2008. The interaction between predation and competition. Nature 456: 235–238.
Chipps, S. R., D. E. Hubbard, K. B. Werlin, N. J. Haugerud, K. A. Powell & J. Thompson, 2006. Association between wetland disturbance and biological attributes in floodplain wetlands. Wetlands 26: 497–508.
Clavero, M., V. López, N. Franch, Q. Pou-Rovira & J. M. Queral, 2015. Use of seasonally flooded rice fields by fish and crayfish in a Mediterranean wetland. Agriculture, Ecosystems and Environment 213: 39–46.
Colautti, R. I., I. A. Grigorovich & H. J. MacIsaac, 2006. Propagule pressure: a null model for biological invasions. Biological Invasions 8: 1023–1037.
Collas, F. P. L., S. K. D. Breedveld, J. Matthews, G. van der Velde & R. S. E. W. Leuven, 2017. Invasion biology and risk assessment of the recently introduced Chinese mystery snail, Bellamya (Cipangopaludina) chinensis (Gray, 1834), in the Rhine and Meuse River basins in Western Europe. Aquatic Invasions 12: 275–286.
Colleran, B. P. & K. E. Goodall, 2014. In situ growth and rapid response management of flood-dispersed japanese knotweed (Fallopia japonica). Invasive Plant Science and Management 7: 84–92.
Connell, J. H., 1978. Diversity in tropical forests and coral reefs. Science 199: 1302–1310.
Crain, P. K., K. Whitener & P. B. Moyle, 2004. Use of a restored Central California floodplain by larvae of native and alien fishes. American Fisheries Society Symposium 39: 125–140.
Crespo, D., M. Dolbeth, S. Leston, R. Sousa & M. A. Pardal, 2015. Distribution of Corbicula fluminea (Müller, 1774) in the invaded range: a geographic approach with notes on species traits variability. Biological Invasions 17: 2087–2101.
Cruz, D. O., R. T. Kingsford, I. M. Suthers, T. S. Rayner, J. A. Smith & A. H. Arthington, 2020. Connectivity but not recruitment: Response of the fish community to a large-scale flood on a heavily regulated floodplain. Ecohydrology. https://doi.org/10.1002/eco.2194
Čuda, J., Z. Rumlerov, J. Bruna, H. Sláková & P. Pysek, 2017. Floods affect the abundance of invasive Impatiens glandulifera and its spread from river corridors. Diversity and Distributions 23: 342–354.
Davis, M. A., J. P. Grime & K. Thompson, 2000. Fluctuating resources in plant communities: a general theory of invasibility. Journal of Ecology 88: 528–534.
Davis, A. M., B. J. Pusey & R. G. Pearson, 2018. Big floods, big knowledge gap: Food web dynamics in a variable river system. Ecology of Freshwater Fish 27: 898–909.
Dawson, S. K., R. T. Kingsford, P. Berney, D. A. Keith, F. A. Hemmings, D. I. Warton, C. Waters & J. A. Catford, 2017a. Frequent inundation helps counteract land use impacts on wetland propagule banks. Applied Vegetation Science 20: 459–467.
Dawson, S. K., D. I. Warton, R. T. Kingsford, P. Berney, D. A. Keith & J. A. Catford, 2017b. Plant traits of propagule banks and standing vegetation reveal flooding alleviates impacts of agriculture on wetland restoration. Journal of Applied Ecology 54: 1907–1918.
De Jager, N. R., B. J. Cogger & M. A. Thomsen, 2013. Interactive effects of flooding and deer (Odocoileus virginianus) browsing on floodplain forest recruitment. Forest Ecology and Management 303: 11–19.
Death, R. G., I. C. Fuller & M. G. Macklin, 2014. Resetting the river template: the potential for climate-related extreme floods to transform river geomorphology and ecology. Freshwater Biology 60: 2477–2496.
Decruyenaere, J. G. & J. S. Holt, 2005. Ramet demography of a clonal invader, Arundo donax (Poaceae), in Southern California. Plant and Soil 277: 41–52.
DeGrandchamp, K. L., J. E. Garvey & R. E. Colombo, 2008. Movement and kabitat selection by invasive Asian carps in a large river. Transactions of the American Fisheries Society 137: 45–56.
Descombes, P., B. Petitpierre, E. Morard, M. Berthoud, A. Guisan & P. Vittoz, 2016. Monitoring and distribution modelling of invasive species along riverine habitats at very high resolution. Biological Invasions 18: 3665–3679.
Díaz, S. & M. Cabido, 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology & Evolution 16: 646–655.
Diez, J. M., C. M. D’Antonio, J. S. Dukes, E. D. Grosholz, J. D. Olden, C. J. B. Sorte, D. M. Blumenthal, B. A. Bradley, R. Early, I. Ibáñez, S. J. Jones, J. J. Lawler & L. P. Miller, 2012. Will extreme climatic events facilitate biological invasions? Frontiers in Ecology and the Environment 10: 249–257.
Díez-del-Molino, D., R. M. Araguas, M. Vera, O. Vidal, N. Sanz & J. L. García-Marín, 2016. Temporal genetic dynamics among mosquitofish (Gambusia holbrooki) populations in invaded watersheds. Biological Invasions 18: 841–855.
Dixon, M. D., C. J. Boever, V. L. Danzeisen, C. L. Merkord, E. C. Munes, M. L. Scott, W. C. Johnson & T. C. Cowman, 2015. Effects of a ‘natural’ flood event on the riparian ecosystem of a regulated large-river system: the 2011 flood on the Missouri River, USA. Ecohydrology 8: 812–824.
Doubledee, R. A., E. B. Muller & R. M. Nisbet, 2003. Bullfrogs, disturbance regime, and the persistence of California red-legged frogs. Journal of Wildlife Management 67: 424–438.
Drago, I. E., M. Marchese & K. M. Wantzen, 2004. Benthos of a large neotropical river: spatial patterns and species assemblages in the Lower Paraguay and its floodplains. Archiv für Hydrobiologie 160: 347–374.
Duncan, R. P., 2011. Propagule Pressure. In Simberloff, D. & M. Rejmánek (eds), Encyclopedia of Biological Invasions. University of California Press, Berkeley: 561–563.
Dong, A., J. Greet, C. J. Walsh & M. J. Sammonds, 2019. Managed flooding can augment the benefits of natural flooding for native wetland vegetation. Restoration Ecology 27: 38–45.
Duquette, M. C., A. Compérot, L. F. Hayes, C. Pagola, F. Belzile, J. Dubé & C. Lavoie, 2016. From the source to the outlet: Understanding the distribution of invasive knotweeds along a North American river. River Research and Applications 32: 958–966.
Dyakov, N. & P. Zhelev, 2013. Alien species invasion and diversity of riparian forest according to environmental gradients and disturbance regime. Applied Ecology and Environmental Research 11: 249–272.
EEA (European Environmental Agency), 2018. Why should we care about floodplains? Briefing no. 14/2018. https://www.eea.europa.eu/themes/water/european-waters/why-should-we-care-about-floodplains.
Elton, C. S., 1958. The Ecology of Invasions by Animals and Plants. London: Methuen (reprinted 2000, Chicago: University of Chicago Press).
Ernandes-Silva, J., F. H. Ragonha, L. C. Rodrigues & R. P. Mormul, 2016. Freshwater invasibility level depends on the population age structure of the invading mussel species. Biological Invasions 18: 1421–1430.
Ernandes-Silva, J., G. D. Pinha & R. P. Mormul, 2017. Environmental variables driving the larval distribution of Limnoperna fortunei in the upper Paraná River floodplain, Brazil. Acta Limnologica Brasiliensia 29:
Espínola, L. A., C. V. Minte-Vera, H. F. Júlio-Jr, L. N. Santos & K. O. Winemiller, 2015. Evaluation of factors associated with dynamics of Cichla ocellaris invasion of the Upper Paraná River floodplain system, Brazil. Marine and Freshwater Research 66: 33–40.
Esselman, P. C., J. J. Schmitter-Soto & J. D. Allan, 2013. Spatiotemporal dynamics of the spread of African tilapias (Pisces: Oreochromis spp.) into rivers of Northeastern Mesoamerica. Biological Invasions 15: 1471–1491
Fernandes, R., A. A. Agostinho, E. A. Ferreira, C. S. Pavanelli, H. I. Suzuki, D. P. Lima & L. C. Gomes, 2009. Effects of the hydrological regime on the ichthyofauna of riverine environments of the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 661–668.
Feyrer, F., T. R. Sommer, S. C. Zeug, G. O’Leary & W. Harrell, 2004. Fish assemblages of perennial floodplain ponds of the Sacramento River, California (USA), with implications for the conservation of native fishes. Fisheries Management and Ecology 11: 335–344.
Flanagan, N. E., C. J. Richardson & M. Ho, 2015. Connecting differential responses of native and invasive riparian plants to climate change and environmental alteration. Ecological Applications 25: 753–767.
Fobert, E., Z. Grzegorz, L. Vilizzi, M. J. Godard, M. G. Fox, S. Stakénas & G. H. Copp, 2013. Predicting non-native fish dispersal under conditions of climate change: case study in England of dispersal and establishment of pumpkinseed Lepomis gibbosus in a floodplain pond. Ecology of Freshwater Fish 22: 106–116.
Fridley, J. D., J. J. Stachowicz, S. Naeem, D. F. Sax, E. W. Seabloom, M. D. Smith, T. J. Stohlgren, D. Tilman & B. Von Holle, 2007. The invasion paradox: reconciling pattern and process in species invasions. Ecology 88: 3–17.
Fridley, J. D., 2011. Invasibility, of communities and ecosystems. In Simberloff, D. & M. Rejmánek (eds), Encyclopedia of Biological Invasions. University of California Press, Berkeley: 356–360.
Fuller, I. C. & R. G. Death, 2018. The science of connected ecosystems: what is the role of catchment-scale connectivity for healthy river ecology? Land Degradation & Development 29: 1413–1426.
Gehrke, P. C. & J. H. Harris, 2001. Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia. Regulated Rivers: Research & Management 17: 369–391.
Gibson-Reinemer, D. K., L. E. Solomon, R. M. Pendleton, J. H. Chick & A. F. Casper, 2017. Hydrology controls recruitment of two invasive cyprinids: bigheaded carp reproduction in a navigable large river. PeerJ 5:
Glenn, E. P. & P. L. Nagler, 2005. Comparative ecophysiology of Tamarix ramosissima and native trees in western U.S. riparian zones. Journal of Arid Environments 61: 419–446.
Gois, K. S., F. M. Pelicice, L. C. Gomes & A. A. Agostinho, 2015. Invasion of an Amazonian cichlid in the Upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia 746: 401–413.
González-Muñoz, N., P. Castro-Díez & O. Godoy, 2014. Lack of superiority of invasive over co-occurring native riparian tree seedling species. Biological Invasions 16: 269–281.
Górski, K., K. J. Collier, D. P. Hamilton & B. J. Hicks, 2014. Effects of flow on lateral interactions of fish and shrimps with off-channel habitats in a large river-floodplain system. Hydrobiologia 729: 161–174.
Greet, J., R. D. Cousens & J. A. Webb, 2013a. Flow regulation is associated with riverine soil seed bank composition within an agricultural landscape: potential implications for restoration. Journal of Vegetation Science 24: 157–167.
Greet, J., R. D. Cousens & J. A. Webb, 2013b. More exotic and fewer native plants species: riverine vegetation patterns associated with altered seasonal flow patterns. River Research and Applications 29: 686–706.
Greet, J., J. A. Webbs & R. D. Cousens, 2015. Floods reduce the prevalence of exotic plant species within the riparian zone: evidence from natural floods. Applied Vegetation Science 18: 503–512.
Grizzetti, B., A. Pistocchi, C. Liquete, A. Udias, F. Bouraoui & W. van de Bund, 2017. Human pressures and ecological status of European rivers. Scientific Reports 7:
Grotkopp, E., M. Rejmánek & T. L. Rost, 2002. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. American Naturalist 159: 396–419.
Gurevitch, J., 2011. Competition, plant. In Simberloff, D. & M. Rejmánek (eds), Encyclopedia of Biological Invasions. University of California Press, Berkeley: 122–125.
Havel, J. E., C. E. Lee & M. J. V. Zanden, 2005. Do reservoirs facilitate invasions into landscapes? BioScience 55: 518–525.
Hejda, M., M. Chytrý, J. Pergl & P. Pyšek, 2015. Native range habitats of invasive plants: are they similar to invaded range habitats and do they differ according to the geographical direction of invasion? Diversity and Distributions 21: 312–321.
Hershner, C. & K. J. Havens, 2008. Managing invasive aquatic plants in a changing system: strategic consideration of ecosystem services. Conservation Biology 22: 544–550.
Ho, M. & C. J. Richardson, 2013. A five year study of floristic succession in a restored urban wetland. Ecological Engineering 61: 511–518.
Ho, S. S., N. R. Bond & R. M. Thompson, 2013. Does seasonal flooding give a native species an edge over a global invader? Freshwater Biology 58: 159–170.
Hobbs, R. J., 2011. Disturbance. In Simberloff, D. & M. Rejmánek (eds), Encyclopedia of Biological Invasions. University of California Press, Berkeley: 165–168.
Hoegh-Guldberg, O., D. Jacob, M. Taylor, M. Bindi, S. Brown, I. Camilloni, A. Diedhiou, R. Djalante, K.L. Ebi, F. Engelbrecht, J. Guiot, Y. Hijioka, S. Mehrotra, A. Payne, S.I. Seneviratne, A. Thomas, R. Warren & G. Zhou, 2018. Impacts of 1.5°C Global Warming on Natural and Human Systems. In: Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (in press).
Höfle, R., S. Dullinger & F. Essl, 2014. Different factors affect the local distribution, persistence and spread of alien tree species in floodplain forests. Basic and Applied Ecology 15: 426–434.
Hood, W. G. & R. J. Naiman, 2000. Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecology 148: 105–114.
Howell, J. & D. Benson, 2000. Predicting potential impacts of environmental flows on weedy riparian vegetation of the Hawkesbury-Nepean River, south-eastern Australia. Austral Ecology 25: 463–475.
Hudon, C., P. Gagnon & M. Jean, 2005. Hydrological factors controlling the spread of common reed (Phragmites australis) in the St. Lawrence River (Québec, Canada). Ecoscience 12: 347–357.
Hughes, J. W. & W. B. Cass, 1997. Pattern and process of a floodplain forest, Vermont, USA: predicted responses of vegetation to perturbation. Journal of Applied Ecology 34: 594–612.
Hulme, P. E., 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. Journal of Applied Ecology 46: 10–18.
Hutchinson, M. J. & P. H. Armstrong, 1993. The invasion of a South-Western Australian river system by Perca fluviatilis: history and probable causes. Global Ecology and Biogeography Letters 3: 77–89.
Iketani, G., M. A. B. Aviz, C. Maciel, W. Valenti, H. Schneider & I. Sampaio, 2016. Successful invasion of the Amazon Coast by the giant river prawn, Macrobrachium rosenbergii: evidence of a reproductively viable population. Aquatic Invasions 11: 277–286.
IUCN – International Union for Conservation of Nature. http://www.issg.org/is_what_are_they.htm
Johnson, S. E., K. L. Amatangelo, P. A. Towsend & D. M. Waller, 2016. Large, connected floodplain forests prone to flooding best sustain plant diversity. Ecology 97: 3019–3030.
Johnson, P. T. J., J. D. Olden & M. J. V. Zanden, 2008. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 359–365.
Jones, M. J. & I. G. Stuart, 2009. Lateral movement of common carp (Cyprinus carpio L.) in a large lowland river and floodplain. Ecology of Freshwater Fish 18: 72–82.
Jones, P. E., S. Consuegra, L. Börger, J. Jones & C. G. de Leaniz, 2020. Impacts of artificial barriers on the connectivity and dispersal of vascular macrophytes in rivers: a critical review. Freshwater Biology (in press). https://doi.org/10.1111/fwb.13493.
Jordan, F., K. Babbitt & C. Mclvor, 1998. Seasonal variation in habitat use by marsh fishes. Ecology of Freshwater Fish 7: 159–166.
Juárez-Escario, A., J. A. Conesaa & X. O. Solé-Senan, 2016. Identifying alien plants linkages between irrigated orchards and adjacent riparian habitats from a trait-based approach. Agriculture, Ecosystems and Environment 225: 173–183.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106: 110–127.
Kettenring, K. M., K. E. Mock, B. Zaman & M. McKee, 2016. Life on the edge: reproductive mode and rate of invasive Phragmites australis patch expansion. Biological Invasions 18(2): 475–2495.
King, A. J., P. Humphries & P. S. Lake, 2003. Fish recruitment on floodplains: the roles of patterns of flooding and life history characteristics. Canadian Journal of Fisheries and Aquatic Sciences 60: 773–796.
Koehn, J. D., C. R. Todd, B. P. Zampatti, I. G. Stuart, A. Conallin, L. Thwaites & Q. Ye, 2018. Using a population model to inform the management of river flows and invasive carp (Cyprinus carpio). Environmental Management 61: 432–442.
Kopéc, D., N. Ratajczyk, A. Wolańska-Kamińska, M. Walisch & A. Kruk, 2014. Floodplain forest vegetation response to hydroengineering and climatic pressure—a five decade comparative analysis in the Bzura River valley (Central Poland). Forest Ecology and Management 314: 120–130.
Košir, P., A. Čarni, A. Marinšek & U. Šilc, 2013. Floodplain forest communities along the Mura River (NE Slovenia). Acta Botanica Croatica 72: 71–95.
Kropf, M., A. S. Huppenberger & G. Karrer, 2017. Genetic structuring and diversity patterns along rivers—local invasion history of Ambrosia artemisiifolia (Asteraceae) along the Danube River in Vienna (Austria) shows non-linear pattern. Weed Research 58: 131–140.
Lasnea, E., S. Lekb & P. Laffaillea, 2008. Patterns in fish assemblages in the Loire floodplain: The role of hydrological connectivity and implications for conservation. Biological Conservation 139: 258–268.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Levine, J. M., 2000. Species diversity and biological invasions: Relating local process to community pattern. Science 288: 852–854.
Lewerentza, A., G. Eggera, J. E. Householdera, B. Reidc, A. C. Braund & V. Garófano-Gómez, 2019. Functional assessment of invasive Salix fragilis L. in north-western Patagonian flood plains: a comparative approach. Acta Oecologica 95: 36–44.
Li, J., A. L. Rypel, S. Y. Zhang, Y. M. Luo, G. Hou, B. R. Murphy & S. G. Xie, 2017. Growth, longevity, and climate–growth relationships of Corbicula fluminea (Müller, 1774) in Hongze Lake, China. Freshwater Science 36: 595–608.
Louback-Franco, N., M. S. Dainez-Filho, D. C. Souza & S. M. Thomaz, 2020. A native species does not prevent the colonization success of an introduced submerged macrophyte, even at low propagule pressure. Hydrobiologia 847: 1619–1629.
Lonsdale, W. M., 1993. Rates of Spread of an Invading Species - Mimosa Pigra in Northern Australia. Journal of Ecology 81: 513–521.
Lunt, I. D., A. Jansen & D. L. Binns, 2012. Effects of flood timing and livestock grazing on exotic annual plants in riverine floodplains. Journal of Applied Ecology 49: 1131–1139.
Luz-Agostinho, K. D. G., A. A. Agostinho, L. C. Gomes, H. F. Júlio-Jr & R. Fugi, 2009. Effects of flooding regime on the feeding activity and body condition of piscivorous fish in the Upper Paraná River floodplain. Brazilian Journal of Biology 69: 481–490.
Lytle, D. A. & N. L. Poff, 2004. Adaptation to natural flow regimes. Trends in Ecology & Evolution 19: 94–100.
Macdonald, J. & D. A. Crook, 2014. Nursery sources and cohort strength of young-of-the-year common carp (Cyprinus carpio) under differing flow regimes in a regulated floodplain river. Ecology of Freshwater Fish 23: 269–282.
McShane, R. R., D. A. Auerbach, J. M. Friedman, G. T. Auble, B. Shafroth, M. F. Merigliano, et al., 2015. Distribution of invasive and native riparian woody plants across the western USA in relation to climate, river flow, floodplain geometry and patterns of introduction. Ecography 38: 1254–1265.
Maiztegui, T., C. R. M. Baigún, J. R. Garcia de Souza, O. L. F. Weyl & D. C. Colautti, 2019. Population responses of common carp Cyprinus carpio to floods and droughts in the Pampean wetlands of South America. NeoBiota 48: 25–44.
Mao, R., T. L. T. Nguyen, O. O. Osunkoya & S. W. Adkins, 2019. Spread pathways of the invasive weed Parthenium hysterophorus L.: The potential for water dispersal. Austral Ecology 44: 1111–1122.
Marengo, J. A., S. C. Chou, G. Kay, L. M. Alves, J. F. Pesquero, W. R. Soares, D. C. Santos, A. A. Lyra, G. Sueiro, R. Betts, D. J. Chagas, J. L. Gomes, J. F. Bustamante & P. Tavares, 2012. Development of regional future climate change scenarios in South America using the Eta CPTEC/HadCM3 climate change projections: climatology and regional analyses for the Amazon, São Francisco and the Paraná River basins. Climate Dynamics 38: 1829–1848.
Mazumder, D., M. Johansen, N. Saintilan, J. Iles, T. Kobayashi, L. Knowles & L. Wen, 2012. Trophic shifts involving native and exotic fish during hydrologic recession in floodplain wetlands. Wetlands 32: 267–275.
Merrin, L. E., C. R. Toddb, D. G. Williamsa, E. S. G. Schreiberb & C. James, 2005. Coupling meta-population models with GIS to predict freshwater biotic invasions. MODSIM 2005: International Congress on Modelling and Simulation: Advances and Applications for Management and Decision Making: 1416-1442.
Mitsch, W. J., Grosselink, J. G. 2015. Wetlands, 5th edition. Wiley, New York
Molina, F. R. & S. J. Paggi, 2008. Zooplankton in the Paraná River floodplain (South America) before and after the invasion of Limnoperna fortunei (Bivalvia). Wetlands 28: 695–702.
Mortenson, S. G., P. J. Weisberg & L. E. Stevens, 2012. The influence of floods and precipitation on Tamarix establishment in Grand Canyon, Arizona: consequences for flow regime restoration. Biological Invasions 14: 1061–1076.
Moyle, P. B. & T. Light, 1996. Biological invasions of fresh water: empirical rules and assembly theory. Biological Conservation 78: 149–161.
Müller, N. & S. Okuda, 1998. Invasion of alien plants in floodplains—a comparison of Europe and Japan. In Starfinger, U., K. Edwards, I. Kowarik & M. Williamson (eds), Plant invasions: ecological mechanisms and human responses. Backhuys Publishers, Leiden: 321–332.
Mumba, M. & J. R. Tompson, 2005. Hydrological and ecological impacts of dams on the Kafue Flats floodplain system, southern Zambia. Physics and Chemistry of the Earth 30: 442–447.
Mworia, J. K., J. I. Kinyamario, J. K. Omari & J. K. Wambua, 2011. Patterns of seed dispersal and establishment of the invader Prosopis juliflora in the upper floodplain of Tana River, Kenya. African Journal of Range & Forage Science 28: 35–41.
Nagler, P. L., O. Hinojosa-Huerta, E. P. Glenn, J. Garcia-Hernandez, R. Romo, C. Curtis, A. R. Huete & S. G. Nelson, 2005. Regeneration of native trees in the presence of invasive saltcedar in the Colorado River Delta, Mexico. Conservation Biology 19: 1842–1852.
Nakayama, N., J. Nishihiro, Y. K. T. Muranaka & I. Washitani, 2007. Seed deposition of Eragrostis curvula, an invasive alien plant on a river floodplain. Ecological Research 22: 696–701.
Neiff, J. J., 1990. Ideas para la interpretacion ecológica del Parana. Interciencia 15: 424–441.
Nico, L. G., W. H. Beamish & P. Musikasinthorn, 2007. Discovery of the invasive Mayan Cichlid fish “Cichlasoma” urophthalmus (Günther, 1862) in Thailand, with comments on other introductions and potential impacts. Aquatic Invasions 2: 197–214.
Nobis, A., K. Rola & M. Węgrzyn, 2017. Detailed study of a river corridor plant distribution pattern provides implications for river valley conservation. Ecological Indicators 83: 314–322.
Norman, J. D. & G. W. Whitledge, 2015. Recruitment sources of invasive Bighead carp (Hypopthalmichthys nobilis) and Silver carp (H. molitrix) inhabiting the Illinois River. Biological Invasions 17: 2999–3014.
Novoa, A., D. M. Richardson, P. Pyšek, L. A. Meyerson, S. Bacher, S. Canavan, J. A. Catford, J. Čuda, F. Essl, L. C. Foxcroft, P. Genovesi, H. Hirsch, C. Hui, M. C. Jackson, C. Kueffer, J. J. Le Roux, J. Measey, N. P. Mohanty, D. Moodley, H. Müller-Schärer, J. G. Packer, J. Pergl, T. B. Robinson, W.-C. Saul, R. T. Shackleton, V. Visser, O. L. F. Weyl, F. A. Yannelli & J. R. U. Wilson, 2020. Invasion syndromes: a systematic approach for predicting biological invasions and facilitating effective management. Biological Invasions 22: 1801–1820.
Nunes da Cunha, C. & W. J. Junk, 2004. Year-to-year changes in water level drive the invasion of Vochysia divergens in Pantanal grasslands. Applied Vegetation Science 7: 103–110.
Oliveira, M. D., A. M. Takeda, L. F. de Barros, D. S. Barbosa & E. K. de Resende. 2006. Invasion by Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) of the Pantanal wetland, Brazil. Biological Invasions 8: 97–104. https://doi.org/10.1007/s10530-005-0331-0
Oliveira, A. G., H. I. Suzuki, L. C. Gomes & A. A. Agostinho, 2015. Interspecific variation in migratory fish recruitment in the Upper Paraná River: effects of the duration and timing of floods. Environmental Biology of Fishes 98: 1327–1337.
Oliveira, M. D., A. M. Takeda, L. F. de Barros, D. S. Barbosa & E. K. de Resende, 2010a. Invasion by Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) of the Pantanal wetland, Brazil. Biological Invasions 8: 97–104.
Oliveira, M. D., S. K. Hamilton & C. M. Jacobi, 2010b. Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Aquatic Invasions 5: 59–73.
Oliveira, A. G., T. M. Lopes, M. A. Angulo-Valencia, R. M. Dias, H. I. Suzuki, I. C. B. Costa & A. A. Agostinho, 2020. Relationship of freshwater fish recruitment with distinct reproductive strategies and flood attributes: A long-term view in the Upper Paraná River floodplain. Frontier in Environmental Science. https://doi.org/10.3389/fenvs.2020.577181
Ondračková, M., J. Fojtů, M. Seifertová, Y. Kvach & P. Jurajda, 2019. Non-native parasitic copepod Neoergasilus japonicus (Harada, 1930) utilizes non-native fish host Lepomis gibbosus (L.) in the floodplain of the River Dyje (Danube basin). Parasitology Research 118: 57–62.
Ooue, K., N. Ishiyama, M. Ichimura & F. Nakamura, 2018. Environmental factors affecting the invasion success and morphological responses of a globally introduced crayfish in floodplain waterbodies. Biological Invasions 21: 2639–2652.
Ortega, J. C. G., H. F. Julio Jr., L. C. Gomes & A. A. Agostinho, 2015. Fish farming as the main driver of fish introductions in Neotropical reservoirs. Hydrobiologia 746(1): 147–158.
Osunkoya, O. O., S. Ali, T. Nguyen, C. Perrett, A. Shabbir, S. Navie, A. Belgeri, K. Dhileepan & S. Adkins, 2014. Soil seed bank dynamics in response to an extreme flood event in a riparian habitat. Ecological Research 29: 1115–1129.
Padial, A. A., F. Ceschin, S. A. J. Declerck, L. de Meester, C. C. Bonecker, F. A. Lansac-Tôha, L. Rodrigues, L. C. Rodrigues, S. Train, L. F. M. Velho & L. M. Bini, 2014. Dispersal ability determines the role of environmental, spatial and temporal drivers of metacommunity structure. PLoS ONE 9:
Paillex, A., S. Dolédec, E. Castella, S. Mérigoux & D. C. Aldridge, 2013. Functional diversity in a large river floodplain: anticipating the response of native and alien macroinvertebrates to the restoration of hydrological connectivity. Journal of Applied Ecology 50: 97–106.
Paillex, A., E. Castella, P. S. E. Z. Ermgassen & D. C. Aldridge, 2015. Testing predictions of changes in alien and native macroinvertebrate communities and their interaction after the restoration of a large river floodplain (French Rhone). Freshwater Biology 60: 1162–1175.
Paillex, A., E. Castella, P. S. E. Z. Ermgassen, B. Gallardo & D. C. Aldridge, 2017. Large river floodplain as a natural laboratory: non-native macroinvertebrates benefit from elevated temperatures. Ecosphere https://doi.org/10.1002/ecs2.1972/full
Pander, J., M. Mueller, M. Sacher & J. Geist, 2016. The role of life history traits and habitat characteristics in the colonisation of a secondary floodplain by neobiota and indigenous macroinvertebrate species. Hydrobiologia 772: 229–245.
Paolucci, E. M., P. Sardiña, F. Sylvester, P. V. Perepelizin, A. Zhan, S. Ghabooli, M. E. Cristescu, M. D. Oliveira & H. J. MacIsaac, 2014. Morphological and genetic variability in an alien invasive mussel across an environmental gradient in South America. Limnology & Oceanography 59: 400–412.
Parsons, M. & M. Southwell, 2015. Flooding and geomorphology influence the persistence of the invasive annual herb Noogoora burr (Xanthium occidentale Bertol.) in the riparian zone of the dryland Darling River. Australia. The Rangeland Journal 37: 433–444.
Pearce, C. M. & D. G. Smith, 2001. Plains cottonwood’s last stand: can it survive invasion of Russian olive onto the Milk River, Montana floodplain? Environmental Management 28: 623–637.
Pearce, C. M. & D. G. Smith, 2003. Saltcedar: distribution, abundance, and dispersal mechanisms, Northern Montana, USA. Wetlands 23: 215–228.
Pegg, M. A., A. M. Lemke & J. A. Stoeckel, 2002. Establishment of bighead carp in an Illinois river floodplain lake: a potential source population for the Illinois River. Journal of Freshwater Ecology 17: 161–163.
Pelicice, F. M., J. D. Latini & A. A. Agostinho, 2015. Fish fauna disassembly after the introduction of a voracious predator: main drivers and the role of the invader’s demography. Hydrobiologia 746: 271–283.
Pelicice, F. M., V. M. Azevedo-Santos, J. R. S. Vitule, M. L. Orsi, D. P. Lima-Junior, A. L. B. Magalhães, P. S. Pompeu, M. Petrere Jr. & A. A. Agostinho, 2017. Neotropical freshwater fishes imperiled by unsustainable policies. Fish and Fisheries 18: 1119–1133.
Pereira, L. S., F. T. Mise, L. F. C. Tencatt, M. T. Baumgartner & A. A. Agostinho, 2007. Is coexistence between non-native and native Erythrinidae species mediated by niche differentiation or environmental filtering? A case study in the upper Paraná River floodplain. Neotropical Ichthyology 15:
Petrášová, M., I. Jarolímek & J. Medvecká, 2013. Neophytes in Pannonian hardwood floodplain forests—history, present situation and trends. Forest Ecology and Management 308: 31–39.
Poff, N. L., D. Allan, M. B. Bain, J. R. Karr, K. L. Prestegaard, B. D. Richter, R. E. Sparks & J. C. Stromberg, 1997. The natural flow regime: a paradigm for river conservation and restoration. BioScience 47: 769–784.
Pollux, B. J. A. & A. Korosi, 2006. On the occurrence of the Asiatic cyprinid Pseudorasbora parva in the Netherlands. Journal of Fish Biology 69: 1575–1580.
Predick, K. I. & M. G. Turner, 2008. Landscape configuration and flood frequency influence invasive shrubs in floodplain forests of the Wisconsin River (USA). Journal of Ecology 96: 91–102.
Price, J. N., C. L. Gross & R. D. B. Whalley, 2010. Prolonged summer flooding switched dominance from the invasive weed Phyla canescens to native species in one small, ephemeral wetland. Ecological Management & Restoration 11: 61–67.
Price, J. N., P. J. Berney, D. Ryder, R. D. B. Whalley & C. L. Gross, 2011a. Disturbance governs dominance of an invasive forb in a temporary wetland. Oecologia 167: 759–769.
Price, J. N., R. D. B. Whalley, R. D. van Klinken, J. A. Duggin & C. L. Gross, 2011b. Periodic rest from grazing provided no control of an invasive perennial forb. The Rangeland Journal 33: 287–298.
Puckridge, J. T., K. F. Walker & J. F. Costello, 2000. Hydrological persistence and the ecology of dryland rivers. Regulated Rivers: Research & Management 16: 385–402.
Pyle, L. L., 1995. Effects of disturbance on herbaceous exotic plant species on the floodplain of the Potomac River. The American Midland Naturalist 134: 244–254.
Quirino, B. A., N. Carniatto, J. V. Gaiotto & R. Fugi, 2015. Seasonal variation in the use of food resources by small fishes habiting the littoral zone in a Neotropical floodplain lake. Aquatic Ecology 49: 431–440.
Quirino, B. A., N. Carniatto, R. Guglielmetti & R. Fugi, 2017. Changes in diet and niche breadth of a small fish species in response to the flood pulse in a Neotropical floodplain lake. Limnologica 62: 126–131.
Rajakaruna, S. L., K. B. Ranawana, A. M. T. A. Gunarathne & H. M. S. P. Madawala, 2017. Impacts of river regulation and other anthropogenic activities on floodplain vegetation: A case study from Sri Lanka. Community Ecology 18: 203–213.
Rayner, T. S., R. T. Kingsford, I. M. Suthers & D. O. Cruz, 2015. Regulated recruitment: native and alien fish responses to widespread floodplain inundation in the Macquarie Marshes, arid Australia. Ecohydrology 8: 148–159.
Rejmánek, M., 2011. Invasiveness. In Simberloff, D. & M. Rejmánek (eds), Encyclopedia of Biological Invasions. University of California Press, Berkeley: 379–385.
Resende, E. K., D. K. S. Marques & L. K. S. G. Ferreira, 2008. A successful case of biological invasion: the fish Cichla piquiti, an Amazonian species introduced into the Pantanal, Brazil. Brazilian Journal of Biology 68: 799–805.
Reshetnikova, A. N. & E. A. Chibilev, 2009. Distribution of the fish rotan (Perccottus glenii Dybowski, 1877) in the Irtysh River Basin and analysis of possible consequences for environment and people. Contemporary Problems of Ecology 2: 224–228.
Reshetnikova, A. N., A. S. Golubtsova, V. B. Zhuravlevc, S. L. Lomakind & A. S. Rezvyie, 2017. Range expansion of rotan Perccottus glenii, Sunbleak Leucaspius delineatus, and bleak Alburnus alburnus in the Ob River Basin. Contemporary Problems of Ecology 10: 612–620.
Richardson, D. M., P. Pyšek & J. T. Carlton, 2011. A compendium of essential concepts and terminology in invasion ecology. In: Richardson, D. M. (ed). Fifty Years of Invasion Ecology: The Legacy of Charles Elton, 1st edition. Blackwell Publishing Ltd. p. 409-420.
Rijal, S. & R. Cochard, 2016. Invasion of Mimosa pigra on the cultivated Mekong River floodplains near Kratie, Cambodia: farmers’ coping strategies, perceptions, and outlooks. Regional Environmental Change 16: 681–693.
Rodrigues, A. C., H. S. de Santana, M. T. Baumgartner & L. C. Gomes, 2018. Coexistence between native and nonnative species: the invasion process and adjustments in distribution through time for congeneric piranhas in a Neotropical floodplain. Hydrobiologia 817: 279–291.
Rood, S. B., L. A. Goater, J. M. Mahoney, C. M. Pearce & D. G. Smith, 2007. Floods, fire, and ice: disturbance ecology of riparian cottonwoods. Canadian Journal of Botany 85: 1019–1032.
Ruaro, R., R. P. Tramonte, P. R. B. Buosi, G. I. Manetta & E. Benedito, 2020. Trends in studies of nonnative populations: Invasions in the Upper Parana River Floodplain. Wetlands 40: 113–124.
Santos, D. A., D. J. Hoeinghaus & L. C. Gomes, 2018. Spatial scales and the invasion paradox: a test using fish assemblages in a Neotropical floodplain. Hydrobiologia 817: 121–131.
Sardina, P., E. Chaves & M. Marchese, 2011. Benthic community responses to invasion by the golden mussel, Limnoperna fortunei Dunker: biotic homogenization vs environmental driving forces. Journal of the North American Benthological Society 30: 1009–1023.
Schmiedel, D. & O. Tackenberg, 2013. Hydrochory and water induced germination enhance invasion of Fraxinus pennsylvanica. Forest Ecology and Management 304: 437–443.
Sepulveda, A. J., M. Layhee, D. Stagliano, J. Chaffin, A. Begley & B. Maxell, 2015. Invasion of American bullfrogs along the Yellowstone River. Aquatic Invasions 10: 69–77.
Sepulveda, A. J., 2018. Novel application of explicit dynamics occupancy models to ongoing aquatic invasions. Journal of Applied Ecology 55: 917–925.
Sharip, Z., A. T. A. Zaki & S. Zakaria, 2014. Flooding effects on the population dynamics of Cabomba furcata and Nelumbo nucifera in a shallow floodplain wetland. Wetlands 34: 713–723.
Shmida, A. & M. V. Wilson, 1985. Biological determinants of species diversity. Journal of Biogeography 12: 1–20.
Simberloff, D., J. L. Martin, P. Genovesi, V. Maris, D. A. Wardle, J. Aronson, F. Courchamp, B. Galil, E. Garcia-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi & M. Vila, 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution 28: 58–66.
Simberloff, D., 2009. The role of propagule pressure in biological invasions. Annual Review of Ecology Evolution and Systematics 40: 81–102.
Simberloff, D. & M. Rejemánek, 2011. Encyclopedia of Biological Invasions. University of California, Berkeley: 765p.
Simberloff, D., 2021. Maintenance management and eradication of established aquatic invaders. Hydrobiologia. https://doi.org/10.1007/s10750-020-04352-5.
Simões, N. R., B. A. Robertson, F. A. Lansac-Tôha, E. M. Takahashi, C. C. Bonecker, L. F. M. Velho & C. Y. Joko, 2009. Exotic species of zooplankton in the Upper Paraná River floodplain, Daphnia lumholtzi Sars, 1885 (Crustacea: Branchiopoda). Brazilian Journal of Biology 69: 551–558.
Simões, N. R., J. D. Dias, C. M. Leal, L. S. M. Braghin, F. A. Lansac-Tôha & C. C. Bonecker, 2013. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a Neotropical floodplain. Aquatic Sciences 75: 607–617.
Slavík, O. & L. Bartoš, 2004. What are the reasons for the Prussian carp expansion in the upper Elbe River, Czech Republic? Journal of Fish Biology 65: 240–253.
Sofkova, M. & S. David, 2016. Invaded plants communities in the Berek floodplain forest (Nové Zámky Distr., Slovakia). Proceedings of the International PhD Student Conference, (MENDELNET 2016): 146–151.
Sommer, T. R., W. C. Harrell & F. Feyrer, 2014. Large-bodied fish migration and residency in a flood basin of the Sacramento River, California, USA. Ecology of Freshwater Fish 23: 414–423.
Sousa, W. T. Z., 2011. Hydrilla verticillata (Hydrocharitaceae), a recent invader threatening Brazil’s freshwater environments: a review of the extent of the problem. Hydrobiologia 669: 1–20.
Sousa, W. T. Z., S. M. Thomaz & K. J. Murphy, 2010. Response of native Egeria najas Planch. and invasive Hydrilla verticillata (L.f.) Royle to altered hydroecological regime in a subtropical river. Aquatic Botany 92: 40–48.
Souza, D. C., E. R. Cunha, R. A. Murillo, M. J. Silveira, M. M. Pulzatto, M. S. Dainez-Filho, L. A. Lolis & S. M. Thomaz, 2017. Species inventory of aquatic macrophytes in the last undammed stretch of the Upper Paraná River. Brazil. Acta Limnologica Brasiliensia 29:
Sprenger, M. D., L. M. Smith & J. P. Taylor, 2001. Testing control of saltcedar seedlings using fall flooding. Wetlands 21: 437–441.
Stoffels, R. J., K. R. Clarke, R. A. Rehwinkel & B. J. McCarthy, 2014. Response of a floodplain fish community to river-floodplain connectivity: natural versus managed reconnection. Canadian Journal of Fisheries and Aquatic Sciences 71: 236–245.
Stoffels, R. J., K. E. Weatherman & S. Allen-Ankins, 2017. Heat and hypoxia give a global invader, Gambusia holbrooki, the edge over a threatened endemic fish on Australian floodplains. Biological Invasions 19: 2477–2489.
Stohlgren, T. J., C. Jarnevich, G. W. Chong & P. H. Evangelista, 2006. Scale and plant invasions: a theory of biotic acceptance. Preslia 78: 05–426.
Stokes, K., K. Ward & M. Colloff, 2010. Alterations in flood frequency increase exotic and native species richness of understorey vegetation in a temperate floodplain eucalypt forest. Plant Ecology 211: 219–233.
Stuart, I. G. & M. Jones, 2006. Large, regulated forest floodplain is an ideal recruitment zone for non-native common carp (Cyprinus carpio L.). Marine and Freshwater Research 57: 333–347.
Sullivan, C. J., M. J. Weber, C. L. Pierce, D. H. Wahl, Q. E. Phelps, C. A. Camacho & R. E. Colombo, 2018. Factors regulating year-class strength of silver carp throughout the Mississippi River Basin. Transactions of the American Fisheries Society 147: 541–553.
Tabacchi, E. & A. M. Planty-Tabacchi, 2005. Exotic and native plant community distributions within complex riparian landscapes: a positive correlation. Écoscience 12: 412–423.
Tabacchi, E., A.-M. Planty-Tabacchi, L. Roques & E. Nadal, 2005. Seed inputs in riparian zones: implications for plant invasion. River Research and Applications 21: 299–313.
Taylor, B. & G. G. Ganf, 2005. Comparative ecology of two co-occurring floodplain plants: the native Sporobolus mitchellii and the exotic Phyla canescens. Marine and Freshwater Research 56: 431–440.
Thébaud, C. & M. Debussche, 1991. Rapid Invasion of Fraxinus ornus L. Along the Hérault River system in Southern France: the importance of seed dispersal by water. Journal of Biogeography 18: 7–12.
Thomas, J. R., D. J. Gibson & B. A. Middleton, 2005. Water dispersal of vegetative bulbils of the invasive exotic Dioscorea oppositifolia L. in southern Illinois. Journal of the Torrey Botanical Society 132: 187–196.
Thomas, J. R., B. Middleton & D. J. Gibson, 2006. A landscape perspective of the stream corridor invasion and habitat characteristics of an exotic (Dioscorea oppositifolia) in a pristine watershed in Illinois. Biological Invasions 8: 1103–1113.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Thomaz, S. M., P. Carvalho, R. P. Mormul, F. A. Ferreira, M. J. Silveira & T. S. Michelan, 2009. Temporal trends and effects of diversity on occurrence of exotic macrophytes in a large reservoir. Acta Oecologica 35: 614–620.
Tibby, J., C. Barr, J. C. Marshall, J. Richards, C. Perna, J. Fluin & H. R. Cadd, 2019. Assessing the relative impacts of land-use change and river regulation on Burdekin River (Australia) floodplain wetlands. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1712–1725.
Tockner, K., M. Pusch, D. Borchardt & M. S. Lorang, 2010. Multiple stressors in coupled river–floodplain ecosystems. Freshwater Biology 55: 135–151.
Tockner, K., F. Malard & J. V. Ward, 2000. An extension of the flood pulse concept. Hydrological Processes 14: 2861–2883.
Tonella, L. H., R. Fugi, O. B. Vitorino Jr., H. I. Suzuki, L. C. Gomes & A. A. Agostinho, 2018. Importance of feeding strategies on the long-term success of fish invasions. Hydrobiologia 817: 239–252.
Tonella, L. H., R. M. Dias, O. B. Vitorino Jr., R. Fugi & A. A. Agostinho, 2019. Conservation status and bio-ecology of Brycon orbignyanus (Characiformes: Bryconidae), an endemic fish species from the Paraná River basin (Brazil) threatened with extinction. Neotropical Ichthyology 17:
Tonkin, J. D., F. Altermatt, D. S. Finn, J. Heino, J. D. Olden, S. U. Pauls & D. A. Lytle, 2018. The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshwater Biology 63: 141–163.
Toth, L. A., 2010. Unrealized expectations for restoration of a floodplain plant community. Restoration Ecology 18: 810–819.
Toth, L. A., 2015. Invasibility drives restoration of a floodplain plant community. River Research & Applications 31: 1319–1327.
Truscott, A. M., C. Soulsby, S. C. F. Palmer, L. Newell & P. E. Hulme, 2006. The dispersal characteristics of the invasive plant Mimulus guttatus and the ecological significance of increased occurrence of high-flow events. Journal of Ecology 94: 1080–1091.
Uowolo, A. L., D. Binkley & E. C. Adair, 2005. Plant diversity in riparian forests in northwest Colorado: effects of time and river regulation. Forest Ecology and Management 218: 107–114.
Van Geest, G. J., H. Coops, R. M. M. Roijackers, A. D. Buijse & M. Scheffer, 2005. Succession of aquatic vegetation driven by reduced water-level fluctuations in floodplain lakes. Journal of Applied Ecology 42: 251–260.
Wang, R., J. F. Wang, Z. J. Qiu, B. Meng, F. H. Wan & Y. Z. Wang, 2011. Multiple mechanisms underlie rapid expansion of an invasive alien plant. New Phytologist 191: 828–839.
Ward, J. V., K. Tockner & F. Schiemer, 1999. Biodiversity of floodplain river ecosystems: Ecotones and connectivity. Regulated Rivers—Research & Management 15: 125-139.
Warren, R. J., D. L. Potts & K. M. Frothingham, 2015. Stream structural limitations on invasive communities in urban riparian areas. Invasive Plant Science and Management 8: 353–362.
Watterson, N. A. & J. A. Jones, 2006. Flood and debris flow interactions with roads promote the invasion of exotic plants along steep mountain streams, western Oregon. Geomorphology 78: 107–123.
Wei, H., S. Cheng, H. Tang, F. Hel, W. Liang & Z. Wul, 2013. The strategies of morphology, reproduction and carbohydrate metabolism of Hydrilla verticillata (Linn.f) Royle in fluctuaging waters. Fresenius Environmental Bulletin 22: 2596–2603.
Welicky, R. L., J. De Swardt, R. Gerber, E. C. Netherlands & N. J. Smit, 2017. Drought-associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health. International Journal for Parasitology: Parasites and Wildlife 6: 430–438.
West, N. M., A. M. Reinhold, G. C. Poole & E. K. Espeland, 2020. Flood dynamics dictate distributions of Elaeagnus angustifolia L. (Russian olive) on a riverine floodplain. Biological Invasions 22: 3493–3499.
Williams, J. E. & S. V. Gregory, 2018. Fish occurrence in a seasonally inundated floodplain of the Willamette River, Oregon. Northwest Science 92: 191–202.
Williams, D. A., N. E. Harmsb, M. J. Grodowitzb & M. Purcell, 2018. Genetic structure of Hydrilla verticillata L.f. Royle in eastern China and the Republic of Korea: Implications for surveys of biological control agents for the invasive monoecious biotype. Aquatic Botany 149: 17–27.
Williamson, M. H. & A. Fitter, 1996. The characters of successful invaders. Biological Conservation 78: 163–170.
Winemiller, K. O., P. B. McIntyre, L. Castello, E. Fluet-Chouinard, T. Giarrizzo, S. Nam, I. G. Baird, W. Darwall, N. K. Lujan, I. Harrison, M. L. J. Stiassny, R. A. M. Silvano, D. B. Fitzgerald, F. M. Pelicice, A. A. Agostinho, L. C. Gomes, J. S. Albert, E. Baran, M. Petrere Jr., C. Zarfl, M. Mulligan, J. P. Sullivan, C. C. Arantes, L. M. Sousa, A. A. Koning, D. J. Hoeinghaus, M. Sabaj, J. G. Lundberg, J. Armbruster, M. L. Thieme, P. Petry, J. Zuanon, G. Torrente Vilara, J. Snoeks, C. Ou, W. Rainboth, C. S. Pavanelli, A. Akama, A. van Soesbergen & L. Sáenz, 2016. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science 351: 128–129.
Wu, N., K. Górski & A. J. Daniel, 2013. Abundance of larval native and nonnative fishes in floodplain habitats of the lower Waikato River, New Zealand. Inland Waters 3: 359–368.
Zaia Alves, G. H., B. R. S. Figueiredo, G. I. Manetta, P. A. Sacramento, R. M. Tófoli & E. Benedito, 2017. Trophic segregation underlies the coexistence of two piranha species after the removal of a geographic barrier. Hydrobiologia 797: 57–68.
Zhang, Y., L. Liu, L. Cheng, Y. Cai, H. Yin, J. Gao & Y. Gao, 2014. Macroinvertebrate assemblages in streams and rivers of a highly developed region (Lake Taihu Basin, China). Aquatic Ecology 23: 15–28.
Zilli, F. L., 2013. Distribution of benthic invertebrate biomass and secondary production in relation to floodplain connectivity in a large river system (Paraná River, Argentina). International Review of Hydrobiology 98: 284–293.
Acknowledgements
I thank R. Fugi and M. S. Dainez-Filho for critical reviewing the first draft of this manuscript. The inclusion of the relative importance of propagule pressure and biotic and abiotic factors in Fig. 1 was the idea of the latter. I also acknowledge three anonymous referees whose comments improved the first version of this manuscript. I also thank J. L. Attayde and R. Panosso for the invitation to present a lecture (which was the basis for this review) at the 10th Shallow Lakes Meeting. I finally acknowledge the National Council for Scientific and Technological Development (CNPq) for continuous funding through a Productivity Research grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: José L. Attayde, Renata F. Panosso, Vanessa Becker, Juliana D. Dias & Erik Jeppesen / Advances in the Ecology of Shallow Lakes.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thomaz, S.M. Propagule pressure and environmental filters related to non-native species success in river-floodplain ecosystems. Hydrobiologia 849, 3679–3704 (2022). https://doi.org/10.1007/s10750-021-04624-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04624-8