Abstract
Astrocytes play essential roles in the central nervous system (CNS), such as the regulation of glutamate metabolism, antioxidant defenses, and inflammatory/immune responses. Moreover, hypothalamic astrocytes seem to be crucial in the modulation of inflammatory processes, including those related to type I interferon signaling. In this regard, the polyphenol resveratrol has emerged as an important glioprotective molecule to regulate astrocyte functions. Therefore, this study aimed to investigate the immunomodulatory and protective effects of resveratrol in hypothalamic astrocyte cultures obtained from mouse depleted of type I interferon receptors (INF-α/β−/−), a condition that can impair immune and inflammatory functions. Resveratrol upregulated glutamate transporter and glutamine synthetase gene expression, as well as modulated the release of wide range of cytokines and genes involved in the control of inflammatory response, besides the expression of adenosine receptors, which display immunomodulatory functions. Resveratrol also increased genes associated with redox balance, mitochondrial processes, and trophic factors signaling. The putative genes associated with glioprotective effects of resveratrol, including nuclear factor erythroid derived 2 like 2 (Nrf2), heme oxygenase 1 (HO-1), sirtuin 1 (SIRT1), and phosphoinositide 3-kinase (PI3K)/Akt, were further upregulated by resveratrol. Thus, our data show that resveratrol was able to modulate key genes associated with glial functionality and inflammatory response in astrocyte cultures derived from IFNα/βR−/− mice. These data are in agreement with previous results, reinforcing its glioprotective effects even in hypothalamic astrocytes with altered inflammatory and immune signaling. Finally, this polyphenol can prepare astrocytes to better respond to injuries, including those associated with neuroimmunology defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes play essential roles for central nervous system (CNS) homeostasis. They participate in the clearance and metabolism of neurotransmitters, synthesize antioxidant defenses, release of trophic factors and contribute to blood–brain barrier (BBB) maintenance (Quincozes-Santos et al. 2021). Moreover, astrocytes have been recognized as essential components of CNS innate immunity because they express many pattern recognition receptors (Han et al. 2021) and secrete cytokines, chemokines, and prostaglandins, which mediate inflammatory responses (Colombo and Farina 2016). In this context, astrocytes produce interferons (especially type I interferon – IFN, also called IFN-α/β) as well as express their receptors. Type I IFN is a family of widely expressed cytokines that have antiviral and immunomodulatory properties, in addition to regulate physiological processes (González-Navajas et al. 2012). Particularly in astrocytes, type I IFN reduces inflammation (Rothhammer et al. 2016) and the lack of type I IFN signaling can be also related to the progression of several diseases (Axtell and Steinman 2008; González-Navajas et al. 2012).
Neuroprotective/glioprotective capacity of astrocytes may decrease with changes in inflammatory responses (Quincozes-Santos et al. 2021; Bobermin et al. 2022), and these cells have emerged as potential therapeutic targets. In line with this, resveratrol, a polyphenolic compound, has been investigated for improving astroglial functions, particularly by the modulation of antioxidant and anti-inflammatory activities (Quincozes-Santos and Gottfried 2011; Quincozes-Santos et al. 2021). Resveratrol is able to modulate several signaling pathways, including nuclear factor erythroid-derived 2-like 2 (Nrf2) and nuclear factor kappa B (NFκB), which are master regulators of inflammatory process in the brain (Quincozes-Santos et al. 2013; Aguilera et al. 2018; Ma et al. 2020; Bhandari et al. 2021; Garrigue et al. 2021; Bobermin et al. 2017, 2022). In this sense, the modulation of inflammatory response by resveratrol has shown to be a critical component in the neuroprotective process elicited by this molecule (Simão et al. 2012; Cai et al. 2018; Miguel et al. 2021). However, the effects of resveratrol under lack of type I IFN signaling in astrocytes have not been investigated.
In recent decades, hypothalamic astrocytes have gained an enormous interest as a potential target for neurotherapies, particularly due to their role in inflammatory process (Sadagurski et al. 2017). In this context, the hypothalamus can integrate peripheral signals and participate in generation and maintenance of chronic inflammation (Burfeind et al. 2016; Färber et al. 2022). In addition, hypothalamus responds to external stressors and undergoes dynamic adaptations (Rosin and Kurrasch 2019), including in neuroinflammatory pathologies. This crucial brain region can also respond to a heterogeneity of immunomodulators, such as IFN, which may alter brain activity to exert feedback on the immune system (Hori et al. 1998). Of note, lack of type I IFN signaling maintains or even exacerbates the expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the hypothalamus (Murray et al. 2015).
Therefore, considering the roles of hypothalamic astrocytes in inflammatory and immune responses, including those related to type I IFN signaling, this study aimed to investigate the glioprotective and anti-inflammatory effects of resveratrol in hypothalamic astrocyte cultures obtained from mice depleted of INF-α/β receptors (IFNα/βR−/− mice). We focused on the expression of genes associated with astrocyte functions and inflammatory response. Our findings may contribute for understanding the effects of resveratrol in astrocytes under different immune conditions and might provide insights on therapeutic control of hypothalamic inflammation.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12), Hank’s balanced salt solution (HBSS), fetal bovine serum (FBS), amphotericin B, gentamicin, TRIzol reagent, ELISA kits for tumor necrosis factor- α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), monocyte chemoattractant protein-1 (MCP-1/CCL2), and anti-rabbit Alexa Fluor 488 were purchased from Gibco/Invitrogen (Carlsbad, CA). High Capacity cDNA Reverse Transcription kit, Taqman Universal PCR Master Mix and TaqMan Assays were purchased from Applied Biosystems (Waltham, MA). Resveratrol, anti-glial fibrillary acidic protein (GFAP), methylthiazolyldiphenyl-tetrazolium bromide (MTT) and ELISA kit for nerve growth factor (NGF) were from Sigma-Aldrich (St. Louis, MO). Glial cell-derived neurotrophic factor (GDNF) ELISA kit was obtained from Abcam (Cambridge, United Kingdom) and brain-derived neurotrophic factor (BDNF) from R&D Systems (Minneapolis, MN). All other chemicals were purchased from common commercial suppliers.
Animals
Neonate IFNα/βR−/− mice (A129/SV-ABR, 3 days old, total of 18 animals) and wild type mice (A129/SV-WT, 3 days old, 6 animals) were obtained from the Institute of Cardiology of Rio Grande do Sul (Porto Alegre, Brazil). The animals were maintained in a constant 12 h light/dark cycle, at a temperature of 24 ± 2 °C, and 50 – 60% relative humidity, with ad libitum access to drinking water and standard food pellets. All animal experiments were performed by following the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Instituto de Cardiologia/Fundação Universitária de Cardiologia (process number IC/FUC-UP 5918/21).
Primary hypothalamic astrocyte cultures preparation and maintenance
Hypothalamic astrocyte cultures were performed based on our previous publication (Santos et al. 2018). The hypothalamus was aseptically dissected, and the meninges removed. The tissue was placed in Hank’s balanced salt solution (HBSS) and then mechanically dissociated for 7 min using a Pasteur pipette and centrifuged at 100 × g for 5 min. The cells were then resuspended in DMEM/F12 (supplemented with 10% fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 2.5 μg/mL amphotericin B, and 0.05 mg/mL gentamicin) and seeded at a density of approximately 2–4 × 105 cells/cm2 in 6- or 24-well plates pre-coated with poly-L-lysine. The cells were cultured at 37 °C in a 5% CO2 incubator. After 24 h, the culture medium was exchanged; the medium supplemented with 10% FBS was replaced once every 2 d until they reached confluence (approximately 14 d).
Resveratrol treatment
Treatment of hypothalamic astrocytes with resveratrol was performed for 7 d, which represent a half of the total culture period (a long-term treatment). After the first week of culture, resveratrol was added to the culture medium (DMEM/F12 10% FBS) at 1 µM. We chose the safely applicable concentration of 1 µM of resveratrol, since it may be more compatible with those obtained in vivo and it is in a range of concentrations that have been used in long-term in vitro studies (Giovannelli et al. 2011; Menicacci et al. 2017, 2019; Bomfim et al. 2020). The culture medium was replaced once every two days until the cells reached confluence, at approximately 14 d, when the analyses were carried out. Resveratrol was diluted in 0.005% ethanol. It is important to mention that cells exposed to ethanol were not different from those obtained at basal conditions without a vehicle, which is in accordance with our previous publication (dos Santos et al. 2006; Bobermin et al. 2012, 2022; Quincozes-Santos et al. 2013).
Immunofluorescence analysis
For actin-labeling analysis, cells were fixed for 20 min with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), rinsed with PBS, and then permeabilized for 10 min in PBS containing 0.2% Triton X-100. After, an incubation with 10 μg/mL rhodamine-labeled phalloidin in PBS for 20 min was performed, followed by two washes with PBS (Santos et al. 2018). For GFAP immunofluorescence, after blocking overnight with 4% albumin, the cells were incubated overnight with anti-GFAP (1:200), at 4 °C, followed by PBS washes and incubation with a specific secondary antibody conjugated with Alexa Fluor 488 (1:1000) for 1 h at room temperature. Cell nuclei were stained with 0.2 μg/mL of 4′, 6′-diamidino-2-phenylindole (DAPI) for further 20 min. Astrocyte cultures were analyzed using a Nikon microscope and photographed with a digital camera DXM1200C and TE-FM Epi-Fluorescence accessory (Nikon Instruments, Melville, NY).
MTT reduction assay
MTT (methylthiazolyldiphenyl-tetrazolium bromide, Sigma-Aldrich) was added to the culture medium at a concentration of 50 μg/mL and cells were incubated for 30 min at 37 °C in an atmosphere of 5% CO2. Subsequently, the medium was removed and the MTT crystals were dissolved in dimethyl-sulfoxide. Absorbance values were measured at 560 nm and 650 nm (SpectraMax i3x, Molecular Devices, San Jose, CA). The results are expressed as percentages relative to the control conditions.
Lactate dehydrogenase (LDH) assay
The release of the enzyme LDH was assessed measuring its activity in the culture medium of astrocytes using a commercial UV assay (Bioclin, Brazil). Results are expressed as percentages of the control value.
Propidium iodide incorporation assay
Astrocyte cultures were incubated with 7.5 μM PI for 30 min in 5% CO2 at 37 °C. The optical density of fluorescent nuclei (labeled with PI), indicative of cell death, was determined with Image J software (National Institutes of Health, Bethesda, MD). Density values obtained are expressed as a percentage of the control value.
RNA extraction and quantitative RT-PCR
Total RNA was isolated from astrocyte cultures (obtained from 9 independent astrocyte cultures treated in duplicate) using TRIzol Reagent. Extracted RNA (1 μg) was submitted to cDNA synthesis by High Capacity cDNA Reverse Transcription Kit. The messenger RNA (mRNA) encoding each target genes was quantified using the TaqMan real-time RT-PCR system with inventory primers and probes purchased from Applied Biosystems (Waltham, MA), as summarized in Table 1. Quantitative RT-PCR was performed using the StepOne System from Applied Biosystems. Target mRNA levels were normalized to β-actin levels. Results were analyzed employing the 2−ΔΔCt method (Livak and Schmittgen 2001).
Inflammatory response and trophic factor measurements
The levels of TNF-α, IL-1β, IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1/CCL2), GDNF, NGF, and BDNF were measured in the culture medium of astrocytes (obtained from 9 independent astrocyte cultures treated in duplicate) using ELISA commercial kits (Table 2).
Statistical analysis
Results are presented as mean ± standard deviation (S.D.). All attempts at replication were successful. The normal distribution was confirmed by Shapiro–Wilk test and variance homogeneity was tested using Bartlett's test. Differences between control and resveratrol were statistically analyzed using Student’s t test. P values < 0.05 were considered significant and are described in the “Results” section, only for significant results. * Indicates differences between control and resveratrol groups (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). All analyses were performed using GraphPad Prism 9.
Results
Effects of resveratrol on cellular viability, metabolism, and astroglial markers in IFNα/βR−/− hypothalamic astrocytes
In order to study the glioprotective effects of resveratrol in cultured hypothalamic astrocytes from IFNα/βR−/− mice, we first analyzed the morphology of these cells, as well as of astrocyte cultures obtained from WT mice. In line with this, cultured hypothalamic astrocytes from both WT and IFNα/βR−/− mice show typical polygonal to fusiform and flat morphology at phase contrast microscopy (Fig. 1A), as it has been widely characterized in vitro (Cechin et al. 2002; Yamamoto et al. 2016; Chaban et al. 2017; Santos et al. 2018; Galland et al. 2019; Bobermin et al. 2020). Furthermore, staining for actin, the major determinant of cellular morphology, revealed the characteristic parallel arrangement of the stress fibers in cultured astrocytes and was not changed between WT and IFNα/βR−/− (Fig. 1A). The cells also express the astrocyte cytoskeleton marker GFAP (Fig. 1A).
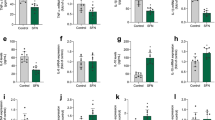
The expression of astroglial markers in IFNα/βR−/− hypothalamic astrocytes and the effects of resveratrol in cellular viability and metabolism. (A) Contrast phase morphology, actin staining and GFAP immunofluorescence of hypothalamic astrocytes (scale bar of 40 µm) obtained from both WT and IFNα/βR−/− mice, in the absence or presence of resveratrol. The gene expressions of GFAP (B), vimentin (C), GS (D), SIRT1 (G), Nrf2 (H), and the extracellular levels of TNF-α (E) and IL-1β (F) were evaluated in hypothalamic astrocytes obtained from WT and IFNα/βR−/− mice. Hypothalamic astrocyte cultures from IFNα/βR−/− were then incubated with resveratrol (1 µM) for 7 d. MTT reduction (I), LDH extracellular activity (J), extracellular lactate levels (K), mRNA levels of GFAP (L), and vimentin (M) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 5–9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between groups. RSV, resveratrol.
We also compared hypothalamic astrocyte cultures from WT and IFNα/βR−/− mice regarding the mRNA levels of astroglial markers, pro-inflammatory cytokine release, and expression of cytoprotective pathways. The mRNA levels of GFAP were increased in hypothalamic astrocytes from IFNα/βR−/− mice compared to WT mice (Fig. 1B; p < 0.001), while mRNA levels of vimentin (Fig. 1C; p < 0.001) and glutamine synthetase (GS) were decreased (Fig. 1D; p = 0.002). Moreover, the extracellular levels of TNF-α (Fig. 1E) did not change in IFNα/βR−/− hypothalamic astrocytes compared to WT astrocytes, but the levels of IL-1β were enhanced (Fig. 1F; p < 0.001). After, we tested the expression of cellular adaptive signaling pathways in IFNα/βR−/− and WT astrocytes at basal conditions, and although we did not observe differences for SIRT1 expression (Fig. 1G), Nrf2 was decreased in IFNα/βR−/− astrocytes (Fig. 1H; p < 0.001). Thus, since IFNα/βR−/− astrocytes present a different gene expression profile of astroglial and inflammatory markers, as well as of signaling pathways, we choose to evaluate the effects of resveratrol specifically on these cells.
Resveratrol did not change cell viability measured by MTT reduction (Fig. 1I), as well as LDH extracellular activity (Fig. 1J), lactate extracellular levels (Fig. 1K), and PI incorporation (data not shown), as well as cellular morphology, actin and GFAP staining (Fig. 1A) in IFNα/βR−/− hypothalamic astrocytes. Although resveratrol also did not change the expression of GFAP (Fig. 1L), it downregulated vimentin (Fig. 1M; p = 0.001), another intermediate filament protein expressed by astrocytes.
Resveratrol modulated inflammatory response
Next, the inflammatory profile of hypothalamic IFNα/βR−/− astrocytes treated with resveratrol was assessed. Resveratrol decreased the mRNA levels and the release of the pro-inflammatory cytokines TNF-α (p < 0.001; Fig. 2A and B) and IL-1β (p < 0.001; Fig. 2D and E). However, the expression of their receptors TNFR1 (Fig. 2C) and IL1R1 (Fig. 2F) was not changed. Both the expression and the release of IL-6 were significantly increased by resveratrol (p < 0.001; Fig. 2G and H), but IL-10 was not modulated (Fig. 2I and J). In addition, the release of the chemokine MCP-1 (p < 0.001; Fig. 2K) was decreased by resveratrol treatment.
Effects of resveratrol on inflammatory response. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. TNF-α expression (A) and release (B), TNFR1 expression (C), IL-1β expression (D) and release (E), IL1R1 expression (F), IL-6 expression (G) and release (H), IL-10 expression (I) and release (J), and MCP-1 release (K) were measured. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
The expression of key genes related to inflammatory processes was further investigated. Resveratrol downregulated NFκB p65 (p < 0.001; Fig. 3A) and its transcriptional targets COX-2 (p < 0.001; 3B), iNOS (p < 0.001; Fig. 3C), and p21 (p < 0.001; Fig. 3D), as well as the expression of the enzyme PARP1 (p < 0.001; Fig. 3E). However, the expression of TLR2 and TLR4, key receptors in innate and adaptive immunity, were not affected (Fig. 3F and G). Since inflammatory processes may be related to BBB dysfunction, the expression of AQP4 (an astrocytic water channel) and VEGF (an inducer of vascular permeability) was evaluated. Resveratrol did not change AQP4 expression (Fig. 3H) but decreased mRNA levels of VEGF (p < 0.001, Fig. 3I).
Effects of resveratrol in the expression of genes related to inflammatory signaling and BBB functionality. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. The mRNA expression of NFκB p65 (A), COX-2 (B), iNOS (C), p21 (D), PARP1 (E), TLR2 (F), TLR4 (G), AQP4 (H), and VEGF (I) was evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
The expression of adenosine receptors, which are recognized for their neuroimmunomodulatory activities, was also evaluated. Resveratrol increased the mRNA levels of A1, A2a, and A3 receptors (p < 0.001; Fig. 4A, B, D), but did not change the expression of the A2b receptor (Fig. 4C).
Effects of resveratrol on adenosine receptors expression. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. The mRNA expression of A1 (A), A2a (B), A2b (C), and A3 (D) receptors was evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
Resveratrol regulated genes associated with redox balance and mitochondrial processes
Inflammation and redox imbalance are closely related. In this regard, the effect of resveratrol treatment on the expression of genes involved in redox balance was also assessed. An upregulation of GCL, SOD1, and SOD2 (p < 0.001; Fig. 5A-C) was observed. Noteworthy, glutathione and superoxide dismutase systems are considered primary antioxidant defenses associated with astrocyte functions in both physiological and pathological conditions. Moreover, resveratrol increased the expression of PGC-1α, citrate synthase, and mitofusin 1, which are associated with crucial mitochondrial processes (p < 0.001; Figs. 5D and 6F).
Effects of resveratrol in the expression of genes associated with redox balance and mitochondrial processes. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. The mRNA expression of GCL (A), SOD1 (B), SOD2 (C), PGC-1α (D), citrate synthase – CS (E), and mitofusin 1 (F) was assessed. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
Signaling mechanisms associated with resveratrol glial immunomodulation. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. The mRNA expression of Nrf2 (A), HO-1 (B), SIRT1 (C), PI3K (D), and Akt (E) was evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
Signaling mechanisms associated with resveratrol glial immunomodulation
Resveratrol upregulated Nrf2, HO-1 and SIRT1 (p < 0.001; Fig. 6A-C), which are key genes involved both in redox and inflammatory regulation. Resveratrol increased the expression of PI3K and Akt, kinases that mediate a crucial cytoprotective pathway (p < 0.001; Fig. 6D and E).
Resveratrol improved trophic functions and changed the expression of genes related to glial glutamate metabolism
The effects of resveratrol on the synthesis and release of trophic factors were also evaluated. Resveratrol increased the mRNA expression and the release of GDNF and NGF (p < 0.001; Fig. 7A-D), but BDNF was not changed (Fig. 7E and F).
Effects of resveratrol on trophic factors. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 days. GDNF expression (A) and release (B), NGF expression (C) and release (D), and BDNF expression (E) and release (F) were evaluated. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
The effect of resveratrol treatment on the expression of specific glial markers related to glutamatergic functionality was also investigated in hypothalamic astrocyte cultures obtained from IFNα/βR−/− mice. Resveratrol significantly increased the mRNA levels of the glutamate transporters GLAST and GLT-1 (p < 0.001; Fig. 8A and B). Moreover, resveratrol increased the expression of GS (p < 0.001; Fig. 8C), an important enzyme of glutamate metabolism that is downregulated in astrocytes from IFNα/βR−/− mice (Fig. 1D).
Effects of resveratrol in the expression of genes related to glutamate metabolism. Primary hypothalamic astrocyte cultures from IFNα/βR−/− were incubated with resveratrol (1 µM) for 7 d. The mRNA expression of GLAST (A), GLT-1 (B), and glutamine synthetase – GS (C) was assessed. Data are presented as mean ± S.D. and differences among groups were statistically analyzed using Student’s t-test (n = 9 independent astrocyte cultures and, at least, a duplicate of treatments). p < 0.05 were considered significant (p values were described for each significant parameter in the “Results” section). * indicates differences between control and resveratrol groups. RSV, resveratrol.
Discussion
Astrocytes actively control multiple aspects of brain health and disease, since they have a wide range of functions (Quincozes-Santos et al. 2021). Although depending on timing and context, astrocyte response may either promote tissue repair or exacerbate inflammatory responses and CNS damage (Colombo and Farina 2016). Recent studies have shown that hypothalamus is a crucial CNS region for modulating immunity (Färber et al. 2022). Therefore, hypothalamic astrocytes may also be potential targets for neurotherapies. In line with this, resveratrol, a glioprotective molecule, has been studied in a variety of experimental conditions, including immunological investigations. Here, for the first time to our knowledge, we described the immunomodulatory effects of resveratrol on hypothalamic astrocyte cultures from IFNα/βR−/− mice.
Type I IFN are pleiotropic cytokines that were originally identified due to their antiviral properties, but they are now recognized as master regulators of innate immunity. In this context, experimental studies have shown that the constitutive activation of type I IFN signaling may confer increased resistance to viral infection (McGlasson et al. 2015). IFNα/βR−/− (A129) mice lack key components of innate immunity and are highly susceptible to infection and/or immunological diseases (Lazear et al. 2016; Rossi et al. 2016). However, these mice may present an upregulation of other signaling inflammatory mechanisms to compensate the absence of IFNα/βR receptor (Kwak et al. 2002; Murray et al. 2015; Rothhammer et al. 2016), but excessive inflammatory responses also contribute to neuropathologies.
IFNα/βR−/− mice show greater dysregulation of inflammatory responses in the brain compared to WT mice. Loss of type I IFN signaling maintain or can even enhance the production of TNF-α, IL-1β and MCP-1 in the brain (Khorooshi and Owens 2010; He et al. 2014; Murray et al. 2015), including in the hypothalamus (Murray et al. 2015), and astrocytes seem to participate in this response (Rothhammer et al. 2016). In agreement to that, we found an increased release of IL-1β in hypothalamic astrocyte cultures obtained from IFNα/βR−/− mice compared to WT mice. Although cytokines and chemokines are essential for resistance against infections and injuries, when produced at high levels they may contribute to brain damage (He et al. 2014). In line with this, resveratrol decreased the mRNA levels and/or release of the pro-inflammatory cytokines TNF-α, IL-1β and MCP-1 in IFNα/βR−/− hypothalamic astrocytes, in agreement with previous data in other cellular experimental models (Bellaver et al. 2014; Bobermin et al. 2019, 2022). Interestingly, resveratrol increased both expression and release of IL-6. Type I IFN seems to play a facilitatory role in IL-6 expression and IFNα/βR−/− mice present a deficient IL-6 response (Murray et al. 2015). Of note, IL-6 has been implicated in dual functions in the CNS, either coordinating neuroimmune responses or mediating non-immunological effects, including neuroprotection (Li et al. 2009; Baune et al. 2012). Particularly considering the hypothalamus, IL-6 regulates neuroendocrine functions (Spangelo and Gorospe 1995). Therefore, the resveratrol-induced increase in IL-6 expression and release in IFNα/βR−/− astrocytes may contribute to protective effects.
NFκB signaling plays a central role in immune and inflammatory responses, inducing the expression of a wide array of pro-inflammatory genes (Cunningham et al. 2019). While exacerbated NFκB activation may have deleterious effects in astrocytes, the attenuation of its signaling can improve neurological outcome in experimental models (Brambilla et al. 2009). Resveratrol decreased not only the expression of NFκB p65, but also of important NFκB transcriptional targets, including cytokines, COX-2, iNOS, and p21, suggesting a downregulation of this pathway. Both COX-2 and iNOS participate in the inflammatory responses in in IFNα/βR−/− mice (Murray et al. 2015; Rothhammer et al. 2016). Regarding p21, although its expression has been related to cellular senescence, immunological roles for this protein have been also described (Tusell et al. 2005). As for PARP-1, previous data showed that it can promote inflammatory responses by positively regulating NFκB signaling (Pazzaglia and Pioli 2019). Thus, resveratrol-mediated downregulation of these genes corroborates anti-inflammatory activity of resveratrol.
Inflammatory processes can drive BBB dysfunction, consequently impairing CNS homeostasis. Resveratrol downregulated VEGF, which is considered the major regulator of vascular permeability and may induce the leakage of the BBB (Argaw et al. 2012). In contrast, resveratrol upregulated GDNF, an important astrocyte-derived trophic factor that may improve the function of BBB (Sano et al. 2007), in addition to promoting neuronal growth (Hamby and Sofroniew 2010). Resveratrol also modulated NGF (expression and release), which can stimulate glial response, including production of antioxidant defenses and inflammatory mediators. Noteworthy, astrocytes are the major source of NGF in neural injuries (Goss et al. 1998). Interestingly, secretion of trophic factors can modulate critical metabolic, immune, and antioxidant properties of astrocytes (Gӧbel et al. 2020). Therefore, it may be speculated that the effects of resveratrol on trophic factors can be a compensatory mechanism to improve glial response in A129 mice that have compromised immune response.
Inflammatory/immune responses and glutamate excitotoxicity are processes closely related, participating in the pathogenesis of neurodegenerative and neuropsychiatric disorders (Quincozes-Santos et al. 2021). Pro-inflammatory cytokines have been demonstrated to decrease glutamate uptake in astrocytes by modulating mRNA expression of glutamate transporters, while IFN-β may reduce this inhibition (Hu et al. 2000). In astrocyte cultures obtained from IFNα/βR−/− mice, resveratrol could compensate such effect by increasing the expression of both glutamate transporters GLAST and GLT-1, in addition to increasing the expression of GS, a key enzyme of glutamate-glutamine cycle. Consistent with this, we previously demonstrated the positive effect of resveratrol on glutamate clearance in astrocytes under lipopolysaccharide (LPS)-induced immune activation (Bellaver et al. 2015). Thus, the present study reinforces the glioprotective effect of resveratrol regarding glutamate homeostasis under different immune conditions.
The glioprotective roles of resveratrol have been related to the modulation of a wide array of signaling pathways. Here, resveratrol increased the expression of adenosine receptors, and recently, we showed the participation of these receptors, which display neuroimmunomodulatory effects, in the anti-inflammatory activity of resveratrol in LPS-stimulated astrocytes (Bobermin et al. 2019). Resveratrol has been also reported as an inducer of genes that control redox homeostasis, including GCL, SOD, and PGC-1α, (Bobermin et al. 2019, 2022; Griñán-Ferré et al. 2021; Dias et al. 2022), and our data are in accordance with these effects previously described. In line with this, GCL participates in the glutathione biosynthesis, an important non-enzymatic antioxidant defense produced and released by astrocytes, while SOD represents a first line antioxidant defense enzyme (Gonçalves et al. 2018). PGC-1α is a transcription co-activator for nuclear receptors and plays a fundamental role in mitochondrial biogenesis (Griñán-Ferré et al. 2021). In addition, we found that resveratrol increased the expression of mitofusin 1, which regulated dynamics events, inducing mitochondrial fusion (Chen et al. 2003). Contributing with this effect on mitochondrial plasticity, resveratrol also upregulated citrate synthase.
Regarding glioprotective mechanisms, we observed that resveratrol increased Nrf2, HO-1, SIRT1, PI3K and Akt signaling pathways. In this sense, Nrf2 mediates cytoprotective responses by activating the expression of genes such as HO-1, GCL, and SOD. Of note, Nrf2/HO-1 axis exerts antioxidant and anti-inflammatory effects, including suppression of NFκB signaling (Ahmed et al. 2017). Moreover, SIRT1 and PI3K/Akt represent important metabolic effectors and have important implications in cell survival and responses to oxidative stress (Zia et al. 2021), which are modulated by resveratrol in several experimental conditions (Bobermin et al. 2019, 2022; Dias et al. 2022). Taken together, these effects on gene expressions modulated by resveratrol in hypothalamic IFNα/βR−/− astrocytes can suggest pivotal cytoprotective targets of resveratrol on brain, reinforcing its glioprotective activity.
It is noteworthy that the maintenance of astrocyte functionality and its endogenous protective properties is essential for CNS homeostasis, since these cells play important roles in the progression and resolution of numerous brain pathologies. Particularly in the hypothalamus, astrocyte dysfunction and inflammatory responses influence feeding and homeostasis regulation, impacting metabolism, body integrity, and immunity (Guijarro et al. 2006). Herein, we showed that resveratrol mediated different protective and immunomodulatory effects in hypothalamic astrocyte cultures obtained from IFNα/βR−/− mice. Recently, the neuroprotective effect of resveratrol in an experimental model of chronic cerebral hypoperfusion was associated with suppression of the expression of pro-inflammatory cytokines likely by mitigating the pathway of the transcription factor interferon regulatory factor 3 (IRF3), which potentiates type I IFN responses (Kang et al. 2022). Since type I IFNs, such as IFN-β, may have dual roles in inflammatory responses associated with CNS diseases models (Kuo et al. 2016; Kang et al. 2022), the ability of resveratrol in modulating inflammatory processes even in excessive and defective type I IFN signaling might be promising in neuroprotective strategies. Thus, our findings reinforce the previously described glioprotective role of resveratrol (Quincozes-Santos et al. 2021; Bobermin et al. 2022), because this molecule could modulate the expression and release of key mediators of astrocyte functions even in the lack of IFNα/βR signaling, a condition that can impair immune and inflammatory functions. Moreover, crucial signaling pathways related to glioprotective effects of resveratrol (Nrf2, HO-1 and SIRT1) had their expression also induced in IFNα/βR−/− astrocytes, and can contribute for preparing astrocytes to better respond to injuries, including those related to immune and inflammatory processes associated with brain pathologies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilera G, Colín-González AL, Rangel-López E et al (2018) Redox signaling, neuroinflammation, and neurodegeneration. Antioxid Redox Signal 28:1626–1651. https://doi.org/10.1089/ars.2017.7099
Ahmed SMU, Luo L, Namani A et al (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis 1863:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Argaw AT, Asp L, Zhang J et al (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122:2454–2468. https://doi.org/10.1172/JCI60842
Axtell RC, Steinman L (2008) Type 1 interferons cool the inflamed brain. Immunity 28:600–602. https://doi.org/10.1016/j.immuni.2008.04.006
Baune BT, Konrad C, Grotegerd D et al (2012) Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain. J Neuroinflammation 9:567. https://doi.org/10.1186/1742-2094-9-125
Bellaver B, Souza DG, Bobermin LD et al (2015) Resveratrol protects hippocampal astrocytes against LPS-induced neurotoxicity through HO-1, p38 and ERK pathways. Neurochem Res 40:1600–1608. https://doi.org/10.1007/s11064-015-1636-8
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol In Vitro 28:479–484. https://doi.org/10.1016/j.tiv.2014.01.006
Bhandari R, Khanna G, Kaushik D, Kuhad A (2021) Divulging the intricacies of crosstalk between NF-Kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol Neurobiol 58:3347–3361. https://doi.org/10.1007/s12035-021-02344-7
Bobermin LD, de Souza Almeida RR, Weber FB et al (2022) Lipopolysaccharide induces gliotoxicity in hippocampal astrocytes from aged rats: insights about the glioprotective roles of resveratrol. Mol Neurobiol 59:1419–1439. https://doi.org/10.1007/s12035-021-02664-8
Bobermin LD, Quincozes-Santos A, Guerra MC et al (2012) Resveratrol prevents ammonia toxicity in astroglial cells. PLoS ONE 7:e52164. https://doi.org/10.1371/journal.pone.0052164
Bobermin LD, Roppa RHA, Gonçalves C-A, Quincozes-Santos A (2020) Ammonia-induced glial-inflammaging. Mol Neurobiol 57:3552–3567. https://doi.org/10.1007/s12035-020-01985-4
Bobermin LD, Roppa RHA, Quincozes-Santos A (2019) Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta Mol Basis Dis 1865:634–647. https://doi.org/10.1016/j.bbadis.2019.01.004
Bobermin LD, Souza DO, Gonçalves C-A, Quincozes-Santos A (2017) Resveratrol prevents ammonia-induced mitochondrial dysfunction and cellular redox imbalance in C6 astroglial cells. Nutr Neurosci 21:276–285. https://doi.org/10.1080/1028415X.2017.1284375
Bomfim GHS, Musial DC, Méndez-López I et al (2020) Chronic resveratrol consumption prevents hypertension development altering electrophysiological currents and Ca2+ signaling in chromaffin cells from SHR rats. Cell Signal 76:109811. https://doi.org/10.1016/j.cellsig.2020.109811
Brambilla R, Persaud T, Hu X et al (2009) Transgenic inhibition of astroglial NF-κb improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182:2628–2640. https://doi.org/10.4049/jimmunol.0802954
Burfeind KG, Michaelis KA, Marks DL (2016) The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin Cell Dev Biol 54:42–52. https://doi.org/10.1016/j.semcdb.2015.10.038
Cai J-C, Liu W, Lu F et al (2018) Resveratrol attenuates neurological deficit and neuroinflammation following intracerebral hemorrhage. Exp Ther Med 15:4131–4138. https://doi.org/10.3892/etm.2018.5938
Cechin SR, Gottfried C, Prestes CC et al (2002) Astrocyte stellation in saline media lacking bicarbonate: possible relation to intracellular pH and tyrosine phosphorylation. Brain Res 946:12–23. https://doi.org/10.1016/S0006-8993(02)02819-6
Chaban YHG, Chen Y, Hertz E, Hertz L (2017) Severe convulsions and dysmyelination in both jimpy and Cx32/47 −/− mice may associate astrocytic L-Channel function with myelination and oligodendrocytic connexins with internodal Kv channels. Neurochem Res 42:1747–1766. https://doi.org/10.1007/s11064-017-2194-z
Chen H, Detmer SA, Ewald AJ et al (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200. https://doi.org/10.1083/jcb.200211046
Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. https://doi.org/10.1016/j.it.2016.06.006
Cunningham C, Dunne A, Lopez-Rodriguez AB (2019) Astrocytes: heterogeneous and dynamic phenotypes in neurodegeneration and innate immunity. Neuroscientist 25:455–474. https://doi.org/10.1177/1073858418809941
Dias FRP, de Souza Almeida RR, Sovrani V et al (2022) Glioprotective effects of resveratrol against BMAA-induced astroglial dysfunctions. Neurotox Res 40:530–541. https://doi.org/10.1007/s12640-022-00492-9
dos Santos AQ, Nardin P, Funchal C et al (2006) Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys 453:161–167. https://doi.org/10.1016/j.abb.2006.06.025
Färber N, Manuel J, May M et al (2022) the central inflammatory network: a hypothalamic fMRI study of experimental endotoxemia in humans. NeuroImmunoModulation 29:231–247. https://doi.org/10.1159/000519061
Galland F, Seady M, Taday J et al (2019) Astrocyte culture models: Molecular and function characterization of primary culture, immortalized astrocytes and C6 glioma cells. Neurochem Int 131:104538. https://doi.org/10.1016/j.neuint.2019.104538
Garrigue P, Mounien L, Champion S et al (2021) Long-term administration of resveratrol at low doses improves neurocognitive performance as well as cerebral blood flow and modulates the inflammatory pathways in the brain. J Nutr Biochem 97:108786. https://doi.org/10.1016/j.jnutbio.2021.108786
Giovannelli L, Pitozzi V, Jacomelli M et al (2011) Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol: Ser A 66A:9–18. https://doi.org/10.1093/gerona/glq161
Gonçalves C-A, Rodrigues L, Bobermin LD et al (2018) Glycolysis-derived compounds from astrocytes that modulate synaptic communication. Front Neurosci 12:1035. https://doi.org/10.3389/fnins.2018.01035
González-Navajas JM, Lee J, David M, Raz E (2012) Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135. https://doi.org/10.1038/nri3133
Goss JR, O’Malley ME, Zou L et al (1998) Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol 149:301–309. https://doi.org/10.1006/exnr.1997.6712
Griñán-Ferré C, Bellver-Sanchis A, Izquierdo V et al (2021) The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res Rev 67:101271. https://doi.org/10.1016/j.arr.2021.101271
Guijarro A, Laviano A, Meguid MM (2006) Hypothalamic integration of immune function and metabolism. In: Progress in Brain Research. Elsevier, pp 367–405
Gӧbel J, Engelhardt E, Pelzer P et al (2020) Mitochondria-endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metab 31:791-808.e8. https://doi.org/10.1016/j.cmet.2020.03.005
Hamby ME, Sofroniew MV (2010) Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 7:494–506. https://doi.org/10.1016/j.nurt.2010.07.003
Han RT, Kim RD, Molofsky AV, Liddelow SA (2021) Astrocyte-immune cell interactions in physiology and pathology. Immunity 54:211–224. https://doi.org/10.1016/j.immuni.2021.01.013
He H, Sharer LR, Chao W et al (2014) Enhanced human immunodeficiency virus type 1 Expression and neuropathogenesis in knockout mice lacking type I interferon responses. J Neuropathol Exp Neurol 73:59–71. https://doi.org/10.1097/NEN.0000000000000026
Hori T, Katafuchi T, Take S, Shimizu N (1998) Neuroimmunomodulatory actions of hypothalamic interferon-α. NeuroImmunoModulation 5:172–177. https://doi.org/10.1159/000026334
Hu S, Sheng WS, Ehrlich LC et al (2000) Cytokine effects on glutamate uptake by human astrocytes. NeuroImmunoModulation 7:153–159. https://doi.org/10.1159/000026433
Kang N, Shi Y, Song J et al (2022) Resveratrol reduces inflammatory response and detrimental effects in chronic cerebral hypoperfusion by down-regulating stimulator of interferon genes/TANK-binding kinase 1/interferon regulatory factor 3 signaling. Front Aging Neurosci 14:868484. https://doi.org/10.3389/fnagi.2022.868484
Khorooshi R, Owens T (2010) Injury-induced type I IFN signaling regulates inflammatory responses in the central nervous system. J Immunol 185:1258–1264. https://doi.org/10.4049/jimmunol.0901753
Kuo P, Scofield BA, Yu I et al (2016) Interferon-β modulates inflammatory response in cerebral ischemia. JAHA 5:e002610. https://doi.org/10.1161/JAHA.115.002610
Kwak M-K, Itoh K, Yamamoto M, Kensler TW (2002) Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22:2883–2892. https://doi.org/10.1128/MCB.22.9.2883-2892.2002
Lazear HM, Govero J, Smith AM et al (2016) A mouse model of zika virus pathogenesis. Cell Host Microbe 19:720–730. https://doi.org/10.1016/j.chom.2016.03.010
Li X, Bai L, Yang Y et al (2009) Effects of IL-6 secreted from astrocytes on the survival of dopaminergic neurons in lipopolysaccharide-induced inflammation. Neurosci Res 65:252–258. https://doi.org/10.1016/j.neures.2009.07.007
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Ma X, Sun Z, Han X et al (2020) Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front Neurosci 13:1400. https://doi.org/10.3389/fnins.2019.01400
McGlasson S, Jury A, Jackson A, Hunt D (2015) Type I interferon dysregulation and neurological disease. Nat Rev Neurol 11:515–523. https://doi.org/10.1038/nrneurol.2015.143
Menicacci B, Laurenzana A, Chillà A et al (2017) Chronic resveratrol treatment inhibits MRC5 fibroblast SASP-related protumoral effects on melanoma cells. J Gerontol: Ser A 72:1187–1195. https://doi.org/10.1093/gerona/glw336
Menicacci B, Margheri F, Laurenzana A et al (2019) Chronic resveratrol treatment reduces the pro-angiogenic effect of human fibroblast “Senescent-Associated Secretory Phenotype” on endothelial colony-forming cells: the role of IL8. J Gerontol: Ser A 74:625–633. https://doi.org/10.1093/gerona/gly175
Miguel CA, Noya-Riobó MV, Mazzone GL et al (2021) Antioxidant, anti-inflammatory and neuroprotective actions of resveratrol after experimental nervous system insults. Special focus on the molecular mechanisms involved. Neurochem Int 150:105188. https://doi.org/10.1016/j.neuint.2021.105188
Murray C, Griffin ÉW, O’Loughlin E et al (2015) Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I:C. Brain Behav Immun 48:274–286. https://doi.org/10.1016/j.bbi.2015.04.009
Pazzaglia S, Pioli C (2019) Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells 9:41. https://doi.org/10.3390/cells9010041
Quincozes-Santos A, Bobermin LD, Latini A et al (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 8:e64372. https://doi.org/10.1371/journal.pone.0064372
Quincozes-Santos A, Gottfried C (2011) Resveratrol modulates astroglial functions: neuroprotective hypothesis: Resveratrol modulates astroglial functions. Ann N Y Acad Sci 1215:72–78. https://doi.org/10.1111/j.1749-6632.2010.05857.x
Quincozes-Santos A, Santos CL, de Souza Almeida RR et al (2021) Gliotoxicity and glioprotection: the dual role of glial cells. Mol Neurobiol 58:6577–6592. https://doi.org/10.1007/s12035-021-02574-9
Rosin JM, Kurrasch DM (2019) Emerging roles for hypothalamic microglia as regulators of physiological homeostasis. Front Neuroendocrinol 54:100748. https://doi.org/10.1016/j.yfrne.2019.100748
Rossi SL, Tesh RB, Azar SR et al (2016) Characterization of a novel murine model to study zika virus. Am J Trop Med Hyg 94:1362–1369. https://doi.org/10.4269/ajtmh.16-0111
Rothhammer V, Mascanfroni ID, Bunse L et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. https://doi.org/10.1038/nm.4106
Sadagurski M, Cady G, Miller RA (2017) Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell 16:652–660. https://doi.org/10.1111/acel.12590
Sano Y, Shimizu F, Nakayama H et al (2007) Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct 32:139–147. https://doi.org/10.1247/csf.07015
Santos CL, Roppa PHA, Truccolo P et al (2018) Age-dependent neurochemical remodeling of hypothalamic astrocytes. Mol Neurobiol 55:5565–5579. https://doi.org/10.1007/s12035-017-0786-x
Simão F, Matté A, Pagnussat AS et al (2012) Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem Int 61:659–665. https://doi.org/10.1016/j.neuint.2012.06.009
Spangelo BL, Gorospe WC (1995) Role of the cytokines in the neuroendocrine-immune system axis. Front Neuroendocrinol 16:1–22. https://doi.org/10.1006/frne.1995.1001
Tusell JM, Saura J, Serratosa J (2005) Absence of the cell cycle inhibitor p21Cip1 reduces LPS-induced NO release and activation of the transcription factor NF-?B in mixed glial cultures. Glia 49:52–58. https://doi.org/10.1002/glia.20095
Yamamoto N, Fujii Y, Kasahara R et al (2016) Simvastatin and atorvastatin facilitates amyloid β-protein degradation in extracellular spaces by increasing neprilysin secretion from astrocytes through activation of MAPK/Erk1/2 pathways: Simvastatin and Atorvastatin Induces Neprilysin Secretion of Astrocytes. Glia n/a-n/a. https://doi.org/10.1002/glia.22974
Zia A, Pourbagher-Shahri AM, Farkhondeh T, Samarghandian S (2021) Molecular and cellular pathways contributing to brain aging. Behav Brain Funct 17:6. https://doi.org/10.1186/s12993-021-00179-9
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Universidade Federal do Rio Grande do Sul, and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sovrani, V., Bobermin, L.D., Sesterheim, P. et al. Glioprotective effects of resveratrol in hypothalamic astrocyte cultures obtained from interferon receptor knockout (IFNα/βR−/−) mice. In Vitro Cell.Dev.Biol.-Animal 59, 366–380 (2023). https://doi.org/10.1007/s11626-023-00777-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00777-z