Abstract
Aging is intrinsically related to metabolic changes and characterized by the accumulation of oxidative and inflammatory damage, as well as alterations in gene expression and activity of several signaling pathways, which in turn impact on homeostatic responses of the body. Hypothalamus is a brain region most related to these responses, and increasing evidence has highlighted a critical role of astrocytes in hypothalamic homeostatic functions, particularly during aging process. The purpose of this study was to investigate the in vitro effects of a chronic treatment with resveratrol (1 µM during 15 days, which was replaced once every 3 days), a recognized anti-inflammatory and antioxidant molecule, in primary hypothalamic astrocyte cultures obtained from aged rats (24 months old). We observed that aging process changes metabolic, oxidative, inflammatory, and senescence parameters, as well as glial markers, while long-term resveratrol treatment prevented these effects. In addition, resveratrol upregulated key signaling pathways associated with cellular homeostasis, including adenosine receptors, nuclear factor erythroid-derived 2-like 2 (Nrf2), heme oxygenase 1 (HO-1), sirtuin 1 (SIRT1), proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and phosphoinositide 3-kinase (PI3K). Our data corroborate the glioprotective effect of resveratrol in aged hypothalamic astrocytes, reinforcing the beneficial role of resveratrol in the aging process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hypothalamus is an important brain region that controls several physiological processes, including metabolism, reproduction, circadian rhythm, and homeostasis [1]. These intricate and complex processes are controlled by appropriate cellular communication at different levels and cellular circuits, which rely on the proper functioning of astrocytes [2]. In addition, changes in hypothalamic function are closely associated with aging and the progression of neurodegenerative diseases, particularly regarding to energy homeostasis, endocrine and inflammatory responses [3,4,5]; thus, hypothalamus has been hypothesized to regulate the process of aging of the body.
Astrocytes are abundant cells throughout the central nervous system (CNS) that make intimate contacts with synapses, blood vessels, and other glial cells. Thus, they are involved in many fundamental processes such as synaptic transmission, maintenance of blood–brain barrier (BBB), and sensing nutrients and hormones [6, 7]. Astrocytes also participate in ionic homeostasis and the metabolism of neurotransmitters, particularly glutamate [8]. In the hypothalamus, all these characteristics are essential for controlling energy homeostasis [9]. However, during metabolic stresses and aging, astrocytes may adopt an activated phenotype, with increased secretion of pro-inflammatory cytokines, excessive production of reactive oxygen/nitrogen species (ROS/RNS), and eventual changes in the expression of glial fibrillary acidic protein (GFAP) [3, 9, 10], which potentially lead to the occurrence of neurotoxicity and neuroinflammation [11].
Resveratrol (3,5,4′-trans-trihydroxy-stilbene) is a polyphenol that naturally occurs in grapes, wines, peanuts, and berries [12, 13] that has been recognized as a multi-target molecule that displays several beneficial effects on the CNS, mainly associated with its antioxidant, anti-inflammatory, and anti-aging properties [14,15,16,17,18,19]. These effects are associated with different signaling pathways, including nuclear factor erythroid-derived 2-like 2 (Nrf2), heme oxygenase 1 (HO-1), sirtuin 1 (SIRT1), phosphoinositide 3-kinase (PI3K), AMP-activated protein kinase (AMPK), nuclear factor kappa B (NFκB), and adenosine receptors [15, 16, 20, 21], which may directly or indirectly influence cellular metabolism and mitochondrial function. Resveratrol can activate SIRT1 and then upregulate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) [22, 23]. In mice, chronic treatment with resveratrol had positive effects on metabolic health [13], but to our knowledge, there are no studies about the chronic effects of resveratrol in hypothalamic astrocytes from aged animals.
Regarding to aging process, mature astrocytes present an overall increase in the expression of inflammatory signaling components and a decrease in cytoprotective pathways [3, 24]. Therefore, we hypothesized that these changes in hypothalamic astrocyte could be prevented by long-term exposure of resveratrol, which mediates glioprotection in several experimental models. In this sense, the aim of this present study was to evaluate the effects of chronic resveratrol treatment in the expression of glial markers and molecular pathways associated with inflammatory response and redox homeostasis in hypothalamic astrocyte cultures of aged Wistar rats. With this approach, we reinforced the glioprotective role of resveratrol contributing to characterize its potential mechanisms in the aging process focusing on hypothalamus.
Methods
Reagents
Resveratrol was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) and other materials for cell culture, TRIzol reagent, and ELISA kits for tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). High Capacity cDNA Reverse Transcription kit, Taqman Universal PCR Master Mix, and TaqMan® Assays were purchased from Applied Biosystems (Foster City, CA, USA). All other chemicals were purchased from common commercial suppliers.
Animals
Male Wistar rats (24 months old) were obtained from the breeding colony of the Department of Biochemistry (Federal University of Rio Grande do Sul, Porto Alegre, Brazil) and maintained under a controlled environment (12 h light/12 h dark cycle; 22 ± 1 °C; ad libitum access to food and water). All animal experiments were performed by following the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees of Federal University of Rio Grande do Sul (process number 35387).
Primary hypothalamic astrocyte cultures preparation and maintenance
Hypothalamic astrocyte cultures were performed with our previous publication [3]. Thirty Wistar rats (24 months old) were anesthetized with isoflurane and after the hypothalamus was aseptically dissected, and the meninges removed. The tissue was enzymatically digested in Hank’s balanced salt solution (HBSS) containing 0.05% trypsin at 37 °C for 7 min. The tissue was then mechanically dissociated for 7 min using a Pasteur pipette and centrifuged at 100×g for 5 min. The pellet was resuspended in HBSS and again mechanically dissociated with a Pasteur pipette until complete homogenization, and then centrifuged at 100 × g for 5 min. After mechanical dissociation and centrifugation, the cells were resuspended in DMEM/F12, supplemented with 10% fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 1% Fungizone®, and 0.04% gentamicin. Cells were seeded in 6- or 24-well plates pre-coated with poly-l-lysine and cultured at 37 °C in a 5% CO2 incubator. The cells were seeded at a density of approximately 2–4 × 105 cells/cm2. After 24 h, the culture medium was exchanged; during the first week, the medium was replaced once every 2 days, and from the second week on, once every 3 days. From the second week on, the astrocytes received medium supplemented with 20% FBS until they reached confluence (at approximately the fourth week). Morphological analysis was performed as control of hypothalamic aged astrocyte cultures in agreement with our previous study [3].
Cellular treatments
Long-term treatment of hypothalamic astrocytes with resveratrol (1 µM) was performed for 15 days [25], a half of the time required for cultured astrocytes reach the confluence. From the second week of culture on, resveratrol was added in the culture medium (DMEM/F12 20% FBS), which was replaced once every 3 days until the cells reached confluence, when the analyses were carried out (Fig. 1). Resveratrol was diluted in 0.005% ethanol. It is important to mention that cells exposed to ethanol were not different from those obtained at basal conditions without vehicle, which is in accordance with our previous publication [26].
Timeline of experimental design. The hypothalamus of male Wistar rats (24 months old) was dissected and processed to obtain the primary astrocyte cultures. Astrocytes were maintained in culture medium (DMEM/F12) for 15 days and then resveratrol (1 µM) was resuspended in this medium for the chronic treatment (15 days). d.i.v. days in vitro
MTT reduction assay
MTT (methylthiazolyldiphenyl-tetrazolium bromide, Sigma-Aldrich) was added to the culture medium at a concentration of 50 μg/mL and cells were incubated for 3 h at 37 °C in an atmosphere of 5% CO2. Subsequently, the medium was removed and the MTT crystals were dissolved in dimethyl-sulfoxide. Absorbance values were measured at 560 nm and 650 nm [16].
Lactate dehydrogenase (LDH) assay
The release of the enzyme LDH was assessed measuring its activity in the culture medium (100 μL) of astrocytes using a commercial UV assay (Bioclin, Brazil).
Extracellular lactate levels
Lactate levels in the extracellular medium were quantified by a commercial kit from Bioclin (Belo Horizonte, MG, Brazil). The results are expressed in mmol/L.
RNA extraction and quantitative RT-PCR
Total RNA was isolated from astrocyte cultures using TRIzol Reagent. Extracted RNA (1 μg) was submitted to cDNA synthesis by High Capacity cDNA Reverse Transcription Kit. Quantitative PCR determination of the messenger RNAs (mRNAs) encoding GFAP (#Rn00566603_m1), vimentin (#Rn00667825_m1), nestin (#Rn00564394_m1), connexin-43 (Cx43; #Rn01433957_m1), glutamate aspartate transporter (GLAST; #Rn00570130_m1), glutamate transporter 1 (GLT-1; #Rn00691548_m1), aquaporin 4 (AQP4; #Rn00563196_m1), glutamine synthetase (GS; #Rn01483107_m1), p21 (#Rn 00589996_m1), tumor necrosis factor-α (TNF-α; #Rn99999017_m1), interleukin-1β (IL-1β; #Rn00580432_m1), NLR family pyrin domain containing 3 (NLRP3; #Rn04244620_m1), interleukin-10 (IL-10 (#Rn00563409_m1), NFκB p65 (#Rn01502266_m1), cyclooxygenase 2 (COX2; #Rn01483828_m1), glucocorticoid receptor (GR; #Rn00561369_m1), toll-like receptor 4 (TLR4; #Rn00569848_m1), toll-like receptor 2 (TLR2; #Rn02133647_s1), receptor for advanced glycation end products (RAGE; #Rn01525753_g1), S100 calcium-binding protein B (S100B; #Rn04219408_m1), high mobility group box 1 (HMGB1; #Rn02377062_g1), adenosine receptor A1 (#Rn00567668_m1), adenosine receptor A2a (#Rn00583935_m1), adenosine receptor A2b (#Rn00567697_m1), adenosine receptor A3 (#Rn00563680_m1), glutamate-cysteine ligase (GCL; #Rn00689046_ m1), superoxide dismutase 1 (SOD1; #Rn00566938_m1), superoxide dismutase 2 (SOD2; #Rn00690588_g1), PGC-1α (#Rn00580241_m1), Poly (ADP-ribose) polymerase (PARP; #Rn00565018_m1), inducible nitric oxide synthase (iNOS; #Rn00561646_m1), HO-1 (#Rn01536933_ m1), Nrf2 (#Rn00582415_m1), SIRT1 (#Rn01428096_ m1), PI3K (#Rn01769524_m1), AMPK (#Rn00576935_m1), β-actin (#Rn00667869_m1) were performed using the TaqMan real-time RT-PCR system with inventory primers and probes purchased from Applied Biosystems (Thermo Fisher Scientific), as referred for each gene. Target mRNA levels were normalized to β-actin levels. Results were analyzed employing the 2−ΔΔCt method [27].
Inflammatory response measurement
Cytokine levels were measured in the extracellular medium using ELISA kits for TNF-α and IL-1β from Gibco/Invitrogen (Carlsbad, CA, USA). The results are expressed in pg/mL and the average minimum sensitivity of the ELISA kits detection was: 25 pg/mL for TNF-α and 12 pg/mL for IL-1β.
Western blot analysis
Astrocytes were solubilized in a lysis solution containing 4% SDS, 2 mM EDTA, and 50 mM Tris–HCL (pH 6.8). Samples were heated at 100 °C for 10 min, fractionated by SDS-PAGE (30 µg protein per sample) and electro-blotted onto nitrocellulose membranes. Protein loading and electro-blotting efficiency were verified through Ponceau S staining. The membrane was blocked in 5% albumin prepared in Tween-Tris-buffered saline (TTBS; 100 mM Tris–HCl, pH 7.6, containing 70 mM NaCl and 0.1% Tween-20), incubated overnight at 4 °C with the primary antibodies anti-NFκB (1:1000, Cell Signaling, Massachusetts, USA), anti-PGC-1α (1:1000, Invitrogen, Massachusetts, USA), anti-β-actin (1:20,000, ProteinTech Group, Illinois, USA) and washed with TTBS. The membrane was incubated with anti-IgG from rabbit or mouse (1:5000, Cell Signaling, Massachusetts, USA) according to the species that originated the primary antibody linked to peroxidase for 2 h at room temperature and washed with TTBS again. The immunoreactivity was detected by enhanced chemiluminescence using Millipore Immobilon™ Western chemiluminescent HRP substrate in a CCD camera (GE Image Quant LAS4000). Densitometric analysis of the membranes was performed with ImageJ software. Blots were developed to be linear in the range used for densitometry. The representative images of Western blot analysis are in the Supplementary Material (Fig. S1).
Statistical analysis
Data were statistically analyzed using Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent astrocyte cultures and, at least, duplicate of treatments). All analyses were performed using GraphPad Prism 9.
Results
Resveratrol modulates important glial parameters
First, we tested three concentrations of resveratrol (1, 10 and 100 µM) in accordance with previous studies in astroglial cells [26, 28]. Chronic treatment of aged hypothalamic astrocytes with 1 and 10 µM of resveratrol did not change cellular viability measured by MTT reduction, neither LDH activity (data not shown). However, 100 µM of resveratrol decreased MTT reduction, but not the activity of LDH (data not shown). Therefore, we choose the safely applicable concentration of 1 µM of resveratrol, since it may be more compatible with those obtained in vivo and it is in a range of concentrations that have been used in long-term in vitro studies [25, 29,30,31]. Moreover, the extracellular levels of lactate, measured as an indicative of metabolic functionality of the cells, were not altered (data not shown).
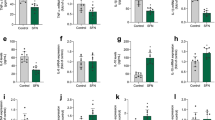
We then determined the effects of resveratrol on glial markers. Chronic resveratrol exposure decreased the mRNA levels of GFAP (p < 0.05; Fig. 2A) but did not change vimentin, nestin, and Cx43 (Fig. 2B–D, respectively), while the expression of AQP4 was decreased (p < 0.0001; Fig. 2E). In addition, the main glutamate transporters, GLAST and GLT-1, had a significant increase after resveratrol treatment (p < 0.05; Fig. 2F, G). GS, an important enzyme in glutamate metabolism, was significantly increased after resveratrol treatment (p < 0.0001; Fig. 2H).
Changes in classical glial parameters after chronic treatment with resveratrol in cultured hypothalamic astrocytes. The cells were incubated with resveratrol (1 µM) in the culture medium for 15 days and the mRNA expression of GFAP (A), vimentin (B), nestin (C), Cx43 (D), AQP4 (E), GLAST (F), GLT-1 (G), and GS (H) were evaluated. The data represent the means ± S.D., analyzed by Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent cultures and, at least, duplicate of treatments). RSV resveratrol
Resveratrol modulates inflammatory response and adenosine receptors
Next, we evaluated the effects of resveratrol on inflammatory response. Resveratrol decreased mRNA levels of p21 (p < 0.0001; Fig. 3A), an important regulator of senescence and inflammation. In addition, resveratrol decreased mRNA expression and extracellular levels of TNF-α (p < 0.05; Fig. 3B and C, respectively), while decreased only the mRNA expression of IL-1β (p < 0.0001; Fig. 3D). The release of IL-1β (Fig. 3E) was not affected. In contrast, resveratrol increased the expression of the anti-inflammatory cytokine IL-10 (p < 0.0001; Fig. 3F) and the mRNA expression of NLRP3 (Fig. 3G), which is involved in IL-1β processing, were not affected.
Effects of chronic resveratrol treatment on inflammatory parameters in cultured hypothalamic astrocytes. The cells were incubated with resveratrol (1 µM) in the culture medium for 15 days and the mRNA of p21 (A) and TNF-α (B), TNF-α release (C), mRNA of IL-1β (D), IL-1β release (E), mRNA of IL-10 (F), NLRP3 (G), NFκB (H), NFκB protein levels (I), mRNA of COX-2 (J), GR (K), TLR4 (L), TLR2 (M), RAGE (N), S100B (O), HMGB1 (P) were evaluated. The data represents the means ± S.D., analyzed by Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent cultures and, at least, duplicate of treatments). RSV resveratrol
Potentially mediating these inflammatory events, we verified a downregulation of NFκB p65 and COX-2 (p < 0.05; Fig. 3H and J, respectively). However, NFκB p65 protein levels were not changed (Fig. 3I). Chronic treatment with resveratrol also modulated the expression of receptors involved in the regulation of inflammatory responses. GR and TLR4 were downregulated (p < 0.05; Fig. 3K and L, respectively), while TLR2 levels were not significantly different (Fig. 3M). Although the mRNA levels of RAGE (Fig. 3N) were upregulated (p < 0.05), resveratrol decreased the expression of S100B (p < 0.05; Fig. 3O), a potential RAGE ligand, and did not change HMGB1 expression (Fig. 3P).
We also evaluated the expression of adenosine receptors, which are recognized for their important inflammatory and neuromodulatory activities. Resveratrol increased the mRNA levels of A1, A2a, and A3 receptors (p < 0.05; Fig. 4A–C), but did not change the expression of A2b receptor (Fig. 4D).
Effects of chronic resveratrol treatment on adenosine receptors in cultured hypothalamic astrocytes. The cells were incubated with resveratrol (1 µM) in the culture medium for 15 days and the mRNA expressions of A1 (A), A2a (B), A3 (C), A2b (D) were evaluated. The data represents the means ± S.D., analyzed by Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent cultures and, at least, duplicate of treatments). RSV resveratrol
Resveratrol changes redox homeostasis
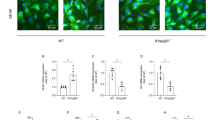
Subsequently, we evaluated the expression of genes associated with redox homeostasis after chronic treatment with resveratrol. We observed an upregulation of GCL, SOD1, SOD2, and PGC-1α (p < 0.0001; Fig. 5A–D). The protein levels of PGC-1α were not significantly changed (Fig. 5E). Furthermore, the enzyme PARP and iNOS were downregulated by resveratrol (p < 0.0001; Fig. 5F and G, respectively).
Changes in redox homeostasis of hypothalamic astrocyte cultures after chronic treatment with resveratrol. The cells were incubated with resveratrol (1 µM) in the culture medium for 15 days. The mRNA expressions of the following enzymes were evaluated: GCL (A), SOD1 (B), SOD2 (C), PGC-1α (D), PARP (F), and iNOS (G), as well as PGC-1α protein levels (E). The data represents the means ± S.D., analyzed by Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent cultures and, at least, duplicate of treatments). RSV resveratrol
Signaling pathways associated with glioprotective effects of resveratrol
Finally, we analyzed important signaling pathways that can mediate the glioprotective effects of resveratrol. The expression of HO-1 (p < 0.05; Fig. 6A), Nrf2 (p < 0.0001; Fig. 6B), SIRT1 (p < 0.05; Fig. 6C), and PI3K (p < 0.05; Fig. 6D) was upregulated by resveratrol. However, resveratrol decreased the mRNA levels of AMPK (p < 0.05; Fig. 6E).
Glioprotective signaling pathways associated with chronic resveratrol treatment. The cells were incubated with resveratrol (1 µM) in the culture medium for 15 days. mRNA expressions of HO-1 (A), Nrf2 (B), SIRT1 (C), PI3K (D), and AMPK (E) were evaluated. The data represents the means ± S.D., analyzed by Student’s t-test. p values < 0.05 were considered significant. *Indicates differences between control and resveratrol groups (n = 10 independent cultures and, at least, duplicate of treatments). RSV resveratrol
Discussion
Aging, a natural biological process, is characterized by a gradual accumulation of oxidized biomolecules and damaged cell organelles, leading to progressive loss of structural and functional integrity of the cells. Metabolism and aging can affect each other mutually; during aging, there are massive changes in body energy metabolism, in which the hypothalamus plays an important role [32]. Accumulating evidence suggests that microinflammatory insults disturb hypothalamic regulation, resulting in metabolic imbalance and aging progression [33]. These processes involve a remodeling of the hypothalamus, which is closely related to astrocytic functionality and changes in gene transcription [3, 19, 34].
Aged human brains display heterogeneous changes in astrocyte morphology and GFAP levels, in addition to mitochondrial/redox homeostasis dysfunction, neuroinflammation, excitotoxicity, and alterations in glucose metabolism [35, 36]. When we evaluated the glial markers in our study, we found a downregulation of GFAP after chronic resveratrol treatment. Because aging astrocytes have shown increased GFAP expression, which may be associated with reactive/activated astrocytes [37, 38], our finding suggests that chronic resveratrol may exert a protective effect by attenuating aging-related astrocyte activation. Another important functional parameter of astrocytes is AQP4, a water channel protein expressed by these cells [39]. Previously, we showed a higher expression of AQP4 in mature astrocytes compared with neonatal cells [24]. In addition to its role in water homeostasis, AQP4 has been related to neuroinflammation and neurodegenerative processes [40, 41], being the downregulation of AQP4 observed in our study possibly related to the role of resveratrol in maintaining astrocyte homeostasis.
Glutamate is the predominant excitatory neurotransmitter in the CNS. Particularly in the hypothalamus, glutamate has been associated with neuroendocrine regulation and feeding behavior [42]. However, it can be neurotoxic when inappropriately remaining at high levels in the synaptic cleft [43, 44], and hypofunction and downregulation of glutamate transporters may be associated with pathological conditions and aging. GLAST is an important glutamate transporter present in glial cells, but the major glutamate transporter in the brain is GLT-1, responsible for more than 90% of glutamate uptake [44]. Here, we observed that chronic treatment with resveratrol increased the expression of both GLAST and GLT-1 in aged hypothalamic astrocytes. Resveratrol-induced improvement in glutamate uptake has been reported in previous studies by our group [14, 28, 45], which may be related to the increased expression of glutamate transporters found in the current study. It is important to note that the activity of glutamate transporters can be impaired by oxidative damage [46] and inflammatory responses, which are also attenuated by resveratrol [47].
In addition to increase glutamate transporters, resveratrol also upregulated the expression of GS, a specific astrocytic enzyme responsible for the conversion of glutamate in glutamine. Glutamine is then forwarded to neurons as an intermediate for recycling glutamate [48]. Our previous works have demonstrated that GS expression decreased in cultured astrocytes in an age-dependent manner, demonstrating that resveratrol may act as a preventive factor in this process [3, 49]. Interestingly, glutamate metabolism can be modulated by glucocorticoids; they are related to decreased glutamate uptake [50] and GS activity and expression [51] by astrocytes. We observed a downregulation of glucocorticoid receptors (GR) after resveratrol treatment, which may represent an additional mechanism by which resveratrol exerts the homeostatic role in glutamate metabolism. In addition, glucocorticoid actions in the hypothalamus may provide an integrative signal linking stress with the regulation of energy homeostasis [52].
With the progression of aging, the expression of senescence markers, including p21, also increases. This protein promotes cell cycle arrest in senescent cells, and the inhibition of this marker is recognized as an important way to treat age-related pathologies [53]. Importantly, we observed a downregulation of p21 by the chronic treatment with resveratrol in hypothalamic astrocytes. Aging progression is also characterized by inflammatory responses throughout the body, according to the concept of “inflammaging” [54]. With this regard, hypothalamic astrocytes from adult and aging animals have presented a pro-inflammatory profile [3], which may lead to a chronic inflammatory background in the hypothalamus [33]. The NFκB pathway is an important player in astrocyte reactivity [32] and age-related hypothalamic inflammation [55], while inhibition of NFκB has been associated with improvement of various aspects of metabolic conditions. Because many inflammatory cytokines are induced through this transcription factor, the downregulation of NFκB p65 expression by resveratrol may be effective in suppressing hypothalamic microinflammation [33]. In agreement with that, astrocytes chronically treated with resveratrol presented a downregulation of several pro-inflammatory mediators, including TNF-α, IL-1β, and COX-2, and an upregulation of the anti-inflammatory cytokine IL-10. Of note, adenosine receptors play neuromodulatory and neuroinflammatory functions [56, 57] that have been associated with resveratrol-mediated anti-inflammatory activity in a previous study by our group [16]. Here, adenosine receptors were also positively modulated by resveratrol chronic treatment, reinforcing their role in the glioprotective effects of resveratrol.
In addition to produce and release a wide range of inflammatory mediators, astrocytes can also respond to them during immune responses through the expression of pattern recognition receptors [58]. In neurodegenerative diseases and CNS injuries, endogenous and exogenous ligands trigger TLR signaling, leading to the transcription of inflammatory cytokines [59]. Thus, the downregulation of TLR4 after treatment with resveratrol may be associated with a negative modulation of inflammatory markers. Moreover, resveratrol decreased S100B, which can act as an extracellular factor that engages RAGE [60]. Activation of RAGE by its ligands induces the release of cytokines, interleukins, and increased reactive oxygen species production in glial cells [61]. However, recent studies demonstrate that RAGE activation also contribute to neuronal-astrocytic communication [62] and promote changes in redox regulation.
Although reactive oxygen/nitrogen species (ROS/RNS) carry out modulatory functions in physiological conditions, excessive production of these species can contribute to the development and progression of pathological processes [63]. Aging process leads to mitochondrial dysfunction and imbalance of redox homeostasis, with significant involvement of oxidative and nitrosative stresses [36, 64]. With this regard, glial cell-related antioxidant defenses display important roles for the protection and repair of the brain, and are essential for controlling energy homeostasis [9]. PGC-1α is a transcription co-activator for nuclear receptors and plays a fundamental role in the control of cellular energy metabolism, redox homeostasis, and neuroinflammation [23, 65]. The astrocytic antioxidant system also involves the synthesis of GSH, which depends on the enzyme GCL. Importantly, neurons are dependent on astrocytic GSH to their own antioxidant defense [66]. In our study, we verified an increase in the expression of PGC-1α and GCL, as well as SOD1 and SOD2, indicating an improvement of antioxidant defenses in hypothalamic astrocytes from aged rats by the chronic treatment with resveratrol. In addition, aging brains show higher levels of PARP and iNOS [64, 67], which was downregulated in our experimental model, reinforcing the antioxidant action of the chronic treatment with resveratrol.
Oxidative stress and inflammation are closely related processes, which are connected by important signaling pathways, such as NFκB and Nrf2 [68]. Signaling pathways act as key regulators of cell survival and responses to different physiological and pathological. Resveratrol is effective to prevent age-related functional alterations of astrocytes by different signaling pathways, including Nrf2/HO-1, PI3K/Akt, AMPK, and NFκB [15, 16, 20, 21]. Nrf2 is an essential transcription factor responsive to metabolic, inflammatory, and redox signals, acting as a regulatory mechanism for the homeostasis of these systems. HO-1, one of the genes regulated by Nrf2, acts to produce cellular responses against stressful conditions [20, 69]. Our data corroborate with these mechanisms since chronic treatment with resveratrol was able to increase the expression of Nrf2 and HO-1. SIRT1 and PI3K, which represent important metabolic effectors, were also upregulated by resveratrol, thus potentially participating in the protective response induced in hypothalamic astrocytes [70, 71]. However, some limitations of this study should be mentioned, including the differences between gene expression and protein levels of NFκB and PGC-1α, probably due to their different regulatory mechanisms.
In addition, data from cultured astrocytes derived from Wistar rats chronically treated with resveratrol will confirm and expand the knowledge about the role of resveratrol on hypothalamus. In line with this, changes in hypothalamus strongly interfere with brain homeostasis, being a critical point in development and progression of age-dependent brain dysfunctions. Therefore, hypothalamic astrocytes may emerge as a therapeutic target for understanding the brain aging under physiological and pathological conditions.
Finally, resveratrol is a recognized anti-inflammatory and antioxidant polyphenol with beneficial effects described in many tissues, but the focus of several studies with this compound is based on the strategy of acute treatments. If we consider long-term treatments, they can have further beneficial effects as a preventive approach. Clinical evidence of food or nutrient supplements chronically administrated in patients suggest that diets rich in polyphenols, for example the Mediterranean diet, could be an effective nutritional strategy [72]. Therefore, in the near future, our findings might contribute for understanding the effects of resveratrol on hypothalamus, including for clinical studies focusing on metabolism and aging. Considering the effects associated with aging on hypothalamic function (Fig. 7), such as oxidative stress and inflammation, it is reasonable to assume that long-term resveratrol treatment could help with these age-dependent changes in astrocytes and consequently the age-related diseases.
Glioprotective effects of long-term resveratrol treatment. Schematic illustration summarizing the effects of aging and chronic treatment with resveratrol (1 µM) for 15 days in hypothalamic astrocytes of aged Wistar rats (24 months old). Chronic treatment attenuated the gliotoxicity caused by the aging process in astrocytes, playing a homeostatic role in the hypothalamus. RSV resveratrol
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kim K, Choe HK (2019) Role of hypothalamus in aging and its underlying cellular mechanisms. Mech Ageing Dev 177:74–79. https://doi.org/10.1016/j.mad.2018.04.008
Murat CDB, García-Cáceres C (2021) Astrocyte gliotransmission in the regulation of systemic metabolism. Metabolites 11:732. https://doi.org/10.3390/metabo11110732
Santos CL, Roppa PHA, Truccolo P et al (2018) Age-dependent neurochemical remodeling of hypothalamic astrocytes. Mol Neurobiol 55:5565–5579. https://doi.org/10.1007/s12035-017-0786-x
Satoh A, Imai S (2014) Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat Commun 5:4211. https://doi.org/10.1038/ncomms5211
Suda Y, Nakashima T, Matsumoto H et al (2021) Normal aging induces PD-1-enriched exhausted microglia and A1-like reactive astrocytes in the hypothalamus. Biochem Biophys Res Commun 541:22–29. https://doi.org/10.1016/j.bbrc.2020.12.086
Tsai H-H, Li H, Fuentealba LC et al (2012) Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337:358–362. https://doi.org/10.1126/science.1222381
Mendes NF, Jara CP, Zanesco AM, de Araújo EP (2021) Hypothalamic microglial heterogeneity and signature under high fat diet-induced inflammation. Int J Mol Sci 22:1–23
Benarroch EE (2016) Astrocyte signaling and synaptic homeostasis. Neurology 87:324–330. https://doi.org/10.1212/WNL.0000000000002875
Douglass JD, Dorfman MD, Fasnacht R et al (2017) Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6:366–373. https://doi.org/10.1016/j.molmet.2017.01.010
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. https://doi.org/10.1007/s00401-009-0619-8
Zhang G, Li J, Purkayastha S et al (2013) Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497:211–216. https://doi.org/10.1038/nature12143
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506. https://doi.org/10.1038/nrd2060
Kulkarni SS, Cantó C (2015) The molecular targets of resveratrol. Biochim Biophys Acta Mol Basis Dis 1852:1114–1123. https://doi.org/10.1016/j.bbadis.2014.10.005
Quincozes-Santos A, Gottfried C (2011) Resveratrol modulates astroglial functions: neuroprotective hypothesis. Ann N Y Acad Sci 1215:72–78. https://doi.org/10.1111/j.1749-6632.2010.05857.x
Bellaver B, Bobermin LD, Souza DG et al (2016) Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim Biophys Acta Mol Basis Dis 1862:1827–1838. https://doi.org/10.1016/j.bbadis.2016.06.018
Bobermin LD, Roppa RHA, Quincozes-Santos A (2019) Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim Biophys Acta Mol Basis Dis 1865:634–647. https://doi.org/10.1016/j.bbadis.2019.01.004
Rosa PM, Martins LAM, Souza DO, Quincozes-Santos A (2018) Glioprotective effect of resveratrol: an emerging therapeutic role for oligodendroglial cells. Mol Neurobiol 55:2967–2978. https://doi.org/10.1007/s12035-017-0510-x
Sovrani V, Bobermin LD, Schmitz I et al (2021) Potential glioprotective strategies against diabetes-induced brain toxicity. Neurotox Res 39:1651–1664. https://doi.org/10.1007/s12640-021-00393-3
Quincozes-Santos A, Santos CL, de Souza Almeida RR et al (2021) Gliotoxicity and glioprotection: the dual role of glial cells. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02574-9
Quincozes-Santos A, Bobermin LD, Latini A et al (2013) Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 8:e64372. https://doi.org/10.1371/journal.pone.0064372
Bastianetto S, Ménard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochim Biophys Acta Mol Basis Dis 1852:1195–1201. https://doi.org/10.1016/j.bbadis.2014.09.011
Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122. https://doi.org/10.1016/j.cell.2006.11.013
Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172. https://doi.org/10.1016/j.bbi.2017.03.003
Bobermin LD, Roppa RHA, Gonçalves CA, Quincozes-Santos A (2020) Ammonia-induced glial-inflammaging. Mol Neurobiol 57:3552–3567. https://doi.org/10.1007/s12035-020-01985-4
Giovannelli L, Pitozzi V, Jacomelli M et al (2011) Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol Ser A 66A:9–18. https://doi.org/10.1093/gerona/glq161
Bobermin LD, de Souza Almeida RR, Weber FB et al (2022) Lipopolysaccharide induces gliotoxicity in hippocampal astrocytes from aged rats: insights about the glioprotective roles of resveratrol. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02664-8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
dos Santos AQ, Nardin P, Funchal C et al (2006) Resveratrol increases glutamate uptake and glutamine synthetase activity in C6 glioma cells. Arch Biochem Biophys 453:161–167. https://doi.org/10.1016/j.abb.2006.06.025
Menicacci B, Laurenzana A, Chillà A et al (2017) Chronic resveratrol treatment inhibits MRC5 fibroblast SASP-related protumoral effects on melanoma cells. J Gerontol Ser A 72:1187–1195. https://doi.org/10.1093/gerona/glw336
Menicacci B, Margheri F, Laurenzana A et al (2019) Chronic resveratrol treatment reduces the pro-angiogenic effect of human fibroblast “senescent-associated secretory phenotype” on endothelial colony-forming cells: the role of IL8. J Gerontol Ser A 74:625–633. https://doi.org/10.1093/gerona/gly175
Bomfim GHS, Musial DC, Méndez-López I et al (2020) Chronic resveratrol consumption prevents hypertension development altering electrophysiological currents and Ca2+ signaling in chromaffin cells from SHR rats. Cell Signal 76:109811. https://doi.org/10.1016/j.cellsig.2020.109811
Liu T, Xu Y, Yi C-X et al (2021) The hypothalamus for whole-body physiology: from metabolism to aging. Protein Cell. https://doi.org/10.1007/s13238-021-00834-x
Cai D, Khor S (2019) “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab 30:19–35. https://doi.org/10.1016/j.cmet.2019.05.021
Kim SK, Nabekura J, Koizumi S (2017) Astrocyte-mediated synapse remodeling in the pathological brain. Glia 65:1719–1727. https://doi.org/10.1002/glia.23169
Jyothi HJ, Vidyadhara DJ, Mahadevan A et al (2015) Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging 36:3321–3333. https://doi.org/10.1016/j.neurobiolaging.2015.08.024
Palmer AL, Ousman SS (2018) Astrocytes and aging. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00337
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Clarke LE, Liddelow SA, Chakraborty C et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci 115:E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Salman MM, Kitchen P, Halsey A et al (2021) Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. https://doi.org/10.1093/brain/awab311
Fukuda AM, Badaut J (2012) Aquaporin 4: a player in cerebral edema and neuroinflammation. J Neuroinflammation 9:771. https://doi.org/10.1186/1742-2094-9-279
Yang J, Zhang R, Shi C et al (2017) AQP4 association with amyloid deposition and astrocyte pathology in the Tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis 57:157–169. https://doi.org/10.3233/JAD-160957
Oliet SHR (2001) Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292:923–926. https://doi.org/10.1126/science.1059162
Maragakis NJ, Rothstein JD (2004) Glutamate transporters: animal models to neurologic disease. Neurobiol Dis 15:461–473. https://doi.org/10.1016/j.nbd.2003.12.007
Rodríguez-Campuzano AG, Ortega A (2021) Glutamate transporters: critical components of glutamatergic transmission. Neuropharmacology 192:108602. https://doi.org/10.1016/j.neuropharm.2021.108602
de Almeida LMV, Piñeiro CC, Leite MC et al (2007) Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol 27:661–668. https://doi.org/10.1007/s10571-007-9152-2
Trotti D, Danbolt NC, Volterra A (1998) Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 19:328–334. https://doi.org/10.1016/S0165-6147(98)01230-9
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol Vitr 28:479–484. https://doi.org/10.1016/j.tiv.2014.01.006
Brusilow SW, Koehler RC, Traystman RJ, Cooper AJL (2010) Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 7:452–470. https://doi.org/10.1016/j.nurt.2010.05.015
Souza DG, Bellaver B, Raupp GS et al (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int 90:93–97. https://doi.org/10.1016/j.neuint.2015.07.016
Virgin CE, Ha TP-T, Packan DR et al (1991) Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem 57:1422–1428. https://doi.org/10.1111/j.1471-4159.1991.tb08309.x
Kazazoglou T, Panagiotou C, Mihailidou C et al (2021) Glutamine synthetase regulation by dexamethasone, RU486, and compound A in astrocytes derived from aged mouse cerebral hemispheres is mediated via glucocorticoid receptor. Mol Cell Biochem. https://doi.org/10.1007/s11010-021-04236-9
Tasker JG (2006) Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity 14:259S-265S. https://doi.org/10.1038/oby.2006.320
Papismadov N, Gal H, Krizhanovsky V (2017) The anti-aging promise of p21. Cell Cycle 16:1997–1998. https://doi.org/10.1080/15384101.2017.1377500
Franceschi C, Garagnani P, Parini P et al (2018) Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. https://doi.org/10.1038/s41574-018-0059-4
Zhang Y, Reichel JM, Han C et al (2017) Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab 25:1091-1102.e4. https://doi.org/10.1016/j.cmet.2017.04.002
Németh ZH, Lutz CS, Csóka B et al (2005) Adenosine augments IL-10 production by macrophages through an A 2B receptor-mediated posttranscriptional mechanism. J Immunol 175:8260–8270. https://doi.org/10.4049/jimmunol.175.12.8260
Pasquini S, Contri C, Borea PA et al (2021) Adenosine and inflammation: here, there and everywhere. Int J Mol Sci 22:7685. https://doi.org/10.3390/ijms22147685
Sofroniew MV (2020) Astrocyte reactivity : subtypes, states, and functions in CNS innate immunity. Trends Immunol 41:758–770. https://doi.org/10.1016/j.it.2020.07.004
Li L, Acioglu C, Heary RF, Elkabes S (2021) Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun 91:740–755. https://doi.org/10.1016/j.bbi.2020.10.007
Donato R, Sorci G, Riuzzi F et al (2009) S100B’s double life: Intracellular regulator and extracellular signal. Biochim Biophys Acta - Mol Cell Res 1793:1008–1022. https://doi.org/10.1016/j.bbamcr.2008.11.009
Kim J, Wan CK, O’Carroll SJ et al (2012) The role of receptor for advanced glycation end products (RAGE) in neuronal differentiation. J Neurosci Res 90:1136–1147. https://doi.org/10.1002/jnr.23014
Kamynina A, Esteras N, Koroev DO et al (2021) Activation of RAGE leads to the release of glutamate from astrocytes and stimulates calcium signal in neurons. J Cell Physiol 236:6496–6506. https://doi.org/10.1002/jcp.30324
Chen Y, Qin C, Huang J et al (2020) The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif 53:1–13. https://doi.org/10.1111/cpr.12781
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503. https://doi.org/10.1016/j.redox.2018.01.008
Katsouri L, Blondrath K, Sastre M (2012) Peroxisome proliferator-activated receptor-γ cofactors in neurodegeneration. IUBMB Life 64:958–964. https://doi.org/10.1002/iub.1097
Mulica P, Grünewald A, Pereira SL (2021) Astrocyte-neuron metabolic crosstalk in neurodegeneration: a mitochondrial perspective. Front Endocrinol. https://doi.org/10.3389/fendo.2021.668517
Jeong Y, Son Y, Han N-K et al (2018) Impact of long-term RF-EMF on oxidative stress and neuroinflammation in aging brains of C57BL/6 mice. Int J Mol Sci 19:2103. https://doi.org/10.3390/ijms19072103
Aguilera G, Colín-González AL, Rangel-López E et al (2018) Redox signaling, neuroinflammation, and neurodegeneration. Antioxid Redox Signal 28:1626–1651. https://doi.org/10.1089/ars.2017.7099
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. https://doi.org/10.1016/j.tibs.2014.02.002
Sarubbo F, Esteban S, Miralles A, Moranta D (2018) Effects of resveratrol and other polyphenols on Sirt1: relevance to brain function during aging. Curr Neuropharmacol. https://doi.org/10.2174/1570159X15666170703113212
Zia A, Pourbagher-Shahri AM, Farkhondeh T, Samarghandian S (2021) Molecular and cellular pathways contributing to brain aging. Behav Brain Funct 17:6. https://doi.org/10.1186/s12993-021-00179-9
Hou CY, Tain YL, Yu HR, Huang LT (2019) The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci 20:1–15. https://doi.org/10.3390/ijms20030535
Acknowledgements
The authors of this study are supported by Universidade Federal do Rio Grande do Sul (UFRGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Funding
This work was supported by Universidade Federal do Rio Grande do Sul (UFRGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Contributions
VS, LDB, CLS, and MB performed the experiments. CAG, GL, and AQS provided resources and materials/chemicals. All authors contributed to the experimental design, interpretation of the results, and writing of the paper. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All animal experiments were performed by following per under the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and Brazil’s National Council for the Control of Animal Experimentation (CONCEA). The experimental protocols were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 35387).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sovrani, V., Bobermin, L.D., Santos, C.L. et al. Effects of long-term resveratrol treatment in hypothalamic astrocyte cultures from aged rats. Mol Cell Biochem 478, 1205–1216 (2023). https://doi.org/10.1007/s11010-022-04585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04585-z