Abstract
The Jimpy mouse illustrates the importance of interactions between astrocytes and oligodendrocytes. It has a mutation in Plp coding for proteolipid protein and DM20. Its behavior is normal at birth but from the age of ~2 weeks it shows severe convulsions associated with oligodendrocyte/myelination deficits and early death. A normally occurring increase in oxygen consumption by highly elevated K+ concentrations is absent in Jimpy brain slices and cultured astrocytes, reflecting that Plp at early embryonic stages affects common precursors as also shown by the ability of conditioned medium from normal astrocytes to counteract histological abnormalities. This metabolic response is now known to reflect opening of L-channels for Ca2+. The resulting deficiency in Ca2+ entry has many consequences, including lack of K+-stimulated glycogenolysis and release of gliotransmitter ATP. Lack of purinergic stimulation compromises oligodendrocyte survival and myelination and affects connexins and K+ channels. Mice lacking the oligodendrocytic connexins Cx32 and 47 show similar neurological dysfunction as Jimpy. This possibly reflects that K+ released by intermodal axonal Kv channels is transported underneath a loosened myelin sheath instead of reaching the extracellular space via connexin-mediated transport to oligodendrocytes, followed by release and astrocytic Na+,K+-ATPase-driven uptake with subsequent Kir4.1-facilitated release and neuronal uptake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amaral et al. [1] encouraged the scientific community to unravel oligodendrocyte interaction with astrocytes and neurons. In response to this challenge we have re-interpreted almost 40 year-old observations in developing Jimpy mice. These animals, which have a gene defect in the Plp gene coding for proteolipid protein (PLP) synthesis, appear to be normal at birth but are deficient in myelination, show greatly increased oligodendrocytic cell death and die by the age of 30 days, often following seizures. Skoff [2] suggested that they also suffer from astrocytic abnormalities. Our group [3–5] has found that astrocytes cultured from newborn Jimpy mice lack a specific astrocyte-characteristic feature, i.e., stimulation of oxidative metabolism by exposure to highly elevated (60 mM) extracellular concentrations of the potassium ion, K+ ([K+]o). However other studied functions, including K+ and glutamate uptake, were normal. At that time it was unknown which activities are reflected by stimulation of astrocytic O2 uptake by [K+]o exceeding 15 mM. However, it is now well established that it is a metabolic response to opening of L-channels for Ca2+. In addition more information about the Jimpy mouse has become available, especially regarding rescue of cultured Jimpy oligodendrocytes by astrocyte-conditioned medium. Relevant aspects of these newer studies on Jimpy mice will initially be reviewed (“The Jimpy Mouse” section). This will be followed by a summary of our previous published and non-published (except for G. Chaban’s thesis [4]) findings in cultured Jimpy astrocytes (“Increased K+ Concentrations Fail to Increase Oxygen Consumption in Jimpy Astrocytes but Other Functions are Normal” section), key points of current knowledge about consequences of L-channel activation in astrocytes (“O2 Uptake Stimulation by High [K+]o Reflects L-Channel Mediated Ca2+ Uptake Stimulating Glycogenolysis, ATP Release and NKCC1” section) and how failing glycogenolysis-dependent ATP release might affect oligodendrocyte maturation and myelination (“Release of Transmitter ATP from Axons and Astrocytes Promotes Oligodendrocyte Development and Myelination” section). It probably also affects formation of connexins and gap junctions, and “Knockout of Oligodendrocytic Connexins Causes Jimpy-like Symptoms” section describes oligodendrocytic and astrocytic connexins and how knockout of two oligodendrocytic connexins leads to virtually similar neurological dysfnction as that seen in Jimpy. Finally a hypothesis is presented how demyelination or connexin knockout may affect K+ homeostasis, in the latter case by compromising K+ transport from K+ after exit from the axon via internodal Kv channels (“Is K+ Homeostasis Compromised in JIMPY and Cx32/47 Knockouts?” section).
The Jimpy Mouse
Studies In Vivo
The Jimpy mouse has an X-linked recessive mutation in Plp, the gene coding for PLP and its shorter splice variant DM20 leading to dysmyelination and seizures. The number of oligodendrocytes in Jimpy is normal at birth and early postnatally, but oligodendrocytes fail to fully differentiate in the central nervous system and the mice die soon after the onset of a rudimentary myelination [6–9]. Neurological symptoms have repeatedly been reported to begin between postnatal days 9 and 15 [10–12]. Nave et al. [6] described a body tremor before motor activity, which appears about postnatal day 11, and 1 week later changes into convulsions. This is consistent with other authors [10, 11, 13, 14] reporting the first seizures between postnatal days 15 and 21. Most authors agree that death occurs around the age of 1 month but survival for up to 50 days has been reported [15]. The seizures can be elicited by handling of the animals, and Fig. 1 shows individual frames from a cinematographic recording of seizure development and recovery [4].
Selected pictures illustrating a 22 s seizure in a 20-day-old Jimpy mouse. From Chaban, 1980 [4]. For a movie of a seizure see website link: https://drive.google.com/file/d/0ByRmcg3mTqsedGRfNFNnN1M4SWs/view?usp=drive_web
The neurological symptoms appear at an ontogenetic stage when cerebral and cerebellar myelination is underway in normal mice [16]. They are associated with a very pronounced deficit in myelin formation in the central nervous system (CNS) combined with a drastic decrease in the number of oligodendrocytes [17–19]. Axonal diameters are reduced [20], whereas there is astroglial hypertrophy with an increased number of cell processes, compromising interaction with oligodendrocytes and axons [2, 18, 21]. In the optic nerve of 21-day-old Jimpy mice the ultrastructural appearance of axons and astrocyte cell bodies seems normal, some oligodendrocytes also look normal but others are filled with lipid inclusions, an indication of dying cells, and myelin is almost absent [22] (Fig. 2). However, impulse propagation occurs since the mice are not blind. More myelinated axons have been observed in spinal cord, but the myelin sheath is morphologically abnormal, with reduced tight contact with the axon [23, 24].
Electron micrographs of optic nerve cross sections from wild-type mouse (a) and Jimpy mutant that does not carry the Tabby mutation (b) shown at high magnification (scale bar 1 µm). The ultrastructural appearance of the axons in the Jimpy mouse seems normal, including the appearance of their mitochondria (M). However, in contrast to the wild type mouse most axons are not myelinated. Astrocytes (A) show normal morphology. From [22]
Myelination in the peripheral nervous system is normal, in agreement with differences between functional characteristics of oligodendrocytes and Schwann cells and of myelin in gray and white matter in normal animals [25, 26]. Normally the potential for regeneration of myelin after demyelination is also different, since white matter is able to regain full functionality after demyelination while cortical gray matter lesions cause permanently altered network function [27].
In the light of the severe symptomatology in Jimpy it is remarkable that several findings indicate that PLP or DM20 are not necessary for myelin formation. Even in the absence of Plp, mice can survive without gross behavioral effects in their first year and they have a fairly normal life span [28, 29], although the compaction of their myelin sheath is abnormal [30]. Different more or less severe neurological problems occur in humans lacking proteolipid protein [31]. For the understanding of the neurological defects in both mouse and man it is very important that PLP plays additional roles in neural development beyond functioning as a structural component of myelin [32]. Replacement in mice optic nerve of PLP with the peripheral nerve myelin protein, P0, leads to mitochondrial pathology and degeneration in the axoplasm of 1-month old mice, which are prominent in the juxtaparanodal region and associated with decreased ATP content [33]. This is different from the normal mitochondrial function in Jimpy astrocytes shown by Best et al. [21]. Moreover, widespread expression of transcripts encoding PLP and DM20 is found during embryonic and early postnatal CNS development, and specificity to the oligodendrocyte cell lineage develops only at later postnatal stages during myelination [34, 35]. PLP is expressed at least 1 week before myelination and its injection before postnatal day 2 leads to secretion of a factor that increases the proliferation of not only oligodendrocyte but also astrocyte lineage cells [36]. In its absence some small-diameter axons lack a compact sheath or show delayed myelination, suggesting that PLP/DM20 is involved in early stages of axon-oligodendrocyte interaction and wrapping of the axon [37]. Jimpy mice show cell cycle abnormalities in both oligodendrocytes and astrocytes and there is a large increase in microglia [34, 38, 39].
In spite of the abnormalities in animals with lack of PLP and a null mutation of its gene they behave much more normally than animals with extra Plp gene copies or missense mutations [40]. Duplication of Plp in rodents disable many functions, and in humans PLP overexpression is the most common etiology of the debilitating Pelizaeus-Merzbacher disease [32, 41]. The overexpression is associated with mitochondrial dysfunction [40], whereas oligodendrocytes cultured from brains of Jimpy mice have normal mitochondrial function [21, 42].
Cultured Oligodendrocytes
Most if not all characteristics of Jimpy oligodendrocytes are maintained in brain oligodendrocytes cultured in conventional ways, including protein supplementation and a low glucose concentration [43–45]. Cell numbers are normal in the cultures and the cells stain for typical glycolipids [9, 43]. However, they fail to develop extensive process networks or large myelin-like membrane sheets or substantial amounts of myelin proteins [15, 45], and only little myelin basic protein (MBP) can be detected [46, 47]. These deficiences are ameliorated when Jimpy oligodendrocytes are grown in medium conditioned by normal astrocytes or by DM20 transfected NIH3T3 cells. When grown in the conditioned media the oligodendrocytes produce myelin-like membranes and MBP and some cells express PLP [8, 43, 45], which is of the Jimpy type [44]. This is consistent with the relative lack of adverse effects of PLP deletion seen in vivo, although Williams and Gard [48] using less traditional procedures for culturing of both oligodendrocytes and astrocytes did nor replicate the beneficial effect of conditioned media. In addition cultured Jimpy oligodendrocytes as well as oligodendrocytes isolated directly from Jimpy brain show an approximately doubling of free cytosolic Ca2+ concentration ([Ca2+]i) [45]. This abnormality is not counteracted by conditioned medium from DM20 transfected NIH3T3 cells and astrocytic [Ca2+]i is not affected.
Increased K+ Concentrations Fail to Increase Oxygen Consumption in Jimpy Astrocytes but Other Functions are Normal
Keen et al. [49] found that the stimulation of oxygen uptake which normally occurs as a result of exposure to high concentrations of potassium ion (K+) is almost absent in brain slices from Jimpy mice. We confirmed this observation [3, 4] and showed that the effect could be reproduced in cultured Jimpy astrocytes which were compared with cultures of their non-affected littermates (Fig. 3). The cells were prepared from new-born animals without use of enzymes and grown in uncoated 30-mm Falcon Petri dishes in a medium very similar to a low glucose (6.5 mM), bicarbonate-buffered Dulbecco’s medium with addition of horse serum and from the age of 2 weeks also dibutyryl cyclic AMP (dBcAMP) [50, 51]. This compound substitutes for the noradrenergic stimulation the cells would have received in vivo. The cultures were at least 3 week-old when used and we never use subculturing. Such cultures from normal mice are with a few exceptions similar to astrocytes freshly isolated from the brain [52]. Ninety-five percent of the cells stain for the astrocytic markers GFAP and glutamine synthetase, neurons are absent, an occasional type 2 astrocyte is rarely seen and macrophages constitute 3% [50, 51]. Both the littermates and Jimpy astrocytes show a pronounced extension of processes in response to treatment with dBcAMP [4] (Fig. 4). This is in contast to oligodendrocytes from Jimpy mice which do not respond to dBcAMP with process extension, although normal oligodendrocyte cultures do extend processes, although of different morphology than the astrocytic processes [53].
Cumulative oxygen uptake by mouse astrocytes from Jimpy animals (left side) and normal littermates (right side) during 3 h of incubation in a HEPES-buffered modified MEM containing 5 mM K+ (circles) or 55 mM K+ (triangles). Results are means of 5–6 experiments, using an O2 electrode inserted into a culture flask filled completely with medium. S.E.M. are indicated by vertical bars, and the results are expressed per 100 mg protein, i.e., approximately 1 g wet weight. Note the initial stimulation by high K+ in control but not in Jimpy cultures. The lowest curve shows the lack of apparent respiration by flasks with medium but no cells (n = 4). Another electrode did show a small drift which was subtracted from the respiratory rates. For graphical reasons, some S.E.M. values are indicated in one direction only. From [3]
Phase contrast micrographs of three-week old astrocyte cultures grown as described in the text and but without (a, c) or with (b, d) dBcAMP addition during the third week. The medium was removed and the cultures fixed with absolute methanol. a, b are from non-Jimpy littermates and c, d from Jimpy mice. Note the homogeneity of the cultures and the similarity between Jimpy mice and their littermates and that the addition of dBcAMP in both cases causes a morphological differentiation from closely packed cells of epithelial appearance to astrocyte-like cells expressing a dense network of processes. This morphological effect of dBcAMP is accompanied by functional differentiation as shown by stimulation of the Na+, K+-ATPase activity (Table 1). The bar represents 30 µm. From [4]
Oxygen uptakes were measured as previously described [54] in at least 4-week-old cultures by aid of an oxygen electrode in the closed flask filled with medium so that no air space functioning as an oxygen reservoir was present. The flask was closed with its usual stopper through which two small holes had been pierced, and placed in a water bath at 37 °C. A needle tip oxygen microelectrode and a reference electrode were inserted through the holes which subsequently were closed with melted beeswax. The signal from the electrodes was amplified and recorded on a Grass recorder, and respiratory rates were calculated. The medium was prepared with 20 mM Hepes (pH 7.3) instead of bicarbonate (which disturbs polarographic measurements due to deposit on the electrode). However, previous experiments with brain slices have shown little difference between oxidative metabolism in media with and without bicarbonate/CO2 [55, 56].
Since the Jimpy mutation is recessive, only half of the offspring will express the Jimpy gene (jp) but new-born animals will not show any signs of the condition. A marker gene for Tabby, ta also known as eda, localized on the same chromosome as jp, was therefore used to identify new-born Jimpy animals. A stock of tajp females was obtained from the Jackson Laboratories, Bar Harbor, Maine and mated with normal C-57 BL males. At the day of birth, the newborn males were separated into groups of Tabby and of non-Tabby animals, based on the presence of one supraorbital vibrissa and no postorbital vibrissa or of two supraorbital vibrissae and one postorbital vibrissa, respectively [57]. Separate batches of cultures were prepared from the Tabby (and Jimpy) and the non-Tabby (and non-Jimpy) animals. Both types of cultures were prepared and grown in the ususal manner. No detailed morphological/histochemical examination was performed, but the overall appearance of the cultures was similar to those grown from normal animals [4].
Rates of Oxygen Uptake During Exposure to 5 and to 55 mM K+
In the medium with 5 mM K+ the rates of oxygen uptake (indicated by the steepness of the graph showing cumulative oxygen consumption) by cells from Jimpy mice (Fig. 3, left side) was initially similar to previous observations in normal cultured astrocytes [54]. The rate of oxygen uptake was fastest (i.e. the slope steepest) at the beginning of the experiment (~250 µmol/hour/100 mg protein) and subsequently showed a small decline, again similar to results from normal astrocytes [54]. In the normal littermates (Fig. 3, right side) the rate of oygen uptake appeared initially to be lower than in the Jimpy animals (and than that previously observed in normal mice [54]) but the difference was not statistically significant, and since the subsequent reduction in respiratory rate was less than in the Jimpy mice the cumulative oxygen uptakes were identical after 3 h in the Jimpy mice and their littermates. In the presence of the high concentration of K+, added to the normal medium, the rate of oxygen uptake by astrocytes from normal littermates (Fig. 3, right side) was initially almost doubled, a statistically significant difference (P < 0.05), but this increase was only maintained for about 1 h. This course is similar to previous observations in normal cultured astrocytes [54] and in brain slices [55], where K+-induced stimulation of metabolism is known to be mainly glial [58]. In contrast, no initial increase was found in the cultures from Jimpy mice (Fig. 3, left side). There was also no subsequent marked decline, so in the long run (3 h) the cumulative oxygen uptake was only slightly lower that in normal littermates in a medium with 5 mM K+. Thus, at normal extracellular K+ ([K+]o) oxidative metabolism is almost identical in Jimpy and non-Jimpy cultures, confirming the normal mitochondrial function in Jimpy mice [21, 22, 42], but the normal transient stimulation by excess [K+]o is absent.
K+ Uptake Rate as a Function of [K+]o and Na+, K+-ATPase Activity
In spite of the absence of any stimulation of O2 uptake by highly elevated [K+]o Fig. 5 shows that the uptake of K+ was similar in Jimpy and non-Jimpy cultures across the entire concentration range between 2.5 and 60 mM [K+]o [4, 5]. As will be discussed in more detail in “O2 Uptake Stimulation by High [K+]o Reflects L-Channel Mediated Ca2+ Uptake Stimulating Glycogenolysis, ATP Release and NKCC1” section, minor increases in [K+]o (up to 10 mM) selectively stimulate the Na+,K+-ATPase, whereas larger increases in addition stimulate NKCC1 [59, 60], a co-transporter of Na+, K+, 2 Cl− and water [61–64]. The Na+,K+-ATPase activity was identical in Jimpy and control astrocytes, and in both it was higher in cultures treated with dBcAMP than in untreated cultures (Table 1). The lack of stimulation of O2 uptake by highly elevated [K+]o indicates that NKCC1 in Jimpy mice is not activated by elevated [K+]o (see “O2 Uptake Stimulation by High [K+]o Reflects L-Channel Mediated Ca2+ Uptake Stimulating Glycogenolysis, ATP Release and NKCC1” section) but this transporter is also stimulated by extracellular hypertonicity [65–70]. Since the high-K+ medium was prepared by addition of excess KCl to a normal, isotonic medium NKCC1 stimulation by hypertonicity probably explains the normal K+ uptake at highly elevated [K+]o.
Rate of K+ uptake (µmol/min per 100 mg protein), measured with 42K during a 30 s. period, into cultured astrocytes from Jimpy animals (open circles) or normal littermates (filled circles) as a function of the external K+ concentration. Since the cultures had been depleted of K+ by incubation in ice-cold K+ free medium before the uptake measurement in order to prevent homoexchange with intracellular K+ the uptakes represent active uptake rates, which up to an extracellular K+ concentration of 10 mM are mediated exclusively by the Na+, K+-ATPase and at higher K+ concentrations jointly by the Na+, K+-ATPase and NKCC1 [59]. Since high K+ concentrations are unlikely to stimulate NKCC1 in the Jimpy mice, it is probably stimulated by hypertonicity in the K+-rich media, which were made by addition of KCl to the isotonic medium. Results are means ± SEM of 3–5 individual experiments. For further details, including statistical tests, see [4]
Glutamate and GABA Uptake, Glutaminase Activity and Contents of Glutamate and Glutamine
Uptake of glutamate (Fig. 6) was identical in Jimpy and control cultures [4] and similar to previously measured uptakes in normal astrocytes [71]. In both types of cultures it was higher at [K+]o of 3–20 mM than in the absence of K+ [4], suggesting similar K+ permeability and pH regulation [72]. The uptake velocity of GABA (measured at 50 mM) was also normal [4]. The contents of glutamate and glutamine showed also no significant difference (Table 2). There was no decrease in glutamine synthetase activity, consistent with a minimal effect on glutamate/glutamine labeling from acetate in Jimpy mice [49].
Rate of glutamate uptake (µmol/min per 100 mg protein), measured with L-[14C]glutamate during a 2 min. period, into cultured astrocytes from Jimpy animals (open circles) or normal littermates (filled circles) as a function of the external glutamate conentration. Since glutamate homoexchange is insignificant [71] no preincubation in glutamate-free medium was performed. Results are means ± SEM of 4–16 individual experiments. For further details, including statistical tests, see [4]
O2 Uptake Stimulation by High [K+]o Reflects L-Channel Mediated Ca2+ Uptake Stimulating Glycogenolysis, ATP Release and NKCC1
An increase in [K+]o is a hallmark of neuronal excitation and it is evoked by several different mechanisms, including action potential generation (reviewed in [60]). The significance of most effects of highly elevated [K+]o on astrocytes were unknown when the effect of elevated [K+]o on oxidative metabolism in Jimpy mice were studied, but are a key to the interpretation of this phenomenon. Overwhelming evidence indicates that elevated [K+]o at concentrations up to ~10 mM is initially accumulated by the K+-stimulated astrocytic Na+,K+-ATPase [64, 73–79] and subsequently released by Kir4.1 channels [76] over a wider area, securing little or no elevation of [K+]o and allowing neuronal re-uptake [60]. Although this uptake can be fueled by oxidative metabolism of pyruvate [80], the rate of lactate production in brain slices [81] and cultured astrocytes [82] is high due to exit of lactate to the large surplus of medium and consequent abolition of the normal feed-back inhibition by pyruvate. A small increase in this glycolysis suffices to support Na+,K+-ATPase-activated K+ uptake and there is no significant stimulation of rate of oxygen uptake in either brain slices or cultured astrocytes until [K+]o becomes high enough to also stimulate the Na+, K+, 2 Cl− and water cotransporter NKCC1.
This cotranporter [61–64], which is metabolically driven by Na+,K+-ATPase-created ion gradients [67], is stimulated in cultured astrocytes by [K+]o ≥15 mM [59, 60] and in brain slices by somewhat higher [K+]o [55, 56, 83]. Expression of NKCC1 or its gene Slc25A12 has been shown in cultured astrocytes [84] and oligodendrocytes [85–87] and in astrocytes in intact brain tissue by Kanaka et al. [88] and MacVicar et al. [89]. mRNA and protein expression of NKCC1 has been determined in astrocytes of the dorsal root and trigeminal ganglia of the rat [90].
That the K+-induced stimulation of O2 uptake in brain slices reflects stimulation of NKCC1 is shown by its inhibition by the non-specific NKCC1 inhibitor ethacrynic acid [91]. Stimulation by highly elevated [K+]o develops between postnatal day 10 and 15 [92], i.e., at the same period when Jimpy mice begin to show their neurological symptoms. This phenomenon has also been observed in microdissected cat astrocytes [93] and in cultured rat and mouse astrocytes [54, 94]. The K+-mediated simulation of O2 uptake in brain slices is abolished in the absence of Ca2+ in the medium [55].
The reason why the K+-stimulated O2 uptake in brain slices requires the presence of Ca2+ is that the stimulation of NKCC1 by elevated [K+]o is due to depolarization-mediated activation of L-channel-mediated Ca2+ uptake. It is often believed that astrocytes have no functional L-channels, partly because Carmignoto et al. [95] reported the absence of voltage-dependent Ca2+ channels in astrocytes in brain slices. However these authors used 2 mM lactate as the only metabolic substrate, and L-channel activity depends upon metabolism [96], in cultured astrocytes specifically degradation of glycogen [59], which is only slowly formed from lactate. Other authors have demonstrated Ca2+ channel activity in retinal glial cells [97], and Duffy and MacVicar [98] described an increase in [Ca2+]i in hippocampal astrocytes in situ which was inhibited by the L-channel blocker verapamil. Finally, mRNA for the L-channels Ca v 1.2 and Ca v 1.3 are expressed both in cultured and freshly isolated mouse astrocytes [99, 100].
In cultured astrocytic depolarization by highly elevated [K+]o leads to nimodipine-inhibited opening of L-channels for Ca2+ [101] (Fig. 7a, b). This activates a signaling pathway that includes transactivation [102–105] of the epidermal growth factor (EGF) receptor and eventually leads to phosphorylation and activation of NKCC1 and astrocytic swelling [106, 107] (Fig. 7c). It is unknown if the astrocytic ion and water changes or the accompanying alterations in the extracellular fluid affect oligodendrocytes or myelination. The growth factor released by K+-induced depolarization is heparin-binding epidermal growth factor (HB-EGF), as shown by ELISA. It is released from its membrane-bound precursor by the matrix metalloproteinase (MMP) ADAM17, and this process is prevented by the metalloproteinase inhibitor GM6001 and by siRNA against ADAM17 [103].
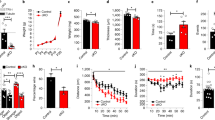
a Uptake of 45Ca into normal astrocytes in primary cultures as a function of time at 37 °C at the normal extracellular K+concentration of 5.4 mM (open squares) or at 60 mM extracellular K+ (filled diamonds) shown as means ± SEM. 45Ca (0.5 µCi) had been added to 1 ml serum-free medium 60 s before the increase in K+ concentration. b Effect of addition 1 h before the measurements of nimodipine, a blocker of L-channels for Ca2+, on unstimulated 45Ca uptake (5.4 mM [K+]o, filled diamonds) and K+-stimulated 45Ca uptake (60 mM K+, open squares) into cultured astrocytes shown as means ± SEM. Note that only the latter is strongly and potently inhibited. c Diagram showing signaling pathways towards ERK1/2 phosphorylation activated by elevation of the extracellular K+ concentration and inhibition of this pathway by specific inhibitors (ovals). Elevation of the extracellular K+ concentration above 10–15 mM depolarizes the cell membrane sufficiently to lead to depolarization-mediated Ca2+ entry through voltage-dependent L-channels. This effect increases with the magnitude of the increase in [K+]o for which reason 60 mM [K+]o was used in this study. The increase in [Ca2+]i is necessary for ERK1/2 phosphorylation, as indicated by its inhibition by BAPTA-AM. Although not shown in this Fig. the [Ca2+]i increase is essential for glycogenolysis (see text) and for release of gliotransmitter ATP (Fig. 8). The increase in [Ca2+]i also leads to a Src-dependent (and PP1-inhibited), release of the growth factor heparin-binding epidermal growth factor (HB-EGF). The released HB-EGF activates (phosphorylates) the EGF receptor (inhibited by AG1478), leading to activation of the MAP kinase cascade, Ras (inhibited by bumetanide), Raf and MEK (inhibited by U0126), with activation of MEK causing phosphorylation of extracellular regulated kinases 1 and 2 (ERK1/2). ERK1/2 phosphorylation is a major signaling event in CNS myelination [131], and it is also stimulated by astrocytic P2Y stimulation by ATP, which activates a pathway similar, but not identical to that shown in this Fig. [128]. ERK1/2 activation also activates (phosphorylates) the cotransporter NKCC1 through pathways that were not studied and are only partly known. This leads to astrocytic influx of Na+ and K+ together with 2 Cl− and water a, b from [101]; c from [60]
Release of gliotransmitter ATP was determined as relative light units (RLU) measured by a luciferin/luciferase technique in cultured astrocytes during incubation for 60 min in glucose-containing (7.5 mM) Dulbecco’s medium supplemented with the ecto-ATPase inhibitor ARL67156 to prevent degradation of released ATP. Addition of 10 mM K+ alone (to a final level of 17.5 mM) or the combined addition of K+ and GABA caused a significant increase in ATP release, although to a smaller degree than higher, more depolarizing K+ concentrations (shown in [114]). This increase was abolished in the additional presence of the L-channel inhibitor nifedipine. The glycogenolysis inhibitor 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) also inhibited K+-induced ATP release (shown in [114]). RLU values are only shown above 1200 RLU because this level was reached also under control conditions. Results are the averages of RLU values from three to five individual cultures. S.E.M. values are indicated by vertical bars. *Statistically significant (P < 0.05) difference from all other groups, but not from each other. From [114]
Figure 7c also shows that the pathway includes an increase in [Ca2+]i and it therefore stimulates glycogenolysis, an effect that is inhibited by nifedipine at high [K+]o, but not at low [K+]o [108, 109]. This is important because glycogenolysis is required for a multitude of astrocytic processes and signaling functions [110–113], including K+-stimulated release of transmitter ATP [114, 115]. Addition of 25 mM K+ to brain slices also causes Ca2+-dependent release of ATP, which mainly is astrocytic as shown by a drastic reduction of the release in the presence of fluoroacetate [116], a mitochondrial astrocyte-specific inhibitor. However, beyond (or perhaps because of) its dependence on glycolysis, K+-induced release of ATP in cultured astrocytes is also dependent on L-channel opening as seen by its abolishment by the L-channel inhibitor nifedipine (Fig. 8). Glycogenolytically generated lactate is released to the extracellular space where it stimulates neuronal signaling [110, 117–119].
It has been disputed whether the increases in [K+]o during normal neuronal stimulation in brain slices are high enough to activate NKCC1 [77]. However, the increase in [K+]o in the narrow space between astrocytes or either neurons or oligodendrocytes might be higher than those generally measured. Moreover, transmitter-mediated inhibition of gap junction coupling may facilitate K+-mediated L-channel opening [60]. This concept is supported by an activity-dependent decrease in extracellular space in rat optic nerve, which paralleled the increase in [K+]o, and ontogenetically developed pari passu with the appearance of glia cells [120]. A comparison between the ontogenetic development of the extracellular shrinkage and the development of astrocytes and oligodendrocytes suggested that swelling of both of these two glia cell types (both of which express NKCC1) may be responsible for the reduced extracellular space.
It is in further support of a physiological role of depolarization-mediated opening of L-channels for Ca2+ that 2 weeks of fluoxetine treatment increases Cav1.2 expression not only in cultured astrocytes but also in astrocytes in brain [100]. This would probably not have been the case if the channel was not operating. Cav1.2 mRNA is also up-regulated in hyperammonemic mice [121], consistent with the ability of NH4 + to potently stimulate NKCC1 via opening of L-channels.
Release of Transmitter ATP from Axons and Astrocytes Promotes Oligodendrocyte Development and myelination
The only demonstrated metabolic defect in Jimpy astrocytes was the absence of stimulation of oxygen uptake by highly elevated [K+]o, which as discussed above is an indication of lack of L-channel-mediated Ca2+ uptake. In turn this will abolish the normal stimulation of glycogenolysis and of ATP release. In the isolated optic nerve action potentials trigger release of ATP, which acts on astrocytic purinergic receptors to further enhance ATP release and to initiate intercellular Ca2+ waves to neighboring glia, including oligodendrocytes and NG2 cells [122, 123]. Combined axonal/astrocytic release of ATP activates oligodendrocytic purinergic receptors and regulates myelination [124–127]. In both astrocytes and oligodendrocytes moderate quantities of ATP act predominantly via the G protein coupled P2Y purinergic receptors. In cultured spinal astrocytes this causes a rise in [Ca2+]i (Fig. 9) through IP3-dependent release of Ca2+ from intracellular stores with further growth factor-mediated down-stream activation of extracellular-regulated kinases 1 and 2 (ERK1/2) [128]. This process reminds of that shown in Fig. 7c, a rather similar pathway also stimulates DNA synthesis in retinal Müller cells although the metalloproteinase is MMP 9 [129]. Growth factor formation may also be essential for oligodendrogenesis and myelination. Thus, in preterm infants intraventricular hemorrhage causes a decline in epidermal growth factor (EGF) and myelination, and in a rabbit model of this condition recombinant human EGF enhances oligodendrocytic proliferation and maturation, myelination and neurological recovery [130]. This may reflect that ERK1/2 phosphorylation is a major signaling event in CNS myelination [131]. It is also consistent with the conclusion that P2Y stimulation generally has beneficial effects on oligodendrocytic maturation and survival and on myelination [127]. Many of the ionotropic P2X receptors are activated at low ATP concentrations, but P2X7 receptors are stimulated at high concentrations of ATP, resulting in sustained influx of Ca2+ which has adverse effects [127].
Proposed signal transduction pathway activated by extracellular ATP in spinal cord astrocytes. ATP binds to the P2Y1 purine receptor, which increases [Ca2+]i and causes phosphorylation of the EGF receptor by a process reminiscent of that shown in Fig. 7c. Activation of the EGF receptor stimulates phosphorylation of ERK1/2 and Akt and stimulation of cPLA2, leading to release of arachidonic acid, all events that seem important for oligodendrocytic proliferation and survival and myelination. Selective inhibitors used to establish the pathway are shown by —|. From [128]
Xia and Zhu [128] also showed that ATP stimulates phospholipase A2 (Fig. 9), which releases arachidonic acid, a component of myelin [132] and that it activates Akt, which in turn decreases phosphorylation of glycogen synthase kinase β (GSKβ) [133]. Akt has many known down-stream effects in oligodendrocytes [76] and a decrease in GSKβ would also be beneficial, since Azim and Butt [134], showed that knock-out of cyclin-dependent kinase 5 (Cdk5) impairs myelin repair by reducing signaling through the Akt pathway which will enhance GSK3β signaling [135]. Azim and Butt [134] investigated effects of GSK3β inhibition on oligodendrocyte development from their precursors in vivo by injection into the lateral ventricle of post-natal mice and in organotypic cultures of intact optic nerve. Their results showed that each of the GSK3β inhibitors ARA-014418, indirubin, L803-mt and Li+ increased oligodendrocyte precursor cells and oligodendrocytes and promoted myelination. Since intact tissue was used it can not be excluded that the target of the inhibitors included astrocytes, where GSKβ inhibition would stimulate glycogen synthesis [136, 137], facilitating ATP release. Absence of β2-adrenergic receptors in white matter astrocytes in multiple sclerosis patients [138] might also affect myelination by inadequate glycogenolysis (although most evidence suggests that glycogenolysis in brain is mediated by stimulation of β1-adrenergic receptors [108, 109]. Reduced glycogenolysis would diminish glycogen-derived increases in extracellular lactate and the associated signaling.
Another major reason for the beneficial effect of ATP on oligodendrocytes and myelination is probably that it greatly increases the expression of mRNA for leukemia inhibitory factor (LIF) in cultured astrocytes [139, 140]. Secretion of LIF from astrocytes is also stimulated in response to transmitter ATP released from axons during firing of action potentials in an intricate communication between axons and myelinating glial cells [139, 141]. Oligodendrocyte precursor co-culturing with astrocytes similarly enhances expression of LIF [142], and LIF−/− mice have a pronounced defect in oligodendrocyte maturation and myelination, with strongly reduced PLP and MBP contents in optic nerve at postnatal day 10 [143]. One mechanism for the favourable effect of LIF is that it induces the neuropeptide galanin which is a survival factor in oligodendrocytes [144].
Knockout of Oligodendrocytic Connexins Causes Jimpy-like Symptoms
Treatment of mouse embryonic stem with LIF together with bone-morphogenic protein-2 (BMP-2) causes a large increase in Cx43 expression in cardiomyocytes [145]. It is unknown if this also applies to astrocytes and/or oligodendrocytes and to different connexins. However, a symptomatology strikingly similar to that in Jimpy mice, except that the mice may live slightly longer, has been found in mice with double knockout of of the oligodendrocytic connexins Cx32 and Cx47. The knockout causes early CNS demyelination and oligodendrocytic death [146, 147]. Vacuoles develop in these mice, mainly in the periaxonal space. They are exacerbated by increased activity, but greatly reduced by tetrodotoxin (TTX) and separate the axons from their myelin sheaths [146, 147]. The dysmyelination and formation of vacuoles is accompanied by the development of a progressive tremor during the third postnatal week followed by tonic seizures, which increase until the animals die, typically during the sixth postnatal week [148, 149]. Menichella et al. suggested that the vacuoles (and thus perhaps also the seizures) reflected pathological accumulation of ions and H2O which the mutant mice are unable to redistribute [148]. They also pointed out that vacuolated myelin similarly has been reported in mice lacking the K+ channel Kir4.1 [150], which is expressed in both oligodendrocytes and astrocytes. They therefore performed an ultrastructural examination in P9 or P10 of Kir4.1−/− mice (which die soon thereafter and thus do not live long enough to show neurological dysfunction), which showed abundant vacuoles in the spinal cord white matter. However, in constrast to the periaxonal localization of the vacuoles in the Cx32/47 double knockouts these vacuoles were generally located on the outside of the myelin sheaths, showing that ions and water had been able to penetrate the myelins sheath but not get any further. Based on these results Menichella et al. [148] proposed a pathway that included oligodendrocyte-astrocyte gap junctions which would be interrupted in the mutants and concluded that oligodendrocytes and their connexins have a critical role in K+ homeostasis in myelinated neurons. To assess this conclusion astrocytic and oligodendrocytic connexins will be described in some detail and they are illustrated in Fig. 10.
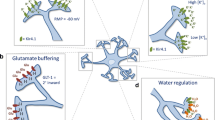
Schematic illustration of proposed involvement of K+ channels, connexins and Na+, K+- ATPase in myelin, oligodendrocytes and astrocytes in K+ homeostasis in normal myelinated neurons, with the exception that oligodendrocyte-astrocyte connection might be slightly more flexible than indicated, and that K2P channels might also play a role. The axon is depolarised by Na+ entry at one node of Ranvier (black arrow). Gradual repolarisation occurs by K+ release via Kv channels under the compacted myelin sheath of the internode (black arrows), a localication which in the CNS may be especially pronounced in the juxtaparanodal region. Released K+ is carried by Cx29 (red arrows) into oligodendrocytes and possibly redistributed into several oligodendrocytes by Cx32 and 47 (red arrows). K+ is transported in or out of oligodendrocytes by Kir channels (green arrows) and it is also released by Cx32 hemichannels, leading to an increase in [K+]o with subsequent uptake into astrocytes by the astrocytic Na+,K+- ATPase (blue arrows), which is stimulated by elevated [K+]o. Redistribution between additional astrocytes occurs via Cx30 and 43. Subsequently accumulated K+ is released from astrocytes via Kir4.1 and due to the redistribution over a larger area [K+]o may not become significantly reduced, furthering re-uptake by the neuronal (axonal) Na+, K+-ATPase (brown arrows), which is fully activated at normal [K+]o. A partly similar pathway has been suggested by Menichella et al. [148], but they suggested Cx-mediated K+ transfer from oligodendrocytes to astrocytes. For reasons indicated in the text we do not believe such a net transport occurs. The oligodendrocytic Cx-mediated K+ release via Kir channels or Cx hemichannels, followed by astrocytic Na+, K+-ATPase-mediated accumulation explains the lack of K+ efflux at the node of Ranvier. It is also consistent with findings by Ransom et al. [73] that K+ released from the optic nerve is initially accumulated by astrocytes and secondarily by the axon, and the subsequent release via Kir4.1 channels is consistent with results by Bay and Butt [76]. In Cx 32/47 knockouts oligodendrocytic redistribution and hemichannel-mediated K+ release is impaired, leading to loosening of myelin and K+ diffusion along the axon and probably also prevention of saltatory impulse propagation. Approximately similar consequences can be expected in Jimpy mice due to failing myelination (Fig. 2) or lack of a compacted myelin sheath
It has long been known that oligodendrocytes express gap junctions which connect them with astrocytes [151–153]. These early authors did not show gap junctions between oligodendrocytes but intensive inter-oligodendrocytic coupling has later been found to occur via Cx47:Cx47 and Cx32:Cx32 homotypic gap junctions [154, 155]. At least Cx32 also forms hemichannels [156] which may allow exit of cytosolic molecules, possibly including K+. In cerebellum of the double Cx47 and Cx32 knockouts, Wasseff and Scherer [157] found increased expression of 348 genes together with decreased expression of 23 genes, many of them oligodendrocytic and two involved in synthesis of myelin lipids. On the other hand co-culture of oligodendrocyte precursor cells with astrocytes induces upregulation or induction of ~50 genes related to myelination, including enhancement of the function of Cx 29 and Cx47 [142].
The main connexin in astrocytes is Cx43 but they also express Cx30 [158–160] together with several other connexins [156], and coupling between astrocytes is mediated by homotypic channels (e.g., Cx43/Cx43 and Cx30/Cx30) [161–163]. Very efficient gap junctions between astrocytes and oligodendrocytes are formed by Cx47/Cx43, whereas transport through Cx32/Cx30 gap junctions is somewhat more restricted, and other Cx combinations do not form gap junctions between the two glia cell types [164, 165]. Somewhat larger variability was found in HeLa cells expressing glial connexins, where Cx47 forms heterotypic channels with astrocyte Cx43 or Cx30, whereas Cx32 can functionally interact with astrocyte Cx30 or Cx26 but not Cx43 [166].
If the reason for the severe seizure activity of mice with double knockout of the oligodendrocytic Cx32 and Cx47 were failing communication between oligodendrocytes and astrocytes via these connexins, double knockout of the astrocytic Cx43 and Cx30 could be expected to have a similar effect, unless their function could be taken over by Cx26. However, in spite of vacuoles in the oligodendrocytes, which were interpreted as possibly due to decreased K+ buffering, such mice did not convulse or die early, although they were impaired in balance and spatial memory tasks [167]. This rather mild symptomatology makes it unlikely that the reason for the severe Jimpy-like symptoms in the Cx32/47 knockouts should be an interruption of K+ trafficking from oligodendrocytes to astrocytes via gap junctions as suggested in [148]. Moreover, K+ uptake, measured by uptake of 42K is much slower in cultured oligodendrocytes than in cultured astrocytes, and so is the final level of 42K reached, an indication that the intracellular K+ concentration also may be much lower than that in astrocytes [168]. If this also is the case in vivo there can be no net transport of K+ from oligodendrocytes to astrocytes via gap junctions. Surprisingly there was also very limited gray matter pathology as a result of the knock-out of the astrocytic connexins, possibly due to the expression of additional connexins in astrocytes, which may have been upregulated.
Cx29 is expressed in oligodendrocytes [149, 164] as well as in myelin throughout the brain and spinal cord [169] where antibody labeling shows its location at internodal and juxtaparanodal regions of small myelin sheaths [170]. Immunofluorescence for Cx29 shows minute puncta within oligodendrocytes and around their periphery. The labeling for Cx29 in oligodendrocyte somata is intense at young ages but it is dramatically shifted to be localized primarily in myelinated fibers in the mature CNS. Cx32 labeling is also localized to oligodendrocyte somata and myelin, but Cx29 and Cx32 are minimally co-localized on oligodendrocytes somata although partly co-localized along myelinated fibers [171]. Ahn et al. also reported that Cx29 does not form gap junctions with other connexins in oligodendrocytes [172] in spite of the oligodendrocytic expression of Cx32 and CX47. In a peripheral nerve Rash et al. [173] found that Cx29 in the juxtaparanodal myelin collars and along the inner mesaxon is precisely aligned with and ultrastructurally coupled in a 1:1 ratio to internodal Kv channels. From these findings [170, 171, 173] it can be concluded that molecules carried from myelin may end up in the oligodendrocytic cytoplasm. This may include K+ released via internodal Kv channels because connexins mediate K+ flux [174].
Is K+ Homeostasis Compromised in Jimpy and Cx32/47 Knockouts?
ATP signalling regulates K+ channels (Arthur Butt, Portmouth University website), which interact with connexins and with the Na+,K+-ATPase in K+ transport between glia cells and extracellular fluid [60, 76, 77]. Wallraff et al. [175] examined changes in K+ homeostasis in hippocampal slices of mouse astrocytes, which were deficient in astrocytic gap junction coupling due to a double knockout of connexins 43 and 30. The knockout increased [K+]o during intense neuronal firing (in some case above the normal ceiling of 12 mM), reduced the threshold for generation of epileptiform events, and caused decreased K+ clearance, especially via the fast component of the K+ uptake. This component has been shown [73] to reflect active uptake into astrocytes, but since the accumulated K+ normally is re-released by Kir4.1 channels [76], possibly after Cx-mediated redistribution [60] it may well be affected by Cx knockout. That even small elevations of [K+]o in hippocampus have epileptogenic effect has repeatedly been observed [176–179]. Potential effects exerted on myelinated nerves or glia cells were not discussed, but Hubbard and Binder [180] have reviewed astrocytic roles in epilepsy, and we have recently discussed roles of astrocytes in the adult brain in K+ homeostasis [60].
Here we will consider possible functions of connexins and K+ channels in myelinated axons and oligodendrocytes and their interaction with Na+,K+-ATPases for Na+/K+ homeostasis, which accounts for a large fraction of brain energy metabolism. Since temporally Jimpy pathology is restricted to the last 3 weeks of the first postnatal month it is important that brain oxygen consumption in all species increases with development [181] and in the rat (which developmentally is rather similar to the mouse) increases 2–3 times between postnatal days 10 and 20. This explains why myelination, which enables saltatory impulse propagation that energetically is much less costly than the contiguous impulse propagation in non-myelinated axons develops postnatally. It is probably also the reason why Jimpy animals show no symptoms during the first 1–2 weeks of life, but develop seizures at a time when myelination is abnormal due to failing myelination and/or compaction of their myelin sheath [30] (Fig. 2). As a result, contiguous impulse propagation probably continues with large passive of Na+ entry and K+ exit and subsequent costly energetic reversal of the passive ion fluxes by the Na+,K+-ATPase which develops with a similar pattern as oxygen consumption [181]. Since astrocytes are relatively scarce in white matter [182], and the initial removal of increased extracellular K+ is astrocytic [60] due to K+-induced stimulation of the astrocytic but not of the neuronal Na+,K+-ATPase, this may possibly lead to increased [K+]o. In turn, the increased [K+]o may evoke seizures. The probable absence of NKCC1 which contributes to K+ accumulation at high [K+]o may further undermine K+ uptake. Alternatively, in partly myelinated axons loss of the tight connection between the axon and the compacted myelin sheath [23, 24] may have similar effect as will be described below for Cx32 and 47 double knockout.
The symptomatology in mice with Cx32 and 47 double knockout of the oligodendrocytic Cx32 and Cx47 cannot be explained in a similar manner, since failing myelination has not been reported in these animals, although myelin sheaths are thinner in mice with double knockout of Cx47 and the astrocytic Cx30 [183]. Since we found it unlikely that the severe Jimpy-like symptoms in the Cx32/47 knockouts should be due to interrupted K+ transport from oligodendrocytes to astrocytes via gap junctions, different possibilities for impaired K+ homeostasis in myelinated axons will be considered. Profound differences exist in characteristics of the myelin producing cells in the central (oligodendrocytes) and peripheral (Schwann cells) nervous systems [184] as well as in myelin structure at the two different locations [26]. Nevertheless, some features could be common, perhaps including the important association between Kv channels and Cx29 recently described by Rash et al. [173], but in general we will focus on K+ handling in the CNS.
The myelin wrapping of the axon is interrupted by nodes of Ranvier, which usually extend for 100 µm~1 µm along the fiber axis and are separated by internodes of ~1 mm length. The axon membrane (the axolemma) is highly differentiated at the nodes of Ranvier, exhibiting different properties in that region compared to other sites along the fiber, including a high density of voltage-dependent Na+ channels (Nav), but no voltage-dependent K+ channels (Kv) [185]. This led to the concept that repolarization exclusively occurs by an outward leakage of K+ [185]. A two-pore domain K+ channel (K2P) has been shown to enable action potential generation in the absence of Kv [186], but this was demonstrated in HEK cells, and this channel has never been shown immunochemically in myelinated neurons. Moreover Kv channels are normally present under the myelin sheath in the paranodal and internodal axon membrane of rabbit nerve fibers, and voltage clamp studies have demonstrated the appearance of Kv conductance in mammalian nerve fibers after sudden disruption of the myelin sheath [187]. These Kv channels might well be of functional importance, on account of the previously described transport of K+ into oligodendrocytes by Cx29 located internodally and contacting Kv channels in a 1:1 ratio [173] without being in direct contact with the oligodendrocytic Cx32 [172]. In the myelinated fibers of the CNS the Kv channels may be clustered at the juxtaparanodal region reached before the next node of Ranvier [26, 188–190], rather than located under the entire internode as shown in Fig. 10. However, immunocytochemistry might more easily reveal juxtaparanodal than internodal channels, and uniform calcium influx along the entire stimulated axon of a fully myelinated mouse optic nerve [191] suggests Kv channel localization under internodal myelin also in the CNS. This is especially the case since cadmium inhibition of the calcium transients suggests that calcium channels are involved, although nifedipine had only a minor inhibitory effect [191], possibly due to limited access. Anyhow, internodal versus juxtaparanodal localization would probably not be of major functional importance, as long as the channels are located before the next node of Ranvier. Myelin compaction and the formation of tight axo-glial junction is required to create and maintain the location of the Kv channels under the myelin sheath [188]. In Jimpy mice nodal expression of Na+ channels is normal, but the paranodes are not intact as indicated by elimination of normal components [192], which suggests abnormal Kv function.
The normal localization of K+ channels and their association with connexins is consistent with the periaxonal vacuoles found in the Cx32 and 47 double knockouts [146, 147]. Subsequently K+ may be transported out of oligodendrocytes by homomeric Kir4.1 or heteromeric Kir4.1/Kir5.1 channels localized at their membrane [193]. Interference with oligodendrocytic efflux would in the long run increase K+ in oligodendrocytes and thereby prevent K+ transport from the outer surface of myelin into oligodendrocytes, which would explain why Kir4.1 −/− mice show vacuoles at the myelin surface [148]. The suggested pathway would also be consistent with the observations that optic nerve activity increases [K+]o and that this increase stimulates Na+,K+-ATPase-mediated K+ uptake, first in astrocytes by the potent K+-stimulated Na+,K+-ATPase in astrocytes [73, 78, 194] and subsequently in neurons after Kir4.1-mediated release of K+ accumulated in astrocytes [60]. Repolarising, gradual K+ exit via Kv channels dispersed along the internodal axon may be important for saltatory impulse propagation, since it is likely to make the axon independent of renewed excitation until full re-polarisation has been achieved immediately before the next node of Ranvier. Such a function of myelin-covered Kv channels would not exclude additional operation of leakage channels at the nodes, especially if full re-polarisation has not been achieved. If normal K+ homeostasis fails, due to inefficient transport across the myelin sheath by Cx29, exit from oligodendrocytes via Kir channels or dysmyelination, it is likely that K+ would accumulate periaxonally and reach the node of Ranvier by diffusion between the axon and a loosened myelin sheath. This may have consequences for impulse conduction, energy metabolism and seizure susceptibility. The risk for development of seizures might be especially pronounced if myelinated neurons within gray matter are affected, and an enhanced metabolic requirement after abrogation of saltatory conduction might expose the tissue to glutamatergic excitation.
Concluding Remarks
Since the stimulation of O2 uptake in Jimpy mice by highly elevated [K+]o represents L-channel opening, the conclusion that depolarisation by high [K+]o is unable to activate L-channel-mediated Ca2+ uptake in Jimpy astrocytes seems valid. Nevertheless this should be confirmed experimentally, probably most easily by determining effects of K+ addition on glycogenolysis in brain tissue, remembering the slow regeneration of glycogen in slices after their preparation [195, 196]. Since most of the K+-induced release of transmitter ATP comes from astrocytes [116], it can also be expected that the effect of elevated [K+]o on ATP release will be greatly reduced. Sub-myelin Kv channels are very rare in Jimpy but occasionally found in one of the few myelinated axons [188].
Jimpy is generally not regarded as a model of human demyelinating disease. However, the childhood leukodystrophies have diverse molecular and genetic etiologies in spite of their common clinical features [197], and some of them are associated with mutations in connexin 47 [198–200]. That neonatal motor disturbances can result from deficient L-channel activity is shown by a higher frequency of motor disturbances in women treated with nifedipine to delay pre-term birth than in those treated with placebo [201]. This effect could equally well be exerted on astrocytes or their precursors as on neurons or oligodendrocytes or their precursors. Jimpy is the convulsant mutant where most knowledge of astrocytic abnormalities is available. Further studies of this mutant may therefore be clinically relevant for elucidation of physiological and pathophysiological roles of interactions between oligodendrocytes and astrocytes and their importance in the pathogenesis of leukodystrophies.
The very close similarity between neurological dysfunction in Jimpy and in mice with double knockout of the oligodendrocytic connexins Cx32 and Cx47 provides information about the possible pathophysiology in both conditions. Literature data about localization, especially of Cx29, and its possible interaction with Kv channels also in oligodendrocytes had led to the model of potential pathways involved in K+ homeostasis shown in Fig. 10, but this pathway obviously needs experimental verification.
References
Amaral AI, Meisingset TW, Kotter MR, Sonnewald U (2013) Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Front Endocrinol 4:54
Skoff RP (1976) Myelin deficit in the Jimpy mouse may be due to cellular abnormalities in astroglia. Nature 264:560–562
Hertz L, Chaban G, Hertz E (1980) Abnormal metabolic response to excess potassium in astrocytes from the Jimpy mouse, a convulsing neurological mutant. Brain Res 181:482–487
Chaban YHG (1980) A biochemical and morphological investigation of astrocytes from the Jimpy mouse, a convulsing neurological mutant. University of Saskatchewan, Saskatoon
Hertz L, Chaban G (1982) Indications for an active role of astrocytes in potassium homeostasis at the cellular level: potassium uptake and metabolic effects of potassium. In: Pfeiffer SE (ed) Neuroscience approached through cell culture. CRC Press, Boca Raton, pp 157–174
Nave KA, Lai C, Bloom FE, Milner RJ (1986) Jimpy mutant mouse: a 74-base deletion in the mRNA for myelin proteolipid protein and evidence for a primary defect in RNA splicing. Proc Natl Acad Sci USA 83:9264–9268
Knapp PE, Skoff RP, Redstone DW (1986) Oligodendroglial cell death in jimpy mice: an explanation for the myelin deficit. J Neurosci 6:2813–2822
Knapp PE, Bartlett WP, Williams LA, Yamada M, Ikenaka K, Skoff RP (1999) Programmed cell death without DNA fragmentation in the jimpy mouse: secreted factors can enhance survival. Cell Death Differ 6:136–145
Ghandour MS, Skoff RP (1988) Expression of galactocerebroside in developing normal and jimpy oligodendrocytes in situ. J Neurocytol 17:485–498
Phillips RJ (1954) Jimpy, a new totally sexlinked gene in the house mouse. Z Indukt Abstamm Vererb 86:322–326
Sidman RL, Appel SH, Fullier JF (1965) Neurological mutants of the mouse. Science 150:513–516
Kurihara T, Nussbaum JL, Mandel P (1969) 2′,3′-cyclic nucleotide 3′-phosphohydrolase in the brain of the “Jimpy” mouse, a mutant with deficient myelination. Brain Res 13:401–403
Zahnd JP, Bonaventure N (1969) Donnees ultrastructurales et ectrophysiologiques obtenues au niveau du systeme nerveux central chez la souris Jimpy. C R Soc Biol 163:1631–1635
Herschkowitz N, Vassella F, Bischoff A (1971) Myelin differences in the central and peripheral nervous system in the ‘Jimpy’ mouse. J Neurochem 18:1361–1363
Wolf MK, Holden AB (1969) Tissue culture analysis of the inherited defect of central nervous system myelination in jimpy mice. J Neuropathol Exp Neurol 28:214–228
Foran DR, Peterson AC (1992) Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci 12:4890–4897
Torii J, Adachi M, Volk BW (1971) Histochemical and ultrastructural studies of inherited leukodystrophy in mice. J Neuropathol Exp Neurol 30:278–289
Farkas-Bargeton E, Robain O, Mandel P (1972) Abnormal glial maturation in the white matter in Jimpy mice. An optical study. Acta Neuropathol 21:272–281
Meier C, Herschkowitz N, Bischoff A (1974) Morphological and biochemical observations in the Jimpy spinal cord. Acta Neuropathol 27:349–362
Robain O, Mandel P (1974) Quantitative study of myelination and axonal growth in corpus callosum and posterior columns of spinal cord in the Jimpy mouse. Acta Neuropathol 29:293–309
Best TT, Skoff RP, Bartlett WP (1988) Astroglial plasticity in hemizygous and heterozygous jimpy mice. Int J Dev Neurosci 6:39–57
Hovhannisyan A, Benkner B, Biesemeier A, Schraermeyer U, Kukley M, Munch TA (2015) Effects of the jimpy mutation on mouse retinal structure and function. J Comp Neurol 523:2788–2806
Meier C, Bischoff A (1975) Oligodendroglial cell development in jimpy mice and controls: an electron-microscopic study in the optic nerve. J Neurol Sci 26:517–528
Omlin FX, Anders JJ (1983) Abnormal cell relationships in Jimpy mice: electron microscopic and immunocytochemical findings. J Neurocytol 12:767–784
Baracskay KL, Duchala CS, Miller RH, Macklin WB, Trapp BD (2002) Oligodendrogenesis is differentially regulated in gray and white matter of jimpy mice. J Neurosci Res 70:645–654
Verkhratsky A, Butt AM (2013) Glial Physiology and Pathophysiology. Wiley-Blackwell, Hoboken
Cerina M, Narayanan V, Gobel K, Bittner S, Ruck T, Meuth P, Herrmann AM, Stangel M, Gudi V, Skripuletz T, Daldrup T, Wiendl H, Seidenbecher T, Ehling P, Kleinschnitz C, Pape HC, Budde T, Meuth SG (2016) The quality of cortical network function recovery depends on localization and degree of axonal demyelination. Brain Behav Immun 59:103–117
Boison D, Stoffel W (1994) Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proc Natl Acad Sci USA 91:11709–11713
Boison D, Bussow H, D’Urso D, Muller HW, Stoffel W (1995) Adhesive properties of proteolipid protein are responsible for the compaction of CNS myelin sheaths. J Neurosci 15:5502–5513
Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA (1997) Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18:59–70
Garbern JY, Yool DA, Moore GJ, Wilds IB, Faulk MW, Klugmann M, Nave KA, Sistermans EA, van der Knaap MS, Bird TD, Shy ME, Kamholz JA, Griffiths IR (2002) Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 125:551–561
Yool DA, Edgar JM, Montague P, Malcolm S (2000) The proteolipid protein gene and myelin disorders in man and animal models. Hum Mol Genet 9:987–992
Yin X, Kidd GJ, Ohno N, Perkins GA, Ellisman MH, Bastian C, Brunet S, Baltan S, Trapp BD (2016) Proteolipid protein-deficient myelin promotes axonal mitochondrial dysfunction via altered metabolic coupling. J Cell Biol 215:531–542
Michalski JP, Anderson C, Beauvais A, De Repentigny Y, Kothary R (2011) The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS One 6:e19772
Harlow DE, Saul KE, Culp CM, Vesely EM, Macklin WB (2014) Expression of proteolipid protein gene in spinal cord stem cells and early oligodendrocyte progenitor cells is dispensable for normal cell migration and myelination. J Neurosci 34:1333–1343
Yamada M, Ivanova A, Yamaguchi Y, Lees MB, Ikenaka K (1999) Proteolipid protein gene product can be secreted and exhibit biological activity during early development. J Neurosci 19:2143–2151
Yool DA, Klugmann M, McLaughlin M, Vouyiouklis DA, Dimou L, Barrie JA, McCulloch MC, Nave KA, Griffiths IR (2001) Myelin proteolipid proteins promote the interaction of oligodendrocytes and axons. J Neurosci Res 63:151–164
Vela JM, Gonzalez B, Castellano B (1998) Understanding glial abnormalities associated with myelin deficiency in the jimpy mutant mouse. Brain Res Brain Res Rev 26:29–42
Ikeda M, Hossain MI, Zhou L, Horie M, Ikenaka K, Horii A, Takebayashi H (2016) Histological detection of dynamic glial responses in the dysmyelinating Tabby-jimpy mutant brain. Anat Sci Int 637:26–30
Appikatla S, Bessert D, Lee I, Huttemann M, Mullins C, Somayajulu-Nitu M, Yao F, Skoff RP (2014) Insertion of proteolipid protein into oligodendrocyte mitochondria regulates extracellular pH and adenosine triphosphate. Glia 62:356–373
Karim SA, Barrie JA, McCulloch MC, Montague P, Edgar JM, Kirkham D, Anderson TJ, Nave KA, Griffiths IR, McLaughlin M (2007) PLP overexpression perturbs myelin protein composition and myelination in a mouse model of Pelizaeus-Merzbacher disease. Glia 55:341–351
Huttemann M, Zhang Z, Mullins C, Bessert D, Lee I, Nave KA, Appikatla S, Skoff RP (2009) Different proteolipid protein mutants exhibit unique metabolic defects. ASN Neuro 1
Bartlett WP, Knapp PE, Skoff RP (1988) Glial conditioned medium enables jimpy oligodendrocytes to express properties of normal oligodendrocytes: production of myelin antigens and membranes. Glia 1:253–259
Knapp PE, Benjamins JA, Skoff RP (1996) Epigenetic factors up-regulate expression of myelin proteins in the dysmyelinating jimpy mutant mouse. J Neurobiol 29:138–150
Knapp PE, Ismaili S, Hauser KF, Ghandour MS (1999) Abnormal Ca(2+) regulation in oligodendrocytes from the dysmyelinating jimpy mouse. Brain Res 847:332–337
Sorg BJ, Agrawal D, Agrawal HC, Campagnoni AT (1986) Expression of myelin proteolipid protein and basic protein in normal and dysmyelinating mutant mice. J Neurochem 46:379–387
Duncan ID, Hammang JP, Goda S, Quarles RH (1989) Myelination in the jimpy mouse in the absence of proteolipid protein. Glia 2:148–154
Williams WC 2nd, Gard AL (1997) In vitro death of jimpy oligodendrocytes: correlation with onset of DM-20/PLP expression and resistance to oligodendrogliotrophic factors. J Neurosci Res 50:177–189
Keen P, Osborne RH, Pehrson UM (1976) Proceedings: respiration and metabolic compartmentation in brain slices from a glia-deficient mutant, the Jimpy mouse. J Physiol 254:22P–23P
Hertz l, Juurlink BHJ, Fosmark H, Schousboe A (1982) Methodological appendix: Astrocytes in primary cultures. In: Pfeiffer SE (ed) Neuroscience approached through cell culture. CRC Press, Boca Raton, pp 175–186
Juurlink BHJ, Hertz L (1992) Astrocytes. In: Boulton AA, Baker GB, Walz W (eds) Neuromethods in cell cultures, also available on the Internet in Springer’s Protocols edn. Humana Clifton, New York, pp 269–321
Hertz L, Chen Y, Song D (2016) Astrocyte cultures mimicking brain astrocytes in gene expression, signaling, metabolism and K+ uptake and showing astrocytic gene expression overlooked by immunohistochemistry and in situ hybridization. Neurochem Res. doi: 10.1007/s11064-016-1828-x
Ghandour MS, Feutz AC, Jalabi W, Taleb O, Bessert D, Cypher M, Carlock L, Skoff RP (2002) Trafficking of PLP/DM20 and cAMP signaling in immortalized jimpy oligodendrocytes. Glia 40:300–311
Hertz E, Hertz L (1979) Polarographic measurement of oxygen uptake by astrocytes in primary cultures using the tissue-culture flask as the respirometer chamber. In Vitro 15:429–436
Hertz L, Schou M (1962) Univalent cations and the respiration of brain-cortex slices. Biochem J 85:93–104
Weiss GB, Hertz L, Goodman FR (1972) Drug-induced alterations in respiration of rat brain cortex and striatum slices in a carbon dioxide-bicarbonate-buffered medium. Biochem Pharmacol 21:625–634
Falconer DS (1953) Total sex-linkage in the house mouse. Z Indukt Abstamm Vererb 85:210–219
Bachelard H, Morris P, Taylor A, Thatcher N (1995) High-field MRS studies in brain slices. Magn Reson Imaging 13:1223–1226
Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485
Hertz L, Chen Y (2016) Importance of astrocytes for potassium ion (K+) homeostasis in brain and glial effects of K+ and its transporters on learning. Neurosci Biobehav Rev 71:484–505
Epstein FH, Silva P (1985) Na–K–Cl cotransport in chloride-transporting epithelia. Ann NY Acad Sci 456:187–197
Dawson DC (1987) Cellular mechanisms for K transport across epithelial cell layers. Semin Nephrol 7:185–192
Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ (2010) Cotransport of water by the Na+–K+–2Cl(−) cotransporter NKCC1 in mammalian epithelial cells. J Physiol 588:4089–4101
Macaulay N, Zeuthen T (2012) Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37:2299–2309
Akar F, Skinner E, Klein JD, Jena M, Paul RJ, O’Neill WC (1999) Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+–K+–2Cl− cotransporter in rat aorta. Am J Physiol 276:C1383–C1390
Qusous A, Geewan CS, Greenwell P, Kerrigan MJ (2011) siRNA-mediated inhibition of Na(+)–K(+)–2Cl− cotransporter (NKCC1) and regulatory volume increase in the chondrocyte cell line C-20/A4. J Membr Biol 243:25–34
Pedersen SF, O’Donnell ME, Anderson SE, Cala PM (2006) Physiology and pathophysiology of Na+/H + exchange and Na+–-K+–2Cl− cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol 291:R1–R25
Hoffmann EK, Schettino T, Marshall WS (2007) The role of volume-sensitive ion transport systems in regulation of epithelial transport. Comp Biochem Physiol A 148:29–43
Sid B, Miranda L, Vertommen D, Viollet B, Rider MH (2010) Stimulation of human and mouse erythrocyte Na(+)-K(+)-2Cl(-) cotransport by osmotic shrinkage does not involve AMP-activated protein kinase, but is associated with STE20/SPS1-related proline/alanine-rich kinase activation. J Physiol 588:2315–2328
Song D, Xu J, Hertz L, Peng L (2015) Regulatory volume increase in astrocytes exposed to hypertonic medium requires beta1 -adrenergic Na(+)/K(+)–ATPase stimulation and glycogenolysis. J Neurosci Res 93:130–139
Hertz L, Schousboe A, Boechler N, Mukerji S, Fedoroff S (1978) Kinetic characteristics of the glutamate uptake into normal astrocytes in cultures. Neurochem Res 3:1–14
Judd MG, Nagaraja TN, Brookes N (1996) Potassium-induced stimulation of glutamate uptake in mouse cerebral astrocytes: the role of intracellular pH. J Neurochem 66:169–176
Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522(Pt 3):427–442
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25:349–365
Dufour S, Dufour P, Chever O, Vallee R, Amzica F (2011) In vivo simultaneous intra- and extracellular potassium recordings using a micro-optrode. J Neurosci Methods 194:206–217
Bay V, Butt AM (2012) Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia 60:651–660
Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N (2014) Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia 62:608–622
Larsen BR, Stoica A, MacAulay N (2016) managing brain extracellular K(+) during neuronal activity: the physiological role of the Na(+)/K(+)-ATPase subunit isoforms. Front Physiol 7:141
Larsen BR, MacAulay N (2014) Kir4.1-mediated spatial buffering of K(+): experimental challenges in determination of its temporal and quantitative contribution to K(+) clearance in the brain. Channels 8:544–550
Hertz L, Gerkau NJ, Xu J, Durry S, Song D, Rose CR, Peng L (2015) Roles of astrocytic Na(+),K(+)-ATPase and glycogenolysis for K(+) homeostasis in mammalian brain. J Neurosci Res 93:1019–1030
Ashford CA, Dixon KC (1935) The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochem J 29:157–168
Walz W, Mukerji S (1988) Lactate release from cultured astrocytes and neurons: a comparison. Glia 1:366–370
Lund-Andersen H, Hertz L (1970) Effects of potassium content in brain-cortex slices from adult rats. Exp Brain Res 11:199–212
Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B 3rd, Reddy PV, Norenberg MD (2008) Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem 283:33874–33882
Wang H, Yan Y, Kintner DB, Lytle C, Sun D (2003) GABA-mediated trophic effect on oligodendrocytes requires Na-K-2Cl cotransport activity. J Neurophysiol 90:1257–1265
Chen H, Kintner DB, Jones M, Matsuda T, Baba A, Kiedrowski L, Sun D (2007) AMPA-mediated excitotoxicity in oligodendrocytes: role for Na(+)–K(+)–Cl(−) co-transport and reversal of Na(+)/Ca(2+) exchanger. J Neurochem 102:1783–1795
Fu P, Tang R, Yu Z, Huang S, Xie M, Luo X, Wang W (2015) Bumetanide-induced NKCC1 inhibition attenuates oxygen-glucose deprivation-induced decrease in proliferative activity and cell cycle progression arrest in cultured OPCs via p-38 MAPKs. Brain Res 1613:110–119
Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K (2001) The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104:933–946
MacVicar BA, Feighan D, Brown A, Ransom B (2002) Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia 37:114–123
Price TJ, Hargreaves KM, Cervero F (2006) Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res 1112:146–158
Hertz L, Peng L, Kjeldsen CC, O’Dowd BS, Dienel GA (2004) Ion, transmitter and drug effects on energy metabolism in astrocytes. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 435–460
Holtzman D, Olson J, Zamvil S, Nguyen H (1982) Maturation of potassium-stimulated respiration in rat cerebral cortical slices. J Neurochem 39:274–276
Hertz L (1966) Neuroglial localization of potassium and sodium effects on respiration in brain. J Neurochem 13:1373–1387
Hertz L, Dittmann L, Mandel P (1973) K+ induced stimulation of oxygen uptake in cultured cerebral glial cells. Brain Res 60:517–520
Carmignoto G, Pasti L, Pozzan T (1998) On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci 18:4637–4645
Kostyuk PG (1984) Metabolic control of ionic channels in the neuronal membrane. Neuroscience 13:983–989
Newman EA (1985) Voltage-dependent calcium and potassium channels in retinal glial cells. Nature 317:809–811
Duffy S, MacVicar BA (1994) Potassium-dependent calcium influx in acutely isolated hippocampal astrocytes. Neuroscience 61:51–61
Yan E, Li B, Gu L, Hertz L, Peng L (2013) Mechanisms for L-channel-mediated increase in [Ca(2+)]i and its reduction by anti-bipolar drugs in cultured astrocytes combined with its mRNA expression in freshly isolated cells support the importance of astrocytic L-channels. Cell Calcium 54:335–342
Du T, Liang C, Li B, Hertz L, Peng L (2014) Chronic fluoxetine administration increases expression of the L-channel gene Cav1.2 in astrocytes from the brain of treated mice and in culture and augments K(+)-induced increase in [Ca(2+)]i. Cell Calcium 55:166–174
Hertz L, Bender AS, Woodbury DM, White HS (1989) Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J Neurosci Res 22:209–215
Luttrell LM, Daaka Y, Lefkowitz RJ (1999) Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11:177–183
Peng L, Du T, Xu J, Song D, Li B, Zhang M, Hertz L (2012) Adrenergic and V1-ergic agonists/antagonists affecting recovery from brain trauma in the Lund project act on astrocytes. Curr Signal Transduct Ther 7:43–55
Lin HH (2013) G-protein-coupled receptors and their (Bio) chemical significance win 2012 Nobel Prize in Chemistry. Biomed J 36:118–124
Peng L (2004) Transactivation in astrocytes as a nivel mnechansm of neuroprotection. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 503–518
Cai L, Du T, Song D, Li B, Hertz L, Peng L (2011) Astrocyte ERK phosphorylation precedes K(+)-induced swelling but follows hypotonicity-induced swelling. Neuropathology 31:250–264
Hertz L, Peng L, Song D (2015) Ammonia, like K(+), stimulates the Na(+), K(+), 2 Cl(−) cotransporter NKCC1 and the Na(+),K(+)-ATPase and interacts with endogenous ouabain in astrocytes. Neurochem Res 40:241–257
Xu J, Song D, Bai Q, Cai L, Hertz L, Peng L (2014) Basic mechanism leading to stimulation of glycogenolysis by isoproterenol, EGF, elevated extracellular K+ concentrations, or GABA. Neurochem Res 39:661–667
Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L (2015) Astrocytic glycogenolysis: mechanisms and functions. Metab Brain Dis 30:317–333
Hertz L, Chen Y (2016) Editorial: all 3 types of glial cells are important for memory formation. Front Integr Neurosci 10:31
Gibbs ME, Lloyd HG, Santa T, Hertz L (2007) Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J Neurosci Res 85:3326–3333
Muller MS, Fox R, Schousboe A, Waagepetersen HS, Bak LK (2014) Astrocyte glycogenolysis is triggered by store-operated calcium entry and provides metabolic energy for cellular calcium homeostasis. Glia 62:526–534
Obel LF, Muller MS, Walls AB, Sickmann HM, Bak LK, Waagepetersen HS, Schousboe A (2012) Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front Neuroenerg 4:3
Xu J, Song D, Bai Q, Zhou L, Cai L, Hertz L, Peng L (2014) Role of glycogenolysis in stimulation of ATP release from cultured mouse astrocytes by transmitters and high K + concentrations. ASN Neuro 6:e00132
Hertz L, Xu J, Peng L (2014) Glycogenolysis and purinergic signaling. Adv Neurobiol 11:31–54
Heinrich A, Ando RD, Turi G, Rozsa B, Sperlagh B (2012) K + depolarization evokes ATP, adenosine and glutamate release from glia in rat hippocampus: a microelectrode biosensor study. Br J Pharmacol 167:1003–1020
Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG (2014) Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun 5:3284
Steinman MQ, Gao V, Alberini CM (2016) The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci 10:10
Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J, Bergersen LH (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res 93:1045–1055
Ransom BR, Yamate CL, Connors BW (1985) Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci 5:532–535
Wang F, Du T, Liang C, Verkhratsky A, Peng L (2015) Ammonium increases Ca(2+) signalling and upregulates expression of Cav1.2 gene in astrocytes in primary cultures and in the in vivo brain. Acta Physiol 214:261–274
Hamilton N, Hubbard PS, Butt AM (2009) Effects of glutamate receptor activation on NG2-glia in the rat optic nerve. J Anat 214:208–218
Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM (2008) Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 56:734–749
Fields RD (2006) Nerve impulses regulate myelination through purinergic signalling. Nos Found Symp 276:148–158; discussion 158–161, 233–147, 275–181
Butt AM (2011) ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol 22:205–213
Butt AM, Fern RF, Matute C (2014) Neurotransmitter signaling in white matter. Glia 62:1762–1779
Rivera A, Vanzulli I, Butt AM (2016) A central role for ATP signaling in glial interactions in the CNS. Curr Drug Targets
Xia M, Zhu Y (2011) Signaling pathways of ATP-induced PGE2 release in spinal cord astrocytes are EGFR transactivation-dependent. Glia 59:664–674
Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A (2003) P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci 44:1211–1220
Vinukonda G, Hu F, Mehdizadeh R, Dohare P, Kidwai A, Juneja A, Naran V, Kierstead M, Chawla R, Kayton R, Ballabh P (2016) Epidermal growth factor preserves myelin and promotes astrogliosis after intraventricular hemorrhage. Glia 64:1987–2004
Gonsalvez D, Ferner AH, Peckham H, Murray SS, Xiao J (2016) The roles of extracellular related-kinases 1 and 2 signaling in CNS myelination. Neuropharmacology 110:586–593
Ansari KA, Shoeman DW (1990) Arachidonic and docosahexanoic acid content of bovine brain myelin: implications for the pathogenesis of multiple sclerosis. Neurochem Res 15:7–11
Tian N, Kanno T, Jin Y, Nishizaki T (2014) Lithium potentiates GSK-3beta activity by inhibiting phosphoinositide 3-kinase-mediated Akt phosphorylation. Biochem Biophys Res Commun 450:746–749
Azim K, Butt AM (2011) GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59:540–553
Luo F, Burke K, Kantor C, Miller RH, Yang Y (2014) Cyclin-dependent kinase 5 mediates adult OPC maturation and myelin repair through modulation of Akt and GsK-3beta signaling. J Neurosci 34:10415–10429
Plenge P, Mellerup ET, Rafaelsen OJ (1970) Lithium action on glycogen synthesis in rat brain, liver, and diaphragm. J Psychiatr Res 8:29–36
Takahashi-Yanaga F (2013) Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol 86:191–199
Cambron M, D’Haeseleer M, Laureys G, Clinckers R, Debruyne J, De Keyser J (2012) White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J Cereb Blood Flow Metab 32:413–424
Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49:823–832
Yamakuni H, Kawaguchi N, Ohtani Y, Nakamura J, Katayama T, Nakagawa T, Minami M, Satoh M (2002) ATP induces leukemia inhibitory factor mRNA in cultured rat astrocytes. J Neuroimmunol 129:43–50
Spiegel I, Peles E (2006) A new player in CNS myelination. Neuron 49:777–778
Iacobas S, Iacobas DA (2010) Astrocyte proximity modulates the myelination gene fabric of oligodendrocytes. Neuron Glia Biol 6:157–169
Ishibashi T, Lee PR, Baba H, Fields RD (2009) Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve. J Neurosci Res 87:3343–3355
Gresle MM, Butzkueven H, Perreau VM, Jonas A, Xiao J, Thiem S, Holmes FE, Doherty W, Soo PY, Binder MD, Akkermann R, Jokubaitis VG, Cate HS, Marriott MP, Gundlach AL, Wynick D, Kilpatrick TJ (2015) Galanin is an autocrine myelin and oligodendrocyte trophic signal induced by leukemia inhibitory factor. Glia 63:1005–1020
Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, Kishore R (2007) STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res 101:910–918
Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2003) Connexins are critical for normal myelination in the CNS. J Neurosci 23:5963–5973
Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K (2003) Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci 23:4549–4559
Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2006) Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci 26:10984–10991
Schiza N, Sargiannidou I, Kagiava A, Karaiskos C, Nearchou M, Kleopa KA (2015) Transgenic replacement of Cx32 in gap junction-deficient oligodendrocytes rescues the phenotype of a hypomyelinating leukodystrophy model. Hum Mol Genet 24:2049–2064
Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P (2001) Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci 21:5429–5438
Massa PT, Mugnaini E (1985) Cell-cell junctional interactions and characteristic plasma membrane features of cultured rat glial cells. Neuroscience 14:695–709
Massa PT, Mugnaini E (1982) Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience 7:523–538
Rash JE, Yasumura T, Dudek FE, Nagy JI (2001) Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci 21:1983–2000
Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H (2010) Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 58:1104–1117
Wasseff SK, Scherer SS (2011) Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol Dis 42:506–513
Giaume C, Leybaert L, Naus CC, Saez JC (2013) Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4:88
Wasseff SK, Scherer SS (2015) Activated immune response in an inherited leukodystrophy disease caused by the loss of oligodendrocyte gap junctions. Neurobiol Dis 82:86–98
Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC, Willecke K (1989) Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci USA 86:10148–10152
Ochalski PA, Frankenstein UN, Hertzberg EL, Nagy JI (1997) Connexin-43 in rat spinal cord: localization in astrocytes and identification of heterotypic astro-oligodendrocytic gap junctions. Neuroscience 76:931–945
Nagy JI, Patel D, Ochalski PA, Stelmack GL (1999) Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–468
Swenson KI, Jordan JR, Beyer EC, Paul DL (1989) Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell 57:145–155
Werner R, Levine E, Rabadan-Diehl C, Dahl G (1989) Formation of hybrid cell-cell channels. Proc Natl Acad Sci USA 86:5380–5384
Dahl E, Manthey D, Chen Y, Schwarz HJ, Chang YS, Lalley PA, Nicholson BJ, Willecke K (1996) Molecular cloning and functional expression of mouse connexin-30,a gap junction gene highly expressed in adult brain and skin. J Biol Chem 271:17903–17910
Nagy JI, Ionescu AV, Lynn BD, Rash JE (2003) Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: implications from normal and connexin32 knockout mice. Glia 44:205–218
Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK (2007) Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci 27:13949–13957
Magnotti LM, Goodenough DA, Paul DL (2011) Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 59:26–34
Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF (2009) Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29:7743–7752
Hertz L, Soliven B, Hertz E, Szuchet S, Nelson DJ (1990) Channel-mediated and carrier-mediated uptake of K+ into cultured ovine oligodendrocytes. Glia 3:550–557
Nagy JI, Ionescu AV, Lynn BD, Rash JE (2003) Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system. J Comp Neurol 464:356–370
Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL (2002) Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci 22:6458–6470
Nagy JI, Rash JE (2003) Astrocyte and oligodendrocyte connexins of the glial syncytium in relation to astrocyte anatomical domains and spatial buffering. Cell Commun Adhes 10:401–406
Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS (2008) Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res 86:992–1006
Rash JE, Vanderpool KG, Yasumura T, Hickman J, Beatty JT, Nagy JI (2016) KV1 channels identified in rodent myelinated axons, linked to Cx29 in innermost myelin: support for electrically active myelin in mammalian saltatory conduction. J Neurophysiol 115:1836–1859
Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045–1056
Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C (2006) The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–5447
Rutecki PA, Lebeda FJ, Johnston D (1985) Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol 54:1363–1374
Yaari Y, Konnerth A, Heinemann U (1986) Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. II. Role of extracellular potassium. J Neurophysiol 56:424–438
Traynelis SF, Dingledine R (1988) Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol 59:259–276
Du M, Li J, Wang R, Wu Y (2016) The influence of potassium concentration on epileptic seizures in a coupled neuronal model in the hippocampus. Cognit Neurodyn 10:405–414
Hubbard JA, Binder DK (2016) Astrocytes and epilepsy. Academc Press, Amsterdam
Erecinska M, Cherian S, I AS (2005) Brain development and susceptibility to damage; ion levels and movements. Curr Top Dev Biol 69:139–186
Wolff JR, Chao TI (2004) Cytoarchitectonics of non-neuronal cells in the central nervous system. In: L H (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 1–51
Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K (2012) Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J Neurosci 32:7499–7518
Nualart-Marti A, Solsona C, Fields RD (2013) Gap junction communication in myelinating glia. Biochim Biophys Acta 1828:69–78
Waxman SG, Ritchie JM (1985) Organization of ion channels in the myelinated nerve fiber. Science 228:1502–1507
MacKenzie G, Franks NP, Brickley SG (2015) Two-pore domain potassium channels enable action potential generation in the absence of voltage-gated potassium channels. Pflugers Arch 467:989–999
Chiu SY, Ritchie JM (1981) Evidence for the presence of potassium channels in the paranodal region of acutely demyelinated mammalian single nerve fibres. J Physiol 313:415–437
Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K (1999) Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K(+) channel clustering. J Neurosci Res 58:752–764
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Rasband MN, Park EW, Zhen D, Arbuckle MI, Poliak S, Peles E, Grant SG, Trimmer JS (2002) Clustering of neuronal potassium channels is independent of their interaction with PSD-95. J Cell Biol 159:663–672
Zhang CL, Wilson JA, Williams J, Chiu SY (2006) Action potentials induce uniform calcium influx in mammalian myelinated optic nerves. J Neurophysiol 96:695–709
Jenkins SM, Bennett V (2002) Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc Natl Acad Sci USA 99:2303–2308
Brasko C, Hawkins V, De La Rocha IC, Butt AM (2016) Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain Struct Funct
Hajek I, Subbarao KV, Hertz L (1996) Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 28:335–342
Lebaron FN (1955) The resynthesis of glycogen by guinea pig cerebral-cortex slices. Biochem J 61:80–85
Fiala JC, Kirov SA, Feinberg MD, Petrak LJ, George P, Goddard CA, Harris KM (2003) Timing of neuronal and glial ultrastructure disruption during brain slice preparation and recovery in vitro. J Comp Neurol 465:90–103
Osorio MJ, Goldman SA (2016) Glial progenitor cell-based treatment of the childhood leukodystrophies. Exp Neurol 283:476–488
Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J (2004) Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet 75:251–260
Hobson GM, Garbern JY (2012) Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders. Semin Neurol 32:62–67
Tesson C, Koht J, Stevanin G (2015) Delving into the complexity of hereditary spastic paraplegias: how unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum Genet 134:511–538
van Vliet E, Dijkema GH, Schuit E, Heida KY, Roos C, van der Post J, Parry EC, McCowan L, Lyell DJ, El-Sayed YY, Carr DB, Clark AL, Mahdy ZA, Uma M, Sayin NC, Varol GF, Mol BW, Oudijk MA (2016) Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta-analysis. BJOG 123:1753–1760
Acknowledgements
Dr. Andrea Rivera, University of Porthmouth, U.K. is thanked for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaban, Y.H.G., Chen, Y., Hertz, E. et al. Severe Convulsions and Dysmyelination in Both Jimpy and Cx32/47 −/− Mice may Associate Astrocytic L-Channel Function with Myelination and Oligodendrocytic Connexins with Internodal Kv Channels. Neurochem Res 42, 1747–1766 (2017). https://doi.org/10.1007/s11064-017-2194-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2194-z