Abstract
In this review, animal cell lines are considered to have two classes of attributes: “before-the-fact” (ante factum) and “after-the-fact” (post factum) properties. Fish cell lines from Actinopterygii (ray-finned fishes) are used to illustrate this distinction and to demonstrate how these properties can be used in various ways to categorize cell lines into groups or invitromes. Before-the-fact properties are set at initiation and are properties of the sample and species from which the cell line arose and of the scientist(s) who developed the cell line. On the basis of the Actinopterygii sample, invitromes exist for embryos, larvae, juveniles, adults, and spawning fish, and for most solid organs but rarely for biological fluids. For species, invitromes exist for only a small fraction of the Actinopterygii total. As to their development, scientists from around the world have contributed to invitromes. By contrast, after-the-fact properties are limitless and become apparent during development, characterization, use, and storage of the cell line. For ray-finned invitromes, cell lines appear to acquire immortality during development, are characterized poorly for differentiation potential, have numerous uses, and are stored formally only sporadically. As an example of applying these principles to a specific organ, the skeletal muscle invitrome is used. For ante factum properties, the cell lines are mainly from trunk muscle of economically important fish from 11 orders, 15 families, 19 genera, and 21 species of ray-finned fishes. For post factum properties, fibroblast-like and myogenic cell lines have been described but epithelial-like FHM is most widely used and curated. Considering cell lines by their before- and after-the-fact properties should facilitate integration of new cell lines into the literature and help incorporate the discipline of cell biology into other research areas, particularly the natural history of fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the last 70 yr, the cells of multicellular animals, such as mammals and fishes, have been put into culture. The cultures can be considered one of two basic types: primary cell cultures and cell lines. Primary cultures are initiated directly from the cells, tissues, or organs of an animal and typically are used within a few days of their preparation, or they can be sub-cultivated or passaged into new culture vessels. The sub-cultivation is the end of a primary culture and the start of a cell line (Bols et al. 2005). Cell lines can be serially sub-cultivated, cryopreserved, and used in a large number of ways. The topic of animal cell lines involves a complex interplay of methodologies, curation, and applications.
In an attempt to make relationships easier to see in a plethora of details, Bols et al. (2017) proposed three terms for in vitro biology: invitromatics, invitroomics, and invitromes. Invitromatics is defined as the science and history of establishing, characterizing, engineering, storing, and distributing animal cell lines. Invitromatics is broadly similar for both vertebrate groups, Mammalia and Actinopterygii (ray-finned fishes). The basal media, serum supplements, antibiotics, and culture vessels are similar, although culture incubation temperatures obviously differ (Bols et al. 1992). On the other hand, the mammalian invitromatics is much more sophisticated in many areas, such as characterization, genetic engineering, and three-dimensional (3D) growth of cell lines. Invitroomics is defined as using cell lines for particular purposes, which range from basic research to commerce. The focus of invitroomics for mammals and ray-finned fishes differs. Most mammalian invitroomics has a medical focus, and although fish cell lines are used in aspects of medical research (Hightower and Renfro 1988), the main focus of ray-finned fish invitroomics has been virology and ecotoxicology (Bols et al. 2005). However, because of the diverse and complex natural histories of Actinopterygii, which have approximately six times more species than Mammalia, ray-finned fish cell lines are progressively being used for more varied purposes.
Invitromes are defined as groups or catalogs of cell lines around themes. The most comprehensive total number of animal cell lines reported in the literature can be obtained from Cellosaurus, an online cell line resource (https://www.cellosaurus.org/) (Bairoch 2018). This would constitute the animal invitrome. According to the Cellosaurus data, the invitromes for mammals and ray-finned fishes differ profoundly. Mammalian cell lines number more than 100,000, whereas fewer than 1000 ray-finned fish cell lines exist. Most mammalian cell lines are from a few species, humans, and rodents, whereas the ray-finned cell lines are from hundreds of species. Subdividing these cell lines into invitromes is an attempt to see “forests among all the trees” and offers several advantages. Firstly, organizing cell lines into invitromes will show where efforts should be put toward developing new cell lines. Secondly, invitromes should stimulate searches for patterns in cell line groupings. The detection of patterns would raise new scientific questions to explore. For example, if the embryo invitrome for a species were found to differ from the adult invitrome in one or more properties, this would generate research into possible explanations, such as an epigenetic one. Another example could be in virology. If cell lines from the invitrome of one species were found to support the replication of a specific virus whereas the invitrome of another species did not, the explanation for this could be sought by using the two cell line groups to study antiviral restriction factors. Thirdly, cell lines as a resource for scientific experimentation would be easier to access. For example, if the experimental interest was in the liver, reviewing the liver invitrome would provide a quick overview of possible cell lines to use. Finally, categorization should help in documenting, storing, and distributing cell lines. In turn, this will help in understanding cell line availability. For example, the informally shared invitromes are cell lines that might be obtained from other researchers but the general usefulness of some of these might suggest that more of these should be in depositories.
This review uses cell lines from ray-finned fishes to develop a general framework for characterizing or categorizing animal cell lines and to illustrate the concepts specifically with cell lines from skeletal muscle. Cell lines are considered to have two types of descriptors: “before-the-fact” or ante factum properties (Fig. 1) and “after-the-fact” or post factum properties (Fig. 2). Ante factum properties are the characteristics of the sample from which the cell line was initiated, of the species from which the sample was obtained, and of the scientists who established the cell line. Post factum properties appear during the development, characterization, use, and storage of the cell line. Thus, the post factum properties arise from both invitromatics (cell line preparation) and from invitroomics (cell line use). With modifications, this framework could be applied to any animal group for which there are a large number of cell lines, such as mammals and insects, and so might be broadly useful for in vitro biology. Invitromatics and invitroomics are not specifically reviewed here but are used to make the case for ante factum and post factum properties of cell lines and to present a broad picture of Actinopterygii cell line diversity.
An informal classification tree for grouping fish cell lines into different invitromes based on “before-the-fact” or ante factum properties. These are properties of the sample that was used to start the cell line (top center oval), of the species from which the sample was taken (middle center oval), and of the scientists who developed the cell line (bottom center oval). The sample properties are the organism from which the sample was taken, life cycle stage of the organism, and the organ or fluid that was sampled. The species properties are its taxonomy, significance to humans, and biological features or natural history. For taxonomy, invitromes could be constructed at any rank from order through to species. The significance of the species to humans could be many, with conservation status and an endangered fish invitrome being one example. Numerous biological features of the species could be used to construct invitromes, and habitat and freshwater fish invitrome is one example. The scientist properties could be the laboratory location, such as India and Indian fish invitrome.
An informal classification tree for grouping fish cell lines into different invitromes based upon properties that become apparent after the start of the cell line. These after-the-fact or post factum properties can emerge as the cell line is being developed (I), characterized (II), experimented on (III), and stored (IV). During development (I), two common features that emerge are cell shape (e.g., epithelial invitrome) and the necessity for a solid support (e.g., anchorage or adherent invitrome). Characterization (II) involves a mix of methodology and endpoints, and here will be considered to proceed along four paths (A, B, C, and D). For (A), variability in the presence, organization, and modifications of specific cytoskeletal and junctional protein can be used to group cell lines. As an example, the α tubulin cytoskeleton is acetylated in some but not all walleye cell lines. For (B), the expression of specific functional or lineage markers during routine cell culturing can define invitromes. An example is RTS11, which belongs to the immune invitrome. For (C), a cell line’s capacity to express stemness or to differentiate and acquire functional properties in response to specific changes in culture conditions would place them in stem cell and progenitor invitromes. An example would be the hepatocyte invitrome. For (D), application of omic procedures should lead to countless new groupings. Experimentation (III) with cell lines leads to them being grouped by their ability to support replication of specific viruses (A), respond to environmental contaminants (B), or to be genetically engineered. Finally, with storage (IV), the availability of cell lines can change, and some might have an uncertain status (zombie invitrome).
“Before-the-fact” or ante factum properties and invitromes
Ante factum properties are the characteristics of the sample, organism, and laboratory that led to the cell line. Figure 1 illustrates how the ray-finned invitrome can be subdivided by applying “before-the-fact” properties. A discussion of this follows, organized around the properties of the samples, organisms, and scientists (laboratory).
Properties of the sample
Cell lines can be grouped into invitromes by using the properties of the biological sample that was used to prepare the primary cultures that initiated the development of the cell line (Fig. 1). These properties come from features of the life cycle stage, of the organ, and of the organism from which the sample was obtained.
Life cycle stage
Fish cell lines are usually described as being derived from either embryos, larvae or adults (Wolf and Mann 1980; Fryer and Lannan 1994; Thangaraj et al. 2021), but other life cycle stages are also of interest. The choice of stage has often been dictated by availability, but in recent years cell lines have been sought specifically from embryos in order to study embryo stem cells (Hong et al. 2011). In the future, comparing cell lines from different life cycle stages might be profitable for several research disciplines, with one being epigenesis. Patterns of global DNA methylation, which is one epigenetic mechanism, have been found to be similar between some human cell lines and their tissue of origin (Rodger et al. 2021). For some fish, global DNA methylation patterns change during their life cycle (Suarez-Bregua et al. 2021). Whether such patterns would be retained in fish cell lines is unknown. Investigating this would be interesting and helped by having a collection of cell lines from all life cycle stages of a single species. Such a life stage invitrome would be expected for rainbow trout because this species has been studied so frequently. Indeed, rainbow trout cell lines have been reported from embryos and adults multiple times (Bols et al. 2017), and at least once from juveniles (Wolf and Mann 1980). Also, spawning rainbow trout have been the source of cell lines from the pituitary, RTP-2 (Bols et al. 1995), and from reproductive fluids, RTmilt5 and RTovfl (Vo et al. 2015a). Yet, no cell line appears to have been reported from rainbow trout larvae. By contrast, larvae have been the source of cell lines for many other species (Thangaraj et al. 2021), such as PHL from Pacific herring (Ganassin et al. 1999) and GML-5 from the Atlantic cod (MacLeod et al. 2018).
Organ
Fish cell lines are usually listed as being from a particular organ or tissue (Wolf and Mann 1980; Fryer and Lannan 1994; Thangaraj et al. 2021), which have nearly always been taken from juvenile or adult fish. Although the intention is usually clear, the semantics can be puzzling. From a histological perspective, an organ is made of four basic tissues: epithelium, connective, muscle, and nervous. The samples that have been used to start nearly all fish cell lines have been started from organs rather than specifically from one of these four tissues. Another possible point of confusion revolves around the term muscle. Sometimes cell lines from skeletal muscle are referred as muscle cell lines, but of course these cell lines are from an organ, because skeletal muscle is in fact an organ (Pedersen 2013). In the first listing of fish cell lines, Wolf and Mann (1980) noted whether the “tissue of origin” was normal or abnormal. Over the subsequent years, the practice has been to start cell cultures only from organs that appear normal on quick inspection. Tumors would be an interesting cell line source, but confirming a tumor in an abnormal-appearing organ requires histological examination, by which time material for starting cell cultures is usually no longer available. Despite this, tumors have been the source of a few fish cell lines, such as RTH149 from a rainbow trout hepatoma (Fryer et al. 1981).
Fish cell lines are from either solid or hollow organs, with the exception of a few from biological fluids. For starting cell lines from organs, the first step is to dissociate or fragment the organs into cells or cell clumps, put them into a growth medium within culture vessels, and allow them to attach and proliferate on the culture surface. For starting cell lines from biological fluids, the dissociation step is not needed but media and culture vessels are the same. Organ dissociation procedures, culture vessels, basal media, and supplementations of media with serum and other factors are the subjects of invitromatics (Bols et al. 2017). Fish invitromatics will not be reviewed here because the basic principles of dissociating organs and of culturing their cells are similar to those for mammals and can be found in basic cell culturing texts (e.g., Freshney 2005). For some of the broad adjustments that have to be made for the ray-finned fishes, some of the early reviews specific to fish are still valuable (Wolf and Quimby 1969; Nicholson 1989; Bols and Lee 1994).
The exact number of fish organs from which cell lines have been developed depends on terminology, whereas the number of cell lines from a specific organ can usually be stated more precisely. According to Thangaraj et al. (2021), the period between 2010 and 2020 saw the development of fish cell lines from twenty different “tissues” (organs). Part of the problem is whether fins from different anatomical sites should be considered as being from one or multiple organs, and similarly, for skin and bone from different body regions. Fish cell lines have been established routinely from the following organs: brain, bone (vertebrae), eye, fin, heart, gill, gonad, intestine, kidney, liver, ovary, pituitary, skeletal muscle, skin, spleen, swim bladder, testis, and thymus (Lakra et al. 2011; Thangaraj et al. 2021; Laizé et al. 2022; Goswami et al. 2022). Thus, conservatively, there are at least 18 fish organ invitromes.
Organ invitromes can be considered to have multiple properties. This idea will be illustrated with an internal organ, the spleen, and an external organ, the fin, and two properties. One property is species diversity, which is the number of species from which cell lines have been developed from the same organ (e.g., spleen). Currently, spleen cell lines have been developed from 13 species: Asian sea bass (Parameswaran et al. 2006), black porgy (Tung et al. 1991), Chinook salmon (Semple et al. 2018), Japanese flounder (Kang et al. 2003), mandarin fish (Dong et al. 2008), orange-spotted grouper (Huang et al. 2018), rainbow trout (Ganassin and Bols 1998), red-spotted grouper (Huang et al. 2009), sea perch (Tong et al. 1998), silver perch (Ellender et al. 1979), walleye (Vo et al. 2015b), white sturgeon (Hedrick et al. 1991), and zebrafish (Xing et al. 2009). The second property is size, the number of cell lines from a particular fish organ (e.g., fin). The fin invitrome appears to be the largest of the organ invitromes, with approximately 133 cell lines (Laizé et al. 2022).
Only a few cell lines have been obtained from biological fluids. Most have been from blood. These include several immune cell lines from the channel catfish (Vallejo et al. 1991; Miller et al. 1994). Others are from the American eel (DeWitte-Orr et al. 2006), catla (Chaudhary et al. 2012), and the common carp (Faisal and Ahne 1990). A few cell lines have been from rainbow trout reproductive fluids, milt (RTmilt5) and ovarian fluid RTovfl (Vo et al. 2015a, b).
Properties of the organism
In addition to these two types of “before-the-fact” properties, the organism or fish species from which the sample was taken to start the cultures has numerous sets of properties. Discussed below are three examples: taxonomy, natural history, and significance to humans.
Taxonomy
Species of origin is a fundamental property of a cell line and can be used to categorize them, beginning with the broadest category “fish.” For the general public, “fish” means cold-blooded aquatic animals that have fins and breathe with gills. Scientifically, “fish” are vertebrates under the phylum Chordata and the term denotes primarily Agnatha (jawless fishes), Chondrichthyes (sharks and rays), Sarcopterygii (lobe-finned fishes), and Actinopterygii (ray-finned fishes) (Ng et al. 2017). No cell lines appear to have developed from Agnatha and Sarcopterygii and only a few from Chondrichthyes (Parton et al. 2010). Therefore, this review is confined to the cell lines from ray-finned fishes, but in this paper fish, ray-finned fish and Actinopterygii will be used synonymously. The number of fish cell lines increases each year (Thangaraj et al. 2021; Laizé et al. 2022), and one way to keep track of the number is to consult Cellosaurus, an online cell line resource that monitors the scientific literature for new cell lines (Bairoch 2018). Based on Cellosaurus (https://www.cellosaurus.org/), the fish invitrome currently has 864 cell lines (release 43, September 2022).
The Actinopterygii or ray-finned fish invitrome could potentially be subdivided around several phylogenetic terms: clade, class, subclasses, and infraclasses. Actinopterygii is a clade, which is a group of classes with a common ancestor. The Actinopterygii is made up of two classes: Cladistia and Actinopteri. The bichirs are the only living species in Cladistia and appear not to have been a source of any cell lines. The Actinopteri are made of two subclasses: Chondrostei and Neopterygii. The sturgeons belong to the Chondrostei and have been the source of several cell lines (Hedrick et al. 1991; Ryu et al. 2018). The Neopterygii is divided into two infraclasses: Holostei and Teleostei. The only members of the Holostei are the bowfin and the gars. For the Holostei, one cell line, GARL, has been reported. GARL remains to be characterized thoroughly but has proven useful in comparative immunology (Liu et al. 2019). The Teleostei contains more than 34,000 species (Dornburg and Near 2021), and except for GARL and the sturgeon cell lines, is the source of all other fish cell lines. Thus, by-and-large, fish cell lines are teleost cell lines.
The teleost invitrome can potentially be subdivided in different ways. With the advent of rapid, large-scale DNA sequencing of teleost genomes, new phylogenetic and taxonomic relationships are being proposed (Santini et al. 2009; Betancur-R et al. 2017; Ravi and Venkatesh 2018). For example, teleosts are now thought to consist of three primary groups: Osteoglossomorpha, Elopomorpha, and Clupeocephala (Takezaki 2021). Subdivisions could also be made around teleost clades, such as Otocephala, and subclades, such as Percomorpha (Dornburg and Near 2021). However, most invitromaticists, who are scientists focused on animal cell culturing (Bairoch 2018), are less familiar with these terms and have by-and-large used taxonomic ranks. A taxonomic hierarchy is a sequence of ranks in increasing or decreasing order and will be used here to categorize teleost cell lines (Fig. 1). This makes categorization easier to integrate with past fish cell line compilations, which have been around a few taxonomic rankings, usually species and family. Therefore, conventional hierarchical ranks will be used to subdivide the teleost invitrome. The precise numbers for each teleost rank vary in the literature because new fish species are found each year and because of taxonomic debates. Consulting the previously mentioned reviews and an online resource for fish data (https://www.fishbase.de/home.htm) will give an idea of the range. Here, the following numbers will be used for each teleost rank: ~ 70 orders, ~ 500 families, ~ 3000 genera, and ~ 34,000 species (Fig. 1). These provide context for where cell lines have been obtained from in the past and for where they should be sought in the future.
The order Salmoniformes and the species Oncorhynchus mykiss (rainbow trout) will be used to illustrate how a taxonomical hierarchy can be used to define invitromes and how most Salmoniformes cell lines come from just a few genera and species. The Salmoniformes has 1 family, the Salmonidae or salmonids, 11 genera, and between 226 and 252 species. The species numbers and number of species in particular genera occasionally are uncertain because teleost taxonomy continues to progress. The Salmoniformes has been the source of 129 cell lines (https://www.cellosaurus.org/; Bols et al. 2017; Pham et al. 2020). Of the 11 salmonid genera, cell lines have been developed from 5 genera: Oncorhynchus (Wolf and Quimby 1962), Salmo (Nicholson and Byren 1973), Stenodus (Follett and Schmitt 1990), Salvelinus (Cheng et al. 1993), and Coregonus (Grunow et al. 2021). The genus Oncorhynchus has between 11 and 16 species, depending on the status of subspecies (https://www.fishbase.de/home.htm). Cell lines from this genus have been developed from 6 species: O. mykiss (rainbow trout) (Wolf and Quimby 1962), O. tshawytscha (Chinook salmon) (Fryer et al. 1965), O. nerka (sockeye) (Lannan et al. 1984), O. keta (chum) (Watanabe et al. 1980), O. kisutch (coho) (Lannan et al. 1984), and O. masou (Masu) (Moritomo et al. 1988). Oncorhynchus invitrome has 99 cell lines. The bulk of these is in the rainbow trout invitrome, with 74 cell lines (https://www.cellosaurus.org/). These are from different life cycle stages and anatomical sites, as described later.
Currently, fish invitromes are expanding rapidly. Several fish cell line compilations have appeared in the literature over the last 45 yr. In 1980, cell lines were reported from 36 species (Wolf and Mann 1980), and by 1994, cell lines had been developed from 74 species, including hybrids (Fryer and Lannan 1994). Relying on Cellosaurus (Bairoch 2018), Goswami et al. (2022) reported that more than 590 species had been the source of cell lines. The ray-finned fishes comprise more than 34,190 species with most being teleosts (Dornburg and Near 2021). Therefore, the species represented in cell lines is roughly 2% of the fish species, whether ray-finned fishes or teleosts. The usual justifications for obtaining cell lines from a new species have been based on the species having some biological properties and/or significance to humans that is not captured with existing cell lines. Clearly, expecting to have cell lines from all fish species is unrealistic. However, with improved understanding of fish phylogenetic relationships, cell lines from one species might be chosen to be representative of higher taxonomic groups.
Natural history
Grouping cell lines around properties of the starting organism is an attempt to begin assimilating information on cells with information on higher levels of biological organization. “An intact and functioning organism encompasses all levels of biological integration” and is a vehicle “into which a vast range of biological data can be fitted” (Bartholomew 1986). The organism properties will include genetics, development, morphology, and physiology but also the natural history of species, such as habitat, distribution, and behavior (Greene 2005). In the past, cell lines have been grouped around habitat, but very few other properties. About 22% of the cell lines were noted in 1980 to be from marine species (Wolf and Mann 1980), but now marine species are slightly more represented in cell lines than freshwater species (Laizé et al. 2022). In the future, habitat could be subdivided further, providing many other kinds of categorizations, with a cave fish invitrome being an exotic example. Another feature is the geographical distribution of the species. This was done by Pham et al. (2020), with two examples being the Great Lakes Basin and North Pacific Ocean invitromes. Considering the diversity and complexity of life histories that arise from approximately 34,000 fish species, cell lines can probably be grouped in hundreds of ways around the attributes of the starting organisms.
Significance to humans
The varying significances of fish species to humans also provide themes around which the cell lines can be grouped. One example is the cell lines established from endangered fish species, which has been done in the hope of preserving their germplasm. The Yangtze sturgeon (Liu et al. 2020) and mahseer (Yadav et al. 2012) are endangered and have been the source of cell lines. These and cell lines from other endangered fishes would constitute the endangered fish invitrome. Many other examples of categorizing cell lines by their import to humans can be imagined. Categories could be the cell lines from fish species that are in aquaculture and the cell lines from fish species under jurisdictional control of a national government. Another could be the significance of the species to the indigenous people of the country of origin; for example, some fish species are considered treasured (taonga) species to the Māori people of New Zealand. Again, these are just a few examples of how invitromes can be organized around the significance of the cell line species to humans.
Properties of the scientists
Cell lines can be grouped by using descriptors of the laboratory or scientists that first established and reported the cell lines. Multiple properties of the laboratory could be considered such as who was the principal investigator or what funding agency supported the cell line development. Lab location is one property that has been used recently to group fish cell lines. For the decade 2010–2020, the Chinese and Indian invitromes for fish cells grew by 78 and 65 respectively (Thangaraj et al. 2021).
“After-the-fact” or post factum properties and invitromes
Properties of a cell line that come into being after the start of the primary cell culture that gave rise to the line are being termed here “after-the-fact” or post factum properties. These properties are revealed through the development and characterization steps of invitromatics and from the results of using the cell lines for different purposes (invitroomics). Post factum properties can range from something very general, like cell shape, to something very specific, like susceptibility to a particular virus. Categorizing cell lines on the basis of these properties gives post factum invitromes. Specific post factum invitromes are based on specific post factum properties. The potential number of post factum properties is probably very large, depending on how much a cell line has been used in the past and will be studied in the future. These properties are realized in different ways. For the sake of simplicity, these ways or processes are identified in this article as being of four types: development, characterization, experimentation, and storage. Figure 2 illustrates how these four processes and post factum properties arising from them can be used to broadly subdivide the ray-finned fish invitrome. The categorization of fish cell lines based on properties acquired during development, characterization, experimentation, and storage is briefly discussed under these headings in the following sections.
Properties that appear during cell line development
From the initiation of primary cultures to begin the development of a cell line to the cryopreservation of the cell line, the cellular morphology and growth characteristics of the cell line become apparent. These simple properties can be used to categorize cell lines.
Cell shape
The cellular morphology of fish cell lines is judged by examining cultures with an inverted phase contrast microscope, and usually is reported as belonging to one of a few shape categories. Most fish cell lines are reported as having either epithelial-like (epithelioid) or fibroblast-like (fibroblastoid) shapes (see lists in Bols et al. (2017) for rainbow trout; Thangaraj et al. (2021) for other species). Yet other morphologies also have been noted frequently, such as spindle-shaped (Gignac et al. 2014) and endothelial-like (Vo et al. 2015a, b). The spindle-shape is associated with myogenic cells, as will be discussed later in the section on trunk skeletal muscle cell lines. The endothelial-like shape is often used synonymously with cobblestone shape and can be accompanied by the cells organizing on the culture surface into capillary-like tubes (Vo et al. 2015a, b). A few cell lines are described as monocyte or macrophage-like (Vallejo et al. 1991; Bols et al. 2003).
Although simple to observe, cell shape as a property of cell lines can be problematic. The reported shape is subjective and depends to a degree on when the culture is observed. During cell line development, early cultures often appear heterogenous, but with passaging, cultures become more homogenous, with one predominant cell shape. This evolution of culture appearance is most obvious when a cell line is started by outgrowth of organ fragments (explant outgrowth), but also is apparent even when attempts have been made to purify a specific cell type from an organ prior to putting them into primary cultures. In the case of the endothelial cell line eelB, the dominant shape did not emerge until late-passage cultures (Bloch et al. 2016). A mixture of cell shapes has been noted for some fish cell lines and appears to be a stable feature of them. This is discussed later in the section on the property of stemness, with RTL-W1 as the example. The shape of cells also changes subtly within the life span of a single culture as cells grow from low cell density at the beginning to form a confluent monolayer at the end of the culture. The rainbow trout spleen stands out for having given rise to cell lines with a diversity of shapes. One is RTS11, which is macrophage-like (Ganassin and Bols 1998). Others are the endothelial-like TSS (Fierro-Castro et al. 2012) and fibroblast-like RBS (Wolf and Quimby 1969). Yet for other spleen cell lines, cellular morphology appears more complex, with RTS being described as pleomorphic (Moritomo et al. 1990), and RTS34st as being a mixture of fibroblast- and epithelial-like cells (Ganassin and Bols 1999).
Growth properties
Growth for most fish cell lines is anchorage dependent and described as continuous or immortal. However, a small invitrome of anchorage-independent cell lines exists. One member is RTS11, which rose from progeny cells being released into the medium from an adherent spleen stromal layer (Ganassin and Bols 1998), and another is a CHSE-214 variant, which was adapted to grow in suspension (Nakano et al. 1993). Some cell lines appear to be capable of growth in agar (Malhao et al. 2013), but this property is not routinely evaluated.
Nearly all fish cell lines appear to have arisen through “spontaneous immortalization” (Bols and Lee 1994), but the necessity for a period of culture deterioration or “crisis” for immortalization to emerge is unclear. A “crisis” has been noted in the establishment of only a few fish cell lines. In the development of catfish leucocyte cell lines, cultures were observed to deteriorate approximately 4 wk after they had been initiated (Barker et al. 2000). This was followed by the reactivation of telomerase activity and continuous cell proliferation (Barker et al. 2000). Culture deterioration was observed later, at 12–15 passages during the establishment of a goldfish cell line (Li et al. 2021) and at 37–43 passages for an Atlantic mackerel cell line (Saad et al. 2022). However, most often a “crisis” has not been mentioned in the context of fish cell line establishment, suggesting a period of culture deterioration either does not occur or fails to be noticed. As a transitory event whose timing, intensity, and duration might vary between species, a “crisis” might be missed. For the development of some rainbow trout and walleye cell lines, small patches of cells appeared to senesce and die in primary cultures but whether this constituted a “crisis” or senescence was difficult to decide (Vo et al. 2015a, b). The results for senescence-associated β-galactosidase (SA β-Gal) staining were complex. During the development of some cell lines, the number of cells staining for SA β-Gal decreased from early to late passage cultures. On the other hand, a few endothelial-like cell lines, WEBA from walleye bulbus arteriosus (Vo et al. 2015c) and eelB1 from the American eel brain (Bloch et al. 2016), continuously stained for SA β-Gal. For other cell lines, cells rarely ever stained for SA β-Gal at any point during their development (Vo et al. 2015a, b). A molecular explanation for the apparent immortal behavior of some fish cells has been offered (Futami et al. 2022). This is the absence of the p16INK4a/ARF locus in the fish genome. Interestingly, senescence can be induced experimentally by treating an immortal cell line with 5-aza-2ʹ deoxycytidine (Futami et al. 2019).
Properties revealed during cell line characterization
The results of cell line characterizations done in the past by simple procedures and in the future with more sophisticated technologies should allow current and future cell lines to be categorized in numerous ways. Characterizations can be considered to proceed along four paths, defined in part by the cellular endpoint being evaluated and in part by the evaluation technology. These four characterization paths are briefly discussed below.
Cytoskeletal and junctional proteins
The cytoskeleton and cell junctions are complex protein structures of most cells but differences in their protein constituents and organization can be revealed by the relatively simple techniques of fluorescent staining, making this one rout to characterizing and categorizing cell lines. The American eel brain cell line EelB-1 is an example of categorization through cytoskeletal and junctional proteins (Bloch et al. 2016). The cytoskeleton usually has been easier to examine because fluorescently conjugated phalloidin detects F-actin in all cells and several commercially available antibodies to mammalian cytoskeletal proteins detect their fish equivalents. However, the distribution of intermediate filament proteins in fish tissues is more complex than in mammals (Herrmann et al. 1996) and so the categorization of cell lines on this basis is less clear. Several monoclonal anti-bodies to α-tubulin and to acetylated α-tubulin work well. When applied to a set of eight walleye cell lines, these revealed a cytoplasmic tubulin network in all cell lines but strong acetylation of this network in only three (Vo and Bols 2016). These might prove to be useful characterization parameters as more information becomes available with other cell lines.
Functional or lineage markers
During routine cell culturing, several fish cell lines appear to continuously express specific functional or lineage markers. Some examples are from the immune system and might be considered to belong to the immune invitrome. These include two from the rainbow trout: the monocyte/macrophage RTS11 from the spleen (Ganassin and Bols 1998), and the T cell HK1532 from the head kidney (Maisey et al. 2016). RTS11 express major histocompatibility (MH) class II polypeptides (Kawano et al. 2010), and HK 1532 are CD4 + (Maisey et al. 2016). Other examples of this category are likely to emerge as more antibodies for cell surface markers and other proteins become available for fish.
Stemness and differentiation
Stem cells and stemness are both simple and sophisticated concepts, and if described more completely for fish cell lines, the cell lines could be categorized into “differentiation” invitromes and their utility improved. For fish, most attention has been given to embryonic stem cells (ESCs). ESCs are cells from early-stage embryos that are pluripotent, which means that they can generate cells of all three germ layers (ectoderm, mesoderm, and endoderm). Although many cell lines have been established from fish embryos (see lists in Bols et al. (2017) for rainbow trout; Thangaraj et al. (2021) for other species), pluripotency has been evaluated in only a fraction of them. A few of these appear pluripotent by several in vitro and in vivo characteristics (Ma et al. 2001; Hong et al. 2011; Verges-Castillo et al. 2021). The status of other fish embryo cell lines remains to be delineated. However, an embryo cell line from the common killifish was found to be myogenic (Gignac et al. 2014), and another, ZEB2J from the zebrafish blastula, appeared to be nullipotent (Xing et al. 2008). With new knowledge being gained about pluripotent marker genes in fish (Li et al. 2022), many more embryo cell lines could be tested in the future for their differentiation potential.
Adult stem cells (ASCs) are intensively studied in mammals where they appear later in development, persist in small numbers in organs throughout life, and retain the capacity to renew and differentiate. They also can be plastic. This makes identifying the origins of ASCs more complex. ASCs could be a stable property of a discrete cell population or a property acquired in a specific context (Zipori 2004; Clevers and Watt 2018). A philosophical analysis by Laplane and Solary (2019) offers four types of properties that constitute stemness: categorical, dispositional, relational, and systemic. As a categorical property, stemness is intrinsic and independent of any interaction with surrounding entities. As a dispositional property, stemness is also intrinsic but becomes apparent upon interaction with external stimuli. As a relational property, stemness requires interaction entities, such as a specific niche. Finally, as a systemic property, stemness is an extrinsic characteristic that is provided and maintained by the system.
Although much less studied in fish, ASCs possibly exist within the population of cells that make up some fish cell lines. The case for this involves four considerations. Firstly, the presence of differentiated cells within cultures of the cell line needs to be established, as this constitutes the strongest evidence for the presence of ASCs or progenitors. The term progenitor cells could be used for situations in which only one differentiated cell type is evident in cultures. Secondly, consideration should be given to possible origins for ASCs/progenitors in cell lines. Thirdly, efforts to identify the ASCs/progenitors within the cell cultures by using molecular markers should be described. Finally, how stemness is maintained within cultures of the cell lines should be considered.
The case for some fish cell lines having ASCs/progenitors will be made by using as an example RTL-W1 which was established from the rainbow trout liver (Lee et al. 1993). Malhao et al. (2013) found that overall RTL-W1 did not share morphological features with differentiated hepatocytes. However, when allowed to aggregate into 3-dimensional (3D) spheroids, which for many mammalian cell systems provides a stem cell niche and this leads to differentiation (Forte et al. 2017), RTL-W1 displayed ultrastructural characteristics similar to those of differentiated hepatocytes (Lammel et al. 2019). Thus, the presence of progenitor cells is implied. As to their origin, at least two possibilities exist. One is bile preductular epithelial cells, which are thought to be stem cells in the rainbow trout liver (Okihiro and Hinton 2000). However, hepatocytes are also possibility because mammalian hepatocytes have been reported to dedifferentiate in culture to become stem cells (Chen et al. 2012). The nature of the stemness in RTL-W1 cultures is open to debate. An intrinsic and dispositional nature is suggested by two observations. Firstly, anti-cytokeratin monoclonal antibodies, AE1/AE3, stained all cells in 2 D RTL-W1 cultures but in the liver stained only biliary epithelial cells (Malhao et al. 2013), which are likely to be stem cells (Okihiro and Hinton 2000). Secondly, hepatocytes formed in spheroid (3D) cultures (Lammel et al. 2019).
However, during the conventional (2D) maintenance cycle of cell trypsinization, plating, and growth to confluency, RTL-W1 cultures show a heterogeneity that is stable, raising a question about the role of stem cells in this. In the original RTL-W1 description (Lee et al. 1993), cell shape was described as predominantly polygonal or epithelial-like, but as cultures became confluent, bipolar or fibroblast-like cells appeared and piled up into ridges between which were monolayers of epithelial-like cells. This behavior has now been observed over approximately 35 yr of RTL-W1 being maintained. Independently, Malhao et al. (2013) found two cell populations based on size and ultrastructure and also commented on the stability of appearance in RTL-W1 cultures. These observations suggest that RTL-W1 cultures maintain an equilibrium among several cell populations and that perhaps the cell line should be considered a system, regulating one or more stem cell populations. Although unraveling these questions is challenging, RTL-W1 is still extremely useful in environmental toxicology (Clemons et al. 1998) and more knowledge about their stemness should enhance their value and serve as a model for understanding the stem cell properties of other fish cell lines.
Omic profiles
Application of omic procedures to the characterization of fish cell lines should lead to innumerable new groupings. Omics includes genomics, epigenomics, transcriptomics, proteomics, metabolomics, and glycomics (Blochl et al. 2021; Nevedomskaya and Haendler 2022), and these techniques are being applied singly or in combinations to characterize large groups (< 500) of mammalian cell lines, especially those from tumors (Salvadores et al. 2020; Yang et al. 2020). At this time, the applications of omics to fish cell lines have been few. However, proteomics has been applied to a few fish cell lines (Wagg and Lee 2005; Martin et al. 2007; Goswami et al. 2015).
Properties revealed during experimentation with cell lines
As cell lines are used for different experimental purposes, new properties of cell lines can become of interest and these properties can be used to categorize cell lines in new ways. This is illustrated below in brief overviews of the kinds of properties that can materialize as fish cell lines are used in virology, ecotoxicology, and genetic engineering.
Relationships to viruses
Adding viruses to cultures of cell lines can reveal the supportive, non-supportive, and partially supportive viral invitromes (Pham et al. 2020). For a particular virus, the supportive invitrome would be all the cell lines that were capable of producing infectious virions. Supportive cell lines would be synonymous with permissive or susceptible cell lines, except that no infection mechanism, such as viral receptors, is implied. The partially supportive invitrome would be cell lines in which one or more genes of a particular virus are expressed but the virus is not produced. The non-supportive viral invitrome would constitute cell lines that express no viral genes and produce no virus and might also be called non-susceptible. For some important fish viral pathogens, such as infectious pancreatic necrosis virus (IPNV), the supportive invitrome is reasonably well known (Lorenzen et al. 1999; Bols et al. 2017; Ruiz-Palacios et al. 2020). By contrast, in fish virology, little attention has been paid to the non-supportive cell lines, which might be noted only in passing but rarely are the focus of investigation. The exception is piscine reovirus (PRV), which has a large non-supportive invitrome that consists of 31 cell lines from 14 species and from multiple organs (Pham et al. 2020). In this case, PRV appears “uncultivable” in cell lines. However, the case can be made that even for “cultivable” viruses attention should be paid to the non-supportive invitrome. Firstly, grouping cell lines into supportive and non-supportive invitromes can give insight into the cell and species tropism of a virus. Secondly, non-supportive cell lines can be used as surrogates for bystander cells (Pham et al. 2020). In the course of an infection, fish will have both infected cells and non-infected cells (bystanders). Non-supportive cell lines will allow study of how bystanders from different organs interact with and respond to virus-infected cells.
Responses to ecotoxicants
Fish cell lines are generally useful for evaluating the relative toxicity of environmental contaminants (Segner 1998; Schirmer 2006), but contaminants belong to different chemical classes and some cell lines share properties that make them uniquely helpful for studying specific classes (Bols et al. 2005; Stadnicka-Michalak et al. 2018). An example is the halogenated aromatic compounds. These include polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins (PCDDs), of which 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin) is the most studied. Many halogenated aromatic compounds exert their toxicity through the aryl hydrocarbon receptor (AhR) signaling pathway and are referred to as dioxin-like compounds (Zhang et al. 2021). Some fish cell lines express the AhR and respond to dioxin-like compounds with the induction of CYP1A1 (Billiard et al. 2002; Bols et al. 1999; Clemons et al. 1998) but others do not (Bloch et al. 2016). Thus, cell lines can be considered to belong or not to the dioxin-responsive invitrome.
Genetic engineering
Cell lines can be grouped on the basis of their common genetically engineered properties. Increasingly sophisticated engineering techniques are being applied to a few fish cell lines (Gratacap et al. 2020). Often the engineered cell lines express a fluorescent marker, such as a form of green fluorescence protein (GFP) (Dehler et al. 2016), and can be used for a variety of purposes, such as in co-culture experiments (Xing et al. 2008), and be considered to belong to the fluorescent reporter invitrome.
Property of cell line availability from storage
Although most fish cell lines are easily cryopreserved, their availability will depend on the administration of their long-term storage. To account for this, Bols et al. (2017) proposed that cell lines can be categorized as belonging to one of three main storage invitromes: curated, informally shared, and zombie. The curated invitrome is all the cell lines that are in national and international repositories, such as the American Type Culture Collection (ATCC), and are available for a fee. The informally shared invitrome is all the cell lines that scientists develop and make available to others through informal agreements. The zombie invitrome is the cell lines that have been noted in the literature but whose storage status and thus availability are uncertain. Zombie cell lines might or might not be in some liquid nitrogen dewar waiting to be thawed so that they can serve science.
Fish skeletal muscle invitromes
Fish skeletal muscle will be used as an example of how the cell lines from a specific organ can be categorized into invitromes. The skeletal muscle of fishes, as in other vertebrates, consists of the head and trunk skeletal muscles. These have different embryonic origins and distinctive functions. Paraxial somitic mesoderm gives rise to the skeletal muscle of the trunk, whereas head skeletal muscle is largely derived from pre-chordal and non-somitic paraxial head mesoderm (Shih et al. 2008). Trunk skeletal muscle is responsible for locomotion. By contrast, the functions of head skeletal muscle are varied. For example, facial musculature is involved in food capture and gill ventilation (Datovo and Rizzato 2018), and extraocular muscles control movements of the eye (Spencer and Porter 2006).
Fish cell lines have been developed from both the head and trunk skeletal muscles. However, the head skeletal muscle invitrome is small. Two cell lines were established from below the mouth. These are SHMS from the snakehead and BTMS from the bluefin trevally (Zhao et al. 2004; Zhao and Lu 2006). Additionally, the extraocular muscle of the eye appears to have been the origin of a snow trout cell line (Kumar et al. 2020). By contrast, the trunk skeletal muscle invitrome is much larger. In the following sections, this invitrome is used to illustrate how “before-the-fact” (Table 1) and “after-the-fact” (Table 2) properties can be used to organize the cell lines from an organ.
Fish trunk skeletal muscle invitromes by before-the-fact or ante factum properties
The samples that have been used to start skeletal muscle cell lines have been from fish of different life cycle stages and from variable regions of the trunk, although the descriptions have sometimes been vague (Table 1). One (KFE-5) was from a late-stage embryo, several were from young or juvenile fish, and most were from adult fish. Trunk sampling sites often have been described rather imprecisely, such as simply “muscle.” However, regional differences can be found along the trunk axis, for example, fewer fast-twitch fibers in the caudal region (Altringham and Ellerby 1999). Also, the distribution of cellular components in trunk skeletal muscle can differ between fish species, for example, adipocytes (Kaneko et al. 2016). In the past, describing the starting material in detail might have been overlooked in the excitement of initiating a cell line or been overwhelmed by all the subsequent details of cell line development. In the future, more careful sample descriptions should enhance the value of the cell lines that arise from trunk skeletal muscle.
The fish trunk skeletal muscle invitrome has a very limited taxonomic range (Table 1). Cell lines have been obtained from 21species. WSBM is from the white sturgeon Acipenser transmountanus, which belongs to the subclass Chondrostei. The Chondrostei has approximately 30 species in two orders and three families. All the other skeletal muscle cell lines are from species in the infraclass Teleostei. These species belong to 19 genera (Table 1, second column), 15 families, and 11 orders (Table 1, last column). Thus, fish skeletal muscle cell lines have been developed from < 0.1% of the species, < 1.0% of the genera, < 3% of the families, and < 15% of the orders for teleosts.
The cell lines from trunk skeletal muscle are from species with different biological features or natural histories. These might allow the establishment of correlations between cell line properties and organismal properties and begin the “integration and reciprocal illumination” between cell and organismal biology, to quote a phrase about molecular biology and organism diversity (Greene 2005). Currently, the number of species, twenty, is too restricted to encompass the full range of some organismal properties, such as swimming behavior. Fish can swim in many different ways (Wardle et al. 1995), with two locomotion groups being median-paired fin (MPF) gaits and body-caudal fin (BCF) gaits. Unfortunately, most of the species in Table 1 have BCF gaits (Friedman et al. 2021) and the status of the other species is uncertain. Instead, Table 1 shows another organismal property, maximum longevity. This shows a much greater range among the species in Table 1, with both short- and long-lived fishes, though this information is not available for some species. For white sturgeon, fathead minnow, zebrafish, goldfish, American shad, turbot, largemouth bass, rainbow trout, bluegill, spotted sea trout, and Atlantic croaker, values for maximum longevity were from the AnAge Database (https://genomics.senescence.info/species/index.html) and the value for the brown bullhead was from the Animal Diversity Web (https://animaldiversity.org). The primary literature was a source of values for Korean rockfish (Kolora et al. 2021) and only an average lifespan is given for the killifish.
Most of the species represented in the trunk skeletal muscle invitrome are important in capture fisheries, in aquaculture, or in sports fishing (Table 2), which has also been noted as recreational or game fishing. The exceptions are a few species widely used as research models: fathead minnow in aquatic toxicology (Ankley and Villeneuve 2006), killifish in evolutionary physiology (Crawford et al. 2020), and zebrafish in multiple disciplines (Meyers 2018b, a).
Fish trunk skeletal muscle invitromes by after-the-fact or post factum properties
Currently, the cell lines from fish trunk skeletal muscle are very poorly characterized and the only parameter consistently noted for all of them is their cellular morphology (Table 2, second column), which is usually described as being either spindle-shaped, fibroblast-like, or epithelial-like. For cell cultures initiated from vertebrate skeletal muscles, spindle-shaped cells are often noted, implying the presence of myoblasts. Thus, on the basis of cellular morphology, the fish trunk skeletal muscle invitrome appears to be made up of myogenic, fibroblastic, and epithelial cell lines. However, this simplicity hides the complexity of trunk skeletal muscle as a source of cell lines.
Adherent cell lines could arise from several compartments of vertebrate skeletal muscle and from different stem cells. Myofibers are one compartment. Beneath the basal lamina of myofibers in both mammals and fishes is a population of stem cells known as muscle stem cells (MuSCs) or satellite cells (Baghdadi and Tajbakhsh 2018; Sultan et al. 2021). Another compartment is the stromal space between myofibers. This contains connective tissue cells, such as fibroblasts, and mesenchymal progenitor cells (Wosczyna et al. 2019; Ritso et al. 2022). Among possible mesenchymal progenitors, most is perhaps known about fibro/adipogenic progenitor cells (FAPs). FAPs can differentiate into adipocytes, myofibroblasts, osteocytes, and chondrocytes (Contreras et al. 2021). A third compartment is the skeletal muscle vascular network (Ritso et al. 2022). This has endothelial and mural cells (Ritso et al. 2022). Mural cells include vascular smooth muscle cells and pericytes. Pericytes are possibly multipotent progenitor cells (Cappellari and Cossu 2013; Birbrair et al. 2017). Except for myogenic cells that differentiate into myotubes, distinguishing these different cell populations in cultures is difficult, even for mammalian cells where multiple markers are available. Despite this, the myogenic, fibroblastic, and epithelial nature of fish skeletal muscle cell lines is discussed below.
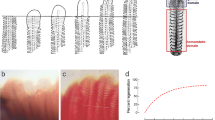
Only six of the fish skeletal muscle cell lines have been characterized sufficiently to be considered myogenic, with most being described as having a spindle shape (Table 2). Figure 3A illustrates an example of cells with a spindle shape in cultures of the myogenic cell line KFE-5 from the killifish (Gignac et al. 2014). Other myogenic cell lines are POMSCS (2n) and POMSCS (3n) from the olive flounder (Peng et al. 2016), OUC-SebSc-Skm-1 from the black rockfish (Kong et al. 2021), and Mack1 and 2 (Mack cells) from the Atlantic mackerel (Saad et al. 2022). KFE-5 expressed α-actinin and desmin, and formed multinucleated, striated cells that stained for myosin. POMSCS (2n) and POMSCS (3n) expressed pax7B, which is a transcription factor associated with myogenesis, and desmin, which is a muscle-specific intermediate filament protein. When the cells were switched to a differentiation medium, which contained basic fibroblast growth factor (bFGF), they formed myotubes and were positive for myosin heavy chain (MyHC). Thus, these cell lines have been thought to represent a mixture of satellite stem cells and committed satellite cells of skeletal muscle (Peng et al. 2016). The black rockfish cell line expressed MyoD, myoblast determination protein 1, and was considered to be predominantly composed of myoblasts (Kong et al. 2021). When cultures were switched to a differentiation medium (DMEM/F12 with 2% horse serum), myotubes were present by 72 h afterwards. Mack1 expressed Pax 7 and MyHC but did not stain for MYOD and MYOG (Saad et al. 2022). In adipogenic medium, Mack1 accumulated lipids and thus appear to be capable of both myogenesis and adipogenesis. For mammals, myogenic cell lines have been found to differ from each other in their transcriptome and metabolism (Abdelmoez et al. 2020). As more fish myogenic cell lines become available in the future, heterogeneity among them will be interesting to watch for.
Phase-contrast appearance of cultures of two muscle cell lines, KFE-5 and CAtmus1PRF. Phase-contrast photomicrographs of a culture of the myogenic KFE-5 from killifish embryos show many spindle-shaped cells (A), whereas in a culture of CAtmus1 PRF from the skeletal muscle of the Australasian snapper, fibroblast-shaped cells dominate (B). Scale bar = 200 μm.
On the basis of shape, most of the fish trunk skeletal muscle cell lines are fibroblast-like (Table 2). Figure 3B illustrates an example of fibroblast-like cells in cultures of the cell line CAtmus1PFR from the Australasian snapper (Chong et al. in press). As noted earlier, this morphology could encompass several cell types from the connective tissue and vascular network of skeletal muscle and could include stem cells. For example, on occasion myoblasts appear to have shapes similar to fibroblasts (Swailes et al. 2006). Efforts to characterize the fibroblast-like cells of Table 2 for cytoskeletal and surface markers and for their capacity to differentiate into different cell types should make the skeletal muscle invitrome much more useful as a research tool. For example, CAtmus1PRF recently has been shown to differentiate into myofibroblasts. This is based on their response to transforming growth factor β1 (TGF β1). TGF β1 increased the expression of smooth muscle actin (SMA) and the formation of actin bundles but impeded cell migration (Chong et al. in press). Myofibroblasts are critical cells in skeletal muscle architecture and repair and thus could contribute to flesh quality in fish aquaculture.
The fish trunk skeletal muscle invitrome has four epithelial-like cell lines (Table 2). These have been subjected to few studies on their basic cellular properties or to little speculation about their possible origins among skeletal muscle cell populations. Two possible origins will be speculated upon here, but research focused on this question will be needed to find answer(s). The first speculation is that one or more of these cell lines started out fibroblast-like but with passaging became epithelial-like. This is the mesenchymal-epithelial transition (MET) and has been observed during the development of some human cell lines (Becerril et al. 2021). However, a MET appears not to be responsible for FHM because during their development the FHM cells were always described as epithelial (Gravell and Malsberger 1965). Another speculation is that one or more of these cell lines could have arisen from vascular smooth cells. In cultures, these cells can have variable shapes and with passaging the dominant shape can change (Chamley et al. 1977). Interestingly, FHM cells were capable of gap-junction mediated-cell communication (Slater et al. 1983). This is a feature of vascular smooth muscle cells, although also a property of other cell types.
At least three of the cell lines from fish trunk skeletal muscle are curated, and one (Mack1) has just been made commercially available, but the availability and storage status for most of the others is uncertain (Table 2). FHM, BB, and BF-2 are held in several cell banks. For example, the American Type Culture Collection (ATCC) sells FHM as CCL-42, BB as CCL-59, and BF-2 as CCL-91. Since their development over 40 yr ago, these cell lines have been used consistently. By contrast, cell lines that appear not to have been used since they were described over 40 yr ago are referred to zombie cell lines. Whether zombie cell lines are still cryopreserved and can be made available to researchers is unknown and difficult to investigate. Cell lines that have been developed in the last 40 yr but have not been curated are listed in Table 2 as being part of the informally shared invitrome. For these cell lines, the scientists who developed them can probably be contacted and asked about their storage status and their availability to other researchers.
To date the main use of the fish trunk skeletal muscle invitrome has been in virology. The Manual of Diagnostic Tests for Aquatic Animals 2021 recommends FHM and BF-2 for the detection of several viral pathogens. Many other fish skeletal muscle cell lines also support the replication of viruses (Table 2). These have been either epithelial or fibroblast-like lines. In the future, myoblast cell lines also might be interesting subjects for virology. Sleeping disease virus (SDV) or salmonid alphavirus 2 causes necrosis and atrophy of red skeletal muscle in rainbow trout (Biacchesi et al. 2016). Through the use of primary cultures, Biacchesi et al. (2016) were able to show that SDV had a tropism for satellite cells. A myoblast cell line might allow this to be investigated further.
Outside of virology, the fish trunk skeletal muscle invitrome has been applied to only a few other disciplines (Table 2, last column). Most applications have appeared in the first publication of the cell line and have been done for the purpose of showing the potential utility of the cell line. For example, the myogenic cell lines from the Atlantic mackerel, olive flounder, and rock fish can be used to study fish muscle growth and differentiation and in cellular agriculture. They could become the fish equivalents of the widely used mammalian myogenic cell lines L6 from the thigh muscle of newborn rats (Yaffe 1968) and C2C12 from the thigh muscle of mice (Blau et al. 1983). A feature of L6 and C2C12 is that they have been used in thousands of studies by hundreds of independent research groups for a diversity of purposes. A few recent review articles illustrate this. These summarize the contribution of L6 and C2C12 to studies on the cell biology of skeletal muscle exercise (Carter and Solomon 2019), the actions of vitamin D in skeletal muscle function and metabolism (Montenegro et al. 2019), and the role of adipokines in skeletal muscle inflammation (Nicholson et al. 2018). By contrast, many publications on cell lines from fish skeletal muscle are cited as examples of cell line development but follow-up studies on their use(s) are few. The exceptions are the skeletal muscle cell lines FHM, BB, and BF-2, which have developed traction in several research communities. All three have been used intensively in toxicology (Babich and Borenfreund 1987; Tan et al. 2008; Poornavaishnavi et al. 2019). Additionally, FHM has been applied to a wide range of research problems, such as cell to cell communication (Slater et al. 1983), heat tolerance (Merz and Laudien 1987), the impact of fish oil and lipid peroxidation (Gregory et al. 2011), the pathogenicity of the intracellular bacteria Nocardia seriolae (Hou et al. 2020), and responses to oxidative stress (Chen et al. 2020).
In the future, the skeletal muscle cell lines should have many more applications but currently several things appear to hold back their use. The applications could range from basic research on muscle metabolism and physiology to applied research on in vitro fish meat. Each of these basic and applied areas would have multiple subdisciplines. For the use of cell lines in these areas/subdisciplines to gain momentum requires more independent researchers to use them. However, researchers are held back from doing this by most of the cell lines being only characterized superficially and being available only informally, although this might change with the availability of Mack cells commercially (Kerafast). In vitro approaches to fish skeletal muscle research would also be enhanced by having more skeletal muscle cell lines from the same species so that they can be used in co-cultures to build up a picture of how they interact in muscle as a whole. Also, skeletal muscle cell lines from multiple anatomical sites with a range of differentiation potentials would allow for the creation of more advanced in vitro models as has been done for human skeletal muscle (Jalal et al. 2021). These could include three-dimensional cultures such as organoids and the use of scaffold-based platforms.
Conclusions
A framework has been developed to categorize the cell lines from ray-finned fishes but it should be applicable to cell lines from other vertebrates, such as mammals, and even invertebrates, such as insects. The categories or groupings are termed invitromes. Invitromes can be defined by two property classes: before-the-fact or ante factum properties and after-the-fact or post factum properties.
Before-the-fact groupings are based on properties of three things: the sample that was used to start the cell line, the species from which the sample was taken, and the cell culturists who did the work. Each of these three can have numerous subdivisions. One that has received relatively little attention to date is the natural history of the species, which includes biological, behavioral, and reproductive characteristics, as well as habitat, ecology, and zoogeography. Grouping cell lines by these properties can help cell line research in the future reach a state of integration and reciprocal illumination with organismal research.
After-the-fact groupings are based on properties that become apparent during cell line development, characterization, experimentation, and storage. The development of nearly all the cell lines arises from spontaneous immortalization. The characterization and experimentation are open ended with many subdivisions possible as new characterization techniques and experimental uses are developed. For example, omic profiles promise to make up possible powerful cell line characterization in the future. One of the fastest growing uses is in toxicology. Storage includes many informally available cell lines but relatively fewer in curated cell banks.
The cell lines from trunk skeletal muscle of ray-finned fishes are used to illustrate how a specific organ invitrome has both before-the-fact and after-the-fact properties. Cell lines have been obtained from both short- and long-lived species. For after-the-fact properties, six cell lines are myogenic: they form myotubes. These are from the Atlantic mackerel, olive flounder, black rockfish, and killifish and were established relatively recently, and so are yet to be widely used. By contrast, the trunk skeletal muscle epithelial invitrome contains four cell lines, and has one of the most widely used fish cell lines, FHM.
References
Abdelmoez AM, Puig LS, Smith JAB, Gabriel BM, Savik M, Dollet L, Chibalin AV, Krook A, Zierath JR, Pillon NJ (2020) Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am J Physiol Cell Physiol 318:C615–C626. https://doi.org/10.1152/ajpcell.00540.2019
Altringham JD, Ellerby DJ (1999) Fish swimming: patterns in muscle function. J Exp Biol 202:3397–3403. https://doi.org/10.1242/jeb.202.23.3397
Ankley GT, Villeneuve DL (2006) The fathead minnow in aquatic toxicology: past, present and future. Aquatic Toxicol 78:91–102. https://doi.org/10.1016/j.aquatox.2006.01.018
Babich H, Borenfreund E (1987) Fathead minnow FHM cells for use in in vitro cytotoxicity assays. Ecotoxicol Environ Saf 14:78–87. https://doi.org/10.1016/0147-6513(87)90086-8
Baghdadi M, Tajbakhsh S (2018) Regulation and phylogeny of skeletal muscle regeneration. Dev Biol 433:200–209. https://doi.org/10.1016/j.ydbio.2017.07.026
Bairoch A (2018) The Cellosaurus, a cell-line knowledge resource. J Biomol Tech 29:25–38. https://doi.org/10.7171/jbt.18-2902-002
Barker KS, Quiniou MA, Wilson MR, Bengten E, Stuge TB, Warr GW, Clem LW, Miller NW (2000) Telomerase expression and telomere length in immortal leukocyte lines from channel catfish. Dev Comp Immunol 24:583–595. https://doi.org/10.1016/s0145-305x(00)00021-5
Bartholomew GA (1986) The role of natural history in contemporary biology. Biosci 36:324–329. https://doi.org/10.2307/1310237
Becerril C, Montano M, Cisneros J, Mendoza-Milla C, Pardo A, Ortiz-Quintero B, Selman M, Ramos C (2021) Mesenchymal–epithelial transition in fibroblasts of human normal lungs and interstitial lung diseases. Biomol 11:378. https://doi.org/10.3390/biom11030378
Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Orti G (2017) Phylogenetic classification of bony fishes. BMC Evol Biol 17(1):1–40. https://doi.org/10.1186/s12862-017-0958-3
Biacchesi S, Jouvion G, Merour E, Boukadir A, Desdouits M, Ozden S, Huerre M, Ceccaldi PE, Bremont M (2016) Rainbow trout (Oncorhynchus mykiss) muscle satellite cells are targets of salmonid alphavirus infection. Vet Res 47:1. https://doi.org/10.1186/s13567-015-0301-1
Billiard SM, Hahn ME, Franks DG, Peterson RE, Bols NC, Hodson PV (2002) Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs). Comp Biochem Phys B 133:55–68. https://doi.org/10.1016/S1096-4959(02)00105-7
Birbrair A, da Terra Borges I, Sena IFG, Almeida GG, da Silva Meire- lles L, Goncalves R, Mintz A, Delbono O (2017) How plastic are pericytes? Stem Cells Dev 26(14):1031–1019. https://doi.org/10.1089/scd.2017.0044
Blau HM, Chiu CP, Webster C (1983) Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32:1171–1180. https://doi.org/10.1016/0092-8674(83)90300-8
Bloch SR, Vo NTK, Walsh SK, Chen CC, Lee LEJ, Hodson PV, Bols NC (2016) Development of a cell line from the American eel brain expressing endothelial cell properties. In Vitro Cell Dev-an 52:395–409. https://doi.org/10.1007/s11626-015-9986-8
Blochl C, Wang D, Madunic K, Lageveen-Kammeijer GSM, Huber CG, Wuhrer M, Zhang T (2021) Integrated N- and O-glycomics of acute myeloid leukemia (AML) cell lines. Cells 10(11):3058. https://doi.org/10.3390/cells10113058
Bols NC, Dayeh VR, Lee LEJ, Schirmer K (2005) Chapter 2 Use of fish cell lines in the toxicology and ecotoxicology of fish. Piscine cell lines in environmental toxicology. In: Mommsen TP, Moon TW (eds) Biochem Mol Biol Fishes, volume 6, Environmental Toxicology, Elsevier, Amsterdam, pp 43–84. https://doi.org/10.1016/S1873-0140(05)80005-0
Bols NC, DeWitte-Orr SJ, Brubacher JL, Dixon B, Ganassin RC (2003) Cell culture approaches in aquatic immunotoxicology. In: Mothersill C, Austin B (eds) In vitro methods in aquatic toxicology. Springer, London, pp 399–420
Bols NC, Lee LEJ (1994) Cell lines: availability, propagation and isolation. In: Hochachka PW, Moon TW (eds) Biochem Mol Biol Fishes, volume 3 Analytical Techniques, Elsevier, Amsterdam, pp 145–159. https://doi.org/10.1016/B978-0-444-82033-4.50019-2
Bols NC, Mosser DD, Steels GB (1992) Temperature studies and recent advances with fish cells in vitro. Comp Biochem Phys A 103:1–14. https://doi.org/10.1016/0300-9629(92)90235-I
Bols NC, Pham PH, Dayeh VR, Lee LEJ (2017) Invitromatics, invitrome, and invitroomics: introduction of three new terms for in vitro biology and illustration of their use with the cell lines from rainbow trout. In Vitro Cell Dev-an 53:383–405. https://doi.org/10.1007/s11626-017-0142-5
Bols NC, Schirmer K, Joyce EM, Dixon DG, Greenberg BM, Whyte JJ (1999) Ability of polycyclic aromatic hydrocarbons to induce 7-ethoxyresorufin-o-deethylase activity in a trout liver cell line. Ecotoxicol Environ Saf 44:118–128. https://doi.org/10.1006/eesa.1999.1808
Bols NC, Yang BY, Lee LE, Chen TT (1995) Development of a rainbow trout pituitary cell line that expresses growth hormone, prolactin, and somatolactin. Mol Mar Biol Biotechnol 4:154–163. https://doi.org/10.1210/en.2002-221005
Cappellari O, Cossu G (2013) Pericytes in development and pathology of skeletal muscle. Circulation Res 113:341–347. https://doi.org/10.1161/circresaha.113.300203
Carter S, Solomon PJ (2019) In vitro experimental models for examining the skeletal muscle cell biology of exercise: the possibilities, challenges and future developments. Eur J Physiol 471:413–429. https://doi.org/10.1007/s00424-018-2210-4
Chamley JH, Campbell GR, McConnell JD (1977) Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tiss Res 177:503–522. https://doi.org/10.1007/bf00220611
Chaudhary DK, Sood N, Pradhan PK, Singh A, Punia P, Agarwal NK, Rathore G (2012) Establishment of a macrophage cell line from adherent peripheral blood mononuclear cells of Catla catla. In Vitro Cell Dev-an 48:340–348. https://doi.org/10.1007/s11626-012-9516-x
Chen X, Wang Q, Guo Z, Zhao Y, Luo S, Yu T, Zhang D, Wang G (2020) Identification of the Nrf2 in the fathead minnow muscle cell line: role for a regulation in response to H2O2 induced the oxidative stress in fish cell. Fish Physiol Biochem 46:1699–1711. https://doi.org/10.1007/s10695-020-00822-8
Chen YX, Wong PP, Sjeklocha L, Steer CJ, Sahin MB (2012) Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatol 55:563–574. https://doi.org/10.1002/hep.24712
Cheng L-L, Bowser PR, Spitsbergen JM (1993) Development of cell cultures derived from lake trout liver and kidney in a hormone-supplemented, serum-reduced medium. J Aquat Anim Hlth 5:119–126. https://doi.org/10.1577/15488667(1993)005%3c0119:DOCCDF%3e2.3.CO;2
Chong GLW, Bohmert B, Lee LEJ, Bols NC, Dowd GC (2022) A continuous myofibroblast precursor cell line from the tail muscle of Australasian snapper (Chrysophrys auratus) that responds to transforming growth factor beta and fibroblast growth factor. In Vitro Cell Dev Biol Anim (2022):1–14
Clemons JH, Myers CR, Lee LEJ, Dixon DG, Bols NC (1998) Induction of cytochrome P4501A by binary mixtures of polychlorinated biphenyls (PCBs) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in liver cell lines from rat and trout. Aquat Toxicol 43:179–194. https://doi.org/10.1016/S0166-445x(98)00048-4
Clevers H, Watt FM (2018) Defining adult stem cells by function, not by phenotype. Annu Rev Biochem 87:1015–1027. https://doi.org/10.1146/annurev-biochem-062917-012341
Contreras O, Rossi FMV, Theret M (2021) Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors — time for new definition. Skeletal Muscle 11:16. https://doi.org/10.1186/s13395-021-00265-6
Crawford DL, Schulte PM, Whitehead A (2020) Evolutionary physiology and genomics in the highly adaptable killifish (Fundulus heteroclitus). Compr Physiol 10:637–671. https://doi.org/10.1002/cphy.c190004
Datovo A, Rizzato PP (2018) Evolution of the facial musculature in basal ray-finned fishes. Front Zool. https://doi.org/10.1186/s12983-018-0285-6
Dehler CE, Boudinot P, Martin SAM, Collet B (2016) Development of an efficient genome editing method by CRISPR/Cas9 in a fish cell line. Mar Biotechnol 18:449–452. https://doi.org/10.1007/s10126-016-9708-6
Dewitte-Orr SJ, Lepic K, Bryson SR, Walsh SK, Lee LEJ, Bols NC (2006) Development of a continuous cell line, PBLE, from an American eel peripheral blood leukocyte preparation. In Vitro Cell Dev-an 42:263–272. https://doi.org/10.1290/0604023.1
Dong C, Weng S, Shi X, Xu X, Shi N, He J (2008) Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res 135:273–281. https://doi.org/10.1016/j.virusres.2008.04.004
Dornburg A, Near TJ (2021) The emerging phylogenetic perspective on the evolution of Actinopterygian fishes. Annu Rev Ecol Evol S 52:427–452. https://doi.org/10.1146/annurev-ecolsys-122120-122554
Dubey A, Goswami M, Yadav K, Mishra A, Kumar A (2015) Establishment of a novel muscle cell line from Wallago attu for in vitro study of pesticide toxicity. Gene Cell Tissue 2(1):e25568. https://doi.org/10.17795/gct-25568
Ellender RD, Wharton JH, Middlebrooks BL (1979) An established spleen cell line from Bairdiella chrysura. In Vitro 15:112–113. https://doi.org/10.1007/BF02618106
Faisal M, Ahne W (1990) A cell-line (Clc) of adherent peripheral-blood mononuclear leukocytes of normal common carp Cyprinus-carpio. Dev Comp Immunol 14:255–260. https://doi.org/10.1016/0145-305x(90)90097-X
Fernandez RD, Yoshimizu M, Kimura T, Ezura KY (1993) Establishment and characterization of seven continuous cell lines from freshwater fish. J Aquatic Animal Hlth. https://doi.org/10.1577/1548-8667(1993)005%3c0137:EACOSC%3e2.3.CO;2
Fierro-Castro C, Barrioluengo L, Lopez-Fierro P, Razquin BE, Carracedo B, Villena AJ (2012) Fish cell cultures as in vitro models of pro-inflammatory responses elicited by immunostimulants. Fish Shellfish Immunol 33:389–400. https://doi.org/10.1016/j.fsi.2012.05.019
Follett JE, Schmitt MK (1990) Characterization of a cell line derived from Inconnu. J Aquat Anim Health 2:61–67. https://doi.org/10.1577/1548-8667(1990)002%3c0061:COACLD%3e2.3.CO;2
Forte E, Chimenti I, Rosa P, Angelini F, Pagano F, Calogero, Giacomello A, Messina E (2017) EMT/MET at the crossroad of stemness, regeneration and oncogenesis: the ying-yang equilibrium recapitulated in cell spheroids. Cancers 9:98. https://doi.org/10.3390/cancers9080098
Friedman ST, Price SA, Wainwright PC (2021) The effect of locomotion mode on body shape evolution in Teleost fishes. Integr Org Biol 3(1):obab016. https://doi.org/10.1093/iob/obab016
Freshney RI (2005) Culture of animal cells. John Wiley & Sons. https://doi.org/10.1002/0471747599.cac024
Fryer JL, Lannan CN (1994) Three decades of fish cell culture: a current listing of cell lines derived from fishes. J Tissue Cult Methods 16:87–94. https://doi.org/10.1007/BF01404816
Fryer JL, McCain BB, Leong JC (1981) A cell line derived from rainbow trout (Salmo gairdneri) hepatoma. Fish Pathol 15:193–200. https://doi.org/10.3147/jsfp.15.193
Fryer JL, Yusha A, Pilcher KS (1965) The in vitro cultivation of tissue and cells of Pacific salmon and steelhead trout. Ann N Y Acad Sci 126:566–586. https://doi.org/10.1111/j.1749-6632.1965.tb14303.x
Futami K, Maita M, Katagiri T (2019) DNA demethylation with 5-aza-2ʹ-deoxycytidine induces the senescence-associated secretory phenotype in the immortal fish cell line, EPC. Gene 697:194–200. https://doi.org/10.1016/j.gene.2019.02.048
Futami K, Sato S, Maita M, Katagiri T (2022) Lack of a p16(INK4a)/ARF locus in fish genome may underlie senescence resistance in the fish cell line, EPC. Dev Comp Immunol 133:104420. https://doi.org/10.1016/j.dci.2022.104420
Ganassin RC, Bols NC (1998) Development of a monocyte/macrophage-like cell line, RTS11, from rainbow trout spleen. Fish Shellfish Immunol 8:457–476. https://doi.org/10.1006/fsim.1998.0153
Ganassin RC, Bols NC (1999) A stromal cell line from rainbow trout spleen, RTS34ST, that supports the growth of rainbow trout macrophages and produces conditioned medium with mitogenic effects on leukocytes. In Vitro Cell Dev-an 35:80–86. https://doi.org/10.1007/s11626-999-0005-9
Ganassin RC, Sanders SM, Kennedy CJ, Joyce EM, Bols NC (1999) Development of a cell line from Pacific herring, Clupea harengus pallasi, sensitive to both naphthalene cytotoxicity and infection by hemorrhagic septicemia virus. Cell Biol Toxicol 15:299–309. https://doi.org/10.1023/A:1007615818427
Gao Y, Zhou H, Gao Z, Jiang H, Wang X, Mai K, He G (2019) Establishment and characterization of a fibroblast-like cell line from the muscle of turbot (Scophthalmus maximus L.). Fish Physiol Biochem 45:1129–1139. https://doi.org/10.1007/s10695-019-00628-3
Gignac SJ, Vo NTK, Mikhaeil MS, Alexander JAN, MacLatchy DL, Schulte PM, Lee LEJ (2014) Derivation of a continuous myogenic cell culture from an embryo of common killifish, Fundulus heteroclitus. Comp Biochem Physiol -Part A : Mol Integr Physiol 175:15–27. https://doi.org/10.1016/j.cbpa.2014.05.002
Goswami M, Dubey A, Yadav K, Sharma BS, Lakra WS (2015) Identification of fish cell lines using 2-D electrophoresis based protein expression signatures. Curr Proteomics 12:245–252. https://doi.org/10.2174/157016461204160119161034
Goswami M, Yashwanth BS, Trudeau V, Lakra WS (2022) Role and relevance of fish cell lines in advanced in vitro research. Mol Biol Rep 49:2393–2411. https://doi.org/10.1007/s11033-021-06997-4
Gratacap RL, Regan T, Dehler CE, Martin SAM, Boudinot P, Collet B, Houston RD (2020) Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system. BMC Biotechnol 20(1):1–9. https://doi.org/10.1186/s12896-020-00626-x
Gravell M, Malsberger RG (1965) A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci 126:555–565. https://doi.org/10.1111/j.1749-6632.1965.tb14302.x
Greene HA (2005) Organisms in nature as a central focus for biology. Trends Ecol Evol 20:23–27. https://doi.org/10.1016/j.tree.2005.04.006
Gregory MK, King HW, Bain PK, Gibson RA, Tocher DR, Schuller KA (2011) Development of a fish cell culture model to investigate the impact of fish oil replacement on lipid peroxidation. Lipids 46:753–764. https://doi.org/10.1007/s11745-011-3558-9
Grunow B, Franz GP, Tonissen K (2021) In vitro fish models for the analysis of ecotoxins and temperature increase in the context of global warming. Toxics 9(11):286. https://doi.org/10.3390/toxics9110286
Hedrick RP, McDowell TS, Rosemark R, Aronstein D, Lannan CN (1991) Two cell lines from white sturgeon. Trans Am Fish Soc 120:528–534. https://doi.org/10.1577/1548-8659(1991)120%3c0528:TCLFWS%3e2.3.CO;2
Herrmann H, Munick MD, Brettel M, Fouquet B, Markl J (1996) Vimentin in a cold-water fish, the rainbow trout: highly conserved primary structure but unique assembly properties. J Cell Sci 109:569–578. https://doi.org/10.1242/jcs.109.3.569
Hightower LE, Renfro JL (1988) Recent applications of fish cell culture to biomedical research. J Exp Zool 248:290–302. https://doi.org/10.1002/jez.1402480307
Hong N, Li Z, Hong Y (2011) Fish stem cell cultures. Int J Biol Sci 7:392–402. https://doi.org/10.7150/ijbs.7.392
Hou S, Wang W, Chen G, Xia L, Wang Z, Lu Y (2020) Identification of a secreted superoxide dismutase (SOD) from Nocardia seriolae which induces apoptosis in fathead minnow (FHM) cells. J Fish Dis 44:63–72. https://doi.org/10.1111/jfd.13268
Huang S-M, Kuo S-T, Kuo H-C, Chang S-K (2018) Assessment of fish iridoviruses using a novel cell line GS-1, derived from the spleen of orange-spotted grouper Epinephelus coioides (Hamilton) and susceptible to ranavirus and megalocytivirus. J Vet Med Sci 80:1766–1774. https://doi.org/10.1292/jvms.18-0078
Huang X, Huang Y, Sun J, Han X, Qin Q (2009) Characterization of two grouper Epinephelus akaara cell lines: application to studies of Singapore grouper iridovirus (SGIV) propagation and virus-host interaction. Aquaculture 292:172–179. https://doi.org/10.1016/j.aquaculture.2009.04.019
Jalal S, Dastidar S, Tedesco FS (2021) Advanced models of human skeletal muscle differentiation, development and disease: three-dimensional cultures, organoids and beyond. Curr Opin Cell Biol 73:92–104. https://doi.org/10.1016/j.ceb.2021.06.004
Kaneko G, Shirakami H, Hirano Y, Oba M, Yoshinaga H, Khieokhajonkhet A, Reiko Nagasaka R, Kondo H, Hirono H, Ushio H (2016) Diversity of lipid distribution in fish skeletal muscle. Zool Sci 33:170–178. https://doi.org/10.2108/zs150096
Kang MS, Oh MJ, Kim YJ, Kawai K, Jung SJ (2003) Establishment and characterization of two new cell lines derived from flounder, Paralichthys olivaceus (Temminck & Schlegel). J Fish Dis 26:657–665. https://doi.org/10.1046/j.1365-2761.2003.00499.x
Kawano A, Kales SC, Fujiki K, DeWitte-Orr SJ, Dixon B, Lee LEJ, Bols NC (2010) A comparison of rainbow trout cell lines for their expression of the major histocompatibility complex genes and the induction of beta-2-microglobulin by dsRNA. Fish Shellfish Immunol 29:312–318. https://doi.org/10.1016/j.fsi.2010.04.007
Kolora SRR, Owens GL, Vazquez JM, Stubbs A, Chatla K, Jainese C, Seeto K, McCrea M, Sandel MW, Vianna JA, Maslenikov K, Bachtrog D, Orr JW, Love M, Sudmant PH (2021) Origins and evolution of extreme life span in Pacific Ocean rockfishes. Sci 374:842–847. https://doi.org/10.1126/science.abg5332
Kong X, Wang X, Li M, Song W, Huang K, Zhang F, Zhang Q, Qi J, He Y (2021) Establishment of myoblast cell line and identification of key genes regulating myoblast differentiation in a marine teleost, Sebastes Schlegelii. Gene 802(2021):145869. https://doi.org/10.1016/j.gene.2021.145869
Kumar A, Singh N, Goswami M, Srivastava JK, Mishra AK, Lakra WS (2016) Establishment and characterization of a new muscle cell line of zebrafish (Danio rerio) as an in vitro model for gene expression studies. Animal Biotech 27:3. https://doi.org/10.1080/10495398.2016.1147455
Kumar MS, Soni P, Singh N, Kumar R, Srivastava S, Mishra AK, Singh VK, Kushwaha B (2020) Establishment and characterization of eye muscle cell line from snow trout, Schizothorax richardsonii (Gray, 1832), a vulnerable coldwater fish, for in vitro studies. Turk J Fish & Aquat Sci 21:95–105. https://doi.org/10.4194/1303-2712-v21_2_05
Lai YS, Chiou PP, Chen WJ, Chen YC, Chenn CW, Chiu IS, Chen SD, Cheng YH, Chang CY (2008) Characterization of apoptosis induced by grouper iridovirus in two newly established cell lines from barramundi, Lates calcarifer (Bloch). J Fish Dis 31:825–834. https://doi.org/10.1111/j.1365-2761.2008.00957.x
Laizé V, Rosa JT, Tarasco M, Cancela ML (2022) Chapter 12 - Status, challenges, and perspectives of fish cell culture—focus on cell lines capable of in vitro mineralization. In Monzón IF and Fernandes JMO (eds) Cellular and molecular approaches in fish biology. Academic Press, pp 381–404. https://doi.org/10.1016/B978-0-12-822273-7.00004-5
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20. https://doi.org/10.1007/s10695-010-9411-x
Lammel T, Tsoukatou G, Jellinek J, Sturve J (2019) Development of three-dimensional (3D) spheroid cultures of the continuous rainbow trout liver cell line RTL-W1. Ecotoxicol Environ Saf 167:250–258. https://doi.org/10.1016/j.ecoenv.2018.10.009
Lannan CN, Winton JR, Fryer JL (1984) Fish cell lines: establishment and characterization of nine cell lines from salmonids. In Vitro 20:671–676. https://doi.org/10.1007/BF02618871
Laplane L, Solary E (2019) Philosophy of biology: towards a classification of stem cells. Elife 8:e46563. https://doi.org/10.7554/eLife.46563
Lee LEJ, Clemons JH, Bechtel DG, Caldwell SJ, Han KB, Pasitschniakarts M, Mosser DD, Bols NC (1993) Development and characterization of a rainbow-trout liver-cell line expressing cytochrome p450-dependent monooxygenase activity. Cell Biol Toxicol 9:279–294. https://doi.org/10.1007/Bf00755606
Li H, Xu W, Xiang S, Tao L, Fu W, Liu J, Liu W, Xiao Y, Peng L (2022) Defining the pluripotent marker genes for identification of teleost fish cell pluripotency during reprogramming. Front Genet 13. https://doi.org/10.3389/fgene.2022.819682
Li N, Guo L, Guo H (2021) Establishment, characterization, and transfection potential of a new continuous fish cell line (CAM) derived from the muscle tissue of grass goldfish (Carassius auratus). In Vitro Cell Dev-an 57:912–931. https://doi.org/10.1007/s11626-021-00622-1
Liu F, Bols NC, Pham PH, Secombes CJ, Zou J (2019) Evolution of IFN subgroups in bony fish - 1: Group I-III IFN exist in early ray-finned fish, with group II IFN subgroups present in the Holostean spotted gar, Lepisosteus oculatus. Fish Shellfish Immunol 95:163–170. https://doi.org/10.1016/j.fsi.2019.10.032
Liu J, Liu X, Zeng Q, Wang B, Xiao K, Tan C, Du H (2020) Establishment and characterization of a cell line derived from fin of the endangered Yangtze sturgeon (Acipenser dabryanus). In Vitro Cell Dev Biol Anim 56:650–658. https://doi.org/10.1007/s11626-020-00488-9
Lorenzen E, Carstensen B, Olesen NJ (1999) Inter-laboratory comparison of cell lines for susceptibility to three viruses: VHSV, IHNV and IPNV. Dis Aquatic Org 37:81–88. https://doi.org/10.3354/dao037081
Ma CG, Fan LC, Ganassin R, Bols N, Collodi P (2001) Production of zebrafish germ-line chimeras from embryo cell cultures. Proc Natl Acad Sci USA 98:2461–2466. https://doi.org/10.1073/pnas.041449398
MacLeod MJ, Vo NTK, Mikhaeil MS, Monaghan SR, Alexander JAN, Saran MK, Lee LEJ (2018) Development of a continuous cell line from larval Atlantic cod (Gadus morhua) and its use in the study of the microsporidian, Loma morhua. J Fish Dis 41:1359–1372. https://doi.org/10.1111/jfd.12830
Maisey K, Montero R, Corripio-Miyar Y, Toro-Ascuy D, Valenzuela B, Reyes-Cerpa S, Sandino AM, Zou J, Wang TH, Secombes CJ, Imarai M (2016) Isolation and characterization of salmonid CD4(+) T cells. J Immunol 196:4150–4163. https://doi.org/10.4049/jimmunol.1500439
Malhao F, Urbatzka R, Navas JM, Cruzeiro C, Monteiro RAF, Rocha E (2013) Cytological, immunocytochemical, ultrastructural and growth characterization of the rainbow trout liver cell line RTL-W1. Tissue Cell 45:159–174. https://doi.org/10.1016/j.tice.2012.10.006
Martin SAM, Mohanty BP, Cash P, Houlihan DF, Secombes CJ (2007) Proteome analysis of the Atlantic salmon (Salmo salar) cell line SHK-1 following recombinant IFN-gamma stimulation. Proteomics 7:2275–2286. https://doi.org/10.1002/pmic.200700020
Merz R, Laudien H (1987) 2 types of heat tolerance in FHM cells- induction of heat shock versus elevated culturing temperature. J Thermal Biol 12:281–288. https://doi.org/10.1016/0306-4565(87)90029-5
Meyers JR (2018) Zebrafish: development of a vertebrate model organism. Curr Protoc Essent Lab Tech 16:e19. https://doi.org/10.1002/cpet.19
Meyers JR (2018b) Zebrafish: development of a vertebrate model organism: zebrafish: development of a vertebrate model organism. Curr Proto- Cols Essent Lab Tech 16:e19. https://doi.org/10.1002/cpet.19
Middlebrooks BL, Ellender RD, Warton JH (1979) Fish cell culture: a new cell line from Cynoscion nebulosus. In Vitro 15:109–111. https://doi.org/10.1007/bf02618105
Middlebrooks BL, Mak PC, Ellender RD (1980) Properties of an established cell line from the Atlantic Croaker (Micropogon undulatus) (40945). Proc Soc Exp Biol & Med 165:123–128. https://doi.org/10.3181/00379727-165-40945
Middlebrooks BL, Stout DL, Ellender RD, Safford S (1981) Fish cell lines: two new cell lines derived from explants of trunk musculature of Cynoscion arenarius. In Vitro 17:427–430. https://doi.org/10.1007/bf02626743
Miller NW, Rycyzyn MA, Wilson MR, Warr GW, Naftel JP, Clem LW (1994) Development and characterization of channel catfish long term B cell lines. J Immunol 152:2180–2189
Montenegro KR, Cruzat V, Carlessi R, Newsholme P (2019) Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev 32:192–204. https://doi.org/10.1017/s0954422419000064
Moritomo T, Anderson DE, Schill WB (1990) Establishment of a cell line with reticuloendothelial characteristics from a rainbow trout spleen explant. Fish Pathol 25:165–170. https://doi.org/10.3147/jsfp.25.165
Moritomo T, Asakawa H, Nakano M, Watanabe T (1988) Establishment and some biological characteristics of a cell line derived from yamame (Oncorhynchus masou) embryonal liver. Bull Jap Soc Sci Fish 54:1881–1887. https://doi.org/10.2331/suisan.54.1881
Nakano H, Wada Y, Hasobe M (1993) Chinook salmon embryo (CHSE-sp) cells for fish virus research. In Vitro Cell Dev-An 29a:265–267. https://doi.org/10.1007/Bf02633951
Nevedomskaya E, Haendler B (2022) From omics to multi-omics approaches for in-depth analysis of the molecular mechanisms of prostate cancer. Int J Mol Sci 23(11):6281. https://doi.org/10.3390/ijms23116281
Ng CKC, Ooi P, Wong W-L, Khoo G (2017) A review of fish taxonomy conventions and species identification techniques. J Surv Fish Sci 4:54–93. https://doi.org/10.18331/SFS2017.4.1.6
Nicholson BL (1989) Fish cell culture: an update. Adv Cell Cult 7:1–18. https://doi.org/10.1016/b978-0-12-007907-0.50007-4
Nicholson BL, Byren C (1973) An established ceil line from the Atlantic salmon (Salmo salar). J Fish Res Board Can 30:913–916. https://doi.org/10.1139/f73-152
Nicholson T, Church C, Baker DJ, Jones SW (2018) The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflammation 15:9. https://doi.org/10.1186/s12950-018-0185-8
Okihiro MS, Hinton DE (2000) Partial hepatectomy and bile duct ligation in rainbow trout (Oncorhynchus mykiss): histologic, immunohistochemical and enzyme histochemical characterization of hepatic regeneration and biliary hyperplasia. Toxicol Pathol 28:342–356. https://doi.org/10.1177/019262330002800215
Parameswaran V, Ravi S, Bhonde RR, Hameed ASS (2006) Splenic cell line from sea bass, Lates calcarifer: establishment and characterization. Aquaculture 261:43–53. https://doi.org/10.1016/j.aquaculture.2006.07.034
Parton A, Bayne CJ, Barnes DW (2010) Analysis and functional annotation of expressed sequence tags from in vitro cell lines of elasmobranchs: Spiny dogfish shark (Squalus acanthias) and little skate (Leucoraja erinacea). Comp Biochem Physiol Part D Genomics Proteomics 5:199–206. https://doi.org/10.1016/j.cbd.2010.04.004
Pedersen BK (2013) Muscle as a secretory organ. Compr Physiol 3:1337–1362. https://doi.org/10.1002/cphy.c120033
Peng LM, Zhen Y, You F, Wu ZH, Tan X, Jiao S, Zhang PJ (2016) Comparison of growth characteristics between skeletal muscle satellite cell line from diploid and triploid olive flounder Paralichthys olivaceus. PeerJ 4:e1519. https://doi.org/10.7717/peerj.1519
Pham PH, Misk E, Papazotos F, Jones G, Polinski MP, Contador E, Russell S, Garver KA, Lumsden JS, Bols NC (2020) Screening of fish cell lines for piscine orthoreovirus-1 (PRV-1) amplification: identification of the non-supportive PRV-1 invitrome. Pathog 9:1–25. https://doi.org/10.3390/pathogens9100833
Poornavaishnavi C, Gowthami R, Srikanth K, Bramhachari PV, Venkatramaih N (2019) Nickel nanoparticles induces cytotoxicity, cell morphology and oxidative stress in bluegill sunfish (BF-2) cells. Appl Surf Sci 483:1174–1181. https://doi.org/10.1016/j.apsusc.2019.03.255
Ravi V, Venkatesh B (2018) The divergent genomes of teleosts. Annu Rev Anim Biosci 6:47–68. https://doi.org/10.1146/annurev-animal-030117-014821
Ritso M, Tung LW, Rossi FMV (2022) Emerging skeletal muscle stromal cell diversity: functional divergence in fibro/adipogenic progenitor and mural cell populations. Exp Cell Res 410:112947. https://doi.org/10.1016/j.yexcr.2021.112947
Rodger EJ, Almomani SN, Ludgate JL, Stockwell PA, Baguley BC, Eccles MR, Chatherjee A (2021) Comparison of global DNA methylation patterns in human melanoma tissues and their derivative cell lines. Cancers 13(9):2123. https://doi.org/10.3390/cancers13092123
Rougee L, Ostrander GK, Richmond RH, Lu Y (2007) Establishment, characterization, and viral susceptibility of two cell lines from goldfish Carassius auratus muscle and swim bladder. Dis Aquat Org 77:127–135
Ruiz-Palacios M, Almeida M, Martins MA, Oliveira M, Esteban MA, Cuesta A (2020) Establishment of a brain cell line (FuB-1) from mummichog (Fundulus heteroclitus) and its application to fish virology, immunity and nanoplastics toxicology. Sci Total Environ 708:134821. https://doi.org/10.1016/j.scitotenv.2019.134821
Ryu JH, Kim MS, Kang JH, Kim DH, Nam YK, Gong SP (2018) Derivation of the clonal-cell lines from Siberian sturgeon (Acipenser baerii) head-kidney cell lines and its applicability to foreign gene expression and virus culture. J Fish Biol 92:1273–1289. https://doi.org/10.1111/jfb.13585
Saad MK, Yuen JSK, Jocye CM, Li X, Lim T, Wolfsonn TL, Wu J, Laird J, Vissapragada S, Calkinns OP, Ali A, Kaplan DL (2022) Continuous fish muscle cell line with capacity for myogenic and adipogenic- like phenotypes. bioRxiv preprint. https://doi.org/10.1101/2022.08.22.504874
Salvadores M, Fuster-Tormo F, Supek F (2020) Matching cell lines with cancer type and subtype of origin via mutational, epigenomic, and transcriptomic patterns. Sci Adv 6(27):eaba1862. https://doi.org/10.1126/sciadv.aba1862
Santini F, Harmon LJ, Carnevale G, Alfaro ME (2009) Did genome duplication drive the origin of teleosts? A comparative study of diversification in ray-finned fishes. BMC Evol Biol 9:194. https://doi.org/10.1186/1471-2148-9-194
Schirmer K (2006) Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicol 224:163–183. https://doi.org/10.1016/j.tox.2006.04.042
Segner H (1998) Fish cell lines as a tool in aquatic toxicology. Fish Ecotoxicol 86:1–38. https://doi.org/10.1007/978-3-0348-8853-0_1
Semple SL, Vo NTK, Poynter SJ, Li M, Heath DD, DeWitte-Orr SJ, Dixon B (2018) Extracellular dsRNA induces a type I interferon response mediated via class A scavenger receptors in a novel Chinook salmon derived spleen cell line. Dev Comp Immunol 89:93–101. https://doi.org/10.1016/j.dci.2018.08.010
Shih HP, Gross MK, Kloussi C (2008) Muscle development: forming the head and trunk muscles. Acta Histochem 110:97–108. https://doi.org/10.1016/j.acthis.2007.08.004
Slater MA, Mosser DD, Bols NC (1983) Established cell lines from different groups of vertebrates undergo metabolic cooperation with one another. In Vitro 19:683–692. https://doi.org/10.1007/bf02628959
Spencer RF, Porter JD (2006) Biological organization of the extraocular muscles. Prog Brain Res 151:43–80. https://doi.org/10.1016/s0079-6123(05)51002-1
Stadnicka-Michalak J, Weiss FT, Fischer M, Tanneberger K, Schirmer K (2018) Biotransformation of benzo [a] pyrene by three rainbow trout (Onchorhynchus mykiss) cell lines and extrapolation to derive a fish bioconcentration factor. Environ Sci Technol 52:3091–3100. https://doi.org/10.1021/acs.est.7b04548
Suarez-Bregua P, Rosendo S, Comesana P, Sanchez-Ruiloba L, Moran P, Planas M, Rotllant J (2021) Dynamic changes in DNA methylation during seahorse (Hippocampus reidi) postnatal development and settlement. Front Zool 18:52. https://doi.org/10.1186/s12983-021-00436-7
Sultan SHA, Dyer C, Knight RD (2021) Notch signalling regulates muscle stem cell homeostasis and regeneration in a teleost fish. Front Cell Dev Biol 9:726281. https://doi.org/10.3389/fcell.2021.726281
Swailes NT, Colegrave M, Knight PJ, Peckham M (2006) Non-muscle myosins 2A and 2B drive changes in cell morphology that occur as myoblasts align and fuse. J Cell Sci 119:3561–3570. https://doi.org/10.1242/jcs.03096
Takezaki N (2021) Resolving the early divergence pattern of teleost fish using genome-scale data. Genome Biol Evol 13(5):evab052. https://doi.org/10.1093/gbe/evab052
Tan F, Wang M, Wang W, Lu Y (2008) Comparative evaluation of the cytotoxicity sensitivity of six fish cell lines to four heavy metals in vitro. Toxicol in Vitro 22:164–170. https://doi.org/10.1016/j.tiv.2007.08.020
Thangaraj RS, Narendrakumar L, Prasannan Geetha P, Shanmuganathan AR, Dharmaratnam A, Nithianantham SR (2021) Comprehensive update on inventory of finfish cell lines developed during the last decade (2010–2020). Rev Aquacult. https://doi.org/10.1111/raq.12566.10.1111/raq.12566
Tong S-L, Miao H-Z, Li H (1998) Three new continuous fish cell lines of SPH, SPS and RSBF derived from sea perch (Lateolabrax japonicus) and red sea bream (Pagrosomus major). Aquaculture 169:143–151. https://doi.org/10.1016/S0044-8486(98)00329-9
Tung LC, Chen SN, Kou GH (1991) Three cell lines derived from spleen and kidney of black porgy (Acanthopagrus schlegeli). Fish Pathol 26:109–117. https://doi.org/10.3147/jsfp.26.109
Vallejo A, Ellsaesser CF, Miller NW, Clem LW (1991) Spontaneous development of functionally active long- term monocyte-like cell lines from channel catfish. In Vitro Cell Dev-an 27A:279–285. https://doi.org/10.1007/bf02630904
Verges-Castillo A, Gonzalez-Vargas IA, Munoz-Cueto JA, Martin-Robles AJ, Pendon C (2021) Establishment and characterisation of single cell-derived embryonic stem cell lines from the gilthead seabream, Sparus aurata. Comp Biochem Phys B 256:110626. https://doi.org/10.1016/j.cbpb.2021.110626
Vo NTK, Mikhaeil MS, Lee LEJ, Pham PH, Bols NC (2015a) Senescence-associated beta-galactosidase staining in fish cell lines and primary cultures from several tissues and species, including rainbow trout coelomic fluid and milt. In Vitro Cell Dev-an 51:361–371. https://doi.org/10.1007/s11626-014-9837-z
Vo NTK, Bender AW, Ammendolia DA, Lumsden JS, Dixon B, Bols NC (2015b) Development of a walleye spleen stromal cell line sensitive to viral hemorrhagic septicemia virus (VHSV IVb) and to protection by synthetic dsRNA. Fish Shellfish Immunol 45:83–93. https://doi.org/10.1016/j.fsi.2015.02.004
Vo NTK, Chen C, Lee LEJ, Lumsden JS, Dixon B, Bols NC (2015c) Development and characterization of an endothelial cell line from the bulbus arteriosus of walleye, Sander vitreus. Comp Biochem Physiol A Mol Integr Physiol 180:57–67. https://doi.org/10.1016/j.cbpa.2014.10.027
Vo NTK, Bols NC (2016) Demonstration of primary cilia and acetylated alpha-tubulin in fish endothelial, epithelial and fibroblast cell lines. Fish Physiol Biochem 42:29–38. https://doi.org/10.1007/s10695-015-0114-1
Wagg SK, Lee LEJ (2005) A proteomics approach to identifying fish cell lines. Proteomics 5:4236–4244. https://doi.org/10.1002/pmic.200401290
Wang G, LaPatra S, Zeng L, Zhao Z, Lu Y (2003) Establishment, growth, cryopreservation and species of origin identification of three cell lines from white sturgeon, Acipenser transmontanus. Methods Cell Sci 25:211–220. https://doi.org/10.1007/s11022-004-9120-x
Wang L, Cao Z, Liu Y, Xiang Y, Sun Y, Zhou Y, Wang S, Guo W (2020) Establishment and characterization of a new cell line from the muscle of humpback grouper (Cromileptes altivelis). Fish Physiol Biochem 46:1897–1907. https://doi.org/10.1007/s10695-020-00841-5
Wardle CS, Videler JJ, Altringham JD (1995) Tuning in to fish swimming waves: body form, swimming mode and muscle function. J Exp Biol 198:1629–1636. https://doi.org/10.1242/jeb.198.8.1629
Watanabe T, Sano M, Ishida Y, Mizusawa Y, Michikawa M (1980) Establishment and properties of the continuous cell-line derived from the embryonated eggs of Chum salmon, Oncorhynchus keta. Bull Jap Soc Sci Fish 46:1203–1209. https://doi.org/10.2331/suisan.46.1203
Wolf K, Gravell M, Malsberger RG (1966) Lymphocystis virus: isolation and propagation in centrarchid fish cell lines. Sci 151:1004–1005. https://doi.org/10.1126/science.151.3713.1004
Wolf K, Mann JA (1980) Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In Vitro 16:168–179. https://doi.org/10.1007/bf02831507
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Sci 135:1065–1066. https://doi.org/10.1126/science.135.3508.1065
Wolf K, Quimby MC (1969) Fish cell and tissue culture. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, p 253–305. https://doi.org/10.1016/s1546-5098(08)60115-6
Wosczyna MN, Konishi CT, Carbajal EEP, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA (2019) Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep 27:2029–2035. https://doi.org/10.1016/j.celrep.2019.04.074
Xing JG, El-Sweisi W, Lee LE, Collodi P, Seymour C, Mothersill C, Bols NC (2009) Development of a zebrafish spleen cell line, ZSSJ, and its growth arrest by gamma irradiation and capacity to act as feeder cells. In Vitro Cell Dev-an 45:163–174. https://doi.org/10.1007/s11626-008-9159-0
Xing JG, Lee LEJ, Fan L, Collodi P, Holt SE, Bols NC (2008) Initiation of a zebrafish blastula cell line on rainbow trout stromal cells and subsequent development under feeder-free conditions into a cell line, ZEB2J. Zebrafish 5:49–63. https://doi.org/10.1089/zeb.2007.0512
Yadav K, Lakra WS, Sharma J, Goswami M, Singh A (2012) Development and characterization of a cell line TTCF from endangered mahseer Tor tor (Ham.). Fish Physiol Biochem 38:1035–1045. https://doi.org/10.1007/s10695-011-9588-7
Yaffe D (1968) Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Nat Acad Sci 61:477–483. https://doi.org/10.1073/pnas.61.2.477
Yang XX, Wen YQ, Song XY, He S, Bo XC (2020) Exploring the classification of cancer cell lines from multiple omic views. Peerj 8:e9440. https://doi.org/10.7717/peerj.9440
Zhang W, Xie HQ, Li Y, Zhou M, Zhou Z, Wang R, Hahn ME, Zhao B (2021) The aryl hydrocarbon receptor: a predominant mediator for the toxicity of emerging dioxin-like compounds. J Hazard Mater 426:128084. https://doi.org/10.1016/j.jhazmat.2021.128084
Zhao Z, Lu Y (2006) Establishment and characterization of two cell lines from bluefin trevally Caranx melampygus. Dis Aquat Org 68:91–100. https://doi.org/10.1577/1548-8667(1993)005%3c0137:EACOSC%3e2.3.CO;2
Zhao Z, Montgomery-Brock D, Lee CS, Lu Y (2004) Establishment, characterization and viral susceptibility of 3 cell lines from snakehead, Channa striatus (Blooch). Methods Cell Sci 25:155–166. https://doi.org/10.1007/s11022-004-3804-0
Zipori D (2004) Opinion - The nature of stem cells: state rather than entity. Nat Rev Genet 5:873–878. https://doi.org/10.1038/nrg1475
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The article reviews the literature, and thus, no animals and human participants were used.
Additional information
Editor: J. Denry Sato
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bols, N.C., Lee, L.E.J. & Dowd, G.C. Distinguishing between ante factum and post factum properties of animal cell lines and demonstrating their use in grouping ray-finned fish cell lines into invitromes. In Vitro Cell.Dev.Biol.-Animal 59, 41–62 (2023). https://doi.org/10.1007/s11626-022-00744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00744-0