Abstract
The literature on cell lines that have been developed from rainbow trout (RT) (Oncorhynchus mykiss) is reviewed to illustrate three new terms: invitromatics, invitrome, and invitroomics. Invitromatics is defined as the history, development, characterization, engineering, storage, and sharing of cell lines. RT invitromatics differs from invitromatics for humans and other mammals in several ways. Nearly all the RT cell lines have developed through spontaneous immortalization. No RT cell line undergoes senescence and can be described as being finite, whereas many human cell lines undergo senescence and are finite. RT cell lines are routinely grown at 18–22°C in free gas exchange with air in basal media developed for mammalian cells together with a supplement of fetal bovine serum. An invitrome is defined as the grouping of cell lines around a theme or category. The broad theme in this article is all the cell lines that have ever been created from O. mykiss, or in other words, the RT invitrome. The RT invitrome consists of approximately 55 cell lines. These cell lines can also be categorized on the basis of their storage and availability. A curated invitrome constitutes all the cell lines in a repository and for RT consists of 11 cell lines. These consist of epithelial cell lines, such as RTgill-W1, and fibroblast cell lines, such as RTG-2. RTG-2 can be purchased from a scientific company and constitutes the commercial RT invitrome. Cell lines that are exchanged between researchers are termed the informally shared invitrome and for RT consists of over 35 cell lines. Among these is the monocyte/macrophage cell line, RTS11. Cell lines whose existence is in doubt are termed the zombie invitrome, and for RT, approximately 12 cell lines are zombies. Invitroomics is the application of cell lines to a scientific problem or discipline. This is illustrated with the use of the RT invitrome in virology. Of the RT invitrome, RTG-2 was the most commonly used cell line to isolate viruses. Fifteen families of viruses were studied with RT invitrome. RT cell lines were best able to support replication of viruses from the Herpesviridae, Iridoviridae, Birnaviridae, Togaviridae, and Rhabdoviridae families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three new terms are proposed here for in vitro biology: invitromatics, invitrome, and invitroomic. They are being proposed in order to more easily consolidate past discoveries and to provide direction for future research. The underpinnings for all three terms are cell lines. A cell line is a population of cells from a multicellular animal or plant that can be propagated outside the organism through the serial transfer (subcultivation) of cells from one fabricated culture vessel to another (Schaeffer 1990). As the production and maintenance of animal and plant cell lines is quite different, this article will deal with only animals, although acknowledging that the definitions could have use in plant in vitro biology as well. Rainbow trout (RT) will be used as an exemplar of how these terms might be applied in animal in vitro biology. RT is chosen because the authors have been involved in developing many cell lines from this species and because the number of RT cell lines is substantial enough for the terms to be illustrated but not so large as to be overwhelming, which might be the case with the human cell lines.
Animal invitromatics is being defined here as the science and history of establishing, characterizing, engineering, storing, and distributing cell lines. An analogy can be found in the discipline of informatics. “Informatics” has been described as the “study and practice of creating, storing, finding, manipulating and sharing information” (University of Washington 2016). This is similar to the one proposed here for invitromatics but “information” has been replaced with cell line. The use of the term animal invitromatics is being restricted to the science and history of the basic establishment, characterization and genetic engineering of cell lines whereas specific uses of cell lines will be described as invitroomics, which will be defined later in this Introduction. The preparation of primary cell cultures might be considered as part of invitromatics because this is a necessary step for establishing cell lines. A primary culture starts with the placement of an animal’s cells from tissues and organs directly into culture and ends when the primary culture is either used in an experiment or subcultured for the first time; at which point, it becomes a cell line (Schaeffer 1990). Primary cultures are excluded here on the basis that they are usually not stored and lack a life after they have been experimented on. The preparation of primary cell cultures certainly has a science, with methods varying with the cell type, tissue or organ being studied, but primary cell cultures are usually “one and done” and have no subsequent history. By contrast, cell lines take on a life of their own that can lead to very diverse and complex issues. An illustration of this complexity is the best-selling book The Immortal Life of Henrietta Lacks that provides among its many achievements a biography on the early life of the human cell line, HeLa (Skloot 2010).
The invitrome is being defined as one or more cell lines grouped around a common theme. Thus, the RT invitrome is all the cell lines that have ever been derived from Oncorhynchus mykiss and is a subset of the fish invitrome, which in turn is a subset of the animal invitrome. However other properties of the animals from which the cell lines were developed might be used to define an invitrome. For example, animal habitat could be used. In this case, the marine invitrome would be all the cell lines that had been developed from marine multicellular animals. Cell lines might be grouped around the organs from which they were initiated so that there would be for example a liver invitrome or brain invitrome. Histological organization might be another descriptor so that invitromes might be clustered around tissue type, such fibroblast or epithelial invitromes, or functional cell type, such as a hepatocyte invitrome or neuronal invitrome. Additionally, all the cell lines from tumors would be a cancer invitrome. Besides their origins, cell lines might be grouped around specific functional properties, such as their ability to support replication of viruses.
Invitroomics is being defined as the use of cell lines to study the cellular and molecular biology of multicellular organisms or to manufacture useful products. Invitroomics would include employing cell lines to understand complex processes in many disciplines, including development biology, physiology, immunology, toxicology, and virology. Therefore, the word might be most easily employed as an adjective, “invitroomic.” For example, invitroomic toxicology would be the use of cell lines to study a toxicology problem; invitroomic cardiology, the use of cell lines to study the heart. Production of commercial products might be called invitroomic manufacturing. At the end of this article, the use of the word invitroomics will be illustrated with the example of RT invitoomic virology.
Rainbow Trout (RT) Invitromatics
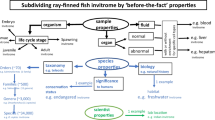
The topics that would encompass the invitromatics for a species are briefly reviewed here for rainbow trout (RT). These involve the history, maintenance, development, characterization, storage, and distribution of RT cell lines (Fig. 1a ).
A schematic that uses rainbow trout (RT) to illustrate the distinctions between three new terms for in vitro biology. Invitromatics is the preparation, storage, and distribution of cell lines (a). All the cell lines that have been cataloged around a theme constitute an invitrome. One theme is the availability of the cell lines. On this basis, this article defines several invitromes, with three of them being the curated invitrome, the informally shared invitrome, and the zombie invitrome (b). From these invitromes, the cell lines can be grouped in many other ways, such around the species from which the cell lines were developed. This article reviews the RT invitrome. Invitroomics is the utilization of the invitrome for investigating scientific problems (c). For example, invitroomic virology and invitroomic toxicology would be the utilization of cells lines to investigate respectively viruses and toxicants.
History
The history of RT invitromatics begins with the establishment of the rainbow trout gonadal cell line, RTG-2 (Wolf and Quimby 1962). RTG-2 was the first fish cell line and so also begins fish invitromatics. To this day, RT cell lines continue to be developed, with perhaps the most recent ones being RT-ovfl and RTmilt5 from reproductive fluids (Vo et al. 2015) and HK1532 from the head kidney (Maisey et al. 2016).
Maintenance of RT cell lines
Detailed maintenance protocols have been presented elsewhere (Wolf and Quimby 1969; Wolf 1979; Wolf and Ahne 1982; Bols and Lee 1994; Dayeh et al. 2005, 2013) and are not that dissimilar from those for mammalian cells so here the emphasis is on some of the considerations that are important for culturing cells from coldwater fish, like RT.
Temperature
RT cell lines are routinely grown at 18–22°C, which is often reported as room temperature. However, cells survive for at least a week in cultures held at any temperature between 0 and 28°C. This is referred to as the RT cellular endurance zone (Bols et al. 1992). The cells will proliferate from 4–5 to 24–25°C (Plumb and Wolf 1971; Mosser et al. 1986; Van Oostrom and Bols 1991), which has been defined as the proliferation zone (Bols et al. 1992). For RTG-2 cultures routinely grown at room temperature, shifting cultures to 24°C and above will initiate heat shock protein (HSP) synthesis (Mosser et al. 1986; Bols et al. 1992) and development of thermotolerance (Mosser et al. 1987; Mosser and Bols 1988).
Media
The complete medium for the growth of RT cell lines consists of a basal medium with a supplement of serum (Bols and Lee 1994), although RTG-2 can survive for several wk in basal medium alone (Lee et al. 1988). A basal medium is a buffered aqueous solution of nutrients that always includes a hexose, bulk ions, amino acids, and vitamins. Basal media were developed for mammalian cells but work well for fish cells and are commercially available. Although RT cell lines can be maintained without antibiotics, most often penicillin and streptomycin are added to complete the medium. The choice of basal media depends on the availability of incubators.
Based on the buffering mechanism, two different classes of basal media have been used to develop and maintain RT cell lines. In one class, pH is maintained by sodium bicarbonate (NaHCO3) and a CO2 rich atmosphere, usually 5% CO2 and 95% air. Eagle’s Minimum Essential Medium (MEM), which was used to develop RTG-2 (Wolf and Quimby 1962), and modifications of MEM belong in this class. These require a CO2-incubator to provide a CO2 atmosphere necessary for the bicarbonate buffering system to work. CO2-incubators have to be modified to maintain temperatures below room temperature so are often not available. To get around this problem, basal media in this class have been modified in several ways to be less dependent on a CO2 atmosphere. Without a CO2-incubator, culture pH can be maintained with these basal media when they have been prepared with Hank’s salts and/or have organic buffers, such as N-2-hydroxyethylpiperazine-N`-2`-ethanesulfonic acid (HEPES) or tris (hydroxymethyl)-aminomethane(tris)-hydrochloride (TRIS). STE-137 from steelhead, which is sea-run RT, grew in a MEM/TRIS based medium without sodium bicarbonate and in free gas exchange with air (Kleeman et al. 1970). In MEM/HEPES, RTG-2 appeared to grow better than in MEM (Wolf and Quimby 1973a).
The second basal media class was formulated specifically for use in free gas exchange with air. Most commonly, this is Leibovitz’s L-15, which maintains physiological pH through a combination of salts, high basic amino acid concentrations, and replacing glucose with galactose. L15 has been the basal medium for developing and using many RT cell lines, including RTL-W1 and RTgill-W1 (Lee et al. 1993; Bols et al. 1994). Another basal media of this class is CO2-Independent Medium, which unlike L-15 contains sodium bicarbonate, and has been used to isolate RT cell lines from the liver and pituitary (Chen et al. 2004, 2010).
Commonly glutamine is added to basal media before use with mammalian cells, but for RT and other fish cell lines, this was not necessary when the basal medium was L15 (Bols et al. 1994). However, when MEM was the basal medium, growth was poor without the addition of glutamine.
Usually, RT cell lines are grown in basal media with 10% fetal bovine serum (FBS) but this has varied with some cell lines. For RTS11, 30% FBS was essential for the early stages of establishment but latter for routine growth was dropped to 15% (Ganassin and Bols 1998). RTL-W1 was maintained for some years with 5% FBS (Lee et al. 1993). Although at various times salmonid sera have been commercially available, they have usually been too expensive to be routine alternative to FBS.
Adherence versus suspension
With the exception of two immune cell lines, all RT cell lines are anchorage dependent: they grow adherent to the surfaces of tissue culture vessels, usually plastic. By contrast, RTS11, which is a monocyte/macrophage cell line (Ganassin and Bols 1998), and HK1532, which is a T cell line (Maisey et al. 2016), grow in suspension or loosely adherent to tissue culture plastic and likely could be grown in suspension bioreactors. Under some conditions, adherent cell lines can form aggregates. In culture vessels that were manufactured not to support cell attachment, RTS34st aggregated to form spheroids (Xing et al. 2008). When added to conventional tissue culture plastic vessels after a week in suspension, the spheroids attached and cells migrated out over the growth surface, indicating that the cells had remained viable in suspension. Some but not all RT cell lines show strict contact-dependent inhibition of growth. When the RT liver cell line, RTL-W1, was maintained for a long time without being passaged, aggregates formed on the monolayer (Malhao et al. 2013).
Subculturing
For the passaging of adherent RT cell lines, cells are removed from the vessel surface with trypsin but alternatives such as TrypLE work as well (Bols and Lee 1994). A usual passaging ratio for such cell lines is 1 to 2 or 3. For difficult to detach cells, alternatives such plant proteolytic extracts (Lee et al. 1986) or non-enzymatic cell dissociation solutions have been used (Bols and Lee 1994). The time between routine subcultivations declines during cell line development and usually stabilizes at between 7 and 14 d (Lannan et al. 1984; Lee et al. 1993). The population-doubling time has been reported at between 3 and 7 d (Chen et al. 2010).
Establishing RT cell lines
ᅟ
Spontaneous immortalization
Most RT cell lines have arisen through spontaneous immortalization. Spontaneous immortalization is the initiation of an indefinitely growing cell line through just the routine procedures of conventional cell culturing, with no addition of biological, chemical or physical agents. RT cell lines are judged to be immortal because lines, such as RTL-W1, have been subcultivated for 2- to 3-yr periods at 1 to 2 or 1 to 3 split ratios, which approximate a population doubling, over a hundred times, without any signs of cultures deterioration. Also continued passaging does not cause the cells to acquire senescence-associated β-galactosidase (SA β-Gal) (Vo et al. 2015), which for mammalian cells is a marker of replicative senescence (Dimri et al. 1995). Spontaneous immortalization occurs for cells from all RT life stages and tissue or organs, although immortalization is rare for cells of the immune system. Two very different general mechanisms can be advanced to explain why cells are so easy to immortalize from apparently normal RT tissues/organs.
One explanation is that cell lines develop from naturally immortal cells. Most tissues and organs of vertebrates have tissue-resident somatic stem cell populations (Chandel et al. 2016). Possibly small populations of immortal stem cells in RT tissues/organs have given rise to the cell lines. Alternatively, in light of the observation that telomerase activity is high in all RT organs (Klapper et al. 1998; Yoda et al. 2002), perhaps all the cells are immortal and any of them can lead to cell lines, which would then be expected to contain telomerase. Telomerase activity has been detected in at least one RT cell line, RTHDF (Ossum et al. 2004). However, the telomerase activity in all RT organs might reflect the indeterminate growth of RT and not the lack of barriers to continuous proliferation.
The second general explanation would be that most RT cells in vivo do have barriers to continuous proliferation, but the barriers are lost spontaneously in some or all of cells upon being placed in culture. One barrier could be replicative senescence, but if so, this barrier must be overcome very early upon the cells being placed in culture. When monitored during the development of RT cell lines from reproductive fluids, SA β-Gal staining was seen in primary cultures from milt but disappeared as the cells were passaged (Vo et al. 2015). For cultures from ovarian fluid, SA β-Gal staining was never seen and like many classic RT cell lines did not reappear on continued passaging of cells (Vo et al. 2015).
Although rare, the spontaneous immortalization of avian and mammalian cells offer some clues as to how proliferation barriers might be breached with RT cells. For the cells of higher vertebrates, changes in the expression and/or activity of genes involved in the p53 and Rb pathways contributed to immortalization (Christman et al. 2006). Therefore, RT cell immortalization might involve changes in the tumor suppressor proteins, p53 and Rb, and their signaling networks. However, recently culture conditions, especially cytokines and growth factors, have thought to be critical in the immortalization of mouse liver endothelial cells (Zhao et al. 2015). Indeed, several observations with RT cells suggest a unique combination and/or concentration of polypeptide growth factors early in the cell culturing might be critical in order for the proliferation to become continuous. Exposure to the recombinant RT Ea4 peptide, which is related to pro-insulin-like growth factor 1 (IGF1), appeared necessary for cells in primary RT pituitary cultures to evolve into immortal cell lines (Chen et al. 2010). Essential to the early development of RTS11 was the use of 30% FBS, which later could be dropped to 15% (Ganassin and Bols 1998). The development of several RT cell lines has started by allowing the primary culture to become over confluent and to let them sit in this state for several weeks without changing the medium before the first sub-cultivation. During this time, autocrine and/or paracrine growth factors might have accumulated that facilitated immortalization.
Directed immortalization
Attempts have been made to directly immortalize RT cells. Heart cells were transduced with polyoma middle T antigen, leading to an endothelial cell line (RTH) (Luque et al. 2014). Whether the T antigen had played a role in RTH development is hard to say, given the ease with which RT cells spontaneously immortalize. However, T antigen has been a common immortalization method for a variety of cell types from different mammalian species (Banerjee et al. 2016).
Basic characterization
ᅟ
Species identification
Species identification of fish cell lines has improved dramatically over the last 10 years but distinguishing between lines from the same species has yet to become routine. Amplifying and sequencing a 653 bp region of the mitochondrial cytochrome c oxidase I gene has been shown to unambiguously identify several thousand species of fish, including RT (Hubert et al. 2008; Ward et al. 2009). This technique, called DNA barcoding, has been used to confirm the species identity of several RT cell lines, including RTgutGC (Kawano et al. 2011). For distinguishing between RT cell lines, several different techniques have been tried. Although having some success at identifying the species origin of a cell line, isoenzyme analysis and proteomics have largely been unsuccessful at distinguishing between different RT cell lines (Lannan et al. 1984; Lidgerding et al. 1984; Wagg and Lee 2005). The most success has been from analyzing microsatellite loci (Perry et al. 2001). Nine RT cell lines could be distinguished from one another when 10 microsatellite loci were analyzed, but this is a technique that is not readily available to every cell culture laboratory.
Mycoplasma
Assessing for mycoplasma is a part of invitroomatics quality control. Mycoplasma refers to any species in the Mollicutes, a class of bacteria with approximately 200 species (Razin and Hayflick 2010). Culturists have long been concerned with mycoplasma because of the ease with which they can contaminate cultures without overtly changing the culture appearance. Over the years, many methods for detecting mycoplasma in animal cell lines have been developed. These fall into three general categories: culture tests, polymerase chain reaction (PCR), and fluorescent DNA staining (Young et al. 2010). Fluorescent staining is technically the easiest method and is done with fluorescent nucleic acid dyes, such as Hoescht H33258 or DAPI (4′,6-diamidino-2-phenylindole). When contaminated cultures are stained, cell nuclei appear as large fluorescent structures; mycoplasma, as small punctate fluorescent staining over and/or around the cell surface (Chen 1977). Staining gives no information as to whether the punctate fluorescent staining over cells are inside or on the surface of cells or to the identity of the contaminating mycoplasma species.
During the course of their development, cell lines either might acquire mycoplasma accidently from within the cell culture facility or have had mycoplasma from the start, being present in the organ from which the primary cultures were initiated. The accidental acquisition is covered later in the invitroomics section, whereas their possible presence in the starting material is considered here. Mucosa membranes of at least some vertebrates contain mycoplasma as commensals and this appears to be the case for the fish gut. Molecular-based methods have revealed multiple mycoplasma species in the intestinal microbiota of RT (Etyemez and Balcazar 2015), Atlantic salmon (Holben et al. 2002; Zarkasi et al. 2014; Llewellyn et al. 2016), and other fish (Bano et al. 2007). Yet, only two mycoplasma species have been grown from fish. Acholeplasma laidlawii was isolated from bass, bluegills, crappies, and sunfish (Francis-Floyd et al. 1998); Mycoplasma mobile, from a tench (Kirchoff et al. 1987). However, the isolation and identification of a mycoplasma species from RT has yet to be reported. We have stained several primary RT cell cultures with H33258 and have seen no signs of mycoplasma. On the other hand, H33258 staining has indicated mycoplasma in several fish cell lines and mycoplasma can be grown from fish cell cultures. H33258 staining revealed mycoplasma in cultures at certain passages for two RT cell lines, STE-137 (Lannan et al. 1984) and RTgill-W1 (Bols et al. 1994). The first report of mycoplasma in any fish cell line was with RTG-2 and the contaminant was identified through culture tests as A. laidlawii (Kunze et al. 1972). Since then, A. laidlawii has been identified by microbiological tests in 11 different fish cell lines (Emerson et al. 1979; Frerichs 1996) and Mycoplasma arginini in three (Frerichs 1996). Overall, an endogenous entry route for the appearance of mycoplasma in fish cell lines is possible but more evidence is needed to support this route.
Several approaches for removing mycoplasma from mammalian cell lines have been developed and many mammalian cell lines have been cleaned up (Uphoff and Drexler 2002; Uphoff et al. 2012), but eradication has been attempted infrequently with fish cell lines. Technically, the simplest eradication methods involve antibiotics and new commercial antibiotic preparations (e.g., Plasmocin) for this purpose continue to become available (Uphoff et al. 2012). One concern about this approach has been the possibility that properties of the cell line change because of treatment with the mycoplasma antibiotics. However, for human embryonic stem cell lines, Plasmocyin was found not to affect pluripotency (Romorini et al. 2013). The only literature report of an RT cell line been cleaned of mycoplasma is for RTgill-W1 (Bols et al. 1994), although some cell repositories (see Table 1) note one or two their fish cell lines have being cleaned. For RTgill-W1, success was achieved by treating cultures in succession with two commercial antibiotic preparations: mycoplasma removal agent (MRA) and BM cyclin. MRA contains a nondisclosed fluoroquinolone; BM cyclin, derivatives of pleuromutilin and tetracycline. Whether other fish cell lines have been cleaned up but have gone unreported would be interesting to know, especially the details of successful treatments.
Although cultures can be cleaned, deciding how to proceed with infected cell lines continues to be difficult. Often the advice has been to discard them, but this is not always practical when cell lines are under development. RT cell lines can take 1 to 2 yr to establish and so discovering mycoplasma late in the process makes discarding them difficult. Such cell lines probably should be cryopreserved, but only thawed when they can be handled separately from other cell lines and attempts can be made to clean them. In the future, better procedures and drugs for cleaning cells of mycoplasma might be developed, allowing contaminated cell lines to be rescued.
Karyotype
Karyotyping has been done on some RT cell lines. The diploid chromosome number for RT can vary with the strain but commonly is reported as being between 58 and 64 (Thorgaard 1983; Colihuegue et al. 2001). RTG-2 had a modal chromosome number of 59 (Wolf and Quimby 1962). However, other RT cell lines have been found to be heterploid, with at least one modal number over 100 (Lee et al. 1993; Bols et al. 1994; Kawano et al. 2011). Early in the history of fish cell line development, karyotyping was done to give some assurance of species of origin, but now species identification can be done more easily and accurately through DNA barcoding. However, determining whether other RT cell lines fall into either diploid or heteroploid classes will be interesting to document because this might suggest two different spontaneous immortalization mechanisms. From work with mammalian cells (Agapova et al. 1996), heteroploidy would suggest the involvement of p53. Indeed, inhibiting p53 in RTgill-W1 induced polyploidy (Zeng et al. 2016).
Cellular morphology
The predominant cell shape in cultures of RT cell lines is usually noted as either being epithelial-like, fibroblast-like or other and is used to suggest a possible origin from either epithelial or connective tissue. In cultures of fibroblast-like lines, the cells are bipolar, usually long, and become more noticeably arranged in parallel as cultures become confluent. By contrast, in cultures of epithelial-like lines, the cells are very flat with outlines varying from irregular to cobblestone. The epithelial cultures can be dynamic with respect to shape. For example, the most common shapes in cultures of the epithelial-like RTP-2 changed as the cells grew from low density to confluency (Bols et al. 1995). Most RT cell lines are epithelial-like (Tables 2, 3, and 4). Attempts to correlate epithelial and fibroblast shapes with expression of cytoskeletal components are frustrated by the complex distribution of intermediate filament proteins in RT tissues (Schaffeld et al. 2002) and the paucity of commercial antibodies that react well with fish cytoskeletal proteins (Malhao et al. 2013). Cultures with the most diverse cellular morphologies are for immune cells. The monocyte/macrophage cell line, RTS11, has a complex mixture of cell shapes in suspension and on the culture surface (Ganassin and Bols 1998).
In-depth characterization of RT cell lines
Increasing the characterization of RT cell lines beyond their basic features can increase their value in research. An example of such an in depth characterization has recently been published for the liver cell line, RTL-W1, for which cell lineage and stem cell properties were assessed (Malhao et al. 2013). RTL-W1 had characteristics of bile preductular epithelial cells (BPDECs), which are considered bipolar progenitor cells capable of differentiating into both hepatocytes and biliary cell lineages (Alison et al. 2001; Malhao et al. 2013). Characterization can also be done to increase the value of the cell line for a particular discipline or purpose. Examples of this will be given for toxicology, endocrinology, and virology. For toxicology, seven different RT cell lines were characterized for the expression of ATB binding cassette (ABC) transport proteins because they might be involved in cellular transport of ecotoxicants (Fischer et al. 2011). ABC transporters were constitutively expressed in all the cell lines. Aryl hydrocarbon receptor (AhR) signaling pathways were characterized in two RT cell lines to improve their utility in studying dioxin-like compounds (Pollenz and Necela 1998). For endocrinology, five RT pituitary cell lines were characterized for the expression of growth hormone and prolactin genes (Chen et al. 2010). For virology, the response of RT cell lines to synthetic double-stranded (ds) RNA, which is a viral mimic, were compared to evaluate their antiviral mechanisms. Double-stranded RNA has multiple effects on rainbow trout cell lines. Morphologically, homotypic aggregation and apoptosis was induced by dsRNA in the monocyte/macrophage cell line, RTS11 (DeWitte-Orr et al. 2005; 2007a). Additionally, dsRNA induced an antiviral state in RTS11 by upregulating interferon-stimulated genes (DeWitte-Orr et al. 2007b); this induction is also dependent on dsRNA length as longer length induced stronger response (Poynter and DeWitte-Orr 2015).
Neoplastic characterization of RT cell lines
RT cell lines have been developed from tumors. RTH-149 was established from an alfatoxin-induced hepatoma (Fryer et al. 1981) and four cell lines (RTH1B1A, RTH1B1D, RTH1B2A, and RTH1B2C) were isolated from liver tumors induced with dibenzo[a,l]pyrene (DBP) (Chen et al. 2004). A RT mesothelioma and a nephrablastoma were the sources for cell lines referred to respectively as RTM (Carter et al. 1996) and RTN (Wolf and Mann 1980). Some of these showed features characteristic of mammalian neoplastic cells in vitro. The liver cell lines formed colonies in soft agar. As judged by immunocytochemistry, RTH-149 and RTM showed higher expression than RTG-2 for the oncogenes ras and c-myc (Carter et al. 1996).
Whether RT cell lines acquire neoplastic properties either through just the act of being continuously cultivated or as a result of carcinogen treatment has been investigated infrequently. In one report RTL-W1 was able to grow in agar without treatment (Malhao et al. 2013) and in another only after the RTL-W1 had been treated with aflatoxin (Bechtel and Lee 1994). In the case of untreated RTL-W1, the growth in agar was thought to reflect the stem cell properties of the cell line rather than neoplastic transformation (Malhao et al. 2013). Overall, the tumorigenicity of RT cell lines from tumors or from normal tissues is unknown because there is no appropriate in vivo tumor assay available to answer the question.
Engineering RT cell lines
Many attempts that have been made to engineer RT cell lines using either virus transduction or transfection produced mixed results. Three cases of virus transduction attempts and two cases of transfection attempts are described below.
Of the three transduction attempts, two were done using baculoviral vectors and one using adenoviral vectors. Both efforts to transduce RTG-2 with baculoviral vectors failed. Leisy et al. (2003) attempted to transduce RTG-2 with a recombinant baculovirus containing a β-galactosidase reporter gene (Ac-CAlacZ) but β-galactosidase activity was not seen in RTG-2. Yokoo et al. (2013) tried to transduce RTG-2 with a baculovirus-Drosophila Mos1 (AcB-GHmos) recombinant system; however, transgene expression was not detected in RTG-2 even though it was detected in CHSE-214 and EPC cells. Transduction of RT cell lines using adenoviral vector, instead of baculoviral vectors, produced more success. All four RT cell lines, RTG-2, RTgill-W1, RTS-34st, and RTS-pBK, expressed the β-galactosidase reporter gene when they were transduced with a recombinant human adenovirus, AdCA35lacZ, containing the lacZ gene controlled by the MCMV-IE promoter (Rainbow and Zacal 2008).
Two transfection attempts of RT cell lines also produced mixed outcomes. After failure to propagate striped jack nervous necrosis virus (SJNNV) on RTG-2 using standard inoculation procedure with infectious virus (Iwamoto et al. 2000), lipofectin transfection of RTG-2 with purified SJNNV RNA also resulted in lack of virus propagation (Iwamoto et al. 2001). However, one of the few successfully engineered RT cell lines is RTG-P1 (Collet et al. 2004). RTG-P1 was created by the transfection of RTG-2 with firefly luciferase gene controlled by the Mx1 gene promoter. The Mx1 gene is an IFN-inducible gene; therefore, this cell line has been used to study the innate immune response. Exposure of RTG-P1 to polyI:C resulted in increased luminescence suggesting an induction of the Mx1 gene; furthermore, exposure of RTG-P1 to viral hemorrhagic septicemia virus (VHSV) also caused increased luciferase activity in the cell line (Collet et al. 2004). In addition to VHSV, other fish viruses, infectious pancreatic necrosis virus (IPNV), infectious hematopoietic necrosis virus (IHNV), and salmonid alphavirus (SAV) also induced luciferase activity in RTG-P1 (Saint-Jean et al. 2010; Park et al. 2011; Collet et al. 2013).
Storing RT cell lines
Cell lines must be stored in some manner for them to be useful as experimental material for invitroomics, and like the cell lines of other vertebrates, RT cell lines can be stored at very low temperatures with cryoprotectants. Most commonly, the cryoprotectant is 10% dimethylsulfoxide (DMSO) in complete growth medium, but other cryoprotectants, such as 10% glycerol, have been used successfully. The procedural steps for freezing and thawing are the same as used for mammalian cells, although thawing at temperatures above room temperature must be watched closely so as not to heat kill them (Bols and Lee 1994). Storage is usually done in liquid nitrogen at −196°C. In our labs, the cryovials are placed overnight in the atmosphere above the liquid nitrogen in the dewar and then lowered into the liquid nitrogen. The presumption is that in liquid nitrogen, the cells can be stored indefinitely, and we have successfully thawed RT cell lines after storage for over 30 yr in liquid nitrogen. RT cell lines in DMSO can also be stored at −80°C, and we have stored them successfully under these conditions 1 to 2 yr but have not tried longer.
On at least one occasion, attempts to cryopreserve a newly developed RT cell line were unsuccessful. Early on in their development, the RT monocyte/macrophage RTS11 failed to be cryopreserved in 10% DMSO and storage in liquid nitrogen (Ganassin and Bols 1998). Different methods of cooling cryovials were tried prior to them being placed in liquid nitrogen but without success. Yet overtime, as RTS11 continued to be grown, the cells began to cyropreserve successfully, by the same standard cryopreservation methods that had been used for other cell lines. Possibly the cell density was higher, up to 10 million cells per 1.5 mL cryovial, contributed to the later success.
Overall cryopreservation of RT cell lines seems to be routine, but procedures likely could still be improved. Different cryoprotectants could be tried, with the natural cryoprotectant trehalose being one possibility (Stokich et al. 2014). A programmable cooler could be used to freeze cells at different controlled rates to identify the optimal freezing rate. Cells could also be stored in cryogenic freezers at −150°C, which would remove the necessity to constantly keep dewars filled with liquid nitrogen.
If the equipment for cryopreservation is not available, RT cell lines can be kept alive at 4–5°C, without having to change the medium. The cells are first grown in L15/FBS at 18 to 21°C in non-vented flasks. After the cultures have reached confluency, the complete volume of the flask is filled with L15/FBS and flask caps are tightened. The flasks are then placed in a laboratory fridge at 4–5°C and can be left unattended for 1–2 yr. When proliferating cells are needed, the flasks are returned to 18–21°C. After a day, the cultures can be subcultivated to being routine growth again.
Distribution of RT cell lines
Repositories, scientific companies, and scientific colleagues make up the distribution network for RT cell lines. The relative merits of each are discussed in the Invitrome section. The repositories with RT cell lines are listed in Table 1. Cells can be shipped frozen in cryovials and/or alive in flasks. Cryovials can be sent in liquid nitrogen dry shippers or on dry ice. Liquid nitrogen dry shippers are perhaps the most convenient method for both the shipping and receiving labs but are expensive. Dry ice is hazardous material for airlines so cryovials being sent this way requires more regulatory hurdles for the shipper and additionally the receiving lab should likely thaw the cryovials immediately on getting them in order to get the best cell recovery. RT cell lines in culture flasks have survived shipment by several commercial couriers. One key to success is to use small flasks (12.5 or 25 cm2) and to fill the whole flask volume with medium so the cells are always covered with medium, even if the package is upside down. Another key to sending RT cell lines in flasks is to ship during periods of cool weather.
Invitrome: Catalog of Cell Lines
The invitrome is being defined as the grouping of cell lines around a theme (Fig. 1b). For this article, the overarching theme is a single species, O. mykiss. Yet cell lines can be cataloged in many other ways in order to help researchers to use and think about them. One way is based on cell line storage and availability and this is discussed after first presenting the RT invitrome.
Rainbow trout (RT) invitrome
All the cell lines reported from O. mykiss constitute the RT invitrome and, currently, consist of approximately 55 cell lines. This number is derived from using scientific indexing services such as the Web of Science and the “cellosaurus” search engine (http://web.expasy.org/cgi-bin/cellosaurus/search). These cell lines can be grouped in numerous additional ways, and below is a way that should help potential users of RT cell lines.
Invitromes based on the storage and availability of cell lines
Invitromes can be defined by the storage and availability of the cell lines. When this was considered for RT cell lines, five invitromes could be distinguished. A similar framework likely can be applied to cell lines from any animal species, from insects to birds.
Curated invitromes
Curated invitromes refer to the cell line collections of agencies that devote resources and personnel to cell line cataloging and quality testing and are committed to the long-term storage and distribution of cell lines. Usually, these agencies are non-profit national and international repositories. The costs of cell line accession, storage, and shipping are recovered by charging a fee for each cell line. Repositories are the best sources for cell lines because any problems of misidentification and mycoplasma usually have been sorted out before they are distributed. Repositories also have standard operating procedures and schedules for shipping and for dealing with cell importation regulations. The movement of cell lines between countries varies with countries (Geraghty et al. 2014).
Repositories with at least one RT cell line are given in Table 1. RT cell lines that are available from repositories are presented in Table 2 and constitute the RT curated invitrome. Choosing a repository is perhaps most often driven by geography but there might be additional factors, such as cost or quality. Other institutions might have curated invitromes, although they are less likely to contain fish cell lines. These include large academic institutions, government laboratories, and pharmaceutical/biotech companies. Like repositories, these agencies can strictly restrict the movement of cells, material, and people into their facilities to help assure the quality of their cell lines. Unlike repositories, their cell lines might not be routinely available for distribution.
Commercial invitromes
Cell lines are available from commercial sources. The cell lines available in this way are usually a small selection of those in repositories. The RT commercial invitrome contains at least one cell line, RTG-2, which is available from Sigma (9010259, St Louis, MO). By contrast, many mammalian cell lines are commercially available and clients can order customized cell lines from some companies, such as SB Drug Discovery (Glasgow, Scotland).
Informally shared invitromes
Many cell lines are cryopreserved but are not in repositories but instead are passed informally between scientists. Excluded from this group are cell lines in repositories and commercially available but other limits are arbitrary and might depend on the species. For RT, the cell lines that have been described or used in the last 20 years are listed in Table 3 and constitute the informally shared invitrome. In this invitrome are some recently developed RT cell lines for which there has not been time to see whether others want to use them and whether they are shareable. Despite this, many RT cell lines clearly fit the definition for an informally shared invitrome. Two examples are RTL-W1 from the liver and RTS11 from the spleen monocyte/macrophage lineage (Lee et al. 1993; Ganassin and Bols 1998). The only cost for these is shipping. However, obtaining cell lines from this invitrome is less regularized. Small source laboratories do not always have the resources to immediately ship cells and the receiving laboratory might have to be more directly involved in negotiating any regulations about importing cell lines into a country. The quality of this invitrome is not as assured as with cell lines from formal repositories. However, the quality is likely adequate for many purposes, and regardless of the cell line source, quality is an ongoing concern during the routine maintenance of any cell line, as discussed later under invitroomics. A final worry about cell lines not in repositories is that they might be lost when laboratories shut down. On the other hand, if cryopreserved in several laboratories, hopefully, a cell line will remain cryopreserved in at least one and be available for future use. However, future scientists might not necessarily know where to find the cells and the cell line could become part of the zombie invitrome.
Zombie invitromes
This refers to all the cell lines that have been described in the literature either in detail or only in passing but have not been placed in repositories and have not been reported as being used much further. As the loss of a cell line is rarely noted in publications, the reader is left to wonder about their status. They might be lost, but alternatively some might still be cryopreserved in liquid nitrogen somewhere, waiting to be thawed. This potential for re-emergence into scientific endeavors suggests the moniker, zombie cell line. Even if they remain dead, they have provided some information about what cell lines are possible. For RT, Table 4 lists the cell lines that have been noted in the literature but have not been mentioned for the last 20 yr and constitute the RT zombie invitrome. Two interesting members are RTM and RTN (Wolf and Mann 1980; Carter et al. 1996) because they are two of only three cell lines from RT tumors. Finally, the zombie invitrome might be the fate of many cell lines that are not deposited in repositories because future scientific interests are hard to predict. Applications for a cell line in common use now might disappear and consequently so might the use of the cell line. Thus, the incentive to keep the cells cryopreserved would disappear and so might the cell line. On the hand, new applications might be found for the cell line much later, and so if the cells have been kept cryopreserved, the cell line could return to the flask of the living.
Cryptic invitromes
Some cell lines might be developed and cryopreserved but never used in the literature. For example, they might be started for projects that subsequently change direction. As long as their cryopreservation is maintained, they could be used but would be known only to the developers and hidden from the larger scientific community. How common this situation is hard to judge but we have cryopreserved new cell lines from fish that have yet to be formally characterized.
Invitroomics: Use of Invitromes
Invitroomics is the application of cell lines to a scientific problem and is done in many disciplines (Fig. 1c ). For example, the RT invitrome is important in aquaculture (Bols 1991), toxicology (Bols et al. 2005) and virology (Wolf and Mann 1980). However, several issues can confound the use of an invitrome and distract from the science. These are briefly illustrated here with the RT invitrome along with several suggestions on how to deal with them, followed by short summary of how the RT invitrome has been used in virology.
Cross contamination
Occasionally, a cell line that is understood to have a particular identity is found subsequently to have a different one. This is cross contamination and was recognized as a problem for mammalian cell lines in the 1960s but continues even to this day (Lucey et al. 2009; Di Girolamo et al. 2016). Although less well documented, cross contamination also has been found with fish cell lines (Winton et al. 2010). The causes of cross contamination can vary and usually are hard to prove retrospectively. One recommendation for reducing the problem is to confirm the cell line identity as often as is possible, although this can be hard if the cell lines are from the same species. However, analyzing microsatellite loci allowed RT cell lines to be distinguished (Perry et al. 2001). Another recommendation is to maintain “good laboratory practice.” “Good practice” for fish cell culturing was presented years ago (Wolf and Quimby 1973b) and specific steps have been listed recently for this issue with mammalian cells (Di Girolamo et al. 2016). For all animal cell culturing, an important habit is to have a separate bottle of medium for each cell line, and after a volume of medium has been drawn out with a pipette, the pipette must never be returned to the medium bottle. This is to avoid introducing cells into the stock bottle of medium, which later may used to culture another cell line. This contamination route might be more likely with cell lines from coldwater fish, such as RT and other salmonids, than with mammalian cell lines. When CHSE-214 cells were deliberately added to a bottle of medium and the bottle put into a fridge, some cells survived and could be cultured from the bottle even after several weeks of storage at 4–5°C (Perry et al. 2001).
Mycoplasma contamination
Mycoplasma are thought to be inadvertently introduced into animal cell cultures in several ways (Drexler and Uphoff 2002), and considering these might help in reducing contamination when working with fish cell lines. One contamination route is media components (Low 1974). This would likely be same for piscine and mammalian cell cultures because media come from the same commercial sources. The researcher themselves can be an importance source of mycoplasma contamination (Drexler and Uphoff 2002). However, this might be less likely for cell lines from coldwater fish, such as rainbow trout, because presumably human mycoplasma would adapt poorly to the low growth temperatures for these cell lines. For human and rodent cells, mycoplasma-infected cell lines are themselves the most important source (McGarrity 1976). The handling of cell cultures creates aerosols, and when cultures are contaminated, the aerosol contains and spreads mycoplasma throughout the tissue culture facility (O’Connell et al. 1964; McGarrity 1976). This is probably how mycoplasma spread into fish cell cultures but two further issues likely exacerbate the problem. As was discussed earlier, only repositories and a few other agencies have the resources to assure regularly that cell lines are free of mycoplasma but relatively few fish cell lines are in repositories. The fish cell culture facilities are often in academic institutions where rigorously restricting the movement of cells, people and material in and out of cell culture facilities is rarely possible.
Starting experiments with cells from a cell line that had been shown to be free of mycoplasma would appear to offer the best assurance that contamination has not compromised the results. Yet contamination might take place at any time, including during the course of the experiment. The mycoplasma in fish cell cultures appear to be very slow growing and confirming their presence might take place wk after some of the cells have been used in an experiment. For example, when RTgill-W1 was being developed and before the cell line had been cleaned, mycoplasma could be consistently detected only in flask cultures that had been allowed to sit undisturbed for 2 to 4 wk without the media having been changed (Bols et al. 1994). If a 1-wk-old flask of these cells had been used to set up an experiment that lasted 3 d, the number of mycoplasma would likely have been vanishingly small and had no effect on the experiment. Rather than throw away the results from such experiments because of possible mycoplasma contamination, we would recommend presenting evidence of the mycoplasma level immediately at the end of an experiment. For this, the most practical test would be H33258 staining because nearly every lab would be able to do this, as the only equipment needed would be a fluorescent microscope. In each experiment, some wells or culture flasks would be set aside, with cells growing on slides or coverslips for staining. Even whole flasks could be stained if an inverted fluorescent microscope were available (Bols and Lee 1994). One set of these would be stained immediately at the end of the experiment and another 1–3 wk later, depending how long the cells could be maintained without media changes. These would be scored for the numbers of cells with multiple small punctate fluorescent staining on or closely around them.
Three general outcomes are possible from the H33258 test. If no punctate fluorescent staining were to be seen at either time point, the cells would be reported as having been mycoplasma negative during the experiments. As all mycoplasma have DNA and most adhere to cells (Drexler and Uphoff 2002), false negatives would be unlikely. Secondly, if no punctate fluorescent staining were seen at the end of the experiment but appeared at the later time point, the cells would be reported as having been negative for the experiment but possibly having been present in the stock culture. Thirdly, if they were present at the end of the experiment and their numbers had increased at the later time point, the cells would be concluded to have had mycoplasma both in the stock culture and during the course of the experiment. We would recommend that such results be published but together with some measure of the level of the contamination, such as the percentage of mycoplasma positive cells as determined with H33258. False positives might be caused by structures such as micronuclei but a fluorescent picture of the stained culture might help the reader to assess this. Also repeating the experiment in the presence of antibiotics that target mycoplasma might confirm whether the experiment had indeed been influenced by mycoplasma. Overall transparency on this issue will help the scientific community build up a more detailed picture of how mycoplasma might influence fish cells and help work to ways to eliminate them and to improve the quality of invitromes and the power of invitroomics.
Stability
Whether characteristics of a cell line are lost or gained with prolonged passaging has long been a worry but has been difficult to document with RT cell lines because of the time that would have to be committed to make a convincing case. During the early development of an RT cell line, the time for a culture to become confluent after a sub-cultivation shortens as the cycle of growth to confluency followed by splitting 1 to 2 or 1 to 3 is repeated. Eventually this time stabilizes after approximately 5 to 20 passages, depending on the cell line. As primary cultures would be expected to start with mainly diploid cells, the karyotype clearly changes during the development of heteroploid RT cell lines but whether the karyotype has stabilized has rarely been investigated. However, for RTH-149, the modal chromosome numbers were different at passage 20 versus passage 141 (Fryer et al. 1981; Lannan et al. 1984). The loss of a specific property has been noted with a pituitary cell line. Expression of growth hormone and prolactin genes failed to be maintained upon the prolonged culturing of RTP-2 (Bols et al. 1995; Chen et al. 2010). Therefore, reporting the passage number range at which RT cell lines are used in experiments is recommended so the stability of a particular property can be established as the cells are used over the years in different labs.
Use of the RT invitrome in virology
Virology was the original application for animal cells lines and was the impetus for RT cell line development as well. Cell lines can be a diagnostic tool for detecting viruses because many viruses on many cell lines cause cytopathic effects (CPE), indicating their presence and allowing them to be isolated and quantified. Some cell lines support the propagation of viruses. This allows the study of the single cell reproductive phase of the virus and agents that might interfere with it, including the antiviral mechanisms of the host cells. Additionally some cell lines produce sufficient virus to allow elucidation of viral structure and generation of diagnostic antibodies and vaccines. In the original description of RTG-2, the cells were shown to develop CPE in response to infectious pancreatic necrosis virus (IPNV) (Wolf and Quimby 1962). Subsequently, many viruses have been studied with the RT invitrome. These are listed in Table 5. In total, viruses from 15 families were studied with RT cell lines; six of these families are double-stranded DNA viruses and two are double-stranded RNA viruses. Three of the families are positive sense ssRNA viruses and three are negative sense ssRNA viruses. In addition, one retrovirus, walleye dermal sarcoma virus was studied with RTgill-W1. RTG-2 was the most used out of all the RT cell lines, likely because it was the first fish cell line developed and the only RT cell line to be recommended by the OiE World Organisation for Animal Health for the isolation of fish viruses (OiE 2016).
Some of the concerns associated with the use of an invitrome have been realized in fish virology. Cross contamination was observed with EPC, which is one of the most widely used cell lines for virus work but is found to be fathead minnow rather than carp (Winton et al. 2010). One of the few documented effects of mycoplasma on an RT cell line activity was on virus propagation (Emerson et al. 1979). By itself, A. laidlawii caused cellular granularity and destruction when added to RTG-2 cultures at high levels but not low ones. At low levels, A. laidlawii appeared to enhance IPNV and IHNV infection. This was seen as a 2- to 100-fold increase in IPNV and IHNV titres. Possibly, A. laidlawii did not increase virus propagation but instead sensitized the RTG-2 to cellular destruction by the viruses so that viral CPE occurred at lower viral numbers than in control cultures. Whether viral susceptibility remains stable or changes as RT cell lines are passaged would be interesting to know as this could improve their use in virology. For the Chinook salmon embryo cell line, CHSE-214, susceptibility varied with different CHSE-214 lineages but did not necessarily correlate with the number of subcultivations (McAllister 1997). Despite these concerns, the RT invitrome has been successfully and widely used in virology, and below, this literature is briefly summarized under the headings for the Baltimore classification of viruses.
Double-stranded DNA viruses (dsDNA)
The RT inivitrome has been mostly used to study Iridoviridae and Herpesviridae families within the dsDNA viruses. Viruses of the Iridoviridae family have mostly been reported to replicate poorly in RT cell lines (Table 5; Ariel et al. 2009; Pham et al. 2015). Of all the RT cell lines examined, RTG-2 appears to be the most capable of supporting these viruses (Table 5). Yet this capacity pales in comparison to cells lines of other species such as fathead minnow EPC, Chinook salmon CHSE-214, and Bluegill fry BF-2 (Ariel et al. 2009; Pham et al. 2015). Interestingly, primary RT monocyte and macrophage cells and the monocyte/macrophage RTS11 cell line appear to be specifically targeted by frog virus 3 (FV3) and grouper iridovirus (GIV) to undergo apoptosis very early during the infection cycle (Pham et al. 2012, 2015).
For the Herpesviridae family, RTG-2 and RTF-1 support the propagation of Salmonid herpesvirus type 1 and 2 (Wolf et al. 1978; Kimura et al. 1983). RTG-2, RTgill-W1, and RTL-W1 allow the replication of cutthroat trout virus (CTV) as judged by increases in genome copy number when measured by RT-qPCR. Yet, no CPE was seen with CTV on the three cell lines (von Nordheim et al. 2016). The responses of RTG-2, RTgill-W1, and RTL-W1 to CTV contrast with the responses of RTS11 to FV3 and GIV. In the first case, virus was produced but no CPE were observed; whereas in the second case, CPE were observed but no viruses produced. These observations offer many potential directions for future studies and illustrate the importance of having a reasonably large invitrome for studying the interactions between viruses and the cells of a potential host.
Double-stranded RNA viruses (dsRNA)
Only two families of dsRNA viruses, Birnaviridae and Reoviridae, have been studied with the RT invitrome. Viruses in the Birnaviridae family are very pathogenic to rainbow trout, causing severe pancreatic necrotic diseases (Crane and Hyatt 2011), whereas those in the Reoviridae family are less pathogenic and typically have no disease association (Winton 1981). The main virus studied in the Birnaviridae family is IPNV. IPNV replicates to titres of at least 108 TCID50/mL in RTG-2, RTH-149, and STE-137 (Wolf and Quimby 1962; Lannan et al. 1984). In contrast, viruses in the Reoviridae family have a very low capacity to replicate in RT cell lines, with many, such as Blue gill virus, Bluetongue virus, and Chum salmon virus, replicating to low titres (Hoffman et al. 1969; Wechsler and McHolland 1988; DeWitte-Orr and Bols 2007), in the range of 102 TCID50/mL in RTG-2 (Table 5). The limited ability of reoviruses and the robust ability of birnaviruses to replicate in RT cell line may be associated with their respectively low and high pathogenicity to RT.
Single-stranded RNA viruses, positive sense (ssRNA(+))
For positive sense ssRNA viruses, the RT invitrome has been used most often to study viruses in the Togaviridae family. Fish togaviruses cause pancreas disease and sleeping disease (Crane and Hyatt 2011). Salmonid alpha virus (SAV), sleeping disease virus (SDV), and salmon pancreas disease virus (SPDV) are all capable of replicating in RTG-2, producing virus yields ranging from 104 TCID50/mL to 107 PFU/mL (Table 5). Interestingly, even mammalian togaviruses, Venezuelan equine encephalomyelitis virus (VEEV), and Eastern equine encephalomyelitis virus (EEEV) were capable of replicating to ~105 and ~106 PFU/mL in RTG-2 when incubated at 22°C. The other two families of positive sense ssRNA viruses studied with RTG-2 are Nodaviridae and Picornaviridae (Fukuda et al. 1996; Delsert et al. 1997; Phelps et al. 2014). However, RTG-2 produced only Dicentrarchus labrax encephalitis virus (DlEV), as measured by immunofluorescence (Delsert et al. 1997). Similar to CTV of the dsDNA family, DlEV appears to replicate without killing the RT cells.
Single-stranded RNA viruses, negative sense (ssRNA(−))
The RT invitrome is used most often to study the Rhabdoviridae family of the ssRNA(−) category. Some viruses in this family cause the most economically important viral diseases of RT. Viral hemorrhagic septicemia is caused by VHSV; infectious hematopoietic necrosis, by IHNV. Most fish rhabdoviruses are capable of replicating in RT cell lines, with VHSV and IHNV having been demonstrated to replicate to at least 105 TCID50/mL in three to four different RT cell lines (Table 5). Although replicating to high titer in RT fibroblast and epithelial cell lines, VHSV does not appear to be able to replicate and produce titer in monocyte/macrophage cell line RTS11. Virus entry into RTS11 is detected through the expression of the N gene; however, inhibition of replication appears to occur before the cells can translate viral mRNA as no virus proteins were detected (Tafalla et al. 2008; Pham et al. 2013). Two other virus families in the ssRNA(−) category studied with the RT invitrome are the Orthomyxoviridae and the Paramyxoviridae. European eel virus (EV-2) and Infectious salmon anemia virus (ISAV) of the Orthomyxoviridae family, and Fer-de-Lance virus (FDLV) of the Paramyxoviridae were unable to replicate in RTG-2 (Table 5). ISAV replication was detected by immunofluorescence in RTgill-W1 at a low titer of 103 TCID50/mL but no CPE was seen (Falk et al. 1997).
RNA viruses that replicate through a DNA intermediate (ssRNA(RT))
Walleye dermal sarcoma virus (WDSV), of the retroviridae family, appears to be the only fish retrovirus to be studied with the RT invitrome. RTgill-W1 became infected with WDSV but was unable to support propagation of the virus (Rovnak et al. 2007).
Summary
In summary, the literature on RT cell lines has been reviewed to illustrate three new terms: invitromatics, invitrome and invitroomics. Succinctly, these terms refer respectively to the development, the catalog, and the use of animal cell lines. The review should aid those considering the use of RT cell lines for investigating a wide range of scientific problems but especially virology ones. Hopefully, the new terms will be broadly helpful and can be applied by those using other animal cell lines. Certainly, they could be applied relatively easily to the species for which only a few cell lines are available.
Although collecting the information for all the cell lines from some species, such as Mus musculus, Cricetulus griseus, and Homo sapiens, might be arduous because of the exceptionally large number of cell lines, the term “invitrome” can still be conveniently used to describe subsets of them. Among the cell lines in the murine invitrome would be all the murine cell lines that had been genetically modified through cell fusion, transfection, and gene editing or obtained from transgenic mice (Obinata 2007) and all the cell lines that had been derived from tumors. All the cell lines from a particular tumor type might constitute a specific invitrome, such as the murine mammary tumor invitrome. The Chinese hamster (C. griseus) invitrome would be large because many variant Chinese hamster ovary (CHO) cell lines have been developed for biotechnology purposes (Xu et al. 2012). These might be called the CHO invitrome. The human invitrome would also be large and include embryonic stem cell lines and all the cell lines that have been derived from patients with various diseases or inherited disorders. These cell lines might be organized around the nature of the patient. For example, the Lesch-Nyhan invitrome would be all the cell lines from patients with an inherited deficiency in hypoxanthine-guanine phosphoribosyltransferase activities. Some repositories, such as the Coriell Institute for Medical Research, offer multiple cell lines from the same individual. Therefore, an individual patient or animal might be considered to have an invitrome.
References
Agapova LS, Ilyinskaya GV, Turovets NA, Ivanov AV, Chumakov PM, Kopnin BP (1996) Chromosome changes caused by alterations of p53 expression. Mutation Res 354:129–138
Ahne W (1979) Fish cell culture: a fibroblastic line (PG) from ovaries of juvenile pike (Esox lucius). In Vitro 15:839–840
Ahne W (1985) Use of fish cell cultures for toxicity determination in order to reduce and replace the fish tests. Untersuchungen über die verwendung von fischzellkulturen für toxizitätsbestimmungen zur einschränkung und ersatz des fischtests. Zentralblatt für Bakteriol Hyg (B) 180:480–504
Alison MR, Poulsom R, Forbes SJ (2001) Update on hepatic stem cells. Liver 21:367–373
Ariel E, Nicolajsen N, Christophersen MB, Holopainen R, Tapiovaara H, Jensen BB (2009) Propagation and isolation of ranaviruses in cell culture. Aquaculture 294:159–164
Banerjee A, Rapin N, Miller M, Griebel P, Zhou Y, Munster V, Misra V (2016) Generation and characterization of Eptesicus fuscus (big brown bat) kidney cell lines immortalized using the Myotis polyomas large T-antigen. J Virol Meth 237:166–173
Bano N, Smith AR, Bennett W, Vasquez L, Hollbaugh JT (2007) Dominance of mycoplasma in the guts of the long-jawed mudsucker, Gillichthys mirabilis, from five California salt marshes. Environ Microbiol 9:2636–2641
Bechtel DG, Lee LE (1994) Effects of aflatoxin B 1 in a liver cell line from rainbow trout (Oncorhynchus mykiss). Toxicol in Vitro 8:317–328
Bols NC (1991) Biotechnology and aquaculture: the role of cell cultures. Biotech Adv 9:31–49
Bols NC, Lee LEJ (1994) Cell lines: availability, propagation and isolation. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 3. Elsevier Science, Amsterdam, pp 145–159
Bols NC, Mosser DD, Steels GB (1992) Temperature studies and recent advances with fish cells in vitro. Comp Biochem Physiol 103A:1–14
Bols NC, Barlian A, Chirino-Trejo M, Caldwell SJ, Goegan P, Lee LEJ (1994) Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J Fish Dis 17:601–611
Bols NC, Yang BY, Lee LEJ, Chen TT (1995) Development of a rainbow trout pituitary cell line that expresses growth hormone, prolactin and somatolactin. Mol Mar Biol Biotechnol 4:154–163
Bols NC, Brubacher JL, Fujiki K, Dixon B, Collodi R, Lamb MP, Lee LEJ (2004) Development and characterization of a cell line from a blastula stage rainbow trout embryo. In Vitro Cell Dev Biol Animal 40:361–371
Bols NC, Dayeh VR, Lee LEJ, Schirmer K (2005) Use of fish cell lines in toxicology of fish. In: Moon TW, Mommsen TP (eds) Biochemistry and molecular biology of fishes—environmental toxicology, vol 6. Elsevier Science, Amsterdam, pp 43–84
Campbell JB, Wolf K (1969) Plaque assay and some characteristics of Egtved virus (virus of viral hemorrhagic septicemia of rainbow trout). Can J Microbiol 15:635–637
Carter CH, Ellington WW, Van Beneden RJ (1996) Confocal laser scanning microscopy of oncogene localization in rainbow trout cell lines derived from normal and tumor tissue. Toxicol Pathol 24:339–345
Chandel NS, Jasper H, Ho TT, Passegue E (2016) Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol 18:823–831
Chen TR (1977) In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 104:255–262
Chen ZX, Zheng JC, Jiang YL (1999) A new iridovirus isolated from soft-shelled turtle. Virus Res 63:147–151
Chen MJ, Choi P, Yang BY, Chieh LO, Son JK, Hendricks J, Bailey G, Chen TT (2004) Development of rainbow trout hepatoma cell lines: effect of PRO-IGF-1 EA4-peptide on morphological changes and anchorage-independent growth. In Vitro Cell Dev Biol 40:118–128
Chen MJ, Chiou PP, Lia YH, Lin CM, Chen TT (2010) Development and characterization of five rainbow trout pituitary single-cell clones capable of producing pituitary hormones. J Endocrinol 205:69–78
Christman SA, Kong BW, Landry MM, Kim H, Foster DN (2006) Contributions of differential p53 expression in the spontaneous immortalization of a chicken embryo fibroblast cell line. BMC Cell Biol 7:27
Clark HF, Soriano EZ (1974) Fish rhabdovirus replication in non-piscine cell culture: new system for the study of rhabdovirus-cell interaction in which the virus and cell have different temperature optima. Infect Immun 10:180–188
Clark HF, Lief FS, Lunger PD, Waters D, Leloup P, Foelsch DW, Wyler RW (1979) Fer de lance virus (FDLV): a probable paramyxovirus isolated from a reptile. J Gen Virol 44:405–418
Colihuegue N, Iturra P, Estay F, Diaz NF (2001) Diploid chromosome number variations and sex chromosome polymorphism in five cultured strains of rainbow trout (Oncorhynchus mykiss). Aquaculture 198:63–77
Collet B, Boudinot P, Benmansour A, Secombes CJ (2004) An Mx1 promoter-reporter system to study interferon pathways in rainbow trout. Dev Comp Immunol 28:793–801
Collet B, Urquhart K, Noguera P, Larsen KH, Lester K, Smail D, Bruno D (2013) A method to measure an indicator of viraemia in Atlantic salmon using a reporter cell line. J Virol Methods 191:113–117
Crane M, Hyatt A (2011) Viruses of fish: an overview of significant pathogens. Viruses 3:2025–2046
Daněk T, Kalous L, Veselý T, Krásová E, Reschová S, Rylková K, Kulich P, Petrtýl M, Pokorová D, Knytl M (2012) Massive mortality of Prussian carp Carassius gibelio in the upper Elbe basin associated with herpesviral hematopoietic necrosis (CyHV-2). Dis Aquat Org 102:87–95
Davidse A, Dijkstra S, An A, Van T (1999) First isolation of herpesvirus of eel (Herpes Virus Anguillae) in diseased European eel (Anguilla Anguilla L.) in Europe. Bull Eur Ass Fish Pathol 19:137–141
Dayeh VR, Schirmer K, Lee LEJ, Bols NC (2005) Rainbow trout gill cell line microplate cytotoxicity test. In: Blaise C, Férard JF (eds) Small-scale freshwater environment toxicity test methods. Kluwer Academic Publishers, Netherlands, pp 473–500
Dayeh VR, Bols NC, Schirmer K, Tanneberger K, Lee, LEJ (2013) Use of fish-derived cell lines for investigation of environmental contaminants: an update following OECD’s fish toxicity testing framework No. 171. In: Current protocols in toxicology. Wiley: New York, 56: 1.5.1–1.5.20
Delsert CL, Morin NA, Comps MI (1997) Fish nodavirus lytic cycle and semipermissive expression in mammalian and fish cell cultures. J Virol 71:5673–5677
DeWitte-Orr SJ, Bols NC (2007) Cytopathic effects of chum salmon reovirus to salmonid epithelial, fibroblast and macrophage cell lines. Virus Res 126:159–171
DeWitte-Orr SJ, Zorzitto JR, Sutton LP, Bols NC (2005) Preferential induction of apoptosis in the rainbow trout macrophage cell line, RTS11, by actinomycin D, cycloheximide and double stranded RNA. Fish Shellfish Immun 18:279–295
DeWitte-Orr SJ, Hsu HC, Bols NC (2007a) Induction of homotypic aggregation in the rainbow trout macrophage-like cell line, RTS11. Fish Shellfish Immun 22:487–497
DeWitte-Orr SJ, Leong JA, Bols NC (2007b) Induction of antiviral genes, Mx and vig-1, by dsRNA and chum salmon reovirus in rainbow trout monocyte/macrophage and fibroblast cell lines. Fish Shellfish Immun 23:670–682
Di Girolamo N, Chow S, Richardson A, Wakefield A (2016) Contamination of primary human corneal epithelial cells with an SV40-transformed human corneal epithelial cell line: a lesson for cell biologists in good laboratory practice. Invest Ophthal Visual Sci 57:611–616
Diago ML, Lopez-Fierro P, Razquin BE, Villena AJ (1995) Establishment and characterization of a pronephric stromal cell line (TPS) from rainbow trout, Oncorhynchus mykiss W. Fish Shellfish Immun 5:441–457
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92:9363–9367
Drexler HG, Uphoff CC (2002) Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39:75–90
Emerson M, Nicholson BL, Bayer R (1979) Effects of Acholeplasma laidlawii and an unidentified mycoplasma on selected fish cell cultures and the replication of fish viruses. J Fish Dis 2:227–238
Etyemez M, Balcazar JL (2015) Bacterial community structure in the intestinal ecosystem of rainbow trout (Oncrohynchus mykiss) as revealed by pryosequencing-based analysis of 16S rRNA genes. Res Vet Sci 100:8–11
Falk K, Namork E, Rimstad E, Mjaaland S, Dannevig BH (1997) Characterization of infectious salmon anemia virus, an orthomyxo-like virus isolated from Atlantic salmon (Salmo salar L.) J Virol 71:9016–9023
Fan L, Crodian J, Collodi P (2004) Culture of embryonic stem cell lines from zebrafish. Method Cell Biol 76:151–160
Fernandez RD, Yoshimizu M, Kimura T, Ezura Y (1993) Establishment and characterization of seven continuous cell lines from freshwater fish. J Aquat Anim Health 5:137–147
Fierro-Castro C, Barrioluengo L, Lopez-Fierro P, Razquin BE, Carracedo B, Villena AJ (2012) Fish cell cultures as in vitro models of pro-inflammatory responses elicited by immunostimulants. Fish Shellfish Immun 33:389–400
Fischer S, Loncar J, Zaja R, Schnell S, Schirmer K, Smital T, Luckenback T (2011) Constitutive mRNA expression and protein activity levels of nine ABC efflux transporters in seven permanent cell lines derived from different tissues of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 101:438–446
Flaño E, López-Fierro P, Álvarez F, Razquin B, Villena A (1998) Splenic cultures from rainbow trout, Oncorhynchus mykiss: establishment and characterization. Fish Shellfish Immun 8:589–606
Francis-Floyd R, Reed P, Gibbs P, Shotts E, Bolon B, Coleman W, Klinger R (1998) Isolation of Acholeplasma laidlawii from centrarchids in a Central Florida Lake. J Aquat Anim Health 10:252–258
Frerichs GN (1996) Identification and elimination of mycoplasmas in fish cell line cultures. J Fish Dis 19:435–439
Frerichs GN, Hill BJ, Way K (1989) Ulcerative disease rhabdovirus: cell-line susceptibility and serological comparison with other fish rhabdoviruses. J Fish Dis 12:51–56
Fryer JL, McCain BB, Leong JC (1981) A cell line derived from rainbow trout (Salmo gairdneri) hepatoma. Fish Pathol 15:193–200
Fukuda Y, Nguyen HD, Furuhash M, Nakai T (1996) Mass mortality of cultured sevenband grouper, Epinephelus septemfasciatus, associated with viral nervous necrosis. Fish Pathol 31:165–170
Galinier R, Van Beurden S, Amilhat E, Castric J, Schoehn G, Verneau O, Fazio G, Allienne JF, Engelsma M, Sasal P, Faliex E (2012) Complete genomic sequence and taxonomic position of eel virus European X (EVEX), a rhabdovirus of European eel. Virus Res 166:1–2
Ganassin RM, Bols NC (1998) Development of a monocyte/macrophage-like cell line, RTS11, from rainbow trout spleen. Fish Shellfish Immun 60:457–476
Ganassin RC, Bols NC (1999) A stromal cell line from rainbow trout spleen, RTS34ST, that supports the growth of rainbow trout macrophages and produces conditioned medium with mitogenic effects on leukocytes. In Vitro Cell Dev Biol Anim 35:80–86
Geraghty RT, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JRW, Meredith J, Stacey GN, Thraves P, Vias M (2014) Guidelines for the use of cell lines in biomedical research. Brit J Cancer 111:1021–1046
Graham DA, Wilson C, Jewhurst H, Rowley H (2008) Cultural characteristics of salmonid alphav.iruses-influence of cell line and temperature. J Fish Dis 31:859–868
Hedrick RP, McDowell T, Eaton WD, Chan L, Wingfield WH (1986) Herpesvirus salmonis (HPV): first occurrence in anadromous salmonids. B Eur Assoc Fish Pat 6:66–67
Heo MS, Sohn SG, Sim DS, Kim JW, Park MA, Lee JS, Choi DL, Jung SH, Kim YJ, Oh MJ (2000) Isolation and characterization of white spot syndrome baculovirus in cultured Penaeid shrimp (Penaeus chinensis). J Fish Pathol 13:7–13
Hoffman GL, Dunbar CE, Wolf K, Zwillenberg LO (1969) Epitheliocystis, a new infectious disease of the bluegill (Lepomis macrochirus). Antonie Van Leeuwenhoek 35:146–158
Holben WE, Williams P, Saarinen M, Särkilahit LK, Apajalahti JHA (2002) Phylogenetic analysis of intestinal microfloa indicates a novel mycoplasma phylotype in farmed and wild salmon. Microb Ecol 44:175–185
Hubert N, Hanner R, Holm E, Mandrak NE, Taylor E, Burridge M, Watkinson D, Dumont P, Curry A, Bentzen P, Zhang J (2008) Identifying Canadian freshwater fishes through DNA barcodes. PLoS One 3:1–8
Iwamoto T, Nakai T, Mori K, Arimoto M, Furusawa I (2000) Cloning of the fish cell line SSN-1 for piscine nodaviruses. Dis Aquat Org 43:81–89
Iwamoto T, Nakai T, Mori K, Arimoto M, Mise K, Furusawa I (2001) Transfection of striped jack nervous necrosis virus (SJNNV) RNA into fish cells. J Fish Dis 24:185–188
Juhasz A, Ahne W (1993) Physicochemical properties and cytopathogenicity of an adenovirus-like agent isolated from corn snake (Elaphe guttata). Arch Virol 130:429–439
Jung SJ, Kim SR, Joung IY, Kitamura SI, Ceong HT, Oh MJ (2008) Distribution of marine birnavirus in cultured olive flounder Paralichthys olivaceus in Korea. J Microbiol 46:265–273
Karger A, Bettin B, Lenk M, Mettenleiter TC (2010) Rapid characterisation of cell cultures by matrix-assisted laser desorption/ionisation mass spectrometric typing. J Virol Methods 164:116–121
Kawano A, Haiduk C, Schirmer K, Hanner R, Lee LEJ, Dixon B, Bols NC (2011) Development of a rainbow trout intestinal epithelial cell line and its response to lipopolysaccharide. Aquac Nutr 17:e241–e252
Kelly RK, Nielsen O, Mitchell SC, Yamamoto T (1983) Characterization of Herpes virus vitreum isolated from hyperplastic epidermal tissue of walleye, Stizostedion vitreum vitreum (Mitchill). J Fish Dis 6:249–260
Kimura T, Suzuki S, Yoshimizu M (1983) In vitro antiviral effect of 9-(2-hydroxyethoxymethyl) guanine on the fish herpesvirus, Oncorhynchus masou virus (OMV). Antivir Res 3:93–101
Kimura T, Yoshimizu M, Gorie S (1986) A new rhabdovirus isolated in Japan from cultured hirame (Japanese flounder) Paralichthys olivaceus and ayu Plecoglossus altivelis. Dis Aquat Org 1:209–217
Kirchoff H, Beyenne P, Fishcer M, Flossdorf J, Heitmann J, Khattab B, Lopatta D, Rosengarten R, Seidel G, Yousefl C (1987) Mycoplasma mobile sp. Nov., a new species from fish. Int J Syst Bact 37:192–197
Klapper W, Heidorn K, Huhne K, Parwaresch R, Krupp G (1998) Telomerase activity in ‘immortal’ fish. FEBS Lett 434:409–112
Kleeman KT, Fryer JL, Pilcher KS (1970) Observed differences in CO2 requirements between mammalian and salmonid fish cell lines. J Cell Biol 47:796–798
Kunze M, Schwanz-Pfitzner I, Ozel M, Laber G (1972) Mykoplasmen (Acholeplasma laidlawii) aus Kaltbluter-Zellkulturen. Zentralblatt fur Bakteriologie, Parasitenkunde Infecktionskrankheiten und Hygiene Abteilung1 Oripnale 227:520–534
Langdon JS, Humphrey JD, Williams LM, Hyatt AD, Westbury HA (1986) First virus isolation from Australian fish: an iridovirus-like pathogen from redfin perch, Perca fluviatilis L. J Fish Dis 9:263–268
Lannan CN, Winton JR, Fryer JL (1984) Fish cell lines: establishment and characterization of nine cell lines from salmonids. In vitro 20:671–676
Lee LEJ, Pochmursky V, Bols NC (1986) Effect of corticosteroids on the morphology and proliferation of two salmonid cell lines. Gen Comp Endo 64:373–380
Lee LEJ, Martinez A, Bols NC (1988) Culture conditions for arresting and stimulating the proliferation of a rainbow fibroblast cell line, RTG-2. In Vitro Cell Dev Biol 24:795–802
Lee LEJ, Clemons JH, Bechtel DG, Caldwell S, Han KB, Pasitschniak M, Mosser DD, Bols NC (1993) Development and characterization of a rainbow trout liver cell line expressing cytochrome P450-dependent monooxygenase activity. Cell Biol Toxicol 9:279–294
Leek SL (1987) Viral erythrocytic inclusion body syndrome (EIBS) occurring in juvenile spring Chinook salmon (Oncorhynchus tshawytscha) reared in fresh water. Can J Fish Aquat Sci 44:685–688
Leisy DJ, Lewis TD, Leong JA, Rohrmann GF (2003) Transduction of cultured fish cells with recombinant baculoviruses. J Gen Virol 84:1173–1178
Li MF (1974) Fish cell cultures. In: Cox GV (ed) Proceedings of a workshop on marine bioassays. Mar Technol Soc, Washington, DC, pp 182–190
Lidgerding BC, Phelps SR, Schill WB (1984) Fish cell lines: characterization by isozyme analysis. In Vitro 20:167–171
Lilley JH, Frerichs GN (1994) Comparison of rhabdoviruses associated with epizootic ulcerative syndrome (EUS) with respect to their structural proteins, cytopathology and serology. J Fish Dis 17:513–522
Lio-Po GD, Traxler GS, Albright LJ, Leaño EM (2000) Characterization of a virus obtained from snakeheads Ophicephalus striatus with epizootic ulcerative syndrome (EUS) in the Philippines. Dis Aquat Org 43:191–198
Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Creer S, Derome N (2016) The biogeography of the Atlantic salmon (Salmo salar) gut microbiota. ISME J 10:1280–1284
Lopez-Doriga MV, Smail DA, Smith RJ, Domenech A, Castric J, Smith PD, Ellis AE (2001) Isolation of salmon pancreas disease virus (SPDV) in cell culture and its ability to protect against infection by the ‘wild-type’ agent. Fish Shellfish Immun 11:505–522
Low IE (1974) Isolation of Acholeplasma laidlawii from commercial, serum-free tissue culture medium and studies on its survival and detection. Appl Microbiol 27:1046–1052
Lucey BP, Nelson-Rees WA, Hutchins GM (2009) Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Path Lab Med 133:1463–1467
Luque A, Granja AG, González L, Tafalla C (2014) Establishment and characterization of a rainbow trout heart endothelial cell line with susceptibility to viral hemorrhagic septicemia virus (VHSV). Fish Shellfish Immun 38:255–264
Maisey K, Montero R, Corripio-Miyar Y, Toro-Ascuy D, Valenzuela B, Reyes-Cerpa S, Sandino AM, Zou J, Wang T, Secombes CJ, Imarai M (2016) Isolation and characterization of salmonid CD4+ T cells. J Immunol 196:4150–4163
Malhao F, Urbatzka R, Navas JM, Cruzeiro C, Monteiro RAF, Rocha E (2013) Cytological, immunocytochemical, ultrastructural and growth characterization of the rainbow trout liver cell line RTL-W1. Tissue Cell 45:159–174
Marcos-Lopez M, Waltzek TB, Hedrick RP, Baxa DV, Garber AF, Liston R, Johnsen E, Forward BS, Backman S, Ferguson HW (2012) Characterization of a novel alloherpesvirus from Atlantic cod (Gadus morhua). J Vet Diagn Investig 24:65–73
McAllister PE (1997) Susceptibility of 12 lineages of Chinook salmon embryo cells (CHSE-214) to four viruses from salmonid fish: implications for clinical assay sensitivity. J Aquat Anim Health 9:291–294
McGarrity GJ (1976) Spread and control of mycoplasmal infection of cell cultures. In Vitro 12:643–648
Moritomo T, Anderson DP, Schill WB (1990) Establishment of a cell line with reticulo-endothelial characteristics from a rainbow trout spleen explant. Fish Pathol 25:165–170
Mosser DD, Bols NC (1988) Relationship between heat-shock protein synthesis and thermotolerance in rainbow trout fibroblasts. J Comp Physiol B 158:457–467
Mosser DD, Heikkila JJ, Bols NC (1986) Temperature ranges over which rainbow trout fibroblasts survive and synthesize heat-shock proteins. J Cell Physiol 128:432–440
Mosser DD, Van Oostrom JJ, Bols NC (1987) Development and decay of thermotolerance by rainbow trout fibroblasts. J Cell Physiol 132:1551–1560
Nagabayashi T, Wolf K (1979) Characterization of EV-2, a virus isolated from European eels (Anguilla anguilla) with stomatopapilloma. J Virol 30:358–364
Nakajima K, Sorimachi M (1994) Biological and physico-chemical properties of the iridovirus isolated from cultured red sea bream, Pagrus major. Fish Pathol 29:29–33
von Nordheim M, Boinay M, Leisi R, Kempf C, Ros C (2016) Cutthroat trout virus—towards a virus model to support hepatitis E research. Viruses 8:289
O’Connell RC, Wittler RG, Farber JE (1964) Aerosols as a source of widespread mycoplasma contamination of tissue cultures. Appl Microbiol 12:337–342
Obinata M (2007) The immortalized cell lines with differentiation potentials: their establishment and possible application. Cancer Sci 98:275–278
Officer JE (1964) Ability of a fish cell line to support the growth of mammalian viruses. Exp Biol Med 116:190–194
Oh MJ, Jung SJ, Choi TJ, Kim HR, Rajendran KV, Kim YJ, Park MA, Chun SK (2001) A viral disease occurring in cultured carp Cyprinus carpio in Korea. Fish Pathol 36:147–151
OiE (2016) Chapter 2.3.0. General information. In: Manual of diagnostic tests for aquatic animals. Retrieved from http://www.oie.int/index.php?id=2439&L=0&htmfile=chapitre_general_information_2_3.htm
Okamoto N, Shirakura T, Sano T (1985) Precision of a plaque assay: eel virus European-and infectious hematopoietic necrosis virus-RTG-2 cell systems. Fish Pathol 19:225–230
Ossum CG, Hoffmann EK, Vijayan MM, Holt SE, Bols NC (2004) Characterization of a novel fibroblast-like cell line from rainbow trout and responses to sublethal anoxia. J Fish Biol 64:1103–1116
Ostrander GK, Blair JB, Stark BA, Marley GM, Bales WD, Veltri RW, Hinton DE, Okihiro M, Ortego LS, Hawkins WE (1995) Long-term primary culture epithelia cells from rainbow trout (Oncorhynchus mykiss) liver. In Vitro Cell Dev-An 31:367–378
Park JW, Moon CH, Harmache A, Wargo AR, Purcell MK, Bremont M, Kurath G (2011) Restricted growth of U-type infectious haematopoietic necrosis virus (IHNV) in rainbow trout cells may be linked to casein kinase II activity. J Fish Dis 34:115–129
Perry GML, McDonald GJ, Ferguson MM, Ganassin RC, Bols NC (2001) Characterization of rainbow trout cell lines using microsatellite DNA profiling. Cytotechnol 37:143–151
Pham PH, Lai YS, Lee FF, Bols NC, Chiou PP (2012) Differential viral propagation and induction of apoptosis by grouper iridovirus (GIV) in cell lines from three non-host species. Virus Res 167:16–25
Pham PH, Lumsden JS, Tafalla C, Dixon B, Bols NC (2013) Differential effects of viral hemorrhagic septicaemia virus (VHSV) genotypes IVa and IVb on gill epithelial and spleen macrophage cell lines from rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun 34:632–640
Pham PH, Huang YJ, Mosser DD, Bols NC (2015) Use of cell lines and primary cultures to explore the capacity of rainbow trout to be a host for frog virus 3 (FV3). In Vitro Cell Dev-An 51:894–904
Phelps NB, Mor SK, Armien AG, Batts W, Goodwin AE, Hopper L, McCann R, Ng TF, Puzach C, Waltzek TB, Delwart E (2014) Isolation and molecular characterization of a novel picornavirus from baitfish in the USA. PLoS One 9:e87593
Plumb JA, Wolf K (1971) Fish cell growth rates: quantitative comparisons of RTG-2 cell growth at 5°-25°C. In Vitro 7:42–45
Plumb JA, Bowser PR, Grizzle JM, Mitchell AJ (1979) Fish viruses: a double-stranded RNA icosahedral virus from a north American cyprinid. J Fish Res Board Can 36:1390–1394
Pollenz RS, Necela B (1998) Characterization of two continuous cell lines derived from Oncorhynchus mykiss for models of aryl-hydrocarbon-receptor-mediated signal transduction: direct comparison to the mammalian Hepa-1c1c7 cell line. Aquatic Toxicol 41:31–49
Poynter SJ, DeWitte-Orr SJ (2015) Length-dependent innate antiviral effects of double-stranded RNA in the rainbow trout (Oncorhynchus mykiss) cell line, RTG-2. Fish Shellfish Immun 46:557–565
Rainbow AJ, Zacal NJ (2008) Expression of an adenovirus encoded reporter gene and its reactivation following UVC and oxidative damage in cultured fish cells. Int J Radiat Biol 84:455–466
Razin S, Hayflick L (2010) Highlights of mycoplasma research—an historical perspective. Biologicals 38:183–190
Ristow SS, de Avila J (1994) Susceptibility of four new salmonid cell lines to infectious hematopoietic necrosis virus. J Aquat Anim Health 6:260–265
Ristow SS, Grabowski LD, Ostberg C, Robison B, Thorgaard GH (1998) Development of long-term cell lines from homozygous clones of rainbow trout. J Aquat Anim Health 10:75–82
Romorini L, Riva DA, Bluguermann C, Richardson GAV, Scassa ME, Sevlever GE, Miriuka SG (2013) Effect of antibiotics against Mycoplasma sp. on human embryonic stem cells undifferentiated status, pluripotency, cell viability and growth. PLoS One 8:e70267
Rovnak J, Casey RN, Brewster CD, Casey JW, Quackenbush SL (2007) Establishment of productively infected walleye dermal sarcoma explant cells. J Gen Virol 88:2583–2589
Saint-Jean SR, Ana I, Prieto SI (2010) The persistence of infectious pancreatic necrosis virus and its influence on the early immune response. Vet Immunol Immunop 136:81–91
Schaeffer WI (1990) Terminology associated with cell, tissue and organ culture, molecular biology and molecular genetics. In Vitro Cell Dev Biol 26:97–101
Schaffeld M, Haberkamp M, Braziulis E, Lieb B, Markl J (2002) Type II keratin cDNAs from the rainbow trout: implications for keratin evolution. Differentiation 70:292–299
Skloot R (2010) The immortal life of Henrietta Lacks. Crown Publishers, New York
Somamoto T, Nakanishi T, Okamoto N (2000) Specific cell-mediated cytotoxicity against a virus-infected syngeneic cell line in isogeneic ginbuna crucian carp. Dev Comp Immunol 24:633–640
Speare R, Smith JR (1992) An iridovirus-like agent isolated from the ornate burrowing frog Limnodynastes ornatus in northern Australia. Dis Aquat Org 14:51–57
Stokich B, Osgood Q, Grimm D, Moorthy S, Chakraborty N, Menze MA (2014) Cryopreservation of hepatocyte (HepG2) cell monolayers: impact of trehalose. Cryobiology 69:281–290
Tafalla C, Sanchez E, Lorenzen N, DeWitte-Orr SJ, Bols NC (2008) Effects of viral hemorrhagic septicemia virus (VHSV) on the rainbow trout (Oncorhynchus mykiss) monocyte cell line RTS-11. Mol Immunol 45:1439–1448
Tapiovaara H, Olesen NJ, Lindén J, Rimaila-Pärnänen E, von Bonsdorff CH (1998) Isolation of an iridovirus from pike-perch Stizostedion lucioperca. Dis Aquat Org 32:185–193
Thorgaard GH (1983) Chromosomal differences among rainbow trout populations. Copiea 3:650–662
Tom DJ, Lee LEJ, Lew J, Bols NC (2001) Induction of 7-ethoxyresorufin-O-deethylase activity by planar chlorinated hydrocarbons and polycyclic aromatic hydrocarbons in cell lines from the rainbow trout pituitary. Comp Biochem Physiol 128A:185–198
University of Washington (2016) Bachelor of Science in informatics: what is informatics? Retrieved from https://ischool.uw.edu/academics/informatics/what-is-informatics
Uphoff CC, Drexler HG (2002) Comparative antibiotic eradication of mycoplasma infections from continuous cell lines. In Vitro Cell Dev Biol-An 38:86–89
Uphoff CC, Denkmann SA, Drexler HG (2012) Treatment of mycoplasma contamination in cell cultures with Plasmocin. J Biomed Biotech 2012:267678
Van Oostrom JA, Bols NC (1991) Influence of temperature on the proliferative response of rainbow trout gonadal fibroblasts to cortisol and RU 486. Fish Physiol Biochem 9:261–269
Villoing S, Béarzotti M, Chilmonczyk S, Castric J, Brémont M (2000) Rainbow trout sleeping disease virus is an atypical alphavirus. J Virol 74:173–183
Vo NTK, Bufalino MR, Hartlen KD, Kitaev V, Lee LEJ (2014) Cytotoxicity evaluation of silica nanoparticles using fish cell lines. In Vitro Cell Dev Biol-An 50:427–438
Vo NTK, Mikhaeil MS, Lee LEJ, Pham PH, Bols NC (2015) Senescence-associated β-galactosidase staining in fish cell lines and primary cultures from several tissues and species, including rainbow trout coelomic fluid and milt. In Vitro Cell Dev Biol-An 51:361–371
Wagg S, Lee LJ (2005) A proteomics approach to identifying fish cell lines. Proteomics 5:4236–4244
Ward RD, Hanner R, Hebert PDN (2009) The campaign to DNA barcode all fishes, FISH-BOL. J Fish Biol 74:329–356
Watanabe T, Nakano M, Asakawa H, Moritomo T (1987) Cell culture of rainbow trout liver. Nippon Suisan Gakkaishi 53:537–542
Watson LR, Yun SC, Groff JM, Hedrick RP (1995) Characteristics and pathogenicity of a novel herpesvirus isolated from adult and subadult white sturgeon Acipensertransmontanus. Dis Aquat Org 22:199–210
Wechsler SJ, McHolland LE (1988) Susceptibilities of 14 cell lines to bluetongue virus infection. J Clin Microbiol 26:2324–2327
Weng SP, He JG, Wang XH, Lü L, Deng M, Chan SM (2002) Outbreaks of an iridovirus disease in cultured tiger frog, Rana tigrina rugulosa, in southern China. J Fish Dis 25:423–427
Winton, JR (1981) Isolation and characterization of a new reovirus from chum salmon (Oncorhynchus keta). PhD dissertation, Oregon State University. Retrieved from ScholarsArchive@OSU
Winton J, Batts W, deKinkelin P, LeBerre M, Bremont M, Fijan N (2010) Current lineages of the epithelioma papulosum cyprini (EPC) cell line are contaminated with fathead minnow, Pimephales promelas, cells. J Fish Dis 33:701–704
Wolf K (1979) Cold-blooded vertebrate cell and tissue culture. In: Jakoby WB, Pasten IH (eds) Methods in Enzymology, vol 58. Academic Press, New York, pp 466–477
Wolf K, Ahne W (1982) Fish cell culture. In: Maramorosch K (ed) Advances in cell culture, vol 2. Academic Press, New York, pp 305–328
Wolf K, Darlington RW (1971) Channel catfish virus: a new herpesvirus of ictalurid fish. J Virol 8:525–533
Wolf K, Mann JA (1980) Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In vitro 16:168–179
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Science 135:1065–1066
Wolf K, Quimby MC (1969) Fish cell and tissue culture. In: Fish physiology. Vol. III. Academic Press, New York, pp 253–305
Wolf K, Quimby MC (1973a) Fish viruses: buffers and methods for plaquing eight agents under normal atmosphere. Appl Microbiol 25:659–664
Wolf K, Quimby MC (1973b) Towards a practical fail-safe system of managing poikilothermic vertebrate cell lines in culture. In Vitro 8:316–331
Wolf K, Quimby MC, Pyle EA, Dexter RP (1960) Preparation of monolayer cell cultures from tissues of some lower vertebrates. Science 132:1890–1891
Wolf K, Gravell M, Malsberger RG (1966) Lymphocystis virus: isolation and propagation in centrarchid fish cell lines. Science 151:1004–1005
Wolf K, Bullock GL, Dunbar CE, Quimby MC (1968) Tadpole edema virus: a viscerotropic pathogen for anuran amphibians. J Infect Dis 1:253–262
Wolf K, Darlington RW, Taylor WG, Quimby MC, Nagabayashi T (1978) Herpesvirus salmonis: characterization of a new pathogen of rainbow trout. J Virol 27:659–666
Xing JG, Lee LEJ, Fan L, Collodi P, Holt SE, Bols NC (2008) Initiation of a zebrafish blastula stem cell line on rainbow trout stromal cells and subsequent development under feeder-free conditions into a cell line, ZEB2J. Zebrafish 5:49–63
Xu X, Nagarajan, Lewis NE, Pan S, Cai Z, Liu X, Chen W, Xie M, Wang W, Hammond S, Anderssen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO, Wang J (2012) The genomic sequence of the Chinese hamster ovary (CHO) K1 cell line. Nat Biotechnol 29:735–741
Yoda M, Takahashi KG, Mori K (2002) Telomerase activity detected in eyed embryos of rainbow trout Oncorhynchus mykiss. Fisheries Sci 68:132–137
Yokoo M, Fujita R, Nakajima Y, Yoshimizu M, Kasai H, Asano SI, Bando H (2013) Mos1 transposon-based transformation of fish cell lines using baculoviral vectors. Biochem Bioph Res Co 439:18–22
Young L, Sung J, Stacey G, Masters JR (2010) Detection of mycoplasma in cell culture. Nat Protoc 5:924–934
Zarkasi KZ, Abell GCJ, Taylor RS, Neuman C, Hatje E, Tamplin ML, Katouli M, Bowman JP (2014) Pyrosequencing-based characterization of gastrointestinal bacteria of Atlantic salmon (Salmo salar L.) within a commercial mariculture system. J Appl Microbiol 117:18–27
Zeng FX, Yu X, Sherry JP, Dixon B, Duncker BP, Bols NC (2016) The p53 inhibitor, pifithrin-α, disrupts microtubule organization, arrests growth, and induces polyploidy in the rainbow trout gill cell line, RTgill-W1. Comp Biochem Phys C 170:1–10
Zhang QY, Tao JJ, Gui L, Zhou GZ, Ruan HM, Li ZQ, Gui JF (2007) Isolation and characterization of Scophthalmus maximus rhabdovirus. Dis Aquat Org 74:95–105
Zhao X, Zhao Q, Luo Z, Yu Y, Xiao N, Sun X, Cheng L (2015) Spontaneous immortalization of mouse liver sinusoidal endothelial cells. Int J Mol Med 35:617–624
Acknowledgements
The research of the authors was supported by grants to NCB and LEJL from the Natural Sciences and Engineering Research Council (NSERC) of Canada. We apologize in advance if we have inadvertently left anyone’s RT cell line(s) out of this review or categorized them incorrectly, but hopefully in the future, they can be incorporated into the framework of this review as cell line categorizations based on storage and use will be expected to change over the years.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Bols, N.C., Pham, P.H., Dayeh, V.R. et al. Invitromatics, invitrome, and invitroomics: introduction of three new terms for in vitro biology and illustration of their use with the cell lines from rainbow trout. In Vitro Cell.Dev.Biol.-Animal 53, 383–405 (2017). https://doi.org/10.1007/s11626-017-0142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-017-0142-5