Abstract
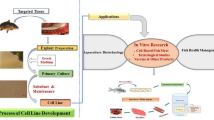
Cell lines provide an important biological tool for carrying out investigations into physiology, virology, toxicology, carcinogenesis and transgenics. Teleost fish cell lines have been developed from a broad range of tissues such as ovary, fin, swim bladder, heart, spleen, liver, eye muscle, vertebrae, brain, skin. One hundred and twenty-four new fish cell lines from different fish species ranging from grouper to eel have been reported since the last review by Fryer and Lannan (J Tissue Culture Methods 16: 87–94, 1994). Among the cell lines listed, more than 60% were established from species from Asia, which contributes more than 80% of total fish production. This includes 59 cell lines from 19 freshwater, 54 from 22 marine and 11 from 3 brackish water fishes. Presently, about 283 cell lines have been established from finfish around the world. In addition to the listing and a scientific update on new cell lines, the importance of authentication, applications, cross-contamination and implications of overpassaged cell lines has also been discussed in this comprehensive review. The authors feel that the review will serve an updated database for beginners and established researchers in the field of fish cell line research and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish comprise 48% of the known vertebrate species (Altman and Dittmer 1972), which represents an enormous resource for the development of vertebrate cell and tissue models for use in biomedical sciences. The physiology and blood plasma constituents of teleost fish are much like those of terrestrial vertebrates; therefore, the methodology for culture of cells is also similar. Nevertheless, fish cell culture differs somewhat from mammalian cell culture in having a wider temperature range for incubation. Also, osmolality must be adjusted upward for fish of marine origin. Because of lower metabolic rates than eurythermic cells, fish cells can be maintained with little care for long periods of time. Thus, permanent fish cell lines, in contrast to the mammalian cells, are easier to maintain and manipulate, and unlike primary cultures, produce highly reproducible results (Wolf and Quimby 1976).
Early cultures of primary cells may represent a more appropriate model of tissues in vivo (Freshney 2005). The production of short-term primary cultures, however, suffers from a lack of reproducibility in the initiation, and homogeneity of cultures that limit their application (Bols et al. 1994). Established cell lines are typically derived from malignant tumors, or through spontaneous transformation, or through oncogenic immortalization and such changes bring about continuously proliferating (immortal) cell lines (Freshney 2005).
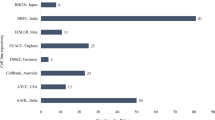
The rainbow trout, Salmo gairdneri gonadal cell line, RTG-2 developed by Wolf and Quimby (1962) was the first permanent cell line of fish origin. Since then many more cell lines have been established. The first review of all fish cell and tissue culture was compiled by Wolf and Mann (1980). A comprehensive global list of freshwater and marine fish cell lines was last published in 1994 by Fryer and Lannan and reported some 159 fish cell lines, established from 74 species or hybrids representing 34 families of fish. We have reviewed the research work carried out since this review and report 124 new established cell lines during this period. Among the cell lines listed, more than 60% were established from Asian region, which contributes more than 80% of total fish production. This include 59 cell lines from 19 freshwater, (Table 1), 53 from 22 marine (Table 2) and 11 from 3 brackish water (Table 3) fishes.
Establishment of cell lines from different tissues
Most fish cell lines originated from normal tissues viz., skin, gill, heart, liver, kidney, spleen, swim bladder, brain, etc. Particularly, embryos or fins are most frequently listed as the source of the tissues used in the primary culture. After ovary, the second most common tissue used for cultivation is fin, due to its high regenerative ability (Fryer and Lannan 1994). Surprisingly, there are not a high number of cell lines originating from gonadal or ovarian tissues since these tissues would also exhibit high levels of mitosis. Cell lines have also been developed from ovary (Kumar et al. 2001), skin and fin (Lakra and Bhonde 1996), vertebrae (Pombinho et al. 2004), scales (Akimoto et al. 2000), etc. However, only one cell line XM (Barnes et al. 2006) was initiated from skin and fin tissue of fish melanoma; and in some cases, these cells remained tumorogenic in vivo following repeated in vitro passage.
Many fish cell lines have been established from fish tissues for the purpose of detection and isolation of fish viruses. The cell lines from different tissues of different species will be valuable for studying species-specific responses to viral infection at the cellular level. Some pathogenic viruses are known to be organ- or tissue-specific, which makes the establishment of additional cell lines from different organs and tissues of a host species essential for proper monitoring of viral diseases.
Embryonic and larval cells are the most easy to cultivate being mitotically activate. In the past, it was difficult to obtain eggs or fry of some fish species because they are pelagic spawners (Wolf and Quimby 1966). However, due to recent advances, many species, which were previously unavailable in embryonic form, are now routinely cultivated for the aquaculture industry. In recent years, a number of embryonic stem-like cells were established by various workers from fish species. To develop embryonic stem (ES) cell lines and gene targeting technique in fish, extensive studies have been done in small model fishes, such as zebra fish (Danio rerio) and medaka (Oryzias latipes), because they offer the possibility of combining embryological, genetic and molecular analysis of vertebrate development. The ES-like cell lines have been established in medaka (Hong et al. 1996, 2000) and zebrafish (Sun et al. 1995). A pluripotent cell line, LJESl, has been established from blastula-stage embryos of Lateolabrax japonicus, and these cells differentiated into different types of cells after retinoic acid treatment (Chen et al. 2003a). A continuous embryonic (SISE) cell line has been established from blastula-stage embryos of sea bass (Lates calcarifer) (Parameswaran et al. 2006b) and a pluripotent embryonic stem cell line SBES from blastula-stage embryos of sea bass (Lates calcarifer) (Parameswaran et al. 2006c) and catfish (Heteropneustes fossilis) (Lakra 2010).
Media and additives
Nowadays, the commercialization of technology like ready to use sterile plastic wares and tissue culture media, enzyme solutions, other reagents could overcome the problems in the media preparations like pH, osmolality, sterility, anchorage of cells, nutrients. In general, most fish cultures use media developed for mammalian cell culture. Eagle’s Minimal Essential Medium (EMEM) supplemented with fetal bovine serum (FBS) comes close to being an all purpose culture medium for the cells of mammals, birds, reptiles, amphibians and of course fish (Wolf and Quimby 1966). Other media routinely used in fish culture are Glasgow MEM, Hank’s MEM (HMEM) and Leibovitz L-15 medium (L-15). An amino acid-rich nutrient medium such as L-15 that does not require CO2 buffering (Leibovitz 1963) has been successfully used with fish cell lines, thus CO2 incubators are not necessary, and cells can be grown conveniently in any undisturbed areas. Due to this advantage, more than 80% of the cell lines established after 1994 used Leibovitz L-15 media. However, some primary cell lines have had specific culture medium designed to optimize growth during development of the primary culture (Wang et al. 1995).
Fetal bovine serum (FBS) seems to be the most popular choice of supplements with the tissue culture media as it is easy to obtain in large volumes and due to the presence of known and unknown growth factors. Wolf and Quimby (1966) quoted instances in which other types of serum have been used but the results appeared to be of mixed benefits. Fish serum was used (<1%) in combination with FBS in developing fish cell lines (Chen et al. 2004; Lakra et al. 2006a). Serum concentration can also have an effect on primary cultures. Throughout the literature, concentration varies from 5% to as high as 20%. Serum concentrations are not usually much higher than this, as there is evidence that high serum concentrations may inhibit cell growth (Freshney 2005).
Most examples of additives to media are in serum-free or reduced media replacing substances that serum would normally provide (Wang et al. 1995). However, that does not entirely discount the possibility of using further additives to media already supplemented with serum. Kumar et al. (2001) used a large list of additives for example, fish muscle extract, sucrose, prawn shell extract, which were explored during developing a primary culture from ovary tissue of African catfish.
Miller et al. (1994) detailed the use of chemical mitogens used to establish suspension cultures from leukocytes. Cell cultures exhibited a strong proliferative response after exposure to the mitogens. Faisal et al. (1995) exposed cultured liver cells to plant-derived mitogens that stimulated DNA synthesis (indicative of cell proliferation). Various growth factors such as mammalian epidermal growth factor (mEGF) (Watanabe et al. 1987), basic fibroblast growth factor (bFGF) (Chen et al. 2004) had been used to stimulate growth of fish cell lines, and bFGF is a potent mitogen for embryonic stem cells derived from Oryzias latipes (Hong et al. 1996) and sea perch (Chen et al. 2003b), lymphoid cells from Penaeus monodon (Hsu et al. 1995), embryonic cells from Paralichthys olivaceus (Chen et al. 2004).
Immortalization of cells
Normal somatic cells have a finite life span and become senescent after a predictable number of cell divisions (Hayflick and Moorehead 1961). Cellular senescence is triggered by two interdependent mechanisms. One induces cell cycle arrest and is controlled by two tumor suppressor pathways, p19ARF/p53 and p16INK4a/Rb (Kiyono et al. 1998). The second is a critical shortening of the telomeres due to the end-replication problem in chromosome replication (Aviv and Harley 2001). It has been documented that a small number of cells arise spontaneously immortalized by a set of genetic alterations. The alterations most frequently observed in immortalized cells are loss of functional Rb (retinoblastoma) or p53 proteins that control two major cell cycle checkpoints (Bodnar et al. 1998). A number of viral oncogenes, including simian virus-40 (SV40) large T-antigen, adenovirus E1A and E1B and polyoma T-antigen, also immortalize cells of a variety of species (Katakura et al. 1998). A catalytic subunit of telomerase ribonucleoprotein (TERT) with reverse transcriptase activity synthesizes and maintains the telomeres, helping cells escape replicative senescence caused by the shortening of telomeres (Bodnar et al. 1998). The preferred method to immortalize cells is through expression of the telomerase reverse transcriptase protein (TERT) (Takakura et al. 1999), particularly those cells most affected by telomere length (e.g., human). Analysis of several telomerase-immortalized cell lines has verified that the cells maintain a stable genotype and retain critical phenotypic markers. A eukaryotic expression plasmid containing the hTERT cDNA is available in ATCC (catalog number ATCC® MBA-141), which will enable researchers to immortalize their own cells.
On the other hand, cell immortalization techniques have attracted enthusiastic attention because they have provided us with cell clones that usually show continual possibility, excellent revitalization after storage and ease of handling in culture. These techniques were mostly used in developing immortal cell lines in humans and other mammalians. But Barker et al. (2000) documented for the first time that the channel catfish long-term leukocyte lines constitutively expressed high levels of telomerase activity. A United States patent (Number. US 6,436,702 B1) was issued for the immortal cell line (spontaneously transformed) derived from grouper Epinephelus coioides to Shau-Chi Chi in 2002. In this patent, the monitoring of the transformation of cells, which was characterized by a change in chromosome number distribution, plating efficiency, FBS requirements and the immortalization, was not induced by any methods.
Cross-contamination and over passage
The ease of handling and simple growth requirements make cross-contamination of cell lines a more likely possibility. Fish cell lines are relatively easy to culture, and most have simple growth requirements that make cross-contamination a potential problem. Cell line contamination is not an uncommon incident in laboratories handling more than one cell lines, and many reports have been made on cross-contamination of mammalian cell lines (Parodi et al. 2002). Although problems of misidentification and cross-contamination of fish cell lines have rarely been reported, these are issues of concern for cell culturists that can make scientific results and their reproducibility unreliable. Human cell lines have been reported contaminated with simian cells or murine cells or even other human cells, most ubiquitously, HeLa cells (Tokiwa et al. 1989). Although cross-contamination of fish cells with other cell types has not been widely reported, Perry et al. (2001) conveyed the identification of a cell line dubbed Clone 1A believed to be derived from rainbow trout as being CHSE-214, a cell line derived from Chinook salmon embryos (Lannan et al. 1984). Accordingly, awareness of good laboratory practices and careful vigilance with fish cell cultures as detailed by Lannan (1994) should be followed to avoid confusion of cell lines. The problem of intraspecies and interspecies cross-contamination among cell lines has been recognized for half a century, and although reviews have been published, evidence of continued use of misidentification and cellular cross-contamination of cell cultures has not declined (Buehring et al. 2004).
In addition, cell lines maintained in culture over a long period of time may experience mutations that alter the original functional characteristics of the cell lines, identified at earlier passage levels (Yu et al. 1997). Furthermore, cell lines do not behave similarly with increased passage number (Hughes et al. 2007). Long-term subculturing places selective pressure on cell line traits leading to, for example, faster growing cells that eventually overrun slower proliferators in the population.
Authentication
When cell lines are obtained from colleagues, they often lack verification or documentation about the condition or passage number of the lines. This practice increases the likelihood that inferior, mal-performing cultures are used, leading to results that may not be accurate or reproducible (Wenger et al. 2004). Methodologies for characterizing fish cell lines have included random amplified polymorphic DNA methods (RAPD) (Matsuo et al. 1999) and microsatellite DNA profiling (Perry et al. 2001), mitochondrial 16S and 18S rRNA and sequence analysis (Ahmed et al. 2009a), which has proven useful for identifying a handful of fish cell lines. A simple proteomic approach has been made to identify several fish cell lines derived from tissues of the same or different species. Protein expression signatures (PES) of the evaluated fish cell lines have been developed using 2-DE and image analysis, and it could thus serve as an additional, valuable and reliable technique for the identification of fish cell lines (Wagg and Lee 2005).
Applications
The availability of fish cell lines, since the 1960s, has begun to make impacts in scientific research, but at a much slower rate than with mammalian cell lines. Early work with fish cell lines was initiated with RTG-2, mainly for virological studies (Wolf and Quimby 1962). Fish cell lines are also finding roles in areas with impacts beyond that of the diseases of fish and are providing important contributions in studies relating to toxicology, carcinogenesis, genetic regulation and expression and DNA replication and repair. In the almost 50 year since then, fish cell lines have grown in number covering a wide variety of species and tissues of origin and an array of applications. Fish immunology (Bols et al. 2001), toxicology (Bahich and Borenfreund 1991), ecotoxicology (Schirmer 2006), endocrinology (Bols and Lee 1991), virology (Wolf 1988), biomedical research (Hightower and Renfro 1988), disease control (Villena 2003), biotechnology and aquaculture (Bols and Lee 1991) and radiation biology (Ryan et al. 2008) are some of the areas in which fish cell lines have made significant contributions.
As exogenous DNA delivery of cultured cell is very useful for both basic research and biotechnological applications, it is necessary to determine the transfection efficiency and gene expression on newly developed cell lines. Various workers observed significant fluorescent signals when the cell lines were transfected with pEGFP vector DNA, indicating their potential utility for transgenic and genetic manipulation studies (Qin et al. 2006; Ye et al. 2006; Parameswaran et al. 2007; Zhou et al. 2007; Ahmed et al. 2008; Ku et al. 2009).
A common method for determining whether a virus is present in a healthy fish population is to attempt to isolate it in an appropriate cell line. A cell line will also allow further study of viruses isolated in disease outbreaks. The different cell lines were tested for the susceptibility to various viruses. Lai et al. (2003) found that besides these four cell lines, previously established grouper brain, kidney and liver cell lines were also used for a viral susceptibility study, which showed that all the cell lines were sensitive to grouper iridovirus, whereas only brain, fin and liver cell lines were susceptible to the yellow grouper nervous necrosis virus (a nodavirus). Five fish viruses were tested on this cell line to determine its susceptibility to these viruses and this was found to be susceptible to MABV NC1 and nodavirus, and the infection was confirmed by RT-PCR and CPE. (Hameed et al. 2006). The SIGE cell line was found to be susceptible to nodavirus, MABV NC-1 and Y6, (Parameswaran et al. 2007). PBLE was susceptible to Chum salmon reovirus (CSV) and supported CSV replication. (Dewitte-Orr et al. 2006). GS cell cultures showed advanced cytopathic effects after infection with a pathogenic grouper iridovirus (Singapore grouper iridovirus, SGIV) or with a grouper nodavirus (Epinephelus tauvina nervous necrosis virus, ETNNV) (Qin et al. 2006). GBC4 cells were susceptible to GSIV and GNNV infection (Wen et al. 2008).
In addition to testing the virus susceptibility on cell lines, several bacterial toxins have also been tested on different cell lines. All three cell lines RGB, RGG and RGH were found sensitive to the extra cellular products of Photobacterium damselae ssp. piscicida (Ku et al. 2009). The bacterial extra cellular products from Aeromonas sp., or Vibrio anguillarum were found to be toxic to this SICH cell line (Ahmed et al. 2009a). The RE and CB cell lines were not susceptible to four marine fish viruses. Extra cellular products from Aeromonas sp. were toxic to the cell lines (Ahmed et al. 2009b).
Repository of cell lines
To date, out of over 3,400 cell lines deposited at the American Type Culture Collection (ATCC), only 43 cell lines could be found that are of aquatic animals, and only 17 fish cell lines are usable and available for dissemination to the researchers globally. The European Collection of Cell Cultures (ECACC) currently holds over 40,000 cell lines representing 45 different species and 50 tissue types. But only 21 fish cell lines have been listed here. The reluctance to use cell lines stems from researcher’s misconception that cell lines are mostly derived from transformed cells and that differentiated characteristics of the tissues of origin are not maintained (Sato 2008). This may be the case for many mammalian cell lines, but most cell lines derived from fish tissues have been from normal tissues with a few exceptions, most notably EPC and RTH-149 cells, which were derived, respectively, from an epithelioma and a hepatoma. Fryer and Lannan (1994) noted that 14 out of 159 fish cell lines reported up to 1994 were initiated from tumorigenic tissues, which is less than 10%. Furthermore, among the fish cell lines listed at ATCC, only three were derived from tumorigenic tissues. This contrasts with mammalian cell lines where over 50% of listed cell lines at the ATCC were derived from cancerous tissues or transformed cells. Altogether ~283 cell lines have been established from finfish around the world but only 43 fish cell lines are being listed in the international cell repository like ATCC, ECACC. If all the established cell lines would have been deposited in that repository, it would be beneficial to the international research community in order to use those cell lines as they are the best alternative to the whole animal research.
Conclusion
Cell cultures, in particular those derived from fish, have been successfully employed as a biological alternative to the use of whole animals. The increasing use and importance of fish cell lines suggest that cell culturists should be encouraged to place these lines with the international cell repositories like ATCC, EACC or other appropriate repository in order to provide a dependable, high-quality source of cells for the benefit of all.
The number of publications containing spurious data as a result of overpassaged, misidentified, or contaminated cell lines is unknown. The basis for any research, development or production program involving cell cultures is the selection of an identity-verified and low-passage cell line. The use of similar and identified passage numbers throughout a project will better ensure reproducible results and comparisons between laboratories. To further ensure the use of authenticated cell lines, full cell line documentation, including the source and passage numbers used during experiments, should be submitted for scientific publications. Cell lines are critical components of experiments and should be considered as standard research reagents and given the same care and quality control measures that surround the use of kits, enzymes and other laboratory products commercially obtained.
References
Ahmed IVP, Chandra V, Parameswaran V, Venkatesan C, Shukla R, Bhonde RR, Hameed ASS (2008) A new epithelial-like cell line from eye muscle of catla (Catla catla): development and characterization. J Fish Biol 72:2026–2038
Ahmed IVP, Chandra V, Sudhakaran R, Rajesh Kumar S, Sarathi M, Sarath Babu V, Ramesh B, Sahul Hameed AS (2009a) Development and characterization of cell lines derived from rohu, Labeo rohita (Hamilton), and catla, Catla catla (Hamilton). J Fish Dis 32:211–218
Ahmed IVP, Sarath Babu V, Chandra V, Nambi KS, Thomas J, Ramesh B, Sahul Hameed AS (2009b) A new fibroblastic-like cell line from heart muscle of the Indian major carp (Catla catla): development and characterization. Aquaculture 293:180–186
Akimoto K, Takaoka T, Sorimachi K (2000) Development of a simple culture method for the tissues contaminated with microorganisms and application to establishment of a fish cell line. Zool Sci 17:61–63
Altman PL, Dittmer DS (1972) Biology data book, 2nd edn. Wiley, Bethesda
Aviv A, Harley CB (2001) How long should telomeres be? Curr Hypertens Rep 3:145–151
Bahich H, Borenfreund E (1991) Cytotoxicity and genotoxicity assays with cultured fish cells: a review. Toxicol In Vitro 5:91–100
Barker KS, Quiniou SMA, Wilson MR, Bengten E, Stuge TB, Warr GW, Clem LW, Miller NW (2000) Telomerase expression and telomere length in immortal leukocyte lines from channel catfish. Dev Comp Immunol 24:583–595
Barnes D, Dowell L, Forest D, Parton A, Pavicevic P, Kazianis S (2006) Characterization of XM a novel Xiphophorus melanoma-derived cell line. Zebrafish 3:371–381
Bejar J, Borrego JJ, Alvarez MC (1997) A continuous cell line from the cultured marine fish gilt-head sea bream (Sparus aurata). Aquaculture 150:143–153
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Shiu CP (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Bols NC, Lee LEJ (1991) Technology and uses of cell culture from tissues and organs of bony fish. Cytotechnology 6:163–187
Bols NC, Barlian AM, Chirino-Trejo M, Caldwell SJ, Goegan P, Lee LEJ (1994) Development of a cell line from primary cultures of rainbow trout, Oncorhynchus mykiss (Walbaum), gills. J Fish Dis 17:601–611
Bols NC, Brubacher JL, Ganassin RC, Lee LEJ (2001) Ecotoxicology and innate immunity in fish. Dev Comp Immunol 25:853–873
Bryson SP, Joyce EM, Martell JD, Lee LEJ, Holt SE, Kales SE, Fujiki K, Dixon B, Bols NC (2006) A cell line (HEW) from embryos of haddock (Melanogrammus aeglefinius) and its capacity to tolerate environmental extremes. Mar Biotechnol 8:641–653
Buehring GC, Eby EA, Eby MJ (2004) Cell line cross-contamination: how aware are mammalian cell culturists of the problem and how to monitor it? In Vitro Cell Dev Biol Anim 40:211–215
Butler R, Nowak BF (2004) A dual enzyme method for the establishment of long and medium-term primary cultures of epithelial and fibroblastic cells from Atlantic salmon gills. J Fish Biol 65:1108–1125
Chang SF, Ngoh GH, Kueh LFS, Qin QM, Che CL, Lam TJ, Sin YM (2001) Development of a tropical marine fish cell line from Asian seabass (Lates calcarifer) for virus isolation. Aquaculture 192:133–145
Chen SL, Sha ZX, Ye HQ (2003a) Establishment of a pluripotent embryonic cell line from sea perch blastula embryo. Aquaculture 218:141–151
Chen SL, Ye HQ, Sha ZX, Hong Y (2003b) Derivation of a pluripotent embryonic cell line from red sea bream blastulas. J Fish Biol 63:795–805
Chen SL, Ren GC, Sha ZX, Shi CY (2004) Establishment of a continuous embryonic cell line from Japanese flounder Pararlichthys olivaceus for virus isolation. Dis Aquat Organ 60:241–246
Chen SL, Ren GC, Zhen-Xia S, Yunhan H (2005) Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus). Aquaculture 249:63–68
Chen MJ, Chiou PP, Liao YH, Lin CM, Chen TT (2010) Development and characterization of five rainbow trout pituitary single-cell clone lines capable of producing pituitary hormones. J Endocrinol 205:69–78
Chi SC, Hu WW, Lo BJ (1999) Establishment and characterization of a continuous cell line (GF-1) derived from grouper, Epinephelus coioides: a cell line susceptible to grouper nervous necrosis virus (GNNV). J Fish Dis 22:173–182
Ciba P, Schicktanz S, Anders E, Siegl E, Stielow A, Klink E, Kruse C (2008) Long-term culture of a cell population from Siberian sturgeon (Acipenser baerii) head kidney. Fish Physiol Biochem 34:367–372
Dannevig BH, Falk K, Namork E (1995) Isolation of the causal virus of infectious salmon anaemia (ISA) in a longterm cell line from Atlantic salmon head kidney. J Gen Virol 76:1353–1359
Devold M, Krossoy B, Asphaug V, Nylund A (2000) Use of RT-PCR for diagnosis of infectious salmon anaemia virus (ISAV) in carrier sea trout Salmo trutta after experimental infection. Dis Aquat Organ 40:9–18
DeWitte-Orr SJ, Lepic K, Bryson SP, Walsh SK, Lee LEJ, Bols NC (2006) Development of a continuous cell line, PBLE, from an American eel peripheral blood leukocyte preparation. In Vitro Cell Dev Biol Anim 42:263–272
Diago ML, López-Fierro P, Razquin B, Villena A (1995) Establishment and characterisation of a pronephric stromal cell line (TPS) from rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol 5:441–457
Dong C, Weng S, Shi X, Shi N, He J (2008) Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res 135:273–281
Faisal M, Rutan BJ, Sami-Demmerle S (1995) Development of continuous liver cell cultures from the marine teleost, spot (Leiostomus xanthurus, Pisces: Sciaenidae). Aquaculture 132:59–72
Flano E, Loupez-Fierro P, Álvarez F, Razquin B, Villena A (1998) Splenic cultures from rainbow trout, Oncorhynchus mykiss: establishment and characterization. Fish Shellfish Immunol 8:589–606
Freshney RI (2005) Culture of animal cells: a manual of basic technique. Wiley, New Jersey
Fryer JL, Lannan CN (1994) Three decades of fish cell culture: a current listing of cell lines derived from fish. J Tissue Culture Methods 16:87–94
Ganassin RC, Bols NC (1998) Development of a monocyte/macrophage-like cell line, RTS11, from rainbow trout spleen. Fish Shellfish Immunol 8:457–476
Hameed ASS, Parameswaran V, Shukla R, Singh IS, Thirunavukkarasu AR, Bhonde RR (2006) Establishment and characterization of India’s first marine fish cell line from kidney of sea bass, Lates calcarifer. Aquaculture 257:92–103
Hayflick L, Moorehead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hightower LH, Renfro JL (1988) Recent applications of fish cell culture to biomedical research. J Exp Zool 248:290–302
Himizu C, Hike H, Denise MM, Breisch E, Westerman M, Buchanan J, Ligman HR, Phillips RB, Carlberg JM, Olst JV, Burns JC (2003) Characterization of a white bass (Morone Chrysops) embryonic cell line with epithelial features. In Vitro Cell Dev Biol Anim 39:29–35
Holen E, Kausland A, Skjærven K (2010) Embryonic stem cells isolated from Atlantic cod (Gadus morhua) and the developmental expression of a stage-specific transcription factor ac-Pou2. Fish Physiol Biochem. doi:10.1007/s10695-010-9381-z
Hong Y, Winkler C, Schartl M (1996) Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev 60:33–44
Hong Y, Chen S, Schartl M (2000) Embryonic stem cells in fish: current status and perspectives. Fish Physiol Biochem 22:165–170
Hsu YL, Yang YH, Chen YC, Tung MC, Wu JL, Engelking MH, Leong JC (1995) Development of an in vitro subculture system for the Oka organ lymphoid tissue of Penaeus monodon. Aquaculture 136:43–55
Huang X, Huang Y, Sun J, Han X, Qin Q (2009) Characterization of two grouper Epinephelus akaara cell lines: application to studies of Singapore grouper iridovirus (SGIV) propagation and virus–host interaction. Aquaculture 292:172–179
Hughes P, Marshall D, Reid Y, Parkes H, Gelber C (2007) The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques 43:575–586
Imajoh M, Ikawa T, Oshima SI (2007) Characterization of a new fibroblast cell line from a tail fin of red sea bream, Pagrus major and phylogenetic relationships of a recent RSIV isolate in Japan. Virus Res 126:45–52
Iwamoto T, Nakai T, Mori K, Arimoto M, Furusawa I (2000) Cloning of the fish cell line SSN-1 for piscine nodaviruses. Dis Aquat Organ 43:81–89
Kang MS, Oh MJ, Kim YJ, Kawai K, Jung SJ (2003) Establishment and characterization of two cell lines derived from flounder, Paralichthys olivaceus (Temminck & Schlegel). J Fish Dis 26:657–665
Karunasagr I, Miller SD, Frerichs GN (1998) A new cell line from Puntius schwanenfeldi sensitive to snakehead fish cell line C-type retrovirus. Asian Fish Sci 8:151–157
Katakura Y, Alam S, Shirahata S (1998) Immortalization by gene transfection. Method Cell Biol 57:69–91
Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA (1998) Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84–88
Kou GH, Wang CH, Hung HW, Jng YS, Chou CM, Lo CF (1995) A cell line (EP-1) derived from “Beko disease” affected Japanese eel elver (Anguilla japonica) persistently infected with Pleistophors anguillarum. Aquaculture 132:161–173
Ku CC, Teng YC, Wang CS, Lu CH (2009) Establishment and characterization of three cell lines derived from the rockfish grouper Epinephelus quoyanus: Use for transgenic studies and cytotoxicity testing. Aquaculture 294:147–151
Kumar GS, Singh IBS, Philip R (2001) Development of a cell culture system from the ovarian tissue of African catfish (Clarias gariepinus). Aquaculture 194:51–62
Lai YS, Murali S, Ju HY, Wu MF, Guo IC, Chen SC, Fang K, Chang CY (2000) Two iridovirus-susceptible cell lines established from kidney and liver of grouper, Epinephelus awoara (Temminck and Schlegel), and partial characterization of grouper iridovirus. J Fish Dis 23:379–388
Lai YS, Murali S, Chiu HC, Ju HY, Lin YS, Chen SC, Guo IC, Fang K, Chang CY (2001) Propagation of yellow grouper nervous necrosis virus (YGNNV) in a new nodavirus susceptible cell line from yellow grouper, Epinephelus awoara (Temminck & Schlegel), brain tissue. J Fish Dis 24:299–309
Lai YS, John JAC, Lin CH, Guo IC, Chen SC, Fang F, Lin CH, Chang CY (2003) Establishment of cell lines from a tropical grouper, Epinephelus awoara (Temminck and Schlegel), and their susceptibility to grouper irido and nodaviruses. J Fish Dis 26:31–42
Lai YS, Chiou PP, Chen WJ, Chen YC, Chen CW, Chiu IS, Chen SD, Cheng YH, Chang CY (2008) Characterization of apoptosis induced by grouper iridovirus in two newly established cell lines from barramundi, Lates calcarifer (Bloch). J Fish Dis 31:825–834
Lakra WS (2010) Pluripotent embryonic stem cell line from catfish. ICAR News 16(1):3
Lakra WS, Bhonde RR (1996) Development of primary cell culture from the caudal fin of an Indian major carp, Labeo rohita (Ham). Asian Fish Sci 9:149–152
Lakra WS, Bhonde RR, Sivakumar N, Ayyappan S (2006a) A new fibroblast like cell line from the fry of golden mahseer Tor putitora (Ham). Aquaculture 253:238–243
Lakra WS, Sivakumar N, Goswami M, Bhonde RR (2006b) Development of two cell culture systems from Asian seabass Lates calcarifer (Bloch). Aquacult Res 37:18–24
Lakra WS, Swaminathan TR, Rathore G, Goswami M, Yadav K, Kapoor S (2010) Development and characterization of three new diploid cell lines from Labeo rohita (Ham.). Biotechnol Progr. doi:10.1002/btpr.418
Lannan CN (1994) Fish cell culture: a protocol for quality control. J Tissue Cult Methods 16:95–98
Lannan CN, Winton JR, Fryer JL (1984) Fish cell lines: establishment and characterization of nine cell lines from salmonids. In Vitro 20:671–676
Lee LEJ, Caldwell SJ, Gibbons J (1997) Development of a cell line from skin of goldfish, Carassius auratus, and effects of ascorbic acid on collagen deposition. Histochem J 29:31–43
Leibovitz A (1963) The growth and maintenance of tissue-cell cultures in free gas exchange with the atmosphere. Am J Hyg 78:173–180
Luc Rougée GK, Ostrander RH, Richmond YL (2007) Establishment, characterization, and viral susceptibility of two cell lines derived from goldfish Carassius auratus muscle and swim bladder. Dis Aquat Organ 77:127–135
Matsuo Y, Nishizaki C, Drexler HG (1999) Efficient DNA fingerprinting method for the identification of cross-culture contamination of cell lines. Hum Cell 12:149–154
Miller NW, Chinchar VG, Clem LW (1994) Development of leukocyte cell lines from the channel catfish (Ictalurus punctatus). J Tissue Cult Methods 16:117–123
Ossum CG, Hoffmann EK, Vijayan MM, Holt SE, Bols NC (2004) Characterization of a novel fibroblast-like cell line from rainbow trout and responses to sublethal anoxia. J Fish Biol 64:1103–1116
Ostrander GK, Blair JB, Everlya SG, Marley AW, Bales ER, Obertw Veltri D, Avide Hinton M, Arko Kihiro LO, Hawkins EW (1995) Long-term primary culture of epithelial cells from rainbow trout (Oncorhynchus mykiss) liver. In Vitro Cell Dev Biol Anim 31:367–378
Parameswaran V, Shukla R, Bhonde RR, Hameed ASS (2006a) Establishment of embryonic cell line from sea bass (Lates calcarifer) for virus isolation. J Virol Methods 137:309–316
Parameswaran V, Shukla R, Bhonde RR, Hameed ASS (2006b) Splenic cell line from sea bass, Lates calcarifer: establishment and characterization. Aquaculture 261:43–53
Parameswaran V, Shukla R, Bhonde RR, Hameed ASS (2006c) Development of a Pluripotent ES-like cell Line from Asian sea bass (Lates calcarifer)—an oviparous stem cell line mimicking viviparous ES cells. Mar Biotechnol 9:766–775
Parameswaran V, Ahmed VPI, Shukla R, Bhonde RR, Hameed ASS (2007) Development and characterization of two new cell lines from milkfish (Chanos chanos) and grouper (Epinephelus coioides) for virus isolation. Mar Biotechnol 9:281–291
Parodi B, Aresu O, Bini D, Lorenzini R (2002) Species identification and confirmation of human and animal cell lines: a PCR-based method. Biotechniques 32:432–440
Perry GML, McDonald GJ, Ferguson MM, Ganassin RC, Bols NC (2001) Characterization of rainbow trout cell lines using microsatellite DNA profiling. Cytotechnology 37:143–151
Pombinho AR, Laize V, Molha DM, Marques SMP, Cancela LM (2004) Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extra cellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res 315:393–406
Qin QW, Wu TH, Jia TL, Hegde A, Zhang RQ (2006) Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J Virol Methods 131:58–64
Ristow SS, De Avila J (1994) Susceptibility of four salmonids cell lines to infectious hematopoietic necrosis virus. J Aquat Anim Health 6:260–265
Ristow SS, Thorgaard GH (1998) Development of long term cell lines from homozygous clones of rainbow trout. J Aquat Anim Health 10:75–82
Ryan LA, Seymour CB, O’Neill-Mehlenbacher A, Mothersill CE (2008) Radiation-induced adaptive response in fish cell lines. J Environ Radioact 99:739–747
Sathe PS, Maurya DT, Basu A, Gogate SS, Banerjee K (1995) Establishment and characterization of a new fish cell line, MG-3, from the gills of mrigal Cirrhinus mrigala. Ind J Exp Biol 33:589–594
Sathe PS, Basu A, Mourya DT, Marathe BA, Gogate SS, Banerjee K (1997) A cell line from the gill tissues of Indian cyprinoid Labeo rohita. In Vitro Cell Dev Biol Anim 33:425–427
Sato G (2008) Tissue cultures: the unrealized potential. Cytotechnology 57:111–114
Schirmer K (2006) Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology 224:163–183
Servili A, Bufalino MR, Nishikawa R, de Melo IS, Muñoz-Cueto JA, Lee LEJ (2009) Establishment of long term cultures of neural stem cells from adult sea bass, Dicentrarchus labrax. Comp Biochem Physiol Part A 152:245–254
Sha ZA, Ren G, Wang X, Wang N, Chen S (2010) Development and characterization of a cell line from the embryos of half smooth tongue sole (Cynoglossus semilaevis). Acta Oceanologica Sinica 29(2):81–87
Sun L, Bradford CS, Ghosh C, Collodi P, Barnes DW (1995) ES-like cell cultures derived from early zebrafish embryos. Mol Mar Biol Biotech 4:193–199
Swaminathan TR, Lakra WS, Gopalakrishnan A, Basheer VS, Khushwaha B, Sajeela KA (2010) Development and characterization of a new epithelial cell line PSF from caudal fin of Green chromide, Etroplus suratensis (Bloch, 1790). In Vitro Cell Dev Biol Anim. doi:10.1007/s11626-010-9326-y
Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M (1999) Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res 59:551–557
Tokiwa T, Kusaka Y, Muraoka A, Sato J (1989) Examination of HeLa cell contamination of human cell lines derived from primary hepatomas using glucose-6-phosphate dehydrogenase and lactate dehydrogenase isozymes. Acta medica Okayama 43:245–247
Tong SL, Lee H, Miao HZ (1997) The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder. Paralichthys olivaceus Aquaculture 156:327–333
Tong SL, Miao HZ, Li H (1998) Three new continuous fish cell lines of SPH, SPS and RSBF derived from sea perch (Lateolabrax japaonicus) and red sea bream (Pagrosomus major). Aquaculture 169:143–151
Villena AJ (2003) Applications and needs of fish and shellfish cell culture for disease control in aquaculture. Rev Fish Biol Fisher 13:111–140
Wagg SK, Lee LEJ (2005) A proteomics approach to identifying fish cell lines. Proteomics 5:4236–4244
Wang R, Neumann NF, Shen Q, Belosevic M (1995) Establishment and characterization of a macrophage cell line from the goldfish. Fish Shellfish Immunol 5:329–346
Wang G, LaPatra S, Zeng L, Zhao Z, Lu Y (2003) Establishment, growth, cryopreservation and species of origin identification of three cell lines from white sturgeon, Acipenser transmontanus. Methods Cell Sci 25:211–220
Wang XL, Wang N, Sha ZX, Chen SL (2010a) Establishment, characterization of a new cell line from heart of half smooth tongue sole (Cynoglossus semilaevis). Fish Physiol Biochem. doi:10.1007/s10695-010-9396-5
Wang N, Wang XL, Sha ZX, Tian YS, Chen SL (2010b) Development and characterization of a new marine fish cell line from turbot (Scophthalmus maximus). Fish Physiol Biochem. doi:10.1007/s10695-010-9402-y
Watanabe T, Nakano M, Asakawa H, Moritomo T (1987) Cell culture of rainbow trout liver. B Jpn Soc Sci Fish 53:537–542
Wen CM, Lee CW, Wang CS, Cheng YH, Huang HY (2008) Development of two cell lines from Epinephelus coioides brain tissue for characterization of betanodavirus and megalocytivirus infectivity and propagation. Aquaculture 278:14–21
Wenger SL, Senft JR, Sargent LM, Bamezai R, Bairwa N, Grant SG (2004) Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci Rep 24:631–639
Wergeland HI, Jakobsen RA (2001) A salmonid cell line (TO) for production of infectious salmon anaemia virus (ISAV). Dis Aquat Organ 44:183–190
Williams LM, Crane MStJ, Gudkovs N (2003) Development and characterisation of pilchard (Sardinops sagax neopilchardus) cell lines derived from liver and heart tissues. Methods Cell Sci 25:105–113
Wolf K (1988) Fish viruses and fish viral diseases. Cornell University Press, New York
Wolf K, Mann JA (1980) Poikilotherm vertebrate cell lines and viruses: a current listing for fishes. In Vitro 16:168–179
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Science 135:1065–1066
Wolf K, Quimby MC (1966) Fish cell and tissue culture. In: Hoar WS, Randall DJ (eds) Fish physiology, vol III. Academic Press, New York, pp 253–305
Wolf K, Quimby C (1976) Primary monolayer culture of fish cells initiated from trypsinized tissues. TCA Manual 2:453–456
Ye HQ, Chen SL, Sha ZX, Xu MY (2006) Development and characterization of cell lines from heart, liver, spleen and head kidney of sea perch Lateolabrax japonicus. J Fish Biol 69:115–126
Yu H, Cook TJ, Sinko PJ (1997) Evidence for diminished functional expression of intestinal transporters in Caco-2 cell monolayers at high passages. Pharm Res 14:757–762
Zhao Z, Lu Y (2006) Establishment and characterization of two cell lines from bluefin trevally Caranx melampygus. Dis Aquat Organ 68:91–100
Zhao Y, Glesne D, Huberman E (2003) A human peripheral blood monocyte derived subset acts as pluripotent stem cells. Proc Natl Acad Sci 100:2426–2431
Zhou GZ, Li ZQ, Yuan XP, Zhang QY (2007) Establishment, characterization, and virus susceptibility of a new marine cell line from red spotted grouper (Epinephelus akaara). Mar Biotechnol 9:370–376
Zhou GZ, Gui L, Li ZQ, Yuan XP, Zhang QY (2008) Establishment of a Chinese sturgeon Acipenser sinensis tail-fin cell line and its susceptibility to frog iridovirus. J Fish Biol 73:2058–2067
Acknowledgments
The authors are grateful to Dr. S. Ayyappan, Director General, Indian Council of Agricultural Research for guidance and encouragement. The Department of Biotechnology, Government of India is thankfully acknowledged for financial support. We also thank Dr. A. Gopalakrishnan of NBFGR Kochi Unit for co-operation and literature support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakra, W.S., Swaminathan, T.R. & Joy, K.P. Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37, 1–20 (2011). https://doi.org/10.1007/s10695-010-9411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9411-x