Abstract
Cell cultures can be a potent and strong tool to evaluate the insecticidal efficiency of natural products. Plant essential oils have long been used as the fragrance or curative products around the world which means that they are safer to be used in close proximity of humans and mammals. In this study, a midgut cell line, developed from Rhynchophorus ferrugineus (RPW-1), was used for screening essential oils from nine different plants. Assays revealed that higher cell mortality was observed at 500 ppm which reached to 86, 65, 60, 59, 56, 54, 54, 53, and 53%, whereas lowest cell mortality at 1 ppm remained at 41, 23, 20, 17, 16, 15, 14, 13, and 10%, for Azadirachta indica, Piper nigrum, Mentha spicata, Cammiphora myrrha, Elettaria cardamomum, Zingiber officinale, Curcuma longa, Schinus molle, and Rosmarinus officinalis, respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay revealed the percentage of cell growth inhibition was highest at 500 ppm and remained at 48, 45, 42, 37, 34, 29, 24, 22, and 18% against A. indica, P. nigrum, M. spicata, C. myrrha, E. cardamomum, Z. officinale, C. longa, S. molle, and R. officinalis, respectively. Lowest LC50 value (7.98 ppm) was found for A. indica, whereas the highest LC50 (483.11 ppm) was against R. officinalis. Thus, in this study, essential oils of A. indica exhibited the highest levels of toxicity, whereas those from R. officinalis exhibited the lowest levels of toxicity toward RPW-1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of rapid assessment tools is required to increase the availability of new control methods for insect pests. Traditional studies involving the entire pest insect life cycle slow the pace of discovery because they often need to be conducted under laboratory as well as field conditions. In this regard, cell cultures from notorious pests can be helpful by providing a quick analysis of the problem. Smagghe et al. (2009) reported that cell lines are perfect tools not only for the screening of insecticides but also for unraveling their mechanism(s) of action. Midgut tissue in insects is important for the production and secretion of luminal enzymes and final digestion by microvillar enzymes. Midgut epithelium consists of columnar absorptive cells with apical microvilli, pear-shaped goblet cells that transport ions, and small round stem cells located at the base of the epithelium (Hakim et al. 2001).
Plant oils are generally considered broad spectrum and safe for the environment as the compounds they contain degrade under field conditions (Yuan et al. 2010). Essential oils are defined as oils with aromatic components which give distinctive odor, flavor, or scent to a plant. These are by-products of plant metabolism and are referred to as volatile plant secondary metabolites. Many plant essential oils show a broad spectrum of activity against pest insects and plant pathogenic fungi ranging from insecticidal, antifeedant, repellent, oviposition deterrent, and growth regulatory activities. Essential oils when mixed with emulsifying agent such as dimethyl sulfoxide become more effective against insects as they can more easily penetrate the waxy insect cuticle (Sampson et al. 2005). Development of insect resistance is an alarming issue for many synthetic pesticides, but it is assumed that resistance will develop more slowly to essential oil-based pesticides because of the complex mixtures of constituents in essential oils. Essential oils are presumed to have greater impact in future integrated pest management because of safety to nontarget organisms and the environment. As their target site is not shared with mammals, most essential oils are relatively nontoxic to mammals and fish and are more compatible with the environment than synthetic pesticides (Isman and Machial 2006).
Rhynchophorus ferrugineus was initially reported on coconut Cocos nucifera in South Asia, but it became an invasive pest of date palm in several Middle Eastern countries (Faleiro 2006). R. ferrugineus is a concealed tissue borer and has been reported to attack 17 palm species worldwide and has especially become a major source of economic loss in date production in the Middle East (Abid et al. 2013). Although integrated control methods have been used to control this pest, the main method is the use of synthetic insecticides (Mahmoud et al. 2013). Increased resistance of this pest against synthetic insecticides urges scientists to look for safer alternatives. Environmental problems caused by overuse of pesticides have been a matter of concern in recent years. About 2.5 million tons of synthetic pesticides are used on crops each year, and the worldwide damage caused by pesticides reaches $100 billion annually because of the high toxicity and residues in soil, water resources, and crops (Koul et al. 2008).
With these perspective in mind, the current study was carried out, using the RPW-1 midgut cell line established during our early studies (Aljabr et al. 2014), to screen potent insecticidal essential oils from different plants (Piper nigrum, Zingiber officinale, Mentha spicata, E. cardamomum, Cammiphora myrrha, Schinus molle, Curcuma longa, Rosmarinus officinalis, Azadirachta indica) as alternative natural pesticides against the red palm weevil.
Material and Methods
Reagents.
All the reagents including cell growth media (Grace’s insect cell media containing l-glutamine (0.6 g/L) and sodium bicarbonate (0.35 g/L)) required for the cell culturing were purchased from Sigma-Aldrich (St. Louis, MO). Essential oils obtained with steam distillation (P. nigrum, Z. officinale, M. spicata, E. cardamomum, C. myrrha, S. molle, C. longa, R. officinalis, A. indica) were purchased from Eden Botanicals (Petaluma, California) (www.edenbotanicals.com).
Essential oil applications.
Five concentrations of each essential oil at 500, 100, 50, 10, and 1 ppm were prepared in 0.01% DMSO. The 1 × 106 cells/mL were seeded in six-well plate containing 3 mL of Grace’s insect cell media (added with antibiotic and antimycotic solution (Sigma-Aldrich A5955)) and incubated for 4 h at 27°C. Cell cultures were then treated with 10 μl/mL media of each oil concentration. Controls were treated with equal volumes of 0.01% DMSO.
Cell mortality percentages.
To assess percent cell mortality, a trypan blue assay was conducted according to Oh et al. (2004) after 24-h exposure to each concentration (500, 100, 50, 10, and 1 ppm) of all the essential oils (P. nigrum, Z. officinale, M. spicata, E. cardamomum, C. myrrha, S. molle, C. longa, R. officinalis, A. indica). After harvesting, 10 μl of cells were mixed with 10 μl of 0.4% trypan blue solution (Sigma-Aldrich) and incubated for 3 min. The numbers of blue (dead) cells were counted under the microscope using neubauer hemocytometer, and percentage of dead and viable cells were calculated.
Formulae:
-
%age of cell mortality = total dead cells (stained) / total cells (viable + dead) × 100
-
Viable cells/mL = average viable cell count per square × dilution factor × 104
LC 50 values of essential oils.
Five concentrations of each essential oil were used to assess their efficacy as RPW-1 cell mortality percentages, and based on the data retrieved, probit regression analysis was carried out to determine the LC50 values of the five essential oil concentrations against RPW-1 (Grimm et al. 2001).
MTT cell proliferation assay.
Vybrant® MTT Cell Proliferation Assay Kit was used to perform 3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay. RPW-1 cells at 1 × 106 cells/mL were seeded in 96-well plates. After 24 h of incubation, 20 μl of each of the five concentrations (500, 100, 50, 10, and 1 ppm) from essential oils (P. nigrum, Z. officinale, M. spicata, E. cardamomum, C. myrrha, S. molle, C. longa, R. officinalis, A. indica) were added and 0.01% DMSO was used as the control. After 24 h of treatment, 10 μL of the 12 mM MTT stock solution was added to each well and incubated for 4 h at 37°C. Then, 100 μL of the SDS-HCl solution was added to each well and mix thoroughly using a pipette. Plate was again incubated at 37°C for 4 h to dissolve the formazan crystals. Later on each sample was mixed properly with a pipette and absorbance was measured at 570 nm by microplate reader. Cytotoxic effect was expressed as a relative percentage of inhibition as follows:
Statistical analysis.
Data analysis was carried out using analysis of variance (ANOVA) and statistical significance was established by using SAS software (SAS 2000). Differences between the treatments were determined by Tukey’s multiple range tests at P < 0.05. All experiments were performed in triplicate with three replications.
Results
Cell mortality percentages.
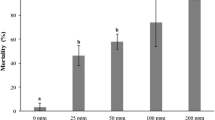
Cell mortality percentage varied with different essential oils ranging from highest (A. indica) to the lowest (R. officinalis). Higher cell mortality was observed at 500 ppm which gradually decreased with concentrations to the lowest at 1 ppm as shown in Table 1. Cell mortality was noted after 24 h which revealed that A. indica caused the highest 86% cell mortality at 500 ppm. At 500 ppm, the cell mortality percentage varied at 65, 60, 59, 56, 54, 54, 53, and 53% against P. nigrum, M. spicata, C. myrrha, E. cardamomum, Z. officinale, C. longa, S. molle, and R. officinalis, respectively, as shown in Table 1. The lowest cell mortality was observed at 1 ppm where percent cell mortality was decreased to 41, 23, 20, 17, 16, 15, 14, 13, and 10% for A. indica, P. nigrum, M. spicata, C. myrrha, E. cardamomum, Z. officinale, C. longa, S. molle, and R. officinalis, respectively, as shown in Table 2. R. officinalis showed a 10% cell mortality at 1 ppm, the lowest of all the treatments. Figure 1 shows the cell viability of RPW-1 increased from higher to lower concentrations of essential oils.
LC 50 values of different essential oils.
Probit regression analysis was carried out to determine the LC50 values of essential oils against RPW-1 cells. The LC50 of A. indica was noted to be the lowest and remained as 7.98 ppm, whereas the highest LC50 was found for R. officinalis reaching to 483.11 ppm, as shown in Table 2.
Cell proliferation by MTT bioassay.
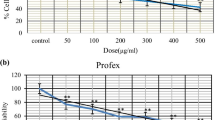
Table 3 reveals the inhibitory effect on RPW-1 cell growth by the nine essential oils (A. indica, P. nigrum, M. spicata, C. myrrha, E. cardamomum, Z. officinale, C. longa, S. molle, R. officinalis) based on the MTT assay. These botanical essential oils acted on percent cell growth in a dose-dependent manner. The level of inhibition of RPW-1 cell proliferation after a 24-h exposure to the plant oils ranged from a high of 32% for A. indica to a low of 6% for R. officinalis at 1 ppm after 24-h exposure. Inhibition of cell proliferation increased with concentrations at 10, 50, and 100 ppm and further showed a maximum inhibition from 48 to 18% at 500 ppm after 24-h exposure (Table 3).
Discussion
Insecticidal bioassays have been useful for identifying insecticidal efficacies; yet, these studies with insect specimens are very laborious and time-consuming. Insect cell cultures from a target species can be a useful tool to screen potential insecticides like essential oils and can be used for biochemical and molecular studies (Grasela et al. 2012). Cytotoxicity has long been studied for DNA damage, morphological changes, and apoptosis in response to the synthetic insecticides (Yoon et al. 2001; Nandi et al. 2006; Sonoda and Tsumuki 2007; Huang et al. 2011). However, little work has been reported relating to the studies of essential oils and insect cells. Previously, midgut epithelial cell culture (RPW-1) from the red palm weevil was developed in our laboratory (Aljabr et al. 2014). As midgut cells have been reported to show high sensitivity to the toxicity (Giner et al. 2012), RPW-1 cell line was used as a tool to screen essential oils derived from different plants as potential insecticidal agents.
The quest to find an alternative for synthetic insecticides against red palm weevil led us to the screening of essential oils belonging to different plant species. Research has focused on the natural products to achieve the most bioactive plant essential oils that have shown good insecticidal properties (Isman 2006). The chemical composition and broad spectrum of biological activity for essential oils can vary with plant age, plant tissues, or organs (Choi et al. 2003; Sedy and Koschier 2003). The action of essential oils against some pests is indicative of a neurotoxic mode of action, and there is evidence for interference with the neuromodulator octopamine (Kostyukovsky et al. 2002) by some oils and with GABA-gated chloride channels by others (Priestley et al. 2003).
The aromatic characteristics of essential oils provide various functions for plants including attracting or repelling insects and utilizing chemical constituents as a defense mechanism (Koul et al. 2008). Biological activity of essential oils is also affected by synergistic and antagonistic interactions among structural components (Chiasson et al. 2001) and that might be a reason of better A. indica and lower R. officinalis efficacy.
Toxicity of some of the essential oils used in this study has also been documented by other researchers against different insect species. Essential oils from E. cardamomum have been proven toxic to other coleopteran insects such as Callosobruchus maculates and Tribolium castaneum and also showed good efficacy as an oviposition deterrent for C. maculates (Abbasipour et al. 2011). Knaak et al. (2012) found that the toxicity of the extracts of Z. officinale caused damage such as vacuolization of cytoplasm, disruption of microvilli, peritrophic membrane destruction, and cell changes in the midgut of Spodoptera frugiperda. Some essential oils like P. nigrum and S. molle have been reported to exhibit the bioactivity on Zabrotes subfasciatus (Oliveira et al. 2005) and Triatoma infestans (Laurent et al. 1997).
Similarly, mode of action of essential oils is based on disruption of several cell functions (Yang et al. 2009; Marchial et al. 2010; Perumalsamy et al. 2010). Anuradha et al. (2007) and Kumar et al. (2007) reported that in Drosophila melanogaster cells, azadirachtin induced depolymerization of actin, leading to cell cycle arrest and subsequently apoptosis in a caspase-independent manner, thus suggesting that actin might be a target of azadirachtin. Furthermore, Jingfei et al. (2011) observed morphological changes in SL-1 cells after treatment with azadirachtin. Essential oils are mainly mixtures of different compounds (Bakkali et al. 2008) that show insecticidal characteristics (Leelaja et al. 2007; Escriba et al. 2009). In general, no adverse effects toward human health or the environment are expected as several essential oils are approved as fragrances and food flavors (Burdock 2010). Cells in the intact insect and cell cultures can behave differently as in intact insect detoxification mechanisms can be more active than in cells cultures. However, this requires more study to fully understand the potential counteractive mechanisms to toxicity in cells and insects.
In conclusion, the data provides a clear picture about the differential insecticidal capabilities of the essential oils against midgut cell line of red palm weevil (RPW-1). Botanical insecticides are good alternatives because they often have lower mammalian toxicity and environmental persistence (Isman 2006). However, further studies with chromatographic analysis are required to fully understand the constituents and potential of the essential oils for controlling red palm weevil.
References
Abbasipour H, Mahmoudvand M, Rastegar F, Hosseinpour MH (2011) Fumigant toxicity and oviposition deterrency of the essential oil from cardamom, Elettaria cardamomum, against three stored–product insects. J Insect Sci 11:165
Abid H, Muhammad R, Ahmed MA, Hassan YA (2013) Managing invasive populations of red palm weevil: a worldwide perspective. J Food Agric Environ 11:456–463
Aljabr AM, Rizwan-ul-Haq M, Hussain A, Al-Mubarak AI, Al-Ayied HY (2014) Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. In Vitro Cell Dev Biol-Animal 50:296–303
Anuradha A, Annadurai RS, Shashidhara LS (2007) Actin cytoskeleton as a putative target of the neem limonoid azadirachtin A. Insect Biochem Mol Biol 37:627–634
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Burdock G.A. (2010) Fenaroli’s handbook of flavour ingredients. Taylor and Francis group. Orlando: CRC Press. 2159 p
Chiasson H, Belanger A, Bostanian N, Vincent C, Poliquin A (2001) Acaricidal properties of Artemisia absinthiumand Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J Econ Entomol 94:167–171
Choi WI, Lee EH, Choi BR, Park HM, Ahn YJ (2003) Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J Econ Entomol 96:1479–1484
Escriba M, Barbut M, Eras J, Canela R, Avilla J, Balcells M (2009) Synthesis of allyl esters of fatty acids and their ovicidal effect on Cydia pomonella (L.). J Agric Food Chem 57:4849–4853
Faleiro JR (2006) A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int J Trop Insect Sci 26:135–154
Giner M, Avilla J, Balcells M, Caccia S, Smagghe G (2012) Toxicity of allyl esters in insect cell lines and in Spodoptera littoralis larvae. Arch Insect Biochem Physiol 79:18–30
Grasela JJ, McIntosh AH, Ringbauer JJ, Goodman CL, Carpenter JE, Popham HJ (2012) Development of cell lines from the cactophagous insect: Cactoblastis cactorum (Lepidoptera: Pyralidae) and their susceptibility to three baculoviruses. In Vitro Cell Dev Biol-Animal 48:293–300
Grimm C, Schmidli H, Bakker F, Brown K, Campbell P, Candol WM, Chapman P, Harrison EG, Mead-Briggs M, Schmuck R, Ufer A (2001) Use of standard toxicity tests with Typhlodromus pyri and Aphidius rhopalosiphi to establish a dose–response relationship. J Pest Sci 74:72–84
Hakim RS, Baldwin KM, Loeb M (2001) The role of stem cells in midgut growth and regeneration. In Vitro Cell Dev Biol- Animal 37:338–342
Huang JF, Tian M, LV CJ, Li HY, Muhammad R, Zhong GH (2011) Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic Biochem Physiol 100:256–263
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Isman MB, Machial CM (2006) Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M, Carpinella MC (eds) Naturally occurring bioactive compounds. Elsevier, BV, pp 29–44
Jingfei H, Kejuan S, Hai-yi L, Meiying H, Guohua Z (2011) Antiproliferative effect of azadirachtin A on Spodoptera litura Sl-1 cell line through cell cycle arrest and apoptosis induced by up-regulation of p53. Pestic Biochem Physiol 99:16–24
Knaak N, Berlitz D.L., Fiuza L.M. (2012) Toxicology of bioinsecticides used in agricultural food production, São Paulo, Brazil, DOI: 10.5772/52070
Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Haaya E (2002) Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag Sci 58:1101–1106
Koul O, Walia S, Dhliwal GS (2008) Essential oils as pesticides: potential and constrainsts. Biopestic Int 41:63–84
Kumar RP, Manoj MN, Kush A, Annadurai RS (2007) In silico approach of azadirachtin binding with actins. Insect Biochem Mol Biol 37:635–640
Laurent D, Vilaseca LA, Chantraine JM, Ballivian C, Gloria S, Ibanez R (1997) Insecticidal activity of essential oils on Triatoma infestans. Phytotherapy Res 11:285–290
Leelaja BC, Rajashekar Y, Reddy PV, Begum K, Rajendran S (2007) Fumigant toxicity of allyl acetate to stored-product beetles in the presence of carbon dioxide. J Stored Prod Res 43:45–48
Mahmoud MA, Hamadttu AE, Ibrahim A (2013) Toxicity of bio-insecticides Abamectin, on red palm weevil, Rhynchophorus ferrugineus (Olivier). Int J Agric Sci Res 2:107–115
Marchial CM, Shikano I, Smirle M, Bradbury R, Isman R (2010) Evaluation of the toxicity of 17 essential oils against Choristoneura rosacerana (Lepidoptera: Tortricidae) and Trichoplusia ni (Lepidotera: Noctuidae). Pest Manag Sci 66:1116–1121
Nandi A, Chandi D, Lechesa R, Pryor SC, Mclaughlin A, Bonventre JA, Flynn K, Weeks BS (2006) Bifenthrin causes neurite retraction in the absence of cell death: a model for pesticide associated neuro degeneration. Med Sci Monit 12:169–17
Oh H, Livingston R, Smith K, Abrishamian GL (2004) Comparative study of the time dependency of cell death assays. MURJ 11:53–62
Oliveira MJ, Campos IFP, Oliveira CBA, Santos MR, Souza PS, Santos SC, Seraphin JC, Ferri PH (2005) Influence of growth phase on the essential oil composition of Hyptis suaveoleni. Biochem Syst Ecol 33:275–285
Perumalsamy H, Chang KS, Park C, Ahn YJ (2010) Larvicidal activity of Asarum heterotropoides root constituents against insecticide susceptible and resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J Agric Food Chem 58:10001–11006
Priestley CM, Williamson EM, Wafford KA, Sattelle DB (2003) Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140:1363–1372
Sampson BJ, Tabanca N, Kirimer N, Demirci B, Baser KHC, Khan IA, Spiers JM, Wedge DE (2005) Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag Sci 61:1122–1128
SAS (2000) Sas user’s guide: statistics. Sas Institute, Cary
Sedy KA, Koschier EH (2003) Bioactivity of carvacrol and thymol, against Frankliniella occidentalis and Thrips tabaci. J Appl Entomol 127:313–316
Smagghe G, Goodman CL, Stanley D (2009) Insect cell culture and applications to research and pest management. In Vitro Cell Dev Biol-Animal 45:93–105
Sonoda S, Tsumuki H (2007) Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic Biochem Physiol 89:185–189
Yang YC, Lee SH, Clark JM, Ahn YJ (2009) Ovicidal and adulticidal activities of Origanum mejorana essential oils constituents against insecticide-susceptible and pyrethroid/malathion-resistant Pediculus humanus capitis (Anoplura: Pediculidae). J Agric Food Chem 57:2282–2287
Yoon JY, Oh SH, Yoo SM, Lee SJ, Lee HS, Choi SJ, Moon CK, Lee BH (2001) N-Nitrosocarbofuran, but carbofuran, induces apoptosis and cell cycle arrest in CHL cells. Toxicology 169:153–161
Yuan HB, Shang LN, Wei CY, Ren BZ (2010) Comparison of constituents and insecticidal activities of essential oil from Artemisia lavandulaefolia by steam distillation and supercritical-CO2 fluid extraction. Chem Res Chinese Univ 26:888–892
Acknowledgment
The authors gratefully acknowledge the role of King Abdul Aziz City of Science and Technology (KACST) for providing research funding under the grant no. 08-BIO 10–6 to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Rizwan-ul-Haq, M., Aljabr, A.M. Rhynchophorus ferrugineus midgut cell line to evaluate insecticidal potency of different plant essential oils. In Vitro Cell.Dev.Biol.-Animal 51, 281–286 (2015). https://doi.org/10.1007/s11626-014-9825-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9825-3