Abstract
Midgut epithelial cell culture was successfully developed from red palm weevil (Rhynchophorus ferrugineus) during this study and named as RPW-1. Optimum conditions for four different commercial media were also worked out to successfully maintain the culture. Grace’s medium was found to be the most effective for RPW-1 culturing which resulted in the highest cell density of 7.5 × 106 cells/ml after 72 h of cell seeding with 96% cell viability. It was followed by Schneider’s medium and TNM-FH medium where cell densities reached up to 7.4 × 106 and 5.9 × 106 cells/ml, respectively, after 72 h having 91 and 89% cell viability. Comparatively, Media-199 was least effective for RPW-1 cell culturing. As a whole, temperature at 27°C and pH 6.3 were the best for RPW-1 culturing where the highest cell density and maximum cell viability were noted. Individually, Grace’s medium, Schneider’s medium, TNM-FH medium, and Media-199 produced better results at 27°C, 27°C, 24°C, and 21°C and pH 6.3, 6.4, 5.3, and 7.1, respectively. The toxicity assay and MTT cell proliferation assay revealed that, out of the ten insecticides used in this study, emamectin benzoate was the most toxic insecticide to RPW-1 cells resulting in 92% cell mortality and 74% cell growth inhibition. Dieldrin was the least potent, causing only 19% cell mortality and 18% cell growth inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects cause huge economic losses of crops and orchards worldwide. Different methodologies have been developed over time and are adopted to control pests, but synthetic insecticides are still the main tool of insect pest control. The problems related to insecticides, such as of resistance, environmental disturbances, and health concerns are increasing over time, and a number of insecticides have been reduced by law in many countries (http://www.irac-online.org; Alford 2000; Ware et al. 2003). Thus, efforts are devoted to the discovery of new bioinsecticidal molecules with lower impact on the environment and high specificity against target pests. Date palm Phoenix dactylifera is the most important fruit tree in the Kingdom of Saudi Arabia, which represents the historic symbol of the country, and approximately 23.5 million trees are planted in the kingdom. Red palm weevil (RPW) Rhynchophorus ferrugineus (Olivier), a concealed tissue borer, is a lethal pest of palms and is reported to attack 17 palm species worldwide. Though the weevil was first reported on coconut Cocos nucifera from South Asia, during the last two decades, it has also been established as a pest of date palm in several Middle Eastern countries (Faleiro 2006). Although integrated control methods are employed to control this pest, the main method is the use of insecticides (Mahmoud et al. 2012).
Among the body tissues of insects, the alimentary canal is one of the most important tissues playing a crucial role in the insect life. Study on the insect alimentary canal is important as it is the site of digestion, detoxification, transport, and semiochemical production, which are important processes in the insect life cycle (Hall et al. 2002a, 2002b; Nardi et al. 2002). The insect gut can be subdivided into three regions with different functional roles. The foregut and hindgut are derived from the embryonic ectodermal layer and are primarily responsible for ingestion of food (foregut), water absorption, and osmoregulation (hindgut), whereas the primary function of the midgut is the absorption of nutrients (Corley and Lavine 2006). Midgut tissue is also important for the production and secretion of luminal enzymes and final digestion by microvillar enzymes. Midgut epithelium consists of columnar absorptive cells with apical microvilli, pear-shaped goblet cells that transport ions in lepidopteran insects, and small round stem cells located at the base of the epithelium (Hakim et al. 2001; Smagghe et al. 2005). Round stem cells are undifferentiated cells that are mitotically active and proliferate to produce additional undifferentiated cells that differentiate through extrinsic or intrinsic signals (Young and Black 2004; Li and Xie 2005).
In the last four decades, cell lines have been established from different insect species. Mostly, these cell lines originated from serious agricultural pests. Noctuid species are the origins of three most popular cell lines such as Spodoptera frugiperda (SF21 and SF9 cells) and the cabbage looper Trichoplusia ni (high five cells). Thus, the use of cell lines for research and commercial applications is currently dominated by three cell lines. Nevertheless, the continued development of new cell cultures from other species is important for the future growth of insect cell studies.
Cell lines are perfect tools not only for the screening of insecticides but also for unraveling their mechanism of action and the mechanism of resistance (Smagghe et al. 2009). So, there is a need to develop and establish cell lines from notorious insect pest species like RPW. The current study was designed to establish the cell culture from the midgut tissues of RPW larvae. The techniques and optimum conditions to maintain the cell culture were also devised during the study. Impacts of synthetic insecticides belonging to pyrethroids (α-cypermethrin, bifenthrin), carbamates (sevin, carbofuran), organophosphates (dursban), avermectins (emamectin benzoate, abamectin), organochlorines (dieldrin), phenylpyrazoles (fipronil), and pyrroles (chlorfenapyr) on the RPW cells were studied to provide a clear picture about the sensitivity to these insecticides.
Material and Methods
Reagents.
All the reagents required for the cell isolation and culturing were purchased from Sigma-Aldrich, St. Louis, MO. Four insect cell culture media (Grace’s insect cell medium containing l-glutamine and sodium bicarbonate; Schneider’s insect cell medium containing l-glutamine and sodium bicarbonate; TNM-FH insect cell medium containing l-glutamine and sodium bicarbonate; and Medium-199 containing Hanks’ salt and l-glutamine) were used in this study. All the insecticides (bifenthrin, dieldrin, emamectin benzoate, fipronil, α-cypermethrin, abamectin, sevin, carbofuran, chlorfenapyr, and dursban) were of technical grade and purchased from Sigma-Aldrich.
Cell isolation.
Fifth-instar larvae were selected from the RPW colony maintained under the laboratory conditions as described by Al-Ayedh (2011). Midgut epithelial cells were isolated according to Lynn (2001) with some modifications as follows:
-
1.
Insect larvae were submerged for 3–5 min in 0.05% sodium hypochlorite and disinfected by submersion in 70% ethanol.
-
2.
Larvae were rinsed twice with sterile distilled water.
-
3.
Larvae were then transferred to a sterile Petri dish containing 3 ml of culture media and antibiotic (gentamicin) at the concentration of 50 μg/ml.
-
4.
Sterile instruments were used to remove the tissue of interest and transferred to a new dish with additional media.
-
5.
Contaminating tissues were teased away, and the dish was left for 2 h to settle, thus diffusing away the contaminating cells and hemocytes.
-
6.
Contents of the midgut lumen were removed, and the tissue of interest was transferred to a new dish containing a drop of media and cut into small pieces.
-
7.
The edge of the dish was sealed with Parafilm and incubated at 25°C.
-
8.
Additional 1 ml of fresh media was added after 48 h.
-
9.
The culture was centrifuged at 400 g for 5 min, and the supernatant was discarded. Cells were resuspended in the media and evenly distributed into six-well plates. The plate was sealed with Parafilm and incubated at 25°C; 0.5 ml of media was added every7 d.
-
10.
Once established, the cell culture was named RPW-1 and subcultured every third day. RPW-1 cells were used for further experiments after 40 passages in the laboratory.
Optimization of conditions for cell cultures.
Optimization of insect cell growth media. Four Insect growth media (Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199) were used to conduct this study. Into six-well plates containing 3 ml of insect cell growth media, 1 × 106 cells/ml were seeded. Cells were counted in triplicate every 24 h for each medium with a Neubauer hemocytometer. Cell density and viable cell percentage were observed up to 72 h to find the optimum growth media for RPW-1 cells. Cell calculations were made as follows:
Optimization of temperature. RPW-1 cells were exposed to a range of five different temperatures: 18°C, 21°C, 24°C, 27°C, and 30°C. Into six-well plates containing 3 ml of insect cell growth medium, 1 × 106 cells/ml were seeded. Each of the four insect cell culture medium, mentioned above, was used separately to be exposed to five different temperatures (18°C, 21°C, 24°C, 27°C, and 30°C). Efficiency of the RPW-1 cells and insect cell culture media at these temperatures was independently assessed by calculating the cell density and percentage of viable cells, every 24 up to 72 h.
Optimization of pH. Based on the preliminary studies (data not included), three different pH levels were set for each insect cell media separately using 1 M NaOH and 1 M HCl. pH 5.8, 6.3, and 6.8 were set for Grace’s medium whereas 5.9, 6.4, and 6.9 for Schneider’s medium. Similarly, pH 4.8, 5.3, and 5.8 were set for TNM-FH medium and 7.1, 7.6, and 8.1 for Medium-199. Into six-well plates containing 3 ml of each insect cell growth media with three different pH levels, 1 × 106 cells/ml were seeded and exposed to a range of five different temperatures (18°C, 21°C, 24°C, 27°C, and 30°C) separately, and data was recorded after every 24 up to 72 h based on the cell density and viability percentage.
Insecticide applications. Ten insecticides from different groups (bifenthrin, dieldrin, emamectin benzoate, fipronil, α-cypermethrin, abamectin, sevin, carbofuran, chlorfenapyr, and dursban) were used during this study. Five concentrations of each insecticide were prepared in 0.01% DMSO as follows: 500, 100, 50, 10, and 1 ppm. In six-well plate containing 3 ml of Grace’s medium, 1 × 106 cells/ml were seeded and incubated for 4 h at 27°C. Cell cultures were then treated with 10 μg/ml of the above concentrations for each insecticide. Controls were treated with equal volumes of 0.01% DMSO. Cells were harvested after 24 h, and trypan blue assay was conducted according to Oh et al. (2004) to assess the percent mortality of the cells. After treatment and incubation of cells, 10 μl of cell solution was mixed with 10 μl 0.4% trypan blue solution (Sigma-Aldrich) and incubated for 3 min. The number of blue (dead) cells was counted under the microscope by using a Neubauer hemocytometer, and the percentage of dead cells was calculated.
Cell mortality percentages and LC 50 values. Cell mortality percentages were noted after 24 h of treatment and worked out separately for each concentration (500, 100, 50, 10, and 1 ppm) of all the ten insecticides. LC50 values were determined using probit regression analysis (Grimm et al. 2001) and represented in a graph.
MTT cell proliferation assay. The Vybrant® MTT Cell Proliferation Assay Kit was used to perform MTT cell proliferation assay (Invitrogen, Life Technologies, www.probes.com). RPW-1 cell suspensions of 180 μl/well (1 × 106 cells/ml) were seeded in 96-well plates. After 24 h of incubation, 20 μl of each of the five insecticide concentrations from ten insecticides was added, and 0.01% DMSO was used as the control. After 24 h of treatment, 10 μL of the 12 mM MTT stock solution was added to each well and incubated for 4 h at 37°C. Then 100 μL of the SDS-HCl solution (10% SDS solution in 0.01 M HCl) was added to each well and mixed thoroughly using the pipette. The plate was again incubated at 37°C for 4 h to dissolve the formazan crystals. Later on, each sample was mixed properly with the pipette, and the absorbance was measured at 570 nm by a microplate reader. Cytotoxic effect was expressed as a relative percentage of inhibition calculated as follows:
Statistical analysis. Data analysis was carried out using analysis of variance (ANOVA), and statistical significance was established by using the SAS software (SAS 2000). Differences between the treatments were determined by Tukey’s multiple range tests at P < 0.05. All experiments were performed in triplicate.
Results
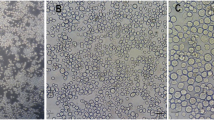
A RPW cell culture was successfully initiated using midgut tissues in serum-free medium. Initial cell growth was observed within 72 h. The first subculture was performed after 15 d followed by subculturing every third day. Initially, cells tended to remain suspended in the medium, but with the continuous subculturing, cells started adhering to the substrate. Cells were maintained through 40 passages (for about 4 mo) before use in the experiments and named RPW-1. Mostly round cells were observed during the passages and used in the experiments (Fig. 1A, B ).
Optimization of condition for RPW-1 cell culture.
Optimization of growth media: Four different insect cell culture media (Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199) were assessed during the study to find the optimum insect cell growth medium for RPW-1 cells. Seeding density of 1 × 106 cells/ml resulted in the increased cell density up to 72 h. The highest cell densities were found to be 7.5 × 106, 7.4 × 106, 5.9 × 106, and 2.2 × 106 cells/ml after 72 h of seeding with 96%, 91%, 89%, and 83% cell viability for Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199, respectively (Fig. 2).
Optimization of temperature. In each of the four insect cell growth media (mentioned above), 1 × 106 cells/ml were seeded and exposed to five different temperatures. Results indicated the optimum temperature for each of the growth medium. The highest cell densities of 7.5 × 106, 7.4 × 106, 5.9 × 106, and 2.2 × 106 cells/ml occurred at 27°C, 27°C, 24°C, and 21°C for Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199 respectively, after 72 h (Fig. 3).
Increase in cell density (106) of RPW-1 cells at different intervals, whereas GT1, GT2, GT3, GT4, GT5; ST1, ST2, ST3, ST4, and ST5; and TT1, TT2, TT3, TT4, TT5; MT1, MT2, MT3, MT4, and MT5 represent different temperatures as T1 = 18°C, T2 = 21°C, T3 = 24°C, T4 = 27°C, and T5 = 30°C along with four different insect cell growth media (G, S, T, and M represent Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199, respectively). Bars with the same letter are not significantly different from each other at P < 0.05 according to Tukey’s test.
Optimization of pH. Three different pH levels were set for each of the four insect cell culture media, and the optimum pH levels were determined based on the cell density and viability. The highest cell densities 7.5 × 106, 7.4 × 106, 5.9 × 106, and 2.2 × 106 cells/ml were found at optimum pH 6.3, 6.4, 5.3, and 7.1 for Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199, respectively, after 72 h (Fig. 4). The results of the optimization experiments revealed that Grace’s medium was the most effective with the highest cell density and cell viability at 27°C and pH 6.3. Therefore, Grace’s medium was used for further experiments (insecticide efficacy on RPW-1 cells, MTT bioassay) at the optimum conditions as determined above.
RPW-1 cell density (106) at different pH levels of the four different media. G, S, T, and M represent insect cell growth media (Grace’s medium, Schneider’s medium, TNM-FH medium, and Medium-199, respectively). Bars with the same letter are not significantly different from each other at P < 0.05 according to Tukey’s test.
Cell mortality percentages and LC 50 . To study the insecticidal effects of ten different insecticides on cell viability, 1 × 106 cells/ml were seeded. Cell mortality was noted after 24 h which revealed that emamectin benzoate caused the highest cell mortality of 92% at 500 ppm. At 500 ppm, the cell mortality percentage varied between different insecticides: 90%, 87%, 85%, 82%, 81%, 80%, 71%, 69%, and 67% against carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin, respectively (Table 1). The lowest cell mortality was observed at 1 ppm where cell mortality percentages decreased to 49%, 47%, 47%, 45%, 41%, 40%, 40%, 35%, 24%, and 19% for emamectin benzoate, carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin, respectively (Table 1). Dieldrin resulted in the lowest cell mortality of 19% at 1 ppm. Five concentrations of each insecticide were used to assess their efficacy based on the RPW-1 cell mortality percentages. Based on the data retrieved, probit regression analysis was carried out to determine the LC50 values of these insecticides against RPW-1 cells. LC50 values were found to be 2.5, 2.7, 3.4, 3.7, 5.3, 6.7, 9.8, 19.2, 39.6, and 87.7 ppm for emamectin benzoate, carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin, respectively as shown in (Table 2).
Cell proliferation. Figure 5 shows the inhibitory effects on RPW-1 cell replication of the insecticides: emamectin benzoate, carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin by MTT assay. These insecticides decreased cell growth in a dose-dependent manner (Fig. 5). The percent decrease in RPW-1 cell numbers from the control were 31%, 31%, 30%, 26%, 31%, 23%, 35%, 23%, 21%, and 18% at 1 ppm of concentration of emamectin benzoate, carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin, respectively, after 24 h of treatment. The percent inhibition of cell proliferation kept on increasing as the concentrations increased, with the maximum inhibition being found at 500 ppm: 74%, 66%, 59%, 55%, 56%, 45%, 50%, 45%, 40%, and 39% against emamectin benzoate, carbofuran, dursban, sevin, fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin, respectively, after 24 h of treatment (Fig. 5).
Discussion
During the last four decades, research about insect cell culture has revealed promising outcomes, leading to the development of high-speed screening technologies for new insect pest management tools. The field of insect cell culture is poised for a very rapid expansion into areas of biology, such as immunity, endocrinology, toxicology, biochemistry, and coevolution (Smagghe et al. 2009). Mostly round cells were noted in RPW-1 cell culture which has been characterized as stem cells by many researchers and might be confirmed as stem cells upon closer inspection in the future. Similarly, round stem cells have been isolated from the midguts of several insect orders including Coleoptera (Hakim et al. 2007) and Lepidoptera (Hakim et al. 2009) both from embryonic and adult tissues (Corley and Lavine 2006). Stem cells are undifferentiated cells that proliferate to produce additional undifferentiated cells or to produce daughters which differentiate via either extrinsic or intrinsic signals (Li and Xie 2005). Midgut cells are regenerated during molting and show rapid cell turnover during active digestion (Loeb et al. 2003). Cells in the midgut epithelium culture developed in this study (RPW-1) initially tended to remain suspended in the medium. With the continuous subculturing of this line, cells started adhering to the substrate, similarities with cells reported in recent studies from different insects (Cermenati et al. 2007; Casartelli et al. 2008; Hakim et al. 2009).
Grace’s medium was the best medium for the culturing of RPW-1 cells. In this medium, densities of 7.5 × 106 cells/ml were found after 72 h, and the highest cell viability was 96%. This was followed by Schneider’s and TNM-FH media which had cell densities of up to 7.4 × 106 and 5.9 × 106 cells/ml after 72 h, respectively (Fig. 2). Medium-199 produced the lowest cell density and percent cell viability for RPW-1 cells as compared with the other media. The temperature optimization experiment revealed that the optimal temperature for the RPW-1 cells in Grace’s and Schneider’s media was 27°C, whereas 24°C and 21°C for TNM-FH medium and Medium-199, respectively (Fig. 3). Shao et al. (1998) observed the optimal temperature range between 24 and 28°C for Sf9 cells, and the highest cell density was observed at 28°C. Our results are in close proximity with these findings having highest cell density of RPW-1 cells at 27°C. The highest cell density was found at pH 6.3 with Grace’s medium, whereas pH 6.4, 5.3, and 7.1 were optimal for Scheneide, TNM-FH, and Media-199 cell culturing media, respectively (Fig. 4). Similarly, Sohi (1980), Hink (1982), and Kurtti and Munderloh (1984) reported that media pH between 6 and 7 is required to culture insect cells. Hild et al. (1992) found maximum cell densities for Sf9 cells between pH range 6.2 and 6.4, which is recommended in the cell culture guideline by Invitrogen (www.invitrogen.com) depending on the cell type.
RPW-1 cell culture was used for the screening of insecticides and revealed that emamectin benzoate (a novel insecticide of avermectin group) was the most toxic against these cells based on the cell mortality and growth inhibition assays. Insecticides from carbamate group (carbofuran, sevin) and organophosphorus group (dursban) were also toxic, but there was no significant difference among LC50 values of these insecticides. Insecticides from other groups—fipronil, chlorfenapyr, bifenthrin, abamectin, α-cypermethrin, and dieldrin—were significantly different with gradually decreasing toxicity and effectiveness against RPW-1 cells. Cell mortality percentage varied with different insecticides, the highest with emamectin benzoate (92%) and lowest cell mortality with dieldrin (19%) (Table 1). The LC50 value was found lowest for emamectin benzoate (2.5 ppm) and highest for dieldrin (87.7 ppm). The impact of insecticides on insect cells has been reported many times. Yoon et al. (2001) reported on DNA damage, morphological changes, and onset of apoptosis in CHL cells of Cricetulus griseus, after treatment with carbofuran. Nandi et al. (2006) noticed less toxicity of bifenthrin to PC12 cells as compared to other pyrethroid insecticides. Sonoda and Tsumuki (2007) reported that chlorfenapyr induced the concentration and time-dependent stress on the Spodoptera exigua cells, which lead to the induction of heat shock proteins. Cell proliferation assays revealed that maximum growth inhibition rate of viable RPW-1 cells (74%) occurred at 500 ppm with emamectin benzoate, after 24 h of treatment (Fig. 5). Zhong et al. (2008) also reported on the cytotoxicity and cell growth inhibition of about 20 chemical insecticides, botanical insecticides, and microbial pesticides on S. frugiperda (Sf9) cells and Spodoptera litura Sl-1 cells. Huang et al. (2011b) also observed the inhibition of Sf9 cell proliferation after treatment with avermectin, whereas Decombel et al. (2004) reported that pyridaben caused the highest cell proliferation inhibitory effect on S. exigua cell lines (BCIRI/AMCY-SeE-CLG1 (Se1), BCIRL/AMCY-SeE-CLG4 (Se4), and BCIRL/AMCY-SeE-CLG5 (Se5)) out of 13 other insecticides.
Although some insecticides used in the study such as dieldrin and carbofuran are banned in Canada and European Union for environmental and resistance issues, these insecticides are still applied in many Asian countries for pest control. Studies based on the cell lines are not only useful for screening potential insecticides but also for elucidating their mode of action. The cytotoxicity of synthetic insecticides has been reported by a number of researchers (Yoon et al. 2001; Decombel et al. 2004; Saito et al. 2005; Huang et al. 2011a), but the question still remains as to why cultured cells are more sensitive to toxins than insects in the field. The heightened sensitivity of cells to toxins may be due to their lack of a secondary barrier (such as the peritrophic membrane in lepidopteran midguts) and their absence of complete detoxification pathways which are present in whole insects.
This study supports the previous findings that demonstrate the development of insect cell cultures, especially for serious agricultural pests, like R. ferrugineus, provide a valuable research tool for molecular studies, examination of pathogens for biological control agents, and the examination of insecticide resistance (Grasela et al 2012).
References
Al-Ayedh H. Y. Evaluating a semi-synthetic diet for rearing the red palm weevil Rynchophorus ferrugineus (Coleoptera: Curculionidae). Int. J. Trop. Insect. Sci. 31: 20–28; 2011.
Alford D. V. Pest and disease management handbook. British crop protection enterprises. Blackwell Science Ltd, Oxford, p 615; 2000.
Casartelli M.; Cermenati G.; Rodighiero S.; Pennacchio F.; Giordana B. A megalin like receptor is involved in protein endocytosis in the midgut of an insect (Bombyx mori, Lepidoptera). Am. J. Physiol. 295: 1290–1300; 2008.
Cermenati G.; Corti P.; Caccia S.; Giordana B.; Casartelli M. A morphological and functional characterization of Bombyx mori larval midgut cells in culture. Invert. Surv. J. 4: 119–126; 2007.
Corley L. S.; Lavine M. D. A review of insec stem cell types. Semin. Cell. Dev. Biol. 17: 510–517; 2006.
Decombel L.; Smagghe G.; Tirry L. Action of major insecticide groups on insect cell lines of the beet armyworm, Spodoptera exigua, compared with larvicidal toxicity. In. Vitro. Cell. Dev. Biol. -Animal. 40: 43–51; 2004.
Faleiro J. R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect. Sci. 26: 135–154; 2006.
Grasela J. J.; McIntosh A. H.; Ringbauer J. J.; Goodman C. L.; Carpenter J. E.; Popham H. J. Development of cell lines from the cactophagous insect: Cactoblastis cactorum (Lepidoptera: Pyralidae) and their susceptibility to three baculoviruses. In. Vitro. Cell Dev. Biol. - Animal. 48: 293–300; 2012.
Grimm C.; Schmidli H.; Bakker F.; Brown K.; Campbell P.; Candol W. M.; Chapman P.; Harrison E. G.; Mead-Briggs M.; Schmuck R.; Ufer A. Use of standard toxicity tests with Typhlodromus pyri and Aphidius rhopalosiphi to establish a dose–response relationship. J. Pest. Sci. 74: 72–84; 2001.
Hakim R. S.; Baldwin K. M.; Loeb M. The role of stem cells in midgut growth and regeneration. In. Vitro. Cell Dev. Biol. - Animal. 37: 338–42; 2001.
Hakim R. S.; Blackburn M. B.; Corti P.; Gelman D. B.; Goodman C.; Elsen K.; Loeb M. J.; Lynn D.; Soin T.; Smagghe G. Growth and mitogenic effects of arylphorin in vivo and in vitro. Arch. Insect. Biochem. Physiol. 64: 63–73; 2007.
Hakim R. S.; Cacci S.; Loeb M.; Smagghe G. Primary culture of insect midgut cells. In. Vitro. Cell Dev. Biol. - Animal. 45: 106–110; 2009.
Hall G. M.; Tittiger C.; Andrews G. L.; Mastick G. S.; Kuenzli M.; Luo X.; Seybold S. J.; Blomquist G. J. Midgut tissue of male pine engraver lps pini, synthesizes mono terpenoid pheromone component ipsdienol de novo. Naturwissenschaflen. 89: 79–83; 2002a.
Hall G. M.; Tittiger C.; Btomquist G. J.; Andrews G. B.; Mastick G. S.; Barkawi L. S.; Bengoa C.; Seybold S. J. Male Jeffrey pine beetle, Dendroctonus jeffreyi, synthesizes the pheromone component frontalin in anterior midgut tissue. Insect. Biochem. Mol. Biol. 32: 1525–1532; 2002b.
Hild H. M.; Emery A. N.; AL-Rubeat M. The effect of pH, temperature, serum concentration and media composition on the growth of insect cells. In: Vlak J. M.; Schlaeger E. J.; Bernard A. R. (eds) Baculovirus and recombinant protein production processes. Editiones Roche, Basel, pp 316–321; 1992.
Hink W. F. Semi-continuous culture of the TN-368 cell line in fermenters with virus production in harvested cells. In: Kurstak E.; Maramorosch K.; Dubendrofer A. (eds) Invertebrate System In Vitro. Elsevier, Holland, pp 27–33; 1982.
Huang J. F.; Shui K. J.; Li H. Y.; Hu M. Y.; Zhong G. H. Antiproliferative effect of azadirachtin A on Spodoptera litura Sl-1 cell line through cell cycle arrest and apoptosis induced by up-regulation of p53. Pestic. Biochem. Physiol. 99: 16–24; 2011a.
Huang J. F.; Tian M.; Lv C. J.; Li H. Y.; Muhammad R.; Zhong G. H. Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic. Biochem. Physiol. 100: 256–263; 2011b.
Kurtti T. J.; Munderloh U. G. Mosquito cell culture. In: Maramorosch K. (ed) Advances in cell culture, vol. 3. Academic, New York, p 259; 1984.
Li L.; Xie T. Stem cell niche: structure and function. Annu. Rev. Cell. Dev. Biol. 21: 605–31; 2005.
Loeb M. J.; Clark E. A.; Blackburn M.; Hakim R. Z.; Elsen K.; Smagghe G. Stem cells from midgets of lepidopteran larvae: clues to the regulation of stem cell fate. Arch. Insect. Biochem. Physiol. 53: 186–98; 2003.
Lynn D. E. Novel techniques to establish new insect cell lines. In. Vitro. Cell. Dev. Biol. -Animal. 37: 319–321; 2001.
Mahmoud M. A.; Saad A. A.; Ibrahim A. B. In vivo toxicity of Beta-cyfluthrin insecticide against the red palm weevil Rhynchophorus ferrugineus (Olivier). J. Agr. Sci. Tech. A. 2: 1322–1331; 2012.
Nandi A.; Chandi D.; Lechesa R.; Pryor S. C.; Mclaughlin A.; Bonventre J. A.; Flynn K.; Weeks B. S. Bifenthrin causes neurite retraction in the absence of cell death: a model for pesticide associated neurodegeneration. Med. Sci. Monit. 12: 169–173; 2006.
Nardi J. B.; Young A. G.; Ujhelyi E.; Tittiger C.; Lehane M. J.; Blomquist G. J. Specialization of midgut cells for synthesis of male isoprenoid pheromone components in two scolytid beetles, Dendroctonus jeffreyi and Ips pini. Tissue. Cell. 34: 221–231; 2002.
Oh H.; Livingston R.; Smith K.; Abrishamian G. L. Comparative study of the time dependency of cell death assays. M.U.R.J. 11: 53–62; 2004.
Saito S.; Sakamoto N.; Umeda K. Effects of pyridalyl, a novel insecticidal agent, on cultured Sf9 cells. J. Pestic. Sci. 30: 17–21; 2005.
SAS. SAS user’s guide: statistics. SAS Institute, Cary; 2000.
Shao H. C.; Hong L. S.; Zuo H. L. Effect of temperature oscillation on insect cell growth and baculovirus replication. Appl. Environ. Microbiol. 64: 2237–2239; 1998.
Smagghe G.; Goodman C. L.; Stanley D. Insect cell culture and applications to research and pest management. In. Vitro. Cell Dev. Biol. - Animal. 45: 93–105; 2009.
Smagghe G.; Vanhassel W.; Moeremans C.; de Wilde D.; Goto S.; Loeb M. J.; Blackburn M. B.; Hakim R. S. Stimulation of midgut stem cell proliferation and differentiation by insect hormones and peptides. Ann. NY. Acad. Sci. 1040: 472–475; 2005.
Sohi S. S. The effect of pH and osmotic pressure on the growth and survival of three lepidopteran cell lines. In: Kurstak E.; Maramorosch K.; Dubendorfer A. (eds) Invertebrate systems in vitro. Elsevier, North Holland, pp 35–43; 1980.
Sonoda S.; Tsumuki H. Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic. Biochem. Physiol. 89: 185–189; 2007.
Ware G. W.; Nigg H. N.; Doerge D. R. Reviews of environmental contamination and toxicology, vol. 176. Springer, New York, p 184; 2003.
Yoon J. Y.; Oh S. H.; Yoo S. M.; Lee S. J.; Lee H. S.; Choi S. J.; Moon C. K.; Lee B. H. N-Nitrosocarbofuran, but carbofuran, induces apoptosis and cell cycle arrest in CHL cells. Toxicology. 169: 153–161; 2001.
Young H. E.; Black Jr. A. C. Adult stem cells. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 276: 75–102; 2004.
Zhong G. H.; Shui K. J.; Huang J. F.; Jia J. W.; Hu M. Y. Induction of apoptosis by botanical components in Spodoptera litura cultured cell line. Acta. Entomol. Sin. 51: 449–453; 2008.
Acknowledgments
The authors gratefully acknowledge the role of King Abdul Aziz City of Science and Technology (KACST) for providing research funding under the grant no. 08-BIO 10-6 to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Ahmed Mohammed Aljabr and Muhammad Rizwan-ul-Haq equally participated in the research work.
Rights and permissions
About this article
Cite this article
Aljabr, A.M., Rizwan-ul-Haq, M., Hussain, A. et al. Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. In Vitro Cell.Dev.Biol.-Animal 50, 296–303 (2014). https://doi.org/10.1007/s11626-013-9694-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-013-9694-1