Abstract
Building on earlier research, insect cell culture began with the successful establishment of one cell line from pupal ovarian tissue. The field has grown to the extent that now over 500 insect cell lines have been established from many insect species representing numerous insect orders and from several different tissue sources. These cell lines are used as research tools in virology, in studies of signaling mechanisms to study insect immunity, hemocyte migration, and to test hypotheses about gene expression, and in screening programs designed to discover new insecticide chemistries. Virology research is revealing fundamentally new information on virus/host cell interactions. Studies in gene expression are uncovering signal transduction pathways that are new to insect science. Research is leading to the development of high-speed screening technologies that are essential in the search for new insect pest management tools. A few insect cell lines are, in routine industrial processes, designed to produce proteins of biomedical significance. Both primary cell cultures and established lines are used in basic biological studies to reveal how insect cells work. This review is designed to briefly cover the history of insect cell culture, recount some recent advances in the field, and offer a vision of the future of insect cell culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last quarter-century of research into insect cell culture has been a period of tremendous growth. The number of established insect cell lines has expanded, along with the number of tissue sources. The utility of insect cell lines for protein production has grown from laboratory-scale experimental work to industrial applications (Elias et al. 2007). Use of cell cultures for production of bioinsecticidal viruses has also advanced. Research in insect cell cultures is now revealing new fundamental information on how insect cells function (Bryant et al. 2008; Soin et al. 2008; Stanley et al. 2008). At first sight, such growth in a field may be taken as a sign that the field has matured. Certainly, the art and science of insect cell culture have developed at impressive rates, yet we would say the field remains in a very early stage of its potential development. While a diversity of insect cell lines has been established (>500; Lynn 2007), creating some of the most interesting lines, such as from endocrine glands or neurons, still remains elusive. A few insect cell lines are now standard industrial tools, yet the potential of other established lines has not yet been fully exploited. While cell lines are now yielding new insights into basic cellular processes, other important processes, such as the mechanisms of cellular resistance to baculovirus replication, still provides important frontiers of our knowledge. In our view, the field of insect cell culture is poised for very rapid expansion into many areas of biology, including immunity, endocrinology, toxicology, biochemistry, and evolution. Our goals in this review article are to briefly recount the history of insect cell culture, to review some of the recent advances in applying cell cultures as working models for research in signaling mechanisms and in applied entomology, and to offer a vision of the future of the field. Perhaps our overarching goal is to persuade our colleagues of the promises and challenges of this rapidly moving field. This review is necessarily selective in literature sources because insect cell culture is successful to the extent that the entire field cannot be captured in a single review, or indeed, in a single volume. In particular, the literature on baculoviruses has been treated extensively in recent reviews (Clem 2007; Condreay and Kost 2007; Elias et al. 2007; Lynn 2007). Except to provide context, baculoviruses are not treated in this chapter.
Brief History of Insect Cell Culture
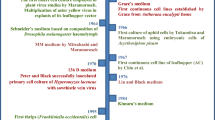
Day and Grace (1959) outlined the history of insect cell culture, to that date, divided into three phases. The first phase involved work primarily on gametogenesis with the tissues cultured in hemolymph and simple salt solutions (Glaser 1917). Mitosis was rarely noted in these studies and cultures usually did not survive beyond several wk. The second phase emphasized culture medium development and refinement, although researchers generally looked to vertebrate tissue composition for their work. These cell cultures did not survive beyond 3 mo; nonetheless, these short periods allowed progress toward the propagation of viruses in cultured cells (Trager 1953). The third phase was considered a breakthrough because of its emphasis on developing media based on insect tissue chemistry. This phase led directly to the establishment of an insect cell line from pupal ovarian tissues (Grace 1962).
Unbeknownst to Grace and his colleagues, a similar breakthrough in insect cell culture occurred a few years earlier in China (Gaw 1958; Vlak 2007). Gao and colleagues used a variety of silk moth tissues and showed the cultivation of virus in these cultures (Gaw 1958; Liu et al. 1959; Vlak 2007). Gao’s and Grace’s successes were followed by the establishment of numerous cell lines from lepidopteran, dipteran, orthopteran, and coleopteran insects, all reviewed elsewhere (Li and Bonning 2007; Lynn 2007; Vlak 2007).
Insect Cell Lines are Tools in Virology
Insect cell lines are important tools in many aspects of virus-related research, including viral propagation and optimization for the development of viral pesticides (Li and Bonning 2007; Lynn 2007). Cell lines play key roles in the study of virus–cell interactions (Gundersen-Rindal and Dougherty 2000; Mudiganti et al. 2006; Schütz and Sarnow 2006; Lennan et al. 2007) and in the production of recombinant proteins/vaccines (Condreay and Kost 2007). Furthermore, health-related products are generated by insect cell lines using vectors developed from insect viruses, notably baculoviruses (with the major genus being the nucleopolyhedrosis viruses [NPVs]; Condreay and Kost 2007). In this review, we focus on the use of insect cells for the propagation of viruses as well as investigating their interactions with host cells. We will draw attention to studies involving non-NPV insect viruses, arboviruses (arthropod-borne vertebrate viruses), and insect-transmitted plant viruses.
Non-NPV insect viruses may be effective in the management of pest insects, including nonlepidopterans that vector plant pathogens such as the whitefly, a vector of the tomato yellow leaf curl virus (Hunter and Polston 2001; Sinisterra et al. 2005). Many of these viruses are produced in cell cultures as production systems for research into host/virus interactions. Representatives of at least 11 virus families replicate in insect cell lines. A summary of selected examples are displayed in Table 1.
A couple of important points in Table 1 highlight the considerable flexibility within some insect cell line systems. First, some insect viruses can replicate in a variety of insect cell lines. For example, the cricket paralysis virus, isolated from crickets (Orthoptera), and the black beetle virus, isolated from black beetles (Coleoptera), replicate in cell lines derived from several insect species and orders (Christian and Scotti 1996). Second, some viruses replicate in cell lines derived from several insect tissues, such as the insect iridescent virus 6 which replicates in lines established from whole larvae, embryonic tissues, fat body, and hemocytes (Miller et al. 1994; Belloncik et al. 2007). This flexibility points to areas of insect cell/virus interactions which are not yet fully understood.
Studies on non-NPV virus replication in insect cell lines are leading to improved understanding of insect virus/host cell interactions, such as those involved in persistent infections (Funk et al. 2001; Sinisterra et al. 2005). Insights from these studies fall into a number of areas, including virus genomics, structure, replication, and assembly. Recent studies have often identified useful model systems with valuable results pending. For example, the nonoccluded virus Hz-1 was discovered in the IMC-Hz1 cell line and, like Hz-2V, replicates only in cell lines of lepidopteran origin (McIntosh et al. 2007). Hz-1 is the first virus shown to infect insect cells in both a productive and persistent manner. Therefore, this virus will serve as a useful tool for deciphering the different mechanisms underlying these two infection strategies. Similarly, the Providence virus (PrV) was discovered in a midgut cell line and is the first known tetravirus able to replicate in insect cell lines. This discovery has lead to a detailed study of the virus genome, structure, and life cycle (Pringle et al. 2003). Genomic results indicate that PrV is similar to viruses in the Betatetravirus genus, whereas structural proteins suggest closer homology to the Omegatetravirus genus. Future studies using this system to develop a reverse genetic system are planned. Two other persistent viruses in lepidopteran cell lines are Galleria mellonella cell line virus (GmCLV) and Perina nuda picorna-like virus. GmCLV replication was induced by infection of Gm120 cells with an unrelated picorna-like virus as well as Autographa californica multiple nucleopolyhedrovirus (AcMNPV; Lery et al. 1997). Upon the induction of GmCLV, neither the picorna-like virus nor AcMNPV replicated. This persistently infected cell line will be used to elucidate the interplay between the host and virus genomes that results in the induction of latent viruses, an important consideration for viral biocontrol agents. Turning to dipteran cell lines, two persistent viruses, Aedes pseudoscutellaris reovirus (APRV) and C6/36 densovirus, were isolated from mosquito cell lines. APRV replicated in a variety of mosquito cell lines (Attoui et al. 2005). Studies of C6/36 densovirus have generally emphasized its structural characterization (Chen et al. 2004). This virus, as well as some of the other persistent viruses, is structurally unique (e.g., novel outer surface morphology and structural proteins) and is still undergoing classification. In Hemiptera, there have been advances in leafhopper cell culture development (Kamita et al. 2005), which supports the replication of ssRNA dicistroviruses (Boyapalle et al. 2006; Hunnicutt et al. 2006; Hunter et al. 2006) and the development of the first psyllid cell cultures, Diaphorina citri (Marutani-Hert et al. 2009), which has yielded a new dsRNA reovirus, as well as is the first such culture within hemipterans to be used to culture endosymbiotic bacteria. Lastly, polydnaviruses produce persistent infections with their DNA being incorporated into the host cell genome (Gundersen-Rindal and Dougherty 2000; Gundersen-Rindal and Lynn 2003). Interest in polydnavirus cell culture systems is related to their potential as vehicles for transforming insect cell lines for the expression of selected proteins. Their main advantage over the NPV expression systems is that they do not stimulate cell lysis. Thus, persistent virus studies using insect cell lines have produced, and will continue to produce, insights into virus/host strategies as well as practical innovations for a broad range of applications.
Many vertebrate and plant viruses replicate in insect cell lines. These generally involve lines developed from the insect vector species which are permissive hosts during part of the virus transmission cycle. Vertebrate viruses from a number of different families, including the Flaviviridae, Reoviridae, Rhabdoviridae, and Togaviridae (especially the Sindbis virus), have been propagated in dipteran cells and have lead to insights into viral entry and replication processes, their effects on cell cycles, host specificity/selection, and mechanisms of persistent infections. For example, Tan et al. (2001) showed that core proteins of the blue tongue reovirus play a part in viral attachment to mosquito cells, distinguishing viral interplay with vector cells from that with mammalian host cells. Additionally, recent work by Mudiganti et al. (2006) has indicated that a Drosophila cell line (drawing upon the well-defined genetics of this organism) can be used as an important tool for understanding Sindbis virus infections. Furthermore, a study on rhabdovirus replication in sandfly cells (LL-5, derived from their vector) and mosquito cells (C6/36) has shown that this virus can rapidly adapt to host cells (Llewellyn et al. 2002). Molecular techniques were used in this study to elucidate the mechanism(s) behind adaptation, although no firm conclusions were drawn at that time. In addition to animal viruses, many plant viruses proliferate in insect cell lines (Creamer 1993; Sinisterra et al. 2005) and have lead to enhanced understanding of viral processes. For example, a study of the rice dwarf virus, a phytoreovirus, involving the lepidopteran cell line Sf-9, showed that a viral capsid protein induces membrane fusion in insect host cells (Zhou et al. 2007), a key step in viral entry and replication. Thus, insect cell lines are important in work involving insect pathogens as well as plant and vertebrate viruses vectored by insects.
Research with insect cell lines has also helped elucidate antiviral strategies of host cells and the counterstrategies evolved by their viral pathogens (Schütz and Sarnow 2006; Weaver 2006). Some insects have evolved apoptosis as a method of clearing viruses, whereas viruses have evolved specific apoptosis inhibitors (Clem 2007; Bryant et al. 2008). RNA interference (RNAi) is another example of coevolution between viruses and host cells (Fritz et al. 2006; Schütz and Sarnow 2006). Hoa et al. (2003) showed that the main components in the RNAi pathway were present in a mosquito cell line. The RNAi pathway acts in the inhibition of both arbovirus and insect virus replication in insect cells (Keene et al. 2004; Sanchez-Vargas et al. 2004; Van Rij et al. 2006). Some viruses circumvent the host RNAi defense system (Sanchez-Vargas 2004; Garcia et al. 2006; Hemmes et al. 2007) by rearranging host cell membranes to “hide” their replicative intermediates from host defenses and by expressing viral-encoded suppressors of components in the RNAi pathway.
Insect Cells as Models for Study of Signal Mechanisms
Research into the signaling mechanisms responsible for mediating and coordinating complex physiological actions is one of the active frontiers in insect biology. Some of the research in this area has revealed signaling systems that at the time were new to science and had far-reaching implications in other areas, including biomedicine. For example, discovery of the Toll receptor in a model insect was very quickly followed by finding Toll-like receptors in mammalian immune systems (Lemaitre and Hoffmann 2007). Prostaglandins and other eicosanoids represent another important signaling system responsible for mediating insect cellular immune reactions to immune challenge. In this section, we highlight research with primary hemocyte cultures and with an established insect cell line to generate new knowledge of eicosanoid actions.
Using primary hemocyte cultures to study migration. Migration is a fundamental property of prokaryotic and eukaryotic cells (Baker et al. 2006; Jin and Hereld 2006) and is an essential process in development and immune reactions. Insects express innate (but not adaptive) immune reactions to microbial, parasitic, and wound challenges. While the distinction is somewhat artificial, two broad categories of immune reactions are recognized, humoral and hemocytic (also called cellular). Hemocytic reactions are characterized by direct interactions between host hemocytes and foreign invaders. Specific cellular defense reactions include phagocytosis, microaggregation, nodulation, and in the case of larger invaders, encapsulation (Lavine and Strand 2002; Stanley and Miller 2006). All of these cellular actions necessarily entail cell migration.
While cellular immune reactions are well-described, far less information is available on the biochemical signaling systems responsible for mediating and coordinating them (Gillespie et al. 1997; Stanley 2006). We suggested that eicosanoids mediate insect cellular immune reactions (Miller et al. 1994; Stanley 2006), which became the basis for two lines of research. One, experiments with a broad sampling of insect taxa and developmental stages support the idea that eicosanoids mediate hemocytic immune reactions in all insect species that express cellular immunity (some do not, such as foraging honeybees; Schmidt et al. 2008). Two, experiments with various species of bacteria, fungi, parasitoids, protozoans, and viruses indicate that eicosanoids act in immune protection against a phylogenetically wide range of challengers (Stanley 2006; Stanley and Miller 2006; Durmus et al. 2007, Stanley and Shapiro 2007). The outcomes of experiments by several research groups indicate that eicosanoids are crucial mediators of phagocytosis, microaggregation, cell spreading, and nodulation reactions (Stanley and Miller 2006). Eicosanoids also mediate whole-animal behavioral fever reactions to infections in locusts, Schistocerca gregaria, and likely in other insect species as well (Bundey et al. 2003).
We hypothesized that insect hemocytes are able to detect and migrate toward a source of N-formyl-Met-Leu-Phe (fMLP), the major chemotactic peptide from Escherichia coli and that pharmaceutical modulation of eicosanoid biosynthesis inhibits hemocyte migration. We used primary hemocyte cultures prepared from tobacco hornworms, Manduca sexta, to test our hypothesis (Merchant et al. 2008). Hemocyte migration assays were performed in a Boyden chamber (Boyden 1962). The outcomes of our experiments revealed three new findings. One, primary hemocyte cultures undergo directed, diapeditic movement through pores. Two, hemocytes respond to fMLP, a bacterial product that stimulates migration in various types of mammalian cells. Three, eicosanoids are key signal moieties in this process. More important to this review, this work demonstrates the usefulness of primary hemocyte cultures in investigations of the signal mechanisms responsible for mediating and coordinating hemocyte immune behaviors.
Insect cell cultures in testing hypotheses about gene expression. While the work on immunity and hemocyte migration treated above implicates prostaglandins (PGs) and other eicosanoids in insect cellular immunity, there is virtually no information on the cellular mechanisms of PG action in insect cells. However, a large amount of literature on the mechanisms of PG actions in mammalian cells exists (Negishi and Katoh 2002), most of which are mediated by means of G protein-coupled (GPCR), rhodopsin-class receptors. The cellular actions driven by these receptors can lead to short-term changes in homeostatic physiology, such as induction of fever seen in mammals and insects (Bundey et al. 2003). PGs also influence gene expression in many mammalian systems. For a single example, PGE2 stimulates the expression of the prolactin gene in the leukemic cell line Jurkat (Gerlo et al. 2004). Overall, PGs can influence immediate cell events regulating homeostasis and long-term changes in gene expression in mammalian cells.
We tested the hypothesis that one mode of PG action in insect cells is their influence on gene expression. We treated an established cell line (BCIRL-HzAM1) with PGA1 and, separately, with PGE1. Relative to controls, these treatments substantially altered the expression of genes encoding a range of proteins (Stanley et al. 2008). In particular, expression of a heat shock protein was upregulated 17-fold by PGE1. Again, for our purposes, the main point is that established insect cell lines are excellent model systems for testing hypotheses about signal transduction mechanisms.
Insect Cell Cultures in Screening for Novel Insecticide Activities, Receptors, and Ligands in Pest Control
To date, an average of ∼10 billion US dollars is spent per year for synthetic insecticides to control agriculturally and medically important insect pests (Beckmann and Haack 2003). The problems associated with these general toxicants, including insect resistance and environmental concerns, have encouraged the development of more insect-specific insecticide screening procedures. The suitability of such strategies is demonstrated by various insect growth regulators that impair endocrine regulation of growth, molting/metamorphosis, and reproduction (Dhadialla et al. 2005). Successes include juvenile hormone analogs (JHAs) (e.g., methoprene, fenoxycarb, and pyriproxyfen), ecdysteroid receptor agonists or molting accelerating compounds (e.g., tebufenozide, methoxyfenozide), and chitin synthesis inhibitors (e.g., benzoylphenylurea). Additionally, there are newer synthetic insecticides that act specifically on insect nervous systems, energy metabolism, and muscle targets (e.g., neonicotinoids, spinosyns, avermectins). In our view, discovering and deploying these modern anti-insect chemistries underscores the potential power of the approach. Nonetheless, this is limited by contemporary screening procedures.
For screening purposes, there is increasing interest in the development of in vitro methods to replace conventional animal toxicity tests. The ultimate goal is to achieve an alternative system that allows for rapid testing of candidate compounds, formulations, and finished products and enables the accurate prediction of toxic efficacy at the whole-animal level. There are certain key requirements in developing an alternative cell-based testing procedure. These include dependable intralaboratory and interlaboratory reproducibility, high predictive power for correct toxicity assessment decisions, relevance to the type of compounds to be tested, and low cost/benefit ratio. Because of the very large inventory of natural and synthetic chemicals potentially useful in insect pest management programs, the ideal systems would be amenable to high-throughput screening technology (HTS). HTS is widely used in private sector drug discovery programs in which thousands of potential pharmaceuticals are screened for desired biological activity on a daily basis. Established insect cell lines, joined with HTS procedures, will contribute to rapid screening of many materials. Tests employing cell cultures can be readily automated. Cell-based assays can enable the discovery of new modes of action for insecticide candidates.
Insect endocrine targets. The concept of interfering with insect endocrine systems as a selective mechanism to manage pest insects was proposed by Williams (1967) who articulated the idea of “third generation pesticides.” The major insect hormones, the terpenoid juvenile hormones (JH) and the steroid molting hormones or ecdysteroids, were the first targets.
Screening on cellular proliferation and differentiation. A large number of insect cell lines from different species and tissue origins respond to ecdysteroids. Although cells express ecdysteroid-responsiveness in several different ways, typical cellular responses to ecdysteroids are elongation and aggregation (Courgeon 1972) plus specific morphological effects (Wyss 1982). These typical responses are the criteria normally used to identify ecdysteroid-responsiveness. D. melanogaster BII tumorous blood cells respond to different ecdysteroids with ecdysteroid agonistic or antagonistic activities, providing changes in morphology and cell densities which can be monitored in a microtiter plate reader (Dinan et al. 2001). Table 2 displays a survey of ecdysteroid-responsive cell lines from a variety of insect orders.
The morphological responses of several insect cell lines to ecdysteroids can be partially antagonized by JH and JHAs. Earlier studies (Lezzi and Wyss 1976; Cherbas et al. 1989) showed the modulation of ecdysteroid-induced effects by JH and JHAs in Drosophila, Chironomus, and Plodia cells. More recently, Soin et al. (2008) tested the three best known JHAs, methoprene, fenoxycarb, and pyriproxyfen, with transformed lepidopteran Bm5 and dipteran S2 cells. They all showed ecdysteroid antagonistic activity; however, additional experiments demonstrated a reduced cell viability that can account for the antagonistic responses by the JHAs. The mechanism of JH action is not well-understood at the molecular level. JH appears to have a dual receptor mechanism with both membrane and intracellular receptors (Goodman and Granger 2005). Interfering with JH action will be an attractive option once the JH receptor is characterized. After this accomplishment, insect cell-based HTS assays will then open the possibility of discovering such novel insecticides.
Reporter gene assays with transformed cells for HTS. The need for HTS to discover novel biorational insecticides which interact with the ecdysteroid or other endocrine receptor systems spurred another line of effort. The concept lies in transfecting insect cell lines with reporter constructs to develop rapid and sensitive detection of interactions between potential insecticides and hormone receptors. For the most part, screening systems have commonly been based on morphological observations and/or growth responses of insect cell lines. Because these older approaches require time and careful monitoring, they have limited applicability to analysis in HTS formats. Useful HTS systems have been developed in Kc (dipteran) and Sf-9 (lepidopteran) cells (Mikitani 1995; Toya et al. 2002). Ecdysteroid-acting compounds bind to ecdysteroid receptors and activate the luciferase gene in a dose–response manner. The gene is located in the ecdysteroid-responsive reporter plasmid. Another cell-based HTS system was constructed with the silkworm ovarian cell line Bm5 with a reporter gene construct encoding green fluorescent protein to detect ecdysteroid receptor agonists and antagonists (Fig. 1) (Swevers et al. 2004). The procedure to screen ecdysteroid-active substances consisted of three simple steps readily adaptable to a robotized HTS format: (1) the distribution of transformed cells in microtiter plates, (2) the addition of compounds/extracts at selected concentrations, and (3) the quantification of fluorescence intensity by an automated fluorescence plate reader. Based on this principle, a similar system was developed with a “pBmbA/ERE.luciferase” construct in other lepidopteran, dipteran, and coleopteran cell lines (Mosallanejad et al. 2008b). These cell systems also allow screening for insecticide resistance development (Spindler-Barth and Spindler 1998; Mosallanejad et al. 2008a). These cell-based protocols will facilitate rapid screening and discovery of new anti-insect chemistries.
Assessment of the primary response to 20-hydroxyecdysone (20E) in silk moth-derived Bm5 cells, transformed with the pBmbA/ERE.gfp construct for induction of green fluorescence. (a) Northern blot analysis of 20E primary response gene BmHR3 expression at intervals of administration of 1 μM of 20E. Actin hybridizations were carried out as control. Molecular weight of hybridizing mRNAs is shown at the right. (b) Induction of CAT activity from reporter construct pBmbA/ERE.cat after treatment with different concentrations of 20E (5–500 nM). Expression levels of induced relative to noninduced cells are indicated. Drawing of the reporter construct is at the top. (c) Observation of induction of green fluorescence by 20E under fluorescence microscopy. Untreated and treated transformed Bm5 cells are shown at the left and right, respectively; 040 objective (redrafted from Swevers et al. 2004).
Using HTS cell systems, chemical libraries can be screened and hormone receptor interactions calculated. Recently, three-dimensional quantitative structure–activity relationship (3D-QSAR) tools were used to describe the Bombyx ecdysteroid receptor interactions (Wheelock et al. 2006). These in silico models helped to provide better fundamental insights in new ligand–receptor interactions allowing predictions for enhanced activities and new lead molecules. Three good examples from the recent years are: 3,5-di-tert-butyl-4hydroxy-N-iso-butyl-4-hydroxybenzamide (DTBHIB) (Mikitani 1996), tetrahydroquinoline [1-aryl-4-(arylamino)-1,2,3,4-tetrahydroquinoline], and α-acylaminoketone (Smith et al. 2003; Tice et al. 2003).
Insect specific metabolic pathways—chitin and cuticle synthesis. The use of insect cell lines to study mode of action and screen for inhibitors of chitin synthesis was first suggested nearly 20 yr ago (Spindler-Barth et al. 1989). In addition to organ cultures of imaginal discs and integument epidermis prepared from different insect species, including representatives of Lepidoptera, Coleoptera, and Orthoptera, two cell lines synthesize or degrade at least parts of the cuticle. These are the epithelial cell line of C. tentans (Spindler-Barth et al. 1989) and the IAL-PID2 cell line from P. interpunctella (Oberlander and Silhacek 1998). Interference with chitin synthesis and chitin degradation can be measured conveniently using insect cell lines, and these assays can be supplemented with tests using homogenates of the same tissue (Palli and Retnakaran 1999). Chitin degradation by chitinases and hexosaminidases can then be measured with high sensitivity and specificity using N-acetylglucosamine (GlcNAc) and its oligomers coupled to a fluorogenic dye (McCearth and Gooday 1992). For the future, with the tools of biotechnology, the availability of both cDNA of the chitin-producing proteins and antibody probes, there is now a real possibility of developing cell-based HTS assays for the discovery of new chitin biosynthesis inhibitors.
The feasibility of cell cultures allows investigators to determine QSARs, aiding in the design and synthesis of newer and more active chitin synthesis inhibitory compounds. For example, the introduction of electron-withdrawing and hydrophobic substituents at the para-position of the phenyl (aniline) moiety of benzoylphenylureas enhanced activity, whereas larger groups reduced activity. In vitro activities and in vivo larvicidal toxicities were correlated after separate consideration of the hydrophobic factors participating in absorption and transport in the insect body (Nakagawa et al. 1989). Moving beyond HTS, this work underscores the rational design of insecticidal compounds.
The ability to culture chitin-producing cells also helps improve understanding of the mechanism of newer compounds. For instance, in the chemical class of 2,4-diphenyl-1,3-oxazolines, the mechanism of etoxazole action could be confirmed as chitin inhibitory using epidermis cell cultures derived from Spodoptera frugiperda (Nauen and Smagghe 2006).
In addition to insect cuticle, the peritrophic matrix in insect midgut is closely associated with specific glycoproteins and the covalent binding of chitin to proteins. In this context, insecticidal lectins can be of great interest with respect to pest insect management. Lectins are proteins of nonimmune origin that interact with cells through sugar-specific binding sites (Van Damme et al. 2007). With lepidopteran midgut cell cultures (CF-203), a series of plant lectins with specificity for mannose, galactose, and GlcNAc oligomers demonstrated that the lectin effects are not correlated with the carbohydrate-binding activity (Smagghe et al. 2005a). This concurs with previous experiments which revealed that lectins can elicit a variety of biological activities such as mitosis stimulation, growth inhibition, and apoptosis. However, it should be emphasized that fine specificity of different lectins toward oligosaccharides and glycans can be very different even though they interact with the same monosaccharide. To complete these studies, tagged lectin was used to visualize lectin binding to membrane receptor(s) and cellular internalization (Vandenborre et al. 2008). The use here of cultured midgut cells facilitates the investigation of the glycosylation process in insects and the interaction of lectin with receptor proteins. New knowledge of these insect-specific systems opens the door to HTS and other approaches to the discovery of novel compounds that inhibit these processes.
Insect cell lines—evaluation of Bt insecticidal proteins. Rapid evaluations of the effects of Bt toxins require established midgut epithelium cultures. In the past decade, significant progress has been made in the preparation of primary cultures of midgut insect stem cells. Midgut epithelial cell cultures from lepidopterans and coleopterans have been established and maintained in vitro for periods up to 3–6 mo while preserving their differentiated characteristics (Sadrud-Din et al. 1996; Smagghe et al. 2005b). These primary midgut cell cultures have been applied to study Bt endotoxin binding to the microvilli of intact epithelial cells from different lepidopteran species (Wang and McCarthy 1997; Garcia et al. 2001; Loeb et al. 2001).
Microscopic observations and electrophysiological studies involving the patch clamp technique and fluorescent probes have been used to investigate the action of Bt toxins on insect cells derived from different species and tissues. However, there is an inherent risk of overinterpreting the results from experiments with insect cells (Gringorten 2001). Continuous cell lines assume morphological and physiological characteristics that can differ from the source of primary cultures. Their response to Bt toxins does not often correlate with responses of the insects from which the cells were derived. Established midgut cell lines bear little resemblance to midgut cells in vivo and their susceptibility to toxins correlates poorly with the susceptibility of the host insect. Generally, they are sensitive to fewer toxins than the host insect. On the other hand, primary midgut cell cultures appear to be susceptible to a broader spectrum of Bt toxins than the host insect, a feature that creates a bias toward overrating insecticide activity based on in vitro assays.
Thus, an obvious drawback in the use of insect cell lines for studying toxin effects in vitro and attempting to draw conclusions about mode of action in vivo has been the inability to reproduce the asymmetric environmental conditions of midgut cells in vivo. In the natural anatomical arrangement within the insect body, the midgut cells experience a steep pH gradient with the apical surface of the plasma membrane exposed to a highly alkaline medium and the basal surface exposed to a neutral or slightly acidic medium of the hemolymph. All experiments with cultured insect cells, including midgut cells, are performed at neutral pH conditions to avoid alkaline injury from the solvent alone. Under such conditions, ion channel activity may be quite difficult to interpret.
Although insect cell cultures may be poor indicators of Bt insecticide activity, they are useful for the characterization of the toxin activity spectra and investigation of the membrane-permeabilizing effects, particularly in determining pore size (Knowles and Ellar 1987; Potvin et al. 1998). As with the columnar cells in vivo, cultured insect cells respond to Bt toxin injury by swelling and lysis (Loeb et al. 2001). In a pivotal study with CF-1, midgut cells from neonates of the spruce budworm C. fumiferana, the cytolytic effect of toxin was studied in the presence of neutral solutes with different hydrodynamic radii. This work helped generate a model for membrane pore formation and colloid osmotic lysis to describe the toxin mechanism of action (Knowles and Ellar 1987).
More recently, in the study of the specific role of cadherin receptors in cytotoxicity of Bt toxins and their interactions with cell membrane, a cell-based system was established utilizing H5 insect cells stably expressing BT-R-1, the cadherin receptor for Cry1Ab toxin (Zhang et al. 2006). In this toxin, oligomers in the cell membrane do not produce lytic pores and do not kill insect cells. Rather, the cell death correlates with the binding of the Cry1Ab toxin monomer to BT-R-1, which apparently activates a previously undescribed Mg2+-dependent cellular signaling pathway. This unique cell screening system is of great use as it provides insights into how insects evolve resistance to Bt toxins and allows screening for newer safer insecticides.
Bt in bacterial and toxin formulations, and as expressed in genetically modified crop and fiber plants, is used globally to protect plants from insect damage. Understanding the modes of Bt action and insect mechanisms of Bt resistance is becoming increasingly important. Attempts to use primary and established cell cultures to generate new knowledge and new hypotheses about Bt will continue to draw increasing attention in these efforts.
Perspectives for Insect Cell Lines in the Future
Let us return now to our introductory comments. The field of insect cell culture is poised for very rapid expansion into areas of biology, such as immunity, endocrinology, toxicology, biochemistry, and coevolution. The expansion process is already underway as seen in the foregoing sections. The future holds promise for continued advances as seen in recent works in signal mechanisms, endocrinology and toxicology, and several other aspects of insect cell biology. The greatest potential relies on our ability to translate this new knowledge into completely novel products and strategies that can be applied to serious insect problems. Work in this area combines advances in insect cell culture with modern tools of biotechnology. We anticipate a future in which the way classical insecticides are used will diminish, as new possibilities are developed. These include exploitation of newly discovered receptors and pathways such as the eicosanoid system in insect immunity along with the new understanding of other critical systems, such as chitin metabolism. Advances in insect science through cell culture systems will be part of the modern vanguard of agricultural science necessary for safer production of healthier foods to meet the demands of a rapidly increasing human population.
References
Attoui, H.; Jaafar, F. M.; Belhouchet, M.; Biagini, P.; Cantaloube, J.-F.; de Micco, P.; de Lamballerie, X. Expansion of family Reoviridae to include nine-segmented dsRNA viruses: isolation and characterization of a new virus designated Aedes pseudoscutellaris reovirus assigned to a proposed genus (Dinovernavirus). Virology 343: 212–223; 2005.
Baker, M. D.; Wolanin, P. M.; Stock, J. B. Systems biology of bacterial chemotaxis. Curr. Opin. Microbiol 9: 187–192; 2006.
Beckmann, M.; Haack, K. J. Chemical pest control—insecticides for agriculture. Chem. Unserer Zeit 37: 88–97; 2003.
Belloncik, S.; Petcharawan, O.; Couillard, M.; Charpentier, G.; Larue, B.; Guardado, H.; Charaeonsak, S.; Imanishi, S. Development and characterization of a continuous cell line, AFKM-On-H, from hemocytes of the European corn borer Ostrinia nubilalis (Hübner) (Lepidoptera, Pyralidae). In Vitro Cell. Dev. Biol. Anim 43: 245–254; 2007.
Berger, E.; Ringler, R.; Alahiotis, S.; Frank, M. Ecdysone-induced changes in morphology and protein synthesis in Drosophila cell cultures. Dev. Biol 62: 498–511; 1978.
Berger, E.; Wyss, C. Acetylcholinesterase induction by β-ecdysone in Drosophila cell lines and their hybrids. Somatic Cell Genet 6: 631–640; 1980.
Boyapalle, S.; Pal, N.; Miller, W. A.; Bonning, B. C. A glassy-winged sharpshooter cell line supports replication of Rhopalosiphum padi virus (Dicistroviridae). J. Invertebr. Pathol 94: 130–139; 2006.
Boyden, S. The chemotacxtic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J. Exp. Med 115: 453–466; 1962.
Bryant, B.; Blair, C. D.; Olson, K. E.; Clem, R. J. Annotation and expression profiling of apoptosis-related genes in the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol 38: 331–345; 2008.
Bundey, S.; Raymond, S.; Dean, P.; Roberts, S. K.; Dillon, R. J.; Charnley, A. K. Eicosanoid involvement in the regulation of behavioral fever in the desert locust, Schistocerca gregaria. Arch. Insect Biochem. Physiol 52: 183–192; 2003.
Chen, S.; Cheng, L.; Zhang, Q.; Lin, W.; Lu, X.; Brannan, J.; Zhou, Z. H.; Zhang, J. Genetic, biochemical, and structural characterization of a new densovirus isolated from a chronically infected Aedes albopictus C6/36 cell line. Virology 318: 123–133; 2004.
Cherbas, L.; Koehler, M. D.; Cherbas, P. Effects of juvenile hormone on the ecdysone response of Drosophila Kc cells. Dev. Genet 10: 177–188; 1989.
Christian, P. D.; Scotti, P. D.; Biopesticides from small RNA viruses of insects: aspects of their in vitro production. In: Maramorosch K.; Loeb M. J. (eds) Invertebrate cell culture: looking toward the twenty first century. Proceedings of the IX International Conference on Invertebrate Cell Culture. Society for In Vitro Biology, San Francisco, pp 73–81; 1996.
Clem, R. J. Baculoviruses and apoptosis: a diversity of genes and responses. Curr. Drug Targets 8: 1069–1074; 2007.
Condreay, J. P.; Kost, T. A. Baculovirus expression vectors for insect and mammalian cells. Curr. Drug Targets 8: 1126–1131; 2007.
Courgeon, A. M. Action of insect hormones at the cellular level. Morphological changes of a diploid cell line of Drosophila melanogaster. Exp. Cell Res 74: 327–336; 1972.
Creamer, R. Invertebrate tissue cultures as a tool to study insect transmission of plant viruses. In Vitro Cell. Dev. Biol. Anim 29: 284–288; 1993.
Day, M. F.; Grace, T. D. C. Cultures of insect tissues. Annu. Rev. Entomol 4: 17–38; 1959.
Decombel, L.; Tirry, L.; Smagghe, G. Action of 24-epibrassinolide on cell line of the beet armyworm, Spodoptera exigua. Arch. Insect Biochem. Physiol 58: 145–156; 2005.
Dhadialla, T. S.; Retnakaran, A.; Smagghe, G. Insect growth and development disrupting insecticides. In: Gilbert L. I.; Iatrou K.; Gill S. (eds) Comprehensive insect molecular science. 6: Pergamon, New York, pp 55–116; 2005.
Dinan, L. Ecdysteroid receptors in a tumorous blood cell line of Drosophila melanogaster. Arch. Insect Biochem. Physiol 2: 295–317; 1985.
Dinan, L.; Bourne, P. C.; Meng, Y.; Sarker, S. D.; Tolentino, R. B.; Whithing, P. Assessment of natural products in the Drosophila melanogaster BII cell bioassay for ecdysteroid agonist and antagonist activities. Cell. Mol. Life Sci 58: 321–342; 2001.
Dübendorfer, A.; Liebig, B. Cell differentiation in vitro and establishment of permanent ecdysone-responsive cell lines from embryonic tissues of the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol 38: 397–407; 1992.
Durmus, Y.; Büyükgüzel, E.; Terzi, B.; Tunaz, H.; Stanley, D.; Büyükgüzel, K. Eicosanoids mediate melatonic nodulation reactions to viral infection in larvae of the parasitic wasp, Pimpla turioinellae. J. Insect Physiol 54: 17–24; 2007.
Eide, P. E.; Caldwell, J. M.; Marks, E. P. Establishment of two cell lines from embryonic tissue of the tobacco hornworm, Manduca sexta (L.). In Vitro 11: 395–399; 1975.
Elias, C. B.; Jardin, B.; Kamen, A. Recombinant protein production in large-scale agitated bioreactors using the baculovirus expression vector system. In: Murhammer D. W. (ed) Methods in molecular biology series. Baculovirus and insect cell expression protocols. Springer, New York, pp. 225–245; 2007.
Fritz, J. H.; Girardin, S. E.; Philpott, D. J. Innate immune defense through RNA interference. Sci. STKE 13: 1–4; 2006.
Funk, C. J.; Hunter, W. B.; Achor, D. S. Replication of insect iridescent virus 6 in a whitefly cell line. J. Invertebr. Pathol 77: 144–146; 2001.
Granados, R. R.; Naughton, M. Replication of Amscata moorei entomopoxvirus and Autographa californica nuclear polyhedrosis virus in hemocyte cell lines from Estigmene acrea. In: Kurstak E.; Maramorosch K. (eds) Invertebrate tissue culture. Applications in medicine, biology, and agriculture. Academic, New York, pp 379–389; 1976.
Garcia, J. J.; Li, G.; Wang, P.; Zhong, J.; Granados, R. R. Primary and continuous midgut cell cultures from Pseudaletia unipuncta (Lepidoptera: Noctuidae). In Vitro Cell. Dev. Biol. Anim 37: 353–359; 2001.
Garcia, S.; Billecocq, A.; Crance, J.-M.; Prins, M.; Garin, D.; Bouloy, M. Viral suppressors of RNA interference impair RNA silencing induced by a Semliki Forest virus replicon in tick cells. J. Gen. Virol 87: 1985–1989; 2006.
Gaw, S.-Y. Culturing all types of silkworm tissues using the monolayer culture. Chin. Sci. Bull 7: 219–220; 1958.
Gerlo, S.; Verdood, P.; Gellersen, B.; Hooghe-Peters, E. L.; Kooijman, R. J. Mechanism of prostaglandin (PG)E2-induced prolactin expression in human T cells: cooperation of two PGE2 receptor subtypes, E-Prostanoid (EP) 3 and EP4, via calcium- and cyclic adenosine 5′-monophosphate-mediated signaling pathways. J. Immunol 173: 5952–5962; 2004.
Gillespie, J. P.; Kanost, M. R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol 42: 611–643; 1997.
Glaser, R. W. The growth of insect blood cells in vitro. Psyche 24: 1–6; 1917.
Goodman, C. L.; El Sayed, G. N.; McIntosh, A. H.; Grasela, J. J.; Stiles, B. Establishment and characterization of insect cell lines from 10 lepidopteran species. In Vitro Cell. Dev. Biol. Anim 37: 367–373; 2001.
Goodman, W. G.; Granger, N. A. The juvenile hormone. In: Gilbert L. I.; Iatrou K.; Gill S. (eds) Comprehensive insect molecular science. 3: Pergamon, New York, pp 319–408; 2005.
Goodwin, R. H.; Adams, J. R.; Shapiro, M. Replication of the entomopoxvirus from Amscata moorei in serum-free cultures of a gypsy moth cell line. J. Invertebr. Pathol 56: 190–205; 1990.
Grace, T. D. C. Establishment of four strains of cells from insect tissues grown in vitro. Nature (London) 195: 788–789; 1962.
Grace, T. D. C. Establishment of a line cells from the silkworm, Bombyx mori. Nature (London) 216: 613; 1967.
Gringorten, J. L. Ion balance in the lepidopteran midgut and insecticidal action of Bacillus thuringiensis. In: Ishaaya I. (ed) Biochemical sites of insecticide action and resistance. Springer, Berlin, pp 167–207; 2001.
Gundersen-Rindal, D.; Dougherty, E. M. Evidence for integration of Glyptapanteles indiensis polydnavirus DNA into the chromosome of Lymantria dispar in vitro. Virus Res 66: 27–37; 2000.
Gundersen-Rindal D.; Lynn, D. E. Polydnavirus integration in lepidopteran host cells in vitro. J. Insect Physiol 49: 453–462; 2003.
Hemmes, H.; Lakatos, L.; Goldbach, R.; Burgyan, J.; Prins, M. The NS3 protein of Rice hoja blanca tenuivirus suppresses RNA silencing in plant and insect hosts by efficiently binding both siRNAs and miRNAs. RNA 13: 1079–1089; 2007.
Hoa, N. T.; Keene, K. M.; Olson, K. E.; Sheng, L. Characterization of RNA interference in an Anopheles gambiae cell line. Insect Biochem. Mol. Biol 33: 949–957; 2003.
Hoshino, K.; Isawa, H.; Tsuda, Y.; Yano, K.; Sasaki, T.; Yuda, M.; Takasaki, T.; Kobayashi, M.; Sawabe, K. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology 359: 405–414; 2007.
Hunnicutt, L. E.; Hunter, W. B.; Cave, R. D.; Powell, C. A.; Mozoruk, J. J. Complete genome sequence and molecular characterization of Homalodisca coagulata virus-1, a novel virus discovered in the glassy-winged sharpshooter (Hemiptera: Cicadellidae). Virology 350: 67–78; 2006.
Hunter, W. B.; Katsar, C. S.; Chaparro, J. X. Nucleotide sequence of 3′-end of Homalodisca coagulata Virus-1. A new leafhopper-infecting virus from the glassy-winged sharpshooter. J. Insect Sci. 6.28. Online: insectscience.org/6.28/; 2006.
Hunter, W. B.; Polston, J. E. Development of a continuous whitefly cell line [Homoptera: Aleyrodidae: Bemisia tabaci (Gennadius)] for the study of begomovirus. J. Invertebr. Pathol 77: 33–36; 2001.
Jin, T.; Hereld, D. Moving toward understanding eukaryotic chemotaxis. Eur. J. Cell Biol 85: 905–913; 2006.
Kamita, S. G.; Do, Z. N.; Samra, A. I.; Hagler, J. R.; Hammock, B. D. Characterization of cell lines developed from the glassy-winged sharpshooter, Homalodisca coagulata (Hemiptera: Cicadellidae). In Vitro Cell. Dev. Biol. Anim 41: 149–153; 2005.
Keene, K. M.; Foy, B. D.; Sanchez-Vargas, I.; Beaty, B. J.; Blair, C. D.; Olson, K. E. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A 101: 17240–17245; 2004.
Kim, M.-K.; Sisson, G.; Stoltz, D. Ichnovirus infection of an established gypsy moth cell line. J. Gen. Virol 77: 2321–2328; 1996.
Knowles, B. H.; Ellar, D. J. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxin with different insect specificity. Biochim. Biophys. Acta 924: 509–518; 1987.
Lan, Q.; Gerenday, A.; Fallon, A. M. Cultured Aedes albopictus mosquito cells synthesize hormone-inducible proteins. In Vitro Cell. Dev. Biol. Anim 29: 813–818; 1993.
Lavine, M. D.; Strand, M. R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol 32: 1295–1309; 2002.
Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol 25: 697–743; 2007.
Lennan, E.; Vandergaast, R.; Friesen, P. D. Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J. Virol 81: 9319–9330; 2007.
Lery, X.; Fediere, G.; Taha, A.; Salah, M.; Giannotti, J. A new small RNA virus persistently infecting an established cell line of Galleria mellonella, induced by a heterologous infection. J. Invertebr. Pathol 69: 7–13; 1997.
Lezzi, M.; Wyss, C. The antagonism between juvenile hormone and ecdysone. In: Gilbert L. I. (ed) The juvenile hormones. Plenum, New York, pp 252–269; 1976.
Li, H.; Bonning, B. C. Evaluation of the insecticidal efficacy of wild type and recombinant baculoviruses. In: Murhammer D. W. (ed) Methods in molecular biology series. Baculovirus and insect cell expression protocols. Springer, New York, pp 379–405; 2007.
Liu, N. T.; Zia, T. U.; Gaw, Z. Y. Tissue culture methods for cultivation of virus grasserie. Wuhan University Journal, Natural Science 3: 98; 1959.
Llewellyn, Z. N.; Salman, M. D.; Pauszek, S.; Rodriguez, L. L. Growth and molecular evolution of vesicular stomatitis serotype New Jersey in cells derived from its natural insect-host: evidence for natural adaptation. Virus Res 89: 65–73; 2002.
Loeb, M. J.; Martin, P. A. W.; Hakim, R. S.; Goto, S.; Takeda, M. Regeneration of cultured midgut cells after exposure to sublethal doses of toxin from two strains of Bacillus thuringiensis. J. Insect Physiol 47: 599–606; 2001.
Long, S. H.; McIntosh, A. H.; Grasela, J. J.; Goodman, C. L. The establishment of a Colorado potato beetle (Coleoptera: Chrysomelidae) pupal cell line. Appl. Entomol. Zool 37: 447–450; 2002.
Lynn, D. E. Available lepidopteran insect cell lines. In: Murhammer D. W. (ed) Methods in molecular biology series. Baculovirus and insect cell expression protocols. Springer, New York, pp 117–144; 2007.
Lynn, D. E.; Hung, A. C. F. Development of continuous cell lines from the egg parasitoids Trichogramma confusum and T. exiguum. Arch. Insect Biochem. Physiol 18: 99–104; 1991.
Lynn, D. E.; Oberlander, H. The establishment of cell lines from imaginal wing discs of Spodoptera frugiperda and Plodia interpunctella. J. Insect Physiol 29: 591–596; 1983.
Maeda, S. Bombyx mori nuclear polyhedrosis virus and their use for expression of foreign genes in insect cells. In: Mitsuhashi J. (ed) Invertebrate cell system applications. CRC, Boca Raton, pp 167–181; 1989.
Marks, E. P. Insect tissue culture—overview 1971–1978. Annu. Rev. Entomol 25: 73–101; 1980.
Marutani-Hert, M.; Hunter, W. B.; Hall, D. G. Establishment of Asian citrus psyllid (Diaphorina citri) primary cultures. In Vitro Cell. Dev. Biol. Anim. in press; 2009.
McCearth, K. J.; Gooday, G. W. A rapid and sensitive microassay for determination of chitinolytic activity. J. Microbiol. Methods 14: 229–237; 1992.
McIntosh, A. H.; Grasela, J. J.; Ignoffo, C. M. In vitro host range of the Hz-1 nonoccluded virus in insect cell lines. In Vitro Cell. Dev. Biol. Anim 43: 196–201; 2007.
Merchant, D.; Ertl, R. L.; Rennard, S. I.; Stanley, D. W.; Miller, J. S. Eicosanoids mediate insect hemocyte migration. J. Insect Physiol 54: 215–221; 2008.
Metakovskii, E. V.; Cherdantseva, E. M.; Gvozdev, V. A. Action of ecdysterone on surface membrane glycoproteins of Drosophila melanogaster cells in culture. Mol. Biol 11: 158–170; 1977.
Mikitani, K. Sensitive, rapid and simple method for evaluation of ecdysteroid agonist activity based on the mode of action of the hormone. Journal of Sericultural Science of Japan 64: 534–539; 1995.
Mikitani, K. A new nonsteroidal chemical class of ligand for the ecdysteroid receptor 3,5-di-tert-butyl-4hydroxy-N-isobutyl-benzamide shows apparent insect molting hormone activities at molecular and cellular levels. Biochem. Biophys. Res. Commun 227: 427–432; 1996.
Miller, J. S.; Nguyen, T.; Stanley-Samuelson, D. W. Eicosanoids mediate insect nodulation responses to bacterial infections. Proc. Natl. Acad. Sci. U. S. A 91: 12418–12422; 1994.
Mitsuhashi, J. A new continuous cell line from larvae of the mosquito Aedes albopictus Diptera Culicidae. Biomed. Res. (Tokyo) 2: 599–606; 1981.
Mosallanejad, H.; Soin, T.; Smagghe, G. Selection for resistance to methoxyfenozide and 20-hydroxyecdysone in cells of the beet armyworm Spodoptera exigua. Arch. Insect Biochem. Physiol 67: 36–49; 2008a.
Mosallanejad, H.; Soin, T.; Swevers, L.; Iatrou, K.; Nakagawa, Y.; Smagghe, G. Non-steroidal ecdysteroid agonist chromafenozide: gene induction activity, cell proliferation inhibition and larvicidal toxicity. Pestic. Biochem. Physiol 92: 70–76; 2008b.
Mudiganti, U.; Hernandez, R.; Ferreira, D.; Brown, D. T. Sindbis virus infection of two model insect cell systems: a comparative study. Virus Res 122: 28–34; 2006.
Nakagawa, Y.; Matsutani, M.; Kurihara, N.; Nishimura, K.; Fujita, T. Quantitative structure–activity studies of benzoylphenylurea larvicides. VIII. Inhibition of N-acetylglucosamine incorporation into the cultured integument of Chilo suppressalis Walker. Pestic. Biochem. Physiol 43: 141–151; 1989.
Nauen, R.; Smagghe, G. Mode of action of etoxazole. Pest Manag. Sci 62: 375–382; 2006.
Negishi, M.; Katoh, H. Cyclopentenone prostaglandin receptors. Prostaglandins Other Lipid Mediat 68–69: 611–617; 2002.
Oberlander, H.; Silhacek, D. L. New perspectives on the mode of action of benzoylphenylurea insecticides. In: Ishaaya I.; Degheele D. (eds) Insecticides with novel modes of action. Springer, Berlin, pp 92–105; 1998.
Palli, S. R.; Caputo, G. F.; Brownwright, A. J.; Sofi, S. S. Studies on apoptosis in a continuous midgut cell line, CF-203, of the spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). In: Maramorosch K.; Mitsuhashi J. (eds) Invertebrate cell culture. Novel directions and biotechnology applications. Science, Enfield, pp 43–51; 1997a.
Palli, S. R.; Retnakaran, A. Molecular and biochemical aspects of chitin synthesis inhibition. In: Jolles P.; Muzzarelli R. A. A. (eds) Chitin and chitinases. Birkhäuser, Basel, pp 85–98; 1999.
Palli, S. R.; Sohi, S. S.; Cook, B. J.; Primavera, M.; Retnakaran, A. Screening of 12 continuous cell lines for apoptosis. In: Maramorosch K.; Mitsuhashi J. (eds) Invertebrate cell culture. Novel directions and biotechnology applications. Science, Enfield1997b.
Peel, D. J.; Milner, M. J. The response of Drosophila imaginal disc cell lines to ecdysteroids. Roux’s Arch. Dev. Biol 202: 23–35; 1992.
Potvin, L.; Laprade, R.; Schwartz, J. L. Cry1Ac, a Bacillus thuringiensis toxin, triggers extracellular Ca2+ influx ad Ca2+ release from intracellular stores in Cf1 cells (Choristoneura fumiferana, Lepidoptera). J. Exp. Biol 201: 1851–1858; 1998.
Pringle, F. M.; Johnson, K. N.; Goodman, C. L.; McIntosh, A. H.; Ball, L. A. Providence virus: a new member of the Tetraviridae that infects cultured insect cells. Virology 306: 359–370; 2003.
Ress, C.; Maas, U.; Dorn, A. The Drosophila tumorous blood cell line l(2)mbn and its response to insect hormones, hormone agonists, and the natural growth regulators azadirachtin and plumbaginoids. In: Maramorosch K.; Mitsuhashi J. (eds) Invertebrate cell culture. Novel directions and biotechnology applications. Science, Enfield, pp 93–103; 1997.
Sadrud-Din, S. Y.; Loeb, M. J.; Hakim, R. S. In vitro differentiation of isolated stem cells from the midgut of Manduca sexta larvae. J. Exp. Biol 199: 319–325; 1996.
Sanchez-Vargas, I.; Travanty, E. A.; Keene, K. M.; Franz, A. W. E.; Beaty, B. J.; Blair, C. D.; Olson, K. E. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res 102: 65–74; 2004.
Schmidt, M. R.; Brockmann, A.; Pirk, C. W. W.; Stanley, D. W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol 54: 439–444; 2008.
Schütz, S.; Sarnow, P. Interaction of viruses with the mammalian RNA interference pathway. Virology 344: 151–157; 2006.
Sinisterra, X. H.; McKenzie, C. L.; Hunter, W. B.; Shatters, R. G. Jr. Differential transcriptional activity of plant pathogenic Begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyroididae). J. Gen. Virol 86: 1525–1532; 2005.
Smagghe, G.; Braeckman, B. P.; Huys, N.; Raes, H. Cultured mosquito cells Aedes albopictus C6/36 (Dip., Culicidae) responsive to 20-hydroxyecdysone and non-steroidal ecdysone agonist. J. Appl. Entomol 127: 167–173; 2003.
Smagghe, G.; Ryckaert, J.; Soin, T.; Caputo, G.; Van Damme, E. J. M. Effect of plant lectins on growth of insect midgut cells. In Vitro Cell. Dev. Biol. Anim 41: 34A; 2005a.
Smagghe, G.; Vanhassel, W.; Moeremans, C.; De Wilde, D.; Goto, S.; Loeb, M. J.; Blackburn, M. B.; Hakim, R. S. Stimulation of midgut stem cell proliferation and differentiation by insect hormones and peptides. Ann. N.Y. Acad. Sci 1040: 472–475; 2005b.
Smith, H. C.; Cavanaugh, C. K.; Friz, J. L.; Thompson, C. S.; Saggers, J. A.; Michelotti, E. I.; Garcia, J.; Tice, C. M. Synthesis and SAR of cis-1-benzoyl-1,2,3,4-tetrahydroquinoline ligands for control of gene expression in ecdysone responsive systems. Bioorg. Med. Chem. Lett 13: 1943–1946; 2003.
Sohi, S. S. Development of lepidopteran cell lines. In: Richardson C. D. (ed) Methods in molecular biology. Baculovirus expression protocols. Humana, New York, pp 397–411; 1995.
Soin, T.; Swevers, L.; Mosallanejad, H.; Efrose, R.; Labropoulou, V.; Iatrou, K.; Smagghe, G. Juvenile hormone analogs do not affect directly the activity of the ecdysteroid receptor complex in insect culture cell lines. J. Insect Physiol 54: 429–438; 2008.
Spindler-Barth, M.; Spindler, K.-D. Ecdysteroid resistant subclones of the epithelial cell line from Chironomus tentans (Insecta, Diptera). I. Selection and characterization of resistant clones. In Vitro Cell. Dev. Biol. Anim 34: 116–122; 1998.
Spindler-Barth, M.; Spindler, K.-D.; Londershausen, M.; Thomas, H. Inhibition of chitin synthesis in an insect cell line. Pestic. Sci 25: 115–121; 1989.
Stanley, D.; Miller, J. S. Eicosanoid actions in insect cellular immune functions. Entomol. Exp. Appl 119: 1–13; 2006.
Stanley, D.; Shapiro, M. Eicosanoid biosynthesis inhibitors increase the susceptibility of Lymantria dispar to nucleopolyhedrovirus LdMNPV. J. Invertebr. Pathol 95: 119–124; 2007.
Stanley, D. W. Prostaglandins and other eicosanoids in insects: biological significance. Annu. Rev. Entomol 51: 25–44; 2006.
Stanley, D. W.; Goodman, C.; An, S.; McIntosh, A.; Song, Q. Prostaglandins A1 and E1 influence gene expression in an established insect cell line (BCIRL-HzAM1 cells). Insect Biochem. Mol. Biol 38: 275–284; 2008.
Stiles, B.; Newman, S. M. Evidence for the induction of cuticle proteins by 20-hydroxyecdysone in 2 established insect cell lines. Arch. Insect Biochem. Physiol 21: 23–40; 1992.
Suchman, E. L.; Carlson, J. O. Production of mosquito densonucleosis viruses by Aedes albopictus C6/36 cells adapted to suspension culture in serum-free protein-free media. In Vitro Cell. Dev. Biol. Anim 40: 74–75; 2004.
Swevers, L.; Kravariti, L.; Ciolfi, S.; Xenou-Kokoletsi, M.; Wong, G.; Ragousis, N.; Smagghe, G.; Nakagawa, Y.; Mazomenos, V.; Iatrou, K. A high-throughput screening system for fast detection of ecdysteroid mimetic and antagonistic substances using transformed Bombyx mori-derived cell lines. FASEB J 18: 134–146; 2004.
Tan, B.-H.; Nason, E.; Staeuber, N.; Jiang, W.; Monastryrskaya, K.; Roy, P. RGD tripeptide of bluetongue virus VP7 protein is responsible for core attachment to Culicoides cells. J. Virol 75: 3937–3947; 2001.
Tice, C. M.; Hormann, R. E.; Thompson, C. S.; Fritz, J. L.; Cavanaugh, C. K.; Michelotti, E. L.; Garcia, J.; Nicolas, Z.; Alberico, F. Synthesis and SAR of alpha-acylaminoletone ligands for control of gene expression. Bioorg. Med. Chem. Lett 13: 475–478; 2003.
Toya, T.; Fukasawa, H.; Masui, A.; Endo, Y. Potent and selective partial ecdysone agonist activity of chromafenozide in Sf-9 cells. Biochem. Biophys. Res. Commun 292: 1087–1091; 2002.
Trager, W. J. Cultivation of virus grasserie in silkworm tissue. J. Exp. Med 61: 501–513; 1953.
Trisyono, A.; Goodman, C. L.; Grasela, J. J.; McIntosh, A. H.; Chippendale, G. M. Establishment and characterization of an Ostrinia nubilalis cell line, and its response to ecdysone agonists. In Vitro Cell. Dev. Biol. Anim 36: 400–404; 2000.
Van Damme, E. J. M.; Rouge, P.; Peumans, W. J. Carbohydrate–protein interactions: plant lectins. In: Kamerling J. P.; Boons G. J.; Lee Y. C.; Suzuki A.; Taniguchi N.; Voragen A. J. G. (eds) Comprehensive glycoscience. From chemistry to systems biology. 3: Elsevier, New York, pp 563–599; 2007.
Vandenborre, G.; Lannoo, N.; Smagghe, G.; Daniel, E.; Breite, A.; Soin, T.; Jacobsen, L.; Van Damme, E. J. M. Cell-free expression and functionality analysis of the tobacco lectin. In Vitro Cell. Dev. Biol. Anim 44: 228–235; 2008.
Van Rij, R. P.; Saleh, M.-C.; Berry, B.; Foo, C.; Houk, A.; Antoniewski, C.; Andino, R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev 20: 2985–2995; 2006.
Vaughn, J. L.; Goodwin, R. H.; Tompkins, G. J.; McCawley, P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae). In Vitro 13: 213–217; 1977.
Vlak, J. M. Professor Shang yin Gao (1909–1989): his legacy in insect cell culture and insect virology. J. Invertebr. Pathol 95: 152–160; 2007.
Wang, P.; McCarthy, W. J. Cytolytic activity of Bacillus thuringiensis Cry1C and Cry1AC toxins to Spodoptera sp. midgut epithelial cells in vitro. In Vitro Cell. Dev. Biol. Anim 33: 315–323; 1997.
Ward, G. B.; Newman, S. M.; Klosterman, H. J.; Marks, E. P. Effect of 20-hydroxyecdysone and diflubenzuron on chitin production by a cockroach cell line. In Vitro Cell. Dev. Biol. Anim 24: 326–332; 1988.
Weaver, S. C. Evolutionary influences in arboviral disease. Curr. Top. Microbiol. Immunol 299: 285–314; 2006.
Wheelock, C. E.; Nakagawa, Y.; Harada, T.; Oikawa, N.; Akamatsu, M.; Smagghe, G.; Stefanou, D.; Iatrou, K.; Swevers, L. High throughput screening of ecdysone agonists using a reporter gene assay followed by 3-D QSAR analysis of the molting hormonal activity. Bioorg. Med. Chem 14: 1143–1159; 2006.
Williams, C. M. Third generation pesticides. Sci. Am 217: 13–17; 1967.
Wu, C.-Y.; Yang, H.-N.; Lo, C.-F.; Wang, C.-H. A Perina nuda cell line (NTU-Pn-HF) from pupal ovary that is persistently infected with a picorna-like virus (PnPV). Appl. Entomol. Zool 37: 171–179; 2002.
Wyss, C. Chironomus tentans epithelial cell lines sensitive to ecdysteroids, juvenile hormone, insulin and heat shock. Exp. Cell Res 139: 309–319; 1982.
Zhang, X. B.; Candas, M.; Griko, N. B.; Taussig, R.; Bulla, L. A. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. U. S. A 103: 9897–9902; 2006.
Zhou, F.; Pu, Y.; Wei, T.; Liu, H.; Deng, W.; Wei, C.; Ding, B.; Omura, T.; Li, Y. The P2 capsid protein of the nonenveloped rice dwarf phytoreovirus induces membrane fusion in insect host cells. Proc. Natl. Acad. Sci. U. S. A 104: 19547–19552; 2007.
Acknowledgments
We gratefully thank Dr. Arthur McIntosh (BCIRL) for his helpful information. Dr. Guy Smagghe acknowledges the support by Ghent University, the Flemish Institute for Promotion of Scientific Research in Industry (IWT), and the Fund for Scientific Research (FWO-Vlaanderen). Research in BCIRL was supported by the USDA/Agricultural Research Service. This article reports the results of research only and mention of a proprietary product does not constitute an endorsement or recommendation for its use by the USDA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Smagghe, G., Goodman, C.L. & Stanley, D. Insect cell culture and applications to research and pest management. In Vitro Cell.Dev.Biol.-Animal 45, 93–105 (2009). https://doi.org/10.1007/s11626-009-9181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-009-9181-x