Abstract
The unintentional introduction of the cactus moth, Cactoblastis cactorum, a successful biological control agent formerly employed in the control of invasive prickly pear cactus species (Opuntia spp.) in Australia, Hawaii, South Africa, and various Caribbean islands, has posed great concern as to the possible threat to native, endangered species of cactus in the southeastern USA as well as with the potential to cause a major infestation of commercial and agricultural cactus crops in Mexico. A number of control measures have been investigated with varying degrees of success including, field exploration for cactus moth-specific parasitoids, insecticides, fungal, bacterial, and nematode agents. Current tactics used by the USA–Mexico binational program to eradicate cactus moth from Mexico and mitigate its westward movement in the USA include host plant removal, the manual removal and destruction of egg sticks and infected cacti stems, and the Sterile Insect Technique. One other approach not taken until now is the development of a cactus moth cell line as a tool to facilitate the investigation of baculoviruses as an alternative biocontrol method for the cactus moth. Consequently, we established C. cactorum cell lines derived from adult ovarian tissue designated as BCIRL-Cc-AM and BCIRL-Cc-JG. The mean cell population doubling time was 204.3 and 112 h for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively, with weekly medium change, while the doubling time was 176.6 and 192.6 h for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively, with a daily change of medium. In addition, the daily versus weekly change in medium was reflected in the percentage viability with both cell lines showing higher levels with a daily medium change. Of the three baculoviruses tested, only the recombinant AcMNPV-hsp70Red and GmMNPV at a multiplicity of infection (MOI) of 1.0 were able to demonstrate significant production of extracellular virus (ECV) in each of the cell lines, whereas both cell lines were refractive to an HzSNPV challenge at an MOI of 10. In this study, we have demonstrated both the successful development of a C. cactorum cell line and its ability to support a complete baculovirus infection. The potential is also there to pursue further investigations to determine the susceptibility of the cactus moth cell line to other viruses. Additionally, the availability of a cactus moth cell line will facilitate the analysis of viruses prior to using the more expensive bioassay test. Finally, it is hoped with the knowledge presented here that baculoviruses may also be considered as an alternative biocontrol method for the cactus moth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A native of South America, the cactus moth, Cactoblastis cactorum (Berg) was historically employed as a cactophagous insect to help control populations of prickly pear cactus (Opuntia spp.) and related species. The cactus moth was introduced into various regions of the world that lack any sort of natural controls to contain these non-indigenous cacti (Zimmerman et al. 2004) C. cactorum inhabits a wide range of climatic conditions both in its native home and countries of introduction and feeds on several Opuntia species as host. A classic example of what can be accomplished by employing an appropriate biological control agent was the introduction to Australia in 1926 and South Africa in 1933 of C. cactorum, which successfully eradicated extensive infestations of the exotic prickly pear cacti in Australia, reclaiming some 16 million acres of land for productive agricultural use. However, the various successful introductions of the moth to control invasive cactus species have been muted by a recent unanticipated event. In 1989, C. cactorum was discovered in Florida to potentially threaten native cacti and has since then expanded its geographical range into the lower southeastern and Gulf regions of the USA (Pemberton 1995; Johnson and Stiling 1996; Hight et al. 2002). How it was able to spread to Florida is unknown, but the possibility exists that multiple introductions either through human-assisted or natural causes (via tropical storms and hurricanes that frequent the area) might be responsible (Simonsen et al. 2008). Empirical evidence from a release–recapture study on comparing the dispersal ability of irradiated versus non-irradiated moths showed that most individuals will remain in close proximity to the release site with only a few moths recaptured some 2.5 km away after a 2-wk period (Hight et al. 2005).
Consideration as to the best possible method to control this insect has ranged from insecticide application (Bloem et al. 2005), the sterile insect technique (Carpenter et al. 2001; Hight et al. 2005; Carpenter et al. 2011), and various biological control agents such as Nosema and several parasitoids including Apanteles alexanderi Brethes, Phyticiplex doddi (Cushman), Phyticiplex eremnus (Porter), Brachymeria cactoblastis Blanchard, and Epicoronimyia mundelli (a braconid, two ichneumonids, a chalicid, and a tachinid, respectively) that are known to attack the moth in its native habitat (Habeck and Bennett 1990). Some expressed concerns and obstacles associated with employing either chemical insecticides or biological control agents have been previously reviewed (Leibee and Osborne 2001; Pemberton and Cordo 2001a, b). However, another biological control approach that so far has not been fully explored involves the possible use of the insect-specific viruses to control the cactus moth. Tamashiro and Huang (1963) described a cytoplasmic polyhedrosis virus with polyhedra 262–880 nm in diameter from C. cactorum from Hawaii, and Marti et al. (2007) observed a similar cytoplasmic polyhedrosis virus as well as a small (19–34 nm in diameter) icosahedral virus in laboratory cultures of C. cactorum originating from South Africa and Florida and reared in the United States Department of Agriculture (USDA) laboratory in Tifton, GA. We are aware of only one investigation to date in which bioassays were used to determine the susceptibility of C. cactorum larvae to the Autographa californica multiple nucleopolyhedrovirus (AcMNPV) (Vail et al. 1984). The results from that study indicated that the cactus moth when fed on an artificial diet inoculated with baculovirus was susceptible with virus-caused mortality ranging from 76.2 % (diet incorporated) to 87.8 % (diet-treated surface). As the cactus moth was highly susceptible to the virus, the employment of this type of insect pathogen to control the cactus moth would appear to show a great deal of promise. However, C. cactorum is a gregarious feeder and the level of mortality caused by viruses was observed to increase when C. cactorum was mass reared in the laboratory on an artificial diet (Marti et al. 2008). Therefore, in light of the past successful establishment of numerous insect cell lines and baculovirus production in many of these cell lines, particularly for many species of Lepidoptera, we decided that an investigation into the development of a cell culture derived from C. cactorum was considered a viable alternative approach to further determine the potential utility of baculoviruses as biological control agents for this moth.
Materials and Methods
Source of cactus moth tissue.
C. cactorum egg sticks, which is a chain of eggs laid by adult moths and cemented together to resemble the naturally occurring spines on prickly pear pads, as well as pupae and a specially developed cactus moth larva diet (Marti et al. 2008) were provided by the USDA Crop Protection & Management Unit, Tifton, Georgia. Egg sticks were placed in 12 oz Dixie cups containing small pieces of diet and incubated at 28 °C. After hatching, the larvae were allowed to feed on the diet until the late-instar stages with daily inspection of cups and replacement of diet if needed to reduce contamination.
Initiation of C. cactorum cell line.
To initiate a cactus moth cell culture, 20 female pupae were maintained at 28 °C and 30 % RH until adults emerged. Four adults were first transferred to a 1.5-ml Eppendorf tube, completely submerged with 5.2 % sodium hypochlorite for 10 min, 70 % ethanol for another 10 min and washed twice with 10 ml sterile ultrapure water. Insects were then pinned to a sterile wax surface with their ventral surface exposed in 5 ml ExCell 420 (SAFC, Biosciences, Lenexa, KS) + 10 % fetal bovine serum (FBS), containing streptomycin and penicillin at 100 μg/ml and 100 units/ml, respectively. A small incision was made the length of the body with sterile scissors and the greenish-colored ovarian tissue removed. The explants were placed onto a sterile Petri dish with 5 ml ExCell 420 + 10 % FBS and minced into small fragments with sterile scissors. The tissue fragments were then transferred to a T-25 cm2 flask containing 5 ml ExCell 420 + 10 % FBS and incubated at 28 °C. Amphotercin B (2.5 μg/ml) was also added initially to the flask to prevent fungal growth. The cultures were inspected weekly for cell attachment, and if there was any indication of new cell growth, then half the medium was replaced with an equivalent amount of standard (without amphotercin B) fresh medium. After the cells were maintained for over a year at 28 °C (passage 9), two establishment C. cactorum cell lines, designated as BCIRL-Cc-AM and BCIRL-Cc-JG, were selected for further cellular studies.
Cell line identification.
DNA amplification fingerprinting (DAF) analysis was employed to obtain primary baseline data on the identity of BCIRL-Cc-AM and BCIRL-Cc-JG and to detect possible future cross-contamination or misidentification (McIntosh et al. 1996). Once the cells reached confluence in T-25 cm2 flasks containing ExCell 420 + 10 % FBS, genomic DNA was extracted employing the Gentra Puregene Core Kit B (Qiagen Inc., Valencia, CA.). The following primers were used to fingerprint the C. cactorum cell lines as well as their larva source: (1) aldolase(F): 5′-CCG GAG CAG AAG AAG GAG CT, (2) aldolase(R) 5′-CAC ATA CTG GCA GCG CTT CA (Integrated DNA Technologies, Inc., Coralville, IA). DNA from Helicoverpa zea (BCIRL-Hz-AM1), Heliothis virescens (BCIRL-Hv-AM1), and Tribolium castaneum (BCIRL-TcA-CLG1) cell lines previously established in our laboratory were used to compare with the cactus moth cell lines.
Cell growth of BCIRL-Cc-AM/JG.
Initially, we established several C. cactorum cell lines from different insect dissections and two of these cell lines, which were designated as BCIRL-Cc-AM and BCIRL-Cc-JG were employed in cell growth studies, each at passage number 25 and 29, respectively. Cell growth was determined under two different experimental conditions. The rationale was that in the first experiment the cells exhibited slow growth with only weekly medium changes and therefore the concern was that metabolic waste may have accumulated in the medium especially during the later time points of the study, compromising cell growth. Consequently, to overcome this concern, a second test was performed; however, this time the spent growth medium was replaced with fresh media on a daily basis. To determine the cell density and doubling time of the two cell lines, 12-well plates were setup with 1 × 105 cells/ml in 2 ml of ExCell 420 + 10 % FBS. Three wells were setup for each time period per cell line. At every time point, cells were detached from the wells by pipetting. All cell density and viability counts were made with the Cellometer Vision automated cell counter using 0.08 % trypan blue (Nexcelon Biosciences, Lawrence, MA). Doubling time was calculated employing the Doubling Time software (Roth 2006; http://www.doubling-time.com/compute.php).
Baculovirus production in C. cactorum moth cells.
The two cactus cell lines were evaluated for their susceptibility to a plaque-purified recombinant AcMNPV expressing a red fluorescent protein under the Drosophila hsp70 promoter (AcMNPV-hsp70Red) (McIntosh et al. 2005), GmMNPV (McIntosh et al. 1985), and HzSNPV (McIntosh et al. 1985). Each cell line was seeded with 1 × 105 cells/ml in each of three T-25 cm2 flasks containing 5 ml growth medium and incubated overnight at 28 °C. Medium was then removed and cells were inoculated at a multiplicity of infection (MOI) of 1.0 with either AcMNPV-hsp70RFP or GmMNPV, while a MOI of 10.0 was used to inoculate cells with HzSNPV and placed on a rocker platform for 2 h. The inoculum was removed and cells were washed twice with 5 ml CMF-PBS to remove any residual inoculum. Fresh medium was then added and cells were incubated at 28 °C for 7 d. Virus was harvested by removing the spent media and centrifuging it at 800×g for 15 min to remove any detached cells. TCID50 values from each replicate were generated using 96-well plates and BCIRL-HS-AM1 as the indicator cell line. The Reed–Muench method was used to calculate the TCID50 values (http://www.med.yale.edu/micropath/pdf/Infectivity%20calculator.xls). A statistical procedure involving the application of bootstrap sampling to compare the difference between TCID50 medians of six cell line–virus groups was employed to determine the degree of susceptibility of the cactus moth cell lines to viruses. The rationale for employing such a resampling procedure is that it avoids the necessary assumptions of traditional parametric tests, as with small samples from non-normal distributions. In addition, resampling has an added advantage of allowing for comparison of medians and ratios derived from data distributions that lack any conformity to standard probability models. The resampling methods for testing means, medians, or other parameters are the same, so one could consider this to be an all-in-one technique that can be applied to these different applications. A bootstrapped sample from each group was produced by randomly generating 10,000 samples with replacement from the observed counts to produce a new sample of the same size as our original sample. In this way, six sampling distributions for the statistic of interest are obtained along with confidence intervals around the observed effect (Resampling Procedures v 1.3. 2007. D. C. Howell, University of Vermont; http://www.uvm.edu/~dhowell/StatPages/Resampling/ResamplingPackage.zip).
Results
Cell morphology.
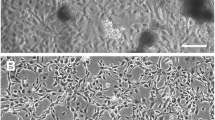
The pictorial chronology of attachment and growth of cactus moth cells is depicted in Fig. 1. Once the ovarian tissue attached to the flask bottom (soon after it was placed in cell culture flask), it was left undisturbed except for a weekly change of medium. Eventually, single cells as well as cell clusters began to emerge from the explants and appeared as either neuronal- and epithelium-shaped cells.
DAF identification of C. cactorum cell lines.
The aldolase molecular marker employed in this study was able to distinguish successfully the cactus cell lines from some other tested insect lines. Most of the major bands (based on relative band density) detected in the cactus moth larvae were also present in the fingerprint patterns of the cactus moth cell lines confirming the origin of the cell line as well as a means to distinguish between other cell lines (Fig. 2).
Cell growth characteristics.
Growth curves for both cell lines are depicted in Fig. 3. The corresponding cell viability data are described in Table 1. In general, the growth pattern of the BCIRL-Cc-AM cell line was slower than BCIRL-Cc-JG in the no-medium-change regime (Fig. 3A ) while the growth in both cell lines was similar with a daily change in medium (Fig. 3B ). The doubling time in experiment 1 was 204 and 112 h for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively. In experiment 2, the doubling time was 176 and 192 h for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively. The percent cell viability was higher when each cell line was exposed to a daily change in medium. The mean percent cell viability without daily change in medium was 81.2 SE ± 4.8 % and 80.7 ± SE 4.0 % for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively, whereas the mean percent cell viability with a daily change in medium was 97.0 SE ± 0.41 % and 95.6 ± SE 0.43 % for BCIRL-Cc-AM and BCIRL-Cc-JG, respectively (Table 1).
TCID50 analysis of virus production.
The difference in susceptibility of the cactus moth cell lines when challenged with each of the MNPVs is shown in Table 2. The production of ECV for both viruses was higher in BCIRL-Cc-AM, with a mean range from 2.54 ± 0.4 × 106 TCID50/ml (GmMNPV) to 5.44 ± 1.6 × 106 TCID50/ml (AcMNPV-hsp70Red), compared to 1.70 ± 0.09 × 106 TCID50/ml (GmMNPV) to 1.83 ± 0.05 × 106 TCID50/ml (AcMNPV-hsp70Red) in BCIRL-Cc-JG. Bootstrap analysis revealed significant differences (P < 0.05 %) in AcMNPV-hsp70Red titer produced in BCIRL-Cc-AM as compared to BCIRL-Cc-JG. GmMNPV production in BCIRL-Cc-JG was also significantly different when compared to BCIRL-Cc-AM (P < 0.05). In contrast, AcMNPV-hsp70Red titer produced in the BCIRL-Cc-JG was not significantly different from the GmMNPV titer in both BCIRL-Cc-AM and BCIRL-Cc-JG (P > 0.05). Expression of the red fluorescent protein by the AcMNPV-hsp70Red in the BCIRL-Cc-JG at 4 d post-inoculation is shown in Fig. 4A , while AcMNPV-hsp70Red occlusion bodies can be observed in BCIRL-Cc-JG at 4 d post-inoculation (Fig. 4B ). The presence of GmMNPV occlusion bodies in BCIRL-Cc-JG cells is shown in Fig. 5A and in BCIRL-Cc-AM cells in Fig. 5B . Both cell lines were nonpermissive to HzSNPV infection.
Discussion
This is the first known report documenting the establishment of continuous cell lines from explants of C. cactorum ovarian tissue. After the C. cactorum cell lines were established, various cellular characteristics were investigated such as DNA fingerprinting (to generate a baseline for cell identification and authentication), cell population growth, and cell viability. Once these goals were achieved the final portion of this study was then to determine the susceptibility of the cactus moth cell lines to different insect viruses. In this study, C. cactorum cell population doubling time was found to be considerably longer than what typically has been observed in other Lepidoptera cell lines, even if we take into consideration varying growth conditions and passage number. For example, an adult ovarian cell line derived from the monarch butterfly (Danaus plexippus, Danaidae) had a population doubling time of 32 h (McIntosh and Grasela 2009). Other studies revealed doubling times of 42 h for Bombyx mori larval ovarian cell line (Khurad et al. 2006), and 31 and 37 h for H. zea and H. virescens pupal ovaries, respectively (McIntosh and Ignoffo 1983). There is the possibility that further experiments with different growth medium might well reduce the population doubling time of C. cactorum. However, each of the cell lines did grow well in the ExCell 420 + 10 % FBS as indicated by the overall high cell viability with a daily change in medium. Results of our TCID50 assays showed that the C. cactorum cell lines were susceptible to infection by both AcMNPV-hsp70Red and GmMNPV. The AcMNPV recombinant was employed to study expression of RFP in the event that C. cactorum cell lines did not support viral replication. Knowledge that a C. cactorum cell line is available and has been shown to support the production of viruses should facilitate future laboratory studies on the susceptibility of the C. cactorum cell lines to other baculoviruses that might be more of an ideal candidate for future field trials. In addition, a prior test of virus infectivity in cell culture would also avoid the more logistically expensive assays with cactus moth larvae. As a previous bioassay experiment has indicated, the potential is there to successfully employ a viral pathogen as an alternative biocontrol agent to moderate infestations of C. cactorum field populations (Vail et al. 1984). The successful establishment of C. cactorum cell lines should provide and facilitate not only the evaluation of baculoviruses as biocontrol agents for this pest but also aid in physiological, biochemical, and molecular studies as well.
References
Bloem S.; Mizell III R. F.; Bloem K. A.; Hight S. D.; Carpenter J. E. Laboratory evaluation of insecticides for control of the invasive Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 88(4): 395–400; 2005.
Carpenter J. E.; Bloem K. A.; Bloem S. Applications of F1 sterility for research and management of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 84: 531–536; 2001.
Carpenter J. E.; Bloem K. A.; Bloem S. Applications of F1 sterility for research and management of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 82(4): 531–536; 2011.
Habeck D. H.; Bennett F. D. Cactoblastis cactorum Berg (Lepidoptera: Pyralidae), a Phycitine new to Florida. Entomol. Circular 333. Florida Dept. of Agriculture and Consumer Ser., Div. of Plant Industry; 1990.
Hight S. D.; Carpenter J. E.; Bloem K. A.; Bloem S.; Pemberton R. W.; Stiling P. Expanding geographical range of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. Sci. Notes 85(3): 527–529; 2002.
Hight S. D.; Carpenter J. E.; Bloem S.; Bloem K. A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective over-flooding ratios and release–recapture field studies. Environ. Entomol. 34(4): 850–856; 2005.
Johnson D. M.; Stiling P. D. Host specificity of Catoblastis cactorum Berg, an exotic Opuntia-feeding moth, in Florida. Environ. Entomol. 25(4): 743–748; 1996.
Khurad A. M.; Kanginakudru S.; Qureshi S. O.; Rathod M. K.; Rai M. M.; Nagaraju J. A new Bombyx mori larval ovarian cell line highly susceptible to nucleopolyhedrovirus. J. Invertebr. Pathol. 92: 59–65; 2006.
Leibee G. L.; Osborne L. S. Chemical control of Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 84: 510–512; 2001.
Marti O. G.; Myers R. E.; Carpenter J. E. Rearing Cactoblastis cactorum (Lepidoptera: Pyralidae) on artificial diet and Opuntia cladodes. J. Entomol. Sci. 43: 95–106; 2008.
Marti Jr. O. G.; Styer E. L.; Myers R. E.; Carpenter J. E. Viruses in laboratory-reared cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 90(1): 274–277; 2007.
McIntosh A. H.; Grasela J. J. Establishment of a monarch butterfly (Danaus plexippus, Lepidoptera: Danaidae) cell line and its susceptibility to insect viruses. Appl. Entomol. Zool. 44(2): 331–336; 2009.
McIntosh A. H.; Grasela J. J.; Materi R. Identification of insect cell lines by DNA amplification fingerprinting (DAF). Insect Mol. Biol. 5(3): 187–195; 1996.
McIntosh A. H.; Grasela J. J.; Popham H. J. R. AcMNPV in permissive, semipermissive, and nonpermissive cell lines from Arthropoda. In Vitro Cell. Dev. Biol. 41: 298–304; 2005.
McIntosh A. H.; Ignoffo C. M. Characterization of 5 cell lines established from species of Heliothis. Appl. Entomol. Zool. 18(2): 262–269; 1983.
McIntosh A. H.; Ignoffo C. M.; Andrews P. L. In vitro host range of five baculoviruses in lepidopteran cell lines. Intervirology 23: 150–156; 1985.
Pemberton R. W. Cactoblastis, cactorum (Lepidoptera: Pyralidae) in the United States: an immigrant biological control agent or an introduction of the nursery industry? Am. Entomol. 41: 230–232; 1995.
Pemberton R. W.; Cordo H. A. Potential and risks of biological control of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 84: 513–526; 2001a.
Pemberton R. W.; Cordo H. A. Nosema (Microsporida: Nosematidae) species as potential biological control agents of Cactoblastis cactorum (Lepidoptera: Pyralidae): surveys for the Microsporida in Argentine and South Africa. Fla. Entomol. 84: 527–530; 2001b.
Roth V. http://www.doubling-time.com/compute.php; 2006.
Simonsen T. J.; Brown R. L.; Sperling F. A. H. Tracing an invasion: phylogeography of Cactoblastis cactorum (Lepidoptera: Pyralidae) in the United States based on mitochondrial DNA. Ann. Entomol. Soc. Am. 101(5): 899–905; 2008.
Tamashiro M.; Huang S.-S. A cytoplasmic polyhedrosis virus of Cactoblastis cactorum (Berg). J. Insect Pathol. 5: 397–399; 1963.
Vail P. V.; Vail S. S.; Summers M. D. Response of Cactoblastis cactorum (Lepidoptera: Phycitidae) to the nuclear polyhedrosis virus isolated from Autographa californica (Lepidoptera: Noctuidae). Environ. Entomol. 13: 1241–1244; 1984.
Zimmerman H.; Bloem S.; Klei H. Biology, history, threat surveillance and control of the cactus moth, Cactoblastis cactorum. (www.pub.iaea.org/mtcd/publications/pdf/faobswc_web.pdf); 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. All programs and services of the US Department of Agriculture are offered on a nondiscriminatory basis without regard to race, color, national origin, religion, sex, age, marital status, or handicap.
Rights and permissions
About this article
Cite this article
Grasela, J.J., McIntosh, A.H., Ringbauer, J. et al. Development of cell lines from the cactophagous insect: Cactoblastis cactorum (Lepidoptera: Pyralidae) and their susceptibility to three baculoviruses. In Vitro Cell.Dev.Biol.-Animal 48, 293–300 (2012). https://doi.org/10.1007/s11626-012-9496-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9496-x