Abstract
A biopolymer electrolyte consisting of iota-carrageenan (I-Carrageenan) and LiCl is prepared using solution casting method. XRD analysis confirms the enhancement in amorphous nature of the prepared polymer electrolytes due to the incorporation of LiCl. Fourier transform infrared (FTIR) spectroscopy is used to analyze the complexation of electrolytes. Electrochemical impedance spectroscopy is utilized to find the ac electrical conductivity of the electrolytes. The combination of 1.0 g I-Carrageenan and 0.3 g LiCl displays highest ionic conductivity value of 5.33 × 10−3 S cm−1 at room temperature. Transference number measurements indicate that the conductivity process is predominantly by Li+ ions. Lithium ion conducting battery is constructed with the highest conducting polymer electrolyte, and its discharge performance is analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portable electronic devices stimulate the development of energy storage devices in compact size. Solid electrolytes based on polymers are one of the best alternatives over liquid electrolytes in energy storage devices [1]. Solid polymer electrolytes (SPEs) as a leak proof electrolyte in the form of a film attract the attention of Scientists and Industrialists due to their large surface area [2, 3]. Moreover, SPEs have good adhesive property, flexibility, adequate conductivity for practical purposes, good mechanical stability and thermal stability [4, 5]. Literature survey shows that most of the works have been carried out with synthetic polymers. Now in researches, biopolymer electrolytes are preferred over synthetic polymers due to its cost-effectiveness and environmental friendliness [6]. There have been very few studies conducted on biopolymer electrolytes. Recent studies show that they provide better electrolyte/electrode contact due to their adhesive nature and their natural abundance appreciates the interest towards them [7]. A few examples of biopolymers are chitosan, cellulose acetate, tamarind seed polysaccharide, starch, agar-agar and Carrageenans. Only a few works have been carried out with Carrageenan as solid polymer electrolyte.
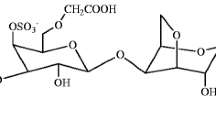
Carrageenans are a family of linear sulphated polysaccharides that are extracted from red edible seaweeds [8]. Carrageenans are made up of repeating galactose units and 3,6-anhydrogalactose [9, 10]. I-Carrageenan (two sulphates per disaccharide) is one of the main commercial classes of Carrageenans, which is soluble in water [11]. Most of the Carrageenans are used for medicines, and this concludes that they will not produce any harm to the mankind. Carrageenans are also used as a thickening agent in foods. They are certified as edible by “The Food and Drug Administration” in the USA to be used as gelling agent in foods. The chemical structure of iota-Carrageenan is shown in Scheme 1.
Mobarak et al. reported the highest ionic conductivity for the carboxymethyl iota carrageenan as 5.51 × 10−3 S cm−1 with lithium salt addition [12]. Shuhaimi et al. have prepared a polymer-salt complex chitosan, κ-Carrageenan and ammonium nitrate, and they have reported the conductivity value of 2.39 × 10−4 S cm−1 [13]. Karthikeyan et al. have studied iota-Carrageenan doped with NH4Br and reported the conductivity value of 1.08 × 10−3 S cm−1 [14]. Jumaah et al. have reported 4.87 × 10−6 S cm−1 by incorporating carboxymethyl with iota Carrageenan [15]. Shamsudin et al. have reported the conductivity value of 5.76 × 10−3 S cm−1 with carboxymethyl κ-Carrageenan doped with imidazolium [16]. A conductivity value of 3.25 × 10−4 S cm−1 has been reported by Ng et al. for κ-carrageenan and cellulose derivatives for solar cell applications [17]. Moniha et al. have produced the highest conductivity of 1.46 × 10−3 S cm−1 for iota-Carrageenan with NH4NO3 [18]. The highest conductivity of 4.83 × 10−4 S cm−1 has been reported for tamarind seed polysaccharide with lithium salt by Premalatha et al. [19]. Perumal et al. have achieved the highest conductivity of 2.08 × 10−3 S cm−1 for pectin biopolymer with lithium chloride [20]. Most of the works that have been tried with I-Carrageenan to obtain high conductivity are done by blending polymers.
This work aims to prepare SPE by using I-Carrageenan as a host polymer. The reason can be stated as it is a polymer with rich hydroxyl groups and oxygen atom which are essential for interaction [18]. With a suitable modification, I-Carrageenan could be used as a successful electrolyte in electrochemical devices. To enhance the potential of I-Carrageenan as an electrolyte, lithium chloride is chosen and incorporated with it. From the literature survey, it is observed that chlorine ions are weakly associated with lithium ions compared to other anions and the dissociation of LiCl is more in a solvent [21]. Also, it is observed that limited studies have been done with lithium salt in biopolymers. Hence, it is intended to prepare a solid biopolymer electrolyte with I-Carrageenan as a host polymer with different concentrations of LiCl. The purpose of this study is to synthesize I-Carrageenan with LiCl as a promising solid polymer electrolyte for electrochemical devices.
Experimental technique
Materials used
Biopolymer I-Carrageenan (Tokyo Chemical Industry, Japan) and lithium chloride (SRL Private Ltd, Mumbai) were used as the source materials for the preparation of biopolymer electrolyte.
Synthesis of biopolymer electrolyte
The biopolymer membranes of I-Carrageenan with various compositions of LiCl were prepared by solution casting technique, and distilled water was used as the solvent. One gram of I-Carrageenan was added in 60 ml of hot water around 60 °C and stirred with a magnetic stirrer for half an hour. Different concentrations of LiCl (0.2 g, 0.3 g, 0.4 g) were dissolved in 10 ml of water separately. After the complete dissolution of polymer, salt solution was added with it and stirred with a magnetic stirrer to get a homogenous solution. Then, it was cast into petri dish and allowed to evaporate the solvent at 60 °C in hot air oven.
Characterization
X-ray diffraction (XRD) analysis
The prepared samples were examined by Philips XPERT-PRO diffractometer at 2θ angles with Cu-Kα (λ = 1.5418 Å) radiation in the range 10° to 90° to investigate the nature of the prepared samples.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of the samples were recorded by SHIMADZU IR Affinity 1 spectrometer in the mid IR range 4000–400 cm−1 at a resolution of 1 cm−1 at ambient temperature, and the IR data were recorded in the transmittance mode.
Differential scanning calorimetry (DSC)
DSC measurements were carried out by TA instrument (Model No. DSC Q20 V24.10 Build 122) for the prepared samples at a heating rate of 10 °C min−1 under nitrogen atmosphere over the temperature range from 5 to 150 °C to determine the glass transition temperature of the samples.
Electrochemical impedance spectroscopy (EIS)
AC impedance analysis was done by HIOKI 3532–50 LCR Hi-Tester in the frequency range between 42 Hz and 1 MHz for the measurement of electrical properties. The sample was sandwiched between a pair of stainless steel electrodes, and impedance data were recorded.
Transference number measurement
The transference number corresponding to lithium ions (t+) was calculated by Wagner dc polarization technique. The prepared polymer electrolytes were subjected to polarization by supplying the dc voltage of 1.5 V. The initial and final currents were noted as a function of time. From the current values, cationic and anionic transport numbers were estimated.
Lithium ion conducting battery
With the highest conducting polymer membrane as electrolyte, a lithium ion conducting battery was fabricated and its discharge characteristics were studied.
Results and discussions
XRD
X-ray diffraction analysis is an informative tool to distinguish the crystalline and amorphous nature of the biopolymer electrolyte. Figure 1 shows the XRD patterns of pure I-Carrageenan and 1.0 g I-Carrageenan with different compositions of LiCl. It is evident that pure I-Carrageenan exhibits amorphous nature with its broad peak at 2θ = 22.17°. In addition, the sharp peaks are observed at 2θ = 31.90° and 45.74°, and the earlier studies show that these sharp peaks are due to impurities [14].
With the addition of 0.2 g LiCl with 1.0 g I-Carrageenan, the peak observed at 22.17° for pure membrane gets broadened with reduction in intensity. Further, with the addition of 0.3 g LiCl with 1.0 g I-Carrageenan, the relative intensity of the peak decreases when compared to 0.2 g LiCl. For 0.4 g LiCl with 1.0 g I-Carrageenan, it is observed that peak intensity again increases suggesting an increase in crystallinity. The relation between the intensity of the peak and the degree of crystallinity is reported by Hodge et al. [22]. Among all the membranes, 0.3 g LiCl with 1.0 g I-Carrageenan exhibits more amorphous nature with extended broadness and reduced intensity.
This increase in amorphous nature is due to the incorporation of salt with the polymer matrix, which in turn disturb the ordered arrangement of polymer network [12, 15]. Mobilization of ions is facilitated by polymer segmental motion in amorphous phase [23]. Hence, the sample with more amorphous phase is expected to yield high conductivity value due to the diffusion of ions. Generally, amorphous nature increases with the increasing concentration of salt. But, the relative increase in peak intensity on addition of 0.4 g LiCl with 1.0 g I-Carrageenan suggests the inability of the polymer to accommodate excess salt [24]. This result suggests the formation of aggregates of ions which decrease the amorphous nature of the polymer membrane. No XRD peaks corresponding to LiCl are found in all the prepared polymer membranes which show the complete dissolution of LiCl.
FTIR
FTIR is used to study the interaction between the polymer and the salt (LiCl) by observing the change in vibrational frequencies of polymer complexes with pure polymer membrane. Figure 2 shows the FTIR spectra of pure I-Carrageenan and 1.0 g I-Carrageenan with various compositions of LiCl polymer membranes.
In all the prepared polymer membranes, the vibrational peak at 928 cm−1 corresponds to C–O–C of 3,6-anhydrogalactose. This proves the existence of galactose of I-Carrageenan. The peaks at 803 and 848 cm−1 are attributed to –O–SO3 stretching vibration at d-galactose-2-sulphate and d-galactose-4-sulphate. It is observed that peak at 803 cm−1 of pure I-Carrageenan gets shifted to 805 cm−1 in all the compositions of LiCl with 1.0 g I-Carrageenan. The vibrational peak corresponding to O=S=O symmetric vibration at 1219 cm−1 of pure I-Carrageenan has been shifted to 1220, 1223, and 1223 cm−1 in the 1.0 g I-Carrageenan:0.2 g LiCl, 1.0 g I-Carrageenan:0.3 g LiCl and 1.0 g I-Carrageenan:0.4 g LiCl polymer membranes, respectively.
In all the complexes, C–O stretching at 1068 and 1155 cm−1 of pure I-Carrageenan has been shifted to 1069 and 1159 cm−1, respectively. The peak assigned to CH2 symmetric stretching at 1026 cm−1 of pure I-Carrageenan has been shifted to 1028 cm−1 in 1.0 g I-Carrageenan:0.3 g LiCl. The peak due to O–H stretching vibration at 3380 cm−1 of pure I-Carrageenan has been shifted to 3362, 3361 and 3356 cm−1 in the 1.0 g I-Carrageenan:0.2 g LiCl, 1.0 g I-Carrageenan:0.3 g LiCl and 1.0 g I-Carrageenan:0.4 g LiCl polymer membranes, respectively. Also, the intensity of these peaks corresponding to O–H stretching increases in all the complexes due to the addition of LiCl. This gives a strong indication of interactions between the oxygen atoms in the hydroxyl groups of the I-Carrageenan and the lithium salt [12]. Also, it is observed that there is reduction in intensity in all the prepared complex membranes from the bands 1500 cm−1 to 1000 cm−1. The change in intensity and shift in vibrational frequencies confirm the interaction between the host polymer and lithium salt. Table 1 provides the FTIR assignments of pure 1.0 g I-Carrageenan and all the prepared complexes.
DSC
Differential scanning calorimetry is a thermo-analytical technique which is used to study phase transitions. DSC measurements are carried out for the pure I-Carrageenan and complex samples at a heating rate of 10 °C/min. The glass transition temperature (Tg) of pure I-Carrageenan and different concentrations of LiCl with 1.0 g I-Carrageenan are observed, and the corresponding thermograms are depicted in Fig. 3. The value of Tg for pure I-Carrageenan is observed as 51.27 °C from DSC analysis. With the addition of 0.2 g, 0.3 g and 0.4 g LiCl with 1.0 g I-Carrageenan polymer, the observed Tg values are 62.97 °C, 57.12 °C and 72.65 °C, respectively.
Normally it is preferred to have a low Tg value for flexibility in polymer backbone [31]. But, with the incorporation of LiCl, Tg increases in the present work. The reason for the increase in Tg value may be explained as follows. This behaviour may be interpreted due to the intermolecular and intra-molecular coordination between Li+ cations and ester oxygen atoms in the I-Carrageenan polymer [32, 33]. As a consequence, polymer segments may not able to rotate freely. Thus, there is reduction in segmental motion, and this may lead hardening in polymer chains. To conclude, the coordination between Li+ and oxygen atom binding decreases the flexibility of the polymer backbone. However, for the highest conducting polymer membrane of 1.0 g I-Carrageenan with 0.3 g LiCl, a slight low Tg is observed than 0.2 and 0.4 g LiCl composition. This may be due to the variation in degree of dipole interaction between the polymer chain segments. There may be reduction in dipole interaction for the polymer (0.3 g LiCl) than other two compositions which may facilitate the ease of chain segment rotation along the chain backbone. Hence, low Tg may occur. Even though Tg value of 0.3 g LiCl is lower than all other polymer compositions, the result is not so appreciable since its value is higher than pure sample. Moniha et al. have reported the similar behaviour of Tg value with increasing salt concentration [18].
Calculation of crystallinity from DSC
The degree of crystallinity of the polymer electrolyte films is calculated from the enthalpy of fusion from the DSC curves, using the following equation [34]:
∆Hm is the enthalpy of the prepared polymer membrane and \( \varDelta {H}_m^p \) is the enthalpy of pure I-Carrageenan.
From the DSC curves, the enthalpy values are noted. The degree of crystallinity for the compositions 0.2 g LiCl, 0.3 g LiCl and 0.4 g LICl with 1.0 g I- Carrageenan are 34%, 15% and 36%. It is observed that % of crystallinity is low for the sample 1.0 g I-Carrageenan with 0.3 g LiCl which is in accordance with XRD result.
AC impedance analysis
Figure 4 shows the complex impedance plot for pure I-Carrageenan and 1.0 g I-Carrageenan with different concentrations of LiCl at ambient temperature. Inset graph shows the complex impedance plot of prepared complexes. Usually, cole-cole plot consists of depressed semicircle in the high frequency region followed by a spike in the low frequency region. Impedance data are usually analyzed by fitting an equivalent electric circuit model with simple electric circuit elements. A semicircle in the high frequency region is ascribed to bulk properties of the electrolyte with a parallel combination of resistor and a capacitor. The ion migration through the polymer matrix is represented by a bulk resistor, and immobile polymer chain is represented by a bulk capacitor. A spike in the low frequency region gives information of an electrical double layer which exists between a polymer electrolyte and electrode.
In this work, semicircle in the high frequency region is not found, and spike is only observed. It suggests that ionic conductivity is mainly due to mobile ions.
With the AC impedance experimental data, the value of bulk resistance is obtained from EQ software developed by Boukmap and EIS parameters are listed in Table 2 [35, 36]. Ionic conductivity of all the prepared polymer electrolytes is calculated by the following equation:
where l and A are the thickness and area of the polymer electrolyte sample. Rb is the bulk resistance of the polymer electrolyte [34, 37].
The measured conductivity value of pure I-Carrageenan is 1.46 × 10−5 S cm−1 which is in accordance with the result produced by Karthikeyan et al. [14]. Ionic conductivity is found to be increasing with increasing concentration of salt. With the addition of 0.2 g LiCl with 1.0 g I-Carrageenan, conductivity is increased to 1.32 × 10−3 S cm−1 which is two orders of magnitude greater than pure polymer. With 0.3 g LiCl composition, conductivity value of 5.33 × 10−3 S cm−1 is achieved. No enormous difference is found in conductivity value when compared to 0.2 g LiCl, but still, conductivity value is found to be increased with 0.3 g LiCl with I-Carrageenan. Further addition of salt decreases the conductivity value to 3.57 × 10−3 S cm−1. This is due to the formation of aggregation of ions or ion pairs. Generally, conductivity increases with amorphous nature and mobilization of charge carriers [38]. The amorphous nature enhances the ionic conductivity by creating free space and more voids for the movement of ions. Thus, addition of salt enhances the amorphous nature of the polymer and facilitates the mobilization of ions in the polymer matrix. This in turn increases the ionic conductivity value. But, when the salt concentration increases beyond a particular limit, the formation of ion pairs may occur. These ion pairs may hinder the mobilization of ions. Hence, ionic conductivity decreases. This is the reason for the decrease in conductivity value for 0.4 g LiCl with 1.0 g I-Carrageenan sample. Among all the prepared polymer electrolytes, 0.3 g LiCl with 1.0 g I-Carrageenan displays the highest conductivity of 5.33 × 10−3 S cm−1 at room temperature due to high amorphous nature of the polymer electrolyte. The conductivity values of all the prepared complexes are listed in Table 3.
Temperature dependence of conductivity
The prepared samples are subjected to temperature over the range of 303 K to 343 K to study the temperature dependence of ionic conductivity. Figure 5 shows the variation of logarithm of electrical conductivity with inverse of temperatures over the range 303–343 K. It has been observed that logarithm of ionic conductivity increases linearly with temperature. Higher temperature expands polymer matrix and produces more free volume. This facilitates the polymer segmental mobility and charge carriers mobility [24, 39].
A graph is plotted between log σ and 1000/T. The linear dependence of ionic conductivity with temperature obeys Arrhenius’ rule. Activation energy is found by the following equation [40]:
where σ0 is the pre-exponential factor, Ea is the activation energy, k is the Boltzmann constant and T is the temperature (K).
Table 3 lists the calculated ionic conductivity and activation energy values for 1.0 g I-Carrageenan:LiCl polymer electrolytes for various compositions at different temperatures. Moniha et al. reported that the activation energy value for pure I-Carraeenan is 0.28 eV [18]. The activation energy values of the prepared complexes vary from 0.19 to 0.23 eV. The highest conducting polymer membrane 1.0 g I-Carrageenan:0.3 g LiCl possesses low activation energy, and its value is 0.19 eV. This low value of Ea facilitates fast Li+ ion movement in the polymer matrix, which effectively increases ionic conductivity.
Conductance spectra analysis
Figure 6 illustrates the relation between logarithmic angular frequency and conductivity. In the plot, two regions are observed within the measured frequency range. The regions are low-frequency dispersion region and plateau region. At low frequencies, space charge polarization occurs at the electrode electrolyte interface [41]. It is assumed that ac conductivity of the complex samples is independent of frequency in the plateau region. Extrapolation of this plateau region to the y-axis gives dc conductivity (σdc) [7].
Plateau region is observed over the frequency range 500,000 Hz to 1,000,000 Hz where the conductivity is independent of frequency. If a line is extrapolated to zero frequency (Y axis) in this frequency range, the line meets Y axis at log σdc = − 2.27 for the highest conducting sample (1.0 g I-Carrageenan:0.3 g LiCl). The corresponding angular frequency value along x axis is 1 MHz and σdc is 5.36 × 10−3 S cm−1. The bulk conductivity value measured for the same sample is 5.33 × 10−3 S cm−1.
Dielectric spectra analysis
Dielectric property of complex polymer electrolytes yields information on ionic transport phenomena. By using the impedance data, the real and imaginary parts of the complex permittivity are calculated.
where ε′ is the real part of the complex permittivity and is known as dielectric constant, and ε″ is the imaginary part of the complex permittivity and is known as dielectric loss.
Figure 7a, b represents the frequency dependence of ε′ (ω) and ε″ (ω) at room temperature. Dielectric permittivity (ε′ and ε″) is high at low frequencies for all the complex polymer electrolytes due to space charge effect at the electrode electrolyte interface. As the frequency increases, dielectric permittivity (ε′ and ε″) is found to decrease. This is because the periodic reversal of the field occurs and it would not be possible for the charge carriers to orient in the field direction and hence less diffusion of charge carriers towards the field direction. A significant high dielectric permittivity is obtained for the highest conductivity polymer electrolyte 1.0 g I-Carrageenan:0.3 g LiCl.
The increase in ε′ and ε″ values at low frequency denotes the high charge carrier density in the low frequency region [20]. The very high ε′ and ε″ values are observed for 0.3 g LiCl based sample than 0.2 and 0.4 g LiCl-based samples in the low frequency region. This result is appreciable. But, difference in conductivity values for these samples is not high. The very high ε′ and ε″ values for the sample (1.0 g I-Carrageenan:0.3 g LiCl) in the low frequency region can be explained as follows: Polymer flexibility plays a vital role in determining the magnitude of dielectric permittivity in low frequency [42]. In the present work, the highest conducting sample (1.0 g I-Carrageenan:0.3 g LiCl) is comparatively flexible than the other two compositions as per the DSC result. This may lead a high value of ε′ and ε″.
Transference number measurement
Transference number is the important parameter to identify the contribution of ions to total electric current in the polymer electrolyte. The most important and simple technique to evaluate ionic transference number is the Wagner’s polarization method [43]. The highest conducting polymer sample is sandwiched between two blocking electrodes. Then, a dc voltage of 1.5 V is applied across the electrodes.
DC current is monitored as a function of time. When the circuit is closed, the instantaneous or initial current gives total conductivity of ions and electrons. The final stabilized current is the electron conductivity. The graph between polarization current and time is depicted in Fig. 8. The transference numbers (t+) and (t−)(t−) are calculated by the following equations [43, 44].
where Ii is the initial current and If is the final current.
t+ and t− are the transference numbers of ions and electrons.
The diffusion coefficients of Li+ (D+) and Cl− (D−) are found by the following equations using the measured values of cationic transference number (t+) and conductivity values [45, 46].
where n is the number of charge carriers related to the salt composition, k is the Boltzmann’s constant and T is the absolute temperature.
The ionic mobility of cations (μ+) and anions (μ−) of all the samples are calculated using the following equations [39]:
By using the above formulae, diffusion coefficient of cations (D+) and anions (D−) for the highest conducting polymer membrane (1.0 g I-Carrageenan: 0.3 g LiCl) are found to be 9.47 × 10−8 cm2/s and 3.94 × 10−9 cm2/s. The mobility of cations (μ+) and anions (μ−) of the sample 1.0 g I-Carrageenan:0.3 g LiCl is 3.62 × 10−6 cm2 Vs−1 and 1.51 × 10−7 cm2 Vs−1. It is observed that D+ is greater than D− and μ+ is greater than μ−. Also, the value of diffusion coefficient and mobility of the sample (1.0 g I-Carrageenan:0.3 g LiCl) are higher than the remaining compositions. The high value of ionic conductivity of this membrane is due to high mobility and diffusivity.
Table 4 lists transference number, diffusion coefficients and mobility values of cations and anions. Transference number for the prepared polymer membrane varies from 0.94 to 0.96. The highest conducting polymer membrane (0.3 g LiCl with 1.0 g I-Carrageenan) exhibits highest cationic transference number of 0.96 which is close to unity, and it is in accordance with conductivity result. This result suggests that conductivity is mainly due to Li+ cations and negligible contribution by anions. (A very low t− value may be due to the following reason: The ionic radius of Cl− ion is 0.181 nm. Due to its higher radius, the mobility of Cl− ion will be very small. The mobility of Li+ ion is high because of its smaller radius 0.076 nm. So, the transference number of Cl− is very low).
Fabrication of Li-ion conducting battery
Li-ion conducting battery is fabricated using the highest conducting polymer membrane as the electrolyte. Anode for the battery is fabricated in the form of a pellet by taking zinc powder, ZnSO4·7H2O and graphite powder in a suitable ratio of 3:1:1. Similarly, cathode pellet is prepared by mixing PbO2, V2O5, graphite and the prepared highest polymer electrolyte membrane (1.0 g I-Carrageenan:0.3 g LiCl) in a suitable ratio of 8:2:1:0.5 [19]. Then, the battery is fabricated by keeping the highest polymer electrolyte membrane (1.0 g I-Carrageenan:0.3 g LiCl) as an electrolyte in-between the anode and cathode pellets.
The anode and cathode reactions are given below [39].
The anode reaction is given as follows:
The cathode reaction is given as follows:
H+ ions produced at the anode site repel Li+ ions in the polymer. Li+ ions from the polymer site reach the cathode site by the process of hopping mechanism [47]. Similar electrochemical testing was reported by using the same anode and cathode material for Li+ ion conduction [19, 20, 39, 47,48,49].
Testing results
Figure 9 shows the voltage versus time performance of a battery. During discharge, the sharp decrease in cell voltage is observed due to polarization [50]. After that, the open circuit voltage (OCV) remains stable at 1.77 V for 95 h. Also, a short circuit current of 670 μA is observed. When a load resistance of 100 KΩ is connected across the cell, the voltage drops to 1.6 V from 1.77 V and remains stable for 72 h. Kingslin et al. have fabricated a battery and produced OCV of 1.7 V with PVA-PAN doped with LiBr [48]. Monisha et al. have reported a OCV of 1.8 V with cellulose acetate lithium nitrate combination [39]. Premalatha et al. have reported a OCV of 1.63 V with LiBr: tamarind seed polysaccharide [19]. Perumal et al. reported OCV of 1.93 V by incorporating lithium chloride with pectin [20]. Sampath Kumar et al. have studied tamarind seed polysaccharide with LiCl and fabricated a lithium ion conducting battery with a OCV of 1.57 V [47]. The measured cell parameters are listed in Table 5.
Conclusion
The new solid biopolymer electrolytes based on I-Carrageenan with LiCl have been prepared by solution casting technique. XRD studies confirm the amorphous nature of the prepared SPEs. Polymer–salt complexation has been studied by FTIR. The highest conductivity of 5.33 × 10−3 S cm−1 has been obtained for the sample (1.0 g I-Carrageenan:0.3 g LiCl) at ambient temperature. EIS study reveals that conductivity increases with temperature. Dielectric permittivity has been studied from the real and imaginary parts of the impedance data. Transference number measurements conclude that conductivity is dominated by cations. The Li-ion conducting battery has been constructed with the highest conducting polymer electrolyte, and parameters were evaluated. All these results conclude that I-Carrageenan and LiCl combination is a suitable combination to use as a promising electrolyte in solid-state devices.
References
Changyu T, Ken H, Qiang F, Pulickel MA, Haleh A (2012) High ion conducting polymer nanocomposite electrolytes using hybrid nanofillers. Nano Lett 12:1152–1156
Virya A, Liana K (2017) Polyacrylamide-lithium chloride polymer electrolyte and its applications in electrochemical capacitors. Electrochem Commun 74:33–37
Rani MSA, Rudhziah S, Ahmad A, Mohamed NS (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bastfiber. Polymers 6:2371–2385
Vijaya N, Selvasekarapandian S, Sornalatha M, Sujithra KS, Monisha S (2017) Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X=Cl, Br). Ionics 23(10):2799–2808
Sohaimy MIH, Isa MIN (2015) Effect of ammonium carbonate salt concentration on structural and ionic conductivity of cellulose based solid polymer electrolytes. Fibers and Polymers 16(5):1031–1034
Rudhziaha S, Rani MSA, Ahmad A, Mohamed NS, Kaddami H (2015) Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind Crop Prod 72:133–141
Chatterjee B, Kulshrestha N, Gupta PN (2016) Nano composite solid polymer electrolytes based on biodegradable polymers starch and poly vinyl alcohol. Measurement 82:490–499
Tuvikenea R, Truusa K, Vaherb M, Kailasb T, Martinc G, Kersenc P (2006) Extraction and quantification of hybrid carrageenans from the biomass of the red algae Furcellaria lumbricalis and Coccotylus truncates. Proc Estonian Acad Sci Chem 55(1):40–53
Yao Z, Wu H, Zhang S, Du Y (2014) Enzymatic preparation of κ-carrageenan oligosaccharides and their antiangiogenic activity. Carbohydr Polym 101(0):359–367
Volery P, Besson R, Schaffer-Lequart C (2004) Characterization of commercial Carrageenans by Fourier transform infrared spectroscopy using single-reflection attenuated total reflection. J Agric Food Chem 52:7457–7463
Tanaka T, Lu T, Yuasa S, Yamaura K (2001) Structure and properties of poly (vinyl alcohol)/κcarrageenan blends. Polym Int 50(10):1103–1108
Mobarak N, Jumaah FN, Ghani MA, Abdullah MP, Ahmad A (2015) Chemical interaction and conductivity of carboxymethyl κcarrageenan based green polymer electrolyte. Electrochim Acta 175:224–231
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting κ-carrageenanchitosan electrolytes. Funct Mater Lett 1:195–201
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2016) Proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23:2775–2780
Jumaah FN, Mobarak N, Ahmad A, Ghani MA, Rahman MYA (2014) Derivative of iota-carrageenan as solid polymer electrolyte. Ionics 21(5):1311–1320
Shamsudin IJ, Ahmad A, Hassan NH, Kaddami H (2016) Biopolymer electrolytes based on carboxymethyl-carrageenan and imidazolium ionic liquid. Ionics 22(6):841–851
Ng CA, Camacho DH (2015) Polymer electrolyte system based on carrageenan-poly(3,4-ethylenedioxythiophene) (PEDOT) composite for dye sensitized solar cell. IOP Conf Series: Mater Sci Eng 79:012020
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2017) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J on-Crystalline solids 481:424–434
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Selvalakshmi S, Vinoth Pandi D (2017) Tamarind seed polysaccharide (TSP)-based li-ion conducting membranes. Ionics 23(10):2677–2684
Perumal P, Christopher Selvin P, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its application to electrochemical devices. Ionics. https://doi.org/10.1007/s11581-018-2507-5
Leena Chandra MV, Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S (2017) Study of PVAc-PMMA-LiCl polymer blend electrolyte and the effect of plasticizer ethylene carbonate and nano filler titania on PVAc-PMMA-LiCl polymer blend electrolyte. J Polymer Eng 37(6):537–645
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of ater in semicrystalline poly (vinyl alcohol) films. Polymer 37(8):1371–1376
Ravi M, Song S, Wang J, Wang T, Nadimicherla R (2015) Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J Mater Sci Mater Electron 2:1370–1377
Hu P, Chai J, Duan Y, Liu Z, Cui G, Chen L (2016) Progress in nitrile-based polymer electrolyte for high performance lithium batteries. J Mater Chem A. https://doi.org/10.1039/C6TA02907H
Chopin T, Whalen E (1993) A new and rapid method for carrageenan identification by FT IR diffuse reflectance spectroscopy directly on dried, ground alga material. Carbohydr Res Res 246:51–59
Jumaah FN, Ahmad A, Mobarak NN (2013) Characterization of iota-carrageenan and its derivative based green polymer electrolytes. AIP Conference Proceedings 1571:768. https://doi.org/10.1063/1.4858748
Maurya K, Neelam Srivastava SA, Hashmi S, Chandra (1992) Proton conducting polymer electrolyte: II poly ethylene oxide+ NH4I system. J Mater Sci 27(23):6357–6364
Pereira L, Amado AM, Critchley AT, van de Velde F, PJA R-C (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FTRaman). Food Hydrocoll 23(7):1903–1909
Campo VL, Kawano DF, da Silva DB Jr, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym 77:167–180
Mobarak N, Ramli N, Ahmad A, Rahman MYA (2012) Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid State Ionics 224(0):51–57
Monisha S, Selvasekarapandian S, Mathavan T, Milton Franklin Benial A, Manoharan S, Karthikeyan S (2016) Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J Mater Sci: Mater Electron 27(9):9314–9324
NirmalaDevi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23(12):3377–3388
Rodrigues LC, Barbosa PC, Silva M, Smith MJ (2007) Electrochemical and thermal properties of polymer electrolytes based on poly (epichlorohydrin-co-ethylene oxide-co-ally glycidyl ether). Electrochim Acta 53(4):1427–1431
Taib NU, Idris NH (2014) Plastic crystal-solid biopolymer electrolytes for rechargeable lithium batteries. J Membr Sci 468:149–154
Boukamp BA (1986) A non linear least squares fit procedure for analysis of immittance data of electrochemical studies. Solid State Ionics 20:31–44
Boukamp BA (1986) A package of impedance admittance data analysis. Solid State Ionics 18&19:136–140
Ramesh S, Shanti R, Morris E (2013) Employment of [Amim] Cl in the effort to upgrade the properties of cellulose acetate based polymer electrolytes. Cellulose 20:1377–1389
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Nithya H, Iwai Y, Kawamura J (2014) Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 21:1017–1029
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Latha PM (2016) Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 23:2697–2706
Malathi J, Kumaravadivel M, Brahmanandhan GM, Hema M, Baskaran R, Selvasekarapandian S (2010) Structural, thermal and electrical properties of PVA-LiCF3SO3 polymer electrolyte. J Non-Crystalline solids 356:2277–2281
Ramesh S, Arof AK (2001) Structural, thermal and electrochemical cell characteristics of poly (vinyl chloride)-based polymer electrolytes. J Power Sources 99:41–47
Popielarz R, Chiang CK, Nozaki R, Obrzut J (2001) Dielectric properties of polymer/ferroelectric ceramic composites from 100 Hz to 10 GHz. Macromolecules 34:5910–5915
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26:1597–1601
Ramlli MA, Isa MIN (2016) Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped ammonium fluoride. J Phys Chem B 120(44):11567–11573
Tan W, Arof AK (2006) Transport properties of hexanoyl chitosan-based gel electrolyte. Ionics 12:149–152
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Arunkumar D, Nithya H, Junichi K (2016) Structural, electrical conductivity, and transport analysis of PAN–NH4Cl polymer electrolyte system. Ionics 22:1085–1094. https://doi.org/10.1007/s11581-016-1645-x
Sampath Kumar L, Christopher Selvin P, Selvasekarapandian S (2018) Tamarind seed polysaccharide biopolymer membrane for Lithium ion conducting battery. Ionics. https://doi.org/10.1007/s11581-018-2541-3
Kingslin Mary Genova F, Selvasekarapandian S, Karthikeyan S et al (2015) Study on blend polymer (PVA - PAN) doped with lithium bromide. Polymer science 57:851–862
Kingslin Mary Genova F, Selvasekarapandian S, Vijaya N, Sivadevi S, Premalatha M, Karthikeyan S (2017) Lithium ion-conducting polymer electrolytes based on PVA–PAN doped with lithium triflate. Ionics 23:2727–2734
Janaki Rami Reddy T, Achari VBS, Sharma AK, Narasimha Rao VVR (2007) Preparation and electrical characterization of (PVC+KBrO3) polymer electrolytes for solid state battery applications. Ionics 13:435–439
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chitra, R., Sathya, P., Selvasekarapandian, S. et al. Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25, 2147–2157 (2019). https://doi.org/10.1007/s11581-018-2687-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2687-z