Abstract
This paper presents results of studies on dc electrical conductivity and transference number measurements on potassium bromate (KBrO3) complexed polyvinyl chloride (PVC) films prepared by solution cast technique. Temperature dependence of dc electrical conductivity and transference number data indicated the dominance of ion type charge transport in these specimens. The magnitude of conductivity increased with increase in concentration of the salt and temperature. Using this (PVC + KBrO3) electrolyte, solid-state electrochemical cells were fabricated, and their discharge profiles were studied under a constant load of 100 kΩ. Several cell profiles such as open circuit voltage, short circuit current, power density, and energy density associated with these cells were evaluated and were reported. The features of complexation of the electrolytes were studied by X-ray diffraction and Fourier transform infrared spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of polymeric systems with high ionic conductivity is one of the main objectives in polymer research. This is due to their potential applications as electrolytes in solid-state batteries [1–4], in view of their high ionic conductivity, high energy density, wide electrochemical stability, and easy processability.
The main aim of the present work is to develop a polymer electrolyte with polyvinyl chloride (PVC) as the host. The widespread use of PVC is attributed mainly to the presence of lone pair electrons at the chlorine atom where inorganic salts can be solvated. The dipole–dipole interaction between the hydrogen and chlorine atoms, which can stiffen the polymer backbone and perhaps make the PVC–salt complexation difficult, can be weakened by the addition of plasticizers. With these properties, it should be possible to turn PVC into a good ion-conducting polymer [5, 6].
Over the past few years, many polymeric electrolytes based on various salts dissolved in polymers have been widely investigated because of their potential viability in high-performance batteries. Such electrolytes have been based mainly on alkali metal salt systems with special emphasis on lithium. A relatively small number of studies have been reported on the development of PVC-based polymer electrolyte systems [7–10]. Limited efforts have been made on solid polymer electrolytes and batteries based on potassium complexed systems. Apart from its scientific interest, the use of potassium has several advantages over lithium counterparts. Potassium is much more abundant and less expensive than lithium. The softness of this metal makes it easier to achieve and maintain contact with other components in the battery. Further, potassium is more moisture-resistant than lithium. Keeping these aspects in view, a study based on a new K+ ion conducting polymer electrolyte was undertaken.
Experimental
Films (thickness, ∼130 μm) of pure PVC + KBrO3 in the wt.% ratios 95:05, 90:10, 85:15, and 80:20 for conductivity measurements and battery discharge profiles were prepared by the solution-cast technique using tetrahydrofuran (THF) as solvent. The mixture of these solutions was stirred for 8–10 h, cast onto polypropylene dishes, and evaporated slowly at room temperature. The final product was vacuum dried thoroughly at 10−3 mbar.
The dc conductivity was studied at room temperature as a function of composition of KBrO3 using a conductivity cell designed in our laboratory [11]. The total ionic transport number (t ion) was evaluated by means of Wagner’s polarization technique [12]. In this technique, freshly prepared polymer electrolyte films were polarized in the configuration K/polymer electrolyte/C under a dc bias (step potential of 1.5 V). The resulting current was monitored as a function of time. After polarizing the electrolyte, the transport number (t ion) was calculated from the initial current I i and the final residual current I f, i.e.,
Using (PVC + KBrO3) as the electrolyte, electrochemical cells with configuration K / (PVC + KBrO3) / (I2 + C + electrolyte) were fabricated. The discharge characteristics of the cells were studied under a constant load of 100 kΩ. Details regarding the circuit and the electrochemical cell are given elsewhere [13].The X-ray diffraction (XRD) studies were performed by means of Seifert X-ray diffractometer in the range 10–65°. Infrared spectra profiles were obtained using Perkin-Elmer Fourier transform infrared (FTIR) spectrophotometer in the range 500–4,000 cm−1 in the transmittance mode.
Results and discussion
XRD analysis
In order to investigate the influence of potassium salt on PVC structure, XRD studies were performed. Figure 1a–f shows the XRD patterns of pure PVC, KBrO3 salt, and PVC complexed with KBrO3 salt. Comparison of the XRD patterns of complexed PVC films with those of pure PVC and KBrO3 reveals the following differences. Peaks observed for 2θ values around 26° are less intense in complexed PVC films compared to those in pure PVC films. This indicates that the addition of KBrO3 salt causes a decrease in the degree of crystallinity of the PVC complex. This is in agreement with the results reported by Uma et al. [14] and indicates that the polymeric films are composed of a combination of semicrystalline and amorphous phases [15]. The crystalline peaks for 2θ values at 20, 27, 29, and 40° corresponding to KBrO3 (Fig. 1f) are absent in complexed PVC. This indicates the absence of any excess (uncomplexed) salt in the complexed polymer electrolyte films. Therefore, it may be confirmed that complexation has taken place in the amorphous phase.
FTIR studies
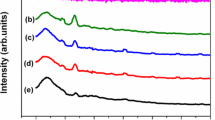
FTIR is a powerful tool to study the local structural changes. FTIR spectroscopic studies on PVC complexes are expected to throw light on mainly two kinds of information: (1) the conformation of the polymer chain and (2) the position of the ion in the polymer chains, which are essentially the finger prints of different molecules and structures [16]. The infrared spectra would be sensitive in both situations whether the complexation has occurred in crystalline or amorphous phase. The FTIR spectra of pure PVC, KBrO3, and polymer complexes are shown in Fig. 2a–f. These bands are of complex origin and depend on the conformational structure of the polymer and on the spatial position of the atoms surrounding the C–Cl bond (Fig. 2a). The bands of the spectrum in the region of 600–700 cm−1 (Fig. 2a) are characteristic of C–Cl stretching vibrations. CH2 deformation vibration band is observed at 1,429 cm−1, while the band at 961 cm−1 is assigned to the weak trans CH wagging. The bands at 696 and 636 cm−1 are attributed to the cis CH wag vibration. The band at 1,074 cm−1 is assigned to the CH rocking vibration in pure PVC, which gets shifted to 1,124, 1,132, 1,076, and 1,068 cm−1 in the polymer complex. This suggests that the K+ ion may be located on the vinyl chloride of the polymer chain [-CH2-CHCl-]n. The peaks at 3,436, 2,919, 2,358, 1,565, 785, and 428 cm−1 corresponding to KBrO3 disappeared in the polymer complex. The bands of PVC at 2,964, 2,872, 1,718, 1,579, and 489 cm−1 were shifted in the polymer complexes. From the above analysis, the complex formation has been confirmed.
DC conductivity studies
The conductivity of pure PVC was found to be about 0.78 × 10−9 S/cm at room temperature. The change in room temperature (303 K) conductivity with composition of KBrO3 is steep up to a composition of 5 wt.%; however, thereafter, it increases slowly. Figure 3 shows the conductivity vs composition plots for pure and different compositions of (PVC + KBrO3) polymer electrolyte in the temperature range 325–423 K. Within the temperature range under study, the conductivity was found to increase with temperature in both pure PVC and (PVC + KBrO3) polymer electrolyte systems. The increase in conductivity with increasing dopant concentration may be explained on the basis of the charge transfer complexes (CTCs) in the host lattice. These charge transfer complexes increase the electrical conductivity by providing additional charges in the lattice.
Figure 4 shows conductivity vs temperature plots, which follow an Arrhenius nature throughout, with two different activation energies in two regions (region I and II). In region-I, the conductivity of pure PVC increased slowly with increase in temperature up to 100 °C. Around a particular temperature range (100–120 °C), there was a sudden increase in the variation of σ leading to region-II. This may be due to a transition from a semicrystalline phase to an amorphous phase. According to Druger et al. [17, 18], in polymer electrolytes, the change of conductivity with temperature is due to segmental motion, which results in an increase in the free volume of the system. This increase in free volume would also facilitate the motion of ionic charge [19].
The linear variation of σ vs 103/T plots can be expressed by the relation,
where σ 0 is a preexponential factor, E a is the activation energy, and k is the Boltzmann constant.
The activation energies in the two regions evaluated from the above plots are tabulated in Table 1. The activation energies show a decreasing trend with increasing dopant concentration, which may be explained in terms of formation of charge transfer complexes.
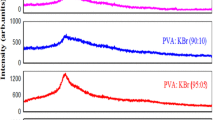
Transference number measurements
The transference number measurements of (PVC + KBrO3) electrolyte systems were evaluated using Wagner’s polarization technique [12]. In this technique, the dc current is monitored as a function of time on application of a fixed dc voltage across the cell in the configuration K/polymer electrolyte/C. After polarization of the cell with 1.5 V dc, the current versus time plots were obtained (Fig. 5). The calculated transference numbers, t ion and t ele, are given in Table 2. These values suggest that the charge transport in these polymer electrolyte films is predominantly due to ions; only a negligible contribution comes from the electrons.
Discharge profiles of solid state battery
Using the (PVC + KBrO3) polymer electrolyte, electrochemical cells were fabricated in the configuration K (anode) / (PVC + KBrO3) / (I 2 + C + electrolyte; cathode). The discharge characteristics of the electrochemical cell for a constant load of 100 KΩ are shown in Fig. 6. The initial sharp decrease in the voltage in these cells may be due to polarization and/or the formation of a thin layer of potassium salt at the electrode–electrolyte interface. The open circuit voltage and short-circuit current of the PVC + KBrO3 (95:05) cell were 2.89 V and 940 μA, respectively. For the PVC + KBrO3 (90:10) cell, these values were 2.97 V and 1,050 μA; for the PVC + KBrO3 (85:15) cell, they were 3.10 V and 1,210 μA; and for the PVC + KBrO3 (80:20) cell, they were 3.22 V and 1,420 μA, respectively. The other parameters for these cells were evaluated and are listed in Table 3. The data indicate that cell parameters are better in cells with composition (80:20).The cell parameters of the present study are superior to these of Na+-based [20–23] and Ag+-based [24] cells reported in literature. Hence, these cells offer interesting alternatives for room temperature solid state batteries.
Discharge characteristics of solid state electrochemical cell in the configuration a K / (PVC + KBrO3) (95:05) / 2 + C +electrolyte; b K / (PVC + KBrO3) (90:10) / I 2 + C + electrolyte; c K / (PVC + KBrO3) (85:15) / I 2 + C+ electrolyte); d K / (PVC + KBrO3) (80:20) / I 2 + C + electrolyte, polymer electrolyte system
Conclusions
The XRD study reveals the amorphous nature of the polymer electrolytes. The complex formation in polymer electrolyte system has been confirmed from FTIR studies. These studies indicate that the dc conductivity of pure PVC and (PVC + KBrO3) films increased with increasing temperature and dopant concentration. Transference number data showed that the conductivity is mainly due to ions rather than electrons. Parameters were evaluated for (PVC + KBrO3) polymer electrolyte cells. These cells exhibit better performance, which indicates that these cells are suitable for fabricating solid-state batteries.
References
Hooper A, North JM (1983) Solid State Ionics 9:1161
Mac Callum JR, Vincent CA (1987/1989) Polymer electrolytes reviews 1 and 2. Elsevier, London
Acosta JL, Morales E (1996) Solid State Ionics 85:85
Kim JY, Kim SH (1999) Solid State Ionics 124(1–2):91
Moryossef A, Bonnat M, Fouletier M, Hitcher P (1991) Proc Ris Int Symp 1:210
Cole M, Latnam RJ, Linford RG, Sheldon MH (1991) Mater Res Soc Symp Proc 1:210
Alamgir M, Abraham K (1993) J Electrochem Soc L96:140
Sukeshini M, Nishimoto A, Watanabe M (1996) Solid State Ionic 86:385
Ramesh S, Arof AK (2000) Solid State Ionics 136/137:1197
Rajendran S, Uma T, Mahalingam T (1999) Ionics 5:227
Lakshmi Narayana K (1995) PhD thesis, S.V. University, September
Wagner JB, Wagner C (1957) J Chem Phys 26:1597
Jaipal Reddy M, Sreenivasa Reddy D, Sreepathi Rao S, SubbaRao UV (1995) Mater Lett 23:129
Uma T, Mahalingam T, Stimming U (2004) J Mater Sci 39:2901
Li J, Huang X, Chen K (2000) J Electrochem Soc 147(7):2653
Nakamoto K (1986) Infrared and raman spectra of inorganic and coordination compounds. Wiley, New York
Druger SD, Nitzam A, Ratner MA (1983) J Chem Phys 79:3133
Druger SD, Ratner MA, Nitzam (1985) A Phys Rev B 31:3939
Miyamoto T, Shibayama K (1973) J App Phys 44:5372
Sreepathi Rao S, Jaipal Reddy M, Laxminarasaiah E, Subba Rao UV (1995) J Mater Sci Eng B 33:173
Sreepathi Rao S, Jaipal Reddy M, Subramanyam S, Subba Rao UV (1999) J Mater Sci Eng B 64:107
Madhu Mohan V, Raja V, Sharma AK, Narasimha Rao VVR (2005) Mater Chem Phys 94:177
Subba Reddy Ch.V, Jin AP, Zhu QY, Maind LQ, Chen W (2006) Eur Phys J E 19:417
Sreepathi Rao S, Rao KVS, Shareefuddin Md, M, Subba Rao UV, Chandra S (1994) Solid State Ionics 67:331
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janaki Rami Reddy, T., Achari, V.B.S., Sharma, A.K. et al. Preparation and electrical characterization of (PVC + KBrO3) polymer electrolytes for solid state battery applications. Ionics 13, 435–439 (2007). https://doi.org/10.1007/s11581-007-0129-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0129-4