Abstract
Novel biopolymer electrolytes based on carboxymethyl kappa-carrageenan (CMҡ-carrageenan) and ionic liquid 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) have been successfully developed. Strong coordination and hydrogen bonding interaction of [Bmim]Cl with the biopolymer were detected by Fourier transform infrared (FTIR) spectroscopy. The efficient function of [Bmim]Cl as the charge carrier in the system was reflected by electrochemical impedance spectroscopy (EIS) where the highest ionic conductivity (σ) of (5.76 ± 0.20) × 10−3 S cm−1 was achieved at ambient temperature (298 K) upon 30 wt.% of [Bmim]Cl inclusion. X-ray diffraction (XRD) analysis confirmed that the addition of ionic liquid did not alter the prominent amorphous phase of CMҡ-carrageenan. Analysis of scanning electron microscopy (SEM) proved the strong interaction of [Bmim]Cl with the biopolymer matrix. The highest conducting biopolymer electrolyte showed an electrochemical stability up to 3.0 V, whereas the transference number measurement revealed that ions are the major elements that contribute to the conductivity with 0.970 ion transference number.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past decades, the studies of electrolytes based on biopolymers have been actively progressing due to the desirable properties of biopolymers and the concern on environmental issues. Biopolymers offer great choices as they are readily biodegradable, renewable, abundant, and non-hazardous compared to synthetic polymers. Numerous studies of biopolymers such as cellulose and its derivatives either as a polymer host or filler in an electrolyte system have been reported [1–6]. Biopolymer electrolytes based on chitosan [7–11], corn starch [12–16], and agar [17–21] have been profusely reported as well. These trends show that biopolymers have the ability to be applied in the electrolyte systems. Earlier investigations have proven that biopolymers are the suitable candidates for the electrolyte system as they possess great thermal stability [6, 15, 17–19], high mechanical property [2, 4, 6], improved flexibility [1, 5, 7, 18], sufficient electrochemical stability [1, 7, 16, 18], and high ionic conductivity [1–21]. These characteristics therefore enable them to be applied in various electrochemical device applications such as dye-sensitized solar cells [11, 20], fuel cells [5, 9], and batteries [1, 5, 18].
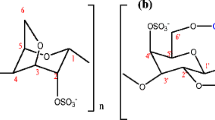
ҡ-Carrageenan is among the biopolymers that have been extensively used in a variety of commercial applications such as gelling, thickening, and stabilizing agents especially in food products [22]. Apart from that, carrageenans are used in pharmaceutical, medicine, cosmetics, and industrial application [23–25]. ҡ-Carrageenan is an anionic sulfated linear polysaccharide extracted from red seaweed (Rhodophyceae) with a linear backbone built of alternating (1 → 3)-linked β-d-galactopyranose and (1 → 4)-linked α-d-galactopyranose [26, 27]. The selection of ҡ-carrageenan in this study rather than other types of carrageenan such as iota-carrageenan and lambda-carrageenan is because its chemical structure has the most hydroxyl group in the polymer chain. The hydroxyl group is expected to be more favorable in reacting with the ionic liquid. Moreover, ҡ-carrageenan has been reported to produce hard gel and has better miscibility with 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) compared to other two carrageenans that gave the formation of soft gels with [Bmim]Cl [28]. Carboxymethyl ҡ-carrageenan (CMҡ-carrageenan) is a derivative of ҡ-carrageenan that is synthesized via chemical modification [29] in which the modification has proven to improve the ionic conductivity [30]. The chemical structure of CMҡ-carrageenan is shown in Fig. 1. The utilization of CMҡ-carrageenan as a polymer matrix in this work is based on the great properties of CMҡ-carrageenan as previously reported [30–32] which comprise of high ionic conductivity, high flexibility, low crystallinity, and most essentially the chemical structure of the carboxymethyl ҡ-carrageenan itself which is rich in electron-donating groups such as hydroxyl (OH−), sulfate (OSO3 −), and carboxylate (COO−). These polar groups are expected to be the active sites for coordination or interaction with the charge carrier in the system. The high number of active sites is expected to favor the enhancement of ionic conductivity as there will be more frequent interaction between the charge carrier and the biopolymer matrix [30, 33].
Herein, ionic liquid 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) has been chosen to act as the charge carrier in this system. [Bmim]Cl has been numerously reported as a dissolution media for highly rigid and crystalline structure biopolymers such as cellulose, chitin, and chitosan [34–39]. The proficiency of [Bmim]Cl in the pretreatment of biomass has also been reported [40, 41]. The dissolution of carrageenan in [Bmim]Cl has also been reported in previous studies [28]. Hence, the effectiveness of [Bmim]Cl to interact with biopolymers is described to be mainly influenced by the anion (Cl−). The anion plays a major role in determining the ability of the ionic liquid to accept hydrogen bond which is characterized by its basicity (β) value measured by Kamlet-Taft parameters [42, 43]. The β value for [Bmim]Cl is 0.890, which is relatively high compared to the β values reported for other 1,3-dialkylimidazolium ionic liquids such as 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][OTf]) (0.483), 1-butyl-3-methylimidazolium dicyamide ([Bmim][N(CN)2]) (0.596), and 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([Bmim]NTF2) (0.240) and also higher compared to common fluorinated ionic liquids such as 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]) (0.376) and 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) (0.210) [40, 42, 43]. Thus, the high basicity of [Bmim]Cl implies that the chloride ion (Cl−) has a strong tendency to accept hydrogen bond by the coordination interaction with hydrogen in the biopolymer network. This type of coordination interaction is also important in a biopolymer-based electrolyte system as the continuous interactions and movement of the charge carrier within the biopolymer network will generate ionic conductivity. Thus, based on this exceptional ability, [Bmim]Cl is proposed to serve as the main conducting ions in the biopolymer network of CMҡ-carrageenan. To date, the investigation on the addition of ionic liquid to CMҡ-carrageenan-based electrolyte system has never been explored. The chemical structure of [Bmim]Cl is shown in Fig. 2.
Apart from that, ionic liquid offers a lot of fascinating characteristics that could be useful in the electrolyte system such as high ionic conductivity, wide electrochemical stability window, low melting point (typically below 100 °C), and high chemical and thermal stability [44, 45]. In addition, the flame-retardant properties and negligible vapor pressure of ionic liquid are the added values that contribute to the enhanced safety features of the electrolytes.
This work describes an electrolyte system based on carboxymethyl ҡ-carrageenan and the interaction of ionic liquid [Bmim]Cl with biopolymer matrix. The physical and chemical changes of the biopolymer with the inclusion of [Bmim]Cl are discussed based on the characterizations performed.
Experiment
Materials
Ҡ-Carrageenan was commercially obtained from Tacara Corporation Sdn. Bhd. (Sabah, Malaysia). Eucheuma cottonii seaweeds are typically found below the low tide mark to the upper subtidal zone of a reef, growing on the sand to rocky seafloor areas along a coral reef, where water movement is slow to moderate. The molecular weight of ҡ-carrageenan was 1.41 × 106 Da. Acetic acid and ionic liquid 1-butyl-3-methylimidazolium chloride were purchased from Sigma-Aldrich (USA). All materials were used without further purification.
Preparation of thin film
ҡ-Carrageenan was used as a precursor to prepare carboxymethyl ҡ-carrageenan (CMҡ-carrageenan) according to a published method [46]. One gram of CMҡ-carrageenan with different concentrations of [Bmim]Cl was prepared via solution casting technique. CMҡ-carrageenan powder was dissolved in 50 ml 1 % (v/v) solution of acetic acid and stirred overnight in order to obtain a complete dissolution of biopolymer. Different molar ratios of [Bmim]Cl (5–30 wt.%) were measured and added to the CMҡ-carrageenan solution separately in a glove box. The mixed solution was further stirred for 24 h. After complete dissolution, the solution was poured into a Teflon petri dish and left to dry for several days under the fume hood. The resulted thin film was then peeled and stored in a desiccator with phosphorous pentoxide (P2O5) powder to prevent any moisture contact. The film was further dried in a vacuum oven until constant weight was obtained to eliminate any remaining moisture.
Characterization
Attenuated total reflectance Fourier transform infrared analyses
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) analyses were performed using the Perkin-Elmer Spectrum 2000 in the range of 4000–650 cm−1. The scanning resolution was 4 cm−1. The analyses were carried out to observe any changes and shifts of the characteristic band due to the chemical interaction of chitosan with the charge carrier.
X-ray diffraction
X-ray diffraction (XRD) measurements were performed using model D5000 Siemens. The data were collected from a range of diffraction angle 2θ from 3° to 60° at a rate of 0.05°s−1 to determine whether the sample is crystalline, semi-crystalline, or amorphous.
Morphology
Investigation on the morphologies of the polymer electrolytes was examined using FESEM Supra 55VP model at 10,000 magnifications.
Impedance spectroscopy
The measurement of the bulk resistance (R b) of the sample was carried out by alternating current (AC) impedance measurement using a high-frequency response analyzer (HFRA; Solartron 1260 Schlumberger) and an electrochemical interface (SI 1286) in the frequency ranging from 1 Hz to 10 MHz with an amplitude of 10 mV. The sample was sandwiched between two blocking stainless steels under the spring pressure with a contact area of 2.0 cm2. The bulk resistance (R b) was determined from the equivalent circuit analysis by using Zview analyzer software. The conductivity values (σ) of electrolyte were calculated based on equation (1)
where t is the thickness of the sample, R b is the bulk resistance, and A is the contact area. All measurements were conducted at ambient temperature (298 K).
Electrochemical stability
Linear sweep voltammetry (LSV) technique was used to determine the electrochemical stability window of the polymer electrolyte. The sample was placed between two stainless steel blocking electrodes using 1 mVs−1 scan rate from 0 to 5 V.
Transference number measurement
Transference number measurement (TNM) was carried out in order to investigate the main conducting element in the polymer electrolyte system using the DC polarization method. The ion transference number t ion in the electrolyte was determined by monitoring the current as a function of time. Fixed 1.0 V dc voltage was applied across the sample sandwiched between two stainless steel electrodes. By applying Wagner’s polarization technique, the value of t ion was calculated from the current versus time plot by using equation (2) [47].
where I i and I ss are the initial and steady-state current, respectively.
Results and discussion
ATR-FTIR
The chemical interaction of [Bmim]Cl with biopolymer can be detected in the FTIR spectra. Specific functional groups in the biopolymer that are predicted to have a chemical interaction with the ionic liquid will result in significant changes either in terms of the shift in the wavenumber, change in the intensity, or change in the band shape. Figure 3 depicts the FTIR spectra of pure CMҡ-carrageenan film and CMҡ-carrageenan with different wt.% of [Bmim]Cl in the region: (a) 3800–3000 cm−1 and (b) 1700–1000 cm−1.
As the basicity (β), which is the ability of an ionic liquid to accept hydrogen bond, depends on the anion, [Bmim]Cl is expected to have a high ability to form coordination interaction with CMҡ-carrageenan matrix due to the high basic feature of the chloride ion (Cl−). The hydrogen bonds are presented in the CMҡ-carrageenan matrix as a result of the interaction between the hydrogen and oxygen of the hydroxyl groups (OH) with another hydroxyl group in the intermolecular and intramolecular network. Based on earlier investigations [30, 31], the hydroxyl group in CMҡ-carrageenan matrix is protonated to OH2 + during the dissolution of the biopolymer in acetic acid and has been proven by the increase of intensity of the OH band as shown in Fig. 3a. Thus, this positively charged OH2 + will be an attractive site for the chloride ion (Cl−) to form coordination interaction. The high basicity of Cl− and the electrostatic attraction of OH2 + towards Cl− are the main cause of the interaction between the biopolymer and the ionic liquid. Accordingly, a shift of about 10 cm−1 of wavenumbers in the OH stretching vibration from 3380 cm−1 in the pure CMҡ-carrageenan to 3370 cm−1 with [Bmim]Cl inclusion can be seen in Fig. 3a. The shift of the band towards lower wavenumber indicates that the OH2 + stretching bond in the CMҡ-carrageenan matrix has been lengthened probably due to the strong coordination interaction of Cl− with the hydrogen in the OH2 + group that eventually pulls the hydrogen away from the center atom and thus requires less energy to vibrate the particular bond. The schematic interaction of the protonated OH and the ionic liquid’s anion (Cl−) is shown in Scheme 1 .
The acidity (α), which is the ability of an ionic liquid to donate hydrogen bond, depends on the cation [43]. Generally, in 1,3-dialkylimidazolium ionic liquid, the α is mainly determined by the hydrogen in the C2 position due to the highly acidic hydrogen (H2) that is in between two ring nitrogen atoms in the imidazolium ring (Fig. 2) [40, 43]. This acidic hydrogen (H2) has the strongest tendency to donate the hydrogen in order to form a hydrogen bond, and thus it is suggested to be the main point of interaction with the polar groups in the CMҡ-carrageenan matrix. As for H4 and H5 located at C4 and C5 position in the imidazolium ring, despite being less acidic than H2, they also have the ability to interact with other polar groups as proven by Avent et al. who stated that a strong hydrogen bonding in imidazolium ionic liquid involves all three imidazolium ring protons (H2, H4, and H5) [48], and thus they are predicted to participate in the hydrogen bonding interaction as well. Hence, the polar groups or active sites in the CMҡ-carrageenan matrix such as carbolylate (COO−) and sulfate (OSO3 −) would be the interesting coordinating sites for imidazolium ring as the hydrogen bonding interaction is accessible to be formed with oxygen that carries available lone pairs of electrons in the groups. This is supported by several significant changes observed in Fig. 3b. The asymmetric stretching of COO− in the CMҡ-carrageenan at 1586 cm−1 [49] was shifted to a higher wavenumber to 1591 cm−1 upon [Bmim]Cl addition. The peak at 1230 cm−1 attributed to the S=O stretching [49, 50] was shifted to 1235 cm−1 while C–O–H and C–O–C stretching at 1104 and 1061 cm−1 [49] were shifted to 1109 and 1068 cm−1, respectively. The shifts in the wavenumbers suggest that hydrogen bonding interactions might have taken place in the system. The strong interaction between the H2 especially, H4, and H5 in the imidazolium ring with the polar groups in the CMҡ-carrageenan matrix might have push the COO−, C–O–H, and C–O–C bonds closer and thus shorten the bond length. As a result, more energy is needed to vibrate the COO−, C–O–H, and C–O–C bonds and resulted in the shift towards higher wavenumbers upon the addition of [Bmim]Cl. Scheme 2 shows the proposed interaction of the asymmetric stretching of COO− with the imidazolium ring.
Another interesting change observed due to the inclusion of ionic liquid [Bmim]Cl into the CMҡ-carrageenan matrix is the decrease in the intensity of several bands as observed in Fig. 4. Significantly, the changes in the intensity occurred in the polar groups in the CMҡ-carrageenan involving the peaks at 1586 and 1416 cm−1 were attributed to the asymmetric and symmetric stretching of COO−, respectively, stretching of sulfate esters in C–O–S=O at 1327 cm−1 and S=O at 1230 cm−1, C–O–H stretching at 1104 cm−1, and ether (C–O–C) stretching at 1061 cm−1 [49, 50]. The intensities of the characteristics peaks were noticed to be significantly reduced as higher wt.% of [Bmim]Cl was incorporated into the system. This might be due to the changes in the dipole moment of the molecule bonds in the polar groups which were resulted from the strong interaction with [Bmim]Cl. As more wt.% of [Bmim]Cl is added, more frequent and continuous interactions of both anion and cation of the ionic liquid with the polar groups might have resulted in the charge imbalance in the molecule and thus limit the bond vibrations of the polar groups. Consequently, the reduced intensities of the peaks were perceived.
Hence, the significant changes observed in the FTIR spectra as the ionic liquid [Bmim]Cl was added into CMҡ-carrageenan suggest the compatibility between the biopolymer matrix and the ionic liquid. This also signifies that [Bmim]Cl is capable of interacting with CMҡ-carrageenan, whereby the coordination and hydrogen bonding interaction of the anion (Cl−) and cation ([Bmim]+) with possible active sites or polar groups in the CMҡ-carrageenan implies that the ions are mobile in the system. Ion mobility is important in an electrolyte system as high ion mobility will favor ionic conductivity. Hence, this has given a suggestion that [Bmim]Cl has the potential to function as a charge carrier in the system. A proposed conduction mechanism of [Bmim]Cl with the biopolymer matrix of CMҡ-car is shown in Scheme 3. A conducting pathway was proposed to be formed as there will be electrostatic attraction between the positively charged OH2 + and the negatively charged sulfate (OSO3 −) with the mobile Cl− and imidazolium ring [Bmim]+. The continuous coordination and hydrogen bonding interactions of the charge carrier with the biopolymer matrix in the conducting pathway and the high mobility of the charge carrier will then generate the development of an electrolyte system.
X-ray diffraction
The effect of [Bmim]Cl inclusion in the biopolymer phase was investigated by XRD analysis. The XRD diffractograms of CMҡ-carrageenan with various weight percentages of [Bmim]Cl are depicted in Fig. 5. CMҡ-carrageenan showed a disordered structure with broad humps, this indicates an amorphous phase of biopolymer. Noticeably, the addition of ionic liquid [Bmim]Cl (5–30 wt.%) has not changed the amorphous properties of the CMҡ-carrageenan as the broad humps seemed to be consistent and tend to flatten. This might be due to the plasticizing effect of ionic liquid where the addition of ionic liquid has proven to contribute to the development of amorphous phase in polymers [7, 12, 51], and thus in this system conserved the amorphous character of CMҡ-carrageenan. In addition, no formation of crystalline and ion multiple peaks were observed, hence suggesting no sign of [Bmim]Cl agglomeration in the system. Thus, this observation suggests that [Bmim]Cl is a suitable charge carrier in the system as it did not alter the amorphous phase of CMҡ-carrageenan and showed compatibility with the polymer host.
Morphology
SEM analysis on the fractured surfaces of the biopolymer electrolyte films were performed in order to identify the physical interaction and the effect of ionic liquid ([Bmim]Cl) inclusion in the CMҡ-carrageenan matrix. The fractured surface morphology of CMҡ-carrageenan in Fig. 6a shows a granulated structure with even surface indicating an amorphous phase of biopolymer which is in accordance with the XRD analysis. Unique changes have been observed upon the inclusion of [Bmim]Cl which can be seen in Fig. 6b–d. The biopolymer started to agglomerate with the addition of only 10 wt.% [Bmim]Cl (Fig. 6b), hence indicates the strong effect of [Bmim]Cl on biopolymer matrix. Higher concentrations of [Bmim]Cl which were 20 wt.% (Fig. 6c) and 30 wt.% (Fig. 6d) tremendously altered the CMҡ-carrageenan morphologies. Greater degree of agglomeration has been observed. This might be due to the highly amorphous character of CMҡ-carrageenan that leads to high flexibility of biopolymer backbone, and thus the stronger interaction of [Bmim]Cl with the CMҡ-carrageenan matrix changes the morphology of the biopolymer matrix. However, despite the agglomeration, the amorphous phase of the biopolymer as shown in the XRD analysis has not changed thus expected to favor the ionic conduction in the biopolymer.
Ionic conductivity
In order to investigate whether the ionic liquid ([Bmim]Cl) has the ability to act as a charge carrier in the CMҡ-carrageenan electrolyte system, electrochemical impedance spectroscopy (EIS) analysis was performed on the biopolymer film. The impedance plots of CMҡ-carrageenan with various wt.% of [Bmim]Cl are shown in Fig. 7. The R b was determined from the interception of the tilted spike at the real impedance axis (Z′). Smaller R b values were observed with increasing wt.% of [Bmim]Cl. The R b values were applied in Eq. (1) in order to calculate the ionic conductivity (σ) of the films.
Figure 8 depicts the σ of CMҡ-carrageenan as a function of [Bmim]Cl wt.% at ambient temperature (298 K). It was noted that the highest σ of (5.76 ± 0.20) × 10−3 S cm−1 was achieved at the utmost addition of [Bmim]Cl (30 wt.%). Thus, this high σ value has proven the efficient function of [Bmim]Cl as a charge carrier in the system as it has improved the σ value of pure CMҡ-carrageenan where the σ measured was (1.71 ± 0.45) × 10−4 S cm−1. In addition, the highest σ recorded in this system was highly comparable to the latest reported studies of the electrolyte system based on CMҡ-carrageenan. The most recent study has reported a maximum σ of 5.51 × 10−3 S cm−1 at 30 wt.% doping of lithium nitrate (LiNO3) salt [31]. Rudhziah et al. have investigated a blend system of CMҡ-carrageenan and carboxymethyl cellulose that exhibited an utmost σ of 2.41 × 10−3 S cm−1 using 30 wt.% ammonium iodide (NH4I) salt [32]. Both studies were carried out at room temperature (298 K). In comparison with other biopolymer/ionic liquid systems, Leones et al. have reported the highest conductivity which was 2.35 × 10−5 S cm−1. It was registered for the agar with ionic liquid 1-ethyl-3-methylimidazolium acetate composition [18]. Meanwhile, biodegradable corn starch-lithium hexafluorophosphate integrated with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate has produced a maximum conductivity of (1.47 ± 0.02) × 10−4 S cm−1 [13]. In another solid polymer electrolyte system, ionic liquid 1-ethyl-3-methylimidazolium dicyamide has enhanced the conductivity of poly(vinylidine fluoride-hexafluoropropylene) (PVdf-HFP) to 3.42 × 10−3 S cm−1 at 250 wt.% of ionic liquid concentration [52]. This indicates that ionic liquid [Bmim]Cl is also suitable to be applied in an electrolyte system as it also has the potential to function as a charge carrier as good as other ionic liquids, inorganic lithium, and ammonium salts. The ability of [Bmim]Cl to effectively function as a charge carrier in this system might be due to the high basicity of the chloride ion and the acidity of the imidazolium ring, [Bmim]+, as previously discussed in the “ATR-FTIR” section. Furthermore, the low melting point (T m) of [Bmim]Cl which is around 80 °C [45] compared to the T m of common lithium and ammonium salts (>250 °C) [53, 54] suggests the weak interaction between [Bmim]+ and Cl−, and thus favors the dissociation of ions in CMҡ-carrageenan. High dissociation leads to free movement of ions in the system which resulted in further interactions between the charge carrier and the biopolymer network.
Noticeably, the σ showed an increasing trend as the wt.% of [Bmim]Cl increased. This is related to the higher number of mobile charge carrier in the system as more frequent ions-biopolymer interactions had taken place in the system. Additionally, the high flexibility of the biopolymer matrix due to the amorphous phase of CMҡ-carrageenan has led to the segmental motion of the biopolymer matrix that helps the conduction process. It is suggested that the plasticizing effect of [Bmim]Cl as observed in the XRD analysis has softened the biopolymer backbone and increased the free volume of the biopolymer matrix, and thus providing a more flexible path for ionic conduction in the electrolyte system [16]. The good homogeneity and compatibility of the biopolymer and the ionic liquid as observed in the SEM analysis suggest that the movement of ions is favorable in the CMҡ-carrageenan matrix even at higher wt.% of [Bmim]Cl. Hence, this has enhanced the σ in the system.
Electrochemical stability
The electrochemical stability of an electrolyte is the potential range where neither oxidation nor reduction is experienced by the electrolyte. It is an important criterion to be identified in order to investigate the working cell potential of an electrolyte. Figure 9a shows the linear sweep voltammetry of pure CMҡ-carrageenan film while the voltammogram of the highest conducting film, CMҡ-carrageenan with 30 wt.% [Bmim]Cl, is depicted in Fig. 9b. Observably, the pure CMҡ-carrageenan film exhibited an electrochemical stability up to ±2.5 V. The anodic current was negligible below ±1.6 V, but then increased gradually which might be due to the decomposition of the anion (Cl−) [7, 55]. A similar observation was noticed in Fig. 9b. However, the decomposition voltage of the film which was taken at the current onset increased up to ±3.0 V. This proved that the addition of [Bmim]Cl has improved the electrochemical stability of the electrolyte, and thus showed its potential to be applied in electrochemical devices or energy storage materials.
Transference number study
Transference number study of the highest conducting film was carried out in order to determine the contribution of the ions to the overall charge transport in the electrolyte system. The plot of current versus time for the highest conducting film, CMҡ-carrageenan with 30 wt.% [Bmim]Cl, is shown in Fig. 10 . The initial total current was found to decrease with time as a result of the depletion of the charge carriers or ionic species in the electrolyte. Thus, in a completely depleted condition, the current becomes constant. Based on the figure, the initial total current was found to fall rapidly with time before being saturated at 0.07 μA. Hence, the steady-state current is then carried by electronic carriers. By applying Eq. (2), the ionic transference number of the film was found to be ±0.970. Therefore, it is suggested that ions are the predominant contributor to the electrolyte conduction as the influence of electrons was only ±0.03 and can be neglected [1, 5].
Conclusion
Solid biopolymer electrolytes based on CMҡ-carrageenan and imidazolium ionic liquid ([Bmim]Cl) as the charge carrier were successfully prepared and characterized. The strong interactions of CMҡ-carrageenan with [Bmim]Cl was proven by the ATR-FTIR analysis, and thus a conduction mechanism of the biopolymer with the charge carrier was proposed. The XRD analysis revealed that the inclusion of [Bmim]Cl did not alter the amorphous phase of CMҡ-carrageenan. Good miscibility and compatibility of [Bmim]Cl with the biopolymer matrix was proven by the SEM analysis. EIS analysis confirmed that [Bmim]Cl is applicable to act as a charge carrier in the system as the ionic conductivity increased up to (5.76 ± 0.20) × 10−3 S cm−1 at ambient temperature. High electrochemical stability up to ±3.0 V was achieved by the highest conducting electrolyte measured by LSV and the transference number measurement showed that the ions are the major contributor to the electrolyte conduction.
References
Samsudin AS, Lai H, Isa M (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Samir MASA, Mateos AM, Alloin F, Sanchez J-Y, Dufresne A (2004) Plasticized nanocomposite polymer electrolytes based on poly (oxyethylene) and cellulose whiskers. Electrochim Acta 49:4667–4677
Rani MSA, Rudhziah S, Ahmad A, Mohamed NS (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6:2371–2385
Jafirin S, Ahmad I, Ahmad A (2013) Potential use of cellulose from kenaf in polymer electrolytes based on MG49 rubber composites. Bioresources 8:5947–5964
Samsudin AS, Khairul WM, Isa M (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non-Cryst Solids 358:1104–1112
Samir MA, Chazeau L, Alloin F, Cavaillé J-Y, Dufresne A, Sanchez J-Y (2005) POE-based nanocomposite polymer electrolytes reinforced with cellulose whiskers. Electrochim Acta 50:3897–3903
Shamsudin IJ, Ahmad A, Hassan N, Kaddami H (2015) Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ionics 278:11–19
Yamada M, Honma I (2005) Anhydrous proton conductive membrane consisting of chitosan. Electrochim Acta 50:2837–2841
Ma J, Sahai Y (2013) Chitosan biopolymer for fuel cell applications. Carbohydr Polym 92:955–975
Osman Z, Ibrahim Z, Arof A (2001) Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Carbohydr Polym 44:167–173
Singh PK, Bhattacharya B, Nagarale R, Kim K-W, Rhee H-W (2010) Synthesis, characterization and application of biopolymer-ionic liquid composite membranes. Synth Met 160:139–142
Ning W, Xingxiang Z, Haihui L, Benqiao H (2009) 1-Allyl-3-methylimidazolium chloride plasticized-corn starch as solid biopolymer electrolytes. Carbohydr Polym 76:482–484
Ramesh S, Liew C-W, Arof A (2011) Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J Non-Cryst Solids 357:3654–3660
Ramesh S, Shanti R, Morris E (2012) Studies on the plasticization efficiency of deep eutectic solvent in suppressing the crystallinity of corn starch based polymer electrolytes. Carbohydr Polym 87:701–706
Pawlicka A, Sabadini AC, Raphael E, Dragunski DC (2008) Ionic conductivity thermogravimetry measurements of starch-based polymeric electrolytes. Mol Cryst Liq Cryst 485:804–816
Liew C-W, Ramesh S (2013) Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellulose 20:3227–3237
Raphael E, Avellaneda CO, Manzolli B, Pawlicka A (2010) Agar-based films for application as polymer electrolytes. Electrochim Acta 55:1455–1459
Leones R, Sentanin F, Rodrigues L, Marrucho I, Esperança J, Pawlicka A, et al. (2012) Investigation of polymer electrolytes based on agar and ionic liquids. Express Polym Lett 6:1007
Raphael E, Avellaneda C, Aegerter M, Silva M, Pawlicka A (2012) Agar-based gel electrolyte for electrochromic device application. Mol Cryst Liq Cryst 554:264–272
Singh R, Jadhav NA, Majumder S, Bhattacharya B, Singh PK (2013) Novel biopolymer gel electrolyte for dye-sensitized solar cell application. Carbohydr Polym 91:682–685
Lima E, Raphael E, Sentanin F, Rodrigues L, Ferreira R, Carlos L, et al. (2012) Photoluminescent polymer electrolyte based on agar and containing europium picrate for electrochemical devices. Mat Sci Eng: B 177:488–493
Saha D, Bhattacharya S (2010) Hydrocolloids as thickening and gelling agents in food: a critical review. J Food Sci Technol 47:587–597
Kadajji VG, Betageri GV (2011) Water soluble polymers for pharmaceutical applications. Polymers 3:1972–2009
Popa G, Gomes ME, Reis RL (2011) Cell delivery systems using alginate–carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 12:3952–3961
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Viebke C, Borgström J, Piculell L (1995) Characterisation of kappa-and iota-carrageenan coils and helices by MALLS/GPC. Carbohydr Polym 27:145–154
Campo VL, Kawano DF, da Silva DB, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis–a review. Carbohydr Polym 77:167–180
Prasad K, Kaneko Y, Kadokawa JI (2009) Novel gelling systems of κ-, ι-and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol Biosci 9:376–382
Leong H, Chung LY, Noordin MI, Mohamad K, Nishikawa M, Onuki Y, et al. (2011) Carboxymethylation of kappa-carrageenan for intestinal-targeted delivery of bioactive macromolecules. Carbohydr Polym 83:1507–1515
Mobarak NN, Ramli N, Ahmad A, Rahman M (2012) Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid State Ionics 224:51–57
Mobarak N, Jumaah F, Ghani M, Abdullah M, Ahmad A (2015) Carboxymethyl carrageenan based biopolymer electrolytes. Electrochimica Acta 175:224–321
Rudhziah S, Ahmad A, Ahmad I, Mohamed N (2015) Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell. Electrochimica Acta 175:162–168
Mobarak N, Ahmad A, Abdullah M, Ramli N, Rahman M (2013) Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim Acta 92:161–167
El S, Koschella A, Fidale LC, Dorn S, Heinze T (2007) Applications of ionic liquids in carbohydrate chemistry: a window of opportunities. Biomacromolecules 8:2629–2647
Zakrzewska ME, Bogel-Łukasik E, Bogel-Łukasik R (2010) Solubility of carbohydrates in ionic liquids. Energy Fuel 24:737–745
Rinaldi R (2011) Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem Commun 47:511–513
Hameed N, Guo Q (2009) Natural wool/cellulose acetate blends regenerated from the ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr Polym 78:999–1004
Xiao W, Chen Q, Wu Y, Wu T, Dai L (2011) Dissolution and blending of chitosan using 1, 3-dimethylimidazolium chloride and 1-H-3-methylimidazolium chloride binary ionic liquid solvent. Carbohydr Polym 83:233–238
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728
Brandt A, Hallett JP, Leak DJ, Murphy RJ, Welton T (2010) The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem 12:672–679
Nam T, Yoon LW, Lee KM, Ngoh G-C, Chua ASM, Lee MG (2011) Efficiency of ionic liquid in the dissolution of rice husk. Bioresources 6:4790–4800
Cláudio FM, Swift L, Hallett JP, Welton T, Coutinho JA, Freire MG (2014) Extended scale for the hydrogen-bond basicity of ionic liquids. Phys Chem Chem Phys 16:6593–6601
Ab Rani M, Brant A, Crowhurst L, Dolan A, Lui M, Hassan N, et al. (2011) Understanding the polarity of ionic liquids. Phys Chem Chem Phys 13:16831–16840
Marsh K, Boxall J, Lichtenthaler R (2004) Room temperature ionic liquids and their mixtures—a review. Fluid Phase Equilib 219:93–98
Zhang S, Sun N, He X, Lu X, Zhang X (2006) Physical properties of ionic liquids: database and evaluation. J Phys Chem Ref Data 35:1475–1517
Sun G-z, Chen X-g, Li Y-y, Zheng B, Gong Z-h, Sun J-j, et al. (2008) Preparation of H-oleoyl-carboxymethyl-chitosan and the function as a coagulation agent for residual oil in aqueous system. Front Mater Sci China 2:105–112
Vest R, Tallan N (1965) High-temperature transference number determinations by polarization measurements. J Appl Phys 36:543–547
Avent G, Chaloner PA, Day MP, Seddon KR, Welton T (1994) Evidence for hydrogen bonding in solutions of 1-ethyl-3-methylimidazolium halides, and its implications for room-temperature halogenoaluminate (III) ionic liquids. J. Chem. Soc., Dalton Trans.:3405–3413
Fan L, Wang L, Gao S, Wu P, Li M, Xie W, et al. (2011) Synthesis, characterization and properties of carboxymethyl kappa carrageenan. Carbohydr Polym 86:1167–1174
Tranquilan-Aranilla C, Nagasawa N, Bayquen A, Dela Rosa A (2012) Synthesis and characterization of carboxymethyl derivatives of kappa-carrageenan. Carbohydr Polym 87:1810–1816
Sankri A, Arhaliass A, Dez I, Gaumont AC, Grohens Y, Lourdin D, et al. (2010) Thermoplastic starch plasticized by an ionic liquid. Carbohydr Polym 82:256–263
Singh K, Sabin K, Chen X (2015) Ionic liquid–solid polymer electrolyte blends for supercapacitor applications. Polym Bull:1–9
Dominey LA, Koch VR, Blakley TJ (1992) Thermally stable lithium salts for polymer electrolytes. Electrochim Acta 37:1551–1554
Perry L (2011) Handbook of inorganic compounds. CRC
Sownthari K, Suthanthiraraj S (2013) Synthesis and characterization of an electrolyte system based on a biodegradable polymer. Express Polym Lett 7:495–504
Acknowledgments
The authors are pleased to acknowledge UKM for the provision of grant FRGS/2/2013/TK04/UKM/02/4, ERGS/1/2013/TK07/UKM/02/4, and ETP2013-027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamsudin, I.J., Ahmad, A., Hassan, N.H. et al. Biopolymer electrolytes based on carboxymethyl ҡ-carrageenan and imidazolium ionic liquid. Ionics 22, 841–851 (2016). https://doi.org/10.1007/s11581-015-1598-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1598-5