Abstract
Poly (acrylonitrile) (PAN) and ammonium chloride (NH4Cl)-based proton conducting polymer electrolytes with different compositions have been prepared by solution casting technique. The amorphous nature of the polymer electrolytes has been confirmed by XRD analysis. The FTIR analysis confirms the complex formation of the host polymer (PAN) with the salt (NH4Cl). DSC measurements show a decrease in Tg with the increase in salt concentration. The conductivity analysis shows that the 25 mol% ammonium chloride doped polymer electrolyte has a maximum ionic conductivity, and it has been found to be 6.4 × 10−3 Scm−1, at room temperature. The temperature dependence of conductivity of the polymer electrolyte complexes appears to obey the Arrhenius nature. The activation energy (Ea = 0.23 eV) has been found to be low for 25 mol% salt doped polymer electrolyte. The dielectric behavior has been analyzed using dielectric permittivity (ε*), and the relaxation frequency (τ) has been calculated from the loss tangent spectra (tan δ). Using this maximum ionic conducting polymer electrolyte, the primary proton conducting battery with configuration Zn + ZnSO4·7H2O/75 PAN:25 NH4Cl/PbO2 + V2O5 has been fabricated and their discharge characteristics have been studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton conducting polymer electrolyte has attracted great attention as the most appropriate choice to fabricate solid state electrochemical devices such as solid state batteries, fuel cells, super capacitors, humidity and gas sensors, and electrochromic displays [1–5].
Many research groups have focused on the host polymer and copolymer such as polyvinyl alcohol (PVA), polyvinyl acetate (PVAc), polymethyl methacrylate (PMMA), polyethylene oxide (PEO), P(VdF-co-HFP)/PVAc, PVC-PEO, and PVAc-PMMA as polymer electrolytes for their possible applications in these solid state electro chemical devices [6–13].
The proton conducting polymer electrolytes based on hydrocarbon polymers such as sulfonated poly (ether ether ketone) have been studied by Rikukawa et al. [14]. Qiao and Okada have reported the proton conducting polymer electrolyte based on polyvinyl pyrrolidone modified polyvinyl alcohol/2 acrylamido-2-methyl/propane sulfonic acid (PVA-PAMS) for the application of low temperature direct methanol fuel cells [15]. Proton conducting polymer electrolyte composed of silicotungstic acid, orthophosphoric acid, and PVA have been investigated by Gao and Lian [16]. PVA with embedded phosphotungstic acid-based polymer electrolytes have been studied by Li et al. [17]. Polyacrylonitrile (PAN) has good flame resistance, good processability, high abrasion resistance, good mechanical and electrochemical stability [18].
PAN-lithium bis(oxalate)borate polymer electrolyte for the application of electric double layer capacitor has been examined by Arof et al. [19]. Kufian and Arof have reported the effect of calix(6)arene charge-discharge of lithium air cell based on PAN-LiBOB polymer electrolytes [20]. Gel polymer electrolyte based on LiBOB–PAN for the application in dye sensitized solar cells has been studied by Arof et al. [21]. PAN-based solid polymer electrolytes with LiCF3SO3 have been investigated by Yoon et al. [22]. Lewandowski et al. have reported PAN/sulfolane/C2H5NB4 polymer electrolytes for the applications of chemical capacitors [23].
Proton conducting polymer electrolytes have been studied using strong inorganic acids such as H3PO4, H3PO3, H2SO4, and HCl as the doping agents. However, such proton conducting polymer electrolytes suffer from chemical degradation and mechanical stability and thus are unsuitable practical applications. Other than these inorganic acids, proton conducting polymer electrolytes have been prepared with ammonium salts. Ammonium salts have already been reported as a very good proton donor to the polymer matrix system [24]. Reports of ammonium salts doped proton conducting polymer electrolyte based on polyacrylonitrile are rare except Nithya et al. and Sikkanthar et al. [25, 26].
For this paper, proton conducting polymer based on PAN containing NH4Cl has been prepared and investigated by X-ray diffraction, FTIR, differential scanning calorimetric, and electrochemical impedance spectroscopy, and the results are discussed and reported.
Experimental
The polymer electrolytes, PAN (MW = 150,000 Sigma Aldrich) with NH4Cl (Merck) in various molwt% (95:05, 90:10, 85:15, 80:20,75:25, and 70:30), were prepared by solution casting technique using DMF (Merck) as a solvent. The solutions of PAN and NH4Cl mixtures have been thoroughly stirred to obtain a homogenous mixture. The mixtures are then poured into a poly petri dishes and are subjected to drying at 60 °C in vacuum oven. The uniform thick films, which are transparent to visible light and having a good mechanical strength, are obtained. X-ray diffraction (XRD) patterns were recorded using Philips X’pert pro diffractometer with Cu-Kα for 2θ measurements from 5 to 90°. FTIR measurements have been made with a Shimadzu-8000 spectrophotometer instrument in the wave number range of 4000–400 cm−1. The electrical conductivity study of the prepared polymer electrolytes has been carried out in the temperature range of 303 to 343 K over a frequency range of 42 Hz–1 MHz using computer controlled HIOKI 3532 LCZ meter with a cell having stainless steel electrodes. Differential scanning calorimetric thermograms have been obtained using a differential scanning calorimeter model DSC-6100, Seiko instruments Inc. in the temperature range 25–300 °C with the heating rate of 5 °C per minute. Primary proton conducting battery has been fabricated using the membrane with the highest ionic conductivity as electrolyte. The configuration of proton conducting battery is Anode (Zn + ZnSO4·7H2O)/polymer electrolyte (75 PAN:25 NH4Cl)/Cathode (PbO2 + V2O5). Its open circuit voltage was measured and its discharge curve is plotted against time.
XRD analysis
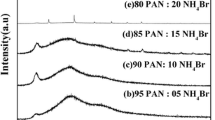
Figure 1 (a–g) shows the XRD spectra of pure PAN and PAN: X NH4Cl (X = 5, 10, 15, 20, 25, and 30 mol%) polymer electrolytes. Figure 1(b) shows the XRD pattern of pure NH4Cl. The characteristic peak of pure PAN at 2θ = 17° shows semi crystalline in nature, corresponding to the orthorhombic PAN reflection (110) [27]. From Fig. 1, it has been observed that the relative intensity of sharp peak decreases with increase of NH4Cl concentration. The decrease in relative intensity with broadening of apparent peak of PAN-NH4Cl polymer electrolyte membranes has been observed when compared with pure PAN. This result can be interpreted by considering the Hodge et al. criteria which establish a correlation between intensity of the peak and the degree of crystallinity [28].
a XRD pattern for (a) pure PAN, (b) 95 PAN: 05 NH4Cl, (c) 90 PAN:10 NH4Cl, (d) 85 PAN:15 NH4Cl, (e) 80 PAN:20 NH4Cl, (f) 75 PAN:25 NH4Cl, and (g) 70 PAN:30 NH4Cl. Fig 1 b XRD pattern for pure NH4Cl
The interaction between salt and polymer results in lowering the crystallinity nature and increasing of amorphous nature. This change of intensity and broadening nature of the peaks in the polymer electrolytes suggests the amorphous nature of the polymer electrolytes. The maximum amorphous nature has been observed for 75 PAN:25 NH4Cl polymer electrolyte system. Peaks corresponding to pure NH4Cl have been found to be absent in the salt doped polymer complexes (95:05), (90:10), (85:15), (80:20), and (75:25) indicating the complete dissociation of salt in the polymer matrix. However, in (70 PAN:30 NH4Cl) polymer complex, the peaks 2θ = 44.3°, 64.67°, and 77.82° (JCPDS file no. 34–0710) corresponding to pure NH4Cl have been observed which indicates the presence of some undissociated salt in that polymer complex. These peaks attributed to the recrystallization of NH4Cl out of the membrane surface. This is because the host polymer was unable to accommodate the salt which leads to the recombination of the ions [29].
FTIR analysis
FTIR spectroscopy has been used to analyze the interactions among atoms or ions in polymer electrolyte systems. Fig. 2 shows the FTIR spectra of pure PAN and different concentrations of NH4Cl doped with PAN recorded at room temperature. FTIR transmission band positions and its assignments of all prepared polymer electrolyte have been listed in Table 1. The vibrational bands at 2243, 1665, 1451, 1388, 1257, and 847 cm−1 in Fig. 2 (a) have been assigned to C≡N stretching, C=C stretching, C–H bending of CH2, C–H plane bending, C–H wagging, and C–H rocking of pure PAN, respectively. The vibrational band at 2243 cm−1 is the most characteristics nitrile band (C≡N) of pure PAN [30, 31]. From Fig. 2b–d, the observed band around 3399–3414 cm−1 has been attributed to N–H asymmetric stretching of NH4 + ions. The peak at 2867 cm−1 has been ascribed to N–H symmetric stretching of NH4 + ions. The peak at 1665 cm−1 corresponding to C = C stretching of PAN has been shifted to 1664, 1663, 1662, 1661, 1660, and 1658 cm−1, respectively, in X NH4Cl (X = 5, 10, 15, 20, 25, and 25) doped polymer electrolyte system. The peak at 1451 cm−1 corresponding to C–H bending of CH2 has been found to be shifted to 1446–1450 cm−1 in 5–30 mol% NH4Cl doped with PAN. The peak observed at 1388 cm−1 assigned to C–H plane bending of pure PAN, gets shifted to 1386–1389 cm−1 in salt doped with PAN. The peak at 1257 cm−1 corresponding to C–H wagging has been found to be shifted to 1246–1256 cm−1 in 5–30 mol% NH4Cl doped with PAN. The peak at 847 cm−1 corresponding to C–H rocking of PAN has been shifted to 873, 872, 875, 870, 872, and 873 cm−1, respectively, in X NH4Cl (X = 5, 10, 15, 20, 25, and 30) doped polymer electrolyte system. The change in peak position and intensity confirms the complex formation between the polymer and the salt.
DSC analysis
Figure 3 (a–g) shows the DSC thermogram of the pure PAN, 5, 10, 15, 20, 25, and 30 mol% NH4Cl doped PAN polymer electrolyte. The Tg of pure PAN has been studied by Dissanayake et al. and its value is found to be 88.1 °C [32]. The addition of ammonium chloride to the pure PAN decreases the glass transition temperature of the polymer electrolyte system. With the addition of the NH4Cl concentration up to 25 mol%, the observed glass transition temperature of the polymer decreased significantly. This may be due to the softening of the polymer by the guest salt (NH4Cl), which is beneficial for easy proton transport. The plasticization effect is related to a weakening of the dipole–dipole interaction between the PAN and NH4Cl. The low glass transition temperature causes the higher segmental motion of the polymer electrolyte. Such segmental motion produces voids, which enable the easy flow of ions through the polymer chain when there is an applied electric field. However, for 30 mol% NH4Cl doped with PAN, the Tg value has been found to increase which may be due to the presence of some undissociated salt in the polymer matrix. The glass transition temperatures have been tabulated in Table 2.
Impedance analysis
The ionic conductivity of the proton conducting polymer electrolyte (PAN:NH4Cl) system has been derived from the complex impedance plots. Cole-Cole plot for PAN–NH4Cl polymer electrolyte samples of different compositions at 303 K is shown in Fig. 4a. A typical cole-cole plot consists of a high-frequency depressed semicircle represented by a frequency-dependent capacitor (Cg) parallel to a bulk resistor (Rb) and tilted spike at low-frequency represented by a constant phase element (CPE).
The migration of ions may occur through the free volume of polymer matrix, which can be represented by a resistor. The immobile polymer chains become polarized in the alternating field, which can be represented by a capacitor. The ionic migration and bulk polarization are physically in parallel and therefore gives a semicircle. The tilted spike at low-frequency region is attributed to the effect of electrode polarization which is characteristics of diffusion process [33]. But in our system, it is observed that semicircle has disappeared and only the spikes appear. The disappearance of semicircular portion in the cole-cole plot leads to a conclusion that the total ionic conductivity is mainly due to the result of ionic conduction [34].
The ionic conductivity values of the prepared polymer electrolyte have been calculated using the relation σ = l/RbA where Rb is the bulk resistance of the material, l is the thickness of the membrane (cm), and A is the contact area of the membrane (cm2). The bulk resistance has been calculated using EQ software developed by Boukamp [35, 36]. The highest ionic conductivity at room temperature is found to be 6.4 × 10−3 Scm-1 for the composition of 75 PAN:25 NH4Cl. The conductivity values of all prepared polymer electrolytes at different temperature have been tabulated in Table 3.
Figure 4b represents the cole-cole plot of the maximum conductivity sample at different temperatures. At all temperatures, the disappearance of semicircle indicates the prevailing of the resistive component of the electrolyte system [37]. The ionic conductivity of the polymer electrolyte increases with the increase of temperature which can easily be understood on the basis of the ionic transport mechanism of proton conducting polymer electrolytes. When the temperature is increased, the ionic mobility of the polymer chain is enhanced and the fraction of free volume in a proton conducting polymer electrolyte increases accordingly, which leads to an increase in the ionic conductivity of the polymer electrolyte [38]. In addition, the mobility of charge carriers increases with increase of temperature resulting in an increase in the ionic conductivity at higher temperatures.
Scheme 1 shows the equivalent circuit for 25 mol% ammonium chloride doped into the 75 PAN. This equivalent circuit is a combination of resistances (Rb) and CPE. Values of the above elements have been extracted by fitting the complex impedance plot using EQ software developed by Boukamp [35, 36] and tabulated (Table 4)
The impedance of the constant phase element (ZCPE) is given as
where Q0 and n are the frequency-independent parameters. n value lies between 0 to 1. If n = 1, it represents a pure capacitor. If n = 0, it denotes a pure resistor. The pure PAN has the values R = 324 Ω, CPE = 146 μF, and n = 0.46. The R value decreases from 307 to 106 Ω when addition of 5–25 mol% of NH4Cl with PAN. The R value increases to 267 Ω for 70 PAN:30 NH4Cl system. The CPE and n values lie between 98 – 146 μF and 0.57–0.75, respectively, for all prepared polymer electrolyte system.
Conductance spectra
The conductance spectra for the maximum conductivity sample (i.e., 75 PAN:25 NH4Cl) with different temperature are shown in Fig. 5. The spectra consist of two regions, a low-frequency dispersive region and plateau region. At high temperatures, low-frequency dispersion is observed due to electrode polarization effects. At these temperatures, the ionic conductivity is high enough to produce a significant build up of charges at the electrodes, which reduces the effective applied field across the sample and hence the apparent conductivity. The plateau is representing the dc conductivity. At higher frequency range, the period of the applied field is too short for the charging to occur and the ac conductivity is generally taken to assume the frequency-independent value, which is equal to the dc conduction. As the frequency decreases, more and more charge accumulation occurs at the electrode and electrolyte interface, which leads to a decrease in number of mobile ions and eventually to a drop in conductivity at low frequency. In a high-frequency region, the mobility of charge carriers is high and hence the conductivity increases with frequency [37]. It has been observed that the dc conductivity increases with increase of temperature which suggests that the free volume around the polymer chain causes the mobility of ions and polymer segments [39].
Temperature dependence of the ionic conductivity
The temperature-dependent ionic conductivity of the proton conducting polymer electrolytes has been evaluated to analyze the mechanism of ionic conduction in polymer electrolyte. Figure 6 shows the variation of ionic conductivity with the reciprocal of temperature for the polymer electrolytes.
The linear variation of log σ versus 1000/T plots suggests an Arrhenius type thermally activated process. The conductivity can be expressed as [40, 41]
where σ0 is the pre-exponential factor, Ea is the activation energy, and T is the absolute temperature in K. The activation energy values have been calculated from the slope of the Arrhenius plot. Table 2 shows the activation energy of proton conducting polymer electrolytes with different compositions, and the results reveal that the activation energy (Ea) decreases effectively with the NH4Cl concentration. As temperature increases, the polymer chain acquires faster internal modes in which bond rotations produce segmental motion. This, in turn, favors inter-chain hopping and intra-chain ion movements, and accordingly, the conductivity of the polymer electrolyte becomes high [42].
Dielectric behavior
The complex permittivity (ε*) or dielectric constant of a system is defined by
where ε’ is the real part of dielectric constant, ε” is the imaginary part of dielectric constant of the material, σ is the real part of conductivity, ω is the angular frequency, and ε0 is the permittivity of the free space.
Figure 7a shows the ε’ vs. log f plots for all prepared PAN-NH4Cl polymer electrolytes at room temperature. The dielectric constant is proportional to ωn–1 for low-frequency values. In Fig. 7a, the observed variation in ε’ with frequency could be attributed to the formation of a space charge region at the electrode and electrolyte interface, which is familiarly known as ωn–1 variation or the non-Debye type of behavior, where the space charge regions with respect to the frequency is explained in terms of ion diffusion [43]. The low-frequency dispersion region is attributed to the contribution of charge accumulation at the electrode–electrolyte interface [44]. The higher values of dielectric constant for 75 PAN:25 NH4Cl system are due to the enhanced charge carrier density at the space charge accumulation region, resulting in an increase in the equivalent capacitance. At high frequencies, the periodic reversal of the electric field occurs so fast that there is no excess ion diffusion in the direction of the field. The polarization due to the charge accumulation decreases, leading to the decrease in the value of ε’.
a Frequency dependence of ε’(ω) for (square) 95 PAN:05 NH4Cl, (circle) 90 PAN:10 NH4Cl, (triangle pointing right) 85 PAN:15 NH4Cl, (triangle pointing down) 80 PAN:20 NH4Cl, (triangle pointing up) 75 PAN:25 NH4Cl, and (triangle pointing left) 70 PAN:30 NH4Cl polymer electrolytes at 303 K. b Frequency dependence of ε” (ω) for 25 mol% of NH4Cl doped with PAN at different temperatures
Figure 7b shows the ε” versus log f plots for different temperatures for the maximum conductivity system (75 PAN:25 NH4Cl). The dielectric loss (ε”) becomes very large at lower frequencies, and it increases with temperature due to free charge motion within the material [45]. These values do not correspond to the bulk dielectric processes but are due to the free charges build up at the interface between the material and the electrodes. For very low frequencies, there is particularly time for charges to build up at the interfaces before the field changes the direction and this contributes to very large apparent values of ε”. This phenomenon leads to the so called “conductivity relaxation” [46].
Electric modulus analysis
The dielectric behavior of the sample can also be studied using the electric modulus. Figure 8 shows the frequency dependence of the imaginary part of the modulus for 75 PAN:25 NH4Cl polymer electrolyte. It is observed that the modulus increases with the increase in frequency. At low frequency, the modulus value approaches to zero indicating that the electrode polarization phenomenon makes a negligible contribution. The appearance of long tail in the low-frequency region is due to the capacitance effect at the electrodes. The increase in modulus spectra at higher frequencies indicates that the polymer electrolyte systems are ionic conductors and this is attributed to the bulk of the material.
Loss tangent spectra
The dielectric relaxation parameter of the polymer electrolytes can be obtained from the study of Tan δ as a function of frequency. The dielectric loss tangent, Tan δ, can be defined by the equation Tan δ = ε″/ε′. The variation of Tan δ with the frequency for all the prepared PAN:NH4Cl polymer complexes at 303 K is presented in Fig. 9.
It has been observed that Tan δ increases with increasing frequency and reaches a maximum. Then it decreases for further increase of frequency. For maximum dielectric loss at a particular temperature, the loss tangent peak is described by the relation ωτ = 1 where τ is the relaxation time and ω is the angular frequency of the applied electric field. The relaxation time has been calculated for all the prepared polymer electrolytes at 303 K and tabulated in Table 5. A low relaxation time is obtained for 25 mol% NH4Cl salt doped polymer electrolyte which is consistent with the conductivity analysis which shows higher conductivity for 25 mol% NH4Cl-doped polymer electrolyte.
Transference number measurements
The transference numbers corresponding to ionic (t+) and electronic (t−) transport have been evaluated in PAN-NH4Cl proton conducting polymer electrolyte systems using Wagner’s polarization technique [47]. In this technique, the dc current is monitored as a function of time on the application of fixed dc voltage across the sample with blocking electrodes. The results of dc polarization measurements on the 75 PAN:25 NH4Cl proton conducting polymer electrolyte taken with stainless steel blocking electrodes by applying 1.0 V dc bias voltage at 303 K are shown in Fig. 10. The transference numbers have been calculated from the polarization current versus time plot using the equations:
where Ii is the initial current, If is the final residual current, D+ is the diffusion coefficient of cation, D− is the diffusion coefficient of anion, μ+ is the ionic mobility of cation, μ− is the ionic mobility of anion, k is the Boltzmann constant, e is the charge of electron, T is the absolute temperature, n is the number of charge carriers, N is the Avagadro number, and ρ is the density of NH4Cl. The transport parameters such as D+, D−, μ+, and μ− have been calculated using the different formula given earlier in the text and they have been given in the Table 6. For all compositions of the PAN–NH4Cl electrolyte systems, the values of the ionic transference numbers (t +) have been found to be in the range of 0.94–0.99. This suggests that the charge transport in these polymer electrolyte films is predominantly due to ions and the contribution to ionic conductivity is mainly due to the loosely bound proton of NH4 + ion. Table 6 shows that the cation mobility (μ+) has greater value than the ionic mobility of anions (μ−). When μ+ decreases, the conductivity also decreases and vice versa. The same behavior also can be detected for D+. Maximum values of μ+ and D+ are found for 75 PAN:25 NH4Cl polymer electrolytes. Hence, the study of transference number measurements leads to the conclusion that the conductivity has been influenced by the μ+ and D+.
Cell fabrication
Among the prepared polymer electrolytes under the study, 75 PAN:25 NH4Cl possess highest ionic conductivity. Hence, the cell has been constructed using 75 PAN:25 NH4Cl polymer electrolyte. The cell configuration
Discharge characteristics
Open circuit voltage (OCV) value of the prepared cell is 1.35 V. The cell has been allowed to stabilize for certain duration to attain a constant voltage. The open circuit voltage for cell is monitored for 100 h and presented in Fig. 11. The stabilized voltage of 1.35 V has been observed for cell. After the attainment of stabilized voltage, the cell has been discharged through 1 MΩ loads at room temperature. Figure 12 shows the cell-potential variation as a function of time for the 1 MΩ load resistances. It has been found that during discharge, the voltage of cell decreases initially, remains constant for a particular duration (time of stable performance of the cell) after which there is a decline in voltage. The initial decrease in cell voltages may be due to polarization [48]. While discharging through 1 MΩ load, the voltage value of cell remains constant at 1.30 V for ∼200 h. The region in which the cell voltage remains constant is called as the “plateau region” [40]. Beyond the plateau region, voltage value of the cell drops again. Table 7 lists few important cell parameters.
Conclusion
The proton conducting polymer electrolytes of PAN-NH4Cl with different mol% have been prepared by solution casting method. The increase in amorphous nature of the polymer electrolytes has been confirmed by XRD analysis. The polymer–salt complex formation has been confirmed from FTIR spectral studies. Thermal analysis indicates that the glass transition temperature is low for the 75 PAN:25 NH4Cl polymer electrolyte. The highest ionic conductivity of 6.4 × 10−3 Scm−1 at 303 K has been observed for 75 PAN:25 NH4Cl polymer electrolyte sample. The temperature dependence of ionic conductivity of these electrolytes exhibited Arrhenius behavior. The low activation energy has been found to be 0.23 eV for the 75 PAN:25 NH4Cl polymer electrolyte using the Arrhenius plot. The transport number measurements reveal the charge transport and the polymer electrolyte films are predominantly due to ions. The dielectric analysis revealed the non-Debye nature of the material. The low-frequency dispersion of dielectric constant reveals the space charge effects arising from the electrodes. The high dielectric loss (ε”) values at lower frequencies are due to the free charges build up at the interface between the material and the electrodes. The primary proton conducting battery has been fabricated, and characterizations are studied using the maximum ionic conductivity polymer electrolyte system. The OCV of prepared cell has been found to be 1.35 V.
References
Bozkurt A (2002) Turk J Chem 26:663–668
Qiao J, Hamaya T, Okada T (2005) Polymer 46:10809–10816
Wieczorek W, Florjanczyk Z, Zukowska G, Borkowska R, Kuźma P, ZygadloMonikowska E, Kuźwa K (2002) Solid State Ionics 154–155:45–49
Lewandowski A, Zajder M, Frackowiak E, Beguin F (2001) Electrochim Acta 46:2777–2780
Ningping C, Liang H (2002) Solid State Ionics 146:377–385
Selvasekarapandian S, Baskaran R, Hema M (2005) Physica B 357:412–419
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Solid State Ionics 177:2679–2682
Choi N-S, Lee Y-G, Jung-Ki P, Yang-Myoun K (2001) Elactrochim Acta 46:1581–1586
Croce F, Persi L, Scrosati B, Serraino-Fiory F, Plichta E, HendRickson MA (2001) Eletrochim Acta 46:2457–2461
Baskaran R, Selvasekarapandian S, Kuwata N, Iwai Y, Kawamura J, Hattori T (2006) Mater Chem Phys 98:55–61
Agarwal SL, Rai N, Natarajan TS, Chand N (2012) Ionics 19(1):145–154
Ramesh S, Winie T, Arof AK (2007) European Polymer Journal 43(5):1963–1968
Dobrowska AW, Wieczorek W (1994) Mater Sci Eng B 22:107–116
Rikukawa M, Sanui K (2000) Prog Polym Sci 25:1463–1502
Qiao J, Okada T (2007) J New Mater Electrochem Syst 10:231–236
Gao H, Lian K (2010) Electrochim Acta 56:122–127
Li L, Xu L, Wang Y (2003) Mater Lett 57(8):1406–1410
Song JY, Wang YY, Wan CC (1999) J Power Sources 77:183–197
Arof AK, Suhaimi NEA, Amiruddin S, Kufian MZ, Woo HJ, MA C (2014) Polym Adv Technol 25:265–272
Kufian MZ, Arof AK (2014) Mater Technol 29:114–117
Arof AK, Jun HK, Sim LN, Kufian MZ, Sahraoui B (2013) Opt Mater 36:135–139
Yoon HK, Chung WS, Jo NJ (2004) Electrochim Acta 50:289–293
Lewandowski A, Galinskin M, Krzyzanowski M (2003) Solid State Ionics 158:367–373
Ramya CS, Selvasekarapandian S, Hirankumar G, Savitha T, Angelo PC (2008) J Non-Cryst Solids 354:1494–1502
Nithya S, Selvasekarapandian S, Karthikeyan S, Inbavalli D, Sikkanthar S, Sanjeeviraja C (2014) Ionics 20(10):1391–1398
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, VinothPandi D, Nithya S, Sanjeeviraja C (2015) J Solid State Electrochem 19(4):987–999
Sawai D, Miyamoto M, Kanamoto T, Ito MJ (2000) J Polym Sci Part B: Polym Phys 38:2571–2579
Rajendran S, Sivakumar M, Subadevi R (2003) J Power Sources 124:225–230
Kadir MFZ, Majid SR, Arof AK (2010) Electrochim Acta 55:1475–1482
Stuff SI, Carr SH (1977) J Polym Sci Polym Phys Ed 15(3):485–499
Coleman MM, Petcavich RJ (1978) J Polym Sci Polym Phys (Ed) 16:821–832
Dissanayake MAKL, Bandara LRAK, Bokalawala RSP, PARD J, OA I, Somasundaram S (2002) Mater Res Bull 37:867–874
Samsudin AS, Khairul WM, Isa MIN (2012) J Non-Cryst Solids 358:1104–1112
Izuchi S, Ochiai S, Takeuchi K (1987) J Power Sources 68:37–4239
Boukamp BA (1986) Solid State Ionics 20:31–44
Boukamp BA (1986) Solid State Ionics 18(19):136–140
Ramesh S, Arof AK (2001) Mater Sci Eng B 85:11–15
Zhu W, Wang X, Yang B, Tang X (2001) J Polym Sci B Polym Phys 39:1246–1254
Miyamoto T, Shibayama K (1973) J Appl Phys 44:5372–5376
Luo J, Jensen AH, Brooks NR, Sniekers J, Knipper M, Aili D, Li Q, Vanroy B, Wübbenhorst M, Yan F, Van Meervelt L, Shao Z, Fang J, Luo Z-H, De Vos DE, Binnemans K, Fransaer J (2015) Energy Environmental Science 8:1276–1291
Luo J, Conrad O, Vankelecom IFJ (2013) J Mater Chem 1:2238–2247
Subbareddy Ch V, Sharma AK, Narasimha Rao VVR (2003) J Power Sources 114:338–345
Macdonald (1987) (Ed) Impedance Spectrocopy- Emphasizing Solid materials and Systems, Wiley –Interscience New York
Howell FS, Bose RA, Macedo PB, Moynihan CT (1974) J Phys Chem 78:639–648
Kyritsis A, Pissis P, Grammatikakis J (1995) J Polym Sci Part B: PolymPhys 33:1737–1750
Dyre JC (1991) J Non-Cryst Solids 135:219
Wagner JB, Wagner C (1957) J Chem Phys 26:1597–1601
Agrawal RC, Hashmi SA, Pandey GP (2007) Ionics 13:295–298
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sikkanthar, S., Karthikeyan, S., Selvasekarapandian, S. et al. Structural, electrical conductivity, and transport analysis of PAN–NH4Cl polymer electrolyte system. Ionics 22, 1085–1094 (2016). https://doi.org/10.1007/s11581-016-1645-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1645-x