Abstract
In this study, we report the discovery of Jahnula dianchia sp. nov., a freshwater lignicolous fungus belonging to the order Jahnulales (Dothideomycetes, Ascomycota), from a submerged woody substrate from Dianchi Lake, Yunnan, China. Morphologically, this new taxon differs from other species of the genus in ascospore morphology; the ascospores of the new species are dark brown with mammiform apices. We also provide a phylogenetic tree showing the molecular relationships between the new taxon with the previously accepted Jahnula species. The analysis of combined ITS, LSU, and SSU sequence dataset places J. dianchia within the Jahnulales sister to J. sangamonensis. The phylogeny also shows the placement of both strains of J. dianchia in a well-supported independent sub-clade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jahnulales (Dothideomycetes), an order of freshwater lignicolous ascomycetes, was established by Pang et al. (2002). This order is phylogenetically related to the order Dothideales, Patellariales, and Pleosporales (Campbell et al. 2007). Most species of the Jahnulales occur on rotting or soft submerged corticated or decorticated wood (Inderbitzin et al. 2001; Pang et al. 2002; Shenoy et al. 2006, 2010; Campbell et al. 2007; Suetrong et al. 2011; Tanaka et al. 2015). The order Jahnulales is characterized by ascomata with peridial walls multilayered, composed of large cells, stalked and (or) sessile bitunicate asci, and one-septate ascospores with gelatinous sheaths or appendages (Pang et al. 2002; Campbell et al. 2007; Suetrong et al. 2011; Jones et al. 2015). Most authors accept two families in this order, Aliquandostipitaceae and Manglicolaceae (Suetrong et al. 2011; Hyde et al. 2013; Jones et al. 2015). The family, Aliquandostipitaceae within this order was proposed based on the genus Aliquandostipite, which contains only tropical species and is characterized by broad septate hyphae, globose to subglobose ascomata, and one-septate ascospores (Inderbitzin et al. 2001). Six genera are accommodated in this family including Aliquandostipite, Jahnula and Megalohypha (Kirschstein 1936; Inderbitzin et al. 2001; Ferrer et al. 2007), known by its sexual morph, whereas Brachiosphaera, Speiropsis and Xylomyces are exclusively asexual morph (Tubaki 1958; Descals et al. 1976; Goos et al. 1977; Campbell et al. 2007; Suetrong et al. 2011). The other family of the order, Manglicolaceae, also present cylindrical asci and appendaged ascospores (Suetrong et al. 2011). The Manglicolaceae, represented by the marine fungus Manglicola guatemalensis (type) and the terrestrial fungus M. samuelsii, has been classified as an independent family based on LSU and SSU rRNA sequence analyses (Kohlmeyer and Kohlmeyer 1971; Huhndorf 1994; Jones et al. 2015). In addition, it differs from the members of the Aliquandostipitaceae by the production of obtusely clavate to fusiform, stipitate ascomata bearing a broad ostiole and unequally one-septate ascospores (Suetrong et al. 2011).
The largest genus within Jahnulales, Jahnula, was introduced by Kirschstein (1936) and is typified by Jahnula aquatica. All 16 species of Jahnula have been reported from wood or decorticated wood in fresh-water habitats (Hawksworth 1984; Hyde 1993; Hyde and Goh 1998; Hyde and Wong 1999; Ho et al. 2002; Pang et al. 2002; Pinruan et al. 2002; Raja and Shearer 2006; Campbell et al. 2007; Raja et al. 2008; Sivichai and Boonyuen 2010; Suetrong et al. 2011; Fournier et al. 2015). The genus is currently polyphyletic, and Jahnula sensu stricto accommodates Jahnula aquatica, J. granulosa, J. potamophila, and J. rostrata (Suetrong et al. 2011).
We are carrying out a survey of freshwater fungi along a north south latitudinal gradient in China and Thailand (Luo et al. 2017; Hyde et al. 2016a). Members of Jahnulales are major fungal species colonizing substrates in freshwater habitats, and recent taxonomic studies in freshwater habitats have revealed new asexual species (Luo et al. 2017). In this study, we report on two collections of Jahnula in China. The specimens were studied morphologically to enable identification, and regions of complete ITS and partial LSU and SSU rRNA gene were analyzed to determine their phylogenetic affinity to previously known Jahnula species.
Materials and methods
Sample collection, morphological studies, and isolation
Submerged dead wood pieces were collected from the Dianchi Lake, Yunnan Province in China in October 2016 and brought to the laboratory in zip lock plastic bags. Incubation of specimens was performed as outlined by Vrijmoed (2000). Fruiting bodies were found growing on decayed wood in a sterile plastic box after 2 weeks and were subsequently isolated based on the method of Chomnunti et al. (2014). Morphological characters were examined using an Olympus SZ61 stereoscope, and ascomata were sectioned by free-hand with a razor-blade. These sections were examined by a Nikon ECLIPSE Ni compound microscope, and images were taken with a Canon EOS 600D digital camera. Measurements were made with Tarosoft® Image Frame Work program v. 0.9.7. The specimens are deposited in the Kunming Institute of Botany, Academia Sinica and duplicated in Mae Fah Luang University (MFLU) Herbarium, Chiang Rai, Thailand. Facesoffungi (FoF) and Index Fungorum (IF) numbers were registered as explained in Jayasiri et al. (2015) and Index Fungorum (2017) (http://www.indexfungorum.org/names/names.asp). New species are established based on the recommendations outlined by Jeewon and Hyde (2016).
DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted directly from mycelium using a Trelief™ Plant Genomic DNA Kit following the instructions of the manufacturer. The genomic DNA was amplified by using polymerase chain reaction (PCR) in a 25 μL reaction mixture. Regions of the internal transcribed spacers (ITS1–5.8S-ITS2), partial large subunit rRNA (LSU), and partial small subunit rRNA (SSU) were amplified using primer pair ITS5 and ITS4 (White et al. 1990), LROR and LR5 (Vilgalys and Hester 1990) and NS1 and NS4 (White et al. 1990) respectively. Each PCR reactions contained 12.5 μL of 2 × Power Taq PCR MasterMix, 10.5 μL ddH2O, and 0.5 μL of each primer (10 μM); and 1 μL genomic DNA extract and amplifications were carried out in an Applied Biosystems 2720 thermocycler (Foster City, CA, USA) with the following profile: an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 50 s, annealing at 50 °C (SSU) or 53 °C (ITS and LSU) for 50 s and extension at 72 °C for 1 min, and a final extension at 72 °C for 8 min. The PCR products were visualized by loading 5 μL on 1% agarose electrophoresis gels and bands viewed in Gel documentation system. The PCR products were sent to a commercial sequencing provider Tsingke Company, Beijing, P.R. China.
Phylogenetic analysis
The quality of our amplified nucleotide sequences were checked by Finch TV version 1.4.0 (www.geospiza.com/finchtv), and subjected to the BLAST search under the National Center for Biotechnology Information (NCBI). Then the closest matches for our strains were retrieved from the NCBI database (Pang et al. 2002; Campbell et al. 2007; Prihatini et al. 2008; Suetrong et al. 2011; Phookamsak et al. 2014; Wanasinghe et al. 2014; Ariyawansa et al. 2015; Hyde et al. 2016b). Sequences were aligned using the default setting of MAFFT v. 7.310 (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley 2016), and manually corrected using Bioedit 7.0.9.0 (Hall 1999).
The phylogenetic analyses of combined gene regions were performed using maximum-likelihood (ML), Bayesian inference (BI), and maximum parsimony (MP) methods. The evolutionary model was obtained using MrModeltest v. 2.3 (Nylander et al. 2008) under the Akaike Information Criterion (AIC) performed in PAUP v. 4.0b10.
The maximum-likelihood analysis was run or performed using RAxML-HPC v.8 on XSEDE in CIPRES Science Gateway (Stamatakis 2014; Miller et al. 2010, 2015) with 1000 rapid bootstrap replicates using the General Time Reversible model incorporating invariant sites and a gamma distribution (GTR + I + G).
Bayesian inference was implemented by MrBayes v. 3.0b4 (Ronquist and Huelsenbeck 2003) with the best-fit model (GTR + I + G for ITS and LSU; SYM + I + G for SSU) of sequence evolution estimated with MrModeltest 2.3 (Nylander et al. 2008). Posterior probabilities (PP) were estimated by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist 2001; Zhaxybayeva and Gogarten 2002; Rannala and Yang 2008). Four simultaneous Markov Chain Monte Carlo (MCMC) chains were run from random trees for 100,000,000 generations and sampled every 1000 generations. The temperature value was lowered to 0.15, burn-in was set to 0.25, and the run was automatically stopped as soon as the average standard deviation of split frequencies reached below 0.01.
Phylogenetic analyses were also performed with maximum parsimony in PAUP v. 4.0b10 (Swofford 2002). Details are outlined as in Jeewon et al. (2002, 2003) and Cai et al. (2006). In brief, ambiguously aligned regions were excluded by hand and gaps were treated as missing data. Trees were inferred with the heuristic search option with TBR branch swapping and 1000 random sequence additions. Maxtrees were unlimited; branches of zero length were collapsed, and all parsimonious trees were saved. Tree length [TL], consistency index [CI], homoplasy index [HI], retention index [RI], and rescaled consistency index [RC] were calculated for the MP tree. Clade stability was assessed using a bootstrap (BT) analyses with 1000 replicates, each with ten replicates of random stepwise addition of taxa.
Phylogenetic trees were viewed with FigTree v1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) and processed by Adobe Illustrator CS5. Alignment and trees were deposited in TreeBASE (submission ID: 20896). The nucleotide sequence data of new taxa have been deposited in GenBank (Table 1).
Results
Phylogenetic analyses
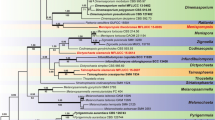
The alignment comprised 2347 total characters including gaps. The tree was rooted with Farlowiella carmichaeliana. The Bayesian analysis resulted in a tree with the same topology and clades as the ML and MP trees. RAxML analysis yielded a best scoring tree with a final optimization likelihood value of − 16,010.965307 (Fig. 1). Parsimony analyses indicated that 1411 characters were constant; 264 variable characters were parsimony-uninformative, and 672 characters were parsimony-informative. The parsimony analysis of the data matrix resulted in a single most parsimonious tree (TL = 2691, CI = 0.544, RI = 0.751, RC = 0.409, HI = 0.456) is shown here. In the phylogenetic tree, the two isolates of Jahnula dianchia constitute a well-supported clade (100%, ML/1.0, PP/100%, MP) that is sister to J. sangamonensis (100%, ML/1.0, PP/100%, MP).
Maximum likelihood phylogenetic tree generated from analysis of a combined ITS, LSU, and SSU sequences dataset for 51 taxa of Dothideomycetes and Farlowiella carmichaeliana as the outgroup taxon. ML support values greater than 70% (BSML, left), Bayesian posterior probabilities greater than 0.95 (BYPP, middle) and MP bootstrap value higher than 70% (BSMP, right) are indicated above the nodes. The strain numbers are noted after the species names. Ex-type strains are indicated in bold. Isolates from this study are indicated in red bold
Taxonomy
Jahnula dianchia S.K. Huang & K.D. Hyde, sp. nov .
Index Fungorum number: IF553200; Facesoffungi number: FoF 03149. Fig. 2
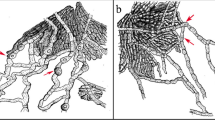
Jahnula dianchia (HKAS96327, holotype). a Material. b Ascoma on host. c Ascoma in vertical section. d Peridium. e Ostiole in vertical section with periphyses. f Asci with pseudoparaphyses. g–i Asci. j–l Ascospores. m Germinating ascospores. Note: g–h stained in Melzer’s reagent, arrow showing mammiform ascospores apex. Scale bars c, d = 100 μm, e–i = 50 μm, j–m = 20 μm
Etymology: The name dianchia refers to the geographic location were the specimen was collected.
Holotype: HKAS96327
Saprobic on dead wood. Sexual morph: Ascomata 307–418 × 248–374 μm (\( \overline{x}=380\times 325\ \upmu \mathrm{m} \), n = 5), perithecial, solitary, superficial to sub-immersed, unilocular, obpyriform to subglobose, dark brown to black, papillate, ostiolate. Ostiole central, short, lined with hyaline periphyses. Peridium 35–75 μm thick, membranous, composed of brown to hyaline cells of textura angularis (Fig. 2d). Hamathecium comprising 2–3 μm wide, septate, branched, filiform pseudoparaphyses, embedded in a gelatinous matrix. Asci 198–238 × 16–20 μm (\( \overline{x}=217\times 19\ \upmu \mathrm{m} \), n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, pedicellate, rounded at apex, with a distinct ocular chamber. Ascospores 24–32 × 10–21 μm (\( \overline{x}=27.5\times 13\ \upmu \mathrm{m} \), n = 50), uniseriate, initially hyaline, becoming dark brown at maturity, oval to broadly ellipsoid, 1-septate, with a mammiform apex, slightly curved, smooth to verruculose, multiguttulate, apiculate, rounded at lower end. Asexual morph: undetermined.
Culture characteristics: Ascospores germinating on potato dextrose agar (PDA) within 2 weeks at 23 °C, colony 1.5 cm diam., hyphae 3–10 μm thick, cream to faint yellow from above and reverse, with concentric zonation, with filamentous mycelium, filiform at margin, with rough surface and raised elevation.
Material examined: CHINA, Yunnan, Kunming, Dianchi Lake (24° 51′ 29.18″ N, 102° 39′ 58.19″ E); on dead wood, 1 October 2016; S.K. Huang (KUN HKAS 96327, holotype), ibid. (MFLU 17-0693, isotype); Ex-type KUMCC 17-0034, MFLUCC 17-0887. ibid. (KUN HKAS 97459, MFLU 17-0698; KUMCC 17-0039, MFLUCC 17-0891).
Key to species of Jahnula based on the sexual morph
-
1. Ascospores with appendages ......................................2
-
1. Ascospores without appendages ...............................10
-
2. Ascospores with long appendages arising from both poles ................................................................................3
-
2. Ascospores surrounded by mucilaginous sheath .........4
-
2. Ascospores with mucilaginous pads at both poles ......7
-
3. Ascospores 47.5–55 × 23.5–26.5 μm4 .....................................................Jahnula appendiculata
-
3. Ascospores 17.5–20 × 5–6.5 μm7 .......................................................................J. morakotii
-
4. Two types of ascospores morphologies2 .........J. systyla
-
4. One type of ascospores morphology ...........................5
-
5. Ascospores light brown2 ......................J. potamophila
-
5. Ascospores dark brown ...............................................6
-
6. Ascospores 26–37.5 × 15–18 μm2 .........J. granulosa
-
6. Ascospores 32–45 × 12–15 μm5 ................J. rostrata
-
7. There are two types of ascospores2 .....J. seychellensis
-
7. There are only one type of ascospores ........................8
-
8. Ascospores dark brown5 .............................J. bipileata
-
8. Ascospores light brown ...............................................9
-
9. Asci cylindrical, ascospores uniseriate2 ......J. bipolaris
-
9. Asci obclavate, ascospores biseriate3, 8 ..J. sunyatsenii
-
10. Ascospores ellipsoid to fusiform..............................11
-
10. Ascospores oval to oblong ......................................14
-
11. Ascospores basal cell shorter than apical cell6 .......................................................................J. apiospora
-
11. Ascospores symmetrical .........................................12
-
12. Ascospores longer than 30 μm5 .........................................................................J. aquatica
-
12. Ascospores shorter than 30 μm ...............................13
-
13. Ascospores with tapering apices2 ...........J. poonythii
-
13. Ascospores with rounded apices5 ..............................................................J. sangamonensis
-
14. Asci cylindrical .......................................................15
-
14. Asci obclavate9 ........................................J. purpurea
-
15. Ascospores without mammiform apex, 19–30 × 6–8 μm1 .......................................................J. australiensis
-
15. Ascospores with mammiform apex, 24–32 × 10.5–21 μm .............................................................J. dianchia
1 Hyde (1993); 2 Hyde and Wong (1999); 3 Inderbitzin et al. (2001); 4 Pinruan et al. (2002); 5 Raja and Shearer (2006); 6 Raja et al. (2008); 7 Sivichai and Boonyuen (2010); 8 Suetrong et al. (2011); 9 Fournier et al. (2015)
Discussion
As part of our studies on freshwater fungi in the Yunnan Province of China, we collected a fungal species that is morphologically similar to the genus Jahnula or Jahnula spp. Species of Jahnula are predominantly collected or isolated from freshwater habitats (Hyde 1993; Hyde and Wong 1999; Tsui et al. 2000; Ho et al. 2001; Pang et al. 2002; Pinruan et al. 2002; Raja and Shearer 2006; Raja et al. 2008; Sivichai and Boonyuen 2010; Suetrong et al. 2011; Fournier et al. 2015). Further, the two specimens in this study fit well with the diagnosis of Jahnula as previously circumscribed, but are morphologically distinct from other Jahnula species such as to justify the description and naming of a new species. In addition, DNA sequence data support that the new taxon, J. dianchia, is phylogenetically distinct from other known species. Cai et al. (2002) reported Jahnula poonythii K.D. Hyde & S.W. Wong from Dianchi Lake, which may be an earlier misidentified collection of our new species. However, fusiform ascospores are seen in J. poonythii whereas they are broadly ellipsoidal to oval in J. dianchia.
The phylogeny (Fig. 1) reveals a close relationship of the newly collected fungus to J. sangamonensis which has been reported from decorticated submerged woody debris in the USA (Raja and Shearer 2006). Jahnula dianchia is similar to J. sangamonensis but differs in the following aspects: (i) most of the ascomata of J. dianchia are superficial and J. sangamonensis is sub-immersed in the wood, (ii) the peridium of J. dianchia is 35–75 μm wide with the inner wall layer of ostiole lined with hyaline periphyses while those of J. sangamonensis are 40–44 μm wide with the inner wall layer of ostiole lined with reddish brown periphyses, (iii) the asci of two taxa are different in that endoascus of J. sangamonensis extends up to 500 μm long in water, and (iv) the ascospores of J. dianchia are similar to those of J. sangamonensis except that these are with mammiform apex. Seventeen species are included in the genus Jahnula (Suetrong et al. 2010, 2011; Fournier et al. 2015). Ten of them are different from J. dianchia by having ascospores with a gelatinous sheath, pads, or appendages (viz. Jahnula appendiculata, J. bipileata, J. bipolaris, J. granulosa, J. morakotii, J. potamophila, J. rostrata, J. seychellensis, J. sunyatsenii and J. systyla) (Fournier et al. 2015; Hyde and Wong 1999; Pang et al. 2002; Pinruan et al. 2002; Sivichai and Boonyuen 2010). The shape of ascospores in Jahnula apiospora, J. australiensis, J. poonythii, and J. purpurea is fusiform or oblong while in our new taxon, it is oval to broadly ellipsoid (Fournier et al. 2015; Hyde and Wong 1999; Raja et al. 2008). Jahnula dianchia is also different from J. aquatica, the type species of Jahnula, collected from Germany and South Africa, in terms of ascospore length (> 30 μm) (Hyde and Wong 1999).
To further support our new taxon, we follow the recommendations by Jeewon and Hyde (2016). Comparison of the 463 base pairs (bp) across the nuclear ribosomal DNA ITS region, including the spacers ITS1 and ITS2 together with the 5.8S rRNA gene reveals that there are 20 bp (4.3%) differences when compared to J. sangamonensis. In the same way, comparison of the 862 bp of the 28S ribosomal RNA gene region reveals 11 bp (1.3%) difference compared to J. sangamonensis that justifies our new species.
Despite having only two strains herein, J. dianchia constitutes a strong monophyletic subclade with high support and therefore establishment of a new species is justified. The phylogeny generated herein (Fig. 1) agrees with other published studies in recovering Jahnula as a non-monophyletic group within the Jahnulales. Five clades are recognized: Clade I comprises species that belongs to Jahnula sensu lato with high statistical support (Campbell et al. 2007; Suetrong et al. 2011) together with those anamorphic genus Speiropsis (typified by S. pedatospora) and Brachiosphaera taxa (typified by B. tropicalis) (Suetrong et al. 2011). Clade II includes species of Aliquandostipite, while clade III comprises species of Jahnula sensu stricto such as J. aquatica, J. granulosa, J. potamophila, and J. rostrata (Campbell et al. 2007, Suetrong et al. 2011). Clade IV represents the genus Manglicola (Suetrong et al. 2010, 2011), whereas Clade V accommodates the genus Megalohypha (Raja et al. 2011).
References
Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Boonmee S, Wanasinghe DN, Phookamsak R, Hongsanan S, Singtripop C, Chukeatirote E, Kang JC, Jones EBG, Hyde KD (2015) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71:85–139
Cai L, Tsui CKM, Zhang KQ, Hyde KD (2002) Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Divers 9:57–70
Cai L, Jeewon R, Hyde KD (2006) Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycol Res 110:137–150
Campbell J, Ferrer A, Raja HA, Sivichai S, Shearer CA (2007) Phylogenetic relationships among taxa in the Jahnulales inferred from 18S and 28S nuclear ribosomal DNA sequences. Can J Bot 85:873–882
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu JC, Liu XZ, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Descals E, Nawawi A, Webster J (1976) Developmental studies in Actinospora and three similar aquatic hyphomycetes. Trans Br Mycol Soc 67(2):207–222
Ferrer A, Sivichai S, Shearer CA (2007) Megalohypha, a new genus in the Jahnulales from aquatic habitats in the tropics. Mycologia 99(3):456–460
Fournier J, Raja HA, Shearer CA (2015) Freshwater Ascomycetes: Jahnula purpurea (Jahnulales, Dothideomycetes), a new species on submerged wood from Martinique Island, Lesser Antilles. MycoKeys 9:29–36
Goos RD, Brooks RD, Lamore BJ (1977) An undescribed hyphomycete from wood submerged in a Rhode Island stream. Mycologia 69(2):280–286
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hawksworth DL (1984) Observations on Jahnula Kirschst., a re-markable aquatic pyrenomycete. Sydowia 37:43–46
Ho WH, Hyde KD, Hodgkiss IJ, Yanna (2001) Fungal communities on submerged wood from streams in Brunei, Hong Kong, and Malaysia. Mycol Res 105:1492–1501
Ho WH, Yanna, Hyde KD, Hodgkiss IJ (2002) Seasonality and sequential occurrence of fungi on wood submerged in Tai Po Kau forest stream, Hong Kong. Fungal Divers 10:21–43
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Huhndorf SM (1994) Neotropical ascomycetes. 5. Hypostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia 86(2):266–269
Hyde KD (1993) Tropical Australian freshwater fungi. V. Bombardia sp., Jahnula australiensis sp. nov., Savoryella aquatica sp. nov. and S. lignicola sp. nov. Aust Syst Bot 6(2):161–167
Hyde KD, Goh TK (1998) Fungi on submerged wood in Lake Barrine, North Queensland, Australia. Mycol Res 102:739–749
Hyde KD, Wong SW (1999) Tropical Australian freshwater Fungi. XV1The ascomycete genus Jahnula, with five new species and one new combination. Nova Hedwigia 68(3–4):489–509
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, de Hoog S, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Fryar S, Tian Q, Bahkali AH, Xu JC (2016a) Lignicolous freshwater fungi along a north-south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol 19:190–200
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, Góes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, Santiago ALCMDA, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar MA, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, da Silva GA, de Lima CLF, de Oliveira RJ, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytӧvuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lücking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L (2016b) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Inderbitzin P, Landvik S, Abdel-Wahab MA, Berbee ML (2001) Aliquandostipitaceae, a new family for two new tropical ascomycetes with unusually wide hyphae and dimorphic ascomata. Am J Bot 88(1):52–61
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, GhobadNejhad M, Nilsson H, Pang KL, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q, Kang JC, Promputtha I (2015) The faces of fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74:3–18
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7(11):1669–1677
Jeewon R, Liew ECY, Hyde KD (2002) Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Mol Phylogenet Evol 25:378–392
Jeewon R, Liew ECY, Simpson JA, Hodgkiss IJ, Hyde KD (2003) Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Mol Phylogenet Evol 27:372–383
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Katoh K, Standley DM (2016) A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 32(13):1933–1942
Kirschstein W (1936) Beiträge zur Kenntnis der Ascomyceten und ihrer Nebenformen besonders aus der Mark Brandenburg und dem Bayerischen Walde. Annales Mycologici 34:180–210
Kohlmeyer J, Kohlmeyer E (1971) Marine fungi from tropical America and Africa. Mycologia 63(4):831–861
Luo ZL, Bhat JD, Jeewon R, Boonmee S, Bao DF, Zhao YC, Chai HM, Su HY, Su XJ, Hyde KD (2017) Molecular phylogeny and morphological characterization of asexual fungi (Tubeufiaceae) from freshwater habitats in Yunnan, China. Cryptogam Mycol 38(1):1–28
Miller MA, Pfeiffer W, Schwartz T (2010) “Creating the CIPRES science gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA pp 1–8
Miller AM, Schwartz T, Pickett BE, He S, Klem EB, Scheuermann RH, Passarotti M, Kaufman S, O’Leary MA (2015) A RESTful API for access to phylogenetic tools via the CIPRES science gateway. Evol Bioinforma 11:43–48
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are wer there yet?): a system for graphical exploration fo MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Pang KL, Abdel-Wahab MA, Sivichai S, El-Sharouney HM, Jones EBG (2002) Jahnulales (Dothideomycetes, Ascomycota): a new order of lignicolous freshwater ascomycetes. Mycol Res 106(9):1031–1042
Phookamsak R, Liu JK, Mckenzie EHC, Manamgoda DS, Chatpapamon C, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Pinruan U, Jones EBG, Hyde KD (2002) Aquatic fungi from peat swamp palms. Jahnula appendiculata sp. nov. Sydowia 54(2):242–247
Prihatini R, Boonyuen N, Sivichai S (2008) Phylogenetic evidence that two submerged-habitat fungal species, Speiropsis pedatospora and Xylomyces chlamydosporus, belong to the order, Jahnulales insertae sedis Dothideomycetes. Microbiol Indones 2(3):136–140
Raja HA, Shearer CA (2006) Jahnula species from north and central America, including three new species. Mycologia 98(2):319–332
Raja HA, Carter A, Platt HW, Shearer CA (2008) Freshwater ascomycetes: Jahnula apiospora (Jahnulales, Dothideomycetes), a new species from Prince Edward Island, Canada. Mycoscience 49(5):326–328
Raja HA, Schoch CL, Hustad VP, Shearer CA, Miller AN (2011) Testing the phylogenetic utility of MCM7 in the Ascomycota. MycoKeys 1:63–94
Rannala B, Yang Z (2008) Phylogenetic inference using whole genomes. Annu Rev Genomics Hum Genet 9:217–231
Ronquist F, Huelsenbeck JP (2003) Mrbayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:916–928
Shenoy BD, Jeewon R, Wang HK, Amandeep K, Ho WH, Bhat DJ, Crous PW, Hyde KD (2010) Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Divers 44:161–169
Sivichai S, Boonyuen N (2010) Jahnula morakotiisp. nov. and J. appendiculata from a peat swamp in Thailand. Mycotaxon 112:475–481
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Suetrong S, Sakayaroj J, Phongpaichit S, Jones EBG (2010) Morphological and molecular characteristics of a poorly known marine ascomycete, Manglicola guatemalensis (Jahnulales: Pezizomycotina; Dothideomycetes, Incertae sedis): new lineage of marine ascomycetes. Mycologia 102:83–92
Suetrong S, Boonyuen N, Pang KL, Ueapattanakit J, Klaysuban A, Sriiindrasutdhi V, Sivichai S, Jones EBG (2011) A taxonomic revision and phylogenetic reconstruction of the Jahnulales (Dothideomycetes), and the new family Manglicolaceae. Fungal Divers 51(1):163–188
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tsui CKM, Hyde KD, Hodgkiss IJ (2000) Biodiversity of fungi on submerged wood in Hong Kong streams. Aquat Microb Ecol 21:289–298
Tubaki K (1958) Studies on the Japanese Hyphomycetes. V. Leaf & stem group with a discussion of the classification of the Hyphomycetes and their perfect stages. J Hattori Bot Lab 20:142–244
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Vrijmoed LLP (2000) Isolation and culture of higher filamentous fungi. In: Hyde KD, Pointing SB (eds) Marine mycology—a practical approach. Fungal Diversity Press, Hong Kong, pp 1–20
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Mortimer PE, Xu JC, Yang JB, Hyde KD (2014) An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogam Mycol 35:323–337
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic press, San Diego, pp 315–322
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3:4
Acknowledgements
We thank Kunming Institute of Botany, Chinese Academy of Sciences providing laboratory facilities for carrying out taxonomic work. Shi-Ke Huang is particularly grateful to Dr. Qi Zhao and Dr. Olivier Raspé for their invaluable suggestions in this paper. Drs. R. Jeewon and D.J. Bhat thank Mae Fah Luang University for the offer of a short term Visiting Professorship in 2016 and keynote speakers for the COEIC 2017 conference. K.D. Hyde is grateful for the position of adjunct Professor at Chiang Mai University.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31360015) and the CAS/SAFEA International Partnership Program for Creative Research Teams, and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-Z-9 KIB2016002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Section Editor: Kevin Hyde and Christiane Baschien
This article is part of the “Special Issue on Freshwater Ascomycota”
Rights and permissions
About this article
Cite this article
Huang, SK., Maharachchikumbura, S.S.N., Jeewon, R. et al. Morphological and molecular taxonomy of Jahnula dianchia sp. nov. (Jahnulales) from submerged wood in Dianchi Lake, Yunnan China. Mycol Progress 17, 547–555 (2018). https://doi.org/10.1007/s11557-018-1390-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-018-1390-9