Abstract
This paper lists the accepted names and classification of marine fungi, updating the scheme presented in 2009. The classification includes 1,112 species (in 472 genera): Ascomycota 805 (in 352 genera), Basidiomycota 21 species (in 17 genera), Chytridiomycota and related phyla 26 species (in 13 genera), Zygomycota three (in two genera), Blastocladiomycota one species (one genus), asexual morphs of filamentous fungi 43 (in 26 genera); and marine yeasts: Ascomycota 138 species (in 35 genera), Basidiomycota 75 species (in 26 genera). These fungi belong to 129 families and 65 orders. The Halosphaeriaceae remains the largest family of marine fungi with 141 species in 59 genera, while the most specious genera are Aspergillus (47 species), Penicillium (39 species) and the yeast genus Candida (64 species). The review includes details of recent higher order nomenclature changes, and accounts of new families, genera and species described over the past 5 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first classification of marine fungi was by Johnson and Sparrow (1961) and this was expanded upon by Kohlmeyer and Kohlmeyer (1979); both treatments were before the incorporation of molecular studies. Jones et al. (2009) provided the first classification of higher orders of marine fungi, which included some phylogenetic data. The past 5 years have seen a dramatic increase in molecular phylogenetic data available for this group. Key papers are by Sakayaroj et al. (2011) and Pang (2012) on Halosphaeriaceae, a family extensively studied with 75 % of species partially sequenced; Suetrong et al. (2009) on marine Dothideomycetes and a major review of the Dothideomycetes by Hyde et al. (2013). Other phylogenetic reviews that inpinge on the taxonomy of marine fungi include those by Wijayawardene et al. (2014) and Ariyawansa et al. (2015) on the Dothideomycetes and Pleosporales; Maharachchikumbura et al. (2015) an outline of Sordariomycetes; and Senanayake et al. (2015) an outline of the Xylariomycetidae (Sordariomycetes). Studies have highlighted new lineages of marine fungi, which has also greatly impacted on the classification of terrestrial taxa. Jones (2011) estimated there were in excess of 10,000 marine fungi and listed habitats/substrates that have not been fully explored: 1. unidentified species on a range of substrata, 2. marine derived fungi isolated from sediments, sand and water, 3. planktonic fungi, 4. deep-sea fungi, 5. unculturable fungi, and 6. cryptic species. With wider sampling, e.g., deep sea hydrothermal vents (Nagahama and Nagano 2012), and application of new techniques, e.g., tag-encoded 454 pyrosequencing, metagenomic,-transcriptomic,-proteomic and -metabolomic studies (Buée et al. 2009; Peršoh 2015), a much wider range of fungi will be identified. Rämä et al. (2014) examined 50 logs washed on to the shores of Norway (intertidal and sea floor logs) and reported half of the OTUs considered to be non-marine. Nine OTUs could not be assigned to any fungal phylum.
Our knowledge on marine fungi has advanced significantly over the last two decades and this has been summarized in recent books and monographs. Jones et al. (2009) reviewed the classification of marine fungi based on available phylogenetic data. In an edited book on marine fungi (Jones and Pang 2012), a comprehensive range of topics was reviewed: phylogeny of marine fungi (Ascomycota, Basidiomycota, yeasts and true zoosporic fungi), their biodiversity (occurrence in mangroves, on marine algae, salt marsh fungi, marine-derived fungi, fungi in deep-sea environments), and their application in industry, as well as the phylogeny of fungal-like organisms. These studies were strongly supported by DNA sequence data and highlighted a number of new lineages of marine fungi. Raghukumar (2012) edited another book on marine fungi, focusing on pathogenic marine fungi of marine animals, their diversity on various substrates/environments, taxonomy of specific groups and their biotechnological potential. Richards et al. (2012), in a paper based on molecular data and largely devoted to the diversity of fungi in the deep-sea, were of the opinion that the “known fungal diversity in marine environments represents a tiny fraction of that from terrestrial environments” and that fungal-specific molecular studies in marine environments are relatively few. Significant advances on the molecular phylogeny of marine fungi and their occurrence in a wide spectrum of habitats/environments have been made (Boonyuen et al. 2011; Jones 2011; Jones and Pang 2012; Singh et al. 2012; Orsi et al. 2013; Jones et al. 2014; Lepelletier et al. 2014a, b; Manohar et al. 2014; Rämä et al. 2014). Richards et al. (2012) also suggested that ‘fungi appear to be rare in the marine environment’, an opinion that did not consider the wide range of published literature on this subject (e.g., Raghukumar, ed. 2012). Marine fungi are associated with organic matter, which is concentrated in coastal areas (Jones et al. 2013). Greater emphasis is now being placed on the fungal diversity in sea water, sediments and deep sea hydrothermal vents (Nagahama and Nagano 2012). These have highlighted a greater diversity with species more similar to those found in terrestrial habitats (Zuccaro et al. 2004; Yu et al. 2013). However, these studies fail to consider their role in marine habitats. An aspect that has to be considered is that many of these fungi may be passively washed into the marine milieu and survive as dormant propagules (Hagler et al. 1982; Araujo and Hagler 2011; Fell 2014). While culture-independent studies of fungal diversity in the sea have discovered new lineages of fungi: a marine Chyridiomycota lineage in deep-sea sediments (Nagano et al. 2010), an “unknown phyllotypes branching within the basidiomycete radiation” in deep-sea hydrothermal vent Le Calvez et al. (2009) and 36 novel marine lineages (Richard et al. 2012), but they fail to acknowledge the role of these fungi.
Kohlmeyer and Kohlmeyer (1979) adopted a very narrow definition of what constitutes a marine fungus, grouping them into obligate and facultative marine fungi. In our opinion, this is too restrictive and in this monograph we adopt a wider concept. Are all documented marine fungi adapted and metabolically active in the marine environment? Pyrosequencing, and other molecular techniques, are highlighting a greater divertsity than studies at the morphological level; therefore their role in marine habitats has to be addressed. Amend (2014) raises the question of whether Malassezia species (Malasseziomycetes) recovered from deep-sea sediments (Lai et al. 2007) and hydrothermal vents (Le Calvez et al. 2009), are truly marine. This is a genus known primarily from both healthy and diseased human skin, yet reported to occur in a great diversity of habitats and locations, from polar regions to deep-sea vents. They may even be dominant in certain marine habitats, and therefore should be regarded as marine fungi (Amend 2014).
Jones et al. (2009) listed 23 Verrucaria species from the littoral/intertidal zone. Various molecular studies have since been undertaken resulting in revisions in the taxonomy of the family Verrucariaceae (Gueidan et al. 2007, 2009, 2011). Currently we list 12 Verrucaria marine species as others have been referred to Wallenbergiella (three species) (Gueidan et al. 2009, 2011) and Hydropunctaria (six species) (Gueidan et al. 2009). Mastodia, previously placed in Mastodiaceae (Unitunicate Ascomycota incertae sedis), has now been shown to group in the Verrucariaceae (Pérrez-Ortega et al. 2010). Molecular data showed that Mastodia tessellata, a fungus associated with the green algal genus Prasiola, nested within the Verrucariaceae, and is a sister group to the marine genus Wahlenbergiella with high statistical support (Pérrez-Ortega et al. 2010).
In this volume we present an up to date classification of marine fungi (Table 1) and marine yeasts (Table 2), followed by notes on new taxa and new combinations from 2009 to 2015. The text is divided into six sections: Ascomycota (sexual and asexual), Marine Yeasts (Ascomycota and Basidiomycota), Marine-derived Fungi, Basidiomycota, Blastocladiomycota and Chytridiomycota.
In the last decades, research on natural products of marine fungi is on the rise and there are over 1000 metabolites described from fungi isolated from marine substrates (Overy et al. 2014). These so-called ‘marine-derived fungi’ were mainly isolated from marine animals, macroalgae, mangrove plants and sediments and are taxonomically diverse (Ebel 2012; Debbab et al. 2012). ‘Marine-derived fungi’ include fungi of marine origin and terrestrial/freshwater taxa (facultative marine fungi) washed off from the terrestrial environment. Some of these terrestrial/freshwater fungi only appeared once in the literature of fungal natural product research, others were repeatedly cultured from the marine environment, suggesting their possible marine occurence, e.g., species in the genera Aspergillus, Penicillium and Trichoderma (Overy et al. 2014). This treatise is the first comprehensive attempt to list these true marine fungi so as to distinguish them from those of terrestrial/freshwater origin. Moreover, secondary metabolites of the marine Eurotiomycetes are the most intensely studied group and this classification paper presents the most updated, highly diverse lineages of marine fungi, highlighting groups of marine fungi for future screening of natural products. It is vital that all fungi isolated from the marine milieu are fully described, sequenced and deposited in international culture collections for future study. Further research is required to determine how these taxa differ from their terrestrial counterparts (Zuccaro et al. 2004).
Materials and methods
The methods and materials for the study of marine fungi are well documented in Vrijmoed (2000) and Jones et al. (2009) for filamentous taxa, while those for zoosporic species are documented by James et al. (2006). Yeasts are currently characterised by sequence data, particularly ITS and D1/D2 regions of the LSU rDNA, which leads to their rapid identification (Kurtzman et al. 2011a, b). Identification and characterization of fungi is by a combination of morphological observations, a constellation of ultrastructural characters and analyses of gene moleucular sequences, which has revolutionized our understanding of the phylogeny of fungi (Powell and Letcher 2012). It is imperative that herbarium material and cultures are deposited in international centers with linked sequence data deposited in Gen Bank. Fungi isolated as part of the search for new bioactive compounds should be sequenced and deposited in international archives so that their taxonomy can be evaluated by other researchers.
Higher order classification
Tables 1 and 2 present our current classification of the Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota, with changes resulting from molecular studies carried out since 2009 (Jones et al. 2009). In these tables we have assigned asexual genera to their higher order position based on molecular studies (Pang et al. 2008; Suetrong et al. 2009; Kurtzman et al. 2011a, b; Lepelletier et al. 2014a, b; Wijayawardene et al. 2014; Ariyawansa et al. 2015; Maharachchikumbura et al. 2015; Senanayake et al. 2015). However, not all marine asexual species have been sequenced, so their placement will be revised when molecular data becomes available. In Table 1 where asexual genera are included (*), those supported by molecular data are indicated as #. The reasons for the various changes are outlined in the following sections.
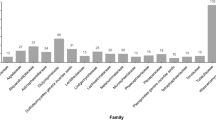
In this monograph of the marine fungi we document 1112 species (in 472 genera): Ascomycota 805 (in 352 genera), Basidiomycota 21 species (in 17 genera), Chytridiomycota and related phyla 26 species (in 13 genera), Zygomycota three (in two genera), Blastocladiomycota one species (one genus), asexual filamentous fungi 43 (in 26 genera); and marine yeasts: Ascomycota 138 species (in 35 genera), Basidiomycota 75 species (in 26 genera) (Fig. 1). These fungi belong to 129 families and 65 orders. The Halosphaeriaceae remains the largest family of marine fungi with 141 species in 59 genera, while the most specious genera are Aspergillus (47 species), Penicillium (39 species) and the yeast genus Candida (64 species). However, this current figure of marine fungi may represent but a fraction of the species that occur in the marine environment, with Jones (2011) estimating there are as many as 10,000 species, especially those occurring on algae. Garzoli et al. (2015) isolated 1,500 marine fungal strains (mainly Ascomycota, but a breakdown to number of species/genera was not given) from various substrates in the Mediterranean Sea, focusing on the algae Flabellia petiolata (green) and Asparagopsis taxiformis (red).
Outline of classification of marine fungi
Table 1.
Outline clasiification of marine yeasts
Table 2.
Notes on new taxa and new combinations 2009–2015
Ascomycota and asexual fungi
Pezizomycotina
Dothideomycetes
The past decade has seen intensive studies of ascomycetes with bitunicate asci (Dothideomycetes) especially at the molecular level, which has resulted in a major revision of the taxa referred to this class (Schoch et al. 2009). Suetrong et al. (2009) reviewed the marine genera and species referred to the Dothideomycetes and introduced the families Aigialaceae, Morosphaeriaceae and placed Verruculina enalia in Testudinaceae. Hyde et al. (2013) undertook a major revision of the Dothideomycetes and accepted 22 orders and 105 families.
Of the orders accepted, Dyfrolomycetales was introduced to accommodate a new family Dyfrolomycetaceae with four marine species, including the new genus Dyfrolomyces (D. tiomanensis) and Saccardoella mangrovei, S. marinospora and S. rhizophorae (Hyde et al. 2013; Pang et al. 2013) (Fig. 2b, f-i).
Morphological features of marine Dothideomycetes: Manglicolaceae and Dyfrolomycetaceae. Manglicola guatemalensis a. Mature ascomata on surface of Nypa fruticans. c. Ascospore in ascus. d-e. 1-septate ascospores. Dyfrolomyces marinospora b. Surface wood cut to show immersed ascoma f. Ascospore. D. rhizophorae. g. Ascospore. i. ascospores. D. mangrovei. h. Ascospores. Ascospores. a, c-e. = Manglicolaceae. b, f-i = Dyfrolomycetaceae. Bars a-b = 500 μm; c = 30 μm; h-i =25 μm; e-g = 20 μm; d =15 μm
Two new families were introduced for marine taxa previously not assigned to any family: Biatriosporaceae and Salsugineaceae (Maharachchikumbura et al. 2015).
Jahnulales K.L. Pang, Abdel-Wahab, El-Shar., E.B.G. Jones & Sivichai, Mycol. Res. 106 (9): 1033 (2002)
The family Aliquandostipitaceae was introduced by Inderbitzin et al. (2001) to accommodate the freshwater fungal genera Aliquandostipite and Jahnula, and subsequently referred to the order Jahnulales (Pang et al. 2002). Pang et al. (2002) included the genera Aliquandostipite (two species), Jahnula (three species) and introduced the new genus Patescospora (one species). Campbell et al. (2007) emended the description of the order to include taxa with wide, brown hyphae and a wider variation in ascospore characters. No marine members of Jahnulales were known until Suetrong et al. (2009, 2010) demonstrated that the marine ascomycete Manglicola (M. guatemalensis) also belonged in the order. Suetrong et al. (2011a) undertook a revision of the Jahnulales (Dothideomycetes), and introduced the new family Manglicolaceae. Currently, Jahnulales includes four sexual genera: Jahnula (15 species), Aliquandostipite (five species), Megalohypha (one) and Manglicola (one), and three asexual genera: Brachiosphaera (two species), Speiropsis (nine species) and Xylomyces (eight species).
Manglicolaceae Suetrong & E.B.G. Jones, Fungal Divers. 51(1): 183 (2011)
This family was introduced to accommodate the marine species Manglicola guatemalensis based on morphological and molecular evidence (Suetrong et al. 2011a) (Fig. 2a, c-e). The genus was previously referred to the Hypsostromataceae by Huhndorf (1994), a family with no previously known relationship to any group in the Dothideomycetes. Manglicola guatemalensis is an infrequently collected species usually on decayed mangrove wood or frond bases of the brackish water palm Nypa fruticans.
Dyfrolomycetales K.L. Pang, K.D. Hyde & E.B.G. Jones, Fungal Divers. 63: 7 (2013)
This new order was described by Hyde et al. (2013) for the family Dyfrolomycetaceae, which includes Dyfrolomyces which accommodated marine Saccardoella species that could not be referred to any family or order in the Ascomycota. Ascus structure in the genus was confusing as they neither appeared unitunicate nor bitunicate, thus taxonomic placement was difficult based solely on morphology. A molecular study of three Saccardoella species and a new Saccardoella-like species showed they grouped with high statistical support in the class Dothideomycetes, but were distinct from any order or family in the class. Pang et al. (2013) referred the four species to Dyfrolomyces, in a new family Dyfrolomycetaceae. Dyfrolomyces is characterized by forming a clypeus on substrata, with immersed perithecial ascomata, bitunicate, fissitunicate asci and multi-septate, hyaline ascospores with or without a sheath (Fig. 2b, f-i).
Pleosporales Luttr. ex M.E. Barr, Prodr. Cl. Loculoasc. (Amherst): 67 (1987)
Aigialaceae Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, Stud. Mycol. 64: 166 (2010)
This family includes the marine genera Aigialus (with five species), Ascocratera (one species) and Rimora (accommodating Lophiostoma mangrovei) (Suetrong et al. 2009), (Fig. 3a-b, f, k, n) and subsequently two new terrestrial genera, Fissuroma and Neoastrosphaeriella (Liu et al. 2011b). Fissuroma and Neoastrosphaeriella were previously referred to the Pleosporales incertae sedis and Lophiostomataceae respectively, based on morphological features (Jones et al. 2009).
Morphological features of marine Dothideomycetes: Aigialaceae, Halojulellaceae and Halotthiaceae. Aigialus parvus a. Surface view of mature ascomata. f. Ascus tip. i. Ellipsoidal to broadly fusiform ascospore. A. mangrovis j. Ellipsoidal to fusiform ascospore. A. grandis k. Ellipsoidal fusiform ascospore. A. rhizophorae l. Broadly fusiform ascospore. Ascocratera manglicola m. Ellipsoidal ascospore. Rimora mangrovei b. Broadly oblong ascomata. n. Fusiform ascospore. Halojulella avicenniae c. Surface view of ascomata on Avicennia wood. g. Clavate to cylindrical ascus. o. Ellipsoidal ascospore. Halotthia posidonia e d. Broadly conical ascomata. p. Ellipsoidal, dark brown ascospore. Pontoporeia biturbinata e. Globose ascoma. h. Ascospores in broadly clavate asci. r. Subellipsoidal ascospore. Mauritiana rhizophora e q. Fusiform ascospore. a-b, f, i-n = Aigialaceae. c, g, o = Halojulellaceae. d-e, h, p-r = Halotthiaceae. Bars a-b, d-e = 1500 μm; c = 250 μm; h = 100 μm; g = 50 μm; p =30 μm; f, i-n, q = 25 μm; o, r = 20 μm
Biatriosporaceae K.D. Hyde, Fungal Divers. 63: 50 (2013)
Hyde (in Hyde et al. 2013) introduced this family to accommodate a monotypic genus/species Biatriospora marina, a unique mangrove ascomycete with dark brown ascospores that are fusiform with hyaline swollen tips, which release mucilage, and 1–4 septa situated near the ends (Hyde and Borse 1986). Phylogenetically, this genus sits independently with Roussoella and Roussoellopsis strains with strong statistical support in the Pleosporales (Hyde et al. 2013).
Halojulellaceae Suetrong, K.D. Hyde & E.B.G. Jones, Phytotaxa 130(1): 5 (2013)
The family Halojulellaceae was introduced by Ariyawansa et al. (2013) to accommodate Jullella avicenniae, which forms a separate clade in the suborder Pleosporineae, order Pleosporales. The type is Halojulella avicenniae initially described as Pleospora avicenniae (Borse 1987) and subsequently transferred to Julella (Hyde 1992). Suetrong et al. (2009) showed that five strains formed a monophyletic clade in Pleosporales, but could not be assigned to any family. Halojullela avicenniae grows on woody substrata, usually the tips of branches of Avicennia marina, and its perithecia form beneath a clypeus; the peridium is composed of a single layer of elongate cells and with cellular, hyphae-like pseudoparaphyses (Fig. 3e, g, o).
Halotthiaceae Ying Zhang, J. Fourn. & K.D. Hyde, Mycologia 105(3): 604 (2013)
The family was introduced by Zhang et al. (2013b) to accommodate three monotypic genera Halotthia, Mauritiana and Pontoporeia, all with dark coloured ascospores (Fig. 3d, e, h, p, r). Suetrong et al. (2009), used four-gene analyses to show they grouped with moderate support in Pleosporales, but did not assign them to any family. Zhang et al. (2013b) subsequently described the new genus Phaeoseptum (type species Ph. aquaticum) which also grouped in the Halotthiaceae with high support (86 %). Halotthia posidoniae and Pontoporeia biturbinata are widely collected on the cast rhizomes of the sea grass Posidonia oceanica in the Mediterranean Sea, while Mauritiana rhizophorae occurs on mangrove wood (Jones et al. 2009). Unlike the other taxa in the family, Ph. aquaticum is a freshwater species found on a submerged branch of Robinia pseudoacacia (Angiospermae: Fabaceae) (Zhang et al. 2013b).
Didymosphaeriaceae Munk, Dansk. bot. Ark. 15(no. 2): 128 (1953)
Jones et al. (2009) referred the genus Tremateia to the Pleosporaceae but recent molecular studies placed it in the Didymosphaeriaceae, forming a sister group to Bimuria with high statistical support (Schoch et al. 2009; Suetrong et al. 2009; Ariyawansa et al. 2014).
Morosphaeriaceae Suetrong, Sakay., E.B.G. Jones & C.L. Schoch, Stud. Mycol. 64: 161 (2009)
The family was introduced to accommodate two genera with marine species of the genera Morosphaeria and Helicascus (Suetrong et al. 2009), previously referred to Lophiostomataceae and Pleosporaceae, respectively (Fig. 4a-e, g-h, j-k,n-q). Two Morosphaeria species are recognised (M. velatispora = Massarina velatispora; M. rammunculicola = Massarina rammunculicola), while six Helicascus species form a sister group with high statistical support (Suetrong et al. 2009; Boonmee et al. 2012; Hyde et al. 2013; Zhang et al. 2013a). Helicascus comprises two marine (i.e., H. kanaloanus and H. nypae) and four freshwater (i.e., H. aegyptiacus, H. aquaticus, H. elaterascus and H. thalassioides) species (Zhang et al. 2013a).
Morphological features of marine Dothideomycetes: Morosphaeriaceae and Trematosphaeriaceae. Morosphaeria velataspora a. Surface view of mature ascoma. g. Ascospores in ascus. n. Ellipsoidal to fusiform ascospore with mucilaginous sheath. Morosphaeria ramunculicola b. Surface view of mature ascoma. h. Mature ascus undergoing fissitunicate dehiscence. o. Ellipsoidal to fusiform ascospore with polar cap-like mucilaginous pads. Helicascus kanaloanus c. Surface view of mature ascomata. d. Tangential section. j. Ascus tip. p. Ascospore. Helicascus nypae e. Tangential section. k. Subcylindrical asci with pseudoparaphyses. q. Obovoidal ascospore. Falciformispora lignatilis f. Surface view of mature ascomata. m. Clavate asci. r-s. Fusiform ascospores surrounded by thin gelatinous sheathe and single scythe-like appendage at the base. Halomassarina thalassiae. i. Mature ascus undergoing fissitunicate dehiscence. l. Ellipsoidal ascospores with gelatinous sheath. a-e, g-h, j-k, n-q = Morosphaeriaceae. f, i, l-m, r-s = Trematosphaeriaceae. Bars c = 1500 μm; a, d-e = 1000 μm; b, f = 250 μm; g-i, r-s = 50 μm; m = 30 μm; j = 25 μm; k-l, n-s = 15 μm
Salsugineaceae K.D. Hyde & S. Tibpromma, Fungal Divers. 63: 227 (2013)
The monotypic genus Salsuginea (S. ramicola) is a mangrove genus similar to Helicascus (Morosphaeriaceae), but does not group in that family (Suetrong et al. 2009). Salsuginea groups with another mangrove genus Acrocordiopsis with moderate support (71 % BS support) (Suetrong et al. 2009) and Hyde et al. (2013) introduced a new family to accommodate them (54/62/90 support). Both genera have a peridium of textura porrecta, hyaline, narrow pseudoparaphyses, and cylindrical-clavate asci, with an ocular chamber and a prominent ring, and hyaline, brown, dark brown to black ascospores, 1-septate, constricted at the septa. Neither genus has a known asexual morph.
Trematosphaeriaceae K.D. Hyde, Y. Zhang, Suetrong & E.B.G. Jones, Cryptog. Mycol. 32(4): 347 (2011)
Cannon and Kirk (2007) do not list this as a family, but it was subsequently introduced by Suetrong et al. (2011b) based on morphological and molecular data (combined SSU, LSU, TEF-1-alpha and RPB2 datasets). This showed that the genera Falciformispora, Halomassarina and Trematosphaeria form a strongly supported cluster within Pleosporales. The main distinguishing characters of the family are medium-sized rounded ascomata with a papillate ostiole, a relatively wide, coriaceous peridium, cellular pseudoparaphyses and cylindro-clavate asci (Fig. 4f, i, l-m, r-s). The ascospores are two-celled or many-celled, hyaline or brown. The genera Falciformispora, Halomassarina (H. thalassiae = Massarina thalassiae) are marine fungi, although F. lignatilis has also been reported from the rachis of the oil palm Elaeis guineensis and in a freshwater environment (Pinruan 2010). Three marine Trematosphaeria species have been described (T. lineolatispora, T. malaysiana, T. mangrovei), but these have not been sequenced so their placement in the family requires confirmation at the molecular level.
Pseudorobillarda phragmitis (Cunnell) M. Morelet, Bull. Soc. Sci. nat. Arch. Toulon et du Var 175: 6 (1968)
Jones et al. (2009) referred this species to the Dothideomycetes incertae sedis based on unpublished molecular data, while Suetrong et al. (2009) showed it was placed in an unresolved clade basal to the Mytilinidiales. Subsequently, Runjindamai et al. (2012) showed that three Pseudorobillarda species formed a monophyletic group in the Pleosporomycetidae (Dothideomycetes) based on LSU, SSU and ITS nrDNA sequence data analysis, but did not show any affinity with any family or order. Further sampling and a wider range of genes are needed to resolve the taxonomic placement of the genus.
Sordariomycetes
Diaporthomycetidae
Diaporthales
Lautosporaceae Kohlm., Volkm.-Kohlm. & O.E. Erkiss., Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 38(2): 169 (1995)
This family was introduced by Kohlmeyer et al. (1995) for two marine Lautospora species thought to possess bitunicate asci. The family was not assigned to any order for lack of molecular data and the genus was assigned to Ascomycotina incertae sedis (Kohlmeyer et al. 1995). LSU sequence data (S. Suetrong, unpublished data) placed Lautospora simillima in Sordariomycetes in a basal clade to the orders Diaporthales, Xylariales, Microascales and an unnamed clade. Lautospora simillima groups with the neotropical ascomycete Mirannulata sameulsii with weak support, but shares few morphological features with this genus (Fig. 5) (Huhndorf et al. 2003). The Lautospora/Mirannulata clade forms a sister group to Verticola confusa and Rhamphoria delicatula (Annulatascaceae) in an unsupported clade. Consequently, it is proposed to emend the diagnosis of the family.
One of most parsimonious trees resulting from maximum parsimony analysis from partial LSU rDNA sequences. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values greater than 50 % are given above the node. Bayesian posterior probabilities greater than 0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap proportions (100 %) and posterior probabilities (1.00) are shown as a thicker line. The tree was rooted to Dothidea sambuci, Elsinoe veneta, Mycosphaerella fijiensis and Stylodothis puccinioides. Bar indicates 10 character stage changes
Lautosporaceae (Kohlm., Volkm.-Kohlm. & O.E. Erikss.) Suetrong & E.B.G. Jones, fam. emend.
MycoBank MB 81975, Faces of fungi number: FoF00859
A family in the Diaporthales, Sordariomycetes. Saprobic in marine habitats, Sexual morph: Ascomata ellipsoidal, ostiolate, brown, coriaceous, solitary. Hamathecium of simple, septate persistent paraphyses. Asci 4-spored, cylindrical, short pedicellate, thick-walled, unitunicate, with an ocular chamber. Ascospores uni- or biseriate, fusiform, muriform, distoseptate, hyaline, outer wall very thick (Fig. 6a-c). Asexual morph: Undetermined.
One of most parsimonious trees resulting from maximum parsimony analysis from combined SSU and LSU rDNA sequences. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values greater than 50 % are given above the node. Bayesian posterior probabilities greater than 0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap proportions (100 %) and posterior probabilities (1.00) are shown as a thicker line. The tree was rooted to Aureobasidium pullulans, Dothiora cannabinae and Elsinoe veneta. Bar indicates 10 character stage changes
Tirisporellales Suetrong, E.B.G. Jones & K.L. Pang, ordo novus
Type family: Tirisporellaceae
MycoBank MB812597
Faces of fungi number: FoF00860
An order in the class Sordariomycetes, subclass Diaporthomycetidae, which includes a single family and three genera. Saprobic in freshwater to brackish habitats. Sexual morph: Ascomata partially immersed to superficial, globose to subglobose, black, coriaceous to carbonaceous, ostiolate, scattered to gregarious, with necks and periphysate. Peridium thick-walled. Paraphyses present. Asci cylindrical to clavate. Ascospores 2–3 seriate, 1–7-septate, fusoid, straight to falcate to lunate, hyaline to brown with pale end cells, cell wall smooth or verrucose with or without appendages. Asexual morph: Craspedodidymum and Phialophora-like when present, or undetermined.
Type species: Tirisporella beccariana (Ces.) E.B.G. Jones, K.D. Hyde & Alias, Can. J. Bot. 74 (9): 1490 (1996)
Tirisporellaceae Suetrong, E.B.G. Jones & K.L. Pang, Cryptog. Mycol. [In Press]
The monotypic genus Tirisporella (type species T. beccariana) was introduced by Jones et al. (1996) to accommodate a mangrove species growing on the bases of the fronds of the palm Nypa fruticans. The new genus was based on a taxon variously referred to Sphaeria, Melanomma, Gibberidea and Tryblidiella, and assigned by Jones et al. (2009) to the Pleosporales incertae sedis. Five isolates of T. beccariana were selected for phylogenetic analyses with SSU and LSU rDNA sequence data, in order to provide information on its molecular phylogeny and relationship with other related genera using maximum parsimony, maximum likelihood and Bayesian analyses. Tirisporella beccariana clustered in the class Sordariomycetes, subclass Sordariomycetidae, order Diaporthales, family incertae sedis. Five T. beccariana isolates formed a monophyletic group with 100 % BSMP, 100 % BSML and 1.00 B.P. support, with Thailandiomyces bisetulosus, a freshwater ascomycete, as the sister group (Suetrong et al. 2015a, b). The Tirisporella/Thailandiomyces clade formed a sister clade to Jobellisia fraterna, J. luteola and J. guangdongensis (Jobellisiaceae) with weak support. Subsequently, a new ascomycete Bacusphaeria (type species B. nypenthi) was collected in Malaysia, also on Nypa fronds. LSU and SSU sequences of this species showed it grouped with T. beccariana, but was considered not to be congeneric (Fig. 5).
Suetrong et al. (2015a) subsequently introduced the family Tirisporellaceae to accommodate the genera Bacusphaeria, Thailandiomyces, and Tirisporella, while the position of Phruensis remains unresolved. Tirisporella and Bacusphaeria share many morphological features in common, especially the brown ascospores with the basal cell pale brown or hyaline (Fig. 8a-f). They differ in that T. beccariana has a single polar appendage.
Morphological features of marine Sordariomycetes: Tirisporellaceae. Tirisporella beccariana a. Partially immersed to superficial ascomata. b. Cylindrical ascus. d. Ascospore with apical appendage. f. Mature and immature ascospores. Bacusphaeria nypenthi c. Cylindrical ascus. e. Ascus tip. Bars a = 1500 μm; b-f = 25 μm
Hypocreomycetidae
Savoryellales Boonyuen, Suetrong, Sivichai, K.L. Pang & E.B.G. Jones, Mycologia, 103(6): 1368 (2011)
Savoryellaceae Jaklisch & Räblovä, in Jaklisch & Räblovä, Icones Fungorum 209 (2015)
The taxonomic placement of the genus Savoryella has been widely debated and Jones et al. (2009) referred it to the Sordariales incertae sedis. Boonyuen et al. (2011), in a combined phylogenetic analysis of Savoryella species (LSU, SSU, 5.8S rRNA genes, rpb1, rpb2, tef1), showed that they formed a monophyletic group in the Sordariomycetes, but showed no affinities with accepted orders. The order Savoryellales was introduced to accommodate Savoryella species, along with the genera Ascotaiwania, Ascothailandia (and its asexual morph Canalisporium), as they formed a new lineage in the Sordariomycetes (Fig. 9a-g) (Boonyuen et al. 2011). Canalisporium has priority as the older name and the more common morphological morph (Maharachchikumbura et al. 2015).
Morphological features of marine Sordariomycetes: Savoryellales Savoryella longispora a. Subglobose or ellipsoidal ascoma. e. Ellipsoidal ascospore. S. paucispora b. Mature, immature and paraphyses. S. melanospora c. Mature and immature ascospores. S. lignicola d. Ellipsoidal ascospore. S. appendiculata f-g. Ascospores with appendages. Bars a = 250 μm; c = 50 μm; d-g = 25 μm; b = 15 μm
Boonyuen et al. (2011) introduced the order Savoryellales, and the family Savoryellaceae was subsequently introduced by Jaklisch and Räblovä (2015) to accommodate the genus Savoryella (Fig. 5).
Torpedosporales E.B.G. Jones, Abdel-Wahab & K.L. Pang, ordo novus
Type family: Torpedosporaceae
MycoBank: MB812596, Faces of fungi number: FoF00861
Saprobic on lignicolous substrates and leaves, in marine habitats. Sexual morph: Ascomata perithecioid, immersed or superficial, subglobose, ostiolate, papillate, subcarbonaceous to coriaceous, brown. Paraphyses narrow, irregular, persistent or early deliquescing. Asci 8-spored, unitunicate, thin-walled, clavate to ellipsoidal, lacking an apical apparatus, deliquescing early or persistent, short pedicellate. Ascospores cylindrical to ellipsoidal, 1¬3-septate, hyaline, with appendages at one or both ends, or with equatorial appendages (Fig. 9). Asexual morph: Undetermined, or with helicoid conidia.
The marine sordariomycetous genera Juncigena, Swampomyces, and Torpedospora, have not been taxonomically assigned to any family or order with Jones et al. (2009) referring them to the Hypocreales incertae sedis. Schoch et al. (2006) noted they formed three clades in the Hypocreomycetidae (1: Torpedospora species; 2: Swampomyces and Etheirophora species; 3: Swampomyces species and Juncigena adarca), and associated with the Coronophorales with good support. They named this new fungal lineage the TBM clade (Torpedospora/Bertia/Melanospora). In a new phylogenetic study, Jones et al. (2014) examined the relationship of the genera Etheirophora, Juncigena, Swampomyces, and Torpedospora, along with the marine species Chaetosphaeria chaetosa and the terrestrial genus Falcocladium, with sequences of two ribosomal nuclear loci. They grouped in four well-supported subclades: 1. Juncigena subclade, 2. Etheirophora and Swampomyces s. s. subclade, 3. Falcocladium subclade, and, 4. Torpedospora subclade. They introduced four new families to accommodate these genera: Juncigenaceae, Etheirophoraceae, Falcocladiaceae (asexual species) and Torpedosporaceae, respectively.
Combined SSU and LSU rDNA sequence analysis shows that taxa in the families Etheirophoraceae, Juncigenaceae and Torpedosporaceae form a highly supported clade in the Hypocreomycetidae and a new order is introduced to accommodate them (Fig. 7). The Torpedosporales forms a sister clade to the orders Falcocladiales, Coronophorales and Melanosporales with high support (Fig. 7).
Etheirophoraceae Rungjindamai, Somrithipol & Suetrong, Cryptog. Mycol. 35(2): 134 (2014)
MycoBank: MB 808178, Faces of fungi number: FoF00863
Saprobic on lignicolous substrates in marine habitats. Sexual morph: Ascomata immersed, ostiolate, periphysate, papillate, clypeate, coriaceous, light coloured, paraphysate. Peridium of textura angularis. Asci 8-spored, cylindrical to oblong, pedicellate, non-amyloid, thin-walled, unitunicate, persistent. Ascospores biseriate, 1-septate, ellipsoidal, with a filamentous appendage at one or both ends. Appendages bristle-like but their origin at the ultrastructural level not determined. Asexual morph: Undetermined.
Included taxa: Etheirophora (E. bijubata, E. blepharospora, E. unijubata) and Swampomyces (S. armeniacus, S. triseptatus).
The genus Etheirophora was assigned to Sphaeriales by Kohlmeyer and Volkmann-Kohlmeyer (1989) and to the Halosphaeriales by Hawksworth et al. (1995), Kirk et al. (2001), and Hypocreales incertae sedis (Jones et al. 2009). However, molecular data clearly shows it does not belong in Halosphaeriaceae, but in a new family and order Torpedosporales.
Juncigenaceae E.B.G. Jones, Abdel-Wahab & K.L. Pang, Cryptog. Mycol. 5 (2): 133 (2014)
MycoBank: MB 808177, Faces of fungi number: FoF00862
Saprobic fungi growing on lignicolous substrates in marine habitats. Sexual morph: Ascomata perithicoid, immersed, ostiolate, papillate, coriaceous, brown to dark-brown, contents apricot coloured in mass. Paraphyses numerous, narrow, unbranched, persistent, connected to the top and bottom of the ascomatal cavity. Asci 8-spored, unitunicate, thin-walled, persistent, cylindrical to fusiform, with apical apparatus, short pedicellate. Ascospores uni- to biseriate, ellipsoidal, clavate to fusiform, 3-septate, hyaline, constricted at the septa, with or without equatorial and polar appendages. Asexual morph: helicoid conidia when present.
Included taxa: Juncigena adarca (= Cirrenalia adarca), Fulvocentrum aegyptiacus (= Swampomyces aegyptiacus), F. clavatisporum (= S. clavatispora), Marinokulati chaetosa (= Chaetosphaeria chaetosa), Moheitospora fruticosae.
Eriksson (1999) referred Juncigena to Magnaporthaceae, but Thongkantha et al. (2009) found no support for this. Schoch et al. (2006) noted an affinity with the Coronophorales and Melanosporales and referred it to the TBM clade, a new lineage in the Hypocreomycetidae. Jones et al. (2014) showed the genus belonged in a new family and order incertae sedis, in the Hypocreomycetidae. The genera Juncigena, Fulvocentrum, Marinokulati and Moheitospora form a strongly supported clade in the Hypocreomycetidae and a sister group to the Etheirophoraceae and the Falcocladiaceae (Jones et al. 2014).
Torpedosporaceae E.B.G. Jones & K.L. Pang, Cryptog. Mycol. 35(2): 135 (2014)
MycoBank: MB 808180, Faces of fungi number: FoF00864
Saprobic on lignicolous substrates and leaves. Sexual morph: Ascomata perithicoid, immersed or superficial, subglobose, ostiolate, papillate, subcarbonaceous to coriaceous, brown. Paraphyses narrow, irregular, persistent or early deliquescing. Asci 8-spored, unitunicate, thin-walled, clavate to ellipsoidal, short pedicellate, lacking an apical apparatus. Ascospores cylindrical to ellipsoidal, 3-septate, hyaline, with several radiating appendages at one or both ends. Asexual morph: helicoid conidia when present.
Included taxa: Glomerulispora mangrovis, Torpedospora ambispinosa, T. radiata.
Torpedospora has been referred to various higher order placements: Hypocreales incertae sedis (Jones et al. 2009), a sister group to the Bionectriaceae (Sakayaroj et al. 2005) and TBM clade (Schoch et al. 2006). Jones et al. (2014) showed it belonged in Torpedosporaceae within the Hypocreomycetidae, but did not group with any order. The marine hyphomycete Glomerulispora mangrovis, with irregularly helicoid conidia forming muriform spores (Fig. 12 b), groups with T. radiata (Fig. 10) with high statistical support (Fig. 7) (Abdel-Wahab et al. 2010). Two Torpedospora species have been described, but they share few phenotypic characters (Jones 1995). Further sampling is required to resolve their phylogenetic relationship in order to determine if the two species are congeneric.
New genera/species (*) introduced since 2009
*Amarenographium solium Abdel-Wahab, Hodhod, Bahkali & K.D. Hyde, Cryptog. Mycol. 33(3): 290 (2012)
This is the second marine species described in Amarenographium, with A. solium described from decaying wood of Avicennia marina collected at Yanbu mangrove, Saudi Arabia (Hodhod et al. 2012). It is characterized by relatively large conidia with an apical appendage. The conidia are muriform, olivaceous-brown, becoming brown at maturity, with 3¬7 transverse septa and 1¬2 longitudinal septa. No sexual morph has been observed. Phylogenetic analysis of SSU and LSU rDNA sequence data showed it grouped consistently with Medicopsis romeroi with high bootstrap support, forming a basal clade to the families Montagnulaceae and Trematosphaeriaceae (Pleosporales, Dothideomycetes). Amarenographium solium differs from A. metableticum, another marine species, in having larger conidial dimensions. Amarenographium metableticum is known from bark, maritime grasses and salt marsh plants with an Amarenomyces ammophilae sexual morph.
Bacusphaeria Norlailatul, Alias and S. Suetrong, Bot. Mar. (In press)
Bacusphaeria nypenthi Norlailatul, Alias and S. Suetrong, Bot. Mar. (In press)
Saprophytic marine ascomycete. Sexual morph: Ascomata globose to subglobose, ostiollate, periphysate, immersed to erumpent, carbonaceous, lacking interascal tissue. Asci 8-spored, cylindrical, unitunicate, short pedicellate, with a conspicuous refractive apical ring. Ascospores uniseriate, ellipsoid, 1–5 septate, central cells brown, end cells hyaline or pale brown without a sheath or appendages. Asexual morph: Undetermined.
Bacusphaeria nypenthi was collected on Nypa fruticans in a mangrove at Kuala Sungai Baru, Malacca, Malaysia and can easily be seen on the frond bases by the large dimensions of the ascomata. Other features of note are the asci with a distinct, large J-, apical ring, which are often thick–walled when immature. Ascospores are quite variable in morphology generally 1¬5 septate, with hyaline to pale brown end cells, and other cells brown (Fig. 8c). The genera Bacusphaeria and Tirisporella share many features, especially the large thick-walled perithecia, cylindrical asci with short pedicels, versicoloured ascospores and their occurrence on Nypa frond bases in brackish waters. Bacusphaeria differs from Tirisporella in lacking polar ascospore appendages and fewer septa (1¬5/ 5¬7), lack of interascal tissue, and asci with a prominent apical ring. Phylogenetic analyses, using combined SSU and LSU rDNA sequence data, show that Bacusphaeria does not have any affinity with Dothideomycetes, but groups in the Diaporthales (Sordariomycetes, Sordariomycetidae) (Suetrong et al. 2015b) with high bootstrap support.
*Corollospora mesopotamica Al-Saadoon, Marsh Bull. 2: 135 (2006) (Figs. 9 and 10)
This species was described from material growing on sand grains of the Khaw Al-Zubair estuary, in Basrah, Southern Iraq. This species most closely resembles C. maritima and C. cinnamomea with their 1-septate ascospores, but differ in ascospore measurement and the fact that C. mesopotamica may also have 2-septate ascospores that are brown (Jones et al. 2009). Only five Corollospora species have brown ascospores: C. cinnamomea, C. fusca, C. californica, C. novofusca and C. mesopotamica. The known distribution of this species includes Iraq and Thailand.
*Dactylospora vrijmoediae K.L. Pang, S.Y. Guo, Alias, Hafellner & E.B.G. Jones, Bot. Mar. 57(4): 317 (2014)
This species differs mainly from other marine Dactylospora species (D. haliotrepha, D. mangrovei, D. canariensis) in having a thick hyaline sheath connected at the septum of the ascospores and flared at both ends (Fig. 11 a) (Pang et al. 2014). All Dactylospora species belong in Eurotiomycetes but D. haliotrepha, D. mangrovei and D. vriijmoediae form an unnamed clade in the class, well-separated from the terrestrial species (D. lobariella, D. imperfecta) (Pang et al. 2014) (Fig. 12).
*Diatrypasimilis J.J. Zhou & Kohlm., Mycologia 102(2): 432 (2010)
*Diatrypasimilis australiensis J.J. Zhou & Kohlm., Mycologia 102(2): 432 (2010) (type species)
Chalkley et al. (2010) introduced this genus based on a culture that was deposited in the American Type Culture Collection (ATCC: MYA 3540) isolated from decaying Rhizophora wood collected in Australian mangroves (Chalkley et al. 2010). Although the ribosomal DNA genes were sequenced and the fungus was characterized in culture, the authors did not fully characterize the morphology of the taxon on natural substrates, apparently because of a lack of material. This species was recently collected on decayed wood in Saudi Arabia and fresh cultures established (Abdel-Wahab et al. 2014). LSU sequence data placed this species in the Diatrypaceae with high support. The two strains isolated from Saudi Arabia formed a monophyletic group with the Diatrypasimilis American strain with 99 % similarity. Abdel-Wahab et al. (2014) illustrated the new collection of this fungus (Fig. 13a-e).
*Dyfrolomyces K.D. Hyde, K.L. Pang, Alias, Suetrong & E.B.G. Jones, Cryptog. Mycol. 34(1): 227 (2013)
*Dyfrolomyces tiomanensis K.L. Pang, Alias, K.D. Hyde, Suetrong & E.B.G. Jones, Cryptog. Mycol. 34(1): 228 (2013) (type species)
Dyfrolomyces was introduced to accommodate three species previously classified in the genus Saccardoella (D. mangrovei, D. marinospora, D. rhizophorae) and a new taxon (D. tiomanensis) collected at Tioman Island, Malaysia (Fig. 2b, f-i) (Pang et al. 2013). Dyfrolomyces tiomanensis has ascospores with 20¬24 septa, compared with 3¬9 septa in other Dyfrolomyces species. Phylogenetically, D. tiomanensis groups with D. rhizophorae based on a combined analysis of SSU, LSU rDNA and TEF1 sequence data in the Dothideomycetes. Dehiscence of the ascus wall was observed in D. tiomanensis, which is consistent with the phylogenetic placement in Dothideomycetes (Pang et al. 2013).
Other included species:
Dyfrolomyces mangrovei (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong
& E.B.G. Jones, Cryptog. Mycol. 34(1): 228 (2013)
Dyfrolomyces marinospora (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong
& E.B.G. Jones, Cryptog. Mycol. 34(1): 228 (2013)
Dyfrolomyces rhizophorae (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong
& E.B.G. Jones, Cryptog. Mycol. 34(1): 228 (2013)
*Ebullia K.L. Pang, Mycoscience 56: 40 (2015)
Chu et al. (2015) undertook a molecular reappraisal of Nimbospora, a genus with three marine species, with recent collections from Taiwan. The genus was shown to be polyphyletic with Nimbospora octonae distantly placed from the type species N. effusa and a second species N. bipolaris. A new genus, Ebullia, was established for E. octonae. The genus has no known asexual morph (Fig. 11c).
Included species:
Ebullia octonae (Kohlm.) K.L. Pang, Mycoscience 56: 40 (2015) (type species)
*Gesasha Abdel-Wahab & Nagah., Nova Hedwigia 92(3–4): 501 (2011)
*Gesasha peditatus Abdel-Wahab & Nagahama, Nova Hedwigia 92:502 (2011) (type species)
*G. unicellularis Abdel-Wahab & Nagahama, Nova Hedwigia 92:505 (2011)
*G. mangrovei Abdel-Wahab & Nagahama, Nova Hedwigia 92:507 (2011)
The genus Gesasha was introduced by Abdel-Wahab and Nagahama (2011) to accommodate three taxa collected on decaying wood in Gesashi mangrove, Okinawa, Japan. The three Gesasha species form a monophyletic group in the Halosphaeriaceae (Microascales) with Arenariomyces trifurcatus as a sister group. The genus is characterised by hyaline to light brown immersed to erumpent, coriaceous ascomata, persistent asci, with a thickened apical pore with cytoplasmic retraction below the ascus apex and 1-septate, globose to widely ellipsoidal ascospores, with or without ephemeral, amorphous polar to sub-polar appendages (Fig.14a-h). Gesasha resembles the genus Aniptodera but differs in: 1. phylogenetically distantly placed within the Halosphaeriacaeae; 2. colour of the ascomata, and 3. nature of the appendages. In Aniptodera ascospore appendages (when present) form hamate structures, which uncoil when mounted in water to form long filaments.
Gesasha spp. a–c. G. peditatus a. Vertical section through ascoma b. Apical part of the ascus c. Ascospore d–f. G. unicellularis d. Vertical section through ascoma e. Apical part of the ascus f. Ascospore g–i. G. mangrovei. g. Vertical section through ascoma . h. Apical part of the ascus i. Ascospore . Bars a, d, g = 50 μm; b–c, e–f, h–i = 5 μm
*Glomerulispora Abdel-Wahab & Nagah., Mycol. Prog. 9(4): 552 (2010)
*Glomerulispora mangrovis Abdel-Wahab & Nagah., Mycol. Prog. 9(4): 552 (2010) (type species)
Phylogenetic analyses of LSU and SSU rDNA sequence data place this new asexual genus in the TBM clade as a sister clade to Torpedospora radiata with high statistical support (Abdel-Wahab et al. 2010). Subsequently, the new family Torpedosporaceae was introduced to accommodate the genera Glomerulispora and Torpedospora (Jones et al. 2014). The molecular data suggested that the sexual morph of G. mangrovis may be T. radiata, but sporulating stages have not been observed in cultures of G. mangrovis. Conidia initially comprise large, globose, hyaline cells which then divide in different planes to form a muriform ball of pale brown cells. Glomerulispora mangrovis differs from Moheitospora fruticosae and M. adarca in having a greater number of conidial cells that are much smaller in size (Fig. 12b). The fungus was described from decaying driftwood collected in Gesashi Bay Mangrove, Okinawa, Japan.
*Halazoon Abdel-Aziz, Abdel-Wahab & Nagah., Mycol. Prog. 9(4): 545 (2010)
*Halazoon melhae Abdel-Aziz, Abdel-Wahab & Nagah., Mycol. Prog. 9(4): 546 (2010) (type species)
This genus was introduced by Abdel-Wahab et al. (2010) for an asexual taxon (H. melhae) that grouped in Lulworthiales with moderate support. Cirrenalia fusca was transferred to Halazoon as it shares some morphological features with Halazoon melhae. Both species have coiled conidia (Fig. 12c-d). H. melhae was isolated from decayed drift wood collected at Port Said, Egypt. No sexual morph was found.
Other included species:
H. fusca (I. Schmidt) Abdel-Wahab, K.L. Pang, Nagah., Abdel-Aziz & E.B.G. Jones, Mycol. Prog. 9(4): 547 (2010)
*Halokirschsteiniothelia Boonmee & K.D. Hyde, Mycologia 104(3): 705 (2012)
The genus Kirschsteiniothelia has been shown to be polyphyletic (Boonmee et al. 2012) based on ITS, LSU and SSU rDNA combined sequence data analysis with species separating into three lineages. Kirschsteiniothelia maritima clustered with Mytilinidion spp. as a sister group in the Mytilinidiaceae clade, with 100 % bootstrap support (BT), and 1.00 posterior probabilities (PP) support (Suetrong et al. 2009; Boonmee et al. 2012). Other Kirschsteiniothelia species group in Morosphaeriaceae (K. elaterascus) and Kirschsteiniotheliaceae (K. lignicola, K. aethiops) (Boonmee et al. 2012). The genus Halokirschsteiniothelia was therefore introduced to accommodate K. maritima, a species that occurs on wood in marine habitats.
Included species:
Halokirschsteiniothelia maritima (Linder) S. Boonmee & K.D. Hyde, Mycologia 104(3): 705 (2012) (type species)
Syn: Amphisphaeria maritima Linder, Farlowia 1: 411 (1944)
Microthelia maritima (Linder) Kohlm., Nova Hedwigia 2: 322 (1960)
Kirschsteiniothelia maritima (Linder) D. Hawksw., Bot. J. Linn. Soc. 91(1–2): 193 (1985)
*Halomassarina Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, Stud. Mycol. 64: 161 (2009).
This genus was introduced to accommodate Massarina species that did not group in Massarinaceae based on a multigene analysis of selected marine ascomycetes (Suetrong et al. 2009) (Fig. 4i, l). Halomassarina grouped in Trematosphaeriaceae with high statistical support with Suetrong et al. (2011b) formally introducing the family in the Pleosporales. The following new combination was proposed by Suetrong et al. (2009).
Included species:
Halomassarina thalassiae (Kohlm. & Volkm.-Kohlm.) Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, Stud. Mycol. 64: 161 (2009) (type species)
*Halosarpheia japonica Abdel-Wahab & Nagah., Mycol. Progr. 11(1): 89 (2012)
This species was described from intertidal driftwood collected at Hakkeijima beach, Yokohama, Japan and isolated into pure culture (Abdel-Wahab and Nagahama 2010). It groups with the type species in the Halosarpheia sensu stricto clade with high statistical support (99/98). However, it differs from the type species (H. fibrosa) in possessing unicellular globose ascospores with a mucilaginous sheath. Halosarpheia unicellularis also has unicellular ascospores but differs from H. japonica in having a periphysate neck and thin-walled ascospores with bipolar apical appendages, initially closely adpressed to the ascospore wall, and unfurling in water to form long thin filaments. Halosarpheia japonica differs from other Halosarpheia species in possessing an asexual morph with conidia that are helicoid, brown, constricted at the septa and thick-walled. The asexual stage is similar to other marine helicoid species such as Cirrenalia, Cumulospora, Halenospora, and Zalerion.
*Hydea E.B.G. Jones & K.L. Pang, anam. gen., Mycol. Prog. 9:549 (2010)
Hydea was introduced for Cirrenalia pygmea as it did not form a monophyletic group with the type species of Cirrenalia. The species formed a basal clade to various taxa in the Lulworthiales (Abdel-Wahab et al. 2010). Morphologically the conidia of Hydea differ from those of Cirrenalia with a pronounced large apical cell in the former genus (Fig. 12e).
Included species:
Hydea pygmea (Kohlm.) E.B.G. Jones and K.L. Pang, Mycol. Prog. 9(4): 549 (2010) (type species)
*Hydropunctaria C. Keller, Gueidan & Thüs, Taxon 58(1): 193 (2009)
*Hydropunctaria oceanica Orange, Lichenologist 44(3): 312 (2012)
*Hydropunctaria orae Orange, Lichenologist 44(3): 314 (2012)
This is a lichen genus, introduced to accommodate species previously assigned to Verrucaria: Hydropunctaria adriatica, and H. maura. Molecular data showed that four Hydropunctaria species formed a well-supported clade distinct from other genera in Verrucariaceae (Gueidan et al. 2007). The genus is characterised by a crustose thallus often interrupted by black punctae or columns, weakly developed upper cortex, medulla not differentiated forming a black basal layer, algal cells arranged in vertical columns, upper surface of involucrellum often unevenly rough, ascospores median length greater than 12 μm and a length/width ratio greater than 2. Conidia are sometimes are present.
Other included species:
Hydropunctaria adriatica (Zahlbr.) C. Keller & Gueidan, Taxon 58(1): 194 (2009)
Hydropunctaria amphibia (Clemente ex Ach.) Cl. Roux, Bull. Soc. linn. Provence, num. spéc. 14: 108 (2011)
Hydropunctaria aractina (Wahlenb.) Orange, Lichenologist 44(3): 305 (2012)
Hydropunctaria maura (Wahlenb.) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
Hydropunctaria rheitrophila (Zschacke) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
Hydropunctaria scabra (Vězda) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
*Kitesporella Jheng & K.L. Pang, Bot. Mar. 55(5): 462 (2012)
*Kitesporella keelungensis J.S. Jheng & K.L. Pang, Bot. Mar. 55(5): 462 (2012)
This genus in Halosphaeriaceae was introduced for Kitesporella keelungensis collected from driftwood at Keelung City, Taiwan. It is characterised by thin-walled, clavate asci that are persistent and ascospores that are kite-like or rhomboid in shape, unicellular, hyaline, with two prominent guttules and lacking appendages. Kitesporella keelungensis resembles Anisostagma, Iwilsoniella and Thalassogena (all members of the Halosphaeriaceae), with unicellular hyaline ascospores, lacking appendages. However, the latter three genera have ascospores that are globose to subglobose (Pang and Jheng 2012a) (Fig. 11b). The asexual morph is unknown.
*Kochiella Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 96 (2011)
A phylogenetic evaluation of Remispora, based on three loci nuclear (LSU, SSU rDNA, the second largest RNA polymerase II subunit (RPB2)), demonstrated that the genus is polyphyletic. Remispora maritima (type species), R. pilleata, R. quadri-remis, R. spitsbergenensis and R. stellata formed a monophyletic group (Remispora sensu stricto) with Sablecola chinensis as a sister taxon with good support, in Halosphaeriaceae (Sakayaroj et al. 2011). Remispora crispa was distantly placed from Remispora in a clade with Ocostaspora apilongissima, but they were not considered as conspecific. The genus Kochiella was therefore introduced to include this species based on morphological and molecular data (Sakayaroj et al. 2011).
Included species:
Kochiella crispa (Kohlm.) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 96 (2011) (type species)
*Lignincola conchicola J.K. Liu, E.B.G. Jones & K.D. Hyde, Mycotaxon 117: 344 (2011)
Lignincola conchicola was described from submerged fronds of Phoenix paludosa (Liu et al. 2011a). Morphologically, this species is similar to the type species of the genus, L. laevis, but L. conchicola consistently occurs on adhesive pads of a marine invertebrate.
*Marinokulati E.B.G. Jones & K.L. Pang, Cryptog. Mycol. 35(2): 132 (2014)
Chaetosphaeria chaetosa was described by Kohlmeyer (1963) and referred to the Sphaeriaceae. However, its placement in Chaetosphaeria was questioned by Jones et al. (1983) as it differs from other species in the genus, primarily in ascospores with both polar and equatorial appendages, formed by fragmentation of an exosporic sheath and its marine occurrence. No asexual morph has been reported for Ch. chaetosa (Fig. 15a-e). Fresh collections of Ch. chaetosa yielded two cultures and enabled assessment of its phylogenetic relationship with other Chaetosphaeria species (Jones et al. 2014). Chaetosphaeria was shown to be polyphyletic with most species grouping in the Chaetosphaeriales. Two sequences of Ch. chaetosa formed a monophyletic group with Juncigena adarca, Moheitospora fruticosa and two Fulvocentrum species, with high bootstrap support in a new family Juncigenaceae (Jones et al. 2014).
Included species:
Marinokulati chaetosa E.B.G. Jones & K.L. Pang, Cryptog. Mycol. 35(2): 133(2014) (type species)
*Matsusporium E.B.G. Jones & K.L. Pang, Mycol. Prog. 9:537–558 (2010)
This genus was introduced to accommodate Cirrenalia tropicale which did not group with the type species Cirrenalia macrocephala, a taxon in Halosphaeriaceae (Abdel-Wahab et al. 2010). Cirrenalia tropicale clustered with Lulworthia grandispora in Lulworthiales as a sister group, but they are not congeneric (Fig. 12f).
Included species:
Matsusporium tropicale (Kohlm.) E.B.G. Jones & K.L. Pang, Mycol. Progr. 9(4): 550 (2010) (type species)
*Moheitospora Abdel-Wahab, Abdel-Aziz & Nagah., Mycol. Prog. 9(4): 551 (2010)
This is an asexual genus that groups in the family Juncigenaceae with the marine genera Juncigena, Fulvocentrum, and Marinokulati (Jones et al. 2014). It forms a sister group to Juncigena with high support. The taxon has coiled conidia with small cells that distinguish it from Cirrenalia species (Abdel-Wahab et al. 2010). No sexual morph has been observed (Fig. 12g).
Included species:
M. fruticosae Abdel-Wahab, Abdel-Aziz & Nagah., Mycol. Progr. 9(4): 551 (2010) (type species)
*Moleospora Abdel-Wahab, Abdel-Aziz & Nagah., Mycol. Prog. 9(4): 547 (2010)
*Moleospora maritima Abdel-Wahab, Abdel-Aziz & Nagah., Mycol. Progr. 9(4): 548 (2010)
A genus introduced for an asexual taxon (M. maritima) that initially started growing as a coiled conidium which soon becomes a mass of cells that are dark brown (Abdel-Wahab et al. 2010). This species groups in Lulworthiales and further collections are required to determine its relationships in the order. The taxon was collected on decaying driftwood collected at Port Said, Egypt. No sexual morph is known (Fig. 12h).
*Moromyces Abdel-Wahab, K.L. Pang, Nagah., Abdel-Aziz & E.B.G. Jones, Mycol. Progr. 9(4):555 (2010)
A phylogenetic study of asexual species assigned to the genera Cirrenalia and Cumulospora showed that Cumulospora varius did not group with the type species C. marina (Lulworthiales), and this genus was introduced to accommodate it (Abdel-Wahab et al. 2010). The genus also clusters in Lulworthiales clade that includes Lulwoana uniseptata (= Zalerion maritimum), but they are not congeneric. No sexual morph is known (Fig. 12i).
Included species:
Moromyces varius (Chatmala & Somrith.) Abdel-Wahab, K.L. Pang, Nagah., Abdel-Aziz & E.B.G. Jones, Mycol. Progr. 9(4):555 (2010) (type species)
*Morosphaeria Suetrong, Sakay., E.B.G. Jones & Schoch, Stud. Mycol. 64: 161 (2009)
This genus was introduced to accommodate two marine Massarina species (M. velatospora, M. ramunculicola) that did not group in Massarinaceae (Suetrong et al. 2009) based on a multigene analysis (Fig. 4a, b, g-h,j-k,n-q).
Included species:
Morosphaeria velatospora (K.D. Hyde & Borse) Suetrong, Sakay., E.B.G. Jones & Schoch, Stud. Mycol. 64: 161 (2009) (type species)
Morosphaeria ramunculicola (K.D. Hyde) Suetrong, Sakay., E.B.G. Jones & Schoch, Stud. Mycol. 64: 162 (2009)
*Natantispora unipolarae K.L. Pang, S.Y. Guo & E.B.G. Jones, In: Liu et al. Fungal Divers. 72:19 (2015b)
This new species was described from drift Phragmites in an estuary in southern Taiwan. This species forms a monophyletic group with N. retorquens in a phylogenetic analysis using SSU and LSU rDNA sequence data (Liu et al. 2015). Natantispora unipolarae morphologically differs from N. retorquens in having only one polar appendage at one end of the ascospores in the former species, which unfurls in sea water to form a long thread (Liu et al. 2015).
*Paradendryphiella Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013)
Jones et al. (2008), with DNA-based studies (ITS, SSU, LSU), concluded that the marine Dendryphiella species, D. arenaria and D. salina, belonged to Pleosporaceae in a sister clade to the Pleospora/Stemphylium complex. They also showed the type species of Dendryphiella, D. vinosa, to be only distantly related to D. arenaria and D. salina (Jones et al. 2008). The marine Dendryphiella species are morphologically different from Scolecobasidium as they lack denticles on the conidiogenous cells as described by Ellis (1976). When the conidia become detached, distinct pores can be seen on the conidiogenous cells (Jones et al. 2008). Jones et al. (2008) pointed out the need for a new genus to accommodate the two species and this has been implemented by Woudenberg et al. (2013).
Included species:
Paradendryphiella arenariae (Nicot) Woudenberg & Crous, Stud. Mycol. 75(1): 208 (2013)
Paradendryphiella salina (G.K. Sutherl.) Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013) (type species)
*Penicillium jejuense M.S. Park & Y.W. Lim, Mycologia 107(1): 212 (2015)
This species was isolated from a marine sponge and sand at Jeju Island, Korea (Park et al. 2015). A phylogenetic study (ITS, BenA, CaM and RPB2 sequences) confirmed its placement in Penicillium (section Aspergilloides) and distinct from those currently known. Penicillium jejuense is most closely related to P. croticola, but differs in reverse colour colony on YES medium, sclerotia production on MEA medium, and the dimensions and shape of the conidia (Park et al. 2015). The species is tolerant of 15 % NaCl concentrations with optimal growth on 3.3 % (w/v) sea salt. All strains were shown to exhibit alginase and β-glucosidase activity, and had fungal activity against the plant pathogens Colletotrichum acutatum and Fusarium oxysporum.
*Pileomyces K.L. Pang & Jheng, Bot. Studies 53: 536 (2012)
*Pileomyces formosanus K.L. Pang & J.S. Jheng, Bot. Studies 53: 536 (2012) (type species)
Pileomyces is a new genus in Halosphaeriaceae, Microascales, described from a bamboo culm trapped in the intertidal zone, collected at Yingkeshih, Taiwan (Pang and Jheng 2012b). Ascospores of P. formosanus are similar to those of Aniptodera and Phaeonectriella and are ellipsoidal, 1-septate, hyaline, thin-walled and appendages cover one end of the spore (Fig. 11d).
*Praelongicaulis E.B.G. Jones, Abdel-Wahab & K.L. Pang, gen. nov.
Mycobank: MB812594, Faceoffungi number: FoF00865
Saprobic. Sexual morph: Ascomata ellipsoidal, dark coloured, membranous to coriaceous, immersed, ostiolate. Necks periphysate. Peridium two layers of cells of textura angularis, outer layer of polygonal dark brown, melanised cells with large lumina, inner layer of elongated, polygonal, light brown cells. Asci 8-spored, thin-walled, unitunicate, clavate, persistent, with a long pedicellate. Catenophyses present. Ascospores hyaline, ellipsoidal, thin-walled, 1-septate, not constricted at the septum. Appendages bipolar, at first hamate that deliquesce in water to form thin flat sheets (Fig. 16a-f).
Praelongicaulis kandeliae comb. nov. a. Dark-coloured ascoma with a long neck. b. Peridium of several layers of elongated cells. c. Neck with periphyses. d. Ascus with a long stalk. e–f. Ascospores with bipolar pad-like appendages which unfurl when mounted in sea water. Bars a = 50 μm; b–c = 30 μm; d, e = 10 μm
Typus generis: Praelongicaulis kandeliae (Abdel-Wahab & E.B.G. Jones) E.B.G. Jones, Abdel-Wahab & K.L. Pang
Etymology: Referred to the very long ‘praelongus’ stalk ‘caulis’ of the asci.
In a multi-gene phylogeny of Halosphaeriaceae, Halosarpheia kandeliae did not group in the Halosarpheia sensu stricto clade, but was distantly placed in a clade with Halosphaeriopsis medisetigera and Saagaromyces species as sister groups (Sakayaroj et al. 2011). Praelongicaulis kandeliae differs from many Halosarpheia species in having asci with a long drawn out stalk (tail-like), and polar appendages that initially appear amorphous and only later form the characteristic thread-like bipolar appendages. Sakayaroj et al. (2011) did not formally introduce a new genus to accommodate H. kandeliae, and this is therefore proposed here. Genera with bipolar unfurling appendages have evolved many times within the family and are found in both freshwater and marine habitats (Pang et al. 2003; Pang and Jones 2004).
Praelongicaulis kandeliae (Abdel-Wahab & E.B.G. Jones) E.B.G. Jones, Abdel-Wahab & K.L. Pang, comb. nov. Fig. 16
Mycobank: 812595, Face of fungi number: FoF 00866
Basionym: Halosarpheia kandeliae Abdel-Wahab & E.B.G. Jones, Mycol. Res. 103(11): 1500 (1999)
Holotype: IMI 379307.
Ex-type culture: CY1492 (City University of Hong Kong).
Known geographic distribution: Hong Kong, Taiwan.
Substrata: Mangrove wood and bark.
*Rimora Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B.G. Jones Stud. Mycol. 64: 166 (2009)
Lophiostoma mangrovei, in a multigene phylogenetic study, was distantly placed from the type species L. macrostomum, and a new genus was introduced to accommodate it (Suetrong et al. 2009). Rimora is assigned to Aigialaceae (Pleosporales) with high statistical support along with the genera Aigialus and Ascocratera.
Included species:
Rimora mangrovei (Kohlm. & Vittal) Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B.G. Jones Stud. Mycol. 64: 166 (2009) (type species)
*Saagaromyces mangrovei Abdel-Wahab, Bahkali & E.B.G. Jones, In: Liu et al. Fungal Divers. 72:32 (2015)
Saagaromyces mangrovei forms a monophyletic group with S. abonnis, S. ratnagiriensis and S. glitra in a phylogenetic analysis of the SSU and LSU rDNA sequence data (Liu et al. 2015). It differs from other species in the genus in having smaller asci and ascospores.
*Sedecimiella K.L. Pang, Alias & E.B.G. Jones, Bot. Mar. 53(6): 495 (2010)
*Sedecimiella taiwanensis K.L. Pang, Alias & E.B.G. Jones, Bot. Mar. 53(6): 495 (2010) (type species)
Pang et al. (2010) described Sedecimiella taiwanensis from twigs of Kandelia obovata collected at Chunan, Taiwan and a further collection on unidentified mangrove wood at Futian Nature Reserve, Shenzhen, China. Phylogenetic analyses did not resolve its familial position in Hypocreales. A distinguishing feature of the ascomycete is its cylindrical unitunicate asci with 16 hyaline globose ascospores.
*Toriella Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 99 (2011)
Sakayaroj et al. (2011) carried out a multi-gene phylogeny of Halosphaeriaceae with 36 taxa. Ceriosporopsis was shown to be polyphyletic and C. tubulifera did not cluster with the type species of the genus (C. halima). The genus Toriella was introduced to accommodate C. tubulifera. Earlier studies noted that morphologically the ascospores did not conform to those described for the type species, where the polar appendages break through an exosporic sheath (Johnson et al. 1987). Ceriosporopsis tubulifera possesses ascospores with an exosporium that forms an annulus-like equatorial appendage and the polar appendage is formed inside an end chamber that consists of two electron-dense layers (Johnson et al. 1987).
Included species:
Toriella tubulifera (Kohlm.) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 100 (2011) (type species)
*Tubakiella Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 97 (2011)
Tubakiella was distantly placed from Remispora sensu stricto with the two sequenced strains forming a monophyletic group with Nautosphaeria cristaminuta and Haligena elaterophora as a sister group with weak support (Sakayaroj et al. 2011). The genus was therefore introduced to accommodate the species T. galerita.
Included species:
Tubakiella galerita (Tubaki) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 99 (2011) (type species)
*Wahlenbergiella Gueidan & Thüs, Taxon 58(1): 199 (2009)
A lichen genus introduced to accommodate two species previously assigned to Verrucaria: Wahlenbergiella mucosa (V. mucosa) and W. striatula (V. striatula) based on a molecular phylogenetic study (Gueidan et al. 2007). A third species Verrucaria tavaresiae (Wahlenbergiella tavaresiae) was transferred to the genus by Gueidan et al. (2011). Phylogenetic inferences from two nuclear and one mitochondrial loci confirmed the placement of Verrucaria tavaresiae within the marine genus Wahlenbergiella. Wahlenbergiella is characterized by small ascospores (median length generally shorter than 12 μm), a subgelatinous thallus, a smooth surface of the involucrellum, a greenish thallus, and the general absence of an upper cortex and a differentiated medulla (Gueidan et al. 2009).
Included species:
Wahlenbergiella mucosa (Wahlenb.) Gueidan & Thüs, Taxon 58(1): 200 (2009) (type species)
Wahlenbergiella striatula (Wahlenb.) Gueidan & Thüs, Taxon 58(1): 200 (2009)
Wahlenbergiella tavaresiae (R.L. Moe) Gueidan, Thüs & Pérez-Ort., Bryologist 114(3): 567 (2011)
New combinations since 2009
Calycina marina (Phill. Ex Boyd) Rämä, Baral & O.E. Erikss., Bot. Mar. (2015) In press.
Syn: Orbilia marina Boyd, Trans. Br. mycol. Soc.: 16 (1908)
Laetinaevia marina (Boyd) Spooner, Kew Bulletin 38 (4): 568 (1984)
Orbilia marina Phill. ex Boyd was described on drift material of the brown seaweed Ascophyllum nodosum (Boyd 1908), but this was a nomen nudum and was later referred to as Calloria marina Phillips in Smith (1908, unpublished manuscript). Subsequently, Kirk and Spooner (1984) transferred the fungus to Laetinaevia Nannf. The ascomycete has frequently been collected on A. nodosum, Fucus serratus, F. vesiculosus, Fucus sp. and Halidrys siliquosa washed ashore and decay at high tide mark or further up on the beach. This taxon has been collected in Scotland, Denmark, Norway and Sweden. More recently it has been collected at Tromsø, Norway and isolated and sequenced (Rämä et al. 2015). Based on morphological characters and ribosomal RNA and protein coding gene sequence data, it is distinct from the genera and families it was earlier placed in, and this species was transferred to Calycina in the Helotiaceae (Helotiales, Leotiomycetidae, Leotiomycetes) (Rämä et al. 2015). Calycina marina was shown to possess a hemi-amyloid apical ring, the absence of croziers and paraphyses were reported for the first time along with a thin gelatinous sheath surrounding the ascospores.
Ceriosporopsis intricata (Jørg. Koch & E.B.G. Jones) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 99 (2011)
Syn: Bovicornua intricata Jørg. Koch & E.B.G. Jones, Can. J. Bot. 71(2): 346 (1993)
Bovicornua intricata was shown in a multigene study to group with the type species of Ceriosporopsis (C. halima) (Sakayaroj et al. 2011) and the above combination was introduced. The two genera share many morphological characteristics but differ in the complexity of the exosporium in B. intricata (Yusoff et al. 1993), a character used to separate the latter from Ceriosporopsis when it was first described.
Dyfrolomyces mangrovei (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong & E.B.G. Jones, Cryptog. Mycol. 34: 228 (2013)
Syn: Saccardoella mangrovei K.D. Hyde, Mycologia 84(5): 803 (1992)
Dyfrolomyces marinospora (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong & E.B.G. Jones, Cryptog. Mycol. 34: 228 (2013)
Syn: Saccardoella marinospora K.D. Hyde, Mycologia 84(5): 806 (1992)
Dyfrolomyces rhizophorae (K.D. Hyde) K.D. Hyde, K.L. Pang, Alias, Suetrong & E.B.G. Jones, Cryptog. Mycol. 34: 228 (2013)
Syn: Saccardoella rhizophorae K.D. Hyde, Mycologia 84(5): 806 (1992)
Ebullia octonae (Kohlm.) K.L. Pang, Mycoscience 56: 40 (2014)
Syn: Nimbospora octonae Kohlm., Can. J. Bot. 63(7): 1122 (1985)
Nimbospora is polyphyletic with N. octonae distantly placed from the type species, N. effusa (Chu et al. 2015). Nimbospora octonae was transferred to Ebullia.
Halazoon fuscus (I. Schmidt) Abdel-Wahab, K.L. Pang, Nagahama, Abdel-Aziz and E.B. G. Jones, Mycol. Prog. 9(4): 547 (2010)
Syn: Cirrenalia fusca I. Schmidt, Mycotaxon 24: 419 (1985)
This genus was introduced to accommodate two marine asexual species: Cirrenalia fusca and a new species Halazoon melhae based on a molecular study of LSU and SSU rDNA sequence data (Abdel-Wahab et al. 2010). They form paraphyletic clades in Lulworthiales with lack of statistical support.
Halomassarina thalassiae (Kohlm. & Volkm.-Kohlm.) Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, Stud. Mycol. 64: 161 (2009)
Syn: Massarina thalassiae Kohlm. & Volkm.-Kohlm., Can. J. Bot. 65(3): 575 (1987)
Helicascus elaterascus (Shearer) H. Zhang & K.D. Hyde, Sydowia 65: 158 (2013)
Syn: Kirschsteiniothelia elaterascus Shearer, Mycologia 85: 963 (1993)
Morosphaeria elaterascus (Shearer) Boonmee & K.D. Hyde Mycologia 104(3): 705 (2012)
Based on a re-evaluation of the genus Helicascus, sequence data of the freshwater ascomycete Morosphaeria elaterascus was included in the phylogenetic analysis and shown to cluster with those from other Helicascus species in a strongly supported monophyletic clade (Zhang et al. 2013a). Therefore, M. elaterascus was transferred to Helicascus.
Hydea pygmea (Kohlm.) E.B.G. Jones and K.L. Pang, Mycol. Prog. 9(4): 549 (2010)
Syn: Cirrenalia pygmea Kohlm., Ber. Deuts. Bot. Gesell. 79: 35 (1966)
Hydropunctaria adriatica (Zahlbr.) C. Keller & Gueidan, Taxon 58(1): 194 (2009)
Syn: Verrucaria adriatica Zahlbr., Denkschriften der Akademie der Wissenschaften (Wien) Mathematisch-naturwissenschaftliche Klasse 92: 303 (1915)
Hydropunctaria amphibia (Clemente ex Ach.) Cl. Roux, Bull. Soc. linn. Provence, num. spéc. 14: 108 (2011)
Syn: Verrucaria amphibia Clemente, Synopsis Methodica Lichenum: 94 (1814) [MB#408679]
Verrucaria striatula Wahlenb., Supplementum species quamplures novas descriptas nec non observationes varias complectens, quod praeviae suae Methodo Lichenum adjunxit Auctor (S. A. et L.): 21 (1803)
Hydropunctaria aractina (Wahlenb.) Orange, Lichenologist 44(3): 305 (2012)
Syn: Verrucaria aractina Wahlenb., in Acharius, Methodus, Suppl.: 17 (1803)
Hydropunctaria maura (Wahlenb.) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
Syn: Verrucaria maura Wahlenb., Methodus qua Omnes Detectos Lichenes Secundum Organa Carpomorpha ad Genera, Species et Varietates Redigere atque Observationibus Illustrare Tentavit Erik Acharius: 19 (1803)
Hydropunctaria rheitrophila (Zschacke) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
Syn: Verrucaria rheitrophila Zschacke, Verhandlungen des Botanischen Vereins der Provinz Brandenburg 64: 108 (1922)
Verrucaria rheithrophila Zschacke (1922)
Verrucula rheitrophila (Zschacke) M. Choisy, Bulletin Mensuel de la Société Linnéenne de Lyon 19: 69 (1950)
Verrucula rheithrophila (Zschacke) M. Choisy (1950)
Hydropunctaria scabra (Vězda) C. Keller, Gueidan & Thüs, Taxon 58(1): 194 (2009)
Syn: Verrucaria scabra Vězda, Folia geobot. Phytotax. 5(3–4): 308 (1970)
Juncigena adarca Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 40(4): 291 (1997)
Syn: Cirrenalia adarca Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 40: 292 (1997)
Moheitospora adarca (Kohlm., Volkm.-Kohlm. and O.E. Erikss.) Abdel-Wahab, Abdel-Aziz & Nagahama, Mycol. Progr. 9(4): 552 (2010)
The asexual Cirrenalia adarca is reduced to synonymy with its sexual morph Juncigera adarca. Moheitospora adarca was proposed by Abdel-Wahab et al. (2010) to include C. adarca and is a rejected epithet in compliance with one name Juncigena is referred to the family Juncigenaceae (order Torpedosporales, Hypocreomycetidae, Ascomycota).
Kochiella crispa (Kohlm.) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 96 (2011)
Syn: Remispora crispa Kohlm., Can J Bot 59(7): 1317 (1981)
Marinokulati chaetosa E.B.G. Jones & K.L. Pang, Cryptog. Mycol. 35(2): 133 (2014)
Syn: Chaetosphaeria chaetosa Kohlm., Nova Hedwigia 6(3–4): 307 (1963)
Matsusporium tropicale (Kohlm.) E.B.G. Jones & K.L. Pang, Mycol. Progr. 9(4): 550 (2010)
Syn: Cirrenalia tropicale Kohlm., Mycologia 60(2): 267 (1968)
Moheitospora adarca (Kohlm., Volkm.-Kohlm. & O.E. Erikss.) Abdel-Wahab, Abdel-Aziz & Nagah., Mycol. Progr. 9(4): 552 (2010)
Syn: Cirrenalia adarca Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 40: 292 (1997)
This name is superfluous under the one name one fungus protocol.
Moromyces varius (Chatmala & Somrith.) Abdel-Wahab, K.L. Pang, Nagah., Abdel-Aziz & E.B.G. Jones, Mycol. Progr. 9(4): 555 (2010)
Syn: Cumulospora varius Chatmala & Somrith., Fungal Divers. 17: 3 (2004)
Morosphaeria ramunculicola (K.D. Hyde) Suetrong, Sakay., E.B.G. Jones & Schoch, Stud. Mycol. 64: 162 (2009).
Syn: Massarina ramunculicola K.D. Hyde, Mycologia 83(6): 839 (1992)
Morosphaeria velatospora (K.D. Hyde & Borse) Suetrong, Sakay., E.B.G. Jones & Schoch, Stud. Mycol. 64: 161 (2009)
Syn: Massarina velatospora K.D. Hyde & Borse, Mycotaxon 27: 161 (1986)
Nohea delmarensis (Kohlm. & Volkm.-Kohlm.) Abdel-Wahab, Mycotaxon 115: 448 (2011)
Syn: Naufragella delmarensis Kohlm. & Volkm.-Kohlm., Syst. Ascom. 16(1–2): 10 (1998)
The transfer of Naufragella delmarensis to Nohea has also to be considered as doubtful in view of the studies by Chu et al. (2015). The genera Naufragella and Nohea share many common characteristics, in particular the ascospore appendages which are of two types: 1. a gelatinous sheath, and 2. a crown of delicate filaments positioned below each apex. No cultures of N. demarensis are available for comparison, while its inclusion in the phylogenetic analysis will determine the validity of the genus Naufragella.
Nohea spinibarbata (Jørg. Koch) Abdel-Wahab, Mycotaxon 115: 448 (2011)
Syn: Remispora spinibarbata Jørg. Koch, Nordic J. Bot. 8(5): 517 (1989)
Naufragella spinibarbata (Jørg. Koch) Kohlm. & Volkm.-Kohlm., Syst. Ascomy. 16(1–2): 11 (1998)
Abdel-Wahab (2011) concluded, based on LSU rDNA sequence data that the genera Naufragella and Nohea were congeneric and Naufragella spinibarbata was consequently transferred to Nohea. However, Chu et al. (2015) in a phylogenetic reappraisal of Nimbospora, showed that N. spinibarbata forms a sister group to two Nimbospora species with high statistical support. Their data placed Nohea umiumi as a sister clade to the Nimbospora/Naufragella clade with weak support. Therefore, the transfer of Naufragella spinibarbata to Nohea requires further sampling of related taxa and a wider range of genes before the relationships of these genera are resolved.
Paradendryphiella arenariae (Nicot) Woudenberg & Crous, Stud. Mycol. 75: 208 (2013)
Syn: Dendryphiella arenariae Nicot, Revue Mycol., Paris 23: 93 (1958)
Paradendryphiella salina (G.K. Sutherl.) Woudenberg & Crous, Stud. Mycol. 75(1): 207 (2013)
Syn: Cercospora salina G.K. Sutherl., New Phytol. 15: 43 (1916)
Dendryphiella salina (G.K. Sutherl.) Pugh & Nicot, Trans. Br. Mycol. Soc. 47(2): 266 (1964)
Scolecobasidium salinum (G.K. Sutherl.) M.B. Ellis, More dematiaceous hyphomycetes (Kew): 192 (1976)
Embellisia annulata de Hoog, Seigle-Mur., Steiman & K.-E. Erikss., Antonie van Leeuwenhoek 51(4): 409 (1985)
Plectosphaerella oratosquillae (P.M. Duc, Yaguchi & Udagawa) A.J.L. Phillips, A. Carlucci & M.L. Raimondo, Persoonia, Fungi 28: 43 (2012)
Syn: Plectosporium oratosquillae P.M. Duc, Yaguchi & Udagawa, Mycopathologia 167(5): 237 (2009)
Duc et al. (2009) described Plectosporium oratosquillae from diseased mantis shrimp in Yamaguchi Pref., Japan. It is an asexual species with hyaline conidiophores with unicellular conidia in slimy heads at the tips of phialides. Plectosporium has been shown to be the asexual morph of the genus Plectosphaerella, a member of the Plectosphaerellaceae. Carlucci et al. (2012) therefore proposed the new combination Plectosphaerella oratisquillae (P.M. Duc, Yaguchi & Udagawa) A.J.L. Phillips, A. Carlucci & M.L. Raimondo, to comply with the one fungus one name principle.
Pleospora halimiones de Gruyter & Verkley, Stud. Mycol. 75: 25 (2012)
Syn: Ascochyta obiones (Jaap) P.K. Buchanan, Mycol. Pap.156: 28 (1987)
De Gruyter et al. (2012) showed that Ascochyta obiones (strain CBS 432.77) grouped in the Pleosporaceae based on sequences of the 28S and internal transcribed spacer regions 1 & 2 and 5.8S nrDNA (ITS), with high statistical support. It grouped in a clade with Pleospora calvescens, Ascochyta hyalospora and Pleospora betae (= anam. Phoma betae). Under the one name one fungus frame work Pleospora halomiones has priority.
Rimora mangrovei (Kohlm. & Vittal) Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B.G. Jones Stud. Mycol. 64: 166 (2009)
Syn: Lophiostoma mangrovei Kohlm. & Vittal, Mycologia 78(3): 487 (1986)
Astrosphaeriella mangrovei (Kohlm. & Vittal) Aptroot & K.D. Hyde, Nova Hedwigia 70(1–2): 154 (2000)
Toriella tubulifera (Kohlm.) Sakay, K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 100 (2011)
Syn: Halosphaeria tubulifera Kohlm., Nova Hedwigia 2: 311 (1960)
Ceriosporopsis tubulifera (Kohlm.) P.W. Kirk ex Kohlm., Can. J. Bot. 50(9): 1953 (1972)
Tubakiella galerita (Tubaki) Sakay., K.L. Pang & E.B.G. Jones, Fungal Divers. 46: 99 (2011)
Syn: Remispora galerita Tubaki, Publ. Seto Mar. Biol. Lab. 15(5): 362 (1967)
Halosphaeria galerita (Tubaki) I. Schmidt, Natur Naturschutz Mecklenberg 12: 49 (1974)
Infrequently collected taxa
Many marine fungi are known only from an initial description while others are infrequently collected, which means that they have not been isolated and studied at the molecular level. These taxa often are difficult to assign to a family/order, and may well be new lineages of fungi that evolved in the marine ecosystem.
Orcadia ascophylli G.K. Sutherl., Trans. Br. Mycol. Soc. 5(1): 151 (1915)
This fungus is only known from limited collections made by Sutherland (1914), Wilson (1951) and Webber (1967) and infects the brown seaweeds Ascophyllum nodosum and Halidrys siliquosa. Webber (1959) reported this species on the “College rocks”, Aberystwyth, “South rocks”, Borth and drift washed up at Ynyslas, Dovey estuary, Cardiganshire, Wales, UK. Orcadia ascophylli infected old and worn thalli at the extreme upper limits of A. nodosum, causing blackening of the host tissue (Webber 1959). However, attempts to isolate the fungus were unsuccessful. Perithecia were immersed in the host thallus, the centrum wall was 5¬6 layers thick of brown textura angularis with cells occluding the ostiole opening, and with downward growing mycelial threads (Webber 1959). Asci deliquesce to release the 3-septate hyaline ascospores lacking appendages.
Trailia ascophylli G.K. Sutherl., Trans. Br. Mycol. Soc. 5(1): 149 (1915)
Trailia ascophylli is another marine ascomycete which has only been collected on a few occasions (Sutherland 1914; Wilson 1951; Webber 1959), and occurs on A. nodosum, often heavily infected by Orcadia ascophylli. Webber (1959) reported that the ascomata are deeply seated in the host thallus, 57–85 μm with very long necks up to 420 μm. Asci measure 60¬80 × 9.5¬13 μm, 8-spored, unitunicate, deliquescing early and ascospores 90¬105 μm, hyaline, 3-septate with their unusual habit of being bent double in the ascus. Ascospores are released passively into the centrum cavity and remain bent, followed by the broad apical region of the spore entering the neck, becoming straight and released through neck opening into the surrounding water. On their release, the ascospores resume their bent habit.
Lautitia danica S. Schatz, Can. J. Bot. 62 (1): 31 (1984)
Syn: Leptosphaeria danica Berl., Icon. Fung. 1(2): 87 (1892)
Didymosphaeria danica (Berl.) I.M. Wilson & Knoyle, Trans. Br. Mycol. Soc. 44: 57 (1961)
According to Wilson and Knoyle (1961), Lautitia danica is found on the red alga Chondrus crispus growing below low water mark of spring tides with some 29 collections made in the Aberystwyth area, and Dale (Wales, UK) and Falmouth (England, UK). Maximum infection of tetrasporic plants was from March to July, while on cystocarpic plants it was September to March, these times corresponding to the peak period of reproduction of Chondrus. This may be advantageous in the infection of newly released gametes. Webber (1959) reported the occurrence of both the sexual and asexual morphs of the taxon, the asexual form being pycnidial. Stanley (1991, 1992) reported that the fungus was most abundant on cystocarpic plants of Ch. crispus, collected at Bembridge, Isle of Wight, UK, and confined to the cystocarps, and present from August through to July. Maximum occurrence of the fungus was during November to February, which corresponds to the peak fertility of the host alga, when nearly 25 % of the fertile fronds examined had fungal perithecia. This species requires fresh collection, isolation and sequencing to determine its taxonomic position.
Lindra I.M. Wilson, Trans. Br. Mycol. Soc. 39(4): 411 (1956)
This genus was described from intertidal wood and test panels exposed at Aberystwyth, UK and characterised by its filiform ascospores with 30–50 septa and a terminal enlarge globose cell or appendage (Wilson 1956). It has been variously collected in Canada, Denmark, Wales, and Norway (Kohlmeyer and Kohlmeyer 1979). Subsequently, further species have been described: L. crassa, L. hawaiiensis, L. obtusa and L. thalassiae (Jones et al. 2009). The genus is polyphyletic with L. obtusa forming a basal group to the order Lulworthiales and distantly placed from L. crassa and L. thalassiae (Jones et al. 2009). Morphologically species differ, with the type species (L. inflata: Fig. 17a-d) possessing carbonaceous ascomata, globose terminal cells on the ascospores and growing on woody substrates, while the second group (L. thalassiae) have membranous ascomata, ascospores with pointed end cells and growing on sea grasses (Campbell et al. 2005). Further collections of L. inflata are required to enable it to be isolated and sequenced.
Bathyascus and other poorly collected species
Many marine fungi have yet to be sequenced so that their taxonomic position can be resolved. For example, the genus Bathyascus was introduced by Kohlmeyer (1977) to accommodate a mangrove species B. vermisporus. Subsequently, four other species (B. avicenniae, B. gransisporus, B. mangrovei, B. tropicalis) have been described and delineated by ascospore septation and their measurements. These species have rarely been collected and therefore no molecular data is available to assign them to a higher taxonomic order. Currently assigned to Halosphaeriaceae, B. tropicalis for example might be better referred to Thalespora (Jones et al. 2006).
The same can also be applied to the cleistothecial genera: Biflua, Crinigera, Dryosphaera and Marisolaris (Koch and Jones 1989), taxa to be found on wood associated with sand. They have proven to be difficult to isolate and their small size does not yield sufficient DNA for sequencing (E.B.G. Jones unpublished data). Preliminary data suggests they are a basal group in the Ascomycota, further studies are required (M.A. Abdel-Wahab unpublished data).
New fungi to marine habitats
Aniptodera lignatilis K.D. Hyde, Aust. Syst. Bot. 5(1): 111 (1992)
Taxa of Halosphaeriaceae are predominantly marine/estuarine but many can also be found in freshwater habitats including, Aniptodera, Halosarpheia, Lignincola and Phaeonectriella (Pang 2002). Aniptodera lignatilis was originally described from a submerged log in a freshwater environment and it has been found on mangrove wood in Taiwan (Pang et al. 2011).
Dictyosporium inflatum (Matsush.) K.L. Pang, E.B.G. Jones & R. Kirschner, Mycol. Progr. 12(1): 31 (2013)
Syn: Cheiromyces inflatus Matsush., Matsush. Mycol. Mem. 3: 3 (1983)
This species is reported for the first time from England, UK on driftwood collected in the intertidal zone (Kirschner et al. 2013). Morphological and DNA analyses (partial SSU, and LSU and ITS of the rRNA gene) indicate placement of this species in Dictyosporium. Further collections are required to confirm a marine attribution for this species.
Catabotrys decidua (Berk. & Broome) Seaver & Waterson, Mycologia 38(2): 184 (1946)
Syn: Hypoxylon deciduum Berk. & Broome, Bot. J. Lin. Soc. 14(74): 120 (1873)
This species is common on monocots in terrestrial environments, forming large black stromata (Huhndorf et al. 2004). Recently this fungus was discovered on intertidal decayed wood in mangroves of Taiwan and Malaysia, and may have recently fallen into the marine milieu.
Marine yeasts
There is no complete or recent list of marine/estuarine yeasts and they were not included in our classification paper on the mycelial fungi Ascomycota, Basidiomycota and asexual fungi in Jones et al. (2009). Johnson and Sparrow (1961) in their volume on “Marine and Estuarine Fungi” listed seven species which they regard as truly marine, while Kohlmeyer and Kohlmeyer (1979) documented 165 species (23 obligate marine yeasts, 142 facultative marine yeasts). Kurtzman et al. (2011a, b) reports 72 sexual and 14 asexual Ascomycota (Taphrinomycotina, Saccharomycotina) and 34 sexual and 27 asexual Basidiomycota (Pucciniomycotina, Agaricomycotina and Ustilaginomycotina), making a total of 147 species. As many are known from a single survey, care must be taken in establishing that they truly are marine taxa. Many yeasts are undoubtedly transient and further documentation is required. There is always the possibility that some originate from “run off” from terrestrial habitats or are the result of pollution (Hagler et al. 1982; Araujo and Hagler 2011). Generally, marine yeasts have been studied by a different group of mycologists, and characterised on their phenotypic features (Fig. 18a-e), particulary morphology and their ability to utilise various carbon sources and nitrogen compounds. Currently, they are characterised by sequence data, particularly ITS and D1/D2 regions of the LSU rDNA, which leads to their rapid identification (Kurtzman et al. 2011a, b).
Yeast cultural and morphological yeast features. a. Cystofilobasidium capitatum CBS 6358. Left panel: Yeast colony; Middle panel: germinating teliospore with basidiospores (courtesy J.F. Fell); Right panel: yeast cells and chlamydospore-like cells. b. Trichosporon cutaneum CBS 2466. Left panel: Yeast colony; Middle panel: yeast-like cells and filaments; Right panel: Scanning electron micrographs of arthroconidia (courtesy J. Stalpers and R. Samson). c. Rhodotorula glutinis CBS 20. Left panel: Yeast colony; Middle pane: yeast cells showing polar budding; Right panel: Scanning electron micrograph of budding yeast cells with scar (courtesy J. Stalpers and R. Samson). d. Kazachstania aestuarii CBS 4438. Left panel: Yeast colony; Middle panel: asci with ascospores; Right panel: yeast cells with multilateral budding. e. Schwanniomyces vanrijiae CBS 3024. Left panel: Yeast colony; Middle panel: asci with ascospores; Right panel: yeast cells with multilateral budding
In Table 2 we present a preliminary list of marine yeasts to which we hope others will be added as they are included within a wider study of marine fungi (Jones and Fell 2012). Recent studies report the isolation of strains which have been identified by molecular data but still there is no data-base that list all known marine yeasts. Since our monograph (Jones et al. 2009) many new genera and species have been described from marine habitats, including water columns, mangroves, intertidal sediments, diseases of marine plants and animals, and deep-sea hydrothermal vents (Fell et al. 2001; Nagahama et al. 2003; Fell et al. 2004; Mahdi et al. 2008; Burgaud et al. 2010; Statzell-Tallman et al. 2010; Wei et al. 2011).
Mangoves are rich in yeast species as recent studies have recorded 149 yeast strains with many new species (Limtong et al. 2007, 2008, 2010; Am-in et al. 2011). Similarily, Fell et al. (2011) have reported on marine yeasts from the mangrove areas of the Everglades in Florida, USA. Many yeasts have been documented from intertidal sands and may be present in high numbers. For example, Loureiro et al. (2005) isolated 250 strains (31 species, 4 genera) from Bairro Novo and Casa Caiada, Brazil, with Brettanomyces bruxellensis, and Candida sake present in high numbers in sand samples. Other studies on sand fungi have been conducted by Paula et al. (1983), Toro and Piontelli (1985) and Vogel et al. (2007), all indicating the presence of yeasts, numbers being high in bathing beaches. Many of the taxa recovered were previously known as human pathogens (Vogel et al. 2007). Hagler et al. (1982) report the highest densities of yeasts in sediments from polluted sites, with Pichia kudriavzevii (= Issatchenkia orientalis) and Pichia membranifaciens the most prevalent. Therefore care has to be exercised in counting these as truly marine species.
New species since 2008
Candida andamanensis Am-In, Limtong, Yongman. & Jndam., Int. J. Syst. Evol. Microbiol. 61(2): 459 (2011)
This species was isolated from estuarine waters of a mangrove forest in Laem Son National Park, Ranong Province, Thailand (Am-In et al. 2011). Sequence data shows that Candida andamanensis groups with two strains of C. butyri with high support, but they are not conspecific and differ in the inability of C. andamanensis to ferment D-glucose and to assimilate citric acid and by its ability to grow in vitamin-free medium. Candida andamanensis differs from other similar species (C. conglobata, C. aaseri) by sequence analysis as well as biochemical and physiological characteristics.
Candida laemsonensis Am-In, Limtong, Yongman. & Jindam., Int. J. Syst. Evol. Microbiol. 61(2): 458 (2011)
Two strains of C. laemsonensis group with C. berthetii with hight support, but are not conspecific. The species can be distinguished from C. berthetii by only a few phentotypic characteristics, including the ability to assimilate sorbose and cellobiose, its inability to grow in 0.01 and 0.1 % cyclohexamide and the ability to grow on 50 and 60 % glucose. The species was isolated from estuarine water collected at Laem Son mangrove, Ranong, Thailand (Am-In et al. 2011).
Candida neustonensis Chang & Liu, Antonie van Leeuwenhoek 97(1): 38 (2010)
Candida neustonensis was introduced for four strains isolated from the sea surface microlayer in Taiwan (Chang et al. 2010). No sexual morph was reported. Phylogenetic analysis of sequence data placed C. neustonensis as a member of the Pichia guilliermondii clade, with C. fukuyamaensis (4.4 % divergence), C. xestobii (4.4 % divergence) and P. guilliermondii (4.5 % divergence) as its closest relatives.
Candida oceani Burgaud & Barbier, Antonie van Leeuwenhoek 100(1): 79 (2011)
The type strain of this species was isolated from deep-sea coral collected near the Rainbow hydrothermal site at the Mid-Atlantic Ridge (2,300 m depth), Atlantic Ocean, while two other strains (MARY089 and CBS 5307) were found respectively in water samples from the Rainbow hydrothermal site and from the stomach of a fish. Phylogenetically C. oceani groups with C. taylorii with high support (72/100/81) and differs from C. atlantica and C. atmosphaerica, respectively, by 1.2 and 1.3 % in the SSU rDNA and 9.1 and 8.2 % in the internal transcribed spacer region. Candida taylorii and C. spencermartinsiae (these are further discussed below) were harvested in seawater near corals. Burgaud et al. (2011) indicated that these yeasts appear to form a cluster of marine species.
Candida ranongensis Am-In, Limtong, Yongman. & Jindam., Int. J. Syst. Evol. Microbiol. 61(2): 459 (2011)
Two strains of C. ranongensis cluster with unidentified Candida spp. in a sister group to C. scorzettiae and differ from the latter on the basis of its inability to assimilate cellobiose, lactose, salicin and citric acid and its ability to assimilate L-sorbose and to grow in 10 % NaCl. The species was isolated from estuarine water from Laem Son mangrove, Ranong, Thailand (Am-In et al. 2011)
Candida rhizophoriensis Fell, M.H. Gutiérrez, Statzell & Scorzetti, Antonie van Leeuwenhoek 99(3): 545 (2011)
Candida rhizophoriensis was isolated repeatedly from stations 4–6 of the Shark River Slough within the Everglades mangrove, Florida, USA. These are saline zones and the species was not encountered at the freshwater stations. Sequence analysis of the D1/D2 LSU rDNA showed that C. rhizophoriensis was related to C. melibiosica (72 % bootstrap support) in the Metschnikowia clade, with 23 Candida species in four divergent subclades (Fell et al. 2011).
Candida sanitii Limtong, Am-In, Kaewwichian, Boonmak, Jindam., Yongmanitchai, Srisuk, H. Kawas. & Nakase, FEMS Yeast Res. 10(1): 118 (2010)
This species was isolated from estuarine water in a mangrove forest at Ranong Province, Thailand and is characterised by phenotypic, physiological and sequence data. Cells are sphaerical to ovoidal and proliferate by multilateral budding; pseudohyphae are present, but no true hyphae (Limtong et al. 2010). Three strains of this species formed a monophyletic group in the Saturnispora clade with moderate support with a strain of C. suwanaritii as a sister subclade (Limtong et al. 2010).
Candida suwanaritii Limtong, Boonmak, Kaewwichian, Am-In, Jindam., Yongman., Srisuk, H. Kawas. & Nakase, FEMS Yeast Res. 10(1): 120 (2010)
Candida suwanaritii can be separated from its closed relative C. saniatii by its ability to ferment glucose and to assimilate trehalose (weak) and soluble starch, and the inability to assimilate glycerol and to grow at 40-42 °C. Phylogenetically it forms a sister group to C. sanitii within the Saturnispora clade, with high bootstrap support. Candida suwanaritii was isolated from estuarine waters from a mangrove forest in Pred Nai village, Trat province, Thailand (Limtong et al. 2010).
Candida spencermartinsiae Statzell-Tallman, Scorzetti & Fell, Int. J. Syst. Evol. Microbiol. 60 (8): 1980 (2010)
This species was isolated from seawater samples taken at 10 m adjacent to corals at Looe Key Reef, Florida, USA. Sequence analysis of the D1/D2 LSU and the ITS1, 5.8S and ITS2 rDNA placed it in a clade of marine yeasts grouping in the Debaryomyces/Loddermyces clade in the Saccharomycetales. Candida spencermartinsiae grouped with a Candida sp. isolated from Taiwan marine habitats and C. atlantica from which it differs by 9 bases in their ITS (Statzell-Tallman et al. 2010).
Candida taylorii Statzell-Tallman, Scorzetti & Fell, Int. J. Syst. Evol. Microbiol. 60(8): 1981 (2010)
Candida taylorii was isolated from reef and mangrove habitats. One isolate came from 10 m adjacent to corals at Looe Key Reef, Florida, USA, and nine strains from mangrove swamps in Belize and the Bahamas. Ascospores were not observed and the species was identified by sequence data (Statzell-Tallman et al. 2010). The nearest taxon to C. taylorii is Candida sp. strain May 089, but the former differs by 18 bases in D1/D2 LSU rDNA and utilises rhamnose.
Cryptococcus keelungensis Chang & Liu, Int. J. Syst. Evol. Microbiol. 58(12): 2974 (2008)
Cryptococcus keelungensis was isolated from the sea-surface microlayer at Keelung on the north-east coast of Taiwan. It is characterized by the lack of sexual spores and ballistoconidia, utilization of D-glucuronate, the absence of fermentative ability, positive stain for Diazonium blue B reaction and positive urease activity, growth at 25-35 °C but not at 40 °C on YM agar, and the main ubiquinone is Q-10 (Chang et al. 2008). Sequence analysis of the D1/D2 domain of the LSU rDNA of strain SN-82 T suggested that this strain is related to the aerius clade in the Filobasidiales, grouping with three unidentified Crytpococcus and three Filobasidium spp.
Cryptococcus mangaliensis Fell, Statzell & Scorzetti, Antonie van Leeuwenhoek 99(3): 548 (2011)
Cryptococcus mangaliensis is a member of the Tremellomycetes that clusters with Bullera pseudoalba and B. hoabinhensis, with four and two base pair differences, respectively, in the D1/D2 region of LSU rDNA (Fell et al. 2011). Cryptococcus mangaliensis does not produce ballistoconidia while the Bullera species do. The species was isolated from a mangrove in Chatman Bend, in the Everglades National Park, Florida.
Kluyveromyces siamensis Am-in, Yongman. & Limtong, FEMS Yeast Res. 8(5): 825 (2008)
Seven strains of this Kluyveromyces species were isolated from seven water samples collected from a mangrove forest in Ranong, Thailand (Am-In et al. 2008). Based on sequences of the D1/D2 domain of the LSU rRNA gene, K. siamensis is a sister species of K. aestuarii and forms a clade with the other six recognized species of Kluyveromyces. Phenotypically the seven strains of K. siamensis have spherical to ellipsoidal cells, proliferated by multilateral budding, and formed one to four spherical ascospores in a conjugated and evanescent ascus: pseudohyphae were produced but not true hyphae, glucose is fermented, nitrate is not assimillated, gave negative results for the Diazonium blue B and urease reactions and had Q-6 as the major ubiquinone, as do other members of the genus Kluyveromyces.
Kwoniella mangrovensis Statzell, Belloch & Fell, FEMS Yeast Res. 8(1): 107 (2008)
Strains were isolated from marine waters in mangrove habitats at six locations at geographically dispersed sites in the Bahamas Islands and Shark River Slough, Everglades (Statzell-Tallman et al. 2008). Kwoniella is distinct from other members of Tremellales by a combination of characteristics: lack of ballistospores, ballistoconidia and basidiocarps, and the presence of morphologically variant basidia, which are globose, ovoid or lageniform with longitudinal to oblique and transverse septa and navicular with transverse septa. Molecular sequence analysis of a partial region (D1/D2 domains) of LSU rRNA shows K. mangrovensis forms a sister group to Cryptococcus bestiolae with 99 % bootstrap support, and is closely related to C. dejecticola, C. bestiolae and Bullera dendrophila, but did not mate with any of those species (Statzell-Tallman et al. 2008). Kwoniella mangroviensis has a widespread occurrence in mangrove habitats, but a specific ecological role in mangrove habitats is unknown.
Pseudozyma abaconensis Statzell-Tallman, Scorzetti & Fell, Int. J. Syst. Evol. Microbiol. 60(8): 1983 (2010)
The genus is a member of the Ustilaginales with close affinities to the sexual genera Ustilago and Sporisorium, and isolated from seawater at a depth of 3–5 m in a coral reef at Green Turtle Key, Abaco, the Bahamas. It has variable-shaped cells that can be fusoid, ovoid, ellipsoid or cylindrical, and conidia are produced on sterigmata. Sugars are not fermented, and starchy-like compounds are not produced. Colonies are often pinkish, orange or brownish yellow.
New combinations
Hannaella surugaensis (Nagah., Hamam. & Nakase) F.Y. Bai & Q.M. Wang, FEMS Yeast Res. 8(5): 805 (2008)
Syn: Cryptococcus surugaensis Nagah., Hamam. & Nakase, Int. J. Syst. Evol. Microbiol. 53(6): 2097 (2003)
In a phylogenetic study based on sequence analyses of the SSU rRNA gene, LSU rRNA gene D1/D2 domain, internal transcribed spacer (ITS) region including 5.8S rRNA gene and mitochondrial cytochrome b gene, Cryptococcus surugaensis did not group in the C. luteolus lineage and along with other species was transferred to a new genus Hannaella (type species H. sinensis) (Wang and Bai 2008). The genus is characterized by budding cells that are oval, ellipsoidal or subglobose, ballistoconidia may or may not be formed, hyphae or pseudohyphae may be present, and lack of clamp connections and fermentation, Diazonium blue B and urease reactions are positive and the major ubiquinone is Q-10 (Wang and Bai 2008).
Marine derived fungi
Many asexual fungi have been isolated from marine habitats, especially seawater and as saprobes of marine substrates (e.g., drift seaweeds, in sediments and as endophytes of both plants and animals, pathogens of marine animals) (Hatai 2012; Raghukumar and Ravindram 2012; Sridhar et al. 2012). These species often are only reported once and herbarium material/cultures are not deposited for future reference. These fungi are often referred to as marine-derived and many are the source of novel bioactive compounds (Schulz et al. 2008; Trisuwan et al. 2009; Sakayaroj et al. 2012). Very few have been subject to a molecular phylogenetic study and it is therefore difficult to verify their identification or classification. Zuccaro et al. (2003) isolated a wide range of fungi, especially Acremonium and Emericellopsis species, from Fucus species. Sequences of Emericellopsis, Stanjemonium and some marine-derived Acremonium species (ITS rRNA gene and intron 3 of the β tubulin gene) identified four clades that were monophyletic. One clade contained isolates originating from marine sources and saline lakes (Zuccaro et al. 2004). Another clade comprised only terrestrial derived strains. They also demonstrated that species from the marine clade grew better on media containing salt, and concluded that members of this clade “are products of an evolutionary radiation into saline environments”.
Previous published reviews and books have focused on marine fungi growing on wood (Jones et al. 2009; Pang et al. 2011), Juncus reoerianus Kohlmeyer et al. 1995, Spartina spp. (Gessner and Kohlmeyer 1976; Da Luz Calado and Barata 2014), while those from other substrates have not been included e.g., yeasts (Fell 2014) and zoosporic fungi (Powell and Letcher 2012; Lepelletier et al. 2014a, b). Bungi and Ireland (2004), in a study of bioactive compounds from marine fungi, showed that marine derived fungi from sponges, and algae accounted for 33 and 24 %, respectively, of the fungal diversity, with only 13 % from woody substrates. The distribution of new compounds reported from marine derived fungi was 28, 27 and 10 % respectively for those isolated from sponges, algae and wood. Therefore we have been ignoring a major component of the marine fungal diversity.
In an attempt to recognise the existence of these fungi we include them in this review of marine fungi, with the objective to encourage others to fully document these fungi and to deposit cultures in fungal/microbiological collections, in order to make phylogenetic assessment possible of the fungi to be found in marine habitats. Species isolated and recorded from identified substrata are included in Table 1. Over 200 so called “marine derived fungi” have been included in this monograph, however, not all are supported with molecular data. Of the 47 Aspergillus species listed in Table 1, 23 species are supported by sequence data. Some new species and genera have been described as the result of molecular phylogenetic studies, e.g., the genera Amorosia (Mantle et al. 2006) and Paradendryphiella (Woudenberg et al. 2013) and the species Penicillium dravuni (Janso et al. 2005). We excluded from our review, fungi isolated as endophytes and those recovered from the Dead Sea (Pang et al. 2008; Chareprasert et al. 2010; Oren and Gunde-Cimerman 2012). Many studies on endophytes from mangrove plants are isolated from the terrestrial parts of the host and therefore can not be considered marine.
Basidiomycota
There have been no major additions to the marine basidiomycetes in recent years, with the exception of the documentation of four taxa reported from less saline mangrove habitats: Henningsomyces spp., Schizophyllum commune, Hyphoderema smabuci and Gramomothele fuligo, all found colonising the basal parts of the fronds of the brackish water palm Nypa fruticans (Fig. 19c-d, 20a-h) (Loilong et al. 2012).
Various basidiomycetes have been reported to cause butt rot of mangrove trees, and those included Ganoderma lucidum, Rigidoporus zonalis, Phellinus mangrovicus, P. pachyphloeus and Inonotus culcatensis (Chalermpongse 1991; Mwangi 2001; Gilbert et al. 2008). However, these generally colonize the aerial parts of trees. Sakayaroj et al. (2012) noted that the root system of the mangrove tree Xylocarpus granatum was infected by a number of bracket polypores (Fulvifomes siamensis, F. halophilus, F. xylocarpicola) (Fig. 21a-f). In a comprehensive molecular study of 46 isolates from fruting bodies collected on X. granatum trees at Hat Khanom-Mu Ko Thale Tai National Park, Thailand, they grouped in two clades, usually occurring on the roots below the tidal level and covered at least once a day by brackish/seawater. They also reported that mycelia of this fungal group were able to grow best on malt extract agar (MEA) containing sea salt (15‰), suggesting that they may be salt tolerant and well adapted to their life in saline mangrove habitats. In a subsequent paper these were described as new species in the genus Fulvifomes: F. halophilus and F. siamensis, while a third species was described for F. xylocarpicola that grew on more aerial part of the tree. Another basidiomycete collected on mangrove trees was Fomes mangrovicus and as this species grouped in the genus Fulvifomes; a new combination was introduced as F. mangrovicus (Hattori et al. 2014).
New species
Fulvifomes Murrill, Northerm polypores, privately printed, New York (1914)
Several Hymenochaetaceae species have been reported from mangrove forests including the following Fulvifomes species: F. fastuosus, F. merrillii, F. rimosus and F. swieteniae (Gilbert and Sousa 2002; Gilbert et al. 2008; Baltazar et al. 2009). In addition, Phellinus mangrovicus is known from Micronesia and Brazil (Imazeki 1941; de Campos and Cavalcanti 2000; Gilbert et al. 2008). In a study of basidiomycetes causing butt rot of the mangrove tree X. granatus at various mangroves in Thailand, a number of species were recorded (Jones and Choeyklin 2008; Sakayaroj et al. 2012). A molecular study, based on LSU and ITS1, 2, 5.8S rDNA analyses, confirmed that all taxa isolated and described belonged to the poroid genus Fulvifomes in the Hymenochaetaceae with high statistical support. Two species always isolated (29 strains) from the roots of X. granatus showed they were salt tolerant growing best at MEA containing sea salt (15 ‰). These strains formed unique phylotypes which did not group with other known Fulvifomes species and were described as new species: F. siamensis, F. halophilus, F. xylocarpicola (Hattori et al. 2014).
Fulvifomes siamensis T. Hatt., Sakay. & E.B.G. Jones, Mycoscience 55(5): 346 (2014)
This species can be characterized by the small pores (7–8 pores per mm2) in the hymenium, lack of a distinct crust, monomitic hyphal system in the context, and subglobose basidiospores (Fig. 20e–f). F. siamensis may be phylogenetically close to F. fastuosus and F. nilgheriensis (Sakayaroj et al. 2012), but both of these species have a distinct crust on the pileus surface, and can be easily discriminated from F. siamensis (Hattori et al. 2014).
Fulvifomes halophilus T. Hatt., Sakay. & E.B.G. Jones, Mycoscience 55(5): 347 (2014)
This species is characterized by the presence of a distinct crust on the pileus surface, a subdimitic to dimitic hyphal system in the context, and subglobose basidiospores. Fulvifomes merrillii is similar, but this species has larger pores (4–5 pores per mm2) and reniform basidiospores (Fig. 20c–d) (Hattori et al. 2014).
Fulvifomes xylocarpicola T. Hatt., Sakay. & E.B.G. Jones, Mycoscience 55(5): 345 (2014)
This species is characterized by the lack of a crust near the pileus and broadly ellipsoid basidiospores measuring 4–5.5 μm long. Fulvifomes rimosus has a similar macro-morphology, but has a monomitic to subdimic hyphal system in the context and larger basidiospores measuring 5.5–7.5 μm (Fig. 20a–b) (Hattori et al. 2014).
Tritirachium Limber, Mycologia 32: 26 (1940)
Tritirachium candoliense Manohar, Boekhout & Stoeck, Fungal Biol. 118(2): 143 (2014)
Tritirachium candoliense was isolated from coastal sediments of the Arabian Sea during the anoxic season (Manohar et al. 2014). Multigene phylogenetic analyses confidently placed the organism as a novel species within the recently defined class Tritirachiomycetes, subphylum Pucciniomycotina (Schell et al. 2011).
Chytridiomycota
Chytrids are poorly represented in marine habitats and consequently are often left out of reviews of marine fungi (Shearer et al. 2007; Jones et al. 2009). However, they were included in the book on marine fungi edited by Jones and Pang (2012) when six species were discussed (Gleason et al. 2012). The only published account of marine chytrids and their classification is by Johnson and Sparrow (1961), who included 22 species in the Chytridiales. Booth (1979) recovered Chytriomyces pocularus, and Phlyctochyrium mangrovei from Spartina and Avicennia substrates from Brazilian and South American waters, and five species from beaches, dune sands and tide flats from Hudson Bay, Churchill and Great Whale river regions, Canada: Chytriomyces multioperculatum, Phlyctochyrium palustre, Rhizophlyctis barderi, Rh. angulsoum and Rh. sphaerotheca (Booth 1981). Chytrids have also been reported by Höhnk (1958) from locations in Germany: Rhizophydium sphaertheca, Olipidium maritimum, and O. pendulum, and by Gaertner (1980, 1982) from the Tay Estuary, Dundee, Scotland, and the Atlantic sea, off Portugal, respectively, but not identified to species level. However, few of these have been collected recently and most have not been isolated and sequenced. Currently, there is much interest in zoosporic fungi and fungal-like organisms, with the description of new species. In order to encourage their study we include a list of marine chytrids in this volume.
Diverse and abundant environmental fungal sequences have also been retrieved from particular marine habitats, such as deep water and anoxic marine ecosystems (Le Calvez et al. 2009; Jebaraj et al. 2010; Orsi et al. 2013).
New families
Lobulomycetales D.R. Simmons, Mycol. Res. 113 (4): 453 (2009)
Lobulomycetaceae D.R. Simmons, Mycol. Res. 113(4): 453 (2009)
Lobulomyces D.R. Simmons, Mycol. Res. 113(4): 454 (2009)
This is a new order, family and genus introduced by Simmons et al. (2009) to accommodate two terrestrial chytrids referred to Chytriomyces: Ch. angularis, Ch. poculatus (= Lobulomyces angularis and L. poculatus, respectively). In a phylogenetic study, Ch. polysiphoniae (a marine species) also grouped with the Lobulomyces species in a well-supported clade. However, no formal transfer was made to Lobulomyces because the culture was lost and no data was available on the ultrastructure of the zoospore (Simmons et al. 2009). Gleason et al. (2012) discussed the ecology and taxonomical history of this species, but made no comment on its classification.
Chytridium polysiphoniae Cohn, Hedwigia 4: 169 (1865)
This species groups in the Lobulomyces and should be referred to this genus as outlined above.
Rhizophydiales Letcher, Mycol. Res. 110 (8): 908 (2006)
Dinomycetaceae Karpov & Guillou, Protist 165: 240 (2014)
This new family, genus and species were introduced by Lepelletier et al. (2014a, b) for a marine chytrid (Dinomyces arenysensis) isolated from a dinoflagellate bloom of Alexandrium minutum in the Arenys de Mar harbour, Mediterraneaen Sea, Spain. A phylogenetic study showed that the species grouped in the Rhizophydiales, but could not be assigned to any family, genus or species. The family is characterised by zoospores with a centriole that is not parallel to the kinetosome, with a bilaminated spur, and a ribosomal core crossed by endoplasmic reticulum.
Dinomyces arenysensis Karpov & Guillou, Protist 165: 241 (2014)
Five strains of Dinomyces arenysensis, in a phylogenetic study of ITS and partial LSU rRNA gene sequences, formed a monophyletic clade in the Rhizophydiales with an environmental sequence (RCC3408) which differed by 164 nucleotides over 1547 positions from the other strains. The species is characterised by possessing inoperculate epibiotic sporangia, zoopores slightly elongate 2–4 μm long with a single lipid globule, released by breakdown of the sporangial wall (Lepelletier et al. 2014a, b).
Uebelmesseromycetaceae M.J. Powell & Letcher, Mycologia 107: 423 (2015)
This family was introduced by Powell et al. (2015) to accommodate a misclassified chytrid Rhizophlyctis harderi, that was described in German without a Latin diagnosis (Uebelmesser 1956). The thalli are monocentric, eucarpic, with sporangia with a single inoperculate discharge pore. Zoospores have a shield-shaped kinetosome-associated structure and a layered cap over the anterior end of the kinetosome and fenestrated cisternae.
Uebelmesseromyces harderi M.J. Powell & Letcher, Mycologia 107: 423 (2015)
The species is described from a culture deposited in the American Type Culture Collection and collected from intertidal soil in British Columbia, Canada (Booth 1981), and studied at the ultrastructural and molecular level by Powell et al. (2015). As the species does not belong in Rhizophlyctis, and the name Rh. harderi is invalid, Powell et al. (2015) described it as a new species. Phylogenetic analyses of nuc rDNA places it in the Rhizophydiales, not in the Rhizophlyctidales, while the distinctive kinetosome-associated structure, a curved shield bridged to two of the kinetosome triplets and a layered cap anterior to the kinetosome, distinguishes from other chytrids (Powell et al. 2015).
Blastocladiomycota
James (2006) introduced the phylum Blastocladiomycota to accommodate zoosporic fungi previously regarded as chytrids. The only marine species that can be referred to this phylum is Catenaria anguillilae, reported from estuarine waters of Adyar, Madras, India (Raghukumar 1977). Optimum growth for the species was 10 % salinity and it failed to grow at 30 % and above. Further studies are required to determine if it is a regular inhabitant of the marine milleu.
References
Abdel-Wahab MA (2011) Marine fungi from Sarushima Island, Japan, with a phylogenetic evaluation of the genus Naufragella. Mycotaxon 115:443–456
Abdel-Wahab MA, Hodhod MS, Bahkali AHA, Jones EBG (2014) Marine fungi of Saudi Arabia. Bot Mar 57:323–335
Abdel-Wahab MA, Nagahama T (2010) Halosarpheia japonica sp. nov. (Halosphaeriales. Ascomycota) from marine habitats in Japan. Mycol Progr 11:85–92
Abdel-Wahab MA, Nagahama T (2011) Gesasha (Halosphaeriaceae, Ascomycota), a new genus from ther Gesashi mangroves in Japan. Nova Hedw 92:497–512
Abdel-Wahab MA, Pang KL, Nagahama T, Abdel-Aziz F, Jones EBG (2010) Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulospora with the description of eight new genera and four new species. Mycol Progr 9:537–558
Amend A (2014) From dandruff to deep-sea vents: Malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog 10(8):1–4
Am-In S, Limtong S, Yongmanichai W, Jindamorakot S (2011) Candida andamanensis sp. nov., Candida laemsonensis sp. nov. and Candida ranongensis sp. nov., anamorphic yeast species isolated from estuarine waters in a Thai mangrove forest. Int J Syst Evol Microbiol 61:454–461
Am-In S, Yongmanitchai W, Limtong S (2008) Kluyveromyces siamensis sp. nov., an ascomycetous yeast isolated from water in a mangrove forest in ranong province, Thailand. FEMS Yeast Res 8:823–828
Araujo FV, Hagler AN (2011) Kluyveromyces aestuarii, a potential environmental quality indicator yeast for mangroves in the State of Rio de Janeiro, Brazil. Antonie Van Leeuwenhoek 100:341–347
Ariyawansa HA, Jones EBG, Suetrong S, Alias SA, Kang JC, Hyde KD (2013) Halojulellaceae a new family of the order Pleosporales. Phytotaxa 130:14–24
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Mapook A, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Thambugala KM, Manamgoda DS et al (2015) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71:85–139. doi:10.1007/s13225-015-0323-z
Baltazar JM, Trierveiler-Pereira L, Loguercio-Leite C, Ryvarden L (2009) Santa Catarina island mangroves 3: a new species of fuscoporia. Mycologia 101:859–863
Boonmee S, Ko-Ko TW, Chukeatirote E, Hyde KD, Chen H, Cai L, McKenzie EHC, Jones EBG, Kodsueb R, Hassan BA (2012) Two new Kirschsteiniothelia species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia 104:698–714
Boonyuen N, Chuaseeharonnachai C, Suetrng S, Sri-Indrasutdhi V, Sivichai S, Jones EBG, Pang KL (2011) Savoryellales (Hypocreomycetideae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia and Savoryella. Mycologia 103:1351–1350
Booth T (1979) Strategies for study of fungi in marine and marine influenced ecosystems. Rev Microb (S Aaulo) 10:123–137
Booth T (1981) Lignicolous and zoosporic fungi in marine environments of Hudson Bay. Can J Bot 59:1867–1881
Borse BD (1987) Marine fungi from India-V. Curr Sci 56:1109–1111
Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F (2009) Pyrosequencing analyses of forest soils reveal an unexpectantly high fungal diversity. New Phytol 184:449–456
Bungi TS, Ireland CM (2004) Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep 21:145–163
Burgaud G, Arzur D, Durand L, Cambon-Bonavita M, Barbier GH (2010) Marine culturable yeasts in deep-sea hypothermal vents, species richness and association with fauna. FEMS Microbiol Ecol 73:121–133
Burgaud G, Arzur D, Sampaio JP, Barbier GH (2011) Candida oceani sp. nov., a novel yeast isolated from a Mid-Atlantic Ridge hydrothermal vent (−2300 m). Antonie Van Leeuwenhoek 100:75–82
Campbell J, Ferrer A, Raja HA, Sivichai S, Shearer CA (2007) Phylogenetic relationships among taxa in the Jahnulales inferred from 18S and 28S nuclear ribosomal DNA sequences. Can J Bot 85:873–882
Campbell J, Volkmann-Kohlmeyer B, Gräfenhan T, Spataofora JW, Kohlmeyer J (2005) A reevaluation of Lulworthiales: relationships based on 18S and 28S rDNA. Mycol Res 109:556–568
Cannon PF, Kirk PM (2007) Fungal families of the world. CABI, England
Carlucci A, Raimondo ML, Santos J, Phillips AJL (2012) Plectosphaerella species associated with root and collar rots of horticultural crops in southern Italy. Persoonia 28:34–48
Chalermpongse A (1991) Fungal diseases in mangrove ecosystem. In: Proceeding, the 5th Silviculture Seminar in Thailand, Division of Silviculture, Royal Forest Department, Bangkok, Thailand, pp 307–338
Chang CF, Lee CF, Liu SM (2008) Cryptococcus keelungensis sp. nov., an anamorphic basidiomycetous yeast isolated from the sea-surface microlayer of the north-east coast of Taiwan. Int J Syst Evol Microbiol 58:2973–2976
Chang CF, Lee CF, Liu SM (2010) Candida neustonensis sp. nov., a novel ascomycetous yeast isolated from the sea surface microlayer in Taiwan. Antonie Leeuw 97:35–40
Chareprasert S, Piapukiew J, Whalley AJS, Sihanonth P (2010) Endophytic fungi from mangrove plant species of Thailand: their antimicrobial and anticancer potentials. Bot Mar 53:555–564
Chalkley DB, Suh SO, Volkmann-Kohlmeyer B, Kohlmeyer J, Zhou JJ (2010) Diatrypasimilis australiensis, a novel xylarialean fungus from mangrove. Mycologia 102:430–437
Chu Y, Alias SA, Rizman-Idid M, Guo SY, Pang KL (2015) A molecular reappraisal of Nimbospora (Halosphaeriaceae, Microascales) and a new genus Ebullia for N. octonae. Mycoscience 56:34–41
Da Luz CK, Barata M (2014) Salt marsh fungi. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 345–281
Debbab A, Aly A, Proksch P (2012) Endophytes and associated marine derived fungi: ecological and chemical perspectives. Fungal Divers 57(1):45–83
de Campos EL, Cavalcanti MAQ (2000) Primeira ocorrencia de Phellinus mangrovicus (Imaz.) Imaz. para o Brasil. Acta Bot Bras 14:263–265
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenwald JZ, Crous PW (2012) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 785:1–36
Duc PM, Hatai K, Kurata O, Tensha K, Uchida Y, Yaguchi T, Udagawa SI (2009) Fungal infection of mantis shrimp (Oratosquilla oratoria) caused by two anamorphic fungi found in Japan. Mycopathologia 167:229–247
Ebel R (2012) Natural products from marine-derived fungi. In: Jones EBG, Pang KL (eds) Marine Fungi and Fungal-Like Organisms. De Gruyter, Berlin, p 411–440
Ellis MB (1976) More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, UK
Eriksson OE (1999) Outline of Ascomycota 1999. Myconet 3:1–88
Fell JW (2014) Yeasts in marine environments. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 91–102
Fell JW, Boekhout T, Fonseca A, Sampaio JP (2001) Basidiomycetous yeasts. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) Mycota, systematics and evolution. Part VII. Springer-Verlag, Berlin Heidelberg, pp 3–35
Fell JW, Statzall-Tallman A, Kurtzman CP (2004) Lachancea meyersii sp. nov., an ascosporogenous yeast from mangrove regions in the Bahama Islands. Stud Mycol 50:359–363
Fell JW, Statzell-Tallman S, Scorzetti G, Gutiérrez MH (2011) Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Antonie Van Leeuwenhoek 99:533–549
Gaertner A (1980) Quantitative studies on the marine phycomycetes, chytrids and higher mycelial fungi of the upper Tay estuary. P Roy Soc Edinb B 78:57–78
Gaertner A (1982) Lower marine fungi from the Northwest African upwelling areas and from the Atlantic off Portugal. Meteor Forsch Ergebn 34:9–30, Reihe D
Garzoli L, Gnavi G, Poli A, Prigione V, Varese GC (2015) Marine fungi in the Mediterranean Sea, hidden biodiversity and taxonomical challenges. Second Inrterntaional Workshop Ascomycete Systemtatics, Abstract p24, CBS, Amsterdam
Gessner RV, Kohlmeyer J (1976) Geographical distribution and taxonomy of fungi from salt marsh Spartina. Can J Bot 54:2023–2037
Gilbert GS, Gorospe J, Ryvarden L (2008) Host and habitat preferences of polypore fungi in Micronesian tropical flooded forests. Mycol Res 112:674–680
Gilbert GS, Sousa WP (2002) Host specialization among wood-decay polypore fungi in a Caribbean mangrove forest. Biotropica 34:396–404
Gleason FH, Frithjof CK, Glockling SL (2012) Zoosporic true fungi. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 101–114
Gueidan C, Roux C, Lutzoni F (2007) Using a multigene analysis to assess generic delineation and character evolution in the Verrucariaceae (Eurotiomycetes, Ascomycota). Mycol Res 111:1147–1170
Gueidan C, Savic S, Thüs H, Roux C, Keller C, Tibell L, Prieto M, Heiðmarsson S, Breuss O, Orange A, Fröberg L, Wynns AA, Navarro-Rosinés P, Krzewicka B, Pykälä J, Grube M, Lutzoni F (2009) Generic classification of the Verrucariaceae (Ascomycota) based on molecular and morphological evidence: recent progress and remaining challenges. Taxon 58:184–208
Gueidan C, Thüs H, Pérez-Ortega S (2011) Phylogenetic position of the brown algae-associated lichenized fungus Verrucaria tavaresiae (Verrucariaceae). Bryologist 114:563–569
Hagler AN, De Olivera RB, Mendonca-Hagler LC (1982) Yeasts in the intertidal sediments of polluted estuary in Rio de Janeiro, Brazil. Antonie Van Leeuwenhoek 48:53–56
Hatai K (2012) Diseases of fish and shellfish caused by marine fungi. In: Raghukumar C (ed) Biology of marine fungi. Springer, Berlin, pp 15–52
Hattori T, Sakayaroj J, Jones EBG, Suetrong S, Preedanon S, Klaysuban A (2014) Three species of Fulvifomes (Basidiomycota, Hymenochaetales) associated with rots on mangrove tree Xylocarpus granatum in Thailand. Mycoscience 55:344–354
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth and Bisby’s Dictionary of the Fungi, 8th edn. CAB International, UK
Hodhod MS, Abdel-Wahab MA, Bahkali AH, Hyde KD (2012) Amergraphium solium sp. nov. from Yanbu mangroves in the Kingdom of Saudi Arabia. Crypto Mycol 33:285–294
Höhnk W (1958) Mykologische Notizen: 1. Mikropilze im Eis 5:193–194
Huhndorf SM (1994) Neotropical ascomycetes. 5. Hypsostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia 86:266–269
Huhndorf SM, Fernando AF, Miller AN, Lodge J (2003) Neotropical Ascomycetes 12. Mirannulata samuelsii gen. et sp. nov. and M. costaricensis sp. nov., new taxa from the Caribbean and elsewhere. Sydowia 55:172–180
Huhndorf SM, Miller AN, Fernández FA (2004) Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96:368–387
Hyde KD (1992) Julella avivenniae (Borse) comb. nov. (Thelenellaceae) from intertidal mangrove wood and miscellaneous fungi from the north east coast of Queensland. Mycol Res 95:939–942
Hyde KD, Borse BD (1986) Marine fungi from Seychelles V. Biatriospora marina gen. et sp. nov. from mangrove wood. Mycotaxon 26:263–270
Hyde KD, Jones EBG, Liu JK et al (2013) Families of dothideomycetes. Fungal Divers 63:1–313
Imazeki R (1941) Materials of the micronesian higher fungi. J Jap Bot 17:175–184
Inderbitzin P, Landvik S, Abdel-Wahab MA, Berbee ML (2001) Aliquandostipitaceae, A new family for two new tropical ascomycetes with unusually wide hyphae and dimorphic ascomata. Am J Bot 88:52–61
Jaklitsch WM, Réblová M (2015) Index Fungorum 209:1
James TY (2006) A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98:860–871
Janso JE, Bernan VS, Greenstein M, Bugni TS, Ireland CM (2005) Penicillium dravuni, a new marine-derived species from an alga in Fiji. Mycologia 97:444–453
Jebaraj CS, Raghukumar C, Behnke A, Stoeck T (2010) Fungal diversity in oxygen-depleted regions of the Arabian Sea revealed by targeted environmental sequencing combined with cultivation. FEMS Microbiol Ecol 71:399–412
Johnson RG, Jones EBG, Moss ST (1987) Taxonomic studies of the Halosphaeriaceae: Ceriosporopsis, Haligena and Appendichordella gen. nov. Can J Bot 65:931–942
Johnson TW, Sparrow FK (1961) Fungi in oceans and estuaries. J. Cramer, Germany
Jones EBG (1995) Ultrastructure and taxonomy of the aquatic ascomycetous order Halosphaeriales. Can J Bot 73:S790–S801
Jones EBG (2011) Are there more marine fungi to be described? Bot Mar 54:343–354
Jones EBG, Alias SA, Pang KL (2013) Distribution of marine fungi and fungus-like organisms in the South China Sea and their potential use in industry and pharmaceutical application. Malays J Sci 32:95–106
Jones EBG, Chatmala I, Klasuban A, Pang KL (2008) Ribosomal DNA phylogeny of marine anamorphic fungi: Cumulospora varia, Dendryphiella species and Orbimyces spectabilis. Raff Bull Zool Suppl 19:11–18
Jones EBG, Chatmala I, Pang KL (2006) Two new genera of the Halosphaeriaceae isolated from marine habitats in Thailand: Pseudoligninicola and Thalespora. Nova Hedwig 83:219–232
Jones EBG, Choeyklin R (2008) Ecology of marine and freshwater basidiomycetes. In: Boddy L, Frankland JC, van West P (eds) Ecology of saprotrophic basidiomycetes. Academic, London, pp 301–324
Jones EBG, Fell JW (2012) Basidiomycota. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 47–61
Jones EBG, Hyde KD, Read SJ, Moss ST, Alias SA (1996) Tirisporella gen. nov., an ascomycete from the mangrove palm Nypa fruticans. Can J Bot 74:1487–1495
Jones EBG, Moss ST, Cuomo V (1983) Spore appendage development in the lignicolous marine pyrenomycetes Chaetosphaeria chaetosa and Halosphaeria trullifera. Trans Br Mycol Soc 80:193–200
Jones EBG, Pang KL (eds) (2012) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston
Jones EBG, Sakayaroj J, Suetrong S, Somrithipol S, Pang KL (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–187
Jones EBG, Suetrong S, Cheng WH, Rungjindamai N, Sakayaroj J, Boonyuen N, Somrithipol S, Abdel-Whahab MA, Pang KL (2014) An additional fungal lineage in the Hypocreomycetidae (Falcocladium species) and the taxonomic re-evaluation of Chaetosphaeria chaetosa and Swampomyces species, based on morphology, ecology and phylogeny. Cryptog Mycol 35:119–138
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth and Bisby’s Dictionary of the Fungi, 8th edn. CABI Publishing, London
Kirk PM, Spooner BM (1984) An account of the fungi of Arran, Gigha and Kintyre. Kew Bull 38:503–597
Kirschner R, Pang KL, Jones EBG (2013) Two cheirosporous anamorphic fungi reassessed based on morphological and molecular examination. Mycol Progr 12:29–36
Koch J, Jones EBG (1989) The identity of Crinigera maritima and three new genera of marine cleistothecial ascomycetes. Can J Bot 67:1183–1197
Kohlmeyer J (1963) Fungi marini novi vel critici. Nova Hedw 6:297–329
Kohlmeyer J (1977) New genera and species of higher fungi from the deep sea (1615-5315m). Rev Mycol 41:189–206
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology. The higher fungi. Academic Press, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1989) Hawaiian marine fungi, including two new genera of Ascomycotina. Mycol Res 92:410–421
Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995) Fungi on Juncus roemerianus. 2. New dictyosporous ascomycetes. Bot Mar 38:165–174
Kurtzman CP, Fell JW, Boekhout T (2011a) Gene sequences analysis and other DNA-based methods for yeast species recognition. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 4th edn. Elsevier, Amsterdam, pp 293–307
Kurtzman CP, Fell JW, Boekhout T (2011b) The yeasts, a taxonomic study, vol 1–3, 5th edn. Elsevier, Amsterdam
Lai X, Cao L, Tan H, Fang S, Huang Y et al (2007) Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J 1:756–762
Le Calvez T, Burgaud G, Mahe S, Barbier G, Vandenkoornhuyse P (2009) Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microbiol 75:6415–6421
Lepelletier F, Karpov SA, Alacid E, Le Panse S, Bigeard E, Garcés E, Jeanthon C, Guillou L (2014a) Dinomyces arenysensis gen. et sp. nov. (Rhizophydiales, Dinomycetaceae fam. nov.), a chytrid infecting marine dinoflagellates. Protist 165:230–244
Lepelletier F, Karpov SA, Alacide E, Le Pansef S, Bigearda E, Garcése E, Jeanthona C, Guilloua L (2014b) Dinomyces arenysensis gen. et sp. nov. (Rhizophydiales, Dinomycetaceae fam. nov.), a chytrid infecting marine dinoflagellates. Protist 165:230–244
Limtong S, Kaewwichian R, Am-In S, Boonmark C, Jindamorakot S, Yongmanitchai W, Srisuk N, Kawasaki H, Nakase T (2010) Three anamorphic yeast species Candida sanitii sp. nov., Candida sekii sp. nov., and Candida suwanaritii, three novel yeasts in the Saturispora clade isolated in Thailand. FEMS Yeast Res 10:114–122
Limtong S, Yongmanitchai W, Kawasaki H, Seki T (2007) Candida thaimueangensis sp. nov., an anamorphic yeast species from estuarine water in a mangrove forest in Thailand. Int J Syst Evol Microbiol 57:650–653
Limtong S, Yongmanitchai W, Kawasaki H, Seki T (2008) Candida phangngensis sp. nov., an anamorphc yeast species in Yarrowia clade, isolated from water in mangrove forests in Phang-Nga Province, Thailand. Int J Syst Evol Microbiol 58:515–519
Liu JK, Jones EBG, Chukeatirote E, Bahkali AH, Hyde KD (2011a) Lignincola conchicola from palms with a key to the species of Lignincola. Mycotaxon 117:343–349
Liu JK, Phhokamsak R, Jones EBG, Zhang Y, Ko-Ko TW, Hu HL, Boonmee S, Doilom M, Chukeatirote E, Bahkali AH, Wang Y, Hyde KD (2011b) Astrosphaeriella is polyphyletic, with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers 51:135–154
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA et al (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Loilong A, Sakayaroj J, Rungjindamai N, Choeyklin R, Jones EBG (2012) Biodiversity of fungi on the palm Nypa fruticans. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 267–284
Loureiro STA, Cavalcanti MADQ, Neves RP, Passavante JZDO (2005) Yeasts isolated from sand and sea water in beaches of Olinda, Pernambuco state, Brazil. Braz J Microbiol 36:333–337
Maharachchikumbura SSN, Hyde KD, Jones EBG, et al. (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. In press
Mahdi LE, Statzell-Tallman A, Fell JW, Brown MV, Donachie SP (2008) Sympodiomycopsis lanaiensis sp. nov., a basidiomycetous yeast (Ustilaginomycotina: Microstromatales) from marine driftwood in Hawaii. FEMS Yeast Res 8:1357–1363
Manohar CS, Boekhout T, Muller WH, Stoeck T (2014) Tritirachium candoliense sp. nov., a novel basidiomycetous fungus isolated from the anoxic zone of the Arabian Sea. Fungal Biol 118:139–149
Mantle PG, Hawksworth DL, Pazoutová S, Collinson SJ, Rassing BR (2006) Amorosia littoralis gen. sp. nov., a new genus and species name for the scorpinoneand caffeine-producing hyphomycete from the littoral zone in The Bahamas. Mycol Res 110:1371–1378
Mwangi JG (2001) A new pest causing decline of mangrove forests in Kenya, Africa. (www.easternarc.org)
Nagahama T, Hamamoto M, Nakase T, Takaki Y, Horikoshi K (2003) Cryptococcus surugaensis sp. nov., a novel yeast species from sediment collected on the deep-sea floor of Suruga Bay. Int J Syst Evol Microbiol 53:2095–2098
Nagahama T, Nagano Y (2012) Cultured and uncultured fungal diversity in deep-sea environments. In: Raghukumar C (ed) Biology of marine fungi. Springer, Germany, pp 173–187
Nagano Y, Nagahama T, Hatada Y, Nunoura T, Takami H, Miyazaki J, Takai K, Horikoshi K (2010) Fungal diversity in dee-saea sediments – the presence of novel fungal groups. Fungal Ecol 3(4):316–325
Oren A, Gunde-Cimerman N (2012) Fungal life in the Dead Sea. In: Raghukumar C (ed) Biology of marine fungi. Springer, Germany, pp 89–114
Orsi W, Biddle JF, Edgcomb V (2013) Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS ONE 8:e56335
Overy DP, Bayman P, Kerr RG, Bills GF (2014) An assessment of natural product discovery from marine (sensu strictu) and marine-derived fungi. Mycology 5:145–167
Pang KL (2002) Systematics of the Halosphaeriales: which morphological characters are important? In: Hyde KD (ed) Fungi in marine environments. Fungal Diversity Press, Hong Kong, pp 35–57
Pang KL (2012) Phylogeny of the marine Sordariomycetes. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin/Boston, pp 35–47
Pang KL, Abdel-Wahab MA, El-Sharouney HM, Sivichai S, Jones EBG (2002) Jahnulales (Dothideomyces, Ascomycota) a new order of lignicolous freshwater ascomycetes. Mycol Res 106:1031–1042
Pang KL, Vrijmoed LLP, Goh TK, PlaingamN JEBG (2008) Fungal endophytes associated with Kandelia candel (Rhizophoraceae) in Mai Po nature reserve, Hong Kong. Bot Mar 51:171–178
Pang KL, Alias SA, Chiang MWL, Vrijmoed LLP, Jones EBG (2010) Sedecimiella taiwanensis gen. et sp. nov., a marine mangrove fungus in the Hypocreals (Hypocreomycetidae, Ascomycota). Bot Mar 53:493–498
Pang KL, Guo SY, Alias SA, Hafellner J, Jones EBG (2014) A new species of marine Dactylospora and its phylogenetic affinities within the Eurotiomycetes, Ascomycota. Bot Mar 57:315–321
Pang KL, Hyde KD, Alias SA, Suetrong S, Guo SY, Idid R, Jones EBG (2013) Dyfrolomycesillaceae, a new family in the Dothideomycetes, Ascomycota. Cryptog Mycol 34:223–232
Pang KL, Jheng JS (2012a) A checklist of marine fungi of Taiwan with a description of Kitesporella keeluingensis gen. et sp. nov. Bot Mar 55:459–466
Pang KL, Jheng JS (2012b) Pileomyces formosanus gen. et sp. nov. (Halosphaeriaceae, Ascomycota) from a rocky shore of Taiwan. Bot Stud 53:535–539
Pang KL, Jones EBG (2004) Reclassification of Halosarpheia and related genera with unfurling ascospore appendages. Nova Hedwigia 78:269–271
Pang KL, Vrijmoed LLP, Kong RYC, Jones EBG (2003) Lignincola and Nais, polyphyletic genera of the Halosphariales (Ascomycota). Mycol Prog 2:29–39
Pang KL, Jheng JS, Jones EBG (2011) Marine mangrove fungi of Taiwan. National Taiwan Ocean University Press. Keelung, Taiwan
Park MS, Fong JJ, Oh SY, Houbraken J, Sohn JH, Hong SB, Lim YW (2015) Penicillium jejuense sp. nov., isolated from the marine environments of Jeju Island, Korea. Mycologia 107:209–216
Paula CR, Purrchio A, Gambale W (1983) Yeasts from beaches in the southern area of São Paulo state. Braxil Rev Microbiol 14:136–143
Peršoh D (2015) Plant-associated fungal communities in the light of meta’omics. Fungal Divers. doi:10.1007/s13225-015-0334-9
Pérez-Ortega S, Spribille T, Palice Z, Elix JA, Printzen C (2010) A molecular phylogeny of the Lecanora varia group, including a new species from western North America. Mycol Progr 9:523–535
Pinruan U (2010) Endophytic and saprophytic palm fungi and their ability as antagonists against the oil palm pathogen, Ganoderma boninense. Ph.D. Thesis, Chiang Mai University, Thailand
Powell MJ, Letcher PM (2012) From zoospores to molecules: the evolution and systematics of Chytridiomycota. In: Misra JK, Tewari JP, Deshmukh SK (eds) Sysytematics and Evolution of Fungi. CRC Press, Boca Raton, pp 29–54
Powell MJ, Letcher PM, Chabers JG (2015) A new genus and family for the miscalassified chytrid, Rhizophlyctis harderi. Mycologia 107:419–431
Raghukumar C (ed) (2012) Biology of marine fungi. Springer, Berlin
Raghukumar C, Ravindram J (2012) Fungi and their role in corals and coral reef ecosystems. In: Raghukumar C (ed) Biology of marine fungi. Springer, Berlin, pp 89–113
Raghukumar S (1977) Studies on an estuarine isolate of Catenaria anguillulae (Phycomycetes) from India. Veröff. Inst Meeresforsch Bremerh 16:167–175
Rämä T, Norden J, Davey ML, Nathiassen GH, Soatafora JW, Kauserud H (2014) Fungi ahoy! Diversity on marine wooden substrata in the high North. Fungal Ecol 8:46–58
Rämä T, Baral H.O, Eriksson, O.E (2015) Morphological update and systematic placement of Calycina marina (Helotiales) comb. nov. Bot, Mar.(In press)
Richards TA, Jones MDM, Leonard G, Bass D (2012) Marine fungi: their ecology and molecular diversity. Annu Rev Mar Sci 4:495–522
Rungjindamai N, Sakayaroj J, Somrithipol S, Plaingam N, Jones EBG (2012) Phylogeny of the appendaged coelomycete genera: Pseudorobillarda, Robillarda, and Xepiculopsis based on nuclear ribosomal DNA sequences. Cryptog Mycolog 33:319–332
Sakayaroj J, Pang KL, Jones EBG (2011) Multi-gene phylogeny of the Halosphaeriaceae: its ordinal status, relationships between genera and morphological character evolution. Fungal Divers 46:87–109
Sakayaroj J, Pang KL, Jones EBG, Vrijmoed LLP, Abdel-Wahab MA (2005) A systematic reassessment of marine ascomycetes Swampomyces and Torpedospora. Bot Mar 48:395–406
Sakayaroj J, Preedanon S, Suetrong S, Klaysuban A, Jones EBG, Hattori T (2012) Molecular characterization of basidiomycetes associated with the decayed mangrove tree Xylocarpus granatum in Thailand. Fungal Divers 56:145–156
Senanayake IC, Maharachchikumbura SSN, Kevin D. Hyde KD et al. (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes) Fungal Diver. In press
Schell WA, Lee AG, Aime MC (2011) A new lineage in Pucciniomycotina: class Tritirachiomycetes, order Tritirachiales, family Tritirachiaceae. Mycologia 103:1331–1340
Schoch CL, Sung GH, López-Giráldez F (2009) The Ascomycota tree of life: a phylum–wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Schoch CL, Sung GH, Volkmann-Kohlmeyer B, Kohlmeyer J, Spatafora JW (2006) Marine fungal lineages in the Hypocreomycetidae. Mycol Res 110:257–263
Schulz B, Draeger S, dela Cruz TE, Rheinheimer J, Siems K, Loesgen S, Bitzer J, Schloerke O, Zeeck A, Kock I, Hussain H, Dai J, Krohn K (2008) Screening strategies for obtaining novel, biologically active, fungal secondary metabolites from marine habitats. Bot Mar 51:219–234
Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanová L, Padgett D, Porter D, Raja H, Schmit JP, Thornton H, Voglmayr H (2007) Fungal biodiversity in aquatic habitats. Biodivers Conserv 16:49–67
Simmons DR, James TY, Meyer AF, Longcore JE (2009) Lobulomycetales, a new order in the Chytridiomycota. Mycol Res 113:450–460
Singh P, Raghukumar C, Verma P, Shouche Y (2012) Fungal diversity in deep-sea sediments revealed by culture-dependent and culture-independent approaches. Fungal Ecol 5:543–553
Sridhar KR, Karamchand KS, Pascoal C, Cássio F (2012) Assemblage and diversity of fungi on wood and seaweed litter of seven Norwest Portuguese beaches. In: Raghukumar C (ed) Biology of marine fungi. Springer, Berlin, pp 209–228
Stanley SJ (1991) The autecology and ultrastructure interaction between Mycosphaerella ascophylli Cotton, Lautitia danica (Berlese) Schatz, Mycaureola dilsea Maire et Chemin and their respective marine algal hosts. PhD Thesis, University of Portsmouth, UK
Stanley SJ (1992) Observations on the seasonal occurrence of marine endophytic and parasitic fungi. Can J Bot 70:2089–2096
Statzell-Tallman A, Belloch C, Fell JW (2008) Kwoniella mangroviensis gen. nov., sp. nov. (Tremellales, Basidiomycota), a teleomorphic yeast from mangrove habitats in the Florida Everglades and Bahamas. FEMS Yeast Res 8:103–113
Statzell-Tallman A, Scorzetti G, Fell JW (2010) Candida spencermartinsiae sp. nov., Candida taylorii sp. nov. and Pseudozyma abaconensis sp. nov., novel yeasts from mangrove and coral reef ecosystems. Int J Syst Evol Microbiol 60:1978–1984
Suetrong S, Boonyuen N, Pang KL, Ueapattanakit J, Klaysuban A, Sri-Indrasutdhi V, Sivichai S, Jones EBG (2011a) A taxonomic revision and phylogenetic reconstruction of the Jahnulales (Dothideomycetes), and the new family Manglicolaceae. Fungal Divers 51:163–188
Suetrong S, Hyde KD, Zhang Y, Bahkali AH, Jones EBG (2011b) Trematosphaeiaceae fam. nov. (Dothideomycetes, Ascomycota). Cryptog Mycolog 332:343–358
Suetrong S, Sakayaroj J, Phongpaichit S, Jones EBG (2010) Morphological and molecular characteristics of a poorly known marine ascomycete, Manglicola guatemalensis. Mycologia 102:83–92
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Suetrong S, Klaysuban A, Sakayaroj J, Preedanon S, Rung-areerate P, Phongpaichit S, Jones EBG (2015a) Tirisporellaceae, a new family in the order Diaporthales (Sordariomycetes, Ascomycota). Boot. Mar. (In press)
Suetrong S, Rung-areerates P, Nor NABM, Alias SA, Jones EBG, Pang KL (2015b) Bacusphaeria nypenthi gen. et sp. nov. from the mangrove palm Nypa fruticans. Phytotaxa (In Press)
Sutherland GK (1914) New marine pyrenomycetes. Trans Br Mycol Soc 5:147–155
Thongkantha S, Jeewon R, Vijaykrishna D, Lumyong S, McKenzie EHC, Hyde KD (2009) Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species, Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Divers 34:157–173
Toro MA, Piontelli LE (1985) Yeast communities in sandy soils a beach of V Region, Chile II. Bol Micologico 2:109–118
Trisuwan K, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Preedanon S, Sakayaroj J (2009) Lactone derivatives from the marine‐derived fungus Penicillium sp. PSU‐F44. Chem Pharm Bull 57:1100–1102
Uebelmesser E-R (1956) Über einige neue chytridineenaus erdboden (Olpidium, Rhizophidium, Phlyctochytrium und Rhizophlyctis). Arch Mikrobiol 25:307–324
Vogel C, Rogerson A, Schatz S, Laubach H, Tallman A, Fell JW (2007) Prevalence of yeasts in beach sand at three bathing beaches in South Florida. Water Res 41:1915–1920
Vrijmoed LLP (2000) Isolation and culture of higher filamentous fungi. In: Hyde KD, Poimting SB (eds) Marine mycology- A practical approach. Fungal Divers Res Ser 1, Fungal Diversity Press, Honbg Kong, pp 1–20
Wang QM, Bai FY (2008) Molecular phylogeny of basidiomycetous yeasts in the Cryptococcus luteolus lineage (Tremellales) based on nuclear rRNA and mitochondrial cytochrome b gene sequence analyses: proposal of Derxomyces gen. nov. and Hannaella gen. nov., and description of eight. FEMS Yeast Res 8:799–814
Webber FC (1959) Marine fungi. PhD Thesis, University of Wales
Webber FC (1967) Observations on the structre, life history and biology of Mycosphaerella ascophylli. Trans Br Mycol Soc 50:583–601
Wei YH, Liou GY, Liu HY, Lee FL (2011) Sympodiomycopsis lanaiensis as Jaminaea lanaiensis comb. nov. Int J Syst Evol Microbiol 61:469–473
Wilson IM (1951) Notes on some marine fungi. Trans Br Mycol Soc 34:540–543
Wilson IM (1956) Some new marine Pyrenomycetes on wood and rope: halophiobolus and Lindra. Trans Br Mycol Soc 39:401–415
Wilson IM, Knoyle JM (1961) Three species of Didymosphaeria on marine algae: D. danica (Berlese) comb. nov., D. pelvetiana Suth. and D. fucicola Suth. Trans Br Mycol Soc 44:55–71
Wijayawardene NN, Crous PW, Kirk PM et al (2014) Naming and outline of Dothideomycetes (2014) including proposals for the protection or suppression of generic names. Fungal Divers 69(1):1–55
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212
Yu Z, Zhang B, Sun W, Fengli Zhang F, Li Z (2013) Phylogenetically diverse endozoic fungi in the South China Sea sponges and their potential in synthesizing bioactive natural products suggested by PKS gene and cytotoxic activity analysis. Fungal Divers 58:127–141. doi:10.1007/s13225-012-0192-7
Yusoff M, Koch J, Jones EBG, Moss ST (1993) Ultrastructural observations on a marine lignicolous ascomycete Bovicornua intricata gen. et sp. nov. Can J Bot 71:346–352
Zhang H, Hyde KD, Abdel–Wahab MA, Abdel–Aziz FA, Ariyawansa HA, KoKo TW, Zhao RL, Alias SA, Bahkali AH, Zhou DQ (2013a) A modern concept for Helicascus with a Pleurophomopsis-like asexual state. Sydowia 65:147–166
Zhang Y, Fournier J, Phookamsak R, Bahkali AH, Hyde KD (2013b) Halotthiaceae fam. nov. (Pleosporales) accommodates the new genus Phaeoseptum and several other aquatic genera. Mycologia 105:603–609
Zuccaro A, Schulz B, Mitchell JI (2003) Molecular detection of ascomycetes associated with Fucus serratus. Mycol Res 107:1451–1466
Zuccaro A, Summerbell RC, Gams W, Schroers HF, Mitchell JI (2004) A new Acremonium species associated with Fucus spp., and its affinity with a phylogenetically distinct marine Emericellopsis clade. Stud Mycol 50:283–297
Acknowledgments
Gareth Jones is supported by the Distinguished Scientist Fellowship Program (DSFP), King Saud University. This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-BIO2840-02.K.L). Pang would like to thank Ministry of Science and Technology, Taiwan for financial support (NSC101-2621-B-019-001-MY3). T. Boekhout is supported by a grant NPRP6-647-1-127 from the Qatar National Research Fund (a member of the Qatar Foundation). This research was also financially supported by The Biodiversity and Training Program (BRT R_251006, BRT R_351004, BRT_R352015) and National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand. We thank Drs. Holger Thüs and Patrick M. McCarthy for their comments on what constitutes littoral/intertidal lichens; Drs. Joyce Longcore, D. Reham Simmons, Serygey K. Karpov, Frank Gleason for their critical comments on marine chytrids; Drs. Teppo Rämä, Hans-Otto Baral and Ove E. Eriksson for allowing us to refer to their publication on Orbilia marina. Jack Fell for useful comments on marine yeasts; Rhiannon Owen for assistance with theses held at the Hugh Lloyd Library, University of Aberystwyth, Wales.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, E.B.G., Suetrong, S., Sakayaroj, J. et al. Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Diversity 73, 1–72 (2015). https://doi.org/10.1007/s13225-015-0339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-015-0339-4