Abstract
Genera assigned to the Jahnulales are morphologically diverse, especially in ascospores equipped with or without appendages, sheaths or apical caps. They are predominantly freshwater fungi occurring on woody substrata, with Manglicola guatemalensis, Xylomyces chlamydosporus and X. rhizophorae the only species known from marine habitats. The order Jahnulales with 4 teleomorphic genera: Jahnula (15 species), Aliquandostipite (5), Megalohypha (1), Manglicola (2) and the anamorphic genera Brachiosphaera (2), Speiropsis (9), Xylomyces (8), amounting to a total of 42 species, is reviewed and nomenclatural changes are proposed. Twenty species are treated at the molecular level, with 94 sequences, 13 of which are newly generated for this review. Three species are rejected (Speiropsis irregularis, Xylomyces aquaticus, X. elegans) while the phylogenetic placement of 6 Xylomyces, 7 Speiropsis, 1 Brachiosphaera and 1 Manglicola require molecular data to confirm their placement in the order. Sequences are derived from ex-holotype isolates and new collections made in Thailand. Most taxa are included in the family Aliquandostipitaceae and a new family Manglicolaceae is erected for the marine ascomycete Manglicola guatemalensis with its large ascomata (1,100–1,750 × 290–640 μm), wide ostioles and ascospores that are fusiform, unequally one-septate with the apical cell larger than the turbinate basal cell and bear apical gelatinous appendages. The genus Jahnula is polyphyletic grouping in three clades with J. aquatica, J. granulosa, J. rostrata, J. potamophila and Megalohypha aqua-dulces in the Jahnula sensu stricto clade. No taxonomical changes are proposed for Jahnula species not grouping in the Jahnula sensu stricto clade, until further species are isolated and sequenced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Jahnulales was proposed by Pang et al. (2002) to accommodate ascomycetes with stalked/sessile and dimorphic ascomata, hyphal stalk cells that are circa 40 μm wide, and ascospores that are unequally 2-celled with or without various types of appendages or sheaths. Pang et al. (2002) presented molecular data for six species: Aliquandostipite sunyatsenii (later transfered to Jahnula), A. khaoyaiensis, Jahnula bipolaris, J. australiensis, J. siamensiae (a new species), and Patescospora separans (a new genus and species) and referred them to the family Aliquandostipitaceae. All taxa assigned to the order were freshwater species, mostly growing on submerged wood, and lacking anamorphs.
The order was based on the genus Jahnula with J. aquatica as the type species (Kirschstein 1936), however, Wegelin (1894) described Amphisphaeria helevetica which may be an earlier epithet and this is discussed later in the paper. Hawksworth (1984) did not refer to this earlier epithet but commented on the diagnostic structure of the ascomatal wall with the “massive pseudoparenchymatous cells up to 30 μm wide”. The concept of the genus has changed with the description of new species to include emphasis on the ascomatal wall structure, stalk, ascal morphology and the variety of caps, sheaths and appendages to the ascospores, as well as the wide hyphae now known to occur in most of the genera in the order. Campbell et al. (2007) emended the ordinal description to include wide, brown hyphae and a wider variation in ascospore characters, not known when the Jahnulales was proposed.
Subsequently, other genera and species were referred to the order: Jahnula (Hyde 1993; Pinruan et al. 2002; Raja and Shearer 2006; Raja et al. 2008; Sivichai and Boonyuen 2010), Aliquandostipite (Raja et al. 2005), and Megalohypha all known from freshwater habitats. Initially, no anamorphs were known until Campbell et al. (2007) and Prihatini et al. (2008) showed that the anamorphic fungi Speiropsis pedatospora and Xylomyces chlamydosporus, had a phylogenetic affinity with the Jahnulales. Subsequently, Sivichai et al. (2011) showed that X. chlamydosporus was the anamorph of Jahnula aquatica. No marine members of the Jahnulales were known until Suetrong et al. (2009, 2010) demonstrated that the marine ascomycete Manglicola (M. guatemalensis) also belonged in the order.
Molecular studies suggest that the genus Jahnula is polyphyletic (Campbell et al. 2007; Shearer et al. 2009; Suetrong et al. 2009) with the type species J. aquatica grouping with J. granulosa, J. rostrata, J. potamophila and Megalohypha aqua-dulces. Other Jahnula species are distally placed and may constitute more than one genus, with Shearer et al. (2009) suggesting 4 or 5 separate lineages and advocating further molecular studies to resolve their phylogenetic relationship. Currently, one family has been described, Aliquandostipitaceae (Inderbitzin et al. 2001) based on the genus Aliquandostipite (type species A. khaoyaiensis), to which most other genera have been referred. However, the taxonomic position of the genus Manglicola warrants further study as to its familial position (Suetrong et al. 2010).
The objective of this contribution is to determine the familial status of the genera assigned to the order Jahnulales.
Materials and methods
Sample sources
Collection, incubation and examination of freshwater and marine members of this group are well established (Vrijmoed 2000). To obtain single spore cultures ascospores were removed from ascomata with sterile forceps and placed in sterile sea water. Small drops of this spore suspension were placed on GYA (10 g glucose, 1 g yeast extract, 18 g agar, 0.5 g chloromephenicol in 1 L sea water) Petri dishes and incubated at 25°C in the dark. Germinated spores were transferred to new GYA/PDA Petri dishes and incubated at 25°C in the dark. Sporulating material was mounted in sea water for all measurements and photography. Voucher slides and type material of the new fungi have been deposited at BIOTEC Bangkok Herbarium (BBH), with cultures deposited in international culture collections and sequences in GenBank (Shearer et al. 2009; Suetrong et al. 2009, 2010). Species used in this study, their isolate numbers, sources and GenBank accession numbers are listed in Supplementary Table 1 for members of the Jahnulales. All isolates for the generation of new sequences were obtained from BCC, all new sequences are in bold and ex-holotype sequences carry the prefix *. Supplementary Table 2 lists representative sequences from selected orders of the Dothideomycetes with Roccella fuciformis and Schismatomma decolorans as the outgroup taxa.
DNA extraction, amplification and sequencing
Fungi were grown in potato dextrose broth with seawater, and or, freshwater at a temperature of 25°C for 14 days or until enough mycelium for DNA extraction obtained. Fungal biomass was harvested for a different set of isolates by filtering through cheesecloth, and washed several times with sterile distilled water. The harvested mycelium was stored at −20°C and ground to a fine powder with a mortar and pestle. Fifty to 100 mg ground fungal mycelium was placed into 400 ml lysis buffer (O’Donnell et al. 1997) and DNA extracted as follows: the tube was incubated at 70°C for 30 min, and an equal volume of phenol-chloroform (PIERCE) added. The upper liquid phase was transferred to a new microtube containing chilled absolute ethanol and 7.5 M ammonium acetate. The mixture was kept at −20°C for 30 min, or until the DNA had precipitated, and then centrifuged at 14,000 rpm, 4°C, for 15 min. The DNA pellet was washed twice with chilled 75% ethanol and air dried.
Sequence alignment and phylogenetic analyses
Sequence data were generated from four loci: partial nuclear SSU rDNA and LSU rDNA, 5.8S rDNA and the translation elongation factor 1-alpha (TEF-1-alpha), using the primers NS1, NS3, NS4 and NS6 for SSU rDNA (White et al. 1990), JS1, JS8, LR7 and LROR for LSU rDNA (Bunyard et al. 1994; Landvik 1996), ITS1 and ITS4 for 5.8S rDNA (White et al. 1990), and 983F, 2218R, CEFR2, CEFF2, 1577F and 1567R (Rehner 2001). DNA sequencing was performed using the primers mentioned above in an Applied Biosystem 3730XL DNA Analyzer at Macrogen, Inc in Korea. Each sequence was checked for ambiguous bases and assembled using BioEdit 6.0.7 (Hall 2004). Sequence homologies were also analysed using the BLAST search engine at the National Center for Biotechnology Information (NCBI) to facilitate the selection of other fungal sequences to be used in the analyses. Sequences with the highest alignment score were selected for phylogenetic analyses. Alignments were checked and manually optimised along with other sequences obtained from the GenBank nucleotide database. The consensus sequences for each DNA region were initially aligned with ClustalW v. 1.6 (Thompson et al. 1994) and improved in MUSCLE (Edgar 2004).
Manual gap adjustments were made to improve the alignment. Ambiguously aligned regions were excluded. Missing data at the 5′-and 3′-end of partial sequences were coded by ‘?’. The final alignment was again optimised by eye and manually corrected using Se-Al v. 2.0a8 (Rambaut 1996). The tree construction procedure was performed in PAUP* 4.0b10 (Swofford 2002) on Window versions and a Power Macintosh G4 (Apple Computer, Inc., Cupertio, California, USA). Phylogenetic trees were visualized using the program Treeview (Page 1996). The phylogenetic analyses of different datasets were performed using maximum parsimony, Bayesian and maximum likelihood algorithms.
-
i)
Maximum parsimony analyses were performed using PAUP v. 4.0b10 (Swofford 2002), with gaps treated as missing data. Trees were generated using 100 replicates of random stepwise addition of sequence and tree-bisection reconnection (TBR) branch-swapping algorithm, with all characters given equal weight. Branch support for all parsimony analyses was estimated by performing 1,000 bootstrap replicates (Felsenstein 1985) with a heuristic search of 10 random-addition replicates for each bootstrap replicate. The consistency indices (CI; Kluge and Farris 1969), retention indices (RI; Farris 1989) and rescaled consistency indices (RC; Farris 1989) were calculated for each tree generated. Tree topologies from parsimony analyses were tested with the Kashino-Hasegawa (K-H) maximum likelihood test (Kishino and Hasegawa 1989) to find the most likely tree.
-
ii)
Bayesian analyses: The model of substitution used for Bayesian analyses was chosen using the program Mrmodeltest 2.2 (Nylander 2004). Independent Bayesian phylogenetic analysis was performed in MrBayes 3.0b4 (Huelsenbeck and Ronquist 2001) using a uniform [GTR+I+G] model, Iset nst = 6 rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). Four Markov chains were run from random starting tree for 2,000,000 generations and sampled every 100 generations. The first 2,000 trees, which represented the burn-in phase of the analysis, were discarded, with 18,000 trees used for calculating posterior probabilities (BYPP) in the consensus tree. Posterior probabilities were obtained for each clade. Confident branch support is defined as Bayesian posterior probabilities equal or more than 0.95.
-
iii)
Maximum likelihood analyses (ML) were conducted in RAxML v. 7.2.2 (Stamatakis 2006). The dataset was partitioned according to each gene and separated codons (two partitions). A general time reversible (GTR+I+G model) plus invariant sites plus gamma distributed model A tree was obtained by simultaneously running a fast bootstrap search of 1,000 pseudoreplicates followed by a search for the most likely tree under functional setting “a”. Maximum likelihood bootstrap value (BSML) equal or greater than 50% are given above each node.
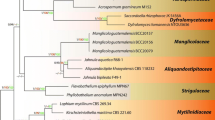
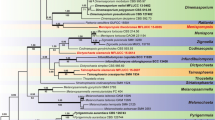
Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap value greater than 50% are given above the node. Bayesian posterior probabilities greater than 0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap proportions (100%) and posterior probabilities (1.00) are shown as a thicker line.
Taxonomy
The order includes 42 species in seven genera (Aliquandostipite, Brachiosphaera, Jahnula, Manglicola, Megalohypha, Speiropsis, Xylomyces) and 20 species are treated in the molecular study with 94 sequences, 13 of which are newly generated for this review. Sequences are derived from ex-holotype isolates and new collections made in Thailand. Most species group in the Aliquandostipitaceae, while a new family is proposed for Manglicola guatemalensis, based on morphological and molecular evidence. Excluded taxa from the order are: Speiropsis irregularis (insertae sedis), Xylomyces aquatica and X. elegans (Pleosporales). Placement of M. guatemalensis in the Hypsostromataceae is rejected. Molecular data are required to confirm the position of 5 Xylomyces, 7 Speiropsis, 1 Manglicola and 1 Brachiosphaera species in the order. Although many Jahnula species do not group in the Jahnula sensu stricto group, no taxonomical changes are proposed at this stage. This is considered premature until other Jahnula species are isolated and sequenced, while others await description (Abdel-Wahab, pers. com.).
Jahnulales K.L. Pang, Abdel-Wahab, El-Sharouney, E.B.G. Jones & Sivichai, Mycol. Res. 106: 1033 (2002).
emend Campbell et al. (2007)
Ascomata globose to subglobose, with a long, wide, brown, septate stalk or sessile, attached to substratum by wide, brown hyphae, immersed or superficial, ostiolate, papillate, coriaceous to sub-carbonaceous, hyaline, pale brown or black. Peridium thick, comprising a few layers of relatively large cells. Hamathecium pseudoparaphyses, hypha-like, filamentous, septate, unbranched between the asci, branching and anastomosing above the asci, persistent. Asci ovoid, cymbiform, saccate, clavate or cylindrical, thick-walled, fissitunicate, persistent or deliquescing, with an apical apparatus. Ascospores ellipsoid-fusiform, 1-septate, becoming 3–4 septate, apical cell slightly larger, hyaline or brown, slightly constricted at the septum, with or without a mucilaginous sheath, gelatinous pads, apical cellular appendages or elongate gelatinous apical appendages. Hyphae in culture wide, brown, septate, not constricted to strongly constricted at the septa. Anamorphs in the genera Brachiosphaera, Speiropsis and Xylomyces.
Order in the Pleosporomycetidae, Dothideomycetes.
Type species: Jahnula aquatica (Plöttner & Kirschst.) Kirschst., Annla. Mycol. 34: 196 (1936).
Family: Aliquandostipitaceae Inderb., Am. J. Bot. 88: 54 (2001).
Genera: Aliquandostipite (5 species), Jahnula (15), Manglicola (2), Megalohypha (1) (teleomorphs), Brachiosphaera (2), Speiropsis (9), Xylomyces (8) (anamorphs).
Aliquandostipitaceae Inderb.
Am. J. Bot. 88: 54 (2001).
Ascomata immersed-erumpent or superficial. Hamathecium comprising pseudoparaphyses. Asci fissitunicate. Mycelium visible on the substratum, comprising up to 50 μm, wide hyphae, which may bear ascomata. Ascospores 1-septate, pale brown, guttulate.
Holotype species: A. khaoyaiensis Inderb.
Jahnula Kirschst. Ann. Mycol. 34: 196 (1936).
= Ascagilis K.D. Hyde Aust. Syst. Bot. 5: 109 (1992).
Ascomata immersed, semi-immersed or erumpent, globose to subglobose, black, coriaceous, ostiolate, short-papillate, easily detached from substratum, solitary or gregarious. Peridium comprising 3–5 layers of relatively large, thin-walled, brown or hyaline angular cells. Hamathecium pseudoparaphyses wide (2–4 μm), hypha-like, filamentous, hyaline, septate, unbranched between the asci, branching and anastomosing above the asci. Asci 8-spored, cylindrical or obclavate, pedicellate, thick-walled, fissitunicate, with an ocular chamber and faint ring. Ascospores uniseriate or 3-seriate, 1-septate, pale to dark brown, guttulate, ellipsoid-fusiform, smooth or verruculose, constricted at the septum, some with a mucilaginous appendage, pads or sheaths.
Type species: J. aquatica (Plöttner & Kirschst.) Kirschst.
Jahnula aquatica (Plöttner & Kirschst.) Kirschst., Ann. Mycol. 34: 196 (1936).
≡ Amphisphaeria aquatica Plöttner & Kirschst., Verh. Bot. ver. Prov. Brandenb. 48: 52 (1906).
≡ Melanopsamma aquatica (Plöttner & Kirschst.) Kirschst., Krypt.-Fl. Brandenburg 7: 226 (1911).
Ascomata arising singly or in small groups, superficial, attached to the substratum by subiculum-like hyphae, subglobose to broadly obpyriform, the base often slightly immersed, mainly 250–400(−500) μm diam, black. Ostiole papillate, scarcely projecting above the substrate. Peridium not membranous, unchanged in potassium hydroxide, variable in thickness, mainly 30–80 μm thick, often thickest in the upper parts, composed of several layers of reddish brown subglobose to polyhedral pseudoparenchymatous cells (textura angularis), variable in size, the outermost 20–30 μm diam with slightly verruculose walls, the inner compressed, paler and mainly 15–20 μm long and 4–8 μm wide; base of the peridium continuous, similar to the sides. Periphyses absent. Hamathecium consisting of trabeculate pseudoparaphyses, persistent, filiform, branched and anastomosing, rarely septate, 1.5–2.5 μm thick, centrum not reacting with iodine. Asci arising from the base of the ascomatal cavity, cylindrical, stalked, thick-walled, fissitunicate, with an internal apical cone when young, when mature with a short apical cylinder or broad rectangular indentation, possibly with some annular apical apparatus, IKI, 180–220 × 15–18 μm, Ascospores with 8 uniseriate, slightly overlapping ascospores, ellipsoid to very broadly fusiform, slightly tapering towards the apices, 1-septate, slightly constricted or scarcely constricted at the septum, reddish brown, moderately thick-walled, generally with numerous small guttules, smooth-walled, without a distinct gelatinous sheath when mature, (30–)32–38(−41) × 11–16 μm. Based on a description by Kirschstein (1936).
Note: Dr. Walter Jaklitsch has drawn our attention to a possible earlier name for Jahnula: Amphisphaeria helvetica which was collected on pine wood, Bischofszell and Heimicswyl, Switzerland (Wegelin 1894). This provided a short description of the species with cylindrical asci and ascospore dimensions of 33–40 × 12.5–15 μm which overlap with those of J. aquatica. In A. helvetica the apical cell is slightly larger than the basal one. Wegelin (1894) also described Amphisphaeria dolioloides with ascospores measuring 34–39 × 14–15 μm but with clavate asci. Scheinpflug (1958) referred A. helvetica as the basionym of Otthia helvetica, a species in the Botryosphaeriaceae, which is distantly related to the Jahnulales. Since no type material of A. helvetica was available, the relationship between it and J. aquatica cannot be resolved. As currently circumscribed, we take J. aquatica as the type of the genus and order, as followed by Hawksworth (1984) and Hyde and Wong (1999).
Anamorph: Xylomyces chlamydosporus Goos, R.D. Brooks & Lamore, Mycologia 69: 282 (1977).
Cultures: Colonies on CMA slow growing, dark brown to black, effuse, hyphae thick-walled, septate and constricted at the septa, chlamydospores developing in culture, fusiform, intercalary, straight or curved, solitary or in chains, occasionally branched, with thickened septa, constricted at the septa, dark brown to blackish, end cells paler.
Sequence data: See Supplementary Table 1.
Material examined: Thailand, Trat province, Mu Ko Chang National Park, Khlong Phlu Waterfall, on submerged wood, 3 April 2006, S. Sivichai & V. Sri-indrasutdhi, BIOTEC SS3895.
Habitat and host range: Submerged wood in freshwater streams.
Geographical distribution: Germany, Hong Kong, South Africa, Thailand, USA.
Phylogenetic study: Molecular data suggest that the genus is polyphyletic with Jahnula sensu stricto including the species J. aquatica (type species), J. granulosa, J. potamophila, J. rostrata and Megalohypha aqua-dulces in a clade with low bootstrap support (Campbell et al. 2007). Other Jahnula species group in a separate clade and may constitute other genera (Campbell et al. 2007; Suetrong et al. 2010).
Concluding remarks: Sivichai et al. (2011) demonstrated by culture techniques that the anamorph of J. aquatica was Xylomyces chlamydosporus, and frequently reported it from freshwater substrates. However, the molecular data do not support this connection (Campbell et al. 2007; current results) and reexamination of the different isolates used in the analysis is required.
Accepted species:
J. apiospora A. Carter, Raja & Shearer, Mycoscience 49: 326 (2008).
*J. appendiculata Pinruan, K.D Hyde & E.B.G. Jones, Sydowia 54: 243 (2002).
*J. aquatica (Plöttner & Kirschst.) Kirschst., Ann. Mycol. 34: 196 (1936).
*J. australiensis K.D. Hyde, Aust. Syst. Bot. 6: 161 (1993).
*J. bipileata Raja & Shearer, Mycologia 98: 321 (2006).
*J. bipolaris (K.D. Hyde) K.D. Hyde, Nova Hedw. 68: 494 (1999).
*J. granulosa K.D. Hyde & S.W. Wong, Nova Hedw. 68: 497 (1999).
J. morakotii Sivichai & Boonyuen, Mycotaxon 112: 476 (2010).
*J. potamophila K.D. Hyde & S.W. Wong, Nova Hedw. 68: 499 (1999).
J. poonythi K.D. Hyde & S.W. Wong, Nova Hedw. 68: 499 (1999).
*J. rostrata Raja & Shearer, Mycologia 98: 325 (2006).
*J. sangamonensis Shearer & Raja, Mycologia 98: 327 (2006).
*J. seychellensis K.D. Hyde & S.W. Wong, Nova Hedw. 68: 504 (1999).
*J. sunyatsenii (Inderb.) K.L. Pang, E.B.G. Jones & Sivichai, Amer. J. Bot. 88: 57 (2001).
J. systyla K.D. Hyde & S.W. Wong, Nova Hedw. 68: 499 (1999).
15 species, * sequenced species.
Jahnula apiospora A. Carter, Raja & Shearer, Mycoscience 49: 326 (2008).
Ascomata scattered, superficial to partially immersed in wood, attached to the wood by broad, brown, superficial, stoloniferous hyphae (growing on the wood surface and linking up between the ascomata), membranous, globose to subglobose, 250–305 × 300–360 μm, black, ostiolate; ostiole circular, depressed. Peridium 40–45 μm wide, composed of textura angularis in surface view; in longitudinal section 6–10 cell layers wide, composed of an outer layer of thick-walled cells 30–33 × 15–19 μm, occluded by black, amorphous material along the upper two-thirds of the ascomata; inner layer of moderately thick-walled, large, brown, isodiametric to angular cells, 12–26 × 5–10 μm. Hamathecium consisting of hyaline, trabeculate, pseudoparaphyses, narrow, branched, and anastomosing above the asci, embedded in a gelatinous matrix. Asci 108–140 × 14–22 μm, basal, cylindrical to narrowly fusoid, pedicellate, fissitunicate, endoascus 18 μm wide, extending to 160–190 μm in length, with eight overlapping uniseriate to biseriate ascospores. Ascospores 30–40 × 8–12 μm, 7–9 μm wide at the septum, fusiform, slightly constricted at the septum, apiculate, unequally 1-septate; septum submedian (0.44–0.64), apical cell 18–27 μm long, basal cell shorter than apical cell, 11–16 μm long, slightly curved, hyaline when young, brown at maturity, multiguttulate, smooth-walled, lacking a gelatinous sheath and appendages. Based on the description by Raja et al. (2008).
Anamorph: None known.
Culture: DAOM 239555 (ex-holotype).
Sequence data: None.
Habitat and host range: Decorticated wood.
Geographical distribution: Canada.
Notes: Raja et al. (2008) considered it similar to J. aquatica, but differs in the shape of the ascomata, and narrower ascospores in J. apiospora.
Jahnula appendiculata Pinruan, K.D. Hyde & E.B.G. Jones, Sydowia 54: 243 (2002).
Ascomata 305–325 μm diam, semi-immersed, becoming erumpent, globose to subglobose, hyaline to pale straw-coloured, membranous, ostiolate, short papillate, solitary, covered by short hyaline setae up to 80 μm long, with a hyaline stalk-like strand attached to the base. Peridium ca. 36 μm wide, comprising 4–6 rows of large angular cells with hyaline cell walls. Pseudoparaphyses hypha-like, septate, unbranched between asci, branching and anastomosing above. Asci 360–410 × 41–43 μm, 8-spored, cylindrical to cylindric-clavata, fissitunicate with shallow ocular chamber and faint ring. Ascospores 47–55 × 23–27 μm, ellipsoid-fusiform, brown, 1-septate, slightly constricted at the septum, wall ornamentation minutely verrucose, surrounded by a mucilaginous sheaths and cellular appendages apically. Based on a description by Pinruan et al. (2002) and further studies.
Anamorph: None known.
Cultures: BCC11400 (ex-holotype), BCC11445, SS2448, SS2900.
Sequence data: See Supplementary Table 1.
Habitat and host range: Submerged trunk of the palm Licuala longecalycata.
Geographical distribution: Thailand.
Notes: This fungus differs from all other Jahnula species in having ascospores with a sheath and bipolar appendages. The only other species having appendages is J. morakotii, which has cap-like appendages and not as long as in J. appendiculata.
Jahnula australiensis K.D. Hyde, Aust. Syst. Bot. 6: 161 (1993).
Ascomata 180–250 μm high, 120–180 μm diam, immersed, erumpent, shedding wood particles and becoming superficial with the base remaining immersed, subglobose or obpyriform, brown above, subhyaline below, coriaceous, ostiolate, short-papillate, solitary or gregarious, associated with algae. Peridium comprising a few layers of relatively large, thin-walled, brown angular cells. Hamathecium consisting of hypha-like pseudoparaphyses up to 3.5 μm wide, filamentous, hyaline, septate, unbranched between the asci, branching and anastomosing above. Asci 90–140 × 14–18 μm, 8-spored, obclavate, pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 19–30 × 6–8 μm, 2-3-seriate, 1-septate, light brown, guttulate, irregularly fusiform, apical cells wider and tapering to a rounded tip, base narrower and more rounded, minutely verruculose, constricted at the septa. Based on a description by Hyde (1993) and further studies in Thailand.
Anamorph: None known.
Culture: BCC12789.
Sequence data: See Supplementary Table 1.
Habitat and host range: On submerged wood.
Geographical distribution: Australia, Thailand.
Material examined: Thailand, Khao Soi Dao Wildlife Sanctuary, 26 March 2000, S. Sivichai & N. Boonyuen, SS665.
Notes: It differs from J. aquatica in possessing smaller ascomata, asci and ascospores.
Jahnula bipileata Raja & Shearer, Mycologia 98: 321 (2006).
Ascomata on wood 395–400 × 200–205 μm, black translucent, membranous, subglobose to obpyriform, ostiolate, superficial with partially immersed base, upright to slightly horizontal, scattered, and connected to the substratum by broad, brown, septate, superficial stoloniferous hyphae. Necks 100–150 × 70–85 μm, pale brown, central; wall of the neck composed of short outwardly diverging globose to cylindrical cells. Peridium 20–30 μm thick, in longitudinal section composed of 3–4 cell layers; inner layer of laterally compressed, hyaline to subhyaline, isodiametric cells, outer layer of large, darkened, moderately thick-walled, globose to angular cells 30–34 × 20–24 μm. Hamathecium consisting of hyaline, septate pseudoparaphyses 2–3 μm wide, slightly constricted at the septa, filamentous, branching and anastomosing above the asci. Asci 170–220 × 10–17 μm, basal, fissitunicate, cylindrical, pedicellate, weakly developed apical chamber; with eight, overlapping uniseriate ascospores. Ascospores 25–30 × 9–10 μm, broadly ellipsoid to fusiform, dark brown, 1-septate, slightly constricted at the septum, upper cell slightly broader and more apiculate than the lower cell, rough-walled in an irregularly striate pattern, with hyaline caps at both apices up to 2 × 2–3 μm, lacking a sheath. Based on the description by Raja and Shearer (2006).
Anamorph: None known.
Cultures: F49-1 (ex-holotype), AF220-1.
Sequence data: See Supplementary Table 1.
Habitat and host range: Decorticated wood.
Geographical distribution: Florida, USA.
Notes: Jahnula bipileata is similar to J. aquatica, but differs in having ascomata with long cylindrical necks, and irregularly striated, rough-walled ascospores with hyaline caps.
Jahnula bipolaris (K.D. Hyde) K.D. Hyde, Nova Hedw. 68: 494 (1999).
≡ Ascagilis bipolaris K.D. Hyde, Aust. Syst. Bot. 5: 111 (1992).
Ascomata 260–325 μm diam, immersed, erumpent, shedding wood particles and becoming superficial with base remaining immersed, globose to subglobose, black, coriaceous, ostiolate, short-papillate, solitary or gregarious, some with algae associations. Peridium up to 34 μm wide, comprising 3–5 layers of relatively large thin-walled, brown angular cells. Hamathecium consisting hyaline, hypha-like pseudoparaphyses to 4 μm wide, filamentous, septate, unbranched between the asci, branching and anastomosing above. Asci 140–200 × 35–40 μm, 8-spored, obclavate, pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 42–52 × 16–23 μm, 2–3 seriate, 1-septate, light brown, guttulate, ellipsoid-fusiform, minutely verruculose, constricted at the septum, with mucilaginous pads at each end. Based on the description by Hyde (1999) and further studies in Thailand.
Anamorph: None known.
Cultures: A421, SS44.
Sequence data: See Supplementary Table 1.
Habitat and host range: Decorticated wood.
Geographical distribution: Australia, Costa Rica, Hong Kong, Malaysia, Thailand.
Notes: Jahnula bipolaris was originally described as Ascagilis bipolaris from Australia and appears to be widely distributed in the tropics.
Jahnula granulosa K.D. Hyde & S.W. Wong, Nova Hedw. 68: 497 (1999).
Ascomata 210–280 μm diam, semi-immersed or erumpent, shedding wood particles and becoming superficial with base remaining immersed, globose to subglobose, brown to dark brown, membranous, ostiolate, short-papillate, with stalk-like strands attached to the base, with a few sparse hair-like projections, solitary. Peridium comprising a few layers of relatively large, thin-walled, light brown angular cells. Hamathecium consisting of hyaline, hypha-like pseudoparaphyses to 3 μm wide, filamentous, septate, unbranched between the asci, branching and anastomosing above. Asci 220–270 × 26–40 μm, 8-spored, obclavate, pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 26–38 × 15–18 μm, 2-seriate at the base of ascus, overlapping uniseriate near apex, 1-septate, dark brown, guttulate, ellipsoid-fusiform, apical cells wider and tapering to an acute apex, possibly with a germ pore, base less tapered and rounded, constricted at the septum, wall granular, surrounded by a thin mucilaginous sheath. Based on the description by Hyde and Wong (1999) and further research in Thailand.
Anamorph: None known.
Culture: SS1567.
Sequence data: See Supplementary Table 1.
Habitat and host range: On submerged wood.
Geographical distribution: South Africa.
Notes: A distinct species with dark brown ascospores, lacking appendages and a granular ornamentation of the spore wall.
Jahnula morakotii Sivichai & Boonyuen, Mycotaxon 112: 476 (2010).
Ascomata 100–180 μm diam, globose to subglobose, superficial with a septate stalk, 18–30 μm wide, or sessile. Peridium of large, thin-walled cells. Hamathecium consisting of hyaline, septate pseudoparaphyses, hyaline, 1.5–2 μm wide, up to 150 μm in length. Asci 107.5–120 × 9–11.5 μm, 8-spored, cylindrical, pedicellate, fissitunicate, with a shallow ocular chamber and faint ring. Ascospores 17.5–20 × 5–6.5 μm, fusiform, brown, 1-septate, uniseriate or biseriate, multi-guttulate, slightly constricted at the septa, straight to curved with cellular bipolar hyaline apical appendages.
Anamorph: None known.
Culture: None.
Sequence data: None available.
Habitat and host range: Submerged wood test block of Azadirachta indica in freshwater stream.
Geographical distribution: Thailand.
Material examined: Thailand, Narathiwat province, Sirindhorn Peat Swamp Forest, on submerged wooden test block of Azadirachta indica, 10 March 2003, S. Sivichai & N. Boonyuen, BIOTEC SS2447.
Notes: Jahnula morakotii differs from all Jahnula species in having the smallest ascospores (17.5–20 × 5–6.5 μm) with bipolar cellular appendages and lacking a sheath. Species close in ascospore size to J. morakotii are J. bipileata (25–30 × 9–10 μm) and J. australiensis (19–30 × 6–8 μm); however, they lack bipolar appendages. Jahnula appendiculata is the only other species with bipolar appendages, but the ascospores of this species are longer and wider (45–52.5 × 22.5–27.5 μm) than those of J. morakotii (17.5–20 × 5–6.5 μm). In addition, ascospores of J. appendiculata have a thick sheath that is absent in J. morakotii.
During a long-term colonization study of freshwater fungi on wood submerged in the Sirindhorn peat swamp forest Narathiwat, in Southern Thailand, J. morakotii (Sivichai and Boonyuen 2010) was collected only once on a test block of Azadirachta indica and it can be considered a rare fungus and may be restricted to this unique habitat.
Phylogenetic study: None.
Jahnula poonythii K.D. Hyde & S.W. Wong, Nova Hedw. 68: 499 (1999).
Ascomata 350–420 μm diam, semi-immersed or erumpent, shedding wood particles and becoming superficial with the base remaining immersed, globose to subglobose, brown to dark brown, membranous, ostiolate, short-papillate, with stalk-like strands attached to the base, with sparse hair-like projections, solitary. Peridium comprising a few layers of relatively large thin-walled, light brown angular cells. Hamathecium consisting of hyaline, hypha-like pseudoparaphyses to 3 mm wide, filamentous, septate, unbranched between the asci, branching and anastomosing above. Asci 120–175 × 16–20 μm, 8-spored, cylindrical, short pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 23–28 × 11–13 μm, mostly uniseriate or overlapping uniseriate, 2-celled, dark brown, guttulate, ellipsoid-fusiform, both cells of approximately equal size, tapering to rounded apices, strongly constricted at the septum, wall verruculose. Based on the description by Hyde and Wong (1999).
Anamorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: Submerged wood in river.
Geographical distribution: Mauritius.
Jahnula potamophila K.D. Hyde & S.W. Wong, Nova Hedw. 68: 499 (1999).
Ascomata up to 450 μm high, 325–390 μm diam, erumpent, shedding wood particles and becoming superficial with base remaining immersed, subglobose, obpyriform or almost conical, hyaline and then metallic grey, coriaceous, ostiolate, short-papillate, solitary or gregarious, with algal associations. Peridium up to 34 μm wide, comprising 3–6 layers of relatively large thin-walled, hyaline angular cells and covered with sparse hyaline hairs. Hamathecium consisting of hyaline, hypha-like pseudoparaphyses to 3.5 μm wide, filamentous, septate, unbranched between the asci, branching and anastomosing above. Asci 200–240 × 26–40 μm, 8-spored, obclavate, pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 46–54 × 17–20 μm, 2-3-seriate near the base, overlapping uniseriate near the apex, 1-septate, light brown, guttulate, ellipsoid-fusiform, constricted at the septum, surrounded by a mucilaginous sheath, wavy in outline. Based on the description by Hyde and Wong (1999).
Anamorph: None known.
Culture: F111-1.
Sequence data: See Supplementary Table 1.
Habitat and host range: Submerged wood in freshwater.
Geographical distribution: Australia.
Notes: This fungus is similar to J. bipolaris in ascospore dimension but differs in having minute guttules, and surrounded by a narrow mucilaginous sheath.
Jahnula rostrata Raja & Shearer, Mycologia 98: 325 (2006).
Ascomata 207–264 × 147–150 μm, hyaline and translucent when young becoming translucent brown to black at maturity, globose to subglobose, solitary, scattered, superficial on wood, connected by stoloniferous hyphae ca. 15–19 μm wide; ostiolate, papillate. Necks 38–40 × 30–32 μm, composed of subglobose cells diverging from the ostiole; periphyses not observed. Peridium 36–38 μm wide, in longitudinal section 3–4 cell layers wide, innermost layer of hyaline cells compressed laterally, outermost layer of large, moderately thick-walled, isodiametric cells. Hamathecium consisting of hyaline, septate pseudoparaphyses 3–4 μm wide, anastomosing above the asci. Asci 152–190 × 32–40 μm, basal, fissitunicate, clavate, short pedicellate, with an apical chamber and eight, overlapping biseriate ascospores. Ascospores 32–45 × 12–15 μm, ellipsoid or broadly fusiform, dark brown, 1-septate, slightly constricted at the septum, upper cell of ascospores broader than lower cell, rough-walled in an irregularly striate pattern, multiguttulate, with or without a thin gelatinous sheath. Based on the description by Raja and Shearer (2006).
Anamorph: None known.
Culture: F4-3.
Sequence data: See Supplementary Table 1.
Habitat and host range: Decorticated wood.
Geographical distribution: Florida, USA.
Notes: This fungus is most similar to J. granulosa with its roughened ascospore cell wall (Raja and Shearer 2006).
Jahnula sangamonensis Shearer & Raja, Mycologia 98: 327 (2006).
Ascomata 468 × 345 μm, globose to obpyriform, reddish brown to black, partially immersed in wood, ostiolate, papillate with subtending brown, septate hypha ca. 160 × 8 μm that attach the base of the ascomata to the wood. Necks 116–120 × 60–65 μm, central, with reddish brown periphyses; wall of the neck composed of chains of elongated cells diverging from the ostiolar canal. Peridium 40–44 mm thick, textura angularis in surface view, in longitudinal section, peridial wall 4–6 cell layers wide; inner layer composed of thin-walled, narrow, flattened, elongated, subhyaline cells 11–16 × 4–5 μm, outer layer of large, moderately thick-walled isodiametric, brown cells 14–34 × 15–20 μm. Hamathecium consisting of hyaline, septate pseudoparaphyses 2–3 μm wide, anastomosing above the asci. Asci 164–200 × 15–20 mm, fissitunicate, endoascus extending in water to ca. 270–500 μm in length, cylindrical, with an apical chamber, pedicellate with eight, overlapping uniseriate ascospores. Ascospores 20–28 × 10–12 μm, broadly ellipsoid, 1-septate, constricted at the septum, dark brown, spore wall smooth or verruculose, multiguttulate, without a sheath. Based on the description by Raja and Shearer (2006).
Anamorph: None known.
Cultures: A402-1B (ex-holotype), A482-1B, F81-1.
Sequence data: See Supplementary Table 1.
Habitat and host range: Submerged decorticated wood.
Geographical distribution: USA.
Jahnula seychellensis K.D. Hyde & S.W. Wong, Nova Hedw. 68: 504 (1999). Figure 3 a-m.
Ascomata 160–300 μm diam, semi-immersed or erumpent with a stalk, subglobose, brown to dark brown, coriaceous, ostiolate, short-papillate, solitary or gregarious, with algal associations. Peridium comprising a few layers of relatively large thin-walled, brown angular cells. Hamathecium pseudoparaphyses to 4 μm wide, hypha-like, filamentous, hyaline, septate, unbranched between the asci, branching and anastomosing above. Two types of asci and ascospores are present in the same ascoma. Asci 125–150 × 17–23 μm, 8-spored, cylindric-clavate, pedicellate, fissitunicate, with an ocular chamber and faint ring. Ascospores 29–36 × 9–12.5 μm, 2-3-seriate, 1-septate, brown, guttulate, irregular fusiform, apical cells wider and tapering to a rounded tip, base narrower and more rounded, constricted at the septum, with mucilaginous pads at each end. Asci 112–137 × 30–35 μm, 8-spored, obclavate, pedicellate, fissitunicate, with an apical apparatus and ring. Ascospores 30–40 × 17–23 μm, 2-3-seriate, 1-septate, hyaline to pale brown, guttulate, ellipsoid, constricted at the septum, with mucilaginous pads at each end. Based on the description by Hyde and Wong (1999) and further observations in Thailand.
Anamorph: None known.
Cultures: A492, SS1536.1, SS1536.2, SS2113.2.
Sequence data: See Supplementary Table 1.
Habitat and host range: Wood submerged in freshwater.
Geographical distribution: Seychelles, Thailand.
Jahnula sunyatsenii (Inderb.) K.L. Pang, E.B.G. Jones & Sivichai, Mycol. Res. 106: 1037 (2002).
≡ Aliquandostipite sunyatsenii Inderb., Am. J. Bot. 88: 57 (2001).
Ascomata sessile, singly erumpent from decorticated branch immersed in small stream, rounded, 300–400 μm diam, papillate, ostiolate, light to dark brown, membranous. Ascomal wall one-layered, 25–40 μm thick, 2–5 cells wide, forming a textura globulosa-angularis in surface view. Outermost cells rounded to elongate, up to 30 μm diam, some protruding up to 13 μm above surrounding cells, inner cells elongate and laterally compressed, cell walls 1–5 μm thick, refractive. Cells at the base and towards papillum dark at times. Ostiole apically lined by elongate cells, ca. 10 × 5 μm. Hamathecium consisting of hyaline, septate pseudoparaphyses persistent, branched, ca. 2.5 μm wide. Asci originating from a cushion shaped ascogenous tissue at the base of the ascomata, 128–193 × 45–57.5 μm, when young saccate with thick-walled apex, ocular chamber and short stalk, fissitunicate, 8-spored. Ascospores straight or slightly curved, (39–) 46–52 × 16–23 μm, 1-septate up to 5 μm above or 4 μm below the median septum, constricted at the septum, light brown, multi-heavily guttulate. Helmet-shaped appendages apically.
Stalked ascoma: one stalked ascoma was found, ca. 350 μm in diameter, originating from the apex of a concolorous stalk. Stalk septate at intervals of 30–40 μm, thick-walled (up to 7.5 μm), 50 μm wide and 0.5 μm long, at the base branching into 15 μm wide hyphae. Asci 137–143 × 45–62 μm. Ascospores 50–52 × 17–20 μm. Based on the description by Inderbitzin et al. (2001) and further observations.
Anamorph: None known.
Culture: UBC-F13876 (ex-holotype).
Sequence data: See Supplementary Table 1.
Habitat and host range: Decaying branches immersed in freshwater.
Geographical distribution: China.
Notes: Described as Aliquandostipite (Inderbitzin et al. 2001), it was referred to Jahnula based on molecular evidence (Pang et al. 2002).
Jahnula systyla K.D. Hyde & S.W. Wong, Nova Hedw. 68: 506 (1999).
Ascomata 320–340 × 290–320 μm diam, semi-immersed and attached to a stalk, or erumpent, shedding wood particles and becoming superficial, with base remaining immersed and attached to a stalk, subglobose, light brown to dark brown, coriaceous, ostiolate, short-papillate, solitary or gregarious, with algal associations. Peridium comprising a few layers of relatively large, thin-walled, brown angular cells. Pseudoparaphyses to 3 μm wide, hypha-like, filamentous, hyaline, septate, unbranched between the asci, branching and anastomosing above. Two kinds of asci and ascospores were presented in the same ascoma. Both types of asci (6-) 8-spored (1) Asci 124–150 × 22–30 μm; ascospores 28–40 × 8–13 μm, 2-3-seriate, 1-septate, brown to dark brown, guttulate, irregularly fusiform, apical cells wider and tapering to a rounded tip, base narrower and more rounded, minutely verruculose, constricted at the septum. (2) Asci 120–190 × 34–46 μm, obclavate, pedicellate, fissitunicate, with an apical apparatus and ring. Ascospores 44–68 × 13–19 μm, 2-3-seriate, 1-septate, hyaline to pale brown, guttulate, ellipsoid, slightly constricted at the septum, surrounded by a wide mucilaginous sheath. Based on the description by Hyde and Wong (1999).
Anamorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: On submerged wood in freshwater.
Geographical distribution: Australia.
Aliquandostipite Inderb. Am. J. Bot. 88: 54 (2001).
Ascomata globose to subglobose, immersed to superficial, papillate, brown to dark brown. Hamathecium consisting of septate, sparsely branched pseudoparaphyses. Asci clavate, fissitunicate, with thickened apical region. Ascospores oval, 1-septate, constricted at the septum, smooth, pale brown with well developed sheath.
Type species: A. khaoyaiensis Inderb., Am. J. Bot. 88: 54 (2001).
Sequences derived from ex-holotype.
Accepted species:
*A. crystallinus Raja, A. Ferrer & Shearer, Mycotaxon 91: 208 (2005).
*A. khaoyaiensis Inderb., Am. J. Bot. 88: 54 (2001).
A. minuta Raja & Shearer, Mycoscience 48: 6 (2007).
*A. separans (Abdel-Wahab & El-Sharouney) J. Campb., Raja, A. Ferrer, Sivichai & Shearer, Can. J. Bot. 85: 881 (2007).
*A. siamensiae (Sivichai & E.B.G. Jones) J. Campb., Raja, A. Ferrer, Sivichai & Shearer, Can. J. Bot. 85: 879 (2007).
5 species, * sequenced species.
Aliquandostipite crystallinus Raja, A. Ferrer & Shearer, Mycotaxon 91: 208 (2005).
Ascomata globose to subglobose, 215–270 × 220–320 μm, hyaline becoming dark brown, papillate, immersed to erumpent, solitary or gregarious, papilla short, 30–36 × 60–90 μm. Peridium 21–25 μm wide, textura angularis, inner layer of hyaline thin-walled cells, outer layer of large thin-walled isodiametric cells. Pseudoparaphyses septate, slightly constricted at the septa, sparsely branched. Asci fissitunicate, clavate to cymbiform (navicular), 8-spored, 132–174 × 30–40 μm, with or without an apical chamber. Ascospores fusiform, 70–86 × 9–15 μm, 1-septate becoming 3-septate, hyaline, with apical appendages 2–5 μm long. Sheath swelling and becoming sigmoid or C shaped, 200–452 μm long, 4–7 μm wide. Based on the description by Raja et al. (2005).
Anamorph: None known.
Cultures: R76-1 (ex-holotype), A514-1, AF007, F83-1.
Sequence data: See Supplementary Table 1.
Habitat and host range: Decorticated wood in freshwater stream.
Geographical distribution: Costa Rica, Panama, USA.
Notes: Ascospores and hyphae contain refractive acicular crystals (Raja et al. 2005).
Aliquandostipite khaoyaiensis Inderb., Am. J. Bot. 88: 54 (2001).
Ascomata singly immersed to erumpent or superficial on old decorticated branch globose to broadly ellipsoid, 216–290 μm high, 220–344 μm wide, papillate, appearing pale brown when young or dark brown with age beneath stereomicroscope. Peridium membranous, one-layered, in surface view pallid brown, forming a textura angularis-globulosa, in transverse section cells rounded to elongate. Wall of outermost cells up to 3.5 thick and refractive, the largest cells protruding up to 8 μm. Papilla ca. 50 μm high, 70 μm wide. Hamathecium consisting of hyaline, septate pseudoparaphyses, sparsely branched, up to 3.5 μm wide. Asci 136–194 × 36–58 μm, 8-spored, clavate, fissitunicate, with thickened apical region, spores variably arranged, small peduncle observed at times. Ascospores oval in outline, 50–70 × 13–20 μm, 1-septate, constricted at the septum, 11–17 μm wide, upper cell slightly longer and narrower than lower cell, smooth, pale brown, guttulate or not, sheathed. Sheath first adpressed to the wall, gradually expanding and detaching from the polar regions towards the septum, then balloon-like at the poles, finally surrounding the entire ascospore, ca. 150 × 50 μm.
Stalked ascomata: stalk to 1.6 mm long and 42 μm wide, wall up to 15 μm thick, arising singly from a superficial hypha, or singly or gregariously from substrate. Based on the description by Inderbitzin et al. (2001) and further observations in Thailand.
Anamorph: None known.
Cultures: UBC-F13875 (ex-holotype), F89-1, SS2843, SS2961, SS3028, SS3321, UAMH10371.
Sequence data: See Supplementary Table 1.
Habitat and host range: On decaying branch in freshwater.
Geographical distribution: Thailand.
Aliquandostipite minuta Raja, A. Ferrer & Shearer, Mycotaxon 91: 208 (2005).
Ascomata 130–150 × 150–160 μm, hyaline to light brown, globose to subglobose, ostiolate, papillate, immersed to superficial and wide hyphae. Peridium with large cells, textura angularis. Hamathecium consisting of septate, hyaline pseudoparaphyses 2 μm wide, slightly swollen at their tips. Asci 740–112 × 36–58 μm, 8-spored, ovoid to broadly clavate, fissitunicate, with or without apical chamber. Ascospores 42–54 × 10–14 μm, hyaline becoming pale brown, 1-septate, upper cell slightly broader than the basal cell, sheath present forming a fringe at the midseptum.
Anamorph: None known.
Culture: F117-1 (ex-holotype).
Sequence data: None.
Habitat and host range: Submerged decorticated wood.
Geographical distribution: Florida, USA.
Notes: Aliquandostipite minuta is similar to A. crystallinus but differs in the dimensions of the ascomata, shape and size of the asci, ascospore sheath and the median filamentous appendages.
Aliquandostipite separans (Abdel-Wahab & El-Sharouney) J. Campb., Raja, A. Ferrer, Sivichai & Shearer, Can. J. Bot. 85: 881 (2007).
≡ Patescospora separans Abdel-Wahab & El-Sharouney, Mycol. Res. 106: 1033 (2002).
Ascomata 200–260 × 280–335 μm diam, immersed to erumpent, dark brown, coriaceous, papillate, solitary, with stalk-like thick hyphae attached to the base, with a few sparse hair-like projections. Peridium 25–45 μm thick, comprising 2–4 layers of cells wide, individual cells 10–16 μm wide. Papilla 80–150 μm high, 80–90 μm wide. Hamathecium consisting of hyaline, septate pseudoparaphyses, branched, up to 7 μm wide. Asci 44–114 × 24–40 μm, 8-spored, ovoid to clavate, with a rounded base, thin-walled, dissolving in water within a few seconds to 1–2 min at most, releasing spores surrounded by a thick and elaborate sheath, the asci scattered in the centrum inside locules formed by disintegration of hamathecial tissue. Ascospores 30–46 × 9–20 μm, 1-septate, variably arranged inside the ascus, deeply constricted at the septum, upper cell slightly longer and narrower than the lower cell, smooth, hyaline, guttulate and surrounded by a multi-layered mucilaginous sheath (110–140 × 22–32 μm), cells of the ascospores separated later in development. Based on the description by Pang et al. (2002) and further observations.
Anamorph: None known.
Culture: CY2787 (ex-holotype).
Sequence data: See Supplementary Table 1.
Habitat and host range: Twigs in river Nile.
Geographical distribution: Egypt.
Aliquandostipite siamensiae (Sivichai & E.B.G. Jones) J. Campb., Raja, A. Ferrer, Sivichai & Shearer, Can. J. Bot. 85: 879 (2007).
≡ Jahnula siamensiae Sivichai & E. B. G. Jones, Mycol. Res. 106: 1037 (2002).
Ascomata 260–350 × 240–350 μm, globose to subglobose, gregarious, superficial with stalk (up to 300 μm long and 40–55 μm width) or sessile. Stalk septate, 25–80 μm wide. Peridium 3–5 μm thick, comprising a few layers of relatively large thin-walled, light brown angular cells. Hamathecium of pseudoparaphyses, ca. 2–3 μm thick, hypha-like, filamentous, hyaline, septate, unbranched between the asci, branching and anastomosing above. Asci 8-spored, fissitunicate, with an ocular chamber and a faint ring, with two spore types: (1) small ascospores formed in obclavate asci, 125–175 × 28–40 μm with a short peduncle; and (2) large ascospores formed in clavate asci, 137–180 × 33–52 μm, with a short pedicel. Ascospores either small, 33–45 × 10–13 μm, brown, or large 58–73 × 15–25 μm, hyaline to pale brown. Both ascospore types are biseriate, 1-septate, guttulate, ellipsoid-fusiform, smooth-walled, apical cell slightly larger, some curved, slightly constricted at the septum. Based on the description by Pang et al. (2002) and further observations in Thailand.
Anamorph: None known.
Culture: SS81.02 (ex-holotype).
Sequence data: See Supplementary Table 1.
Habitat and host range: On submerged soft wood.
Geographical distribution: Thailand.
Brachiosphaera Nawawi, in Descals, Nawawi & Webster, Trans. Br. Mycol. Soc. 67: 213 (1976).
Colonies effuse, hypha varying in width, septate, hyaline. Conidium round with 4–5 conidial arms, each 1–4 septate.
Type species: B. tropicalis Nawawi in Descals, Nawawi & Webster, Trans. Br. Mycol. Soc. 67: 213 (1976).
No ex-holotype culture.
Accepted species:
*B. tropicalis Nawawi in Descals, Nawawi & Webster, Trans. Br. Mycol. Soc. 67: 213 (1976).
Species requiring molecular study:
B. jamaicensis (J.L. Crane & Dumont) Nawawi, in Descals, Nawawi & Webster, Trans. Br. Mycol. Soc. 67: 216 (1976).
2 species, * sequenced species.
Brachiosphaera tropicalis Nawawi in Descals, Nawawi, and Webster Trans. Br. Mycol. Soc. 67: 213 (1976).
Cultures: Colonies effuse, mycelium mostly submerged in culture media. Hypha varying in width, septate, hyaline at first, turning olivaceous brown with age. Within hyphae a few cells in a row becoming slightly widened to form ellipsoid or round-shaped conidia in a cluster. Spherical central body of conidium 44–58 μm diam, each conidial arm mostly 4–5, 82–120 μm and 1–4 septate, in nature conidia mostly with two arms. Conidia frequently formed chains in half-strength corn meal agar and in water agar (Chang 1994: Isolate WL0604 from Wulai, Taipei) and further observations in Thailand.
Teleomorph: None known.
Cultures: E192-1, BCC14538, BCC16746, BCC24051.
Sequence data: See Supplementary Table 1.
Material examined: Thailand, Narathiwat province, Khlong Ai-Kading Waterfall, Hala Bala Wildlife Sanctuary, in foam, 22 February 2003, S. Sivichai & N. Boonyuen, BIOTEC SS2522, and SS2523.
Habitat and host range: Foam samples and leaves in freshwater streams.
Geographical distribution: China, Dominican Republic, Hong Kong, Malaysia, Panamá, Puerto Rico, South Africa, Southern-Western France, Taiwan, Thailand, Venezuela.
Notes: Many Brachiosphaera tropicalis specimens (SS2522, SS2523, SS2944) were obtained from foam samples of several tropical freshwater streams in Thailand. The species is regarded as common in freshwater streams, especially in foam samples.
Morphology: Brachiosphaera is characterized by producing round shaped central cell from which radiate 4–5 arms, each 1–4 septate. In Brachiosphaera tropicalis, conidia are brown and tetraradiate, consisting of 4–8 appendages slightly constricted at the origin, much longer than 1.5 times diam of the central part; central part globose to pyramidal. Colonies are characterized by effuse, septate mycelium, mostly submerged in culture media. Brachiosphaera tropicalis has conidia that are very similar to those of Actinosporella megalospora, the anamorph of the pezizalean discomycete Miladina lechithina (Pyronemataceae), Pezizales which has hyaline and tetraradiate conidia. The two species are sometimes confused with each other and repeatedly misidentified.
Phylogenetic study: The placement of Brachiosphaera within Jahnulales (Campbell et al. 2007) was based on a single locus (LSU) and confirmed its unrelatedness to Actinosporella (Descal and Webster 1978). Additionally, Prihatini et al. (2008) demonstrated that B. tropicalis had phylogenetic affinities to the Jahnulales, sharing a unique pattern of dark brown to black and thick-walled mycelia and closely related to Speiropsis pedatospora. More recently, Shearer et al. (2009) have shown that B. tropicalis has phylogenetic affinities within the Aliquandostipitaceae, Jahnulales, based on two loci (SSU and LSU rDNA).
Brachiosphaera jamaicensis (J.L. Crane & Dumont) Nawawi, in Descals, Nawawi & Webster, Trans. Br. Mycol. Soc. 67: 216 (1976).
≡ Actinospora jamaicensis J.L. Crane & Dumont, Can. J. Bot. 53: 843 (1975).
Cultures: Colonies effuse on half-stength cornmeal agar. Mycelia immersed, hyphae initially hyaline, becoming olivaceous brown when aged, septate. Conidiophores erect, not well differentiated from hyphae. Conidia produced at the apex of conidiophores, and secondary conidia usually produced sympodially by renewed branching of the conidiophore just below the initial conidium. They were also produced in succession under laboratory conditions. Conidia comprising a central spherical body and 6–10 bulbous, short arms 1 or 2 septate, 20–40 μm long, and 6.8–9.5 μm and 3–5 μm wide at basal and upper ends, respectively. Chang (1994) reported that conidia produced on natural substrata were larger than those produced in culture.
Teleomorph: None known.
Culture: None.
Sequence data: None available.
Habitat and host range: Decaying twigs, leaves, branches and foam in freshwater streams.
Geographical distribution: Jamaica, Poland, Puerto Rico, Taiwan.
Phylogenetic study: None.
Notes: Because of the lack of cultures and sequence data, the position of B. jamaicensis in the Jahnulales cannot be confirmed.
Megalohypha A. Ferrer & Shearer, in Ferrer, Sivichai & Shearer, Mycologia 99: 456 (2007).
Ascomata superficial on wood, globose to obpyriform, ostiolate, papillate, stalked or sessile, connected to the substratum by broad, brown, septate, stoloniferous hyphae. Peridium wall of large, hyaline, thin-walled cells. Hamathecium pseudoparaphysate, pseudoparaphyses septate, branched, anastomosing above the asci. Asci fissitunicate, broadly clavate or fusiform, short pedicellate, 8-spored. Ascospores pale brown to dark brown, 1-septate, symmetrical, rough-walled, longitudinally striate.
Type species: M. aqua-dulces A. Ferrer & Shearer, in Ferrer, Sivichai & Shearer, Mycologia 99: 458 (2007).
No ex-holotype culture.
Accepted species:
M. aqua-dulces A. Ferrer & Shearer, in Ferrer, Sivichai & Shearer Mycologia 99: 458 (2007).
Ascomata on wood, 240–300 × 200–250 μm, superficial, globose to obpyriform, ostiolate, papillate, hyaline, membranous, connected to substrate by a brown, septate stalk, strongly constricted at the septa, 20–25 wide and up to 450 μm long; sessile ascomata connected to the substrate by superficial, wide, brown, septate, stoloniferous hyphae. Papillae 55 × 45 μm, short, cylindrical, periphysate; wall composed of thin-walled cells. Peridium about 15 μm thick, in longitudinal section composed of 1–2 layers of hyaline, thin-walled cells. Hamathecium consisting of hyaline, septate pseudoparaphyses 3–4 μm wide, filamentous, branched and anastomosing above the asci. Asci 110–160 × 35–60 μm, fissitunicate, clavate or ellipsoid, short pedicellate, with or without an apical chamber, 8-spored. Ascospores 40–55 × 19–22 μm, irregularly arranged, ellipsoid, tapered to acute apices, brown to dark brown, 1-septate, septum appearing as a dark band, both cells of equal shape and size, rough walled with longitudinal sulcate striations, lacking appendages or a gelatinous sheath.
Anamorph: None known.
Cultures: AF005-2a, AF005-2b.
Sequence data: See Supplementary Table 1.
Habitat and host range: Decorticated wood submerged in freshwater.
Geographical distribution: Panama, Thailand.
Notes: Megalohypha differs from the genera assigned to the Jahnulales in having mycelium and ascomatal stalks that are strongly constricted at the septa, ascospores that are characterized by bipolar symmetry, acute apices, a dark band at the median septum and sulcate striations on the spore wall. Additionally, molecular data support the erection of a new genus (Campbell et al. 2007). Based on the description by Ferrer et al. (2007) and further observations in Thailand.
Manglicola Kohlm. & E. Kohlm., Mycologia 63: 840 (1971).
Ascomata solitary, obtusely clavate to fusiform, stipitate, superficial, seated in the substrate with a hypostroma, coriaceous, olive brown, with a blunt periphysate ostiole, ostiolate, epapillate. Pseudoparaphyses numerous, septate. Asci 8-spored, cylindrical, thick-walled, developing at the base of the ascoma. Ascospores fusiform, apiculate, 1-septate; apical cell larger, dark brown; basal cell small, light brown; deliquescent appendages apically.
Type species: M. guatemalensis Kohlm. & E. Kohlm., Mycologia 63: 841 (1971).
No ex-holotype culture.
Accepted species:
M. guatemalensis Kohlm. & E. Kohlm., Mycologia 63: 841 (1971).
Species requiring a molecular study:
M. samulesii Huhndorf, Mycologia 86: 266 (1994).
Manglicola guatemalensis Kohlm. & E. Kohlm., Mycologia 63: 841 (1971). Figure 4 a-t.
Ascomata 835–1,275 × 185–387 μm, obtusely clavate to obtusely fusiform; stipitate, ostiolate, epapillate, coriaceous, olive brown, solitary or aggregated, ascoma wall differentiated into several layers of polygonal, thick-walled cells; ascoma superficial seated on the substratum on a hypostroma, of pseudoparenchymatous cells and brown hyphae 10–20 μm in diam. Peridium 36–50 μm thick, composed of three to five layers of cells. Ostiole obtuse surrounded by hyaline, clavate hyphae, 4–6 μm diam, periphyses 2–3 μm in diam, simple or ramose. Hamathecium comprising hyaline, septate, trabeculate pseudoparaphyses 2–4 μm wide, numerous, simple, between asci, arising from the base of the centrum, and anastomosing in the upper part. Asci 275–326 × 24–28 μm (Kohlmeyer and Kohlmeyer 1971, 1979), to 440–640 × 30–50 μm (Suetrong et al. 2010), 8-spored, cylindrical, thick-walled, with apical apparatus and ocular chamber. Ascospores 80–109 × 18–34 μm (Kohlmeyer and Kohlmeyer 1971, 1979) to 92.5–125 × 22.5–40 μm (Suetrong et al. 2010), fusiform, apiculate, unequally one-septate, constricted at the septum; apical cell larger, orange brown, light brown, dark brown to chestnut brown, basal cell, turbinate, light brown; gelatinous appendages cover both apices.
Anamorph: None known.
Cultures: BCC20079, BCC21218, BCC24217, BCC24200-BCC24204, BCC24275, BCC24296-BCC24302, BCC25032-BCC25042, BCC25052-BCC25053.
Sequence data: See Supplementary Table 1.
Material examined: Thailand, Trat Province: Mu Ko Chang National Park, Ban Salak Phet, on base frond of Nypa fruticans, 5 October 2005, S. Suetrong, E.B.G. Jones, J. Sakayaroj, R. Choeyklin, U. Pinruan, K.L. Pang, BBH17801; Thailand, Trang Province: Ban Bang Sak, on base frond of Nypa fruticans, 14 November 2005, E.B.G. Jones, R. Choeyklin, A. Pinnoi, U. Pinruan; Thailand, Trat Province: Mu Ko Chang National Park, Ban Salak Phet, on base frond of Nypa fruticans, 14 December 2006, S. Suetrong, J. Sakayaroj, R. Choeyklin, O. Supaphon, BBH23409.
Habitat and host range: Superficial on frond bases of Nypa fruticans and Rhizophora mangle.
Geographical distribution: Brunei, Guatemala, Thailand.
Phylogenetic study: Suetrong et al. (2009, 2010) confirmed the placement of M. guatemalensis in the Jahnulales.
Notes: The taxonomic position of M. samuelsii remains unresolved as morphologically it differs from M. guatemalensis, a rare tropical ascomycete currently referred to Hypsostromataceae, a family in the Pleosporales (See Fig. 1).
One of two MPTs inferred from combined SSU and LSU rDNA sequences of Jahnulales and other taxa of the Dothideomycetes, generated with maximum parsimony. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values greater than 50% are given above the node. Bayesian posterior probabilities greater than 0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap proportions (100%) and posterior probabilities (1.00) are shown as a thicker line. Taxa in red are generated from ex-holotype cultures, those in blue are from verified cultures
Speiropsis Tubaki, J. Hattori. Bot. Lab. 20: 171 (1958).
Conidiophores macronematous, mononematous, with short, pale branches at the apex; stipe straight or curved, mid to dark brown, smooth septate. Conidiogenous cells discrete or integrated formed at the ends of the branches, polyblastic, determinate, clavate, cylindrical or ellipsoidal, usually with 2 or 3 terminal protuberances or denticles on which branched chains of conidia are formed. Conidia cylindrical or cuneiform, hyaline or brown, smooth, aseptate.
Cultures: Colonies effuse, brown or blackish brown, hairy or velvety. Mycelium partly superficial, partly immersed. Stroma absent. Setae and hyphopodia absent.
Type species: Speiropsis pedatospora Tubaki, J. Hattori, Bot. Lab. 20: 171 (1958).
No ex-holotype cultures.
Accepted species:
*S. pedatospora Tubaki, J. Hattori, Bot. Lab. 20: 171 (1958).
Species requiring a molecular study:
S. aquatica Aramb., Cabello & Mengasc, Boln. Soc. Argent. Bot. 25: 222 (1987).
S. belauensis Matsch., Matsush, Mycol. Mem 4: 15 (1985).
S. hyalospora Subram. & Ledha, Can. J. Bot. 42: 1062 (1964).
S. ixorae Subram. & Sudha., Kavka 14: 37 (1987).
S. rogergoosensis T.S.K. Prasad & Bhat, Mycotaxon 82: 127 (2002).
S. scopofromis Kuthub. & Nawawi, Trans. Br. Mycol. Soc. 89: 584 (1987)
S. simplex Marsush., in Kabayasi et al., Bull. Natn. Sci. Mus., Tokyo 14: 475 (1971).
Eight species,*sequenced species.
Rejected species:
S. irregularis R.H. Petersen, Mycologia 55: 26 (1963).
Current name: Arbusculina irregularis (R.H. Petersen) Marvanová & Descals, Trans. Br. Mycol. Soc. 89: 499 (1987).
Speiropsis pedatospora Tubaki, J. Hattori, Bot. Lab. 20: 171 (1958). Figure 5 a-g.
Colonies hyaline, olivaceous to brown, Conidiophores 60–130 μm high, 3.5–5 μm wide, brown, apical region subhyaline to pale brown. Conidia branched with 2–6 arms, arms 65–85 μm long, each with 3–5 septa. Colonies on malt agar, olivaceous to fuscous, aerial hyphae sparse, septate, 1.5–2.5 μm wide. Conidiophores hyaline 50–65 μm long rarely 100 μm. Conidia 3–5 arms, 80–90 μm long, 4.5–6.5 μm wide, pale coloured. Based on Tubaki (1958) and further observations in Thailand.
Teleomorph: None known.
Culture: SS2229.
Sequence data: See Supplementary Table 1.
Habitat and host range: Dead leaves.
Geographical distribution: Japan, Thailand.
Notes: This is the only species currently included in the Jahnulales, as it is the only species with available molecular data. The remaining species listed above require study at the molecular level. This genus may be polyphyletic as indicated by the transfer of S. irregularis to Asbusculina (Marvanovà and Descals 1987). Therefore the only species accepted in the Jahnulales is S. pedatospora.
Xylomyces Goos, R.D. Brooks & Lamore, Mycologia 69: 282 (1977).
Colonies on natural substrate thin, effused. Mycelium immersed and superficial; hyphae branched, septate, hyaline to fuscous. Conidiophores and conidia lacking. Chlamydospores intercalary, multi-septate, fusiform, fuscous.
Known teleomorphs: in Jahnulales Pleosporales.
Type species: Xylomyces chlamydosporus Goos, Brooks & Lamore, Mycologia 69: 282 (1977).
No ex-holotype cultures.
Accepted species:
*X. chlamydosporus Goos, R.D. Brooks & Lamore, Mycologia 69: 282 (1977).
Species requiring molecular study :
X. foliicola W.B. Kendr. & R.F. Castañeda, Univ. Waterloo, Biol. Ser. 33: 54 (1990).
X. giganteus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1324 (1997).
X. punctatus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1328 (1997).
X. pusillus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1328 (1997).
X. rhizophorae Kohlm. & Volkm. -Kohlm., Fungal Divers. 1: 160 (1998).
five species, * sequenced species.
Rejected species :
X. aquaticus (Dudka) K.D. Hyde & Goh, Mycol. Res. 103: 1573 (1999).
≡ Camposporium aquaticum Dudka, Ukr. Bot. Zh. 23: 91 (1966).
≡ Vargamyces aquaticus (Dudka) Tóth, Acta Bota. Acade. Sci. Hung. 25: 403 (1979).
≡ Sporidesmium ontariense Matsush., Matsush. Mycol. Mem. 3: 16 (1983).
X. elegans Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1324 (1997).
Both species are rejected as molecular data places them in the Pleosporales (Prihatini et al. 2008)
Xylomyces chlamydosporus Goos, R.D. Brooks & Lamore, Mycologia 69: 282 (1977). Figure 6 a-b.
Colonies on natural substrates effused, thin, reddish brown. Mycelium immersed and superficial, composed of branched, septate, dark, anastomosing hyphae, (5–) 7–15 (−25) μm diam. Conidiophores and conidia lacking. Chlamydospores abundant, intercalary, solitary or in chains of 2 to 5, occasionally branched, with 5 to 9 septa, constricted at the septa, fusiform, brown to blackish, 100–450 × 35–42 μm. Based on Goos et al. (1977) and observations in Thailand.
Teleomorph: in the Jahnulales.
Cultures: SS2917, H58-4.
Sequence data: See Supplementary Table 1.
Habitat and host range: Submerged wood in freshwater streams.
Geographical distribution: Thailand, USA.
Notes: Sivichai et al. (2011), based on a culture study, demonstrated that Xylomyces chlamydosporus is the anamorph of Jahnula aquatica. However, this is not supported by sequence data in our study (Fig. 1).
Xylomyces foliicola W.B. Kendr. & R.F. Castañeda, Univ. Waterloo, Biol. Ser. 33: 54 (1990).
Colonies spreading, hypophyllous, brown to dark brown. Mycelium mostly superficial, but some immersed in the substrate, composed of septate, branched, anastomosing, smooth-walled, brown hyphae, 2–3 μm wide. Conidiophores and conidiogenous cells absent. Chlamydospores present, resembling phragmoconidia, intercalary, fusiform, brown, sometimes pale brown at the ends, in chains or solitary, usually unbranched, smooth-walled, 4-11-septate, mostly 11-septate, 126–152 × 7–9 μm. Based on a description by Carstañeda and Kendrick (1990).
Teleomorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: Decaying leaves.
Geographical distribution: Cuba.
Xylomyces giganteus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1324 (1997).
Colonies on natural substrate effuse, thin, brown. Mycelium mostly immersed and partly superficial, composed of branched, septate, 4–15 μm wide, dematiaceous, anastomosing hyphae. Stromata lacking. Setae and hyphopodia absent. Conidiophores and conidia not developed. Chlamydospores narrowly fusiform or long-fusiform, intercalary, straight or curved, solitary or in chains, occasionally branched, (140–) 191–575 × 25–50 μm, with 6–26 septa, yellowish brown to mid brown, uniform in colour or end cell paler, thick-walled with scarce irregular longitudinal striations. Based on the description by Goh et al. (1997).
Teleomorph: None known.
Cultures: SAPR15 (HKU(M) 2167), SAPR40 (HKU(M) 2211), ENG6A (HKU(M) 3228).
Sequence data: None.
Habitat and host range: Submerged wood in freshwater streams.
Geographical distribution: Australia.
Xylomyces punctatus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1328 (1997).
Colonies on natural substrate scattered, effuse, dark brown. Mycelium mostly immersed or partly superficial, composed of pale olivaceous brown, branched, septate, 2–3 μm wide, smooth or sometimes punctuate hyphae. Stromata lacking. Setae and hyphopodia absent. Conidiophores and conidia not developed. Chlamydospores intercalary or terminal, solitary, fusiform or slightly clavate, straight or slightly curved, 44–74 × 10–16 μm, with 3–7 septa, constricted at the septa, guttulate, pale reddish brown, end cells paler, with distinctive blackish punctuate wall ornamentation. Based on the description by Goh et al. (1997).
Teleomorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: Submerged wood in freshwater streams.
Geographical distribution: Hong Kong.
Xylomyces pusillus Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1328 (1997).
Colonies on natural substrate scattered, effuse, dark brown. Mycelium mostly immersed and partly superficial, composed of subhyaline to pale olivaceous brown, branched, septate, 1.5–3 μm wide, smooth hyphae. Stromata lacking. Setae and hyphopodia absent. Conidiophores and conidia not developed. Chlamydospores intercalary or terminal, solitary, fusiform or slightly clavate, usually slightly curved, 42–56 × 7–11 μm, with 3–7 septa, not constricted or sometimes slightly at the septa, guttulate, smooth, pale olivaceous brown, uniform in colour or sometimes central cells slightly darker. Based on the description by Goh et al. (1997).
Teleomorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: Submerged wood in freshwater streams.
Geographical distribution: Hong Kong.
Xylomyces rhizophorae Kohlm. & Volkm.-Kohlm, Fungal Divers. 1: 160 (1998).
Hyphae 1.5–4 μm diam, mostly superficial, septate, branched, light to dark brown. Conidiophores and conidia absent. Chlamydospores mostly terminally, rarely intercalary, single or in chains, rarely branching, filamentous, straight or curved, mostly widest at the tips, tapering towards the base, 95–370(−500) × (8–) 9–16 μm, 5.5–8 μm wide at the base, with 11-43(−64) transverse septa, rarely with longitudinal or oblique septa, constricted at some of the septa, smooth, dark brown, more or less uniform in colour throughout.
Teleomorph: None known.
Cultures: None.
Sequence data: None.
Habitat and host range: Washed up prop root of a mangrove tree.
Geographical distribution: Martinique.
Notes: Xylomyces, typified by X. chlamydosporus, is characterized by lacking conidiophores and conidiogenous cells but producing large, dark, multi-septate, fusiform chlamydospores (Goos et al. 1977). Castañeda and Kendrick (1990) described a second species of Xylomyces, X. foliicola from Cuba on decaying leaves of Quercus oleoides var. sagraeana. However, Xylomyces foliicola was concluded to be atypical of the genus in having a terrestrial occurrence and the propagules of this species might be genuine ‘conidia’, which are borne singly or in short unbranched chains (Goh et al. 1997). In the same study, Goh and co-workers described four more Xylomyces species (X. elegans, X. giganteus, X. punctatus and X. pusillus) from submerged wood in freshwater habitats. Xylomyces rhizophorae, discovered on dead submerged roots and branches of Rhizophora mangle, differs from other Xylomyces species in having narrower chlamydospores with consistently more septa and in its marine occurrence (Kohlmeyer and Volkmann-Kohlmeyer 1998). Hyde and Goh (1999) added the last species Xylomyces aquaticus, a taxon transferred from Vargamyces aquaticus. This species was originally described as Camposporium aquaticum by Dudka (1966) and later transferred to Vargamyces aquaticus (Tóth 1979).
Phylogenetic study: Nuclear ribosomal RNA genes of only three Xylomyces species have been sequenced (X. aquaticus, X. chlamydosporus and X. elegans). Both combined analysis of SSU and LSU rDNA genes (Campbell et al. 2007; Shearer et al. 2009) and ITS regions (Prihatini et al. 2008) put X. chlamydosporus in the Jahnulales. However, X. aquaticus and X. elegans were inferred to be related to the Pleosporales (Prihatini et al. 2008) and this placement of X. elegans was confirmed by Shearer et al. (2009) using sequence of the SSU rRNA gene.
Concluding remarks: Since both X. aquaticus and X. elegans did not group with the type species of the genus (X. chlamydosporus), the taxonomic placement of the two species needs to be reexamined. Xylomyces aquaticus is unusual in the genus in producing true conidia (Gönczöl et al. 1990), which clearly does not conform to the generic description of the genus. On the other hand, X. elegans is unique in the genus in having broadly fusiform chlamydospores. Whether this character is important in the delineation between X. chlamydosporus and X. elegans will require addition Xylomyces species in the phylogenetic analysis.
Results
Molecular phylogenies of combined SSU and LSU rDNA sequence data
The BLAST search based on SSU and LSU rDNA revealed the closest match with taxa in the subclass Pleosporomycetidae, order Jahnulales (genera Aliquandostipite, Brachiosphaera, Jahnula, Manglicola, Megalohypha, Speiropsis and Xylomyces). Sequences were aligned and analyzed separately by maximum parsimony, maximum likelihood and Bayesian inference, and the resulting trees compared. The phylogenetic analyses of combined SSU and LSU rDNA sequence data were performed, along with various orders of the Dothideomycetes (Botryosphaeriales, Capnodiales, Dothideales, Hysteriales, Myriangiales, Mytilinidiales and Pleosporales) from sequences retrieved from GenBank (Supplementary Table 2). Major insertions were present in the genes of twelve species (Botryosphaeria stevensii, Dendryphiopsis atra, Guignardia bidwellii, Halomassarina thalassia, Herpotrichia juniperi, Kirschsteiniothelia aethiops, Neottiosporina paspali, Phaeosphaeria avenaria, Phaeosphaeria eustoma, Quadricrura septentrionalis and Roccella fuciformis, SSU; Capnodium salicinum, LSU) inclusion or exclusion of these insertions did not affect the overall tree topology.
All major taxonomic clades presented in the multigene phylogeny of Campbell et al. (2007), Schoch et al. (2006, 2009), Shearer et al. (2009) and Suetrong et al. (2009, 2010) yielded the same topology. The final alignment of combined SSU and LSU rDNA sequences for 164 taxa was 2639 characters long, with Roccella fuciformis and Schismatomma decolorans as the outgroup. The alignment is available in TreeBASE No. 11942 (http://www.treebase.org/treebase-web/home.html).
The unweighted parsimony dataset consisted of 2,639 total characters, 1,573 (59.6%) were constant characters, 740 (28.0%) parsimony informative characters and 326 (12.4%) parsimony uninformative characters. Heuristic searches were run for 100 replicates of random stepwise addition of sequence that treated gaps as missing data. Independent Bayesian phylogenetic analysis was performed using a uniform GTR + I + G model, as selected by hLRT in Mrmodeltest 2.2: [GTR + I + G] Prset statefreqpr = dirichlet (1,1,1,1), Lset nst = 6 rates = invgamma. The maximum parsimony resulted in a single MPT in a length of 4,189 steps (CI = 0.372, RI = 0.819, RC = 0.304, HI = 0.628). One hundred successive searches using a rapid hill-climbing algorithm from distinct randomised starting trees in RAxML yielded a best scoring likely tree (data not shown) with a log likelihood −26515.129553. Phylogenetic trees obtained from maximum likelihood, Bayesian and maximum parsimony analyses yielded trees with similar overall topology at subclass, order and family relationship in agreement with previous work based on maximum parsimony and maximum likelhood (Campbell et al., 2007; Schoch et al. 2006, 2009; Shearer et al. 2009; Suetrong et al. 2009, 2010). However, the internal node relationships of some taxa were resolved differently between the maximum parsimony, maximum likelihood and Bayesian trees (Fig. 1).
Five clades are identified in Fig. 1, clade I comprising a group of species regarded as Jahnula sensu stricto by Campbell et al. (2007) and Shearer et al. (2009): J. aquatica (type species), J. granulosa, J. rostrata, with Megalohypha aqua-dulces and J. potamophila as a sister group. This clade has high support, while the remaining Jahnula species group in clades II and III. Clade II comprises seven Jahnula species, while J. australiensis, Brachiosphaera tropicalis and Speiropsis pedatospora group in clade III. Species grouping in Clade II (Jahnula sensu lato) may comprise more than one genus, for example, J. bipolaris, J. seychellensis and J. sunyatsensii all have ascospores with apical gelatinous pads and could be referred to the genus Ascagilis (Hyde 1992). However, until a wider molecular study is undertaken with 4–6 loci, we believe it to be premature, and regard all species in Clades I-III as belonging in Jahnula. This is a conclusion also drawn by Shearer et al. (2009) with fewer species sampled.
Four Aliquandostipite species form a well supported monophyletic group and this is in agreement with Shearer et al. (2009), although in their study A. separans is on a long branch. Strains of A. khaoyaiensis and A. siamensiae group together, with ascospore measurements in the same range. They differ in that the sheath in A. khaoyaiensis ballons out while in A. siamensiae it is narrow and ascospores in the latter are dimorphic: small and brown (33–45 × 10–13 μm) or large (58–73 × 15–25 μm) and hyaline to pale brown (Pang et al. 2002). Further studies are required to determine whether they may be conspecific. All Aliquandostipite species have well developed ascospore sheaths, with the exception of A. siamensiae. Clade V is a monophyletic group comprising four strains of Manglicola guatemalensis, a marine mangrove taxon reported from Brunei, Guatemala, and Thailand. This clade is distinct from taxa assigned to Aliquandostipitaceae, and a new family is proposed.
Molecular phylogenies of combined LSU and 5.8S rDNA and TEF-1-alpha sequence data
The BLAST search based on 5.8S and LSU rDNA revealed the closest match with taxa in the subclass Pleosporomycetidae, order Jahnulales (Aliquandostipite sunyatsenii, Brachiosphaera tropicalis, Jahnula granulosa, Speiropsis pedatospora and Xylomyces chlamydosporus) with 84-98% similarity.
The combined LSU and 5.8S rDNA and TEF-1-alpha data set consisted of 35 taxa, with Massarina eburnea as the outgroup. The phylogenetic analyses of combined LSU and 5.8S rDNA and TEF-1-alpha sequence data were performed, along with various taxa of the Jahnulales from GenBank. Sequences were aligned and analyzed separately by maximum parsimony, maximum likelihood and Bayesian analysis. The maximum parsimony dataset consisted of 2,218 total characters, 1,649 (74.3%) characters were constant, 283 (12.7%) characters are parsimony informative and 286 (13.0%) characters were parsimony uninformative. Heuristic searches were run for 100 replicates of random stepwise addition of sequence that treated gaps as missing data. The maximum parsimony resulted in eithty-two MPTs in a length of 871 steps (CI = 0.785, RI = 0.833, RC = 0.654, HI = 0.215). Independent Bayesian phylogenetic analysis was performed using a uniform GTR + I + G model, as selected by hLRT in Mrmodeltest 2.2: [GTR + I + G] Prset statefreqpr = dirichlet (1,1,1,1), Lset nst = 6 rates = invgamma. One hundred successive searches using a rapid hill-climbing algorithm from distinct randomised starting trees in RAxML yielded a best scoring likely tree (data not shown) with a log likelihood −7413.460154. Phylogenetic trees obtained from maximum likelihood, Bayesian and maximum parsimony analyses yielded trees with similar overall topology at subclass, order and family relationship in agreement with previous work based on maximum parsimony and maximum likelihood (Campbell et al. 2007; Prihatini et al. 2008; Shearer et al. 2009; Suetrong et al. 2009, 2010). The alignment is available in TreeBASE No 11942 (http://www.treebase.org/treebase-web/home.html). A single MPT is shown as a phylogram, representing the best topology with the best K-H-likelihood scores (Fig. 2).
One of 82 MPTs inferred from combined LSU + 5.8S rRNA+TEF-1-alpha sequences of Jahnulales generated with maximum parsimony. Maximum parsimony (BSMP, left) and likelihood (BSML, right) bootstrap values greater than 50% are given above the node. Bayesian posterior probabilities greater than 0.95 are given below each node (BYPP). The internodes that are highly supported by all bootstrap proportions (100%) and posterior probabilities (1.00) are shown as a thicker line
The dataset of combined LSU and 5.8S rDNA and TEF-1-alpha also comprises five clades but the topology of the tree differs from that in Fig. 1. Clades A, B, D and E correspond to clades V, I, III and IV respectively in Fig. 2, while the resolution of subclades in Clade C differs in their support at the nodes. In this tree, Aliquandostipite species are not monophyletic while Manglicola guatemalensis groups with moderate support with Jahnula, Megalohypha, Brachiosphaera and Speirospsis species (73BSMP and 56BSML) Figs. 3, 4, 5 and 6.
Manglicola guatemalensis on the surface of Nypa fruticans, partially immersed in mud; b Ascoma seated on the substrata a hypostroma, composed of pseudoparenchymatous cells (PC) and dark ascospores (AS) in the mature asci (AC), visible through thin wall; spores exuded at ostiole (OS); c, d Longitudinal section of ascoma. c. Ascoma covered with mud (arrowed MU). Periphyses (PP) are simple; reticulate (arrowed RPR) and anastomosing; pseudoparaphyses in the upper part of the centrum arising from the venter wall; ostiolar canal (OC), trabeculate pseudoparaphyses (PR) arise between the asci from the base of the centrum; d Longitudinal section of ascoma with stalk, asci and pseudoparaphyses; e Narrow pseudoparaphyses; f Cylindrical ascus; g Ascus tip with ocular chamber (arrows); h, i Ascospore in ascus with apical and basal appendages (arrow). Ascospore in ascus (h) with apical (AA) and basal (BA) appendages; j–t Bicelled ascospores. Scale bars a, b = 250 μm; d = 100 μm; c, f, g, j = 50 μm; k–t = 20 μm; e, h, i = 10 μm
Taxonomical changes
-
1.
Manglicolaceae Suetrong & E.B.G. Jones, fam. nov. MycoBank 563225
Etymology: Named after the type genus.
Family in the Jahnulales, Dothideomycetes, Ascomycota. Ascomata obtusely clavate to fusiform, stipitate, solitary or aggregated, superficial, ostiolate, epapillate, brown, coriaceous and large (1,100–1,750 × 290–640 μm). Ostiole wide ca. 100–200 μm at the apex. Hamathecium comprising trabeculate, hyaline pseudoparaphyses simple, numerous, and septate. Asci cylindrical, bitunicate, thick-walled, with an ocular chamber. Ascospores uniseriate, pale brown to brown, fusiform, apiculate, unequally 1-septate, constricted at the septum, apical cell larger, basal cell turbinate, with gelatinous appendages at both ends.
Type genus: Manglicola Kohlm. & E. Kohlm., Mycologia 63: 840 (1971).
Notes: The Manglicolaceae shares a number of features with the Aliquandostipitaceae: ascomata with short stalks, cylindrical asci, appendaged ascospores, wide hyphae (ca. 40 μm wide), and all are saprobic species in aquatic habitats. However, they differ in that Manglicolaceae ascomata are large, with a wide ostiole, have large unequally 1-septate ascospores that are pale brown with a turbinate basal cell, few asci per ascoma, and they differ by their marine mangrove habitat.
Huhndorf (1994) referred Manglicola to the Hypsostromataceae, a family with no previously known relationship to any group in the Dothideomycetes, but “probably with affinities to the Melanommatales” (Mugambi and Huhndorf 2009). Characterisitics that unite Manglicola and the Hypsostromataceae include superficial, large, elongate ascomata (stalked) with a soft texture, trabeculate pseudoparaphyses, stipitate asci attached in a basal arrangement in the centrum and fusiform, septate ascospores (Huhndorf 1994). Our dataset places Hypsostroma saxicola and H. caimitalense (Hypsostromataceae) in the Pleosporales with high bootstrap support (Fig. 1).
-
2.
Genera in the Aliquandostipitaceae
Genera that are well circumscribed include Aliquandostipite, Megalohypha, and the anamorphs Speiropsis, Brachiosphaera and Xylomyces. Jahnula potamophila might be better placed in Megalohypha, and the position of J. australiensis requires further consideration based on the molecular evidence presented in this study.
Taxa rejected from inclusion in the family are:
Speiropsis irregularis R.H. Petersen, Mycologia 55: 26 (1963).
Current name:
Arbusculina irregularis (R.H. Petersen) Marvanová & Descals, Trans. Br. Mycol. Soc. 89: 499 (1987).
Xylomyces aquaticus (Dudka) K.D. Hyde & Goh, Mycol. Res. 103: 1573 (1999).
X. elegans Goh, W.H. Ho, K.D. Hyde & K.M. Tsui, Mycol. Res. 101: 1324 (1997).
Both species are referred to the Pleosporales based on molecular evidence.
Conclusions
The fact that taxa in the Jahnulales share many morphological features, such as wide brown hyphae, ascomata with stalks, bicelled ascospores with or without polar appendages/apical caps/pads, makes it difficult to delineate genera using morphology alone. Manglicola with its large ascomata and wide ostioles, apiculate appendaged ascospores with polar appendages is clearly defined, as are most Aliquandostipite taxa with their clavate/ovoid asci, fusiform, smooth-walled ascospores with a large gelatinous sheath that balloons out in water. However, A. siamensiae has a narrow sheath.
Within the Jahnula clades, there is considerable variation in the morphology of the different taxa. Species in Jahnula sensu stricto share the following features: cylindrical to clavate asci, brown to dark brown ascomata, 1-septate ascospores lacking a sheath or polar caps or appendages; that distinguish them from other Jahnula-like species.
A notable feature of the Jahnulales is the procession of stalked ascomata, with the stalks varying in length from 18–30 × 450–500 μm, to 1.6 mm. However, this is not a constant feature, as illustrated in Fig. 7 of an undescribed Jahnula species which has both sessile and stalked ascomata. Indeed it is interesting to speculate as to the function of these long stalks. Does it raise the ascomata from the substratum (Fig. 7c) above the water level for the discharge of ascospores, or to keep it clear from the debris on the surface of the wood?
Jahnula seychellensis and J. systyla possess dimorphic asci and ascospores, with one type larger than the other (J. seychellensis-Asci 1: 125–150 × 17–23 μm, Asci 2: 112–137 × 30–35 μm, Ascospores 1: 29–36 × 9–12.5 μm, Ascospores 2: 30–40 × 17–23 μm; J. systyla- Asci 1: 124–150 × 22–30 μm, Asci 2: 120–190 × 34–46 μm, Ascospores 1: 28–40 × 8–13 μm, Ascospores 2: 44–68 × 13–19 μm). Isolates were made of both types of ascospores in J. seychellensis and sequenced to determine if they were the same species. Sequence data confirmed that they are identical as shown in Fig. 1, sequence SS1536.1 has large spores, while SS1536.2 has smaller spores.
The Jahnulales are a unique group of ascomycetes with their stalked ascomata, variously appendaged ascospores, and wide vegetative hyphae, predominantly aquatic, tropical and growing on wood or palm fronds. However, little is known of their role in nature other than they are saprobes of submerged wood and palm fronds in aquatic habitats. Simonis et al. (2008) indicated that Jahnula bipileata is able to cause soft rot of wood and thus they may play a role in the decomposition of organic matter in freshwater and marine habitats. In a study of long term submergence of 9 timber test blocks in a stream and a peat swamp in Thailand, Jahnula appendiculata and Xylomyces chlamydosporus were recovered on eight of the timbers at the peat swamp site after a three year exposure period. A Jahnula sp. was only recovered on one test block. No Jahnula species was recovered on the test blocks in a fast flowing river at the Hala Bala Wildlife Sanctuary. The marine species Manglicola guatemalensis was known only from two previous collections (Hyde 1988; Kohlmeyer and Kohlmeyer 1971) until further collections were made on the brackish water palm Nypa fruticans (Suetrong et al. 2010). Ascomata are often covered in mud on the bases of fronds of the palm.
Key to the genera in the Jahnulales
-
1. Conidial genera…………………….........................2
-
1. Ascomatal genera…………………….....................4
-
2. Conidiophores and conidia lacking, chlamydospores intercalary, multiseptate…………….............Xylomyces
-
2. Conidiophores and conidia present….......……………3
-
3. Conidia tetraradiate with 4–8 appendages (arms)………………………………………..Brachiosphaera
-
3. Conidia catenate, branche.............................Speiropsis
-
4. Ascomata larger than 1,150 μm, wide ostioles (100–200 μm), marine….……………………….Manglicola
-
4. Ascomata smaller than 1,000 μm, ostioles circa smaller than 100 μm wide, not marine………...……..5
-
5. Ascospores with a thin or broad gelatinous sheath...........…………………………Aliquandostipite
-
5. Ascospores with apical caps, appendages, or lacking such structures…..........………………………………6
-
6. Ascospores with longitudinal sulcate striations, lacking appendages or a gelatinous sheath........Megalohypha
-
6. Ascospores lacking longitudinal sulcate striations……………………………………………........Jahnula
Key to Aliquandostipite species
-
1. Ascospores with broad gelatinous sheaths…..............2
-
1. Ascospores with thin gelatinous sheath………………………………..........................................A. siamensis
-
2. Ascospores with arcicular crystals and broad, fusiform gelatinous sheath……………………………3
-
2. Ascospores lacking arcicular crystals……….............4
-
3. Ascospores sheath small, constricted at the septum, with numerous median filamentous appendages…………………............................................A. minuta
-
3. Ascospores sheath long and sigmoid, not constricted at the septum, with two short tapering appendages apically…………………………...........................A. crystallinus
-
4. Ascospores 30–40 × 9–20 μm..…………A. separans
-
4. Ascospores 50–70 × 13–20 μm……..A. khaoyaiensis
-
Key to Jahnula species
-
1. Ascospores with a sheath and polar appendages………………...………………………J. appendiculata
-
1. Ascospores lacking pads, appendages or a sheath......…………………………………………………….2
-
1. Ascospores with appendages, caps or gelatinous apical pads……………………....……………………5
-
2. Ascospore wall verrculose...............……………….3
-
2. Ascospore wall smooth…………..………………..4
-
3. Ascospore 20–28 × 10–12 μm, wall minutely verruculose………………………………….J. sangamonensis
-
3. Ascospores 23–28 × 11–13 μm, wall verruculose…………………………………………J. poonythii
-
4. Ascospores narrow, 6–8 μm…...……J. australiensis
-
4. Ascospores wider than 8 μm…….......…………….5
-
5. Ascospores 30–41 × 11–16 μm, cells equal in length………………………………………J. aquatica
-
5. Ascospores 30–40 × 8–12 μm, apical cell larger……………………………………........…….J. apiospora
-
6. Ascospores with gelatinous apical pads……...……7
-
6. Ascospores with a sheath……….………..……….10
-
6. Ascospores 18–20 × 5–6.5 μm, with polar appendage……...….………………………………….J. morakotii
-
7. Ascomata dimorphic, ascospores 39–49 × 17–23 μm.....................................................J. seychellensis
-
7. Ascomata not dimorphic……………..……………8
-
8. Ascospores shorter than 30 μm...............J. bipileata
-
8. Ascospores longer than 30 μm................................9
-
9. Ascospores 42–52 × 16–33 μm………….J. bipolaris
-
9. Ascospores 39–52 × 16–23 μm, helement shaped……………....…………………….J. sunyatsenii
-
10. Ascospores with thin sheaths.……..……....…….11
-
10. Ascospores with wide sheaths…………..………12
-
11. Ascospores 26–37 × 15–18 μm, ascomata with short papilla………………………………J. granulosa
-
11. Ascospores 32–45 × 12–15 μm, ascomata with a neck……………...………………………….J. rostrata
-
12. Ascospores 46–54 × 17–20 μm, sheath margin wavey…...……………………………..J. potamophila
-
12. Ascospores 44–68 × 13–19 μm, sheath margin not wavey…………..…………………………….J. systyla
Key to Xylomyces species
-
1. Width of chlamydospores at least 25 μm…….……2
-
1. Width of chlamydospores less than 20 μm…......…5
-
2. Chlamydospores with fewer than 15 septa, 110–455 × 25–45 μm……………………X. chlamydosporus
-
2. Chlamydospores with more than 15 septa…...…….3
-
3. Chlamydospores with 6–26 septa, 140–575 × 25–50 μm……………….……………………X. giganteus
-
3. Chlamydospores with 11–43 (68) septa, 95–370(−500) × (8–) 9–16 μm……..…………X. rhizophorae
-
4. Chlamydospores with distinct punctuate wall ornamentations, 44–74 × 10–16 μm……...……X. punctatus
-
4. Clamydospores lack distinct punctuate wall ornamentations……………………………………………5
-
5. Chlamydospores 3–7 septate, 42–56 × 7–11 μm………………………………………...……….X. pusillus
-
5. Chlamydspores 4–11 septate predominantly 11, 126–152 × 7–9 μm………………………….X. foliicola
References
Bunyard BA, Nicholson MS, Royse DJ (1994) A systematic assessment of Morcella using RFLP analysis of the 28S ribosomal RNA gene. Mycologia 86:762–772
Campbell J, Ferrer A, Raja HA, Sivichai S, Shearer CA (2007) Phylogenetic relationships among taxa in the Jahnulales inferred from 18S and 28S nuclear ribosomal DNA sequences. Can J Bot 85:873–882
Castañeda RF, Kendrick B (1990) Conidial fungi from Cuba: II. Univ Waterloo Biol Ser 33:1–61
Chang HS (1994) Notes on the genus Brachiosphaera from Taiwan. Bot Bull Acad Sin 35:125–127
Descal EC, Webster J (1978) Miladina lechithina (Pezizales), the ascigerous state of Actinospora megalospora. Trans Br Mycol Soc 70:466–472
Dudka IA (1966) New and rare species of fungi imperfecti from the basins of the southern part of Kiev Polessye. Ukrain Bot J 23:91–95
Edgar R (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res 32:1792–1797
Farris JS (1989) The retention index and the rescaled consistency index. Cladistics 5:417–419
Felsenstein J (1985) Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Ferrer A, Sivichai S, Shearer CA (2007) Megalohypha, a new genus in the Jahnulales from aquatic habitats in the tropics. Mycologia 99:456–460
Goh TK, Ho WH, Hyde KD, Tsui KM (1997) Four new species of Xylomyces from submerged wood. Mycol Res 101:1323–1328
Gönczöl J, Révay Á, Fisher PJ (1990) Notes on Vargamyces aquaticus, a water borne dematiaceous hyphomycete. Mycotaxon 39:301–310
Goos RD, Brooks RD, Lamore BJ (1977) An undescribed hyphomycete from wood submerged in a Rhode Island stream. Mycologia 69:280–286
Hall T (2004) Bioedit version 6.0.7. Department of Microbiology, North Carolina State University. http://www.mbio.ncsu.edu/BioEdit/bioedit.html
Hawksworth D (1984) Observations on Jahnula Kirschst., a remarkable aquatic pyrenomycete. Sydowia 37:43–46
Huelsenbeck JP, Ronquist FR (2001) MRBAYES: Bayesian inference of 380 phylogenetic trees. Biometrics 17:754–755
Huhndorf SM (1994) Neotropical Ascomycetes 5. Hypsostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia 86:266–269
Hyde KD (1988) Studies on the tropical marine fungi of Brunnei. Bot J Linn Soc 98:135–151
Hyde KD (1992) Tropical Australian Freshwater Fungi. II. Annulatascus velatispora gen. et sp. nov., A. bipolaris sp. nov. and Nais aquatica sp. nov. (Ascomycetes). Aust Syst Bot 5:117–124
Hyde KD (1993) Tropical Australian Freshwater Fungi. V* Bombardia sp., Jahnula australiensis sp. nov., Savoryella aquatica sp. nov. and S. lignicola sp. nov. Aust Syst Bot 6:161–167
Hyde KD, Goh TK (1999) Fungi on submerged wood in the River Coln, the Cotswolds. UK Mycol Res 103:1561–1574
Hyde KD, Wong SW (1999) Tropical Australian freshwater fungi. XV. The ascomycetes genus Jahnula, with five new species and one new combination. Nova Hedw 68:489–509
Inderbitzin P, Landvik S, Abdel-Wahab MA, Barbee ML (2001) Aliquandostipitaceae, a new family for two new tropical ascomycetes with unusually wide hyphae and dimorphic ascomata. Am J Bot 88:52–61
Kirschstein W (1936) Beiträge zur Kenntnis der Ascomyceten und ihrer Nebenformen besonders aus der Mark Brandenburg und dem Bayerischen Walde. Annales Mycologici 34:180–210
Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order of Hominoidea. J Mol Evol 29:170–179
Kluge AG, Farris JS (1969) Quantitative physics and the evolution of anurans. Syst Zool 18:1–32
Kohlmeyer J, Kohlmeyer E (1971) Marine fungi from tropical America and Africa. Mycologia 63:831–861
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology. The higher Fungi. Academic, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1998) A new marine Xylomyces on Rhizophora from the Caribbean and Hawaii. Fungal Divers 1:159–164
Landvik S (1996) Neolecta, a fruit–body–producing genus of the basal ascomycetes, as shown by SSU and LSU rDNA sequence. Mycol Res 100:199–202
Marvanová L, Descals E (1987) New taxa and new combinations of aquatic Hyphomycetes. Trans Br Mycol Soc 89:499–507
Mugambi GK, Huhndorf SM (2009) Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Plesporomycetidae), Dothideomycetes, Ascomycota). Stud Mycol 64:103–121
Nylander JAA (2004) MrModeltest 2.2: Program distributed by the author. Evolutionary Biology Centre Uppsala University, Sweden
O’Donnell K, Cigelnik E, Weber NS, Trappe JM (1997) Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89:48–65
Page RMD (1996) Treeview: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Pang KL, Abdel-Wahab MA, Sivichai S, El-Sharouney H, Jones EBG (2002) Jahnulales (Dothidiomycetes, Ascomycota): a new order of lignicolous freshwater ascomycetes. Mycol Res 106:1031–1042
Pinruan U, Jones EBG, Hyde KD (2002) Aquatic fungi from peat swamp palms: Jahnula appendiculata sp. nov. Sydowia 54:242–247
Prihatini R, Boonyuen N, Sivichai S (2008) Phylogenetic evidence that two submerged-habitat fungal species, Speiropsis pedatospora and Xylomyces chlamydosporus belong to the order Jahnulales Incertae Sedis Dothideomycetes. Microbiol Indonesia 2:136–140
Raja H, Shearer CA (2006) Jahnula species from North and Central America, including three new species. Mycologia 98:319–332
Raja HA, Carter A, Platt HW, Shearer CA (2008) Freshwater ascomycetes: Jahnula apiospora (Jahnulales, Dothideomycetes), a new species from Prince Edward Island, Canada. Mycoscience 49:326–328
Raja HA, Ferrer A, Shearer CA (2005) Aliquandostipite crystallinus, a new ascomycete species from submerged wood in freshwater habitats. Mycotaxon 91:207–215
Rambaut A (1996) Sequence alignment editor. Version 2.0. Department of Zoology, University of Oxford, Oxford, Available from http://evolve.zoo.ox.ac.uk/Se-Al/Se-Al.html
Rehner S (2001) Primers for Elongation Factor 1-a (EF1-a). http://ocid.NACSE.ORG/research/deephyphae/EF1primer.pdf
Scheinpflug H (1958) Untersuchungen über die Gattung Didymosphaeria Fuck. und einige verwandte Gattungen. Berichte der schweizerischen Botanischen Gesellschaft 68:325–385
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multiple phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Schoch CL, Sung GH, Lopez-Giraldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny PB, Kauff F, Wang Z, Gueidan C, Andrie R, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson B, Bonito G, Groenewald JZ, Arzanlou M, de Hoog GS, Crous PW, Hewitt D, Pfister DH, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman AY, Lumbsch TH, Lucking RL, Budel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O’donnell K, Sipman H, Rogers JD, Shoemaker RA, Sugiyama J, Summerbell RC, Untereiner W, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW (2009) The Ascomycota Tree of Life: A Phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, Hirayama K, Marvanova L, Hyde KD, Zhang Y (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153
Simonis JL, Raja HA, Shearer CA (2008) Extracellular enzymes and soft rot decay: Are ascomycetes important degraders in fresh water? Fungal Diver 31:135–146
Sivichai S, Boonyuen N (2010) Jahnula morakotii, sp. nov. and J. appendiculata from a peat swamp in Thailand. Mycotaxon 112:475–481
Sivichai S, Sri-indrasudhi V, Jones EBG (2011) Jahnula aquatica and its anamorph Xylomyces chlamydosporus on submerged wood in Thailand. Mycotaxon 116:137–142
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Suetrong S, Sakayaroj J, Phonpaichit S, Jones EBG (2010) Morphological and molecular characteristics of a poorly known marine ascomycete, Manglicola guatemalensis (Jahnulales: Pezizomycotina; Dothideomycetes, incertae sedis): new lineage of marine ascomycetes. Mycologia 102:83–92
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B (2009) Molecular systematic of the marine Dothideomycetes. Stud Mycol 64:155–173
Swofford DL (2002) PAUP: Phylogenetic analysis using parsimony, Version 4.0b10. Sinauer Associates, Inc. Publishers, Sunderland
'Thompson JD, Higgin DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Tóth S (1979) Vargamyces, a new genus of hyphomycetes on submerged plant debris. Acta Bot Acad Sci Hung 25:403–410
Vrijmoed LLP (2000) Isolation and culture of higher filamentous fungi. In: Hyde KD, Pointing SB (eds) Marine mycology—A practical approach. Fungal Diversity Press, Hong Kong, pp 1–20
Wegelin H (1894) Beitrag zur Pyrenomycetenflora der Schweiz. Mitteilungen der Thurgauischen Naturforschenden Gesellschaft 11:1–12
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA gene for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JS, White TJ (eds) PCR Protocol: A guide to methods and applications. Academic, San Diego, pp 315–322
Acknowledgements
We are particularly grateful to Dr Walter Jaklitsch for his thorough review of this paper, his invaluabale suggestions, and especially for drawing our attention to the earlier name for Jahnula aquatica. K.L. Pang acknowledges the financial support by National Science Council of Taiwan (NSC98-2621-B-019-002-MY3). Satinee Suetrong thanks Dr. Jariya Sakayaroj for advice on phylogenetic analysis. Miss Juntima Chanprasert is thanked for RAxML analysis. This work was supported by Fungal DNA Barcoding Grant P-10-11328 and the TRF/BIOTEC program Biodiversity Research and Training Grant BRT R_251006, R_325015, R_252057 and R_251009 to study marine and freshwater fungi of Thailand. For their continued interest and support we also thank BIOTEC: Prof. Morakot Tanticharoen; Dr. Kanyawim Kirtikara; and Dr. Lily Eurwilaichitr.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Sequences of members of the Jahnulales used in this study with taxonomic placement, fungal taxa, culture numbers and GenBank sequence numbers (newly generated sequences in bolda) (DOC 148 kb)
Supplementary Table 2
Sequences from the GenBank of taxa from selected order of the Dothideomycetes (DOC 232 kb)
Rights and permissions
About this article
Cite this article
Suetrong, S., Boonyuen, N., Pang, KL. et al. A taxonomic revision and phylogenetic reconstruction of the Jahnulales (Dothideomycetes), and the new family Manglicolaceae . Fungal Diversity 51, 163–188 (2011). https://doi.org/10.1007/s13225-011-0138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-011-0138-5