Abstract
Purpose
Cobalt (Co) is a toxic metal to the environment and human’s health. The purpose of the study is to achieve an investigation into the efficacy of calcium carbonate and cow dung for Co immobilization in fluvo-aquic soil, as well as their effects on the antioxidant system in plants.
Materials and methods
Calcium carbonate and cow dung were incorporated with the Co-polluted fluvo-aquic soil where pakchois (Brassica chinensis L.) were grown. Co concentration, superoxide dismutase (SOD) activity, catalase (CAT) activity, and malondialdehyde (MDA) concentration in the shoots of the mature plants were inspected.
Results and discussion
As calcium carbonate concentration rose (0 to 12 g kg−1), Co concentration in shoots of the plants decreased firstly and then increased again (P < 0.05), while the accumulation level of Co kept decreasing with cow dung concentration rising (P < 0.05). Under the amendment treatments, the SOD activity, CAT activity, and MDA concentration in the shoots were all positively correlated to the Co concentration in the plant tissue (r = 0.792, 0.904, and 0.807, P < 0.01), indicating the antioxidant system receptivity to the Co accumulation. The amendments in soil can alleviate the oxidative stress in pakchois owing to Co pollution. As calcium carbonate concentration ranged from 5.64 to 7.86 g kg−1, the parameters reached a maxima (minimum), respectfully.
Conclusions
Calcium carbonate and cow dung in fluvo-aquic soil are effective for Co immobilization and mitigating any pertinent oxidative stress in pakchoi plants. Calcium carbonate concentration within a range of 5.64 to 7.86 g·kg−1 will achieve optimum efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cobalt (Co) is an element that is naturally present in the environment, occurring in the Earth’s crust at approximately 0.002% primarily in the form of sulfides, oxides, and arsenides (Jensen and Tuchsen 1990). Cobalt (Co) is an important industrial raw material and strategic resource (Chagnes and Pospiech 2013), which has multiple industrial applications, including aircraft engines, magnetic products, high-strength steels, rechargeable batteries, catalysts, pigments, hip implants, etc. (Lison et al. 2001; Smith et al. 2014; Suh et al. 2016). However, Co is also the type of heavy metal which is toxic to human beings and will show poisoning effects inside the body if excessive levels are met. Cobalt (Co) is a respiratory tract sensitizer, the exposure to which may lead to allergies, several serious lung diseases (Sauni et al. 2010), or even cancer (Jensen and Tuchsen 1990). A wide range of industrial manufacturing businesses (e.g., laptops, cell phones, medical devices, pigments) may permit Co accessibility to soil environments and result in serious environmental consequences.
The previous studies on the environmental behaviors of heavy metals in soil mainly focused on the common heavy metals such as chromium (Cr), arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) (Ojuri et al. 2016; Yang et al. 2016; Birke et al. 2017; Evseev and Krasovskaya 2017; Shrivastava et al. 2017). One reason why heavy metals in soil environments reach considerable concern is due to the effortless accumulation in crops (Gupta et al. 2008; Chang et al. 2014; Liu et al. 2015). Heavy metals in crops may lead to serious oxidative stress and deterioration in the quality. Therefore, the antioxidant system in plants, including superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), etc., was always applied as the biomarker for the evaluation of heavy metal accumulation (Liu et al. 2010; Barrameda-Medina et al. 2014; Beals and Byl 2014; Jakovljević et al. 2014; Li et al. 2014). Also, these heavy metals in crops may enter a human’s body through the food chain, resulting in serious health risk (Zheng et al. 2007; Zhuang et al. 2009; Chang et al. 2014) and heightened bodily intoxication concerns. Therefore, not only does the total concentration pose importance, but also the phytoavailability of heavy metals in soil environments need further scientific investigation.
Chemical immobilization is an effective way to reduce the heavy metal phytoavailability in soil and diminish environmental risks. The amendments can lower the phytoavailability of heavy metals through adsorption, precipitation, complexation, and chelation (Guo et al. 2006). Chemical immobilization is widely used in the agricultural practice because of its practical laboratory convenience and economical merit. For common heavy metals (i.e., Cr, As, Cd, Pb, and Hg), it has been proven that many chemical materials can be applied as amendments. Inorganic materials include calcium ion (Lwalaba et al. 2017), lime (Khan and Jones 2008; Guo et al. 2011), zeolite (Gheshlaghi et al. 2008), phosphate (Zhang and Ryan 1999; Melamed et al. 2003; Cao et al. 2009), fly ash (Jala and Goyal 2006), metal oxides (Kumpiene et al. 2008), etc. Organic materials include citrate (Wasay et al. 2001), biochar (Zhang et al. 2013; Ahmad et al. 2014), excrements of livestock (Narwal and Singh 1998; Rosen and Chen 2014), etc. However, in the previous studies, the methods were mainly applied to common heavy metals, and there are limited studies concentrating on Co immobilization in soil environments.
Calcium carbonate can provide ions which can precipitate heavy metal ions in soil or compete with the metal ions for uptake by plants. Cow dung contains organic matter whose functional groups can complex or chelate with the heavy metal ions in soil (Baghaie et al. 2016; Zhu et al. 2017). They are both accessible and cheap materials. The efficacy of the two materials (i.e., calcium carbonate and cow dung) for Co immobilization in fluvo-aquic soil was studied. Fluvo-aquic soil is a typical soil obtained from the Yellow River Plain in central China, which is very important for the food production in several important areas, such as Beijing, Hebei Province, Henan Province, Jiangsu Province, Anhui Province, etc. Therefore, the quality of fluvo-aquic soil needs attention and fluvo-aquic soil is employed in the present study. Pakchoi (Brassica chinensis L.) is a kind of leafy vegetable which generally grow faster with higher transpiration rates than non-leafy vegetables (Luo et al. 2011). Thus, metal uptake by plant roots can be enhanced in pakchois, resulting in the translocation of metals from roots to other vegetable tissues (Chang et al. 2014). What is more, pakchoi is easy to grow and the growth period is short. Given the properties, pakchoi is appropriate for the study on Co phytoavailability in soil. In the present study, pakchois were grown in the soil mixed with exogenous Co and the amendments (i.e., calcium carbonate and cow dung). Soil properties, the Co concentration, and the antioxidant system (i.e., SOD, CAT, and MDA) in shoots of pakchois were analyzed. This study is designed to provide the chemical remediation of heavy metal-polluted soil while producing scientific evidence towards Co immunization experimentation.
2 Materials and methods
2.1 Soil and amendments
Fluvo-aquic soil was collected in a schoolyard at the Beijing Institute of Technology (E: 116° 18′ 40.05″, N: 39° 57′ 35.47″, depth of 0–20 cm). The soil properties include a pH = 7.98, organic carbon concentration = 14.702 g kg−1, total Co = 8.535 mg kg−1, total nitrogen (N) = 1.035 g kg−1, total phosphor (P) = 0.687 g kg−1, total potassium (K) = 0.027 g kg−1, clay particles content = 11.63%, silt particles content = 15.12%, and sand particle content = 73.25%. The soil was air-dried at room temperature for approximately 2 days, while stones and plant material were removed. The soil was then sieved through a 2-mm nylon mesh for subsequent experimentation. The seeds of pakchoi (B chinensis L.) were purchased from the Dasenlin Flower Market at the Chinese Academy of Agricultural Sciences in the Haidian District, Beijing, China. The amendments in the experiment were calcium carbonate and cow dung. Calcium carbonate (AP) was purchased from Beijing Chemical Works, located in the Daxing District, Beijing, China. The cow dung was purchased from Beijing Dahuanshunxin Organic Fertilizer Works, located in the Shunyi District, Beijing, China, whose pH = 7.021 and organic carbon concentration = 181.253 g kg−1. The cow dung was air-dried and sieved through a 2-mm nylon mesh before the experiment was initiated.
2.2 Pot experiment
A pot experiment was carried out in a greenhouse at the Beijing Academy of Agriculture and Forestry Sciences in the Haidian District, Beijing, China. Prepared soil was introduced to plastic pots (12 × 10 cm) with a mass = 300 g·pot−1. Holes on the bottom of each pot were sealed using glass cement to avoid loss of the simulative pollutant. In total, 27 treatments with various concentrations of exogenous Co and amendments in soil concluded the experiment phase, which are shown in Table 1. Exogenous Co was added to the soil through a CoCl2 solution, while the amendments were added in powder form and mixed with soil uniformly. To command the plant growth requirement, N, P, and K were added within the soil to reach the following levels: 3.067, 1.027, and 0.157 g kg−1, respectively (Long et al. 2003). N, P, and K were added in the form of urea, NaH2PO4 and K2SO4 solutions, respectively, while the treatments were conducted in triplicate. The treated soil was incubated in the dark for 1 month, being irrigated with deionized water to 70% of the water holding capacity, to obtain a stable state (Zhao et al. 2016).
Thenceforth, the pakchois were seeded and the density was five seeds·pot−1. The growth period of the plants was 28 days. Plants were regularly watered using distilled water to maintain the moisture content between 60 and 70% water holding capacity (Siebers et al. 2013). The plants were kept under the condition of 28 °C 14-h and 15 °C 10-h cycles (Liu et al. 2011). Ten days after sowing, the seedlings were thinned to three seedlings·pot−1. Shoots of the plants (edible part) were sampled for analysis at the 28th day of growth.
Following the sampling stage, shoot samples were prepared for chemical analysis. After cleaning all the surface contaminants and weighting, the shoots were divided into two groups, one of which was dried at a 105 °C temperature constraint until a constant weight was obtained. Dried samples were grounded into powder for Co concentration analysis. The other group was kept at a 4 °C temperature constraint to retain freshness for analysis of the antioxidant system.
2.3 Analytical procedure
Soil pH (1:2.5, soil/water) was determined with a combination electrode (NY/T 1121.2-2006, proposed by the Ministry of Agriculture of P. R. China). Soil organic carbon concentration was analyzed with the potassium dichromate oxidation spectrophotometric method (HJ 615-2011, proposed by the Ministry of Environmental Protection of P. R. China). All the processes were conducted in triplicate. Soil microbial activity was represented with fluorescein diacetate (FDA) hydrolysis which was measured applying the methods of Choi (2009) and Schnürer and Rosswall (1982). The results were expressed as the fluorescein concentration in soil after the reaction (μmol·fluorescein g−1 soil).
Weights of shoots determined before pre-preparation were used as the biomass. To assay the total Co concentrations in dried plant samples, the prepared samples were digested with concentrated nitric acid (65%), perchloric acid (70%), and hydrofluoric acid (40%) (8:2:8 v/v). The samples and acid (i.e., nitric acid, perchloric acid, and hydrofluoric acid) were introduced into digestion tanks made of polytetrafluoroethylene and heated with a Graphite Digestion System for 2 hours. The residue was then dissolved with diluted nitric acid (1%) for subsequent analysis. Cobalt (Co) concentrations were determined with Agilent 7500C inductively coupled plasma mass spectrometry (ICP-MS). All the processes were conducted in triplicate.
SOD activity in the shoots of the plants was determined by measuring the inhibition of the photochemical reduction of nitro-blue tetrazolium (NBT) using the method of Beauchamp and Fridovich (1971). One unit of SOD activity was defined as the amount of enzyme causing the half-maximal inhibition of NBT reduction under the assay conditions, and the results were expressed as enzyme units·per gram (FW) units. Catalase (CAT) activity was determined by decomposition of H2O2 and was measured spectrophotometrically by assessing the decrease in absorbance at 240 nm (Aebi 1984), and the results were expressed as the nanomole of H2O2 decomposed·per milligrams fresh weight·per minute. MDA concentration in the tissues were determined based on the methods of Heath and Packer (1968), and the results were expressed as nanomoles MDA per·milligram fresh weight. All the processes were conducted in triplicate.
During the analysis process, replicates, blanks, and certified reference material (provided by the National Institute of Metrology, China) were included for quality assurance.
2.4 Data analysis
All the results in the study were expressed as the means of replicates. Data was graphed with the Origin 9.1 scientific graphing program, and statistical analysis was conducted with the SPSS Statistics 20.0 software.
3 Results
3.1 Soil properties
The soil pH under exogenous Co and amendment treatments is shown in Table 2. It can be seen that the soil pH was maintained at a stable level (7.78 to 8.16), which would not change with exogenous Co and amendment concentrations. Organic carbon concentration in soil under each treatment is shown in Fig. 1. With exogenous Co, the concentration of organic carbon in soil significantly decreased (P < 0.05). Based on Fig. 1a, when calcium carbonate concentration was lower than 6 g kg−1, the organic carbon concentration increased in tandem with the calcium carbonate concentration, and the increase rate ranged from 12.5 to 38.7% (P < 0.05). If the calcium carbonate concentration continued rising, the organic carbon concentration would decrease in response. It can be seen from Fig. 1b that the organic carbon concentration kept increasing with the cow dung concentration (0 to 12 g kg−1) by 41.2 to 55.5% (P < 0.05). To investigate the relationship between Co phytoavailability and the toxicity of Co to soil microorganisms, the FDA hydrolysis was employed to represent the activity of microorganisms in soil and to study the Co toxicity to microorganisms. The FDA hydrolysis under each treatment is shown in Fig. S1 (Electronic Supplementary Material), and there was a positive correlation between the FDA hydrolysis and the organic carbon concentration in soil (r = 0.787, P < 0.01).
Organic carbon concentration in soil under each treatment. a Calcium carbonate. b Cow dung. Results of Duncan Multiple Comparison are expressed as letters in the figure. Different letters in each group (each exogenous Co level) indicate a significant difference (P < 0.05). The same letters indicate differences were not significant, as the same is applicable below
3.2 Biomass of pakchois
The biomass of the shoots (edible part) of the plant under each treatment is shown in Fig. 2. When exogenous Co was added, the biomass significantly decreased (P < 0.05). With the concentration of calcium carbonate increasing, the biomass increased firstly but then decreased again (P < 0.05), while the biomass kept increasing with the cow dung concentration (P < 0.05). There was a positive correlation between the biomass and soil organic carbon concentration (r = 0.720, P < 0.01).
3.3 Cobalt accumulation in pakchois
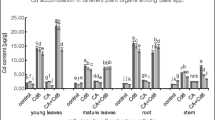
Cobalt (Co) concentrations in the shoots (edible part) of pakchois are shown in Fig. 3. With the exogenous Co concentration increasing, the Co accumulation level in the shoots also increased (P < 0.05). When calcium carbonate concentration in soil was lower than 6 g kg−1, the Co concentration in the shoots kept decreasing while the calcium carbonate concentration increased, and the decrease rate ranged from 41.3 to 55.3% (P < 0.05). However, as calcium carbonate concentration continued increasing, the Co accumulation level increased again (P < 0.05). As can be seen in Fig. 3b, with the cow dung concentration increasing (0 to 12 g kg−1), the Co concentration in the shoots kept decreasing, and the decreased rate was in the range of 31.6 to 57.5% (P < 0.05), indicating good efficacy for Co immobilization. What is more, Co concentration in the shoots was negatively correlated to the soil organic carbon concentration (r = −0.814, P < 0.01) as well as to the FDA hydrolysis (r = −0.665, P < 0.01).
To conclude, both calcium carbonate and cow dung were effective for the Co immobilization in fluvo-aquic soil, but calcium carbonate had an optimum concentration range, which will be discussed in another section.
3.4 Antioxidant system in pakchois
The antioxidant system (SOD, CAT, and MDA) condition in the shoots of pakchois is shown in Fig. 4. The exogenous Co in soil led to increases in SOD activity, CAT activity, and MDA concentration (P < 0.05). When the calcium carbonate concentration was lower than 6 g kg−1, the SOD activity, CAT activity, and MDA concentration all decreased as calcium carbonate concentration rose (P < 0.05). However, as the concentration continued increasing, the three parameters began to increase (P < 0.05).
In order to investigate the mechanism of changes in the antioxidant system, correlation analysis between the Co concentrations in plants, the biomass, and enzyme activities was conducted. Principal component analysis (PCA) was conducted for further inspection. The results of the statistical analysis are shown in Table 3 and Fig. 5. As the table depicts, the Co concentration in shoots was negatively correlated to the biomass (r = −0.526, P < 0.01). Moreover, it can be noticed that Co concentration in shoots were positively correlated to the SOD and CAT activity, as well as the MDA concentrations (r = 0.792, 0.904, and 0.807, respectively, P < 0.01). As illustrated in Fig. 5, two principal components (PC1 and PC2) with eigenvalues higher than 1.0 were extracted from the PCA. PC1 contributed 72.174% of the total variance and is characterized by loadings of soil organic carbon (r = −0.904), biomass (r = −0.832), Co concentration in shoots (r = 0.891), SOD activity (r = 0.950), CAT activity (r = 0.963), and MDA concentration (r = 0.872). PC2 contributed 15.073% of the total variance and is characterized by loadings of soil pH (r = 0.990).
3.5 Optimum concentration of calcium carbonate
Represented in Figs. 1 to 4, the soil organic carbon concentration, Co concentration in plant shoots, SOD activity, CAT activity, and MDA concentration decreased firstly and then rose again with the calcium carbonate concentration increasing, while the variation tendency of soil organic carbon concentration and biomass was completely opposite. In order to investigate the optimum concentration, a quadratic regression analysis between the parameters and calcium carbonate concentration was conducted, and the results are shown in Table 4. It is revealed that when the calcium carbonate concentration was in a range of 5.64 to 7.86 g kg−1, the parameters reached their maxima (minimum) (i.e., extreme point x in Table 4). Therefore, the optimum efficacy could be achieved.
4 Discussion
It can be seen from Fig. 1 that exogenous Co in soil could lead to a significant decrease in organic carbon concentration in soil, which might result from the toxicity of Co to the soil microorganisms. According to the results of Kızılkaya et al. (2004), there was a positive correlation between soil organic carbon and the activity of microorganism. Singh et al. (2014) and Yang et al. (2017) investigated the soil microbial community structure under heavy metal contamination. They found that the heavy metals could severely restrain microbial growth, because of their known toxicity to microorganisms. They also found that phospholipid fatty acid (PLFA) contents and substrate utilization rates were negatively correlated to the heavy metal concentration in soil, while positively correlated to organic carbon concentration. What is more, based on the results of Sitte et al. (2015) and Vittori Antisari et al. (2016), Co in soil could reduce the microbial activity as well as lead to community structure variation. Therefore, it can be speculated that the toxicity of Co to the microorganisms in soil lead to a reduction of microbial activity and variation of community structure which was the reason why soil organic carbon concentration decreased significantly. Under calcium carbonate and cow dung treatments, the correlation analysis result showed that both soil organic carbon concentration and the FDA hydrolysis were negatively correlated to the Co concentration in the plant tissue, which was in agreement with the results of Wang et al. (2007) that the phytoavailability of heavy metals in soil was positively correlated to the toxicity to microorganisms. The application of calcium carbonate and cow dung could contribute to reduce the devastation to organic carbon as well as the FDA hydrolysis in soil, illustrating that these amendments could not only change the phytoavailability of Co in soil, but also reduce the Co toxicity to microorganisms.
Based on Fig. 2, the exogenous Co in soil lead to a significant decrease in the biomass of the plant, and there was a negative correlation between the biomass and the Co concentration in plant tissues (Table 3), indicating the negative influence of Co on the growth of plants. Cobalt (Co) may inhibit shoot growth directly by inhibition of cell division, cell elongation, or a composite of both, resulting in the limited exploration of soil volume for uptake and translocation of nutrients, water, and induced mineral deficiency (Jaleel et al. 2009). On the other hand, according to Table 3, there was a positive correlation between biomass and soil organic carbon concentration (P < 0.05). The soil organic carbon could reflect the fertility of the soil; therefore, the higher soil organic carbon concentration could promote the growth of crops and lead to a larger biomass.
According to Fig. 3, when calcium carbonate concentration was lower than the optimum concentration, the Co accumulation level in the plant kept decreasing while the calcium carbonate concentration increased. The reason for this phenomenon might be based on calcium carbonate providing CO3 2− which could bound to the free Co ion in soil, resulting in a precipitate that was unable to be uptaken by the plants (Zhao and Saigusa 2007). However, as calcium carbonate concentration continued increasing, the Co accumulation level increased again, which might be because of the excessive Ca2+ from calcium carbonate. The excessive Ca2+ might compete with the Co ion for the adsorption sites in soil, leading to more free Co ion and higher phytoavailability (Smičiklas et al. 2015). With the cow dung concentration increasing (0 to 12 g kg−1), the Co concentration in the shoots kept decreasing, which might result from the organic matter whose functional groups can complex or chelate with the Co ions in soil (Baghaie et al. 2016; Zhu et al. 2017).
Statistical results revealed that the oxidative stress caused by Co accumulation in plant tissue was the reason of the variation of the antioxidant parameters (Table 3 and Fig. 5). Heavy metals that accumulated in the plant tissue could lead to increases in reactive oxygen species (ROS) (Liu et al. 2010). SOD is considered as the first defense against ROS as it acts upon superoxide radicals, which are produced in different compartments of the cell and act as a precursor to other ROS (Alscher et al. 2002). CAT is present in peroxisomes and mitochondria where it converts H2O2 to water and molecular oxygen, in order to maintain the H2O2 level as an adaptive mechanism of the plants (Shao et al. 2005). MDA concentration in plant tissue always applied to represent the lipid peroxidation, an increase which could result from the ROS-induced membrane lipid peroxidation (Mahmood et al. 2016; Venkatachalam et al. 2017). Therefore, it can be speculated that the Co accumulation in plant tissue could lead to an increase in ROS levels. The increase in SOD activity, CAT activity, and MDA concentration was the response of the antioxidant to protect the cells from the oxidative damage. Whence, SOD, CAT, and MDA could be used as biomarkers when evaluating the Co pollution in soil and pakchois. An application of the amendments could effectively relieve the oxidative stress caused by Co accumulation.
5 Conclusions
Calcium carbonate and humic acid are both effective amendments for reducing the Co phytoavailability to pakchois (Brassica chinensis L.) in flvuo-aquic soil. With the calcium carbonate concentration rising (0 to 12 g kg−1), the Co accumulation levels within the shoots of pakchois decreased firstly and then proceeded to increase, while the Co concentrations in the plants keep decreasing as cow dung concentration rose. The Co accumulation in pakchois leads to significant oxidative stress and makes the SOD activity, CAT activity, and MDA concentration increase. Under amendment treatments, the three antioxidant parameters are all positively correlated to the Co accumulation level in the plants, indicating calcium carbonate and cow dung can effectively relieve the oxidative stress. When calcium carbonate concentration ranged from 5.64 to 7.86 g kg−1, the parameters reach their maxima (minimum).
In conclusion, calcium carbonate and cow dung are effective amendments for Co immobilization in fluvo-aquic soil, and can also relieve the oxidative stress caused by Co accumulation. The calcium carbonate concentration should be in the range of 5.64 to 7.86 g kg−1 to achieve optimum efficacy.
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Ahmad M, Lee SS, Lim JE, Lee S, Cho JS, Moon DH, Hashimoto Y, Ok YS (2014) Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 95:433–441
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Baghaie A, Khoshgoftarmanesh A, Afyuni M (2016) Phytoavailability of lead (Pb) for corn and sunflower as affected by Pb-enriched sewage sludge and cow manure. J Residuals Sci Tech 13:251–257
Barrameda-Medina Y, Montesinos-Pereira D, Romero L, Ruiz JM, Blasco B (2014) Comparative study of the toxic effect of Zn in Lactuca sativa and Brassica oleracea plants: I. Growth, distribution, and accumulation of Zn, and metabolism of carboxylates. Environ Exp Bot 107:98–104
Beals C, Byl T (2014) Chemiluminescent examination of abiotic oxidative stress of watercress. Environ Toxicol Chem 33:798–803
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to PAGE. Anal Biochem 44:276–287
Birke M, Reimann C, Rauch U, Ladenberger A, Demetriades A, Jähne-Klingberg F, Oorts K, Gosar M, Dinelli E, Halamić J (2017) GEMAS: cadmium distribution and its sources in agricultural and grazing land soil of Europe—original data versus clr-transformed data. J Geochem Explor 173:13–30
Cao X, Wahbi A, Ma L, Li B, Yang Y (2009) Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J Hazard Mater 164:555–564
Chagnes A, Pospiech B (2013) A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J Chem Technol Biot 88:1191–1199
Chang CY, Yu HY, Chen JJ, Li FB, Zhang HH, Liu CP (2014) Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ Monit Assess 186:1547–1560
Choi J (2009) Adsorption, bioavailability, and toxicity of cadmium to soil microorganisms. Geomicrobiol J 26:248–255
Evseev AV, Krasovskaya TM (2017) Toxic metals in soils of the Russian North. J Geochem Explor 174:128–131
Gheshlaghi ZT, McLaren RG, Adams JA (2008) Effect of treated zeolite, iron waste, and liming on phytoavailability of Zn, Cu, and Ni in long-term biosolids-amended soils. Aust J Soil Res 46:509–516
Guo G, Zhou Q, Ma LQ (2006) Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: a review. Environ Monit Assess 116:513–528
Guo XF, Wei ZB, Wu QT, Qiu JR, Zhou JL (2011) Cadmium and zinc accumulation in maize grain as affected by cultivars and chemical fixation amendments. Pedosphere 21:650–656
Gupta S, Nayek S, Saha RN, Satpati S (2008) Assessment of heavy metal accumulation in macrophyte, agricultural soil, and crop plants adjacent to discharge zone of sponge iron factory. Environ Geol 55:731–739
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jakovljević T, Bubalo MC, Orlović S, Sedak M, Bilandžić N, Brozinčević I, Redovniković IR (2014) Adaptive response of poplar (Populus nigra L.) after prolonged Cd exposure period. Environ Sci Pollut R 21:3792–3802
Jala S, Goyal D (2006) Fly ash as a soil ameliorant for improving crop production-a review. Bioresour Technol 97:1136–1147
Jaleel CA, Jayakumar K, Zhao CX, Azooz MM (2009) Antioxidant potentials protect Vigna radiata (L.) Wilczek plants from soil cobalt stress and improve growth and pigment composition. Plant Omics 2:120–126
Jensen AA, Tuchsen F (1990) Cobalt exposure and cancer risk. Crit Rev Toxicol 20:427–437
Khan MJ, Jones DL (2008) Chemical and organic immobilization treatments for reducing phytoavailability of heavy metals in copper-mine tailings. J Plant Nutr Soil Sci 171:908–916
Kızılkaya R, Aşkın T, Bayraklı B, Sağlam M (2004) Microbiological characteristics of soils contaminated with heavy metals. Eur J Soil Biol 40:95–102
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments - a review. Waste Manag 28:215–225
Li Y, Wang L, Yang L, Li H (2014) Dynamics of rhizosphere properties and antioxidative responses in wheat (Triticum aestivum L.) under cadmium stress. Ecotox Environ Safe 102:55–61
Lison D, De BM, Verougstraete V, Kirschvolders M (2001) Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup Environ Med 58:619–625
Liu D, Zhang S, Chen Z, Qiu W (2010) Soil cadmium regulates antioxidases in sorghum. Agr Sci China 9:1475–1480
Liu W, Zhou Q, Zhang Z, Hua T, Cai Z (2011) Evaluation of cadmium phytoremediation potential in Chinese cabbage cultivars. J Agr Food Chem 59:8324–8330
Liu B, Huang Q, Cai H, Guo X, Wang T, Gui M (2015) Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem 188:294–300
Long XX, Yang XE, Ni WZ, Ye ZQ, He ZL, Calvert DV, Stoffella PJ (2003) Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun Soil Sci Plan 34:1421–1434
Luo CL, Liu CP, Wang Y, Liu X, Li FB, Zhang C, Li XD (2011) Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 186:481–490
Lwalaba JLW, Zvobgo G, Fu L, Zhang X, Mwamba TM, Muhammad N, Mundende RPM, Zhang G (2017) Alleviating effects of calcium on cobalt toxicity in two barley genotypes differing in cobalt tolerance. Ecotox Environ Safe 139:488–495
Mahmood S, Ishtiaq S, Yasin G, Irshad A (2016) Dose dependent rhizospheric Ni toxicity evaluation: membrane stability and antioxidant potential of Vigna species. Chil J Agr Res 76:378–384
Melamed R, Cao X, Chen M, Ma LQ (2003) Field assessment of lead immobilization in a contaminated soil after phosphate application. Sci Total Environ 305:117–127
Narwal RP, Singh BR (1998) Effect of organic materials on partitioning, extractability and plant uptake of metals in an alum shale soil. Water Air Soil Pollut 103:405–421
Ojuri OO, Taiwo OA, Oluwatuyi OE (2016) Heavy metal migration along a rural highway route: Ilesha-Akure roadside soil, southwestern, Nigeria. Global NEST J 18:742–760
Rosen V, Chen Y (2014) The influence of compost addition on heavy metal distribution between operationally defined geochemical fractions and on metal accumulation in plant. J Soils Sediments 14:713–720
Sauni R, Linna A, Oksa P, Nordman H, Tuppurainen M, Uitti J (2010) Cobalt asthma - a case series from a cobalt plant. Occup Med 60:301–306
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261
Shao HB, Liang ZS, Shao MA, Sun Q (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.) Chemosphere 60:97–104
Shrivastava A, Barla A, Singh S, Mandraha S, Bose S (2017) Arsenic contamination in agricultural soils of Bengal deltaic region of West Bengal and its higher assimilation in monsoon rice. J Hazard Mater 324:526–534
Siebers N, Kruse J, Leinweber P (2013) Speciation of phosphorus and cadmium in a contaminated soil amended with bone char: sequential fractionations and XANES spectroscopy. Water Air Soil Pollut 224:1564–1576
Singh BK, Quince C, Macdonald CA, Khachane A, Thomas N, Al-Soud WA, Sørensen SJ, He Z, White D, Sinclair A, Crooks B, Zhou J, Campbell CD (2014) Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ Microbiol 16:2408–2420
Sitte J, Löffler S, Burkhardt E, Goldfarb KC, Büchel G, Hazen TC, Küsel K (2015) Metals other than uranium affected microbial community composition in a historical uranium-mining site. Environ Sci Pollut R 22:19326–19341
Smičiklas I, Dimović S, Jović M, Milenković A, Šljivić-Ivanović M (2015) Evaluation study of cobalt(II) and strontium(II) sorption–desorption behavior for selection of soil remediation technology. Int J Environ Sci Technol 12:3853–3862
Smith LJ, Holmes AL, Kandpal SK, Mason MD, Zheng T, Wise JP (2014) The cytotoxicity and genotoxicity of soluble and particulate cobalt in human lung fibroblast cells. Toxicol Appl Pharm 278:259–265
Suh M, Thompson CM, Brorby GP, Mittal L, Proctor DM (2016) Inhalation cancer risk assessment of cobalt metal. Regul Toxicol Pharmacol 79:74–82
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Bioch 110:59–69
Vittori Antisari L, Carbone S, Gatti A, Ferrando S, Nacucchi M, Pascalis FD, Gambardella C, Badalucco L, Laudicina VA (2016) Effect of cobalt and silver nanoparticles and ions on Lumbricus rubellus health and on microbial community of earthworm faeces and soil. Appl Soil Ecol 108:62–71
Wang YP, Shi JY, Lin Q, Chen XC (2007) Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J Environ Sci 19:848–853
Wasay SA, Barrington S, Tokunaga S (2001) Organic acids for the in situ remediation of soils polluted by heavy metals: soil flushing in columns. Water Air Soil Pollut 127:301–314
Yang H, Turner S, Rose NL (2016) Mercury pollution in the lake sediments and catchment soils of anthropogenically-disturbed sites across England. Environ Pollut 219:1092–1101
Yang W, Zhang T, Lin S, Ni W (2017) Distance-dependent varieties of microbial community structure and metabolic functions in the rhizosphere of Sedum alfredii Hance during phytoextraction of a cadmium-contaminated soil. Environ Sci Pollut R 24:14234–14248
Zhang P, Ryan JA (1999) Formation of chloropyromorphite from Galena (PbS) in the presence of hydroxyapatite. Environ Sci Technol 33:618–624
Zhang Z, Solaiman ZM, Meney K, Murphy DV, Rengel Z (2013) Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J Soils Sediments 13:140–151
Zhao XL, Saigusa M (2007) Fractionation and solubility of cadmium in paddy soils amended with porous hydrated calcium silicate. J Environ Sci 19:343–347
Zhao B, Xu R, Ma F, Li Y, Wang L (2016) Effects of biochars derived from chicken manure and rape straw on speciation and phytoavailability of cd to maize in artificially contaminated loess soil. J Environ Manag 184:569–574
Zheng N, Wang Q, Zheng D (2007) Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc Plant in China via consumption of vegetables. Sci Total Environ 383:81–89
Zhu W, Du W, Shen X, Zhang H, Ding Y (2017) Comparative adsorption of Pb2+ and Cd2+ by cow manure and its vermicompost. Environ Pollut 227:89–97
Zhuang P, McBride MB, Xia H, Li N, Li Z (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407:1551–1561
Acknowledgements
We greatly appreciate the Beijing Key Laboratory Construction Project, Beijing Municipal Education Commission Joint Construction Program (20160939023). We are also grateful to Beijing Academy of Agriculture and Forestry Sciences and Research Center of Eco-environmental Sciences, Chinese Academy of Sciences for providing facilities for the experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Xilong Wang
Electronic supplementary material
ESM 1
(DOCX 168 kb)
Rights and permissions
About this article
Cite this article
Liu, B., Huang, Q., Su, Y. et al. Cobalt accumulation and antioxidant system in pakchois under chemical immobilization in fluvo-aquic soil. J Soils Sediments 18, 669–679 (2018). https://doi.org/10.1007/s11368-017-1804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1804-3