Abstract
Phytoextraction is a cost-effective and eco-friendly technique for the removal of pollutants, mainly heavy metal(loids) especially from polluted water and metal-contaminated soils. The phytoextraction of heavy metals is, in general, limited due to the low availability of heavy metals in the growth medium. Organic chelators can help to improve the phytoextraction by increasing metal mobility and solubility in the growth medium. The present research was carried out to examine the possibility of citric acid (CA) in improving chromium (Cr) phytoextraction by Lemna minor (duckweed). For this purpose, healthy plants were collected from nearby marsh and grown in hydroponics under controlled conditions. Initial metal contents of both marsh water and plant were measured along with physico-chemical properties of the marsh water. Different concentrations of Cr and CA were applied in the hydroponics in different combinations after defined intervals. Continuous aeration was supplied and pH maintained at 6.5 ± 0.1. Results showed that increasing concentration of Cr significantly decreased the plant biomass, photosynthetic pigments, leaf area, and antioxidant enzyme activities (like catalase, ascorbate peroxidase, superoxide dismutase, peroxidase). Furthermore, Cr stress increased the Cr concentrations, electrolyte leakage, hydrogen peroxide, and malondialdehyde contents in plants. The addition of CA alleviated the Cr-induced toxicity in plants and further enhanced the Cr uptake and its accumulation in L. minor. The addition of CA enhanced the Cr concentration in L. minor by 6.10, 26.5, 20.5, and 20.2% at 0, 10, 100, and 200 μM Cr treatments, respectively, compared to the respective Cr treatments without CA. Overall, the results of the present study showed that CA addition may enhance the Cr accumulation and tolerance in L. minor by enhancing the plant growth and activities of antioxidant enzymes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing urbanization, broad-scale industrialization, and rapid population emergence since the last few decades are considered as dominant factors in deteriorating the quality of the environment and leading to heavy metal contamination in all spheres of the environment (Qu et al. 2011; Ali et al. 2013a). Discharge of heavy metal-enriched industrial effluents to exposed water channels is posing a highly deleterious impact on flora and fauna which is grabbing the scientists’ attention worldwide (Sood et al. 2012; Júnior et al. 2015). Among all heavy metals, chromium (Cr) is highly toxic, carcinogenic, and persistent in nature (Knasmüller et al. 1998; Sharma and Pandey 2014; Adrees et al. 2015a). Sources of Cr contamination in the environment mainly include metal finishing, leather processing, leachate from sanitary landfills, tobacco emissions, oxidative dyeing, cement plants, timber processing, chromic acid manufacturing, metal plating, and paper and pulp production (Nagajyoti et al. 2010; Afshan et al. 2015; Ali et al. 2015b). Trivalent Cr and hexavalent Cr are the most stable forms of Cr among the variety of valence states ranging from −2 to +6. Furthermore, hexavalent Cr is identified as the more toxic form of Cr compared to trivalent Cr (Becquer et al. 2003). Hexavalent Cr possesses highly mutagenic and carcinogenic impacts on humans and animals (Gerber et al. 1980; Sun et al. 2008; Mondol et al. 2011). Hexavalent Cr toxicity interferes in various physiological processes of plants and ultimately leads to reduction in seed germination, root growth, dry weights, number of leaves, photosynthesis, and induced changes in leaf protein profiles and root microRNA expression (Ali et al. 2011; Bukhari et al. 2015, 2016a, b; Zaheer et al. 2015). Therefore, scientists are more concerned about the remediation techniques to reduce the Cr pollution in a sustainable way to protect the agricultural land for food crops. Phytoextraction is the extraction of heavy metals from soil and water by plants’ natural mechanism of uptake and can be used to decontaminate the polluted soil and water bodies (Adrees et al. 2015a, 2015b; Rizwan et al. 2016a). Phytoremediation is a simple, cost-effective, environment-friendly, economically suitable, and self-sustaining substitute of traditional remediation methods (Rizwan et al. 2016b, 2017). The degree of metal translocation in plants depends upon several factors including heavy metal type, plant species, and surrounding conditions (Jadia and Fulekar 2008; Farooq et al. 2013). Duckweed plant species due to their capability to hyperaccumulate heavy metals are gaining attention around the globe (Radić et al. 2010). Lemna minor, Lemna gibba, and Spirodela polyrhiza are the potential candidates for this technique (Axtell et al. 2003; Charles et al. 2006; Zhang et al. 2011). Lemna minor (duckweed) can be useful in phytoextraction of heavy metals because of its high metal accumulation capability, natural occurrence, rapid growth, tolerance toward cold, and ease of harvesting (Sharma and Gaur 1995; Radić et al. 2010). The average lifespan of L. minor is about 5–6 weeks with a production rate of 0.45 fronds per day and doubling its mass in 2–3 days (Isaksson et al. 2007). Its rapid growth rate can make it a potential candidate for phytoremediation. For the bioavailability of heavy metals in the medium, their mobility and solubility must be ensured. Addition of synthetic chelators like diethylenetriaminepentaacetic acid (DTPA) and ethylenediaminetetraacitic acid (EDTA) and organic chelators like citric acid can solve this problem by solubilizing the heavy metals in the growth medium (Sinhal et al. 2010; Szczygłowska et al. 2011; Farid et al. 2015). In addition, chelators also enhance nutrient uptake by plants, which are supportive to plants and help them maintain their normal physiological activities under heavy metal stress (Freitas et al. 2014). Application of synthetic chelators is not so eco-friendly as they can contaminate the ground water because of their persistence in the environment due to their non-biodegradable nature (Anwer et al. 2012; Bareen 2012; Rizwan et al. 2016b). Citric acid (CA), a highly biodegradable low atomic weight organic acid, can serve as an alternate to EDTA in improving metal solubility in the growth medium and their uptake by plants (Ding et al. 2005; Farid et al. 2013; Shakoor et al. 2014). Previous studies have identified the capability of CA for metal uptake and accumulation in plants (Luo et al. 2005; Sinhal et al. 2010). However, scarce information is available regarding the use of CA for phytoextraction of Cr with L. minor.
Thus, by knowing the importance of Cr in plants, the present study aimed to investigate the effects of exogenous CA on L. minor growth under Cr stress. Furthermore, physiological and biochemical approaches were used to identify the CA-induced Cr tolerance mechanisms in L. minor by evaluating the roles of key components such as (i) plant growth and biomass, (ii) photosynthesis, (iii) Cr accumulation, and (iv) oxidative stress and activities of antioxidant enzymes.
Material and methods
Experimental growth conditions and treatments
Already grown healthy and uniform plants of L. minor were collected from the domestic wastewater pond and thoroughly rinsed with distilled water and transferred to glass platters of 1 L capacity with complete randomized design (CRD). The physico-chemical properties of wastewater and Cr concentration in L. minor collected from pond are given in Table 1. Aeration was continuously provided through pumping. An experimental setup was placed in a laboratory with sunlight and air and at normal room temperature varying between 20 and 25 °C. After transplanting, plants were supplied with Cr as K2CrO4 and citric acid and sprayed with 10% of nutrient solution prepared by the recipe of Hoagland and Arnon (1950) consisting of (in μM) KNO3 3000; KH2(PO4) 100; Ca(NO3)2 2000; MgSO4 1000; H3BO3 50; ZnSO4·7H2O 0.8; MnCl2·4H2O 0.05; CuSO4·H2Mo4·H2O 0.10; 5H2O 0.3; and FeNa-EDTA 12.5. The treatments were as follows: T1, control; T2, CA (2.5 mM); T3, Cr (10 μM); T4, Cr (100 μM); T5, Cr (200 μM); T6, Cr (10 μM) + CA (2.5 mM); T7, Cr (100 μM) + CA (2.5 mM); and T8, Cr (200 μM) + CA (2.5 mM) with three replications. The control system was provided with neither K2CrO4 nor citric acid. The solutions were renewed after every 3 days by draining the previous solution completely. The abovementioned treatments were maintained for 6 weeks. The solutions were stirred up with the help of spatula after every 10 h.
Physiological assessment
After 6 weeks of treatment application, the plants were sampled for biomass (fresh and dry weight), leaf color, and leaf area. The whole plant samples were kept at 70 °C for at least 72 h for the measurement of dry biomass. For biochemical analysis, fresh whole plant samples were used.
Biochemical assessment
Determination of chlorophyll content
After continuous application of treatments for 6 weeks, carotenoid and chlorophyll contents were measured in fully expanded fresh plants by following the method of Metzner et al. (1965) with certain amendments. The fresh leaves were incubated till discoloration in aqueous acetone (85%, v/v) at 4 °C under darkness with continuous shaking for pigment extracts. Further, the extract was centrifuged at 4 °C and 4000 rpm for the next 10 min and the supernatant was collected from the surface for the measurement of light absorbance at 452.5, 644, and 663 nm by using a Halo DB-20/DB-20S (Dynamica Company, London, UK) spectrophotometer. Finally, calculations were made by using an equation and adjusted extinction coefficients given by Lichtenthaler (1987).
Equation for measuring chlorophyll and carotenoid contents:
Determination of protein and antioxidant enzymes
After continuous application of treatments for 6 weeks, the content of soluble protein was measured by following the protocol of Bradford (1976) using a standard (bovine serum albumin) and dye (Coomassie Brilliant Blue G-250). In detail, fresh whole plants were grounded with mortar and pestle under chilled conditions and then mixed in 10 mL of buffer solution made by 50 mM of sodium phosphate containing polyvinylpyrolidine 40 (2%,w/v) and 1 mM EDTA. Further, the supernatant was collected after centrifuging the mixture at 4 °C and 11,000 rpm for 15 min and was used for the measurement of antioxidant enzymes (POD and SOD) and proteins.
To estimate the entire protein concentration, 100 μL of sample extract was further homogenized with 1 mL of Bradford solution and 595 nm wavelength of the spectrophotometer was selected to measure light absorbance.
The protocol described by Aebi (1984) was followed to measure the activity/concentration of catalases (CAT, EC 1.11.1.6). For this, 3 mL of assay mixture consisted of 2.8 mL solution of phosphate buffer (50 mM with 2 mM citric acid, pH 7.0), 100 μL of hydrogen peroxide (300 mM), and 100 μL of enzyme extract. The concentration of CAT was estimated by recording the changes in absorbance at 240 nm which occurred due to the disappearance of hydrogen peroxide (ε = 39.4 mM−1 cm−1).
The activity/concentration of ascorbate peroxidase (APX, EC 1.11.1.11) was estimated by following the protocol presented by Nakano and Asada (1981). An assay mixture of 3 mL contained 2.7 mL phosphate buffer solution (50 mM with 2 mM citric acid, pH 7.0), 100 μL ascorbate (7.5 mM), 100 μL of enzyme extract, and 100 μL of hydrogen peroxide (300 mM). The concentration of APX was estimated by recording the changes in wavelength at 290 nm occurred due to its oxidation (ε = 2.8 mM−1 cm−1).
Hydrogen peroxide contents
To estimate the content of H2O2, the assay mixture was prepared by homogenizing the fresh whole plant (50 mg) with 3 mL of phosphate buffer solution (pH 6.5, 50 mM) and centrifuging at 6000×g and 4 °C for 25 min. Further, 3 mL of extracted supernatant was mixed with 1 mL of sulfuric acid (20%, v/v) and titanium sulfate (0.1%), then again the mixture was centrifuged at 6000×g and 4 °C for 15 min. The concentration of H2O2 was estimated at 410 nm by recording changes in the intensity of the supernatant’s yellow color and calculated by the following extinction coefficient of 0.28 μmol−1 cm−1.
Malondialdehyde content
Malondialdehyde concentration (a product of lipid peroxidation) in whole plant tissues was estimated by following the reaction method of thiobarbituric acid (TBA) as documented by Heath and Packer (1968) along with some modifications added by Zhang and Kirham (1994) and Dhindsa et al. (1981).
Assessment of electrolyte leakage
After completing the 6 weeks of treatments, whole plants were put in test tubes having 8 mL of distilled water. The initial electrical conductivity (EC1) was recorded after incubating the test tubes for 2 h at 32 °C in a water bath. For the measurement of subsequent electric conductivity (EC2), the sample test tubes were autoclaved for 20 min at 121 °C to discharge maximum electrolytes and then were cooled at room temperature, and electrolyte leakage (EL) was determined by following the method described by Dionisio-Sese and Tobita (1998).
Electrolyte leakage was computed by the following formula:
Chromium concentration
Plants were dried at 90 °C and then turned to ash in a muffle furnace at 600 °C for 6 h. The ash samples were dissolved in concentrated solution of HNO3 and HCl (3 mL each) and filtered in a volumetric flask, then distilled water was added to make a final volume of 50 mL. The Perkin Elmer AAnalyst 100 (USA) method was adopted to determine the Cr concentration. Data was analyzed according to Analytical Software, Tallahassee, USA.
Chromium concentration was calculated for the plant as a whole by the following formula:
Metal (μg g−1) in plant = metal reading of digested sample (mg L−1) × dilution factor
where
The metal uptake in whole plant was calculated using the following formula: (Zayed et al. 1998)
Metal uptake = metal concentration in plant (μg g−1) × plant’s whole dry weight (g)
which was used to calculate the bio-concentration factor.
The actual Cr concentration in plant tissues (Cra) = Cr conc. in plant organ (Crb) is subtracted by the already available concentration in plant (Crc) (0.098 ± 0.001):
Statistical analysis
Data presented in this paper are the average of three replicates ± S.D. Analysis of variance (ANOVA) and graphical representation were performed with software Statistix 10.0, further followed by Tukey’s test to find significant differences among mean values of treatments.
Results
Agronomic traits
The variations in L. minor growth parameters including fresh weight and dry weight per hundred plants as a whole are given in Table 2. Chromium application reduced both fresh and dry biomass of plants as compared to the control treatment. The weight was gradually decreased with increasing Cr concentrations in culture medium. Application of CA (2.5 mM) along with Cr 10, 100, and 200 μM significantly increased the fresh and dry biomass of plants compared to those plants treated with respective Cr treatments alone. A similar trend was found in the leaf area of L. minor under Cr and CA application (Table 2). Addition of CA increased the fresh weight by 20.2, 21.8, 22.2, and 24.9% at 0, 10, 100, and 200 μM Cr treatments compared to the respective Cr treatments alone. The dry weight was dramatically decreased under CA by 11.53 and 16.66% at Cr 10 and 200 μM, respectively.
Leaf greenness of plants was also reduced with increasing concentrations of Cr in the nutrient solution (Fig. 1). Plants applied with 200 μM Cr showed the lowest greenness while the highest leaf greenness was observed for the plants treated with CA compared to the control plants. Application of CA in combination with Cr enhanced the leaf greenness compared to the plants treated with Cr only.
Photosynthetic pigments
Chlorophyll (Chl a, Chl b, and total Chl) and carotenoid contents significantly decreased with the addition of Cr in the solution compared to the control (Fig. 2). The maximum total chlorophyll and carotenoid contents were observed in plants treated with CA alone while the highest reduction in photosynthetic pigments was observed in plants treated with 200 μM Cr. Exogenous application of CA along with Cr significantly enhanced the Chl a, Chl b, total Chl, and carotenoid contents in plants as compared to Cr only-treated plants. The addition of CA increased the total chlorophyll contents by 2.14, 14.65, 14.54, and 24.86% at 0, 10, 100, and 200 μM Cr treatments compared to the respective Cr treatments without CA application.
Effect of Cr and citric acid on chlorophyll a, b, total chlorophylls, and total carotenoids in L. minor grown in solution culture with increasing Cr concentrations (10, 100, and 200 μM) treated or not with 2.5 mM citric acid. Values are demonstrated as means of three replicates along with standard deviation. Different normal or italicized small and capital letters indicate that values are significantly different at P < 0.05
Antioxidant enzyme activity
Four key antioxidant enzymes, APX, SOD, POD, and CAT, were analyzed to investigate the impact of Cr and CA amendments on antioxidant capacity of L. minor (Fig. 3). Chromium stress alone significantly inhibited the antioxidant enzyme activities in a dose-additive manner except in 10 μM Cr where antioxidant enzyme activities were increased compared to the control. Exogenous CA + Cr addition further enhanced the antioxidant enzyme activities and showed synergetic effects compared to the respective Cr treatments alone. The antioxidant enzyme activities were boosted up under CA at initial concentrations of Cr, i.e., 10 and 100 μM, while at the highest concentration of 200 μM, a reduction of the activities of antioxidant enzymes was observed.
Antioxidative enzyme activities in L. minor grown in solution culture with increasing Cr concentrations (10, 100, and 200 μM) treated or not with 2.5 mM citric acid. Values are demonstrated as means of three replicates along with standard deviation. Different small normal and italicized letters indicate that values are significantly different at P < 0.05
Electrolyte leakage, hydrogen peroxide, and malondialdehyde content
Oxidative stress in L. minor was evaluated by measuring the EL and H2O2 and MDA contents in plants (Fig. 4a, b, d). Increasing Cr concentrations significantly enhanced the oxidative damage (MDA and H2O2) and EL. The highest concentrations of EL and H2O2 and MDA were observed in the plants treated with the highest concentration of Cr (200 μM) while the lowest contents were observed in the control plants. Application of CA (2.5 mM) alone and/or in combination with Cr significantly alleviated the oxidative stress as was observed by the reduced production of EL and H2O2 and MDA in L. minor under Cr stress compared to the respective Cr treatments without CA application. With CA application under Cr stress, the maximum reduction in H2O2 and MDA contents was by 12.73 and 23.16% at 10 and 100 μM Cr, respectively.
Effect of Cr and citric acid on electrolyte leakage, H2O2 concentration, protein contents, and MDA contents in L. minor grown in solution culture with increasing Cr concentrations (10, 100, and 200 μM) treated or not with 2.5 mM citric acid. Values are demonstrated as means of three replicates along with standard deviation. Different letters indicate that values are significantly different at P < 0.05
Soluble protein
Chromium-stressed L. minor showed the reduced soluble protein contents compared to the control (Fig. 4c). The highest reduction in protein contents was observed in the plants treated with 200 μM Cr treatment alone. However, CA application gradually enhanced the protein contents in Cr-stressed plants compared to the plants treated with respective Cr treatments alone. Addition of CA increased the soluble protein content by 5.0, 10, 18, and 11.6% at 0, 10, 100, and 200 μM Cr treatments compared to the respective Cr treatments alone.
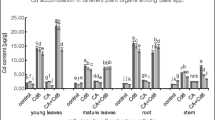
Chromium concentration
Chromium concentration in L. minor significantly increased with increasing Cr concentrations in the nutrient solution (Table 2). Application of CA further enhanced the Cr concentrations and uptake by plants compared to the respective treatments without CA application. The highest Cr concentrations were observed in the plants treated with 200 μM Cr along with CA. Addition of CA increased the Cr concentration by 6.1, 26.5, 20.5, and 20.2% at 0, 10, 100, and 200 μM Cr compared to the respective Cr treatments alone. The correlations among all attributes of L. minor studied are given in Table 3. The negative sign showed a significant decrease in the parameter with respect to the other parameter. The correlation among Cr concentrations to all attributes showed significant decrease except antioxidant enzymes, reactive oxygen species, and electrolyte leakage.
Discussion
A possible mechanism for Cr uptake and tolerance in L. minor by the addition of CA during Cr stress was probed in the present research. Chromium stress reduced the plant biomass (Table 2) and leaf color (Fig. 1). It has been reported in previous studies that Cr uptake and accumulation in plant reduced the plant growth and biomass by suppressing mineral uptake which interrupts normal metabolic processes (Ali et al. 2015a, b; Dheeba et al. 2015; Gill et al. 2015). The Cr-induced stress is responsible for the generation of reactive oxygen species (ROS) which deteriorated the growth and development of plants (Singh et al. 2012; Das et al. 2014). The addition of CA significantly improved the growth and biomass of L. minor under Cr treatments, indicating its promotive role in metal stress mitigation (Figs. 1, 2 and 3; Table 2). A similar role of CA in nutrient uptake under metal stress was reported by several researchers (Najeeb et al. 2011; Ehsan et al. 2014; Zaheer et al. 2015).
Increased Cr uptake ultimately reduced the Chl a, b, total Chl, and carotenoid contents in L. minor (Fig. 2). Deformation of chloroplast ultrastructure causes altered shape and enlargement of thylakoids which is responsible for lowering the Chl a, b, and carotenoid contents in plant leaves (Parmar et al. 2013). The reduced plant growth might be the consequence of low photosynthetic performance owing to decreased chlorophyll and carotenoid contents. Citric acid alleviated Cr-induced toxicity in L. minor possibly by enhancing the photosynthetic rate and recovering chloroplast ultrastructure damaged by Cr toxicity. The promoting role of CA in enhancing chlorophyll and carotenoid content was observed in many plant species under stress caused by different heavy metals like Brassica napus under Cd stress (Ehsan et al. 2014), Cr stress (Afshan et al. 2015), and Pb stress (Shakoor et al. 2014).
The toxicity caused by Cr and other metals usually induces oxidative stress and negatively affects plant growth and development (Afshan et al. 2015; Habiba et al. 2015). In the present study, Cr toxicity increased oxidative stress which resulted in elevated level of EL, as well as enhanced production of MDA and H2O2 (Fig. 4a, b, d). According to Mittler (2002), plants have the natural ability to combat the toxic impacts of ROS. These results are endorsed with recent studies which identified that many plant species such as wheat, B. napus, barley, and tobacco bear oxidative stress under Cr application (Diwan et al. 2012; Ali et al. 2011; Ali et al. 2015a; Gill et al. 2015; Bukhari et al. 2016a, b). The present results suggested the assisted role of CA which considerably mitigated the toxic effect of Cr and reduced MDA and H2O2 accumulation by decreasing EL. Cr and other metals usually induce oxidative stress and then cause injuries in plants (Ali et al. 2011, 2015a; Habiba et al. 2015).
Production of ROS needs to be controlled by ROS-scavenging mechanisms. Plants are naturally capable to mitigate and repair the ROS-induced damage by activating the antioxidant enzyme defense system (Mittler et al. 2004). In this study, it is identified that low concentration of Cr (10 μM) enhanced POD, SOD, APX, and CAT activities, while their activation was slowed down with the increase in Cr concentrations (Fig. 3a, b). This phenomenon indicates that at higher accumulation of metals in plants, the over-expression of antioxidant enzymes might be an intense mechanism for plants’ survival (Haouari et al. 2012). Similar findings have been reported by Bukhari et al. (2016a, b) about the behavior of the plant enzymatic defense system under Cr stress. However, application of CA helped the plants to survive under Cr stress by increasing activities of antioxidative enzymes in L. minor (Fig. 3a, b).
Chromium stress has been reported for reduced soluble protein in plants (Singh et al. 2012; Das et al. 2014). Our results revealed the same decreasing trend of soluble protein in L. minor under Cr stress (Fig. 4c). It might be the result of increased oxidative damages induced by Cr which suppressed the protein contents (Jabeen et al. 2016). Citric acid application enhanced protein contents in L. minor (Fig. 4c) and decreased H2O2 production and EL which protected the plants from further damage (Fig. 4a, b). The improved plant biomass, leaf greenness, and chlorophyll contents with CA application with Cr stress might be due to enhanced antioxidant enzymatic activity (Figs. 1, 2, and 3; Table 2) and suppression of MDA and H2O2 generation (Fig. 4b, d).
The decline in plant agronomic traits and biomass accumulation might be the result of decreased nutrient uptake and increased metal accumulation by plants under metal stress (Rizwan et al. 2012; Pradas-del-Real et al. 2013; Keller et al. 2015; Khaliq et al. 2016). Addition of CA significantly increased Cr uptake by B. napus (Afshan et al. 2015) and Cd uptake by Sedum alfredii (Lu et al. 2013). According to Shakoor et al. (2014), CA is capable of making heavy metals bioavailable in both soil and aqueous media because of its chelating ability. In this study, CA application to Cr-stressed plants enhanced the Cr uptake as compared to Cr only-treated and control system plants (Table 2). In this work, results are being evaluated for the Cr concentration for the whole plant including its root and two to three fronds. Metal distribution in plants’ tissue is a necessary characteristic as it can be indirectly useful to indicate detoxification mechanism.
The results of the present study are in line with the previous work that the role of L. minor as phytoextraction agent for heavy metals is quite obvious in aqueous media also identified by Bokhari et al. (2016). Improved biomass, metal uptake by plant, and bioavailability of heavy metals in the rhizosphere are the key parameters to be judged for successful phytoextraction plan stated by McGrath and Brooks (1998). However, the efficiency of L. minor to hyperaccumulate Cr as well as the role of CA in enhancing Cr bioavailability in the soil system still bear a question mark, which needs further experimental evidence.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-ur-Rehman M, Irshad MK, Bharwana SA (2015a) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015b) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Safe 119:186–197
Aebi H (1984) Catalase in vitro methods. Enzymology 105:121–126
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689
Ali B, Wang B, Ali S, Ghani MA, Hayat MT, Yang C, Xu L, Zhou WJ (2013) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regulate 32:604–614
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015a) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.) Environ Sci Pollut Res 22:10669–10678
Ali S, Zeng F, Cai S, Qiu B, Zhang G (2011) The interaction of salinity and chromium in the influence of barley growth and oxidative stress. Plant Soil Environ 57:153–159
Ali Z, Malik RN, Shinwari ZK, Qadir A (2015b) Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Inter J Environ Sci Technol 12:537–550
Anwer S, Ashraf MY, Hussain M, Ashraf M, Jamil A (2012) Citric acid mediated phytoextraction of cadmium by maize (Zea mays L.) Pak J Bot 44:1831–1836
Axtell NR, Sternberg SP, Claussen K (2003) Lead and nickel removal using Microspora and Lemna minor. Bioresour Technol 89:41–48
Bareen FE (2012) Chelate assisted phytoextraction using oilseed brassicas. Environ Pollut 21:289–311
Becquer T, Quantin C, Sicot M, Boudot JP (2003) Chromium availability in ultramafic soils from New Caledonia. Sci Total Environ 301:251–261
Bokhari SH, Ahmad I, Mahmood-Ul-Hassan M, Mohammad A (2016) Phytoremediation potential of Lemna minor L. for heavy metals. Int J Phytoremed 18:25–32
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem
Bukhari SA, Shang S, Zhang M, Zheng W, Zhang G, Wang TZ, Shamsi IH, Wu F (2015) Genome-wide identification of chromium stress-responsive microRNAs and their target genes in tobacco (Nicotiana tabacum) roots. Environ Toxicol Chem 34:2573–2582
Bukhari SA, Wang R, Wang W, Ahmed IM, Zheng W, Cao F (2016a) Genotype dependent effect of exogenous 24-epibrassinolide on chromium-induced changes in ultrastructure and physicochemical traits in tobacco seedlings. Environ Sci Pollut Res 23:18229–18238
Bukhari SA, Zheng W, Xie L, Zhang G, Shang S, Wu F (2016b) Cr-induced changes in leaf protein profile, ultrastructure and photosynthetic traits in the two contrasting tobacco genotypes. Plant Growth Regul 79:147–156
Charles AL, Markich SJ, Ralph P (2006) Toxicity of uranium and copper individually, and in combination, to a tropical freshwater macrophyte (Lemna aequinoctialis). Chemosphere 62:1224–1233
Das BC, Panda A, Sahoo PK, Jena S, Padhi P (2014) Effect of chromium (VI) on wheat seedlings and the role of chelating agents. Curr Sci 106:1387–1395
Dheeba B, Sampathkumar P, Kannan K (2015) Fertilizers and mixed crop cultivation of chromium tolerant and sensitive plants under chromium toxicity. J Toxicol, 2015.
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Ding YZ, Li ZA, Zou B (2005) Low-molecular weight organic acids and their ecological roles in soil. Soils 37:243–250
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Diwan H, Ahmad A, Iqbal M (2012) Characterization of chromium toxicity in food crops and their role in phytoremediation. J Bioremed Biodeg 3:159. doi:10.4172/2155-6199.1000159
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Safe 106:164–172
Farid M, Ali S, Ishaque W, Shakoor MB, Niazi NK, Bibi I, Dawood M, Gill RA, Abbas F (2015) Exogenous application of ethylenediamminetetraacetic acid enhanced phytoremediation of cadmium by Brassica napus L. Inter J Environ Sci Technol 12:3981–3992
Farid M, Shakoor MB, Ehsan S, Ali S, Zubair M, Hanif MS (2013) Morphological, physiological and biochemical responses of different plant species to Cd stress. IJCBS 3:53–60
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Safe 96:242–249
Freitas EV, Nascimento CW, Silva WM (2014) Citric acid-assisted phytoextraction of lead in the field: the use of soil amendments. Water Air Soil Pollut 225:1–9
Gerber GB, Leonard A, Jacquet P (1980) Toxicity, mutagenicity and teratogenicity of lead. Mutat Res-Rev Genet 76:115–141
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
Haouari CC, Nasraoui AH, Bouthour D, Houda MD, Daieb CB, Mnai J, Gouia H (2012) Response of tomato (Solanum lycopersicon) to cadmium toxicity: growth, element uptake, chlorophyll content and photosynthesis rate. African J Plant Sci 6:001–007
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. University of California, College of Agriculture, Agricultural Experiment Station, Berkeley, Calif
Isaksson R, Balogh SJ, Farris MA (2007) Accumulation of mercury by the aquatic plant Lemna minor. Inter J Environ Stud 64:189–194
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2016) Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662
Jadia CD, Fulekar MH (2008) Phytoremediation: the application of vermicompost to remove zinc, cadmium, copper, nickel and lead by sunflower plant. Environ Eng Manag J 7:547–558
Júnior CA, de Sousa BH, Galazzi RM, Koolen HH, Gozzo FC, Arruda MA (2015) Evaluation of proteome alterations induced by cadmium stress in sunflower (Helianthus annuus L.) cultures. Ecotoxicol Environ Safe 119:170–177
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM cu. Planta 241:847–860
Khaliq A, Ali S, Hameed A, Farooq MA, Farid M, Shakoor MB, Mahmood K, Ishaque W, Rizwan M (2016) Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch Agron Soil Sci 62:633–647
Knasmüller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, Paschke A, Göd R, Kundi M (1998) Detection of genotoxic effects of heavy metal contaminated soils with plant bioassays. Mutat Res-Gen Tox En 420:37–48
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Lu LL, Tian SK, Yang XE, Peng HY, Li TQ (2013) Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: the impact of citric acid and tartaric acid. J Zhejiang Univ Sci B 14:106–114
Luo C, Shen Z, Li X (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
McGrath SP, Brooks RR (1998) Phytoextraction for soil remediation. Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining 261–287
Metzner H, Rau H, Senger H (1965) Untersuchungenzursynchronisierbaketieinzel-nerpigmentmangel-mutation von chlorella. Planta 65:186–194
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004). Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mondol MN, Chamon AS, Faiz B, Elahi SF (2011) Seasonal variation of heavy metal concentrations in water and plant samples around Tejgaon industrial area of Bangladesh. J Bangladesh Acad Sci 35:19–41
Nagajyoti PC, Lee KD, Sreekanth TV (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Najeeb U, Jilani G, Ali S, Sarwar M, Xu L, Zhou W (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186:565–574
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54:1
Pradas-del-Real AE, García-Gonzalo P, Alarcón R, González-Rodríguez A, Lobo MC, Pérez-Sanz A (2013) Effect of genotype, Cr (III) and Cr (VI) on plant growth and micronutrient status in Silene vulgaris (Moench). Span J Agric Res 11:685–694
Qu J, Yuan X, Cong Q, Wang L (2011) The effect of sodium hydrogen phosphate/citric acid mixtures on phytoremediation by alfalfa & metals availability in soil. J Plant Nutr 11:86–96
Radić S, Stipaničev D, Cvjetko P, Mikelić IL, Rajčić MM, Širac S, Pevalek-Kozlina B, Pavlica M (2010) Ecotoxicological assessment of industrial effluent using duckweed (Lemna minor L.) as a test organism. Ecotoxicology 19:216–222
Rizwan M, Meunier JD, Davidian JC, Pokrovsky OS, Bovet N, Keller C (2016a) Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ Sci Pollut Res 23:1414–1427
Rizwan M, Ali S, Rizvi H, Rinklebe J, Tsang DCW, Meers E, Ok YS, Ishaque W (2016b) Phytomanagement of heavy metals in contaminated soils using sunflower—a review. Crit Rev Environ Sci Technol 46:1498–1528
Rizwan M, Ali S, Qayyum MF, Ok YS, Rehman MZ, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd contaminated soils: a critical review. Environ Geochem Health 39:259–277
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Shakoor MB, Ali S, Hameed A, Farid M, Hussain S, Yasmeen T, Najeeb U, Bharwana SA, Abbasi GH (2014) Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Safe 109:38–47
Sharma P, Pandey S (2014) Status of phytoremediation in world scenario. J Bioremed Biodeg 2:178–191
Sharma SS, Gaur JP (1995) Potential of Lemna polyrrhiza for removal of heavy metals. Ecol Eng 4:37–43
Singh D, Gupta R, Tiwari A (2012) Potential of duckweed (Lemna minor) for removal of lead from wastewater by phytoremediation. J Pharm Res 5:1578–1582
Sinhal VK, Srivastava A, Singh VP (2010) EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta).
Sood A, Uniyal PL, Prasanna R, Ahluwalia AS (2012) Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 41:122–137
Sun HW, Li LX, Qiao FX, Liang SX (2008) Availability of lead and cadmium in farmland soil and its distribution in individual plants of dry-seeded rice. Commun Soil Sci Plan 39:450–460
Szczygłowska M, Piekarska A, Konieczka P, Namieśnik J (2011) Use of Brassica plants in the phytoremediation and biofumigation processes. Int J Mol Sci 12:7760–7771
Zaheer IE, Ali S, Rizwan M, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Safe 120:310–317
Zayed A, Gowthaman S, Terry N (1998) Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J Environ Qual 27:715–721
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Zhang X, Hu Y, Liu Y, Chen B (2011) Arsenic uptake, accumulation and phytofiltration by duckweed (Spirodela polyrhiza L.) J Environ Sci 23:601–606
Acknowledgments
The authors are highly thankful to the University of Gujrat, Gujrat, Pakistan, and the Higher Education Commission of Pakistan for financial and technical support during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Sallah-Ud-Din, R., Farid, M., Saeed, R. et al. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ Sci Pollut Res 24, 17669–17678 (2017). https://doi.org/10.1007/s11356-017-9290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9290-0