Abstract
Purpose
Metal distribution patterns among geochemical fractions are informative for metal phytoavailability. Compost added to polluted soils may adsorb metals on the less phytoavailable fractions. A bioassay experiment was conducted to establish possible correlations between metal concentrations in different soil fractions and metal contents in edible plant parts and to investigate the influence of different compost loads on heavy metal availability to plants.
Materials and methods
Chinese cabbage plants were grown in pots with sandy and clayey soils and soils mixed with different doses of biosolid compost spiked with soluble heavy metal salts (Cd, Cu, and Pb). The metals’ distribution pattern in the soil and mixed samples was determined by sequential extraction procedure (modified BCR protocol). The studied fractions, from most to least bioavailable, were water-extractable (WE), exchangeable-adsorbed (EXC), associated with carbonates and acetic acid-soluble forms (CARB), occluded by reducible (hydro)oxides of Fe and Mn (RO), and associated with organic matter (OM) and a residual fraction (RES). Metal concentrations in soil extracts and in the digested plant tissue were measured by ICP-AES.
Results and discussion
The highest compost doses (72 and 115 Mg ha−1) enhanced cabbage yield significantly. No excessive phytoaccumulation of metals was observed in plants grown in the clayey soil or its mixtures with compost. The compost dose of 72 Mg ha−1 was optimal in decreasing Cu accumulation by plants grown in sandy soil, and 28.8 Mg ha−1 was found to be effective in reducing Cd and Pb uptake. Metals were accumulated in plants primarily from the WE, EXC, and CARB fractions, whereas other fractions decreased phytoaccumulation. Compost addition suppressed heavy metal mobility, but different fractions were active in pollutant sorption, depending on soil type and metal.
Conclusions
Compost addition increased metal proportions in the RO and OM fractions, reducing metal phytoavailability. This is especially important for sandy soils with low adsorption ability and higher vulnerability to metal pollution than clayey soils. A compost dose of 20% v/v (or 28.8 Mg ha−1) effectively reduced plant accumulation of Cd and Pb. We propose using the first three steps of the modified BCR protocol as a three-step sequential-extraction procedure for the most phytoavailable fractions of heavy metal: WE, EXC, and CARB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In environmental sciences, “trace” or “heavy” metals are of great ecological concern. The native content of trace elements in soils is very low, but they are essential as micronutrients for plants (Kabata-Pendias and Pendias 2001). In modern agriculture, an important factor for heavy metal input into soils is the use of sewage sludges and their composts as soil organic amendments (OA) and of improperly pretreated wastewater for irrigation (Alvarez et al. 2002; Basta et al. 2005; He et al. 2005). Metals, unlike hazardous organics, cannot be degraded once they have entered the soil (McLean and Bledsoe 1992). The fate of heavy metals in the soil environment is governed by several main parameters: (a) pH and Eh, (b) fine granulometric soil components, (c) organic matter (OM), (d) oxide and hydroxide content, and (e) microorganisms (Kabata-Pendias and Pendias 2001). In the present study, the heavy metals of interest were Cu, Cd, and Pb.
Compost is a product of composting—a partially controlled bio-oxidative process through which highly heterogeneous OM in its solid state is transformed into a humified material (Zmora-Nahum et al. 2007). Compost application plays an important role in heavy metal behavior in soils because of the influence of compost properties on adsorption processes. Compost usually contains a large amount of sorbents: solid OM (SOM); Fe, Mn, and Al oxides; carbonates; and phosphates. At high application doses, compost can dominate the metal-binding chemistry of the soil–compost mixture (Basta et al. 2005).
Many single and sequential extraction (SE) procedures have been developed to determine the forms or phases of heavy metals (e.g., “bioavailable” forms of metals) in soils or sediments. The SE procedures are based on the rational use of a series of more or less selective reagents chosen to solubilize successively different mineralogical fractions thought to be responsible for retaining the larger part of the heavy metals (Gleyzes et al. 2002). The SE procedures are useful for obtaining information on the phases that most affect metal leachability, thereby distinguishing among easily remobilized elements (e.g., Cd and Zn), elements highly affected by reduction processes (e.g., Pb), elements released by oxidation of OM or sulfides (e.g., Cu and Cr), and metals that basically remain in the residual fraction (e.g., Cr) (Sahuquillo et al. 2003).
In Europe, as a result of the Standards, Measurements and Testing Program (SM&T, formerly BCR), a three-step extraction protocol was established, interlaboratory-tested and standardized, including certified reference material preparation and a certification process (Quevauviller 1998a, b and 2002, parts I, II, and III, respectively). In the scientific literature, this protocol is widely known as the BCR procedure. It is applied by many researchers for different purposes, and some useful modifications have been introduced. Developed for soil and sediments, the BCR protocol is successfully applied to substrates with high organic content, such as composts and sludges. The oxidizable step of the BCR scheme is more effective than that employed in the classic Tessier method (Gleyzes et al. 2002).
In general, in addition to plant-specific abilities, metal uptake by plants is affected by soil factors, the most significant being pH, Eh, water regime, clay content, OM content, cation exchange capacity (CEC), nutrient balance, and concentration of other trace elements. Phytoavailable metals are usually extracted by the following methods: (a) deionized water (DW) extraction or soil solution leaching to determine free cations of metals, (b) extraction of exchangeable metals adsorbed on negatively charged sites of soil colloids (clay minerals, SOM, and (hydro)oxides of Fe and Mn, and (c) extraction of specifically adsorbed metals and metals precipitated as barely soluble compounds (Gat 2006). At the same time, according to some investigations, the labile pool concept should be considered independently of the concept of phytoavailability (Kukier et al. 2010).
In this paper, we summarize our investigation of the influence of compost addition on heavy metal distribution among various soil fractions using a novel modified extraction procedure.
2 Materials and methods
2.1 Bioassay experiment
The bioassay experiment was carried out in the phytotron of the Faculty of Agriculture, Food and Environment in Rehovot, Israel. Two soils were chosen and sampled for the incubation experiment: a sandy soil (brown-red sand, Rehovot) and a clayey soil (alluvial soil, clay, Beith-Elazary) (Singer 2010). Selected properties of these soils are presented in Table 1.
The water extract (1:10) of the soils and mixtures sampled on the day of planting (after incubation with metal spike and leaching procedure) was analyzed for metal concentration and other parameters. The routine analyses of soil and mixture extracts were conducted according to classical methods (Chen et al. 1991). The dissolved organic carbon (DOC) content was determined on a Shimadzu carbon analyzer. The soils were dried in the open air and were crushed and sieved to a size <2 mm. The biosolid (sewage sludge) compost was used as a soil OA. The obtained OA was air-dried and sieved (pore diameter of 5 mm). The >5 mm fraction, which mostly consisted of wood chips, was removed. Selected properties of the compost are presented in Table 2.
Compost was added to each soil at the following loading rates: 0 (control), 5, 20, 50, and 80% (v/v), corresponding to the following rates expressed in megagram per hectare: 0 (control), 7.2, 28.8, 72, and 115, respectively (assuming a plough-layer depth of 25 cm). Four replicates were conducted for each treatment. Plastic pots of 15 cm diameter were filled with the air-dried mixture. The weight was equivalent to 1 kg of 105 °C dry weight (dry wt). The solutions of water-soluble salts of heavy metals (chlorides of Cd and Cu and nitrate of Pb) were added to all pots at a concentration of 100 mg kg−1 dry wt for Cu and Pb and 10 mg kg−1 dry wt for Cd. For Cu and Pb, these are the maximum permitted concentrations (MPC) of heavy metals in arable soil, according to Israeli standards (Israel Ministry of Environmental Quality 2005), and for Cd, we used fivefold the MPC. Additional controls (soil without compost or metal addition) were used to demonstrate the metal distribution pattern in native soil. All pots were wetted with tap water to 100% of field capacity without drainage, then mixed and incubated in the phytotron at a constant temperature of 28 °C for 1 month. During the incubation period, the water content was maintained at 100% field capacity by repeated tap water irrigation according to weight loss. After the incubation, all pots were leached out to reach the EC25 drainage value from each treatment of <2 mS cm−1. This procedure took 18 days, and 3.45 L of tap water was used for each pot. After the leaching out procedure, the soil in each pot was mixed thoroughly and samples were taken. The soil samples were dried at 40 °C, finely crushed, and stored air-dried for further analyses.
Chinese cabbage (Brassica chinensis L.) seedlings (about 30 days old) were bought from “Hishtil” company (Nehalim, Israel). Two seedlings were planted per pot. The pots with plants were situated randomly on the three wheel-equipped tables, 16 pots per table. The tables were placed in the phytotron at a constant 22 °C, with natural sunlight during the daylight hours and artificial light during the night hours. The pots were irrigated to leaching once a day: one day with tap water and the next with a fertilizer solution. After 18 days of growth, one plant from each pot was cut, and the pots were placed on four tables in random order. Seven weeks after planting, the plants were cut. The Chinese cabbage leaves were washed in tap water and DW and dried in the phytotron at 28 °C, then in the oven at 65 °C for several days. The dry yield was measured, then the plants were finely crushed, packed into a closed polypropylene flask, and stored for metal analysis.
2.2 Determination of heavy metals in soil and plant samples
We studied the distribution pattern of heavy metals in soil samples using a standardized BCR protocol (Pueyo et al. 2001). We modified this procedure by adding the water-extractable (WE) and exchangeable (EXC) fraction extractions, which were processed as follows: WE—extraction with DW, solid-to-liquid ratio 1:10, and 1 h shaking at ambient temperature; EXC—extraction with 1 M NH4NO3, pH 7 adjusted with NH4OH, solid-to-liquid ratio 1:30, and 1 h shaking at the ambient temperature. These fractions, together with the carbonates and acetic acid-soluble forms (CARB) step of the standardized protocol (acetic acid-soluble species), were positively correlated with the metal content in the tissue of plants grown in spiked soil. We concluded that these three steps (WE, EXC, and CARB), applied sequentially, can be used to evaluate the phytoavailable pool of heavy metals in soil that includes different species, namely water-soluble salts, exchangeable-adsorbed ions, specifically adsorbed metals, metals bound to carbonates, and different acid-soluble species. The rest of the fractions (OM and RO) were extracted according to the standardized procedure (Pueyo et al. 2001).
The water bath pseudo-total metal content analysis (PTA) was carried out according to a protocol developed in our laboratory and was processed as follows: 10 mL of Aqua Regia (HNO3:HCl 1:3) was added to 1 g of sample and digested in a 70 °C water bath for 16 h with occasional manual shaking. Then, digestate was filled with DW, mixed, and centrifuged. This procedure was used for the residue from the SE procedure (residual fraction (RES) fraction extraction) and for the direct sample (PTA metal concentration) as well.
The samples of Chinese cabbage leaves were prepared for metal analysis by the microwave-assisted acid digestion method. The digestion method was based on the standard EPA 3052 Method (1996) as follows: a batch of the sample (about 500 mg dry wt) was digested in 5 mL of 65% HNO3 and 2 mL of 37% HCl in a closed Teflon vessel. We used an “Ethos 1” (Milestone, Italy) sample digestion system (SK-12 Medium Pressure Rotor Application Book 2011). Elemental concentration was measured in the clear solutions by axial inductively coupled plasma atomic emission spectrometer model “ARCOS” from Spectro Gmbh, Germany. The measurement was performed according to EPA Standard Method 6010c (2007).
3 Results and discussion
3.1 Chinese cabbage yield
No difference was observed in the dry yields of plants grown in the two soils, but increasing compost dose significantly enhanced the yield at 50 and 80% compost loading rate (19.1 ± 2.9 and 18.9 ± 0.9 vs. 10.9 ± 1.5 g dry wt at 0% compost in the sandy soil and 23.1 ± 3.5 and 22.5 ± 4.5 vs. 13.7 ± 2.3 g dry wt at 0% compost in the clayey soil, respectively). It can be concluded that metal addition did not lead to phytotoxicity, and Chinese cabbage plants grew under optimal conditions in every treatment studied (see Figs. 1, 2, and 3 in the Electronic Supplementary Material).
3.2 WE fraction
As shown in Table 3, compost addition significantly reduced clayey soil pH but had almost no influence on that of the sandy soil. This is likely a result of the leaching out procedure during which more soluble substances were leached out of the sandy soil than out of the clayey one. For the same reason, EC25 increased significantly in the clayey soil mixtures but did not change in the sandy soil ones. The concentration of DOC increased significantly in the two soils at compost loading rates higher than 20%, with the clayey soil fixing more DOC than the sandy one. At the high loading rates, the compost OM became dominant in the soils, especially the sandy one. At the highest rate, the organic carbon (Corg) content was almost equal in the two soils studied (Table 3).
3.3 Metal distribution pattern
The heavy metal concentrations in the operationally defined geochemical fractions of the sandy and clayey soils and soil–OA mixtures were determined on day of planting by SE procedure. The distribution patterns of each metal are presented in Figs. 1 and 2 as percent of the summed metal fractions and in Tables 2, 3, and 4 in the Electronic Supplementary Material as milligrams per kilogram dry wt.
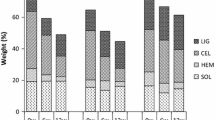
Distribution pattern of Cu, Cd, and Pb in sandy soil treated with different compost doses (percent of all fractions summed). The operationally defined extracted fractions are: water-extractable (WE), exchangeable (EXC), carbonates (CARB), reducible oxides (RO), organic matter (OM), and residual (RES)
Distribution pattern of Cu, Cd, and Pb in clayey soil treated with different compost doses (percent of all fractions summed). The operationally defined extracted fractions are: water-extractable (WE), exchangeable (EXC), carbonates (CARB), reducible oxides (RO), organic matter (OM), and residual (RES)
Copper
In the sandy soil, increasing compost addition reduced Cu in the CARB fraction and increased it the OM fraction. In the clayey soil, Cu also increased the OM fraction (at the expense of that in the RO fraction). Compost addition significantly reduced the metal concentration in most bioavailable fractions of the sandy soil (WE, EXC, and CARB) and increased it in the more stable fractions (RO, OM, and RES), whereas in the clayey soil, Cu concentration in the WE fraction increased but that of the RO fraction decreased. In general, according to these data, compost addition reduces Cu mobility, but different fractions were active in metal sorption depending on the soil type.
Cadmium
No detectable concentration of Cd was found in the native soils. In the sandy soil, Cd was adsorbed mostly by the CARB fraction, and at high compost doses, the portion in the RO fraction increased. In the clayey soil, the RO fraction was the main scavenger of Cd in the absence of compost, and its addition increased the proportion in the RO fraction. Water-soluble Cd decreased with an increase in compost dose in both soils. In the sandy soil, the pattern of Cd concentration in each fraction was similar to that of Cu. In the clayey soil, compost addition significantly reduced the concentration of Cd in the EXC and CARB fractions and increased it in the RO fraction, whereas it did not change in the other fractions. It can be postulated that in the two soils studied, the reducible component of the compost is an important scavenger of Cd.
Lead
In the absence of compost, Pb was distributed mainly between the CARB and RO fractions in the clayey and sandy soils. Increasing compost addition increased the proportion of Pb in the OM fraction in both soils. In the sandy soil, the proportion in the RO fraction also increased, but not in the clayey soil. In the latter, Pb was found only in the CARB and other less soluble fractions. Compost addition influenced only the increase in Pb in the OM fraction. In the sandy soil, Pb was found in the WE fraction but not in the EXC one. Compost addition to the clayey soil reduced the proportions of Pb in the WE and CARB fractions and increased it in the RO and OM fractions.
3.4 Metal phytoaccumulation and metal distribution pattern in soil
Heavy metal concentration in Chinese cabbage tissue calculated per fresh and dry plant weights is presented in Table 4. It can be seen from the reference data in Table 1 of the Electronic Supplementary Material that Cd bioaccumulation exceeded MPC only in the sandy soil at 0 and 5% compost. Copper did not accumulate in any treatment to either the phytotoxic concentration or above the generally expected level. Lead only accumulated in plants grown in sandy soil and accumulation exceeded a MPC at 0 and 5% doses of compost. Compost application also reduced Cd in plants grown in the sandy soil and did not have a significant influence on those grown in the clayey soil. Lead phytoaccumulation in the sandy soil was reduced when a 20% or higher compost dose was used. The same was true for Cu in plants grown in the sandy soil, whereas in the clayey soil, an equal metal concentration accumulated in all treatments. A high and significant negative correlation was found between metal concentration in the plant and compost dose for all cases (Table 5). The same trend was observed by Gat (2006), who grew Chinese cabbage on similar Israeli soils amended with biosolid compost, but in that case, the compost was the only metal source for the plants. This proves relatively low phytoavailability of the heavy metals bound by compost.
The results obtained from the correlation analysis of metal concentration in Chinese cabbage leaves versus metal content in different operationally defined geochemical fractions are also presented in Table 5. A positive correlation between metal content in the plant and that in a particular fraction suggested that the plant accumulated the metal from this geochemical form during the vegetation period. It is apparent from the table that fractions WE, EXC, and CARB, in most cases, served as an important source of heavy metals, whereas RO, OM, and RES could be defined as metal scavengers that reduce bioaccumulation. However, in the clayey soil, a negative correlation was observed between Cu in the WE fraction and metal content in plant leaves. The Cu concentration in the WE fraction increased with increasing compost dose, but metal phytoavailability was reduced. A similar phenomenon was observed by Tandy et al. (2009). Those authors proposed significantly higher humic substance concentrations in the soil solution in the presence of compost. Thus, while the total concentration of Cu in the soil solution may increase, the concentration of free Cu is very likely to decrease due to complexation with organic ligands, which are less phytoavailable than free ion. Hence, while compost may stimulate the migration of Cu in soil, it also reduces its bioavailability (Tandy et al. 2009).
It is possible to use the first three steps of the modified BCR protocol as a short, but at the same time, detailed procedure for extraction of the fractions with the most bioavailable heavy metals in soil and soil–OA mixtures: WE, EXC, and CARB.
4 Conclusions
Heavy metals added to soil even at MPC levels may accumulate to dangerous concentrations in plants. Compost addition generally increased the proportion of metals in the RO and OM fractions, reducing their phytoavailability. This is especially important for sandy soils with low adsorption capacity and higher vulnerability to metal pollution than clayey soils. The correlation found between metal concentrations in different geochemical fractions and plant tissue proved that the most phytoavailable fractions are WE, EXC, and CARB, whereas metal in OM, RO, and RES fractions was not available to plants. We propose to use the first three successive steps of the modified BCR protocol as a three-step SE procedure for extraction of the most phytoavailable fractions for heavy metals: WE, EXC, and CARB. It was found that a compost dose of 28.8 Mg ha−1 is optimal for reducing Cd and Pb accumulation by Chinese cabbage plants.
References
Alvarez EA, Mochon MC, Sanchez JC, Rodriguez MT (2002) Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 47:765–775
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual 34:49–63
Chen Y, Inbar Y, Barak P (1991) Soil testing methods (in Hebrew). Hebrew University of Jerusalem, Rehovot
EPA Method 3052 (1996) Microwave assisted acid digestion of siliceous and organically based matrices. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/3052.pdf. Accessed 22 Sept 2011
EPA Method 6010c (2007) Inductively coupled plasma–atomic emission spectrometry. http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/6010c.pdf. Accessed 24 Aug 2010
Gat P (2006) The effect of dissolved organic matter (DOM) originating from biosolid on metals binding, solubilization and uptake by plants. Dissertation, Hebrew University of Jerusalem, Rehovot
Gleyzes C, Tellier S, Astruc M (2002) Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trends Anal Chem 21:451–467
He ZL, Yanga XE, Stoffellab PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Israel Ministry of Environmental Quality (2005) Regulations for the treatment of soils polluted with heavy metals (in Hebrew). Tel-Aviv, Israel
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC LLC, London
Kukier U, Chaney RL, Ryan JA, Daniels WL, Dowdy RH, Granato TC (2010) Phytoavailability of cadmium in long-term biosolids-amended soils. J Environ Qual 39(2):519–530
McLean JE, Bledsoe BE (1992) Behavior of metals in soils. EPA Ground Water Issue, EPA/540/S-92/018
Pueyo M, Rauret G, Luck D, Yli-Halla M, Muntau H, Quevauviller P, Lopez-Sanchez JF (2001) Certification of the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn in a freshwater sediment following a collaboratively tested and optimised three-step sequential extraction procedure. J Environ Monit 3:243–250
Quevauviller P (1998a) Operationally defined extraction procedures for soil and sediment analysis. I. Standardization. Trends Anal Chem 17:289–298
Quevauviller P (1998b) Operationally defined extraction procedures for soil and sediment analysis: II. Certified reference materials. Trends Anal Chem 17:632–642
Quevauviller P (2002) Operationally defined extraction procedures for soil and sediment analysis. III. New CRMs for trace-element extractable contents. Trends Anal Chem 21:774–785
Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends Anal Chem 22:152–159
Singer A (2010) The soils of Israel. Springer, Berlin
SK-12 Medium Pressure Rotor Application Book (2011) Milestone Srl, Italy. http://www.milestonesrl.com/analytical/customer-care-library-digestion.html
Tandy S, Healey JR, Nason MA, Williamson JC, Jones DL (2009) Remediation of metal polluted mine soil with compost: co-composting versus incorporation. Environ Pollut 157:690–697
Zmora-Nahum S, Hadar Y, Chen Y (2007) Physico-chemical properties of commercial composts varying in their source materials and country of origin. Soil Biol Biochem 39:1263–1276
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Bini
Rights and permissions
About this article
Cite this article
Rosen, V., Chen, Y. The influence of compost addition on heavy metal distribution between operationally defined geochemical fractions and on metal accumulation in plant. J Soils Sediments 14, 713–720 (2014). https://doi.org/10.1007/s11368-013-0819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0819-7