Abstract
To understand the links between the long-term impact of uranium and other metals on microbial community composition, ground- and surface water-influenced soils varying greatly in uranium and metal concentrations were investigated at the former uranium-mining district in Ronneburg, Germany. A soil-based 16S PhyloChip approach revealed 2358 bacterial and 35 archaeal operational taxonomic units (OTU) within diverse phylogenetic groups with higher OTU numbers than at other uranium-contaminated sites, e.g., at Oak Ridge. Iron- and sulfate-reducing bacteria (FeRB and SRB), which have the potential to attenuate uranium and other metals by the enzymatic and/or abiotic reduction of metal ions, were found at all sites. Although soil concentrations of solid-phase uranium were high, ranging from 5 to 1569 μg·g (dry weight) soil−1, redundancy analysis (RDA) and forward selection indicated that neither total nor bio-available uranium concentrations contributed significantly to the observed OTU distribution. Instead, microbial community composition appeared to be influenced more by redox potential. Bacterial communities were also influenced by bio-available manganese and total cobalt and cadmium concentrations. Bio-available cadmium impacted FeRB distribution while bio-available manganese and copper as well as solid-phase zinc concentrations in the soil affected SRB composition. Archaeal communities were influenced by the bio-available lead as well as total zinc and cobalt concentrations. These results suggest that (i) microbial richness was not impacted by heavy metals and radionuclides and that (ii) redox potential and secondary metal contaminants had the strongest effect on microbial community composition, as opposed to uranium, the primary source of contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining and processing of uranium for nuclear weapon and fuel production resulted in the widespread contamination by uranium and other heavy metals in soils and subsurface sediments worldwide. Uranium-contaminated sites are often co-contaminated with heavy metals (Jakubick et al. 1997), sulfate (Chang et al. 2005; Jakubick et al. 1997) or nitrate and technetium (Michalsen et al. 2006). Metals can be hazardous to human health, affecting the functionality of several organs as well as increasing the potential for carcinogenesis due to metal toxicity (e.g., Craft et al. 2004; Denkhaus and Salnikow 2002; Miller and McClain 2007). Consequently, the migration of such toxic aqueous metal cations to the groundwater and their infiltration into adjacent ecosystems is a significant environmental problem. Several microorganisms may contribute to uranium and metal attenuation by direct and indirect mechanisms, usually via the enzymatic reduction of metal ions into their insoluble and chemically inert forms (Kostka and Green 2011; Lloyd 2003; Wall and Krumholz 2006). Dissimilatory iron- and sulfate-reducing bacteria (FeRB and SRB) in particular are known for these reactions and may further contribute to metal retention by abiotic reduction via their Fe(II) and sulfide metabolic products (Fude et al. 1994; Liger et al. 1999).
Less microbial species are present at uranium-contaminated sites in comparison to pristine soils as determined by 16S ribosomal RNA (rRNA) gene microarray and pyrosequencing analyses (e.g., Rastogi et al. 2010; Roesch et al. 2007), likely as a consequence of the inhibitory to lethal effects of metals and radionuclides (Bruins et al. 2000). At uranium-contaminated sites, the numbers of operational taxonomic units (OTU) detected using 16S rRNA gene microarray range from 978 to 1715 (Brodie et al. 2006; DeSantis et al. 2007; Rastogi et al. 2010), while uncontaminated soils contain 1917 to about 5400 OTU (Acosta-Martınez et al. 2008; DeAngelis et al. 2009; Roesch et al. 2007). Gans et al. (2005) calculated that a metal amendment of cadmium, copper, nickel, and zinc reduces species richness by 99.9 %, mainly by eliminating rare taxa, while another study shows that a fourfold increase in low-level uranium concentrations results in a 22 % decrease in OTU (Rastogi et al. 2010). Indigenous bacteria in both uranium-contaminated and pristine sites mostly belong to the Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Acidobacteria phyla, and contamination does not appear to affect the relative occurrence of species (e.g., Brodie et al. 2006; DeAngelis et al. 2009; Rastogi et al. 2010; Roesch et al. 2007). To date, most attention has been paid to a metal contaminant’s solid-phase concentrations; however, it is the bio-available fraction and not the total concentration that seems to correlate best with toxicity parameters (Vig et al. 2003).
In this study, microbial communities of soils of the former Ronneburg uranium-mining district (Thuringia, Germany) contaminated since decades to varying degrees by uranium were investigated using a 16S rRNA gene microarray approach that allowed direct comparison to similar investigations at other contaminated sites. Pyrite oxidation and the leaching of low-grade black shale by acid mine drainage and sulfuric acid from 1971 to 1989 resulted in a large-scale contamination by heavy metals and radionuclides (Jakubick et al. 1997). Although the district was remediated by removing the contaminated top soil, uranium and metal accumulation locally remains, particularly in the former leaching heap area and towards the drainage system of the catchment area (Carlsson and Büchel 2005). This study focused on the comparison of microbial communities inhabiting soils and sediments in this catchment affected by different levels of contaminated groundwater and/or surface water. We hypothesize that the exposure to uranium and other metals over four decades had a long-term impact on the indigenous microbial community composition reflected in fundamental changes on DNA basis, particularly when the metal fractions are analyzed that are available to microorganisms.
Material and methods
Selection and description of sampling sites
Soil and sediment samples were obtained from six locations in the former Ronneburg uranium-mining district (Thuringia, Germany). Of these sites, low metal concentrations were expected at site I, the Gessenwiese test site (Grawunder et al. 2009), located on the former, remediated leaching heap (Fig. 1). The dump material and up to 10 m of the underlying material were removed during remediation (carried out from 1991 to 1995 and 2002 to 2003, respectively), and the basement area was recontoured using allochthonous soil as fill (Carlsson and Büchel 2005; Grawunder et al. 2009). Quaternary sediments below the dumped black shales are still contaminated due to former leachate penetration through drainage and barrier layers and contaminated groundwater flow. We focused on two metal contaminated groundwater affected layers now located in 55- to 75-cm soil depth (Fig. 1) of a sediment profile near a trench, which has been studied in more detail earlier (Burkhardt et al. 2009). The upper layer is characterized as manganese rich (site I layer V) and the lower as iron rich (site I layer VI/VII). Due to the remediation activities, site I presented a drastic alteration of the original soil structure.
Scheme of sampling sites at the former uranium-mining district Ronneburg with (i) the former leaching heap (quaternary sediment; site I), where top soil was removed up to 10 m due to remediation, and (ii) three Luvic Gleysols associated with the Gessen Creek, where the sites II and III were located at the heap-facing creek bank (sites II and III) and site VI was on the heap-opposite area as well as (iii) the creek sediments (sites IV and V). Arrows indicate direction of water flow and exchange. Additionally, site I is connected to the creek-associated sites by surface run-off that is transported via the Gessen Creek
The east-west-lying Gessen valley is the hydrological relaxation zone of the basement and is located to the north of the excavated leaching heap and to the northwest of the former waste rock dump Nordhalde (Geletneky and Fengler 2004). The Gessen Creek therefore represents the main drainage system for leachate as well as upcoming mine and seepage waters (Carlsson and Büchel 2005).
At the Gessen creek, we studied a catena of five sites including the sediment of the Gessen Creek (Fig. 1). We started with an inbound soil profile at site II located at the heap-associated site, approximately 4 m in distance orthogonally to the Gessen Creek bank (Fig. 1). Further, two creek bank soils located at the heap-associated creek bank (site III) and the heap-opposite creek bank (site VI) were studied. Previous studies showed that the soil profile at site III contained two contaminated upper iron-rich, oxidized horizons (site III BElc and Btlc) and two lower reduced horizons (site III Br1 and Br2) reaching to 110 cm in depth that were characterized by high uranium and metal concentrations (Burkhardt et al. 2010; Sitte et al. 2010). The creek bank soil of the heap-opposite area located on the other site of Gessen Creek (site VI) showed similar pedological characteristics and served as a control site for the heap-associated bank soils (site III). In contrast to site III, soils at site VI should have received metal contact only by water of the Gessen Creek which should have resulted in reduced metal load compared to site III. All groundwater-influenced horizons at both creek banks (sites III and VI) are temporarily in direct water exchange because of fluctuating creek water levels or affected indirectly via influence/effluence processes and capillary rising. Thus, we also studied the creek sediments (sites IV and V) which are directly impacted by surface water of the Gessen Creek. The two surface sediment samples were taken from the heap adjacent (site IV, sediment A) and the heap-averting site (site V, sediment B) with a distance of about 2 m.
Creek-associated soils (sites II, III, and VI) were all characterized as Luvic Gleysol, and the focus was upon temporarily (Blc, Bl, BElc, Btlc) and permanent reduced (Br1, Br2, Br) groundwater-influenced horizons but not the topsoil, since the contamination plume reached only the deeper horizons and discharge was likely. Geochemical investigations of these six groundwater- and surface water-affected sites showed a broad range of metal contamination which could have been affected the microbial community composition.
Soil and sediment sampling
Soil was sampled with sterilized spatulas into sterile plastic vials and bags, respectively. Creek sediment was directly scooped with sterile tubes. Triplicate samples were then combined and homogenized as a composite sample. Sample aliquots were stored at 4 °C in the dark for geochemical analyses or at −20 °C prior to extraction of genomic DNA for PhyloChip analysis. Samples for geochemistry were air-dried and sieved for the fraction <2 mm.
Geochemical analysis of soil and sediment samples
Determination of geochemical parameters
Soil pH was determined by the CaCl2 method (Forster 1995) using a pH meter pH330 (WTW, Weilheim, Germany) with a micro-electrode (Inlab 423; Mettler-Toledo, Gießen, Germany). Redox potential was determined by inserting a redox electrode (PT 4800-M5-S7/80; Mettler-Toledo, Gießen, Germany) directly into the soil, and values were reported relative to the SHE. For CNS analysis, soil fractions <2 mm were finely ground to <63 μm with a centrifugal ball mill (S100, Retsch, Hahn, Germany) for 45 min and analyzed in a CHNOS elementary analyzer VARIO EL (Elementar Analysensysteme, Hanau, Germany). Soil organic carbon content was determined by loss-on-ignition at 500 °C for 5 h after samples were dried at 105 °C for 24 h. Soil texture was determined by separation of sand, clay, silt, and gravel using a laser diffraction particle size analyzer LS 13320 (Beckmann Coulter GmbH, USA). Granulometry was performed using the soil mapping manual (Sponagel et al. 2005).
Determination of soil metal concentrations
Finely ground soils (<63 μm) were digested with concentrated hydrofluoric acid, nitric acid, and perchloric acid at 150 to 170 °C in a pressure digestion system (DAS, PicoTrace, Bovenden, Germany) for total soil metal measurements. Single elements were measured in the resulting solutions using inductively coupled plasma-optical emission spectroscopy (ICP-OES; Spectroflame P FAV05; Spectro Analytical Instruments, Kleve, Germany) for Fe and Mn and inductively coupled plasma-mass spectrometry (ICP-MS; X-SeriesII; Thermo Fisher Scientific, Bremen, Germany) for As, Cd, Co, Cr, Cu, Ni, U, Zn, and Pb.
Sequential extractions to determine the binding forms of metals were determined on air-dried and sieved (<2 mm) soil samples according to Zeien and Brümmer (1989). The resulting fractions were analyzed with ICP-OES and ICP-MS as described above. The water-soluble (fraction 1, F1) and specifically adsorbed (fraction 2, F2) fractions were analyzed for all soil and sediment samples. Remaining fractions based on the sequential extraction (bound to manganese oxides [fraction 3, F3], organic material [fraction 4, F4], amorphous iron oxides [fraction 5, F5], crystalline iron oxides [fraction 6, F6] or to the residual fraction, presumably mainly silicates [fraction 7, F7]) were determined for soil samples of site III with the highest metal concentrations. Values of the Btlc horizon at site III were obtained from Burkhardt et al. (2011a) and for site I from Burkhardt et al. (2009).
Amplification of 16S ribosomal RNA genes
Genomic DNA of soil and creek sediments was isolated in triplicate using the PowerSoil DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA) and purified DNA from triplicates were combined for each sample. Amplification of 16S rRNA genes was carried out in triplicate using the universal bacterial primers fd1 (Weisburg et al. 1991) and 1492R (Eden et al. 1991) or universal archaeal primers 4Fa (Hershberger et al. 1996) and 1492R. PCR reactions contained 0.21 μM of each primer, 1× Premix F (Epicentre Biotechnologies, Madison, WI), and 5 U μL−1 Taq polymerase (Jena Bioscience, Jena, Germany). Thermal cycling was performed using an initial denaturation step for 3 min at 95 °C, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at eight temperatures ranging from 48 to 58 °C for 25 s, elongation at 72 °C for 2 min, and a final elongation step at 72 °C for 10 min.
Microbial community analysis by 16S rRNA PhyloChip
16S rRNA gene amplicons were identified using a PhyloChip approach (high-density 16S rRNA microarray) that has been successfully applied to other uranium-contaminated sediments and soils (Brodie et al. 2006; Rastogi et al. 2010; Tokunaga et al. 2008). Within 121 demarcated prokaryotic families, abundant as well as rare species of 8935 bacterial and archaeal OTU can be detected with high resolution (Brodie et al. 2006). It allows a rapid profiling of microbial populations, although unknown OTU remain undetected. Bacterial (500 ng) and archaeal (15 ng) 16S gene amplicons were concentrated, fragmented, and biotin labeled as previously described (DeSantis et al. 2007). Biotin-labeled DNA was hybridized to PhyloChips (Affymetrix GeneChips; Affymetrix, Santa Clara, CA) according to DeSantis et al. (2007) with washing and staining steps performed according to Affymetrix standard protocols.
Statistical analysis of bacterial and archaeal communities
Operational taxonomic units (OTU) were classified at level of phylum, class, order, and family according to sequence similarity cut-off values of 80, 85, 90, and 92 %, respectively (DeSantis et al. 2007), and microbial richness at different taxonomic levels was determined. The impact of environmental variables on microbial OTU distribution was analyzed by redundancy analysis (RDA) and forward selection performed in the R software environment version 2.10.1 (R Development Core Team 2005) using the Vegan package version 1.17-2 (Oksanen et al. 2008) and packfor package version 0.0-7 (Dray 2007). For RDA analysis, subsets of total archaea, total bacteria, and sulfate- and Fe(III)-reducing bacteria were constructed. Redox potential, pH, and concentrations of bio-relevant metals (both bio-available and total) with a ratio standard deviation to average concentrations ≥0.48 were chosen as environmental variables (Tables 1 and 2). Detrended correspondence analysis (DCA) was performed prior to RDA to confirm linearity of species data. RDA results were plotted with species ordination scores scaled using scaling −1 default function, where species data were divided by standard deviation and multiplied by compensative constant. Forward selection was performed with a reduced set of environmental variables to minimize correlation (inflation factor <10) between the parameters. Hierarchical cluster analysis was performed using the Euclidean distance function with average linkage. The function heatmap was used within the made4 package with its default settings (Culhane et al. 2005) for metal concentrations/pH/redox potential as well as the HybScores for microbial OTU to investigate whether single groups of microorganisms follow a pattern of environmental variables.
Results
Geochemical characteristics of soil and sediment
Leaching processes likely acidified the oxidized, glacial sandy and silty layers at site I (soil pH ~4), whose total nitrogen and sulfur contents in the solid phase were below detection limits (Burkhardt et al. 2009). Very low concentrations of organic carbon were found at the excavated leaching heap (≤0.9 % dry weight [dw]) (Table 1), where the upper horizons were removed by remediation processes, and vegetative cover has only developed sparsely. Creek-associated soils were moderately acidic, and redox potentials (220 to 570 mV) indicated oxidizing conditions for site II and the upper creek bank soil horizons (site III BElc and Btlc, site VI Blc; Table 1) while reducing conditions (−30 to 180 mV) were observed for the lower creek bank soil horizons (site III Br1 and Br2, site VI Br). Organic carbon (≤5.4 % [dw]) and, in part, total nitrogen (≤0.36 % [dw]) accumulated at the heap-associated creek bank (site III) and total sulfur was highest at site III, especially in the lower, reduced horizon (site III Br2 1.98 % [dw] Stotal). The gravel creek sediment (sites IV and V) was characterized by circum-neutral pH and low redox potentials, indicating reducing conditions. Organic carbon content was 1.2 and 1.9 %, respectively (Table 1).
Metal content of soil and sediment
Total metal concentrations of all soil samples exceeded background levels given for non-contaminated reference soils (Table 1). Uranium was highest in the heap-associated creek bank (site III) with concentrations up to 1569 μg·g−1, while Quaternary sediments at the excavated leaching heap (site I) contained comparably lower concentrations (≤11 μg·g−1) and sites II, IV, V, and VI all had uranium concentrations ≤85 μg·g−1. Arsenic and manganese were highest at the excavated leaching heap (site I layer VI/VII and layer V, respectively; Burkhardt et al. 2009) but were found at lower concentrations at the other sites. Other heavy metals were also found at their highest concentrations in the heap-associated creek bank soil (site III), and in particular, copper (942 μg·g−1), zinc (564 μg·g−1), and nickel (229 μg·g−1) were detected with high concentrations for all horizons at site III (Table 1). Thus, metals were significantly higher in the heap-associated soil (site III) as compared to the heap-opposite soil (site VI), especially with the upper horizon showing even a 1000-fold higher uranium concentration (Table 1). The sediment samples (sites IV and V) did not show a significant difference, since water turbation processes might cover up any effects of heavy metal enrichment at the heap-associated creek bank.
In addition to iron, bio-available uranium and manganese (F1 and F2) were detected in the soils as potentially used electron acceptors for microorganisms, while arsenic and chromium concentrations were mostly below detection limits (Table 2). Bio-available uranium was found in the highest concentrations at site III, primarily in the specifically adsorbed fraction (≤471 μg·g−1), representing up to 36.4 % of the total uranium present. It was also found in the samples of the other sampling sites, but to a lower extent (≤23.32 μg·g−1). Water-soluble and specifically adsorbed copper (≤83.71 μg·g−1), zinc (≤53.83 μg·g−1), and nickel (≤57.10 μg·g−1) were also highly accumulated at the heap-associated creek bank (site III), whereas lead (≤6.56 μg·g−1), copper (≤10.15 μg·g−1), and cadmium (≤2.79 μg·g−1) were found in relatively low concentrations (Table 2). Bio-available fractions at sites I, II, IV, V, and VI were generally less enriched in metals than site III (Table 2). In general, bio-available concentrations followed the pattern observed for the soil solid phase.
Sequential extractions were performed from the heap-associated creek bank soils (site III) with the highest metal concentrations. Fe-oxides were important sorbents with amorphous Fe phases being slightly more adsorbed to (max. 38 % for uranium and nickel in BElc) than crystalline Fe-oxides (max. 21 % for nickel in BElc) (Fig. 2). The role of organic matter as a sorbent for metals was negligible (≤12 %) with the exception of copper, where 33 % was bound to organic material in the BElc horizon. Additionally, manganese oxides appeared not to be an important sorbent for heavy metals (≤13 %) with the exception of cobalt in the Btlc horizon with 31 % bound (Fig. 2).
Concentration of selected metals in fractions determined by sequential extraction from soils of the heap-associated creek bank (site III; BEw/Ah, BElc, Btlc, Br1, and Br2 horizons). Btlc values were recently published by Burkhardt et al. (2011a)
Microbial communities
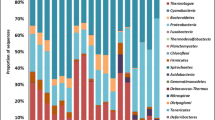
In total, 2393 OTU were positively detected at the Ronneburg site (Table 3), with 2358 bacterial OTU found within 45 phyla and 35 archaeal OTU found within 2 phyla. Total OTU numbers were lower for the heap sediment at site I (≤1455 OTU) as well as the reduced soil horizons (site III Br1 and Br2) of the heap-associated creek bank (≤1439 OTU) (Table 3). In comparison, the oxidized Btlc horizon at site III, which has higher concentrations of heavy metals, contained 1708 OTU (Table 3). Numbers of all taxonomic levels were in a similar range for the oxidized soil horizons of both creek banks (site III BElc and Btlc and site VI Blc, respectively), while numbers of the reduced horizons were higher at the heap-opposite site (site VI Br) compared to the heap-associated bank (site III Br1 and Br2) (Table 3). Phylogenic phyla and classes of Proteobacteria and Firmicutes were rather homogenously distributed among all soil and sediment samples (data not shown). However, the detected OTU affiliated to the Actinobacteria were 13 % above average and numbers of Bacilli OTU were lowered by 80 % in the glacial sediment samples (site I). Gammaproteobacteria OTU numbers were less in the reduced creek bank soils (130 ± 38 OTU compared to an average of 210 ± 69 OTU), and highest OTU number of Bacteroidetes were found in both creek sediments at sites IV and V, respectively (125 ± 4 OTU compared to an average of 95 ± 28 OTU). The low total OTU number in the site III Br2 soil sample was mainly due to the absence or strong reduction of representatives belonging to the Actinobacteria (e.g., Cellulomonadaceae, Dermabacteriaceae, Nocardiaceae, and Micrococcaceae), Alphaproteobacteria (Bradyrhizobiaceae, Caulobacteraceae, Sphingomonadaceae, and several families within the Rhizobiales), and Gammaproteobacteria (Alteromonadaceae, Pseudoalteromonadaceae, Shewanellaceae, and Chromatiaceae).
Heatmap patterns of the environmental variables and microbial groups illustrated only negligible similarities between the microbial communities of the soil and sediment samples (data not shown): Desulfobulbaceae were present where low redox potentials occurred, whereas Alteromonadaceae, Acidobacteriaceae, Actinomycetales, Acidimicrobiales, and Rhizobiales were detected at sites with comparatively higher redox potentials. Bacillaceae, Clostridiaceae, Peptococcaceae, and different families within the Actinomycetales were found mainly at sites with a pH ≤4.1. Exclusively total cobalt concentration was negatively correlated with the presence of Bacillaceae, Halobacteriaceae, Aerococcaceae, and Enterococcaceae.
Bacteria
The majority of bacterial clones were related to Proteobacteria (1081 OTU), Firmicutes (416 OTU), Actinobacteria (208 OTU), Bacteroidetes (160 OTU), and Acidobacteria (78 OTU). The 2358 OTU were found in 100 phylogenetic orders, 55 classes, and 45 phyla. Hierarchical cluster analysis revealed rather similar bacterial communities sampled from the creek-associated oxidized and reduced soils (sites III and VI), which clustered with the communities originated from the excavated leaching heap at site I (Fig. 3). Contrarily, OTU composition of both sediments (sites IV and V) and two of the reduced creek bank horizons (sites III Br2 and VI Br) separated out from this cluster (Fig. 3a). RDA-biplots indicated that the redox potential was strongly correlated to the first axis (Figs. 4a and 5a). Further, bio-available and total manganese concentrations were correlated with the second RDA axis, while bio-available and total cobalt concentrations correlated with the first and second axis, respectively (Figs. 4a and 5a). Forward selection revealed that the redox potential and the bio-available manganese significantly explained 13.3 and 17.0 % variance in OTU distribution, respectively (Table 4). Once the redox potential and total cobalt concentrations were selected, total cadmium concentrations explained 19.0 % of spatial OTU distribution (Table 4), although soil concentrations were low relative to other metals (Table 1). Mainly, the bacterial III Br2 community was correlated to low redox potentials, as expected, and to high concentrations of nickel in the soil solid phase in addition to most of the bio-available metals (Figs. 4a and 5a). In contrast, communities originated from VI Br, III Btlc, and I layer V samples were correlated to low concentrations of most metals in their bio-available form (Fig. 4a).
RDA plots showing the relationships between environmental variables (metal concentrations reflecting the bio-available fraction, redox potential, and pH) and microbial communities at the different sites along the first two RDA axes for a bacteria, b archaea, and c iron-reducing and d sulfate-reducing bacteria
FeRB
Iron-reducing bacteria were present in all samples with a total of 45 OTU detected within the known groups of dissimilatory FeRB (e.g., Geobacteraceae, Acidithiobacillaceae, Shewanella, or Clostridiaceae) and groups of bacteria with the ability to reduce iron without gaining energy (e.g., Enterobacterales, Rhodobacterales, Comamonadaceae, or Acidobacteriaceae). The lowest number of OTU (20 OTU) was detected at the excavated leaching heap (site I), while the highest number of 37 OTU inhabited the creek sediment (sites IV and V). Soils of the creek bank harbored 29 and 28 FeRB OTU at sites III and VI, respectively (data not shown). Hierarchical cluster analysis showed rather similar FeRB OTU communities within the investigated samples as observed for the total bacterial communities. The FeRB communities from the oxidized and reduced creek-associated soils (sites II to V) and sediment layers from the excavated leaching heap clustered (site I). In addition to the creek sediments (sites IV and V), the lowest, reduced creek bank horizons (III Br2 and VI Br) separated from this cluster (Fig. 3c). According to RDA, the redox potential correlated with the second axis and cobalt (both bio-available and total) as well as bio-available manganese concentrations correlated with the first axis (Figs. 4c and 5c). Once the variations in OTU distribution due to bio-available manganese concentrations were considered, the redox potential significantly explained 17.3 % of the observed OTU variability within the samples (Table 4). Similarly, total cadmium concentrations and redox potential explained 28.0 and 43.6 % of spatial OTU variability, respectively, after the consideration of total cobalt concentrations (Table 4). In particular, the FeRB community of the Br2 horizon of site III was correlated to bio-available cobalt, cadmium, nickel, and uranium and very low redox potentials (Fig. 4c). In contrast, this FeRB community was correlated only to soil solid phase nickel and zinc (Fig. 5c). FeRB communities of the Btlc horizon at site III and the Br horizon of site VI were negatively correlated with several bio-available metals (cobalt, uranium, and nickel), although they were positively correlated with metals in the soil solid phase, such as copper, arsenic, and chromium (Figs. 4c and 5c).
SRB
Sulfate-reducing bacteria were detected with a total of 64 OTU at all soil and sediment samples. The majority of clones belonged to the Desulfobacteraceae, Desulfobulbaceae, Syntrophobacteraceae, and Desulfovibrionaceae. The soils of the creek bank (sites III and VI) harbored the highest numbers of SRB OTU (57 OTU at site III, 60 OTU at site VI), and differences in depth were marginal at all locations. According to OTU composition, sediment B (site V) and the Br2 horizon of the heap-associated creek bank (site III) separated out from the cluster of the other creek-associated samples (sites II to VI) and the sediment layers of the excavated leaching heap (site I) (Fig. 3d). RDA indicated that the redox potential as well as cobalt concentrations of the soil solid phase correlated with the second RDA axis (Figs. 4d and 5d). No distant environmental variable correlated with the first RDA axis that explained 72.6 % of the variance in SRB composition within the samples (Figs. 4d and 5d). Forward selection showed that the bio-available manganese and copper concentrations accounted for 24.8 and 17.9 % of the variability in OTU composition, respectively, once the variation due to the redox potential was accounted (Table 4). The redox potential is suggested to explain 18.4 % OTU variability analyzing the environmental dataset including the total metal concentrations and after the removal of variance potentially explained by the cadmium concentrations (Table 4). Metal concentrations were highly correlated with each other, and bio-available ones were negatively correlated with the redox potential and pH, respectively (Figs. 4d and 5d). SRB communities of the heap-associated creek bank (site III Br1 and Br2) correlated with most metals, such as nickel or zinc, and a low redox potential as indicated by the RDA biplot (Figs. 4d and 5d). In addition, communities of other samples clustered and did not correlate with any of the bio-available metals (Fig. 4d).
Archaea
Archaeal OTU were likely not to be present at the excavated leaching heap (site I), since results of the archaea-specific PCR were negative for both sediment layers of site I. Crenarchaeota (total of 15 OTU) were equally distributed along the other sediment and soil samples, while Euryarchaeota (total of 20 OTU), with mainly Methanomicrobia and Halobacteria, were primarily detected only in the reduced horizons of the creek bank soil (sites III and VI). According to hierarchical cluster analysis, archaeal communities from the creek sediment and the Btlc horizon of site III were separated from the other creek-associated soil samples (Fig. 3b). RDA analyses revealed that soil solid manganese and cobalt correlated with the first axis, while the second RDA axis correlated with the redox potential in addition to either the total cadmium and zinc concentrations (Fig. 5b) or the bio-available cobalt concentration. Most of the bio-available metal concentrations were highly correlated with each other (Fig. 4b), so any of the environmental measured variable could be clearly determined to correlate with the first RDA axis that explained 62.1 % of variability in archaeal OTU distribution (Fig. 4b). Once the variability due to the redox potential was accounted, bio-available lead concentrations as well as the pH were suggested to explain 21.0 and 31.5 % of variance in archaeal OTU distribution (Table 4). Additionally, total cobalt and zinc concentrations accounted for 21.9 and 38.5 %, respectively, for the observed variability in archaeal OTU composition (Table 4). Archaeal communities of the Br2 horizon at the heap-associated site (site III) correlated with a low redox potential and most of the bio-available as well as total metal concentrations (Figs. 4b and 5b). Contrarily, archaea originated from site II, and the oxidized horizon Blc at the heap-opposite creek bank (site VI) were not correlated or did not response to metal concentrations, considering both the bio-available and total ones (Figs. 4b and 5b).
Discussion
Metal contamination
Nearly all metals exceeded background levels in both the soil and sediment, although total and bio-available concentrations showed a high spatial heterogeneity within sampling sites providing advantageous conditions for the investigation of metal-microbe interaction. This same tendency has been observed at the US Department of Energy’s Oak Ridge Field Research Center (ORFRC), TN, USA, where total uranium concentrations range from background levels to about 740 μg·g−1 in the soil (Brooks 2001; Watson et al. 2004), less than half the maximum concentrations observed at the Ronneburg site. Higher accumulations in the soil solid phase were also detected in a limited area for most of the other metals (e.g., 6-fold more zinc and 13-fold more cobalt) compared to other uranium-contaminated sites that also have the risk of downstream contamination (Brooks 2001; Rastogi et al. 2010; U.S. Department of Energy 1999). Although iron oxides served as important sorbents for metals, uranium, zinc, manganese, nickel, and cobalt remained available for microorganisms and plants and therefore constituting a potential risk for life in adjacent soils and waters. Downstream uranium contamination has also been observed in the Weiße Elster river sediments (Zerling et al. 2003), into which the Gessen Creek empties, about 12 km below the former mining site.
The heap-associated creek bank (site III) was enriched with high concentrations of uranium and other heavy metals in the soil solid phase due to extensive uranium mining. Iron oxides, identified as relevant metal adsorbents, show the potential to adsorb and co-precipitate with metal cations (Cooper et al. 2006; Cornell and Schwertmann 2003). Metal cations were predominantly adsorbed to amorphous Fe-oxide forms in the creek bank soil (site III). Contrary, crystalline Fe-oxides as well as silicates (maximum values up to 49.2 % for cobalt and 59.8 % for nickel, respectively) were more important metal sorbents at the excavated leaching heap (site I) as compared to amorphous Fe-oxides with a maximum of 19.0 % for cobalt (Burkhardt et al. 2009). Divalent metals could also be absorbed and co-precipitated by Fe sulfides (Morse and Arakaki 1993; Özverdi and Erdem 2006) that were not distinguishable by sequential extraction. However, acid volatile sulfur is present at the creek bank in concentrations of up to 126 mmol·kg [dw] soil−1 in the reduced horizons (Sitte et al. 2010). In addition to chemical transformations, microbially mediated Fe(II) oxidation as well as sulfate reduction might have also resulted in the metal-capturing during biogenic Fe-oxide or metal sulfide precipitation (Emerson and Moyer 1997; Muyzer and Stams 2008).
Influence of contaminants on microbial communities
Both, bacterial and archaeal community compositions, did not appear to be significantly affected by bio-available and total concentrations of uranium, the primary contamination source, which can be harmful due to its metal toxicity but with negligible effect because of radioactivity at the detected concentrations (Sani et al. 2006; Vanengelen et al. 2011). Microbial community distribution was rather influenced by the secondary contaminants, such as manganese, zinc, or copper, as well as by the redox potential. Redox potential is known as a main driver for shifts in microbial community structures especially in sediments where electron acceptors can be readily depleted with increasing depth (e.g., Jørgensen 2006; Tokunaga et al. 2008). Thus, differences between soils of more oxidized (site I) and more reduced sites (sites III and VI) were expected. However, no profound impact was detected at sites with similar reduced horizons (site III and site VI) but different uranium concentrations.
A differentiation of effects due to a single metal species on in situ microbial communities is difficult; microbes are never confronted with one single contaminant only in the field (Bååth 1989). Furthermore, applications of metals to soil microbial communities in the field and laboratory have been shown to have different effects on community composition: (i) addition of zinc tested up to 13 mg·g−1 soil, results in changes of soil microbial phospholipid fatty acid (PLFA) profiles with a decrease of indicator PLFAs for gram-positive bacteria and Actinomycetes (Kelly et al. 2003); (ii) Cu application (250 μg·g−1 soil) causes no community shift by denaturing gradient gel electrophoresis (Girvan et al. 2005), while opposingly even lower concentrations have an effect on bacterial composition determined by terminal restriction fragment length polymorphism patterns (Tom-Petersen et al. 2003); and (iii) metal mixtures, such as cadmium, zinc, copper, or lead, cause shifts in microbial community structures (Frostegård et al. 1993; Griffiths et al. 1997; Macdonald et al. 2008). Bacterial species affiliated to the, e.g., Streptomycetaceae, Alcaligenaceae, Microbacteriaceae, or Rhizobiaceae show resistance towards metals in the millimolar range (Abou-Shanab et al. 2007; Schmidt et al. 2009) and representative OTU were present at Ronneburg, however, without any correlation between the detected OTU number and metal concentrations among the samples (data not shown). Especially microbial communities of site III Br2 soil were suggested to tolerate metal stress in addition to preferring low redox potentials. Little information exists about cobalt tolerances of Bacillaceae and Enterococcaceae (Amoozegar et al. 2005; Kimiran-Erdem et al. 2007), which seemed to avoid the presence of cobalt at the Ronneburg site (data not shown). These results obtained at Ronneburg are in contrast to observations at the Rifle and ORFRC sites, respectively, where the uranium is accounted for the microbial functional gene structure (Liang et al. 2012) or for the microbial 16S rRNA gene diversity (Cho et al. 2012; Gihring et al. 2011) among redox conditions and the presence of other metals.
As FeRB and SRB are involved in in situ bioremediation processes (Gadd 2000), an impact of certain metals, such as uranium or chromium, on community composition was expected. However, as observed for the total bacterial community, both bio-available as well as total uranium concentrations did not explain variability within FeRB and SRB communities. At the excavated leaching heap (site I), there is no indication of either sulfate or iron reduction likely due to the deficiency in organic carbon (Burkhardt et al. 2009), although representative OTU capable of carrying out these processes were present. In contrast, sulfate- and Fe(III)-reducing microorganisms are shown to carry out the dominating electron-accepting processes in the heap-associated creek bank soil at site III (Burkhardt et al. 2010; Sitte et al. 2010). Microcosm studies showed that indigenous bacteria present at the heap-associated creek bank soil (site III) can tolerate metals in the millimolar range; Fe(III)-reducing bacteria could tolerate 2.5 mM Zn and sulfate-reducing bacteria 30 mM Ni and even 40 mM Co. The microbial communities in the microcosms showed a shift towards the genera of Geobacter, Desulfosporosinus, Clostridium, Sedimentibacter, and Citrobacter (Burkhardt et al. 2011b; Sitte et al. 2013). Whereas nickel and cobalt are attenuated from site III Br2 soils during sulfate-reducing conditions likely by precipitation of metal sulfides (Sitte et al. 2010), in site III Btlc soils, uranium, zinc, and cobalt are unexpectedly released in parallel with manganese and iron(III) reduction (Burkhardt et al. 2010). Therefore, SRB but not FeRB could be involved in metal attenuation at the Ronneburg site, especially at the heap-associated creek bank, resulting in the prevention of metal migration.
The abundance of OTU detected at all samples was higher at all examined phylogenetic levels than at other uranium-contaminated sites (DeSantis et al. 2007; Rastogi et al. 2010). The maximum OTU number was detected in the Btlc horizon at the heap-associated creek bank (site III), which also had the highest uranium concentration in the soil solid phase. Species richness of site III Btlc was similar to OTU numbers found in the uncontaminated soil of the Avena fatua (wild oat) rhizosphere (DeAngelis et al. 2009) and was even 38 % higher than uranium-contaminated subsurface soils at the ORFRC site (DeSantis et al. 2007). This suggests that the very high metal load had no overall impact on species richness at the Ronneburg site. The low OTU abundances at the ORFRC and Hanford sites (Johnson et al. 2001; Kaplan et al. 2012; Lin et al. 2012) might be explained by low concentrations of sediment organic carbon and, therefore, a lack of variable energy sources. The reduced OTU numbers at the former heap (site I) could be likely explained by the low organic carbon content that resulted from the removal of topsoil during physical remediation. Most of the indigenous phyla (Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Acidobacteria) were similar to those found previously at a number of different uranium-contaminated sites using the PhyloChip (Brodie et al. 2006; DeSantis et al. 2007; Handley et al. 2012; Rastogi et al. 2010) or further 16S rRNA gene analysis techniques (Bondici et al. 2013; Cho et al. 2012), including recently observed relatives within the Thermodesulfobacteria, Dictyoglomi, Synergists, Thermotogae, Deferribacter, OP3, TM6, and SR1 divisions (Gihring et al. 2011; Rastogi et al. 2010; Tokunaga et al. 2008). Members of the Sphingomonadaceae, Xanthomonadaceae, Bacteriodaceae, and Bacillaceae, for example, have been found in the metabolically active fraction of the indigenous microbial communities in uranium-contaminated sediments from the ORFRC site (Akob et al. 2007; Leigh et al. 2014). Sandaa et al. (1999) previously discussed archaeal diversity in contaminated soils. The methanogen Methanocalculus pumilus as well as the sulfur-oxidizer Sulfolobus metallicus have been shown to resist high concentrations of copper, zinc, or nickel (Huber and Stetter 1991; Mori et al. 2000). Interestingly, few representatives of the Halobacteria were present in reduced, heap-associated creek bank soil (site III), even though this archaeal class is generally restricted to hypersaline environments, and high concentrations of divalent cations are supposed to be of considerable ecological importance to this group (Oren 2006). Otherwise, Halobacteria sp. is recently described in sulfate-reducing wells of the uranium-contaminated Rifle site by the detection of its cellulase genes (Liang et al. 2012). This suggests that these indigenous, archaeal OTU inhabits a wider range of non-saline environments than generally assumed rather than being a remnant from the active leaching period.
Summary
This study showed that uranium concentrations alone did not explain variability within indigenous microorganisms at Ronneburg, suggesting (i) their adaptation towards metal stress during the last decades was due to the constant release of acid mine drainage and/or (ii) that the present microbial communities were rather robust towards metal and radionuclide exposure with relatively high tolerances against multiple metals. The discrepancy between high energy costs for metal homoeostasis and low energy gain due to anaerobic respiration might have resulted in the loss of microbial OTU in the lowest, reduced soil horizon at the heap-associated creek bank (site III Br2 horizon), since neither the high metal concentrations (site III Btlc horizon) nor the low redox potential (site VI Br horizon) alone resulted in the reduction in OTU numbers observed with a soil-based 16S PhyloChip analysis. However, many OTU will not be detected by this approach. Thus, next generation sequencing techniques are needed to investigate in more detail differences in the diversity of microorganisms affected by long-term metal exposure. In addition, fungal DNA sequences should be also included to obtain a more holistic view on the adaption of microorganisms to uranium and heavy metal exposure at the former uranium-mining site Ronneburg.
References
Abou-Shanab RAI, van Berkum P, Angle JS (2007) Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 68:360–367
Acosta-Martınez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770
Akob DM, Mills HJ, Kostka JE (2007) Metabolically active microbial communities in uranium-contaminated subsurface sediments. FEMS Microbiol Ecol 59:95–107
Amoozegar MA, Hamedi J, Dadashipour M, Shariatpanahi S (2005) Effect of salinity on the tolerance to toxic metals and oxyanions in native moderately halophilic spore-forming bacilli. World J Microbiol Biotechnol 21:1237–1243
Bååth E (1989) Effects of heavy metals in soil on microbial processes and populations. Water Air Soil Pollut 47:335–379
Bondici V, Lawrence J, Khan N, Hill J, Yergeau E, Wolfaardt G, Warner J, Korber D (2013) Microbial communities in low permeability, high pH uranium mine tailings: characterization and potential effects. J Appl Microbiol 114:1671–1686
Brodie E, Desantis T, Joyner D, Baek S, Larsen J, Andersen G, Hazen T, Richardson P, Herman D, Tokunaga T, Wan J, Firestone M (2006) Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol 72:6288–6298
Brooks SC (2001) Waste characteristics of the former S-3 ponds and outline of uranium chemistry relevant to NABIR Field Research Center studies. NABIR Field Research Center Oak Ridge, Tennessee
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Burkhardt EM, Meißner S, Merten D, Büchel G, Küsel K (2009) Heavy metal retention and microbial activities in geochemical barriers formed in glacial sediments subjacent to a former uranium mining leaching heap. Chem Erde Geochem 69:21–34
Burkhardt EM, Akob DM, Bischoff S, Sitte J, Kostka JE, Banerjee D, Scheinost AC, Küsel K (2010) Impact of biostimulated redox processes on metal dynamics in an iron-rich creek soil of a former uranium mining area. Environ Sci Technol 44:177–183
Burkhardt EM, Bischoff S, Akob DM, Büchel G, Küsel K (2011a) Heavy metal tolerance of Fe(III)-reducing microbial communities in contaminated creek bank soils. Appl Environ Microbiol 77:3132–3136
Burkhardt EM, Bischoff S, Akob DM, Büchel G, Küsel K (2011b) Heavy metal tolerance of Fe(III)-reducing microbial communities in contaminated creek bank soil. Appl Environ Microbiol 77:3132–3136
Carlsson E, Büchel G (2005) Screening of residual contamination at a former uranium heap leaching site, Thuringia, Germany. Chem Erde Geochem 65:75–95
Chang YJ, Long PE, Geyer R, Peacock AD, Resch CT, Sublette K, Pfiffner S, Smithgall A, Anderson RT, Vrionis HA, Stephen JR, Dayvault R, Ortiz-Bernad I, Lovley DR, White DC (2005) Microbial incorporation of C-13-labeled acetate at the field scale: detection of microbes responsible for reduction of U(VI). Environ Sci Technol 39:9039–9048
Cho K, Zholi A, Frabutt D, Flood M, Floyd D, Tiquia S (2012) Linking bacterial diversity and geochemistry of uranium-contaminated groundwater. Environ Technol 33:1629–1640
Cooper DC, Picardal FF, Coby AJ (2006) Interactions between microbial iron reduction and metal geochemistry: effect of redox cycling on transition metal speciation in iron bearing sediments. Environ Sci Technol 40:1884–1891
Cornell RM, Schwertmann U (2003) The iron oxides. WILEY-VCH, Weinheim
Craft ES, Abu-Qare AW, Flaherty MM, Garofolo MC, Rincavage HL, Abou-Donia MB (2004) Depleted and natural uranium: chemistry and toxicological effects. J Toxicol Environ Health Part B 7:297–317
Culhane AC, Thiolouse J, Perriere G, Higgins DG (2005) MADE4: an R package for multivariate analysis of gene expression data. Bioinformatics 21:2789–2790
DeAngelis KM, Brodie EL, DeSantis TZ, Andersen GL, Lindow SE, Firestone MK (2009) Selective progressive response of soil microbial community to wild oat roots. ISME J 3:168–178
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42:35–56
DeSantis TZ, Brodie EL, Moberg JP, Zubieta IX, Piceno YM, Andersen GL (2007) High-density universal 16S rRNA microarray analysis reveals broader diversity than typical clone library when sampling the environment. Microb Ecol 53:371–383
Dray S (2007) Packfor: forward selection with permutation, R package version 0.0–7. http://pbil.univ-lyon1.fr/members/dray/software.php
Eden P, Schmidt TM, Blakemore R, Pace N (1991) Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16S rRNA-specific DNA. Int J Syst Bacteriol 41:324–325
Emerson D, Moyer C (1997) Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol 63:4784–4792
Forster JC (1995) Soil sampling, handling, storage and analysis. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press Ltd, San Diego, pp 49–121
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Fude L, Harris B, Urrutia MM, Beveridge TJ (1994) Reduction of Cr(VI) by a consortium of sulfate-reducing bacteria (SRB III). Appl Environ Microbiol 60:1525–1531
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11:271–279
Gans J, Wolinsky M, Dunbar J (2005) Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309:1387–1390
Geletneky JW, Fengler HJ (2004) Quartäre Talentwicklung des oberen Gessentales im ehemaligen Ronneburger Uranbergbaugebiet, Ostthüringen. Beitr. Geologie Thüringen 11:35–67
Gihring T, Zhang G, Brandt C, Brooks S, Campbell J, Carroll S, Criddle C, Green S, Jardine P, Kostka J, Lowe K, Mehlhorn T, Overholt W, Watson D, Yang Z, Wu W, Schadt C (2011) A limited microbial consortium is responsible for extended bioreduction of uranium in a contaminated aquifer. Appl Environ Microbiol 77:5955–5965
Girvan MS, Campbell CD, Kilham K, Prosser JI, Glover LA (2005) Bacterial diversity promotes community stability and functional resilience after perturbation. Environ Microbiol 7:301–313
Grawunder A, Lonschinski M, Merten D, Büchel G (2009) Distribution and bonding of residual contamination in glacial sediments at the former uranium mining leaching heap of Gessen/Thuringia, Germany. Chem Erde Geochem 69S2:5–19
Griffiths BS, Diaz-Raviña M, Ritz K, McNicol JW, Ebblewhite N, Bååth E (1997) Community DNA hybridisation and %G + C profiles of microbial communities from heavy metal polluted soils. FEMS Microbiol Ecol 24:103–112
Handley K, Wrighton K, Piceno YM, Andersen GL, DeSantis TZ, Williams KH, Wilkins M, N'Guessan A, Peacock A, Bargar J, Long PE, Banfield JF (2012) High-density PhyloChip profiling of stimulated aquifer microbial communities reveals a complex response to acetate amendment. FEMS Microbiol Ecol 81:188–204
Hershberger KL, Barns S, Reysenbach AL, Dawson SC, Pace N (1996) Wide diversity of Crenarchaeota. Nature 384:420
Huber G, Stetter KO (1991) Sulfolobus metallicus, sp. nov., a novel strictly chemolithoautotrophic thermophilic archaeal species of metal-mobilizers. Syst Appl Microbiol 14:372–378
Jakubick A, Gatzweile R, Mager D, Mac A, Robertson G (1997) The Wismut waste rock pile remediation programme of the Ronneburg district, Germany, Abstr. 4th International Conference on Acid Rock Drainage, Vancouver B.C. 1285–1301
Johnson D, Knoepp J, Swank W, Shan J, Morris L, Van_Lear D, Kapeluck R (2001) Effects of forest management on soil carbon: results of some long-term resampling studies. Environ Pollut 116:S201–S208
Jørgensen BB (2006) Bacteria and marine biogeochemistry. In: Schulz HD, Zabel M (eds) Marine geochemistry. Springer, Berlin Heidelberg
Kaplan DI, Yeager C, Denham ME, Zhang S, Xu C, Schwehr KA, Li H, Brinkmeyer R, Santschi PH (2012) Biogeochemical considerations related to the remediation of 129I plumes. U.S. Department of Energy 59
Kelly JJ, Häggblom MM, Tate RT (2003) Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter as indicated by analysis of microbial community phospholipid fatty acid profiles. Biol Fertil Soils 38:65–71
Kimiran-Erdem A, Arslan EO, Yurudu NOS, Zeybek Z, Dogruoz N, Cotuk A (2007) Isolation and identification of enterococci from seawater samples: assessment of their resistance to antibiotics and heavy metals. Environ Monit Assess 125:219–228
Kostka J, Green S (2011) Microorganisms and processes linked to uranium reduction and immobilization. In: Stolz J, Oremland R (eds) Microbial metal and metalloid metabolism. ASM Press, Washington, pp 117–138
Leigh MB, Wu W, Cardenas E, Uhlik O, Carroll S, Gentry T, Marsh TL, Zhou J, Jardine P, Criddle CS, Tiedje JM (2014) Microbial communities biostimulated by ethanol during uranium (VI) bioremediation in contaminated sediment as shown by stable isotope probing. Frontiers of Environmental Science & Engineering: 5
Liang Y, Van Nostrand J, N'guessan L, Peacock A, Deng Y, Long P, Resch C, Wu L, He Z, Li G, Hazen T, Lovley D, Zhou J (2012) Microbial functional gene diversity with a shift of subsurface redox conditions during in situ uranium reduction. Appl Environ Microbiol 78:2966–2972
Liger E, Charlet L, Van Cappellen P (1999) Surface catalysis of uranium(VI) reduction by iron(II). Geochim Cosmochim Acta 63:2939–2955
Lin X, Kennedy D, Peacock A, Mckinley J, Resch C, Fredrickson J, Konopka A (2012) Distribution of microbial biomass and potential for anaerobic respiration in Hanford site 300 area subsurface sediment. Appl Environ Microbiol 78:759–767
Lloyd J (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425
Macdonald CA, Campbell CD, Bacon JR, Singh BK (2008) Multiple profiling of soil microbial communities identifies potential genetic markers of metal-enriched sewage sludge. FEMS Microbiol Ecol 65:555–564
Michalsen MM, Goodman BA, Kelly SD, Kemner KM, McKinley JP, Stucki JW, Istok JD (2006) Uranium and technetium bio-immobilization in intermediate-scale physical models of an in situ bio-barrier. Environ Sci Technol 40:7048–7053
Miller AC, McClain D (2007) A review of depleted uranium biological effects: in vitro and in vivo studies. Rev Environ Health 22:75–89
Mori K, Yamamoto H, Kamagata Y, Hatsu M, Takamizawa K (2000) Methanocalculus pumilus sp. nov., a heavy-metal-tolerant methanogen isolated from a waste-disposal site. Int J Syst Evol Microbiol 50:1723–1729
Morse JW, Arakaki T (1993) Adsorption and coprecipitation of divalent metals with mackinawite (FeS). Geochim Cosmochim Acta 57:3635–3640
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454
Oksanen J, Kindt R, Legendre P, O'Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H (2008) Vegan: community ecology package. R package version 1.15-1. http://cran.r-project.org/, http://vegan.r-forge.r-project.org/
Oren A (2006) The order Halobacteriales. Prokaryotes 3:113–164
Özverdi A, Erdem M (2006) Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J Hazard Mater B137:626–632
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org
Rastogi G, Osman S, Vaishampayan P, Andersen GL, Stetler L, Sani RK (2010) Microbial diversity in uranium mining-impacted soils as revealed by high-density 16S microarray and clone library. Microb Ecol 59:94–108
Roesch LFW, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, Daroub SH, Camargo FAO, Farmerie WG, Triplett EW (2007) Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1:283–290
Sandaa R, Enger O, Torsvik V (1999) Abundance and diversity of archaea in heavy-metal-contaminated soils. Appl Environ Microbiol 65:3293–3297
Sani RK, Peyton BM, Dohnalkova A (2006) Toxic effects of uranium on Desulfovibrio desulfuricans G20. Environ Toxicol Chem 25:1231–1238
Schmidt A, Haferburg G, Schmidt A, Lischke U, Merten D, Ghergel F, Büchel G, Kothe E (2009) Heavy metal resistance to the extreme: Streptomyces strains from a former uranium mining area. Chem Erde Geochem 69(S2):35–44
Sitte J, Akob DM, Kaufmann C, Finster K, Banerjee D, Burkhardt EM, Kostka JE, Scheinost AC, Büchel G, Küsel K (2010) Microbial links between sulfate reduction and metal retention in uranium- and heavy metal-contaminated soil. Appl Environ Microbiol 76:3143–3152
Sitte J, Pollok K, Langenhorst F, Küsel K (2013) Nanocrystalline nickel and cobalt sulfides formed by a heavy metal-tolerant, sulfate-reducing enrichment culture. Geomicrobiol J 30:36–47
Sponagel H, Grottenthaler W, Hartmann K-J, Hartwich R, Janetzko P, Joisten H, Kühn D, Sabel K-J, Traidl R (2005) Bodenkundliche Kartieranleitung. Bundesanstalt für Geowissenschaften und Rohstoffe. Schweizerbartsche Verlagsbuchhandlung, Stuttgart
Tokunaga TK, Wan J, Kim Y, Daly RA, Brodie EL, Hazen TC, Herman D, Firestone MK (2008) Influences of organic carbon supply rate on uranium bioreduction in initially oxidizing, contaminated sediment. Environ Sci Technol 42:8901–8907
Tom-Petersen A, Leser TD, Marsh TL, Nybroe O (2003) Effects of copper amendment on the bacterial community in agricultural soil analyzed by the T-RFLP technique. FEMS Microbiol Ecol 46:53–62
U.S. Department of Energy (1999) Final site observational work plan for the UMTRA project Old Rifle site GJO-99-88-TAR. U.S. Department of Energy, Grand Junction
Vanengelen MR, Szilagyi RK, Gerlach R, Lee BD, Apel WA, Peyton BM (2011) Uranium exerts acute toxicity by binding to pyrroloquinoline quinone cofactor. Environ Sci Technol 45:937–942
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135
Wall JD, Krumholz LR (2006) Uranium reduction. Annu Rev Microbiol 60:149–166
Watson DB, Kostka JE, Fields MW, Jardine PM (2004) The Oak Ridge field research center conceptual model. NABIR Field Research Center Oak Ridge, Tennessee
Weisburg W, Barns S, Pelletier D, Lane D (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Zeien H, Brümmer GW (1989) Chemische Extraktionen zur Bestimmung von Schwermetallbindungsformen in Böden. Mitt Dt Bodenkundl Ges 59:505–510
Zerling L, Hanisch C, Junge FW, Müller A (2003) Heavy metals in Saale sediments—changes in the contamination since 1991. Acta Hydrochim Hydrobiol 31:368–377
Acknowledgments
The authors thank Dirk Merten and Denise M. Akob for the valuable discussion, Ingo Schöning for soil horizon nomenclature, Michael Rzanny for the helpful suggestions with the statistical analyses, Katy Hartwig and Sylvia Meißner for the technical assistance, and Peter Bouwma for proofreading the manuscript. This project was part of the graduate research school “Alteration and element mobility at the microbe-mineral interface” financially supported by the German Research Foundation (DFG 1257) and embedded in the Jena School of Microbial Communication (JSMC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sitte, J., Löffler, S., Burkhardt, EM. et al. Metals other than uranium affected microbial community composition in a historical uranium-mining site. Environ Sci Pollut Res 22, 19326–19341 (2015). https://doi.org/10.1007/s11356-015-4791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4791-1