Abstract
Water hyacinth (WH) is used as the substrate for biogas production due to its high lignocellulosic composition and natural abundance. The present study used thermal and chemical (alkali) pretreatment techniques to enhance biogas production from water hyacinth used as a substrate by anaerobic digestion. Thermal pretreatment was done using an autoclave at 121 °C and 15 lb (2 bar) pressure and alkali pretreatment by NaOH at two concentrations (2% and 5% w/v). The inoculum:substrate ratio for biogas production was 2:1, where cow dung was used as inoculum. Results indicated that the pretreatments increased biomass degradability and improved biogas production. Water hyacinth pretreated with 5% NaOH produced the highest amount of biogas (142.61 L/Kg VS) with a maximum methane content of 64.59%. The present study found that alkali pretreatment can modify the chemical structure and enhance WH hydrolysis, leading to enhanced energy production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Researchers have mainly concentrated on the bioenergy sector as a step towards a low carbon future due to its rising energy demand. It is noteworthy to mention that the biogas sector is receiving greater attention compared to other renewable sources because of the availability of feedstocks, effectiveness in greenhouse gas (GHG) mitigation, and value addition to biomass waste (Show et al. 2022; Chan et al. 2019; Loy et al. 2018; Luo and Zhou 2012). Yan and Guo (2017) reported that water hyacinth (WH), also known as Eichhornia crassipes, is an invasive plant species native to the Amazon Basin of South America (Ilo et al. 2021) and is linked to its distinguishing biological structures apart from intense eutrophication of water bodies.
WH is considered a promising feedstock for anaerobic digestion (AD) due to its high reproductive capability, no risk to food security worldwide, easy hydrolyzable sugars, and lower lignin content (Koley et al. 2023a, b; Basu et al. 2021; Ilo et al. 2021). Numerous researches have been carried out on AD of WH using various types of inoculums such as cow dung (Bhui et al. 2018; Ali et al. 2022), buffalo dung, poultry litter (Castro and Agblevor 2021), and anaerobic sludge (Barua and Kalamdhad 2019) in various inoculum:substrate (I:S) ratios (Show et al. 2023). The availability of nutrients is directly related to the degradation of substrates (Zhou et al. 2017). The addition of cow dung ensures the carbon-nitrogen (C/N) balance, enriches the microbial consortia, and enhances the degradation kinetics. This method needs future and critical investigations. A challenge encountered in using lignocellulosic biomass for the bio-conversion process is the structural resistance of lignocellulosic biomass cell wall components, which needs to be pretreated before the bioconversion process (Banerjee et al. 2022; Show et al. 2022; Sinha et al. 2021).
Pretreatment is essential when utilizing biomass (Sheng et al. 2021) as a feedstock for the bioenergy process. It is vital for accelerated biomass conversion into hydrolyzable sugars (Sankaran et al. 2020). Bioenergy and biofuels derived from lignocellulosic and microalgal biomass are substitute renewable energy sources (Bajpai and Nemade 2023; Roy et al. 2022). Previously, WH was pretreated with a wide variety of methods like acid (Sathyanagalakshmi et al. 2011), alkali (Aswathi et al. 2013), biological (Sinegani et al. 2005), hot water (Saha et al. 2014), microwave-alkali (Zhang et al. 2016), ultrasound combined alkali (Soontornchaiboon et al. 2016), catalytic hydrothermal liquefaction (Singh et al. 2015), calcium peroxide (Cheng et al. 2015), thermo-chemical conversion (Huang et al. 2016), and microwave-assisted alkali-organosolvent (Das et al. 2016) to increase their biodegradability for enhancing bioenergy production capability with increasing their methane yield (Patil et al. 2011; Lin et al. 2015; Zhang et al. 2018). Mathew et al. (2011) suggested that alkaline pretreatment is more energy efficient than acid pretreatment. NaOH pretreatment has shown an effective ability to remove amorphous substances than other techniques (Singh and Singh 2021). The biogas production from WH can be accelerated by the thermal process (Barua and Kalamdhad 2017a, b) using an autoclave (Kurniawan et al. 2014). It is still necessary to fine-tune pretreatment technologies for various biomass types and improve an economically feasible technique (Sankaran et al. 2020). The objective of the present study encompasses the effect of thermal (autoclave) and alkali (5% and 2% NaOH) pretreatment on WH to enhance the biogas production through various analytical techniques, such as pH, COD, reducing sugar, production of volatile fatty acids (VFA) like acetic acid, and methane concentration.
Materials and methods
Feedstock collection and preparation
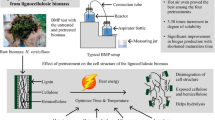
In this study, fresh WH was collected from ponds in Santiniketan, West Bengal, India (23.6800° N, 87.6800° E). The ponds were clean and received no pollutants from the nearby area. The harvested WH plants were carried to the laboratory in polyethylene plastic bags and cleaned to eliminate impurities, like dead plant parts, and the root portions were also removed before milling before the pretreatment. Wet biomass was milled in a mixer blender and kept at 4 °C for 48 h prior to anaerobic digestion. Locally collected and stored cow manure was used for anaerobic digestion. WH and cow dung’s total solids (TS) and volatile solids (VS) contents were separately calculated, and the digester was loaded based on the volatile solids concentration (Table 1) (Mathew et al. 2015). The TS content of pretreated and untreated WH varied between 3.7 and 4.8%. The pH was determined employing the Thermo Scientific Orion ROSS electrode and electrical conductivity by DuraProbe 4-Electrode Conductivity Cells using the Thermo Scientific ORION Star A329 meter. Total nitrogen content, in the form of Kjeldahl nitrogen, was determined using the N-Kjeldahl method developed by Kjeldahl (1883). Organic carbon content was measured through the Walkley and Black method (Walkley and Black 1934).
Pretreatments of water hyacinth
Chemical pretreatment
Sodium hydroxide (NaOH) was used for chemical pretreatment. The NaOH was purchased from Sigma-Aldrich with a purity of ≥ 97%. Two different strengths of NaOH were used: 2% (w/v) and 5% (w/v). The pretreatment was conducted for 48 h. This chemical pretreatment aims to break down and solubilize organic matter in the WH, making it more accessible for bacterial degradation and subsequent biogas generation.
Thermal pretreatment
An autoclave was used for thermal preparation. The thermal treatment was carried out at 121 °C (approximately 250 °F). The pressure during thermal preparation was 15 lb (2 bar) for 15 min. The thermal preparation involves subjecting the WH to high temperature and pressure conditions. This helps in sterilizing the material and further breaking down complex organic compounds.
Anaerobic digestion of aquatic weeds
Cow dung was employed as an inoculum and WH as a substrate in a biogas generation process with a 2:1 inoculum to substrate ratio using the Mathew et al. (2015) technique, employing a 2-L digester bottle (Tarsons, India) (Fig. 1) in a batch culture. The anaerobic environment in the digesters was created by flushing them with nitrogen gas for 5 min and then sealing them. A temperature-regulating water shaking bath at 37 ± 2 °C was used to maintain the anaerobic digesters. Experiments were carried out in triplicate for each treatment.
Analytical methods
The COD was analyzed by the method of Yadav et al. (2006). COD tests were conducted using the reflux method and determined by oxidizing the organic matter in the sample with the K2Cr2O7 solution. Titration with a ferrous ammonium sulfate solution determines the oxygen required for this oxidation, comparable to the COD. The procedure guarantees that all organic matter is oxidized, and the findings are represented as COD, which measures the quantity of organic and inorganic compounds in a sample that chemical agents may oxidize.
The biogas volume was measured by the water displacement method (Sunarso et al. 2010). The volume of gas produced was equal to the volume of water displaced from the measuring cylinders.
The methane and carbon dioxide percentage in the biogas was measured by gas chromatograph (GC) with a thermal conductivity detector (Mathew et al. 2015) using helium as the carrier gas. The column used was HP-molesieve; its dimensions were 30 m in length, a diameter of 0.32 mm, and a film thickness of 12 m. The injector and detector temperatures were maintained at 200 °C and 250 °C, respectively. The temperature range was set to rise from 40 °C for 5 min to 250 °C at a rate of 20 °C for 10 min. The GC was calibrated using a synthetic gas combination of 3 standards like (i) 75% methane, 5% carbon dioxide, 5% hydrogen, and 15% nitrogen; (ii) 50% methane, 10% carbon dioxide, 2% hydrogen, and 38% nitrogen; and (iii) 25% methane, 20% carbon dioxide, 1% hydrogen, and 54%.
The VFA sample was extracted using the method of Manni and Caron (1995). The volatile fatty acid (VFA) was analyzed by GC-FID using an EBX-70 column (length of 60 m, a diameter of 0.25 mm, and a film thickness of 0.25 m) with helium as a carrier gas and hydrogen as a combustion gas. One microliter of the sample was purged using an auto-injection system. The injector and detector were set at a temperature of 250 °C. The column temperature was designed to start at 70 °C with a holding period of 3 min, which was raised to 180 °C at a rate of 10 °C with a holding period of 6 min. The VFA standard (Volatile Free Acid Mix) was procured from Sigma-Aldrich (Supelco Bellefonte, USA).
Results and discussion
Changes in pH in various treatments
The pH value decreased significantly over the first few days of incubation in all treatments. It recovered as a result of the hydrolysis/acidogenesis process using VFA (Wang et al. 2017). The pH range of 6.5–8.2 is known to be optimum for methane generation (Kothari et al. 2014). In the present study, the pH range was found to be optimum in all the treatments. The pH range for pretreatment with 5% NaOH was 8.32 to 6.86; for pretreatment with 2% NaOH, it was 8.13 to 6.92; for thermal pretreatment, it was 7.51 to 6.9 (Fig. 2). Adding NaOH will increase the concentration of hydroxide ion which raises the pH, so that 5% NaOH has the maximum pH value in the present study. In all the treatments, the maximum pH was reduced on day 5 as organic acids were produced. After that, the pH again increased as the concentration of ammonia increased due to the digestion of nitrogen (Angelidaki and Ahring 1993). A similar trend in pH change was observed in a study conducted by Dai et al. (2018), where maximum pH reduction was found on day 6 and increased pH.
Changes in COD in various treatments
COD is a crucial metric in the AD process. Syaichurrozi and Sumardiono (2013) state that biogas production is directly proportional to COD removal. There, the maximum removal of COD was 38%, where vinasse was used as a substrate. The present study found a maximum COD reduction in 5% NaOH alkali pretreatment (68.17%). The initial COD was 208.65 mg/L; at the digestion end, the COD was 66.4 mg/L. The COD reduction in 2% NaOH pretreatment was from 198.35 to 72.56 mg/L (63.41%), and in thermal pretreatment, it was 190.5 to 90.44 mg/L (52.52%) (Fig. 3). In a previous study, WH pretreated with 121 °C and pH 13 (pH adjusted by NaOH) can solubilize 66.64% of COD (Patel et al. 1993). However, the previous study suggests that biological pretreatment can help in more COD reduction (Sinha et al. 2021). Studies suggested that COD removal depends upon various physiological parameters like pH, temperature, hydraulic retention time, and organic loading (Chen et al. 2014; Jiang et al. 2014; Fu et al. 2015; Wu et al. 2016).
Changes in day-wise reducing sugar production in various treatments
In the initial process of biogas production, complex polymers of lignocellulosic material are converted into reducing sugars which play a crucial role in the hydrolysis and acidogenesis step in AD (Xu et al. 2019). Finally, the concentration of these reducing sugars aids in determining the degree of hydrolysis (Vanegas et al. 2015), which leads to VFA formation and the final product as biogas. Across the present study, maximum reducing sugar production was observed at day 2 in 5% NaOH alkali pretreatment, leading to maximum biogas production (294.74 mg/mL). In 2% NaOH pretreatment, the reducing sugar production was (212.42 mg/mL), while in thermal pretreatment, it was (261.58 mg/mL) (Fig. 4).
Changes in day-wise VFA production in various treatments
VFA concentration plays a vital role as a sensitive indicator in the AD process. Accumulation of VFA could inhibit methanogenesis. The present study monitored the total VFA production during the anaerobic digestion of WH pretreated with different concentrations of NaOH and autoclaving. A maximum VFA accumulation was found in 5% NaOH alkali pretreatment (1876.12 ppm), followed by thermal (1641.82 ppm), and 2% NaOH pretreatment (1614.32 ppm) (Fig. 5). Maximum amount of VFA was found at day 9, and the beginning of the methanogenesis phase might indicate the progressive decrease of VFA. According to Barua and Kalamdhad (2017a, b), the highest VFA production levels for untreated and hot air oven-pretreated WH were found on day 14 in concentrations of 1491 mg/L and 1758 mg/L, respectively. The present study reports higher VFA production than WH pretreated with dilute acid-thermal pretreatment and cattle dung biochar (Suthar et al. 2022). This study justified the methanogenic activity as the previous study suggests a VFA concentration of more than 6000–8000 mg/L inhibits methanogenesis (Karthikeyan and Visvanathan 2013). In this study, acetic acid production with alkali and thermal (autoclave) pretreated WH has been monitored; it was observed that with the 5% NaOH pretreatment, the maximum accumulation of acetic acid was 380 ppm and 350 ppm, 326 ppm with the 2% alkali and thermal pretreatment on the 9th day with the gradual decrease till the end of the fermentation period (Fig. 6). Although the AD process took 25 days, no significant changes were seen after day 9. The considerable decline in acetic acid suggests that the methane produced from the VFAs is created during hydrolysis (Sinha et al. 2021). Consequently, the reactors’ VFA production was within the available range; thus, there was no operational imbalance brought on by VFA buildup.
Comparison of methane and biogas production in various treatments
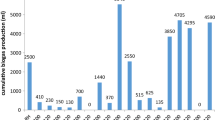
Biogas and methane production from WH with different pretreatment techniques was monitored for 25 days. Barua and Kalamdhad (2017a, b) previously reported that WH pretreated with hot water produced 193 ± 22 mL CH4/g VS on the 14th day with 67.4 ± 0.3% methane content. In this present study, the pretreatment process with the most significant methane concentrations was 5% NaOH alkali (64.59%), followed by thermal pretreatment (54.24%), and 2% NaOH alkali (51.47%) (Fig. 7). On day 12, the methane concentration was highest in 5% alkali treatment. Still, the methane concentration peaked on day 25 for the other treatments, which was not more significant than the control. Pretreated WH also shows maximum biogas production with 5% NaOH (142.61 L/Kg VS), while 2% NaOH alkali treatment results in lower biogas production (137.47 L/Kg VS) than thermally treated WH (139.11 L/Kg VS) (Fig. 8). The model’s variance analysis (ANOVA) result produces an F value of 300.091 and predicted R2 values of 0.995 and 0.998, indicating a well-fitted model. Table 2 provides a summary of the ANOVA results that were used to determine the regression validation for modifying the biogas production. The significance of regression, as determined by the F test, indicates the model’s suitability, reliability, and accuracy. According to the present analysis, total biogas production has increased considerably within 25 days. Overall, 5% NaOH and thermal treatment enhanced biogas production significantly (P ≤ 0.01) compared to 2% NaOH treatment.
The structure of the WH cell wall was altered by alkali pretreatment using 5% NaOH; the organic matter content is made more vulnerable to bacterial assault, which enhances the biogas generation process (Martin and Hadiyanto 2020). An increase in the intensity of NaOH pretreatment can lead to higher lignin reduction (Jung et al. 2020). However, it is important to note that an increase in the intensity of NaOH pretreatment can also cause a greater loss of total biomass (Fahmayanti and Abtokhi 2018). A similar trend was in Barua and Kalamdhad (2017a, b), where hot water pretreated WH produced maximum biogas and methane at day 14. This performance supports past findings by other researchers that pretreatment of WH with NaOH catalyzes the production of biogas, which results in a higher yield (Patil et al. 2011). In this study, the final methane concentration was higher in 5% alkali treatment than the untreated WH reported by Chanakya et al. (1993) and pretreated with dilute acid-thermal pretreatment and cattle dung biochar (0.5 and 1.5% of biochar) reported by Suthar et al. (2022). Yet, methane yield was similar, as reported by Ferrer et al. (2010). However, a study by Bhui et al. (2018) suggested that inoculum to substrate ratio of 3:1 can produce a maximum biogas of up to 383 L/Kg. Still, the methane concentration was lower than in this current study with the 5% NaOH alkali pretreatment technique.
Energy equivalent: an environmentally friendly strategy
Using the fundamental energy equivalents, the potential of WH-derived biogas for power generation with alkali treatment was estimated. In this present study, the total biogas production from 5% NaOH pretreated WH was 142.61 L/Kg VS with 64.59% methane content. To estimate the electrical potential, it was assumed that 1 m3 of biogas has a calorific value of 22 MJ, and 1 m3 of methane has a calorific value of 36 MJ. One cubic meter of biogas will produce 2.14 kWh of power, and 1 m3 of methane will produce 10 kWh of electricity under the premise that the electrical conversion efficiency is 35%. Therefore, according to this current study, it was estimated that 0.305 kWh of electricity from biogas and 0.92 kWh of electricity from the methane content could be generated from 5% NaOH pretreated WH. Families may benefit from using the generated biogas instead of the polluting firewood, kerosene, and dung cake, and the electricity will help in human welfare.
Conclusion
The research emphasized the potential of the aquatic weed Eichhornia crassipes, used to produce biogas. The pretreatment process with alkali and thermal was utilized to increase biogas generation. Results show that the pretreatments boosted gas generation and increased biomass degradability. Thermal pretreatment did not result in any additional enhancement or appreciable changes to WH digestion. The methane concentration is highest in the 5% NaOH alkali pretreatment (64.59%), which can produce 0.92 kWh of electricity, followed by thermal pretreatment (54.22%) and 2% NaOH alkali pre-treatment (51.47%). Pretreated WH with 5% NaOH shows maximum biogas production (142.61 L/Kg VS) than WH pretreated with thermal treatment (137.56 L/Kg VS).
References
Ali SS, Elsamahy T, Abdelfattah A, Mustafa AM, Khalil MA, Mastropetros SG, Kornaros M, Sun J, Azab M (2022) Exploring the potential of anaerobic co-digestion of water hyacinth and cattle dung for enhanced biomethanation and techno-economic feasibility. Fuel 329:125397. https://doi.org/10.1016/j.fuel.2022.125397
Angelidaki I, Ahring BK (1993) Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl Microbial Biotechnol 38(4):560–564. https://doi.org/10.1007/BF00242955
Awasthi M, Kaur J, Rana S (2013) Bioethanol production through water hyacinth, Eichhornia crassipes via optimization of the pretreatment conditions. Int J Emerg Technol Adv Eng 3(3):42–46
Bajpai S, Nemade PR (2023) An integrated biorefinery approach for the valorization of water hyacinth towards circular bioeconomy: a review. Environ Sci Pollut Res 30(14):39494–39536. https://doi.org/10.1007/s11356-023-25830-y
Banerjee A, Show BK, Chaudhury S, Balachandran S (2022) Biological pretreatment for enhancement of biogas production. Cost effective technologies for solid waste and wastewater treatment 101-14. https://doi.org/10.1016/B978-0-12-822933-0.00020-6
Barua VB, Kalamdhad AS (2017a) Biochemical methane potential test of untreated and hot air oven pretreated water hyacinth: a comparative study. J Clean Prod 166:273–284. https://doi.org/10.1016/j.jclepro.2017.07.231
Barua VB, Kalamdhad AS (2017b) Effect of various types of thermal pretreatment techniques on the hydrolysis, compositional analysis and characterization of water hyacinth. Bioresour Technol 227:147–154. https://doi.org/10.1016/j.biortech.2016.12.036
Barua VB, Kalamdhad AS (2019) Biogas production from water hyacinth in a novel anaerobic digester: a continuous study. Process Saf Environ Prot 127:82–89. https://doi.org/10.1016/j.psep.2019.05.007
Basu A, Hazra AK, Chaudhury S, Ross AB, Balachandran S (2021) State of the art research on sustainable use of water hyacinth: a bibliometric and text mining analysis. In Informatics 8(2):38. https://doi.org/10.3390/informatics8020038
Bhui I, Mathew AK, Chaudhury S, Balachandran S (2018) Influence of volatile fatty acids in different inoculum to substrate ratio and enhancement of biogas production using water hyacinth and salvinia. Bioresour Technol 270:409–415. https://doi.org/10.1016/j.biortech.2018.09.055
Castro YA, Agblevor FA (2021) Effect of poultry litter biochar on the biomethanation of alkaline wet air-oxidized water hyacinth at high feed to inoculum (F/I) ratio. Biomass Convers Biorefin 29:1–0. https://doi.org/10.1007/s13399-021-01724-2
Chan YH, Cheah KW, How BS, Loy AC, Shahbaz M, Singh HK, Shuhaili AF, Yusup S, Ghani WA, Rambli J, Kansha Y (2019) An overview of biomass thermochemical conversion technologies in Malaysia. Sci Total Environ 680:105–123. https://doi.org/10.1016/j.scitotenv.2019.04.211
Chanakya HN, Borgaonkar S, Meena G, Jagadish KS (1993) Solid-phase biogas production with garbage or water hyacinth. Bioresour Technol 46(3):227–231. https://doi.org/10.1016/0960-8524(93)90125-U
Chen Z, Wang Y, Li K, Zhou H (2014) Effects of increasing organic loading rate on performance and microbial community shift of an up-flow anaerobic sludge blanket reactor treating diluted pharmaceutical wastewater. J Biosci Bioeng 118(3):284–288. https://doi.org/10.1016/j.jbiosc.2014.02.027
Cheng YS, Chen KY, Chou TH (2015) Concurrent calcium peroxide pretreatment and wet storage of water hyacinth for fermentable sugar production. Bioresour Technol 176:267–272. https://doi.org/10.1016/j.biortech.2014.11.016
Dai BL, Guo XJ, Yuan DH, Xu JM (2018) Comparison of different pretreatments of rice straw substrate to improve biogas production. Waste Biomass Valorization 9(9):1503–1512. https://doi.org/10.1007/s12649-017-9950-9
Das SP, Gupta A, Das D, Goyal A (2016) Enhanced bioethanol production from water hyacinth (Eichhornia crassipes) by statistical optimization of fermentation process parameters using Taguchi orthogonal array design. Int Biodeterior Biodegradation 109:174–184. https://doi.org/10.1016/j.ibiod.2016.01.008
Fahmayanti Y, Abtokhi A (2018) The addition effect of (NaOH and KOH) to the biogas purification. Jurnal Neutrino: Jurnal Fisika dan Aplikasinya 10(2):40–44. https://doi.org/10.18860/neu.v10i2.4410
Ferrer I, Palatsi J, Campos E, Flotats X (2010) Mesophilic and thermophilic anaerobic biodegradability of water hyacinth pre-treated at 80° C. Waste Manag 30(10):1763–1767. https://doi.org/10.1016/j.wasman.2009.09.020
Fu SF, Wang F, Yuan XZ, Yang ZM, Luo SJ, Wang CS, Guo RB (2015) The thermophilic (55 0C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour Technol 175:203–208. https://doi.org/10.1016/j.biortech.2014.10.072
Huang L, Liu J, He Y, Sun S, Chen J, Sun J, Chang K, Kuo J (2016) Thermodynamics and kinetics parameters of co-combustion between sewage sludge and water hyacinth in CO2/O2 atmosphere as biomass to solid biofuel. Bioresour Technol 218:631–642. https://doi.org/10.1016/j.biortech.2016.06.133
Ilo OP, Simatele MD, Nkomo SP, Mkhize NM, Prabhu NG (2021) Methodological approaches to optimizing anaerobic digestion of water hyacinth for energy efficiency in South Africa. Sustainability 13(12):6746. https://doi.org/10.3390/su13126746
Jiang J, Wu J, Zhang J, Poncin S, Li HZ (2014) Multiscale hydrodynamic investigation to intensify the biogas production in upflow anaerobic reactors. Bioresour Technol 155:1–7. https://doi.org/10.1016/j.biortech.2013.12.079Getrightsandcontent
Jung W, Savithri D, Sharma-Shivappa R, Kolar P (2020) Effect of sodium hydroxide pretreatment on lignin monomeric components of Miscanthus× giganteus and enzymatic hydrolysis. Waste Biomass Valori 11:5891–5900. https://doi.org/10.1007/s12649-019-00859-8
Karthikeyan OP, Visvanathan C (2013) Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: a review. Rev Environ Sci 12(3):257–284. https://doi.org/10.1007/s11157-012-9304-9
Kjeldahl C (1883) A new method for the determination of nitrogen in organic matter. Z Anal Chem 22:366
Koley A, Bray D, Banerjee S, Sarhar S, Thakur RG, Hazra AK, Mandal NC, Chaudhury S, Ross AB, Camargo-Valero MA, Balachandran S (2023a) Water hyacinth (Eichhornia crassipes) a sustainable strategy for heavy metals removal from contaminated waterbodies. In Bioremediation of Toxic Metal (loid) s, CRC Press, pp 95-114
Koley A, Mukhopadhyay P, Gupta N, Singh A, Ghosh A, Show BK, GhoshThakur R, Chaudhury S, Hazra AK, Balachandran S (2023b) Biogas production potential of aquatic weeds as the next-generation feedstock for bioenergy production: a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-30191-7
Kothari R, Pandey AK, Kumar S, Tyagi VV, Tyagi SK (2014) Different aspects of dry anaerobic digestion for bio-energy: an overview. Renew Sustain Energy Rev 39:174–195. https://doi.org/10.1016/j.rser.2014.07.011
Kurniawan T, Putra Y, Murni D (2014) Study of biogas production rate from water hyacinth by hydrothermal pretreatment with buffalo dung as a starter. Waste Technol 2(2):26–30. https://doi.org/10.14710/2.2.26-30
Lin R, Cheng J, Song W, Ding L, Xie B, Zhou J, Cen K (2015) Characterisation of water hyacinth with microwave-heated alkali pretreatment for enhanced enzymatic digestibility and hydrogen/methane fermentation. Bioresour Technol 182:1–7. https://doi.org/10.1016/j.biortech.2015.01.105
Loy AC, Yusup S, Lam MK, Chin BL, Shahbaz M, Yamamoto A, Acda MN (2018) The effect of industrial waste coal bottom ash as catalyst in catalytic pyrolysis of rice husk for syngas production. Energy Convers Manag 165:541–554. https://doi.org/10.1016/j.enconman.2018.03.063
Luo Z, Zhou J (2012) Thermal conversion of biomass. In: Chen W-Y, Seiner J, Suzuki T, Lackner M (eds) Handbook of Climate Change Mitigation. Springer US, New York, pp 1001–1042. https://doi.org/10.1007/978-1-4419-7991-9_27
Manni G, Caron F (1995) Calibration and determination of volatile fatty acids in waste leachates by gas chromatography. J Chromatogr A 690(2):237–242. https://doi.org/10.1016/0021-9673(94)01081-O
Martin HHA, dan Hadiyanto S (2020) Effect of Naoh on biogas production under SSAD conditions along with kinetics studies. Jurnal Ilmu Lingkungan 18(2):319–322. https://doi.org/10.14710/jil.18.2.319-322
Mathew AK, Chaney K, Crook M, Humphries AC (2011) Dilute acid pretreatment of oilseed rape straw for bioethanol production. Renew Energy 36(9):2424–2432. https://doi.org/10.1016/j.renene.2011.01.030
Mathew AK, Bhui I, Banerjee SN, Goswami R, Chakraborty AK, Shome A, Balachandran S, Chaudhury S (2015) Biogas production from locally available aquatic weeds of Santiniketan through anaerobic digestion. Clean Technol Environ Policy 17(6):1681–1688. https://doi.org/10.1007/s10098-014-0877-6
Patel V, Desai M, Madamwar D (1993) Thermochemical pretreatment of water hyacinth for improved biomethanation. Appl Biochem Biotechnol 42(1):67–74. https://doi.org/10.1007/BF02788902
Patil JH, AntonyRaj M, Gavimath CC (2011) Study on effect of pretreatment methods on biomethanation of water hyacinth. Int J Adv Biotechnol Res 2(1):143–147
Roy A, Gogoi N, Yasmin F, Farooq M (2022) The use of algae for environmental sustainability: trends and future prospects. Environ Sci Pollut Res 29(27):40373–40383. https://doi.org/10.1007/s11356-022-19636-7
Saha P, Alam MF, Baishnab AC, Khan MR, Islam MA (2014) Fermentable sugar production and separation from water hyacinth using enzymatic hydrolysis. Sustain Energy 2(1):20–24. https://doi.org/10.12691/rse-2-1-4
Sankaran R, Cruz RA, Pakalapati H, Show PL, Ling TC, Chen WH, Tao Y (2020) Recent advances in the pretreatment of microalgal and lignocellulosic biomass: a comprehensive review. Bioresour Technol 298:122476. https://doi.org/10.1016/j.biortech.2019.122476
Satyanagalakshmi K, Sindhu R, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Bioethanol production from acid pretreated water hyacinth by separate hydrolysis and fermentation. J Sci Ind Res 70:156–161 http://nopr.niscpr.res.in/handle/123456789/10978
Sheng Y, Lam SS, Wu Y, Ge S, Wu J, Cai L, Huang Z, Van Le Q, Sonne C, Xia C (2021) Enzymatic conversion of pretreated lignocellulosic biomass: a review on influence of structural changes of lignin. Bioresour Technol 324:124631. https://doi.org/10.1016/j.biortech.2020.124631
Show BK, Banerjee S, Banerjee A, GhoshThakur R, Hazra AK, Mandal NC, Ross AB, Balachandran S, Chaudhury S (2022) Insect gut bacteria: a promising tool for enhanced biogas production. Rev Environ Sci 1:25. https://doi.org/10.1007/s11157-021-09607-8
Show BK, Panja S, GhoshThakur R, Basu A, Koley A, Ghosh A, Pramanik K, Chaudhury S, Hazra AK, Dey N, Ross AB (2023) Optimisation of anaerobic digestate and chemical fertiliser application to enhance rice yield—a machine-learning approach. Sustainability 15(18):13706. https://doi.org/10.3390/su151813706
Sinegani AS, Emtiazi G, Hajrasuliha S, Shariatmadari H (2005) Biodegradation of some agricultural residues by fungi in agitated submerged cultures. Afr J Biotechnol 4(10) https://www.ajol.info/index.php/ajb/article/view/71367
Singh NK, Singh R (2021) Evaluation of pretreatment potential and hydrogen recovery from lignocellulosic biomass in an anoxic double-staged bioelectrochemical system. International J Hydrog Energ 46(79):39122–39135. https://doi.org/10.1016/j.ijhydene.2021.09.155
Singh R, Balagurumurthy B, Prakash A, Bhaskar T (2015) Catalytic hydrothermal liquefaction of water hyacinth. Bioresour Technol 178:157–165. https://doi.org/10.1016/j.biortech.2014.08.119
Sinha D, Banerjee S, Mandal S, Basu A, Banerjee A, Balachandran S, Mandal NC, Chaudhury S (2021) Enhanced biogas production from Lantana camara via bioaugmentation of cellulolytic bacteria. Bioresour Technol 340:125652. https://doi.org/10.1016/j.biortech.2021.125652
Soontornchaiboon WA, Kim SM, Pawongrat R (2016) Effects of alkaline combined with ultrasonic pretreatment and enzymatic hydrolysis of agricultural wastes for high reducing sugar production. Sains Malays 45(6):955–962 http://www.ukm.edu.my/jsm/pdf_files/SM-PDF-45-6-2016/13%20Waesarat.pdf
Sunarso S, Budiyono B, Sumardiono S (2010) Biogas production using anaerobic biodigester from cassava starch effluent. Int J Sci Eng 1(2):33–37 https://ejournal.undip.ac.id/index.php/ijse/article/view/1230/1024
Suthar S, Sharma B, Kumar K, Banu JR, Tyagi VK (2022) Enhanced biogas production in dilute acid-thermal pretreatment and cattle dung biochar mediated biomethanation of water hyacinth. Fuel 307:121897. https://doi.org/10.1016/j.fuel.2021.121897
Syaichurrozi I, Sumardiono S (2013) Predicting kinetic model of biogas production and biodegradability organic materials: biogas production from vinasse at variation of COD/N ratio. Bioresour Technol 149:390–397. https://doi.org/10.1016/j.biortech.2013.09.088
Vanegas CH, Hernon A, Bartlett J (2015) Enzymatic and organic acid pretreatment of seaweed: effect on reducing sugars production and on biogas inhibition. Int J Ambient Energy 36(1):2–7. https://doi.org/10.1080/01430750.2013.820143
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil science 37(1):29–38
Wang D, Liu Y, Ngo HH, Zhang C, Yang Q, Peng L, He D, Zeng G, Li X, Ni BJ (2017) Approach of describing dynamic production of volatile fatty acids from sludge alkaline fermentation. Bioresour Technol 238:343–351. https://doi.org/10.1016/j.biortech.2017.04.054
Wu J, Afridi ZU, Cao ZP, Zhang ZL, Poncin S, Li HZ, Zuo JE, Wang KJ (2016) Size effect of anaerobic granular sludge on biogas production: a micro scale study. Bioresour Technol 202:165–171. https://doi.org/10.1016/j.biortech.2015.12.006
Xu N, Liu S, Xin F, Zhou J, Jia H, Xu J, Jiang M, Dong W (2019) Biomethane production from lignocellulose: biomass recalcitrance and its impacts on anaerobic digestion. Front Bioeng Biotechnol 7:191. https://doi.org/10.3389/fbioe.2019.00191
Yadav AK, Sreekrishnan TR, Satya S, Kohli S (2006) A modified method for estimation of chemical oxygen demand for samples having high suspended solids. Bioresour Technol 97(5):721–726. https://doi.org/10.1016/j.biortech.2005.04.013
Yan S, Guo JY (eds) (2017) Water hyacinth: environmental challenges, management and utilization. CRC Press
Zhang Q, Weng C, Huang H, Achal V, Wang D (2016) Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front Microbiol 6:1411. https://doi.org/10.3389/fmicb.2015.01411
Zhang Q, Wei Y, Han H, Weng C (2018) Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour Technol 251:358–363. https://doi.org/10.1016/j.biortech.2017.12.085
Zhou Y, Li C, Nges IA, Liu J (2017) The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour Technol 224:78–86. https://doi.org/10.1016/j.biortech.2016.11.104
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Binoy Kumar Show, Gaayathri Shivakumaran, Apurba Koley, and Anudeb Ghosh. The first draft of the manuscript was written by Binoy Kumar Show and Gaayathri Shivakumaran, and conceptualisation, methodology, validation, project administration, supervision, and writing—review and editing were performed by Shibani Chaudhury, Amit Kumar Hazra, and Srinivasan Balachandran. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
The authors thank BBSRC, United Kingdom [Grant Ref: BB/S011439/1] for financial support and research fellowship.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Show, B.K., Shivakumaran, G., Koley, A. et al. Effect of thermal and NaOH pretreatment on water hyacinth to enhance the biogas production. Environ Sci Pollut Res 30, 120984–120993 (2023). https://doi.org/10.1007/s11356-023-30810-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30810-3