Abstract
Solid-state chemical pretreatment was carried out on rice husk (RH) for biogas production with hydrochloric acid (HCl), sodium hydroxide (NaOH) and ethanol (C2H5OH), at three concentrations i.e., 1, 3 and 5% at 100 and 120 °C for 60 min. Ethanol (organosolv) pretreated sample with 3% concentration and pretreatment temperature of 100 °C (3E100) had the highest biogas production of 5545 mL followed by NaOH pretreated substrate (3N100) with 4705 mL while acid pretreated sample (5H100) produced 700 mL which was lower than the control (raw RH) which was 2500 mL. The biogas yields values were 67.32, 60.89, 32.26 and 9.32 mL/gVS for 3E100, 3N100, RH and 5H100 respectively. Alkaline and organosolv pretreatments improved biogas quality with methane contents of 50.27 and 50.68%. A confirmation that solid state chemical pretreatment of RH with NaOH and C2H5OH could significantly enhance biogas production. The viability of the digestates as biofertilizer was evaluated with the conclusion that they could be used as low grade fertilizers based on their mineral contents and heavy metal concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The research is undertaken to evaluate the impact of solid state chemical pretreatments on biogas yields from rice husk which is an abundant agricultural waste in Nigeria. Solid state chemical pretreatment which addresses the problem of wastewater generation often associated with wet pretreatment method has not gained prominence among researchers. The research seeks therefore to promote the use of the method in order to make chemical pretreatment methods more cost effective by reducing the amount of chemical reagent that will be needed to reduce the recalcitrant nature of lignocellulosic biomass for anaerobic digestion. Changes in structural and chemical composition of the substrates confirmed by FTIR, SEM and XRD testify to the impact of solid state chemical pretreatment in making the substrates more amenable to bacterial attack. This is attested to by the 121.80% and 89% increase in biogas production of both organosolv and alkaline pretreatments at 3% concentration over the untreated sample. Potentials of the digestates as biofertilizer was also highlighted. The information obtained from the research work is expected to be useful in providing troubleshooting tips for biogas operators.
Introduction
The quest for cleaner and sustainable renewable energy resources is one of the propelling forces that has encouraged numerous research efforts. Renewable energy production from biomass has been adjudged one of the ways to tackle challenges associated with the use of fossil fuels. These challenges range from their depleting nature, threat to the environment which is currently noticed in climate change and global warming [1, 2]. Though a major oil producer, Nigeria still struggles to meet the energy demands of her teeming population [3]. Nigeria is yet to take her position on the continent in investing massively in renewable energies for sustainable development [4]. This is due partly to overdependence on fossil fuels and lack of political will to venture into the ‘unknown’. Apart from being an oil producing nation, Nigeria is also blessed with vast arable lands which have encouraged agricultural practices in some parts of the country leading to generation of several hundred million tonnes of agricultural wastes annually [5].
Rice husk (RH) is a biowaste that is generated in large quantity annually through the activities of rice harvesting and processing. Rice as an important staple food for the average Nigerian is cultivated in large quantity to cater for the need of the consumers. It has been estimated that Nigeria produces 2.0 million tonnes of rice yearly [6]. The RH wastes generated are often disposed indiscriminately; at times by open burning and in some cases they are dumped in landfills where they could be a source of methane emission [7]. All these invariably lead to environmental pollution and degradation posing a formidable threat to human health. Production of renewable energy from agricultural wastes through AD provides an environmentally friendly alternative that can mitigate disposal problems often associated with wastes generated from agricultural activities. RH which is a lignocellulosic material could be used to produce biogas, however, there is a major drawback in the use of lignocellulosic materials for anaerobic digestion (AD) which is their complex nature that makes them resistant to enzymatic or bacterial attack [8]. Pretreatment of biomass prior to AD is therefore imperative to improve biodegradability, biogas yield and overall efficiency of the process. There are four types of pretreatments namely physical pretreatment, physico-chemical pretreatment, biological pretreatment and chemical pretreatment. Chemical pretreatment in comparison to other pretreatment methods has the advantage of easiness, fastness, and effectiveness [9]. The physical structures and chemical compositions of lignocellulosic materials could be altered through various chemical pretreatments, making the compositions in lignocellulosic materials more accessible and more readily biodegradable to anaerobic microorganisms, thus increasing digestion efficiency and biogas production [10]. Chemical pretreatment methods used in this research were acid (HCl), alkaline (NaOH) and organosolv (C2H5OH) pretreatments. Large amount of chemicals are usually consumed in wet chemical pretreatment method which makes the process quite expensive due to the need for recycling and treatment of wastewater generated before discharging into the environment. Besides, the likelihood of explosion or fire hazard and the need for sophisticated reactor associated with wet chemical pretreatment when a volatile solvent like ethanol is used as the pretreatment agent at a high temperature [11] can be avoided through solid-state pretreatment method. Despite these notable benefits, little has been done on solid-state chemical pretreatments compared to wet chemical pretreatment. However, some pioneering studies have been reported with promising results. He et al. [12] in their work on rice straw for biogas production used alkaline solid state chemical pretreatment at four concentrations of 4, 6, 8 and 10% and four loading rates of 35, 50, 65 and 80 g/L. They reported that 6% NaOH-pretreated substrate had a biogas yield of 64.5% higher than the untreated sample. In another work done by Pang et al. [13] on corn stover using also solid state chemical pretreatment method, 6% NaOH-pretreated sample had an increase of 48.50% higher than the raw sample. The effects of various chemical pretreatment methods on biogas yield, production rate, morphologies, chemical composition, and biodegradability were the focus of this research.
Materials and Methods
Sample Collection and Preparation
The rice husk (RH) used in the study was obtained from a rice mill in Auchi, Etsako West Local Government Area of Edo State, Nigeria. The RH was pulverized and screened through a 250 microns sieve and stored in an airtight plastic bottles at room temperature prior to physico-chemical analysis and AD experiments.
Solid-State Chemical Pretreatment
Solid-state chemical pretreatment method was employed using three chemicals namely; hydrochloric acid, sodium hydroxide and ethanol. These represent acid, alkaline and organosolv chemical pretreatments. In this research, 500 g of RH was mixed thoroughly with 500 mL of each of the chemical reagents which was equivalent to solid-to-liquid ratio of 1:1 leaving no wastewater but enough water in the substrates to keep them in the saturated state [12]. Pretreatments were performed by using 1, 3 and 5% of the chemical reagents at 100 and 120 °C for 60 min. Eighteen samples were generated as depicted in Table 1. Organosolv pretreatment was catalyzed by adding 1% (w/w) H2SO4 [14]. Thereafter the samples were sun-dried for three days and then used directly for AD without washing [15].
Experimental Design
The raw and pretreated samples were digested in batch anaerobic digesters. One litre Buchner flasks with a working volume of 0.75 L were used as digesters. 100 g each of the pretreated samples were placed in the digesters and mixed with 800 mL of distilled water to achieve a solid-to-liquid ratio (SLR) of 1:8 while raw RH digested at the same dilution ratio was used as the control. The choice of the SLR was based on our previous study [16].The pretreated samples were labeled based on the concentration of the chemical used and the pretreatment temperature as depicted in Table 1. The experiment was done for a retention time of 21 days at mesophilic temperature of 37 ± 2 °C. Cumulative biogas produced was measured by downward displacement of brine solution every 24 h while the production rate was calculated [17]. Methane contents were determined by the method described by De la Rubia et al. [18]. Flammability test (Fig. 7) was conducted according to the method described by Mokobia et al. [19].
Methanogenic Inhibition
Methanogenic inhibition of the pretreated samples was calculated based on Eq. 1
where CH4; end pretreatment and CH4; end untreated represent percentage of methane in the pretreated and the untreated substrates. Negative values indicate methanogenic enhancement [20].
Analytical Methods
The raw and pretreated samples were analyzed for moisture content (MC), ash content, carbon, nitrogen, total solid, volatile solid, lignin, cellulose and hemicellulose according to standard methods [21, 22] and the results are summarized in Table 2. The pH values of the slurries before and after were determined using a hand-held digital pH meter (Hanna Instruments, Italy). A Fourier-transfer infrared spectrophotometer (Shimadzu 8400s) was used to determine changes in the functional groups of the substrate after pre-treatment. Scanning electron microscopy (SEM, Hitachi S4800) was used to assess the morphology of raw and pretreated samples while X-ray diffraction (XPert PRO model, Netherlands) was used to determine the crystallinity index of the pretreated samples.
Results and Discussion
Physico-Chemical Properties
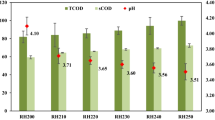
Based on our previous works, 3N100 and 3E100 were the best substrates from acid and organosolv solid state pretreatment categories [23, 24] while 5H100 was the best of the acid-pretreatment category based on cumulative biogas production (Fig. 1). These most promising substrates from each of the categories were compared with the raw RH. Physicochemical properties of the substrates are shown in Table 2. The various components of the samples were differently affected by the different pretreatment methods. The initial pH values ranged from 2.10 to 8.90 while the final pH values ranged from 2.60 to 4.80. Generally, there was a decrease in pH values at the end of the AD process except for 5H100 with a slight increase. The decrease may be due partly to volatile fatty acid (VFA) accumulation [25] and it showed that the buffering capacities of the digesters were poor.
There was a decrease in moisture content after pretreatment except for 5H100. Ash content increased for samples 5H100 and 3N100 but a decrease was observed in 3E100. The increase may be attributed to the Na+ and Cl− ions in the chemicals used in the other two pretreatments [12]. Crude protein ranged between 6.69 ± 0.00 and 18.19 ± 0.09% (RH) with RH having the highest value while 3N100 had the least. The C/N ratios of the raw sample was within the recommended range while 3E100 and 3N100 were slightly higher than the range. C/N ratio for 5H100 was significantly (p < 0.05) higher than the control and other pretreated samples. This could be one of the reasons for the low biogas yields recorded for the acid pretreated sample. The recommended C/N ratio for AD ranges from 10 to 30:1 [26].
There was marked increase in both TS and VS for both 3N100 and 3E100 compared to raw sample (Table 2). NaOH and C2H5OH pretreatments therefore could increase the amount of biodegradable materials which would be readily available to anaerobic microorganisms for biogas production. This was confirmed by the significant (p < 0.05) increase in biogas production in 3N100 and 3E100 compared to RH. The VS content of 3E100 was similar to 83.60% reported for ethanol pretreated rice straw by Mirmohamadsadeghi et al. [14]. 5H100 had lower values than the raw sample for both TS and VS which was evident in the lower biogas production than RH (Fig. 1). The lower yield could also be as a result of formation of inhibitors during pretreatment process. Inhibitors such as furfural and hydromethylfurfural have been reported to inhibit hydrolysis which is the rate-limiting step in anaerobic digestion [27, 28]. This observation was confirmed by the prolonged lag phase of 5H100 digesters compared to others.
Lignin
There was an increase in lignin content after pretreatment except for 5H100. This could be as a result of formation of pseudo-lignin during pretreatment [29]. It has also been reported that there could be redeposition/relocalization of lignin upon cooling after pretreatment [30]. It has also been noticed that lignin could be moved away from much of the cellulose microfibril surfaces without being removed entirely from the biomass. This re-localization enhances accessibility and could be the reason for improved digestibility as observed in 3E100 and 3N100 despite the increase in lignin content. Donohoe et al. [29] have observed that the disruption and relocalization of lignin could be as important as actual removal of lignin to improve biomass degradation. Thus modification of lignin due to pretreatment could have made the substrates more amenable to AD. Taherzadeh and Karimi, [31] have pointed out that the composition (i.e., the type of lignin) and distribution of lignin in a biomass could be as important as the concentration of lignin. Invariably, alteration of lignin composition by chemical pretreatment could improve biomass digestibility though there might be increase in lignin content [32]. Increase in lignin content after pretreatments has been documented in the literature [33, 34]. The lignin content of raw RH was similar to 36.10% reported for rice straw by Bharathiraja et al. [35] while that of the pretreated samples were lower than 58.30% for palm kernel shell by Marrugo et al. [36].
Cellulose and Hemicellulose
The amount of cellulose and hemicellulose decreased after chemical pretreatment except for 5H100 in which hemicellulose increased after pretreatment. This showed that pretreatment caused degradation of these polysaccharides, however, the rate of degradation differed. Degradation of hemicellulose was higher in 3N100 than 3E100 while degradation of cellulose was highest in 5H100. Contrary to the trend observed for hemicellulose degradation in other samples, hemicellulose increased in 5H100. This has been attributed to stripping of lignin and some soluble substances leading to the increase of the relative ratio of hemicellulose in the acid-pretreated substrate [37].
Effect of Chemical Pretreatment Methods on Morphological Properties
Scanning Electron Microscopy (SEM)
Figure 4 shows the SEM images of raw and pretreated samples. Structural deformation after chemical pretreatments was established. The smooth and compact surface of the raw sample was changed in the different pretreated samples to various degrees. Pretreated samples surfaces became uneven, loosened and roughed. Crack development and sponge-like structure of the pretreated samples indicated increase in porosity (surface area) which would make them prone to enzymatic attack. Overall, the structures of the pretreated samples were disrupted significantly compared to the raw sample. This shows that solid state chemical pretreatment at these concentrations could effect structural changes in the biomass.
Fourier Transform Infrared (FTIR)
FTIR spectra of raw and pretreated samples are presented in Fig. 4. The band at 3431 cm−1 was assigned to the OH stretching and the intensity of absorption was decreased for all the samples, an indication that intramolecular hydrogen bonds were disrupted [38]. The bands at position 2926 and 2855 cm−1 attributed to C–H stretching decreased in intensity after pretreatments showing that methylene portions of cellulose were ruptured [39]. The absorption band at 1710 cm−1 attributed to hemicellulose, was found only in raw RH and 5H100 but disappeared after alkaline and organosolv pretreatments indicating that these pretreatment methods were efficient in degrading hemicellulose. This band increased in intensity after acid pretreatment confirming the result of hemicellulose content reported in Table 2 for 5H100. The peak at 1159 cm−1 assigned to hemicellulose increased in sample 5H100 but decreased in other samples. This is corroborated by the physico-chemical analysis data that revealed that hemicellulose content increased in 5H100. The peaks at 1465 and 1649 cm−1 assigned to skeletal aromatics generally found in lignin increased in all the pretreated samples except for 5H100 which was supported by the increase in lignin content after alkaline and organosolv pretreatments (Table 2). Conclusively, the FTIR spectra confirmed changes in chemical composition of the samples after solid-state chemical pretreatments.
X-Ray Diffraction Pattern (XRD)
The X-ray diffraction patterns of raw and pretreated samples are depicted in (Fig. 5). The effectiveness of the pretreatments on cellulose crystallinity was confirmed by calculating the crystallinity index (CrI) and the results revealed that CrI decreased after pretreatments. This shows that the pretreatment methods were able to reduce the crystallinity of the sample making more amorphous cellulose available for enzymatic attack which could improve AD process. It has been reported that the amorphous cellulose were more susceptible to microbial attack than the crystalline cellulose [39]. Similar results were previously reported for pine, poplar and berry woods pretreated with concentrated phosphoric acid [40]. Farahani et al. [41] also reported a decrease in CrI after pretreatment but contrary to that obtained by Li et al. [25] for solid state alkaline pretreated corn stover in which there was no significant difference in CrI of the raw and pretreated sample. The results were 41.80, 27.45, 19.59 and 39.68% for RH, 5H100, 3N100 and 3E100 respectively. There was no direct relationship though between biogas production and CrI. This observation is in agreement with the findings of Kim et al. [42] in their work on aqueous ammonia pretreatment of corn stover.
The sample with the lowest CrI did not produce the highest biogas which is an indication that other factors might have contributed to the overall effect of pretreatment on AD process. It has been observed that the impact of increased surface area accompanying pretreatment could be more important than changes in CrI [43]. However, in comparison with the raw sample decrease in CrI improved biogas yields except for 5H100 which lower pH and/or formation of inhibitors could have overshadowed the impact of decrease in CrI as earlier mentioned.
Comparison of Anaerobic Digestion of Raw and Selected Pretreated Rice Husk
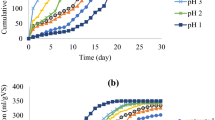
Figures 1 and 2 show cumulative and daily biogas production of the samples. Cumulative biogas production of the samples are: RH = 2500 ± 40, 3E100 = 5545 ± 125, 3N100 = 4705 ± 135 and 5H100 = 700 ± 30 mL respectively. It can been seen that 3E100 and 3N100 produced significantly (p < 0.05) higher cumulative biogas than RH. An opposite trend was observed for 5H100 which produced 700 mL which was significantly lower than the raw sample. All the digesters had zero lag phase except 5H100 with a lag phase of 6 days. Lower lag phase indicates a quick start-up which is beneficial for AD process. It is worthy of note that no inoculum addition was made in order to estimate indigenous methanogenic activities or behaviours of the substrates. This is in line with the work of Vrieze et al. [44] in which indigenous methanogenic activities of some agro-industrial substrates were determined by non-inclusion of inoculum. Inocula are mainly used to startup anaerobic digestion in order prevent process failure [45]. Several works on biogas production from different organic wastes with or without inoculum additives have been reported with various results obtained for startup time. In the study conducted by Alkanok et al. [46] on anaerobic digestion of supermarket wastes inoculated with industrial anaerobic water treatment plant, the lag phase was zero. In the same vein, Pandey et al. [47] reported zero lag phase for dairy waste inoculated with effluent from digester treating potato starch waste. On the other hand, lag phases of 4 and 9 days were reported by Li et al. [25] and Cheng and Zhong [48] for cotton stalk and kitchen waste with inoculum additives. For anaerobic digestion without inoculum, Aiwonegbe et al. [49] reported a lag phase of 1 day for plantain leaves while Ofoefule et al. [50] reported zero lag phase for anaerobically digested unpowdered rice husk. The lag phase recorded for the LMs in this research was relatively low (except 5H100) which was an indication of a good startup in the digesters despite the fact that no inoculum was added.
The low pH of acid-pretreated sample could be one of the reasons for the extended lag phase. Rapid degradation of hemicellulose has been reported to take place at low pH leading to formation of inhibitors which could have impaired biogas production [11]. This was contrary to the finding of Liang et al. [51] in which they observed improvement in biogas production rate but not in biogas production. Figure 3 shows the biogas yields of the samples. The values ranged from 9.32 to 67.31 mL/gVS with the highest recorded for 3E100 while 5H100 had the least value. The 67.31 mL/gVS biogas yield reported for 3E100 was higher than 55 mL/gVS reported for NaOH pretreated yard waste by Zhang et al. [52]. It is worth emphazing that the optimum pretreatment temperature was 100 °C rather than 120 °C in all the chemical pretreatments which would mean less consumption of energy [53]. Daily biogas production of raw and pretreated samples of RH is shown Fig. 2. The substrates attained their peak values at different times. Samples RH and 3E100 reached their peaks on day 1 while 3N100 was on day 3. Though both RH and 3E100 attained their peak values (885 and 870 mL) earlier than 3N100, the amount produced by 3N100 was significantly higher i.e. 1670 mL. This increase would have offset the delay in reaching the peak day earlier. The results revealed that organosolv pretreatment improved cumulative biogas production in 3E100 while alkaline pretreatment improved biogas production rate in 3N100. Attaining peak value in a short time with high amount of biogas would be of immense benefit when AD process is run on a continuous basis. The finding in 3E100 was contrary to that of Liang et al. [51] in which they observed improvement in biogas production rate but not in biogas production (Figs. 4, 5, 6, 7).
Biogas Quality
Methane and Carbon Dioxide Contents
The quality of biogas, which is the percentage of methane, was determined according to the method described by De la Rubia et al. [18]. Table 3 shows methane and carbon dioxide contents of raw RH and pretreated substrates. The methane contents were 46, 1.43, 50.27 and 50.68% for RH, 5H100, 3N100 and 3E100. The methane contents of 3E100 and 3N100 were higher than that of the raw sample and 5H100 but no significant difference in methane content of 3E100 and 3N100. The carbon dioxide content of 5H100 was far higher than other substrates. The methane contents with the exception of 5H100 were higher than 25.27% recorded for organic fraction of municipal waste (OFMSW) by Fdez-Guelfo et al. [54] but close to 57.3% dairy product waste reported by Alkanok et al. [46]. The volume of methane was also highest for 3E100 followed by 3N100. In comparing biogas quantity and quality of the substrates it was observed that both 3E100 and 3N100 improved these two parameters. This observation was similar to that of Zhou et al. [55] in their work on biogas production from pretreated corn stover and cow dung. However, 3E100 performed better than 3N100 in terms of biogas quantity.
All the substrates produced flammable biogas except 5H100. It has been reported that biogas with less than 45% methane would not produce flame which is an indication that the carbon dioxide content was higher than methane content [50]. Methanogenic inhibition of the pretreated samples was calculated by using Eq. 3.1. The results were 96.89, − 9.28 and − 10.00% for 5H100, 3N100 and 3E100 respectively. The negative values indicate that there was methanogenic enhancement in digesters 3N100 and 3E100 as evidenced in biogas production whereas 5H100 had a positive value an indication that there was methanogenic inhibition [20] which could be one of the reasons for the low yield.
Biofertilizer Potentials of Digestates
The digestates from the AD process were evaluated to determine their suitability as biofertilizer. In comparison to raw RH, there was loss in mineral contents (N, P and K) after AD in all the digestates as shown in Table 4. The decrease in nitrogen content has been attributed to release of ammonia during AD process [56, 57]. Among the digestates, rice husk digestate (RHD) had the best value for nitrogen while 5H100 had the best values for potassium and phosphorus. Reduction in phosphorus level in digestate has been reported in literature [58]. Generally, the nitrogen, phosphorus and potassium (NPK) contents of all the digestates were lower than the German Standard for biofertilizers namely: greater than 0.5% (N), 0.3% (P) and 0.5% (K) [59]. The results of this work were contrary to the increase in contents of digestates reported by Bouallagui et al. [60] for the digestates of fruit and vegetable waste and Ali et al. [61] for Jatroph curcas defatted waste. There was also decrease in sodium, calcium and magnesium contents in all the samples in comparison to RH but for 3E100D there was increase in these minerals after anaerobic digestion. The increase or decrease in mineral composition of digestates produced after AD has been attributed to complexation reactions that take place in the digester. Precipitation has also been identified as a possible reason for this phenomenon. However, further research has been recommended to fully understand the effect of AD process on the mineral composition of digestates [62, 63].
The heavy metal contents lead (Pb), cadmium (Cd), chromium (Cr) and nickel (Ni) for RHD, 5H100, 3N100 and 3E100 were determined and compared with Turkish standard on soil pollution control [64] for plants (Table 5). The concentrations of Pb, Cd and Cr in the digestates varied between 0.05–0.11, 0.03–0.05, and 0.02–0.04 and 0.01 ppm respectively while the concentration for Ni was the same in all the digestates. The concentrations of the heavy metals studied were all below the limit values by Turkish standard for soil pollution control. These concentrations were also below the range reported by Demirel et al. [64] in their work on heavy metal contents of digestates from batch anaerobic codigestion of sunflower hulls and poultry manure. The range for cadmium was lower than 0.9 ppm reported for digestate of municipal solid waste (MSW) by Alibardi and Cossu [65] and 0.17 ppm reported for Acacia modesta leaves by Nazir et al. [66].
The effectiveness of a pretreatment is not only determined by the efficiency of AD process but also in terms of economic performance. The financial success of a biogas plant depends among other things on the quality and biogas yield of the feedstock used [67]. A simplistic economic assessment of the pretreatment method vis-à-vis biogas yield was carried out according to the method described by Song et al. [67]. The economic assessment was done based on the exchange rate as at the time of the research which was 281 naira to a dollar. The unit prices of HCl and C2H5OH were per 500 mL while NaOH was per 500 g. Table 6 compares the economic performance at optimum conditions and biogas yields. C2H5OH showed the lowest cost and the highest biogas yield compared to other pretreatment methods followed by alkaline pretreatment method with 270 naira to produce 60.63 mL/gVS. Though the cost of alkaline pretreatment was higher than acid pretreatment but the biogas yield was significantly higher than 9.32 mL/gVS produced from acid pretreated substrate. It is noteworthy that the cost of material for construction of pretreatment reactor will be cheapest for C2H5OH since it is the mildest of the chemicals used and may not require sophisticated reactor that is resistant to corrosion, which makes the pretreatment process relatively inexpensive. Going by all the afore-mentioned, C2H5OH can be considered the most preferred of all the reagents used from economic point of view.
Conclusion
Biogas production and methane contents of RH was enhanced through solid-state chemical pretreatments. Pretreatments also had remarkable effects on the chemical compositions and morphologies of the substrates as evident by the results of physico-chemical analysis, FTIR, SEM and XRD. Solid-state chemical pretreatment at the concentrations considered modified the structure of lignocellulosic biomass thus making them more amenable to attack by anaerobic microorganisms for biogas production. The results from this research revealed that 3% concentration of NaOH and EtOH solid-state pretreatments at 100 °C for 60 min were the optimum conditions for maximum biogas production from RH for alkali and organosolv pretreatments. Acid pretreatment on the other hand, had a negative effect on biogas production despite the fact that there was reduction in lignin content, crystallinity and increase in surface porosity as confirmed by FTIR, XRD and SEM. It is therefore imperative to establish appropriate concentration that is sufficient to cause structural changes in the substrate without inhibiting AD process when solid state chemical pretreatment method is adopted.
References
Midilli, A., Dincer, I., Ay, M.: Green energy strategies for sustainable development. Energy Policy 34, 3623–3633 (2006)
Dias, M.O., Modesto, M., Ensinas, A.V., et al.: Improving bioethanol production from sugarcane: evaluation of distillation, thermal integration and cogeneration systems. Energy 36(6), 3691–3703 (2010)
Akinbami, J.F., Ilori, M.O., Oyebisi, T.O., et al.: Biogas energy use in Nigeria: current status, future prospects and policy implications. Renew. Sustain. Energy Rev. 5(1), 97–112 (2001)
Oyedepo, S.O.: On energy for sustainable development in Nigeria. Renew. Sustain. Energy Rev. 16, 2583–2598 (2012)
Damisa, D., Ameh, J.B., Umoh, V.J.: Effect of chemical pre-treatment of some lignocellulosic wastes on the recovery of cellulose from Aspergillus niger AH3 mutant. Afr. J. Biotechnol. 7(14), 2444–2450 (2008)
Olawale, O., Oyawale, F.A.: Characterization of rice husk via atomic absorption spectrophotometer for optimal silica production. Int. J. Sci. Technol. 2(4), 210–213 (2012)
Chungsangunsit, T., Gheewala, S.H., Patumsawad, S.: Environmental profile of power generation from rice husk in Thailand. The Joint International Conference on Sustainable Energy and Environment, 1–3 December, 2004, Hua Hin, Thailand pp. 739–742. (2004)
Mtui, G.Y.S.: Recent advances in pretreatment of lignocellulosic wastes and production of value added products. Afr. J. Biotechnol. 8(8), 1398–1415 (2009)
Pavlostathis, S.G., Gossett, J.M.: Alkaline treatment of wheat straw for increasing anaerobic biodegradability. Biotechnol. Bioeng. 27, 334–344 (1985)
Ariunbaatar, J., Panico, A., Esposito, G., et al.: Pretreatment methods to enhance anaerobic digestion of organic solid wastes. Appl. Energy 123, 143–156 (2013)
Agbor, V.B., Cicek, N., Sparling, R., Berlin, A., et al.: Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29(6), 675–685 (2011)
He, Y., Pang, Y., Liu, Y., et al.: Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 22(4), 2775–2781 (2008)
Pang, Y.Z., Liu, Y.P., Li, X.J., et al.: Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment. Energy Fuels 22(4), 2761–2766 (2008)
Mirmohamadsadeghi, S., Karimi, K., Zamani, A., et al.: Enhanced solid-state biogas production from lignocellulosic biomass by organosolv pretreatment. Biomed. Res. Int. 2014, 1–6 (2014)
He, Y., Pang, Y., Li, X., et al.: Investigation on the changes of main compositions and extractives of rice straw pretreated with sodium hydroxide for biogas production. Energy Fuels 23, 2220–2224 (2009)
Olugbemide, A.D., Lajide, L., Adebayo, A., et al.: Production of second-generation biofuel from five tropical lignocellulosic materials: effects of particle size and dilution ratio. Chem. Eng. Process. Technol. 2(2), 1030 (2016)
Shilpkar, P., Shah, M., Chaudhary, D.R.: An alternate use of Calotropis gigantea: biomethanation. Curr. Sci. 92(4), 435–437 (2007)
De la Rubia, M.A., Fernández-Cegrí, V., Raposo, F., et al.: Anaerobic digestion of sunflower oil cake: a current overview. Water Sci. Technol. 67(2), 410–417 (2013)
Mokobia, K., Ikhuoria, E.U., Olugbemide, A.D., et al.: Production and characterization of biogas obtained from sugarcane leaves. Int. J. Basic Appl. Sci. 1, 258–262 (2012)
Di Girolamo, G.: Methane production through anaerobic digestion of dedicated energy crops. Ph.D Thesis submitted to the Department of Agricultural Sciences, University of Bologna, Viale Fanin, Bologna, Italy (2014)
AOAC, Association of Official Analytical Chemists: Official Methods of Analysis of the Association of Official Analytical Chemists. (14th ed.), AOAC, Washington, DC (1990)
ASTM, American Society for Testing and Materials. Standard Methods of Test for Alcohol-Benzene Solubility of Wood ASTM D1 107-56. ASTM, West Conshohocken (1972)
Olugbemide, A.D., Lajide, L., Adebayo, A., et al.: Kinetic study of biogas production from raw and solid-state organosolv pretreated rice husk. J. Biofuels 7(2), 110–118 (2016)
Olugbemide, A.D., Lajide, L., Adebayo, A., et al.: Optimization and kinetic study of biogas production from rice husk through solid-state alkaline pretreatment method. Invertis J. Renew. Energy 6(4), 175–180 (2016)
Li, Y., Zhang, R., Liu, X., et al.: Evaluating methane production from anaerobic mono-and co-digestion of kitchen waste, corn stover, and chicken manure. Energy Fuels 27(4), 2085–2091 (2014)
Teghammar, A., Castillo, M.P., Ascue, J., et al.: Improved anaerobic digestion by addition of paper tube residuals: pretreatment, stabilizing and synergetic effects. Energy and Fuels 27, 277–284 (2012)
Juanga, J.P.: Optimizing dry anaerobic digestion of organic fraction of municipal solid waste. M. E. Thesis. Asian Institute of Technology, Bankok, Thailand (2005)
Wyman, C.E.: Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. 1st Edn. Edited by Charles E. Wyman. Fundamentals of Biomass Pretreatment by Fractionation. Wiley, New York (2013)
Donohoe, B.S., Decker, S.R., Tucker, M.P., et al.: Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 101(5), 913–925 (2008)
Karp, E.M., Resch, M.G., Donohoe, B.S., et al.: Alkaline pretreatment of switchgrass. Sustain. Chem. Eng. 3, 1479–1491 (2015)
Taherzadeh, M.J., Karimi, K.: Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9(9), 1621–1651 (2008)
Welker, C.M., Balasubramanian, V.K., Petti, C., et al.: Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies 8(8), 7654–7676 (2015)
Teghammar, A., Karimi, K., Horváth, I.S., et al.: Enhanced biogas production from rice straw, triticale straw and softwood spruce by NMMO pretreatment. Biomass Bioenergy 36, 116–120 (2012)
Pu, Y., Hu, F., Huang, F., et al.: Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 6(1), 15 (2013)
Bharathiraja, B., Sudharsanaa, T., Bharghavi, A., et al.: Insight on lignocellulosic pretreatments for biofuel production- SEM and reduction of lignin analysis. Int. J. Chem. Technol. Res. 6, 4334–4445 (2014)
Marrugo, G., Valdes, C.F., Chejne, F.: Characterization of Colombian agro industrial biomass residues as energy resources. Energy Fuels 30, 8386–8398 (2016)
Li, F., Hu, H., Yao, R., et al.: Structure and saccharification of rice straw pretreated with microwave-assisted diluted lye. Ind. Eng. Chem. Res. 51, 6270–6274 (2012)
Yao, Y., He, M., Ren, Y., et al.: Anaerobic digestion of poplar processing residues for methane production after alkaline treatment. Bioresour. Technol. 134, 347–352 (2012)
Zhao, Q., Leonhardt, E., MacConnell, C., et al.: Purification Technologies for Biogas Generated by Anaerobic Digestion. Compressed Biomethane CSANR, Washington, DC, pp. 1–24 (2009)
Mirmohamadsadeghi, S., Karimi, K., Horváth, I.S.: Improvement of solid state biogas production from wood by concentrated phosphoric acid pretreatment. Bioresources 11(2), 3230–3243 (2016)
Farahani, S.V., Kim, Y.W., Schall, C.A.: A coupled low temperature oxidative and ionic liquid pretreatment of lignocellulosic biomass. Catal. Today 269, 2–8 (2016)
Kim, J., Park, C., Kim, T.H., et al.: Effects of various pre-treatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 95, 271–275 (2003)
Yue, Z., Liu, R., Yu, H., et al.: Anaerobic ruminal degradation of bulrush through steam explosion pretreatment. Ind. Eng. Chem. Res. 47, 5899–5905 (2008)
Vrieze, J., Raport, L., Willems, B., et al.: Inoculum selection influences the biochemical methane potential of agro-industrial substrates. Microb. Biotechnol. 8, 776–786 (2015)
Shah, F.A., Mahmood, Q., Shah, M.M., et al.: Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci. World J. 2014, 1–21 (2014)
Alkanok, G., Demirel, B., Onay, T.T.: Determination of biogas generation potential as a renewable energy source from supermarket wastes. Waste Manag. 34(1), 134–140 (2013)
Pandey, P.K., Ndegwa, P.M., Soupir, M.L., et al.: Efficacies of inocula on the startup of anaerobic reactors treating dairy manure under stirred and unstirred conditions. Biomass Bioenergy 35(7), 2705–2720 (2011)
Cheng, X.Y., Zhong, C.: Effects of feed to inoculum ratio, co-digestion, and pretreatment on biogas production from anaerobic digestion of cotton stalk. Energy Fuels 28(5), 3157–3166 (2014)
Aiwonegbe, A.E., Akinyomi, J.O., Ikhuoria, E.U.: Utilization of plantain (Musa species) leaves for biogas production. Int. J. Pure Appl. Sci. 9(2), 1–7 (2015)
Ofoefule, A.U.: Investigation of the biogas production potentials of Bambara nut chaff (Vigna subterranea). Adv. Appl. Sci. Res. 2(2), 55–61 (2011)
Liang, Y., Yin, S., Si, Y., et al.: Effect of pretreatment and total solid content on thermophilic dry anaerobic digestion of spartina alterniflora. Chem. Eng. J. 237, 209–216 (2014)
Zhang, Z., Li, W., Zhang, G., et al.: Impact of pretreatment on solid state anaerobic digestion of yard waste for biogas production. World J. Microbiol. Biotechnol. 30(2), 547–554 (2013)
Menardo, S., Airoldi, G., Balsari, P.: The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 104, 708–714 (2012)
Fdez-Guelfo, L.A., Alvarez-Gallego, C., Marquez, D.S., et al.: Start-up of thermophilic-dry AD of OFMSW using adapted modified SEBAC inoculums. Bioresour. Technol. 101, 9031–9039 (2000)
Zhou, S., Zhang, Y., Dong, Y.: Pretreatment for biogas production by anaerobic fermentation of mixed corn stover and cow dung. Energy 46(1), 644–648 (2012)
Lin, Y., Wang, D., Li, Q., et al.: Kinetic study of mesophilic anaerobic digestion of pulp & paper sludge. Biomass Bioenergy 35(12), 4862–4867 (2011)
Risberg, K.: Quality and function of anaerobic digestion residues. Doctoral Thesis Swedish University of Agricultural Sciences Uppsala (2015)
Nkoa, R.: Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron. Sustain. Dev. 34(2), 473–492 (2014)
Bouallagui, H., Cheikh, R.B., Marouani, L., et al.: Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 86(1), 85–89 (2003)
Ali, N., Kurchania, A.K., Babel, S.: Biomethanasation of Jatropha curcas defatted waste. J. Eng. Technol. Res. 2(3), 38–43 (2010)
Masse, D.I., Croteau, F., Masse, L.: The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Bioresour. Technol. 98(15), 2819–2823 (2007)
Möller, K., Torsten, M.: Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng. Life Sci. 12(3), 242–257 (2012)
Demirel, B., Göl, N.P., Onay, T.T.: Erratum to: evaluation of heavy metal content in digestate from batch anaerobic co-digestion of sunflower hulls and poultry manure. J. Mater. Cycles Waste Manag. 3(15), 409–409 (2013)
Alibardi, L., Cossu, R.: Stabilization of digestates from wet and dry anaerobic digestion. Proceedings Venice: Third International Symposium on Energy from Biomass and Waste, Venice, Italy; 8–11 November (2010)
Nazir, R., Khan, M., Masab, M., et al.: Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plant and analysis of physico-chemical parameters of soil and water collected from Tanda Dam kohat. J. Pharm. Sci. Res. 7(3), 89 (2015)
Mahnert, P., Linke, B.: Kinetic study of biogas production from energy crops and animal waste slurry: effect of organic loading rate and reactor size. Environ. Technol. 30(1), 93–99 (2008)
Song, Z., Zhang, C., Yang, G., et al.: Comparison of biogas development from households and medium and large-scale biogas plants in rural China. Renew. Sustain. Energy Rev. 33, 204–213 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olugbemide, A.D., Lajide, L., Adebayo, A. et al. Enhanced Biogas Production from Rice Husk Through Solid-State Chemical Pretreatments. Waste Biomass Valor 11, 2397–2407 (2020). https://doi.org/10.1007/s12649-018-00567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-00567-9