Abstract
The presence of lignin in the biomass makes it difficult for the microorganisms to decompose during the anaerobic digestion process, resulting in a prolonged hydrolysis phase and decreased biogas production. This study investigates the effect of four different types of thermal pretreatment techniques, i.e., hot air oven, microwave, autoclave, and hot water bath, on the hydrolysis of a lignocellulosic biomass Hydrilla verticillata to find the best pretreatment method that enhances solubilization. The degree of substrate solubilization imparted by different pretreatments was evaluated based on variations in soluble chemical oxygen demand (sCOD) and volatile fatty acid (VFA) concentrations. Variable parameters, time, and temperature were optimized for each pretreatment technique based on the degree of solubilization. Among all pretreatment techniques, the hot air oven showed the most significant improvement with a tremendous 3.38 times solubilization when operated at optimized conditions of 100 °C and 70 min. The biochemical methane potential (BMP) test revealed that pretreatment considerably decreased maturation time and increased methane yield by 34.27%. The energy balance demonstrated a net positive specific energy of 6918.39 kJ/kg, asserting the effectiveness of hot air oven pretreatment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anaerobic digestion of aquatic weed biomass could be highly promising, especially Hydrilla verticillata, which proliferates and regenerates naturally [1]. The aquatic weed H. verticillate is native to Asia and Australia but distributed worldwide due to its invasive nature and rapid growth. The weed is notoriously known as an invisible menace as it grows rooted and submerged in still and slightly flowing waters and stays unnoticed until it covers the whole water body with its dense mats of vegetation and tops out at the water surface [2]. The weed’s average growth rate is 2.5 cm per day [3, 4]. Once invaded, this weed can severely affect the aquatic ecosystem by depleting the dissolved oxygen and nutrients, limiting the resources to the native species. Also, it hinders human beings’ livelihood and recreational activities [4,5,6].
The lignocellulosic biomasses are very economical as they are renewable and naturally abundant [7]. However, a higher percentage of rigid crystalline lignin content in lignocellulosic biomass makes it difficult for the anaerobes to access the easily digestible carbohydrates cellulose and hemicellulose [8, 9]. Besides, the presence of lignin affects a prolonged hydrolysis period and low methane yield. Various pretreatment techniques benefit the de-crystallization of biomass structure, making it more accessible to microbes. It will shorten the hydrolysis period and improve the conversion rate to enhance methane yield [8, 10,11,12]. Different pretreatment techniques on substrates include physical, chemical, and biological processes [13,14,15]. Optimization of these process strategies is crucial to achieving the maximum output from any substrate. Thermal pretreatment is a physical technique that provides heat energy by various means to solubilize the substrate. Various thermal pretreatment methods include hydrothermal, microwave, and autoclave. A combination of thermal and chemical pretreatment methods is also very effective [14,15,16].
1.1 Pretreatment techniques
In the thermal pretreatment study, heat is transferred in different forms to melt down the weed biomass’s lignin layer. The primary modes of heat transfer are conduction, convection, and radiation [17]. The heat transfer mechanism in a hot air oven is initially convection, followed by conduction. Chopped hydrilla is placed in glassware and kept in a hot air oven. With the help of a fan, dry air is circulated equally throughout the chamber. The outer layer first absorbs the dry heat and then goes through the middle layer, tearing the hydrilla cell wall. The dry heat is delivered to the glassware holding the substrate via convection and subsequently from the glassware to the substrate via conduction. Heat transmission in a hot water bath pretreatment occurs first through convection, then through conduction, as in a hot air oven pretreatment [18]. The key distinction lies in the heat transfer mechanism, where water is utilized instead of hot air. Microwave pretreatment involves using electromagnetic radiation to break down the hydrogen bonds within the biomass’s cell wall [19]. On the other hand, autoclave pretreatment utilizes moist heat to break down the robust lignin layer of hydrilla and solubilize the cellulose present[20]. Various studies are available on the effect of thermal pretreatment on biogas production and methane yield for different lignocellulosic biomasses. Thermal pretreatment was conducted in two stages to get the optimum condition required to solubilize the substrate. They are as follows:
-
(a)
Temperature study in which samples were kept at different temperatures for a fixed time. The optimum temperature was fixed after treatment according to the sCOD and VFA values.
-
(b)
Time study in which the samples were kept at optimum temperature got from temperature study for different time variations. After treatment, the optimum time was confirmed from the sCOD and VFA values [17, 21].
In a study on the effect of hydrothermal pretreatment on biogas production from rice straw, it was observed that an increase in the methane yield of 3% occurred at 180 °C for 15 min and gradually decreased at higher temperatures [22]. Another study on the hydrothermal pretreatment of sunflower residues reported an increment of 43–63% in biodegradability at 180 C for 30 min [23]. Contrary to that, improved methane yield was reported for studies with extended periods at lower temperatures [24].
Despite the existing body of research on the thermal pretreatment of various lignocellulosic biomasses, there is a dearth of literature that investigates and compares specific thermal pretreatment techniques applied to H. verticillata, such as hot ovens, microwaves, hot water baths, and autoclave pretreatment. This study addresses a significant gap in the literature by highlighting the novel use of aquatic weed biomass for biogas production via anaerobic digestion. Using sCOD and VFA as performance markers to determine the degree of solubilization appears to be a methodical analytical tool for distinguishing four thermal pretreatment methods. In addition, it uses the biomethane potential (BMP) tool to systematically evaluate and compare the effects of pretreatment on biogas potential and substrate degradability.
2 Methodology
2.1 Substrate and inoculum
H. verticillata was collected from lakes inside the Indian Institute of Technology Guwahati (IITG), India. Fresh cow dung for anaerobic inoculum was collected from a cattle shed in Amingaon near the IITG campus. An active inoculum with well-balanced microbial communities is critical for decreasing the initiation time of anaerobic digestion (AD) and ensuring its steady and efficient operation [25]. The fresh H. verticillata was ground uniformly and mixed with distilled water in a 1:1 ratio to ensure consistency.
2.2 Pretreatment study
Different pretreatment studies were conducted to increase biogas production and decrease the hydrolysis period. Temperature and time studies were done to get the optimum condition in thermal pretreatment. The parameters, VFA and sCOD, were studied to determine the optimum condition. Freshly ground hydrilla mixed with distilled water in a 1:1 ratio was used for pretreatment. Temperature and time range for each equipment were selected based on the previous works of literature [9, 10, 17, 21, 22, 26, 27].
2.2.1 Hot air oven
Fifty grams of a sample containing ground hydrilla and distilled water (1:1) was placed in a sealed conical flask and heated at 70, 80, 90, 100, and 110 °C for 20 min each to study the effect of different temperatures. To study the effect of time, the sample was heated at optimized temperatures for 60,70, 80, 90, 100, 110, 120, 130, and 140 min. The range of time and temperature of operation of hot air oven was selected based on previous literature available [28, 29]. This pretreatment uses the conduction and convection principles to transfer heat energy through the sample.
2.2.2 Autoclave
To study the effect of autoclave pretreatment on hydrilla, the sealed conical flask containing 50 g of a sample containing ground hydrilla and distilled water (1:1) was heated at 80, 90, 100, 110, and 120 °C for 20 min each. From the temperature study, the optimum temperature was selected. The samples were kept in an autoclave at optimized temperature for 20, 40, 60, and 80 min. Steam is a conduit transporting heat to the hydrilla’s refractory matrix via conduction and convection [17, 22, 28].
2.2.3 Microwave
To study the effect of microwave on the hydrolysis of H. verticillata, the sealed flask containing 50 g of hydrilla was heated at 160, 180, 200, 220, and 250 °C for 10 min each. From the temperature study, the optimum temperature was selected. To investigate the effect of time, the samples were kept in the microwave at optimized temperatures for 5, 10, 15, and 20 min. In the microwave, samples were placed in the center of the plate. As the sample spins inside the oven’s electromagnetic field, it allows consistent energy absorption in the sample. Temperature and time were decided based on available literature on the same type of substrate [20, 26, 30].
2.2.4 Hot water bath
To study the effect of a water bath on the hydrolysis of H. verticillata, the sealed flask containing 50 g of weed was heated at 70, 80, 90, and 100 °C for 30 min each. From the temperature study, the optimum temperature was selected. To investigate the effect of time, the samples were kept in a water bath at optimized temperatures for 30, 60, 90, and 120 min. The temperature and time were taken based on the available literature [22, 28]. Conduction and convection principles were responsible for heat transfer throughout the biomass samples and hydrating H. verticillata’s complex structure. The effect of treatment on digestibility was determined by using a BMP setup to assess the substrate’s hastened methane potential for various F/M ratios (0.5, 1.0, 1.5, 2, 2.5, 3) [26].
2.3 BMP setup
The BMP test was carried out in batch mode using 1000 mL glass reagent bottles. Rubber cork and Teflon tape were used to seal the openings. Each bottle was connected to a specific aspirator bottle containing 1.5 N NaOH solutions through long silicon tubing. Based on volatile solids (VS), the amount of hydrilla and cow dung was decided. Different food-to-microorganism (F/M) ratios F/M 5, 1.0, 1.5, 2.0, and 2.5 were studied in triplicates for each ratio. The composition of each digester, showing the amount of substrate and inoculum corresponding to the F/M ratios, is provided in the supplementary data. Two controls (cow dung and H. verticillata) were also studied separately in triplicate. The F/M ratio is the amount of VS of H. verticillata divided by VS of cow dung. The batch reactors were fed with different amounts of H. verticillata and cow dung and essential macro- and micronutrients (phosphate buffer, ferric chloride, calcium chloride, magnesium chloride, nickel chloride, cobalt nitrate) [31]. The level of inoculum plus substrate in each reagent bottle was maintained at 700 mL using distilled water. Nitrogen gas was pumped into reactor bottles for 3 min each to ensure the reactors’ prevailing anaerobic conditions by purging out any trapped air if present. Then, the reactor bottles were closed with airtight butyl rubber corks, followed by connecting to aspirator bottles having 1.5 N NaOH. Methane production was quantified daily using the water displacement method (Fig. 3). 1.5 N NaOH was used in place of water because NaOH can absorb the CO2 produced in biogas. Thymol blue was added to the aspirator bottle as an alkali indicator [32, 33].
2.4 Analytical methods
Initial characterization of the sample was done on the fresh substrate cut and ground to thick paste. Weekly sampling was performed for the analysis of volatile solids (VS), volatile fatty acids (VFA), and soluble chemical oxygen demand (sCOD). The sample’s pH was determined by a pH meter with an electrode (Thermo Fisher Scientific). The total solid (TS) content was calculated by weighing a fresh sample amount before and after drying at 105 °C according to standard methods [34]. The volatile solid (VS) content was determined by measuring the weight of the dried sample before and after burning at 550 °C. Total chemical oxygen demand (COD), acid-soluble lignin, hemicellulose, and cellulose were estimated by titrimetric analysis according to standard methods [34]. VFA was analyzed by a modified titrimetric method [35]. Five grams of well-mixed sample was taken for sCOD and VFA, and the volume was brought up to 100 mL with distilled water. The sample was filtered after being shaken for 2 h at 150 rpm in a horizontal shaker. After that, the filtered sample was used straight for analysis. Biogas was measured every day by the water displacement method [29]. 1.5 N NaOH was used instead of water to absorb the CO2 produced as CO2 reacts with NaOH to form Na2CO3 [36]. Thymol blue was added as an alkali indicator. The volume of NaOH displaced represents the amount of methane produced. The displaced NaOH was measured using a cylinder [37].
2.5 Characterization
To determine structural changes of the sample, FESEM (Zeiss, Sigma), FTIR (PerkinElmer Spectrum 2), and XRD (Rigaku TTRAX III) were used for characterization analysis. Samples of untreated weed, hot air oven pretreated biomass, and the digestate were prepared by drying at room temperature and then ground to make a fine powder. FESEM images were clicked at 3 kV for different magnifications, i.e., 300X, 500X, and 1000X. An XRD diffractometer was used to record X-ray diffractograms from 0 to 70 °C at a diffraction angle (2θ) at a scanning speed of 4°/min. To produce the sample pellets for FTIR, the sample was thoroughly mixed with 300 mg of KBr, then pressed it to sample discs using a hydraulic jack for 1 min at 20 Mpa. With 16 scans at 4 cm−1, the FTIR spectra were collected from 4000 to 400 cm−1.
2.6 Energy balance assessment
The specific energy utilized, Eu (kJ/kg), for various thermal pretreatment techniques adopted in this study was determined using the following Eq. 1.
where P denotes the power rating of the equipment used, te denotes the exposure time, and W denotes the amount of substrate that can be treated at once. Furthermore, the specific energy, Ea (kJ/kg), made available by the pretreatment technique is calculated based on the sCOD increment from the pretreatment and conversion of sCOD to biogas followed by converting to electrical energy. Conversion factors for sCOD to biogas as well as biogas to electric energy are assumed throughout the calculation based on the available literature and equipment efficiency ratings [28, 38].
where sCODi indicates the sCOD increment caused due to the pretreatment, Φε sCOD removal efficiency of the reactor, Β denotes the sCOD to biogas conversion rate, Η is the lower heating value of biogas, and λε represents the biogas generator efficiency [39].
The net specific energy of each pretreatment technique is calculated by differencing the specific energy utilized (Eu) by the equipment from the specific energy made available by the corresponding pretreatment as given in Eq. 3 [40].
3 Results and discussion
3.1 Initial characterization
The physicochemical characterizations of the substrate and inoculum tested in the current study are shown in Table 1.
3.2 Pretreatment studies
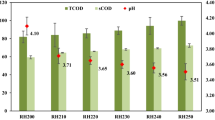
The best thermal pretreatment techniques were determined based on the variation in sCOD and VFA. VFA exhibits a direct relationship with sCOD for the hot air oven pretreatment, as the soluble products formed in the first step of anaerobic digestion convert into short-chain VFA in the second step. Figure 1a shows that with the increase in sCOD, there is an increase in VFA at 100 °C. At this temperature, the sCOD and VFA gave maximum values (9472 mg/L and 2250 mg/L, respectively), after which they started decreasing. The observed decline in soluble chemical oxygen demand (sCOD) and volatile fatty acids (VFA) following a specific temperature threshold can be attributed to the disruption of cell walls at higher temperatures. This disruption leads to the release of the maximum available organic compounds at that temperature. Additionally, volatile organic matter may be released through evaporation above these elevated temperatures [20, 41].
Figure 1b depicts the temporal variations of hot air oven pretreatment in which the highest sCOD and VFA values were observed at 70 min, which were 12160 mg/L and 2025 mg/L, respectively. These values indicate a significant 238.82% (3.38-fold) solubilization increment compared to untreated biomass. Hence, the optimum conditions for hot air oven pretreatment are 70 min at 100 °C for H. verticillata. A similar study on papermill sludge pretreatment using a hot air oven stated significant improvement in solubilization at 80°C for 90 min [27].
For the autoclave pretreatment temperature study (Fig. 2a), the increase and decrease of VFA and sCOD were evident. Up to 100 °C, the VFA and sCOD increase and then decrease. The soluble carbohydrates in the suspension decompose into a mixture of long-chain fatty acids or organic acids, increasing VFA concentrations. It is evident from the data that the optimal temperature for the process is 100 °C. This temperature disassembles complex molecules into simpler monomers. By analyzing the results of a time study, it was determined that a pretreatment duration of 60 min produced the best results with sCOD of 11776 mg/L and VFA 3240 mg/L (Fig. 2b). Therefore, the recommended autoclave pretreatment conditions are 100 °C for 60 min. This pretreatment significantly increased the soluble chemical oxygen demand (sCOD) of H. verticillata by 3.27 times compared to the sCOD of untreated H. verticillata.
For the microwave pretreatment, the temperature study revealed that both the sCOD and VFA concentration showed maximum values of 6912 mg/L and 870 mg/L, respectively, at 180 °C, as shown in Fig. 3a. After the time studies, it further increased to 9728 mg/L sCOD and 780 mg/L VFA which accounted for an increment of 170% (2.70-fold) solubilization with 15 min at 180 °C as the optimum conditions (Fig. 3b).
Similarly, for the hot water bath pretreatment, the temperature study revealed that both the sCOD and VFA concentration increased and gave the maximum values 6400 mg/L and 1140 mg/L, respectively, at 90 °C (Fig. 4a). In the time study, samples pretreated for 90 min gave the best results 6560 mg/L and 1200 mg/L of sCOD and VFA respectively (Fig. 4b). The sCOD increases by 82.22% through this pretreatment technique, i.e., 1.82 times increase in the sCOD than that of untreated H. verticillata.
The percentage increment in sCOD for the pretreated biomass from the untreated biomass delineates the degree of solubilization. Among the four thermal pretreatment techniques, the hot air oven pretreatment showed maximum solubilization with an increment of 237.77% in sCOD, followed by autoclave pretreatment (227.11%), microwave (170.22%), and hot water bath 82.22%). However, in terms of VFA variations, autoclave pretreatment elevated to the highest with 3240 mg/L, while the minimum was for microwave pretreatment with 780 mg/L. This may be due to varying degrees of evaporation across equipment. Thermal pretreatment aids in shortening the hydrolysis period of weed biomass in two ways. Firstly, the water available in the sample with heat energy disrupts the organic compound’s hydrogen bonds that hold the crystalline lignocellulosic structure. Secondly, it actuates cellulose and hemicellulose depolymerization to short-chain monomers [42, 43].
3.3 BMP study of the untreated H. verticillata
For the untreated hydrilla biomass, it was observed that the untreated hydrilla of F/M 2.5 produces the highest amount of biogas (180 ± 14 mL) on the 33rd day of anaerobic digestion (Fig. 5a). Figure 5b depicts that for the cumulative methane production after 50 days of BMP study, F/M 2.5 is highest (4857 mL) among all the F/M ratios. During the BMP study of untreated H. verticillata, the lag phase was observed due to the lignin layer, which acts as a barrier for the microorganisms to access the cellulose.
Reduction of VS indicates mass loss in anaerobic digestion and biogas production. At day zero, the microbial activity is less. Hence, the VS reduction starts after that. The analysis of the results of the BMP study of untreated HV is shown in Fig. 5c.
A decreasing trend of volatile solids was observed over time. Higher VS reductions are observed to increase biogas production. After the initial week, VS decreased considerably. It persisted until the fourth week of anaerobic digestion. The delay in degradation may result from lower microbe activity in the initial days and lower availability of cellulose to microbes due to the presence of a dense lignin layer surrounding the cellulosic portion of the substrate [44]. F/M 2.5 has the most significant volatile solid reduction at 41.46%, followed by F/M 2.0 at 36.34%. The decreasing order of VS reduction is 2.5>2.0>1.5>1.0>3.0>0.5, indicating that the maximum biogas production must occur at F/M 2.5, which is consistent with the experimental findings. The graph demonstrates that the most significant VS reduction occurred within the first 28 days of the BMP study.
Change in sCOD concentration with time for untreated H. verticillata is shown in Fig. 5d. Different F/M ratios provide varied substrate removal rates in the form of sCOD. The F/M ratio 2.5 shows the highest sCOD (6357 ± 147 mg/L) on the 28th day. After 28 days, there was no further increase in sCOD concentration. This may be due to the lower availability of organic substrate, which was already degraded by microbes till that time. While studying the VFA concentration variation of the digesters, it was observed that the concentrations were moderately low in the initial days due to the lower availability of solubilized substrate. Later, due to microorganisms’ hydrolytic and acetogenic activity, the VFA concentration began to increase. After organic matter is converted to volatile fatty acids (VFA), the removal can be viewed in terms of gas production. The highest amount of VFA production was observed (1610 ± 148 mg/L) on the 28th day (Fig. 5e). It can be considered that up to the 28th day, acidogens were active and led to the production of VFA, followed by conversion of these acids into methane and carbon dioxide with the help of methanogens.
3.4 BMP study of the hot air oven pretreated H. verticillata
Hot air oven pretreatment gave the best solubilization among the four thermal pretreatment techniques applied to H. verticillata. A BMP study has been done to compare the biogas production of pretreated H. verticillata with the untreated one. The study produced maximum biogas (227 ± 16 mL) on the 16th day of the BMP study by the F/M 2.0, as shown in Fig. 3.6a. The untreated H. verticillate took 33 days to provide the maximum biogas production. Hence, it is clear that through hot air oven pretreatment, delignification occurred, and hydrolysis occurred faster. This may also be due to the application of heat, which converted the crystalline structure of cellulose into an amorphous nature, making the weed biomass easily digestible for the hydrolytic bacteria.
Rapid VS reduction was seen in this study. This indicates better biogas production. F/M 2.0 of pretreated H. verticillata showed the highest VS reduction of 64.78% (Fig. 6c). This justifies the daily and cumulative biogas generated during the study. As F/M 2.0 showed maximum VS reduction, biogas produced from the particular ratio was also maximum. For untreated H. verticillata, the maximum reduction was for F/M 2.5 of 41.46%. From this, it can be inferred that this treatment enhanced substrate biodegradability. F/M 2.5 also had a VS reduction of 56.60% in the hot air oven study. The VS reduction during BMP test of pretreated H. verticillata for different F/M ratios followed the order 2.0>2.5>1.5>1.0>3.0>0.5, with F/M 2.0 having the most significant VS reduction and F/M 0.5 having the least.
In a hot air oven, pretreated H. verticillata, sCOD increased up to the 14th day of the BMP study, which decreased rapidly. F/M 2.0 showed maximum sCOD concentration (7274 ± 352 mg/L) on the 14th day (Fig. 6d) because of better substrate solubilization. Particulate COD was converted to soluble COD. Hot air oven pretreatment also helped to decrease the prolonged hydrolysis period. As a result, particulate matter was converted to soluble within a brief period. The maximum sCOD produced on the 28th day of anaerobic digestion of untreated H. verticillata was 6357 ± 147 mg/L.
Biogas production, volatile solid (VS) reduction, and soluble chemical oxygen demand (sCOD) values differ significantly between BMP studies of untreated H. verticillata and H. verticillata that have been pretreated in a hot air oven. For untreated H. verticillata, the highest biogas yield (180±20 mL) was obtained on the 33rd day at an F/M ratio of 2.5, whereas the highest cumulative methane production (4857 mL) was obtained after 50 days. Whereas, in the BMP study of hot air oven pretreated H. verticillata, the highest daily methane yield (227 ± 16 mL) was obtained much earlier on day 16, and a cumulative yield of 152.23 mL CH4/gVS biogas was generated within 35 days (F/M 2). Compared to the cumulative methane production from untreated H. verticillata at 35 days (113.37 mL CH4/gVS for F/M 2.5), the biogas production increased by 34.27%. In addition, cumulative biogas production analysis revealed that the pretreated BMP produced the same amount of biogas in a significantly shorter period, whereas the untreated biomass took 50 days to mature. The cumulative methane yield on the 50th day for untreated biomass (157.69 mL CH4/gVS) was comparable to that of pretreated biomass produced by just 35 days. A study on lawn grass with thermal, alkaline, and acidic pretreatment and validation with BMP reported an increase of 25.7% in methane yield [45]. Similar results were reported with enhancement in methane yield for the range of lignocellulosic substrates with various pretreatment techniques obtaining methane yield in the range of 20–40% [46,47,48,49,50].
Due to its barrier effect on cellulose accessibility, the presence of lignin induces a lag phase, with VS reduction indicating anaerobic digestion progress. For untreated biomass, F/M 2.5 results in the highest VS reduction (41.46%), followed by F/M 2.0 (36.34%), indicating that F/M 2.5 is optimal for biogas production. In contrast, pretreatment with a hot air oven accelerates delignification and hydrolysis, resulting in a quicker VS reduction (e.g., F/M 2.0 with 64.78%). Additionally, the sCOD concentration rises rapidly in pretreated biomass due to improved substrate solubilization, with F/M 2.0 exhibiting the highest sCOD (7274 ± 352 mg/L) on day 14. This result indicates increased substrate biodegradability and decreased hydrolysis times. Compared to untreated biomass, which reaches a maximum sCOD concentration of 6357 ± 147 mg/L on the 28th day, the hot air oven pretreatment proves to be exceptionally effective.
3.5 Spectroscopic characterization
Spectroscopic characterization analysis was performed using FESEM, FTIR, and XRD to understand the structural and morphological changes on the substrate due to hot air oven pretreatment. Figure 7 shows the images FESEM (Zeiss, Sigma 300) took at ×1000 magnification. Structural changes to the untreated H. verticillata by hot air oven pretreatment followed by BMP test are evident as the rigid lignocellulosic biomass ruptured after the process. As per the visual inspection of the electron-microscopic images, it is evident that the cell wall is bound and rigid for the untreated biomass (Fig. 7a), whereas for the pretreated biomass, it gets cracked open (Fig. 7b). The digested biomass further deteriorated with significant breaks on the cell wall due to microbial activity (Fig. 7c). A significant 34.27% increment in methane yield from pretreated biomass over that of untreated biomass can be attributed to the breakdown of cell wall during the hot air oven pretreatment. It may have allowed the microbes to access easily digestible cellulose and hemicellulose of the lignocellulosic biomass [51]. FTIR and XRD spectra of untreated, hot air oven pretreated, and the digestate biomass are provided in the supplementary data.
3.6 Energy balance assessment
The specific energy utilized, energy available, and net specific energy were calculated for each pretreatment technique based on the optimized pretreatment conditions, power ratings of equipment, sCOD increment caused by the pretreatment, and other conversion factors by applying Eqs. 1–3. The outcomes of the energy balance analysis are determined and compiled in Table 2.
Except for the hot water bath, every pretreatment method exhibited a positive net specific energy value. It was because the sCOD increment by the hot air oven was extremely low. Pretreatment in a hot air oven yielded the highest En (6918.39 kJ/kg), whereas autoclave pretreatment also yielded comparable results (6608.04 kJ/kg). Even though power consumption was higher for hot air oven pretreatment, the amount of substrate treatable at once and sCOD increment by the treatment were significantly higher which added to the net specific energy. Interestingly, microwave pretreatment also showed a net positive specific energy, which can be attributed to the low time requirements for the pretreatment.
4 Conclusions
The research has emphasized the comparative efficacy of various thermal processes, highlighting hot air oven pretreatment as the most promising technique. The application of hot air at 100 °C for 70 min and autoclave treatment at 100 °C for 60 min significantly increased the solubility of biomass by 3.38 and 3.27 factors, respectively. In contrast, the hot air oven method exhibited superior solubilization at 100 °C and 70 min. Analyses of comparative biomethane potential (BMP) highlighted the efficacy of hot air oven pretreatment in enhancing methane production from hydrilla biomass. On the 33rd day at an F/M ratio of 2.5, untreated biomass yielded a maximum of 180 ± 14 mL CH4, while pretreated biomass yielded 227 ± 16 mL CH4 on the 16th day for F/M 2. Comparing cumulative biogas yields on the 35th day revealed a 34.27% increase in methane yield for pretreated biomass. These results highlight the significance of refining pretreatment techniques in order to increase methane yields and promote efficient anaerobic degradation of lignocellulosic substrates.
References
Amulya K, Morris S, Lens PNL (2023) Aquatic biomass as sustainable feedstock for biorefineries. Biofuels Bioprod Bioref 17:1012–1029. https://doi.org/10.1002/bbb.2471
Sousa WTZ (2011) Hydrilla verticillata (Hydrocharitaceae), a recent invader threatening Brazil’s freshwater environments: a review of the extent of the problem:1–20. https://doi.org/10.1007/s10750-011-0696-2
Pal DK, Nimse SB (2006) Little known uses of common aquatic plant, Hydrilla verticillata (Linn. f.) royle. Indian J Nat Prod Resour 5:108–111
Hershner C, Havens KJ (2008) Managing invasive aquatic plants in a changing system: strategic consideration of ecosystem services. 22:544–550. https://doi.org/10.1111/j.1523-1739.2008.00957.x
Parker IM, Lonsdale WM, Goodell K et al (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Jayan PR, Sathyanathan N (2012) Aquatic weed classification, environmental effects and the management technologies for its effective control in Kerala, India. Int J Agric Biol Eng 5:76–91. https://doi.org/10.3965/j.ijabe.20120501.010
Kumar A, Rapoport A, Kunze G et al (2020) Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: a review. Renew Energy 160:1228–1252. https://doi.org/10.1016/j.renene.2020.07.031
Wang F, Ouyang D, Zhou Z et al (2021) Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J Energy Chem 57:247–280. https://doi.org/10.1016/j.jechem.2020.08.060
Ayub A, Hassan M (2021) Enhancing biogas production through co-digestion and thermal pretreatment of wheat straw and sun fl ower meal. Renew Energy 168:1–10. https://doi.org/10.1016/j.renene.2020.11.149
Abraham A, Mathew AK, Park H et al (2020) Bioresource Technology Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour Technol 301:122725. https://doi.org/10.1016/j.biortech.2019.122725
Haldar D, Purkait MK (2021) Chemosphere A review on the environment-friendly emerging techniques for pretreatment of lignocellulosic biomass: mechanistic insight and advancements. Chemosphere 264:128523. https://doi.org/10.1016/j.chemosphere.2020.128523
Fabbri D, Torri C (2016) ScienceDirect Linking pyrolysis and anaerobic digestion (Py-AD) for the conversion of lignocellulosic biomass. Curr Opin Biotechnol 38:167–173. https://doi.org/10.1016/j.copbio.2016.02.004
Anwar Z, Gulfraz M, Irshad M (2014) ScienceDirect Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Atelge MR, Atabani AE, Banu JR et al (2020) A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 270:117494. https://doi.org/10.1016/j.fuel.2020.117494
Kor-bicakci G, Eskicioglu C (2019) Recent developments on thermal municipal sludge pretreatment technologies for enhanced anaerobic digestion. Renew Sustain Energy Rev 110:423–443. https://doi.org/10.1016/j.rser.2019.05.002
Khan MU, Ahring BK (2021) Anaerobic digestion of digested manure fibers: influence of thermal and alkaline thermal pretreatment on the biogas yield. Bioenerg Res 14:891–900. https://doi.org/10.1007/s12155-020-10190-z
Barua VB, Kalamdhad AS (2017) Effect of various types of thermal pretreatment techniques on the hydrolysis, compositional analysis and characterization of water hyacinth. Bioresour Technol 227:147–154. https://doi.org/10.1016/j.biortech.2016.12.036
Kim Y, Hendrickson R, Mosier NS, Ladisch MR (2009) Liquid hot water pretreatment of cellulosic biomass. In: Mielenz JR (ed) Biofuels. Humana Press, Totowa, NJ, pp 93–102
Usmani Z, Sharma M, Tripathi M et al (2023) Converting biowaste streams into energy–leveraging microwave assisted valorization technologies for enhanced conversion. J Energy Inst 107:101161. https://doi.org/10.1016/j.joei.2022.101161
Appels L, Houtmeyers S, Degrève J et al (2013) Bioresource Technology Influence of microwave pre-treatment on sludge solubilization and pilot scale semi-continuous anaerobic digestion. Bioresour Technol 128:598–603. https://doi.org/10.1016/j.biortech.2012.11.007
Veluchamy C, Kalamdhad AS (2017) Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: a review. Bioresour Technol 245:1206–1219. https://doi.org/10.1016/j.biortech.2017.08.179
Wang D, Shen F, Yang G et al (2018) Bioresource Technology Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass ? Bioresour Technol 249:117–124. https://doi.org/10.1016/j.biortech.2017.09.197
Lee J, Park KY (2020) Chemosphere Impact of hydrothermal pretreatment on anaerobic digestion efficiency for lignocellulosic biomass: influence of pretreatment temperature on the formation of biomass-degrading byproducts. Chemosphere 256:127116. https://doi.org/10.1016/j.chemosphere.2020.127116
Chen X, Xiang X, Dai R et al (2017) Bioresource Technology Effect of low temperature of thermal pretreatment on anaerobic digestion of textile dyeing sludge. Bioresour Technol 243:426–432. https://doi.org/10.1016/j.biortech.2017.06.138
Kainthola J, Kalamdhad AS, Goud VV (2020) Optimization of process parameters for accelerated methane yield from anaerobic co-digestion of rice straw and food waste. Renew Energy 149:1352–1359. https://doi.org/10.1016/j.renene.2019.10.124
Kainthola J, Shariq M, Kalamdhad AS, Goud VV (2019) Enhanced methane potential of rice straw with microwave assisted pretreatment and its kinetic analysis. J Environ Manage 232:188–196. https://doi.org/10.1016/j.jenvman.2018.11.052
Veluchamy C, Kalamdhad AS (2017) Enhancement of hydrolysis of lignocellulose waste pulp and paper mill sludge through different heating processes on thermal pretreatment after pretreatment. J Clean Prod 168:219–226. https://doi.org/10.1016/j.jclepro.2017.09.040
Saha B, Khwairakpam M, Kalamdhad AS (2021) Thermal pre-treatment – a prerequisite for the reduction of hydrolysis stage during anaerobic digestion of Ageratum conyzoides. Mater Sci Energy Technol 4:34–45. https://doi.org/10.1016/j.mset.2020.12.002
Bharati V, Kalamdhad AS (2017) Biochemical methane potential test of untreated and hot air oven pretreated water hyacinth: a comparative study. J Clean Prod 166:273–284. https://doi.org/10.1016/j.jclepro.2017.07.231
Jackowiak D, Bassard D, Pauss A, Ribeiro T (2011) Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour Technol 102:6750–6756. https://doi.org/10.1016/j.biortech.2011.03.107
Heerenklage J, Rechtenbach D, Atamaniuk I et al (2019) Development of a method to produce standardised and storable inocula for biomethane potential tests – preliminary steps. Renew Energy 143:753–761. https://doi.org/10.1016/j.renene.2019.05.037
Esposito G, Frunzo L, Panico A, Pirozzi F (2012) Enhanced bio-methane production from co-digestion of different organic wastes. Environ Technol (United Kingdom) 33:2733–2740. https://doi.org/10.1080/09593330.2012.676077
Moset V, Al-zohairi N, Møller HB (2015) The impact of inoculum source, inoculum to substrate ratio and sample preservation on methane potential from different substrates. Biomass Bioenergy 83:474–482. https://doi.org/10.1016/j.biombioe.2015.10.018
Rice EW, Bridgewater L (2012) American public health association (eds) Standard methods for the examination of water and wastewater (Vol. 10). American public health association: Washington, DC
DiLallo R, Albertson OE (1961) Volatile acids by direct titration. J Water Pollut Control Fed 356–365
Salmón I, Cambier N, Luis P (2018) CO2 Capture by alkaline solution for carbonate production: a comparison between a packed column and a membrane contactor. Appl Sci 8:996. https://doi.org/10.3390/app8060996
Veeken A, Hamelers B (1999) Effect of temperature on hydrolysis rates of selected biowaste components. Biol Technol 69(3):249–254
Saha B, Barua VB, Khwairakpam M et al (2023) Thermal pretreatment of Lantana camara for improved biogas production: process parameter studies for energy evaluation. Environ Res 216:114661. https://doi.org/10.1016/j.envres.2022.114661
Singh P, Kalamdhad AS (2022) Assessment of agricultural residue-based electricity production from biogas in India: resource-environment-economic analysis. Sustainable Energy Technol Assess 54:102843. https://doi.org/10.1016/j.seta.2022.102843
Uthirakrishnan U, Godvin Sharmila V, Merrylin J et al (2022) Current advances and future outlook on pretreatment techniques to enhance biosolids disintegration and anaerobic digestion: a critical review. Chemosphere 288:132553. https://doi.org/10.1016/j.chemosphere.2021.132553
Abelleira-pereira JM, Sara IP, Cruz RD et al (2014) ScienceDirect Enhancement of methane production in mesophilic anaerobic digestion of secondary sewage sludge by advanced thermal hydrolysis pretreatment. 1. https://doi.org/10.1016/j.watres.2014.12.027
Garrote GDHP, Dominguez H, Parajó JC (1999) Hydrothermal processing of lignocellulosic materials. Holz als Roh- und Werkstoff 57(3):191–202
Nguyen VK, Kumar D, Hari R et al (2021) Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 285:119105. https://doi.org/10.1016/j.fuel.2020.119105
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577. https://doi.org/10.1128/MMBR.66.3.506-577.2002
Antonopoulou G, Vayenas D, Lyberatos G (2020) Biogas production from physicochemically pretreated grass lawn waste: comparison of different process schemes. Molecules 25:296. https://doi.org/10.3390/molecules25020296
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
Hernández-Beltrán JU, Hernández-De Lira IO, Cruz-Santos MM et al (2019) Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: current state, challenges, and opportunities. Appl Sci 9:3721. https://doi.org/10.3390/app9183721
Zhao L, Sun Z-F, Zhang C-C et al (2022) Advances in pretreatment of lignocellulosic biomass for bioenergy production: challenges and perspectives. Bioresour Technol 343:126123. https://doi.org/10.1016/j.biortech.2021.126123
Başar İA, Eskicioglu C, Perendeci NA (2022) Biochar and wood ash amended anaerobic digestion of hydrothermally pretreated lignocellulosic biomass for biorefinery applications. Waste Manag 154:350–360. https://doi.org/10.1016/j.wasman.2022.10.014
Ahmed B, Aboudi K, Tyagi VK et al (2019) Improvement of anaerobic digestion of lignocellulosic biomass by hydrothermal pretreatment. Appl Sci 9:3853. https://doi.org/10.3390/app9183853
Sethupathy S, Morales GM, Li Y et al (2021) Harnessing microbial wealth for lignocellulose biomass valorization through secretomics: a review. Biotechnol Biofuels 14:154. https://doi.org/10.1186/s13068-021-02006-9
Acknowledgements
The authors thank the Centre for the Environment, Indian Institute of Technology Guwahati, Assam, India, for providing institutional facilities. Also, the Waste Management Research Group (WMRG) people working at the solid waste management laboratory have supported immensely for the smooth conductance of the experimental works.
Availability of data and materials
Any data sets used for the study will be made available on request.
Funding
Funding agency: Department of Science and Technology, Government of India, New Delhi.
Receiver: Prof. Ajay S Kalamdhad, Department of Civil Engineering, IIT Guwahati.
The authors acknowledge the Department of Science and Technology, Government of India, New Delhi, for the financial support (File No.- DST/TDT/WMT/2017/153 (G)).
Author information
Authors and Affiliations
Contributions
Arun Sathyan: conceptualization; data curation; investigation; methodology; resources; visualization; writing—original draft; writing—review and editing
Ajay S. Kalamdhad: conceptualization, data curation, investigation, methodology, project administration, funding acquisition, supervision, review and editing
Sumona S. Koley: data curation, investigation
Meena Khwairakpam: supervision, review and editing
Corresponding author
Ethics declarations
Ethics approval
Not applicable (no human/animal study was included).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 346 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathyan, A., Koley, S., Khwairakpam, M. et al. Effect of thermal pretreatments on biogas production and methane yield from anaerobic digestion of aquatic weed biomass Hydrilla verticillata. Biomass Conv. Bioref. 13, 16273–16284 (2023). https://doi.org/10.1007/s13399-023-04890-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04890-7