Abstract
Silver nanoparticles (AgNPs) are applied in diverse industries due to their biocide and physicochemical properties; therefore, they can be released into aquatic systems, interact with environmental factors, and ultimately exert adverse effects on the biota. We analyzed AgNPs effects on Ceriodaphnia reticulata (Cladocera) through mortality and life-history traits, considering the influence of food (Tetradesmus obliquus, Chlorophyceae) presence and concentration. C. reticulata was exposed to AgNPs in acute (absence and two algae concentrations plus five AgNPs treatments) and chronic assays (two algae concentrations plus three AgNPs treatments). AgNPs did not affect algae flocculation but increased Ag+ release, being these ions less toxic than AgNPs (as proved by the exposure to AgNO3). A reduction in AgNPs acute toxicity was observed when algae concentration increased. Acute AgNP exposure decreased C. reticulata body size and heart rate. The chronic AgNP exposure reduced C. reticulata molt number, growth, heart rate, and neonate size:number ratio, being these effects mitigated at the highest algae concentration. Increases in relative size and number of neonates were observed in AgNP treatments suggesting energy trade off. The increased Ag+ release with food presence suggests that the AgNP-algae interaction might be responsible of the decreased toxicity. Although algae reduced AgNP toxicity, they still exerted adverse effects on C. reticulata below predicted environmental concentrations. Since algae presence reduces AgNP effects but increases Ag+ release, studies should be continued to provide evidence on their toxicity to other organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nanotechnology industry is constantly increasing and diversifying due to its wide potential applications (Jeevanandam et al. 2018; Turan et al. 2019). Silver nanomaterials are one of the most produced due to their biocide capacity and physicochemical properties such as electrical and thermal conductivity, catalytic activity, and surface plasmon resonance (Jones et al. 2018; Ahmad et al. 2020; Corsi et al. 2022). Currently, more than 50% of the commercial products inventoried by the Woodrow Wilson Project on Emerging Nanotechnologies contain silver nanoparticles (AgNPs) (http://www.nanotechproject.org/). AgNPs are applied in several fields such as medicine, cosmetics, food industry, electrical and optical devices, catalysis, textiles, paints, agriculture, and water treatment (Antezana et al. 2021; Islam et al. 2021; Municoy et al. 2021; Zahoor et al. 2021). The extensive application of AgNPs inevitably leads to their release into aquatic environments making it a rising issue of concern for eco-toxicologists (Bour et al. 2015; Skjolding et al. 2016; Zhang et al. 2019; Devasena et al. 2022). Although quantification methods for nanoparticles in the environment are still limited, AgNPs are expected to reach about 8.8 × 10–5 – 10 μg/L on surface water (Nowack and Mueller 2008; Gottschalk et al. 2013; Maurer-Jones et al. 2013).
AgNPs released in aquatic systems could affect aquatic microorganisms such as zooplankton in different ways (Baun et al. 2008; Gutierrez et al. 2021). This community plays a key role as is a direct link between primary producers and consumers and contributes to organic matter and nutrient cycling (Mano and Tanaka 2016). Within zooplankters, micro-crustaceans are particularly sensitive to environmental disturbances as they have small size and short generation times (Resh 2008; Ferdous and Muktadir 2009). AgNPs have been shown to impair freshwater cladoceran survival, growth, and reproduction in a wide range of concentrations from 1 to > 100 µg/L (Gaiser et al. 2011; Völker et al. 2013; Ribeiro et al. 2014). These effects are usually attributed to the “Trojan horse” mechanism as Ag+ released by AgNPs into organism cells cause oxidative stress as reviewed by de Souza et al. 2019, who pointed out the need for developing new AgNPs considering their toxicity and environmental behavior. Nevertheless, these studies mainly focus on Daphnia magna, which do not represent neither the different functional groups of micro-crustaceans (copepods and cladocerans) nor holotropic region scenarios (Gutierrez et al. 2021). In this sense, there is a gap of knowledge as model test species are usually holarctic and little is known about species representatives from other regions. Particularly, Ceriodaphnia reticulata (Jurine) is a planktonic Daphniidae that was recorded in Palearctic, Nearctic, Neotropical, Oriental-Indomalaya, and Afrotropical regions (Kotov and Forró 2019). It inhabits large streams, reservoirs, and lakes and is easily grown under laboratory conditions. It has a short life cycle that allows completing a three-brood life cycle test in seven days (Mount and Norberg 1984). Toxicity tests of different compounds have been reported for C. reticulata (e.g., Jaser et al. 2003; Mangas-Ramirez et al. 2007; Mano et al. 2010); nevertheless, the effects of nanomaterials have not been previously evaluated for this species. In this scenario, there is a need for studies focusing on chronic endpoints over species that inhabit different regions under environmentally relevant AgNP concentrations to understand the population consequences of this kind of pollution (Baun et al. 2008; Gutierrez et al. 2021).
Several physical, chemical, and biological factors in aquatic systems may alter AgNP behavior, in terms of aggregation, chemical speciation, and adsorption (Corsi et al. 2022; Kansara et al. 2022), which in turn, can modify their toxicity to aquatic organisms. Nevertheless, few studies have analyzed the incidence of environmental factors in AgNPs toxicity on zooplankton leading to a gap in the knowledge of potential attenuators (Zhang et al. 2019; Gutierrez et al. 2021). For instance, dissolved organic matter (DOM) such as humic and fulvic acids are known to reduce AgNP toxicity, as DOM can be adsorbed and reduce their dissolution (Wang et al. 2015; Jung et al. 2018; Ale et al. 2021). The presence of algae as food supply has also been proposed to be an attenuator of AgNP toxicity for some cladocerans, but the underlying mechanisms remain under discussion. Ribeiro et al. (2014) attributed this toxicity reduction to the alga-AgNP interaction as the particles can be agglomerated by DOM or adsorbed to the algae, and also algae can uptake Ag+ released by AgNPs. Besides, the attenuation of AgNPs toxicity could be explained due to both interaction alga-AgNPs and better nutritional condition of exposed cladocerans, as in the presence of food organisms may be more tolerant due to their greater energy status compared to standardized bioassays without food (Harmon et al. 2017). Algal exudates and metabolic products (such as oxygen peroxide) could also contribute to the dissolution of AgNPs (Navarro et al. 2015; Sigg and Lindauer 2015; Chen et al. 2019; Ponton et al. 2019), and thus, modify their toxicity.
In this article, we assessed the effects of AgNPs on Ceriodaphnia reticulata (Cladocera) through mortality and life-history traits, considering the influence of food (Tetradesmus obliquus -before Scenedesmus obliquus-, Chlorophyceae) presence and concentration. We expected that AgNPs would be toxic for C. reticulata by affecting their mortality and life-history traits such as growth, reproduction, and heart rate. According to previous findings reported in the bibliography, we hypothesized that these toxic effects would be reduced by algae presence and it would depend on its concentrations.
Materials and methods
Materials and reagents
Silver nanoparticles were obtained from Nanotek S.A. (20—40 nm, colloidal suspension of 1% w/v, nanArgen®, CAS no. 7440–22-4), and the main ingredient of the product was stated as silver (purity ≥ 99.0%). The capping agent was made of glucose oligomers (mainly nanocrystalline cellulose) and the stabilizing agent was made of polyvinyl pyrrolidone (PVP). Silver nitrate was purchased from Tetrahedron® (CAS no. 7761–88-8), purity ≥ 99.0%.
Test organisms
Ceriodaphnia reticulata was collected from a shallow lake of the middle Paraná River flood plain and gradually adapted to laboratory conditions by maintaining it for several months as the initial stock. One parthenogenetic female was isolated from this initial stock and cultured for more than ten consecutive generations in laboratory to prepare the unique strain for the toxicological experiments. C. reticulata was identified under an optical microscope (Nikon E100), and the species was confirmed through observation of post-abdominal claw ornamentations (Rogers et al. 2020). This culture was maintained at 21 ± 1 °C, 12/12 day/night photoperiod, in dechlorinated and aerated tap water (pH: 7.1, conductivity: 1020 µs/cm, total hardness: 180 mg/L CaCO3, alkalinity 120 mg/L CaCO3, 39 mg/L Ca++, 20 mg/L Mg++, and 146 mg/L HCO3−). Culture water was changed three times a week and the organisms were fed with Tetradesmus obliquus every other day.
The pure strain of Tetradesmus obliquus (Turpin) MJ Wynne (before Scenedesmus obliquus) was isolated from a natural shallow lake of the middle Paraná River flood plain. The species was confirmed by Sanger sequencing and defined based on the NCBI database (www.ncbi.nlm.nih.gov) and taxonomic keys (Wynne and Hallan 2015). The axenic culture was growth in Detmer modified medium for green algae (Watanabe 1960) (KCl: 50, KH2PO4: 50, Ca (NO3)2-4H2O: 360, MgSO4-7H2O: 360, Cl3Fe+: 5, C4H6O6: 5, H3BO3, 2.86, MnCl2-4H2O: 1.81, ZnSO4-7H2O: 0.23, Cl2Cu: 0.05 mg/L), at 25 °C with warm-white LED light (50 μmol/m2.s, 2600 lx) and constant aeration. The culture was cropped in the exponential growth phase and algae were resuspended in sterile distilled water and stocked at -4 °C in the dark. Cell concentration was estimated under an optical microscope (Nikon E100) with a Neubauer chamber.
Exposure conditions
Nanoparticle characterization
In order to assess the optical properties of AgNPs, a suspension of the particles was monitored by UV–vis spectroscopy using a Jasco V-730 Spectrophotometer (Jasco Analytica Spain, Madrid, Spain). Fourier transform infrared (FTIR) spectra of AgNPs were obtained over the range of 4000–500 cm−1 using an FTIR-Raman Nicolet iS 50 (Thermo Scientific). A dynamic light scattering (DLS) analysis was performed to calculate the AgNP average hydrodynamic diameter (Z-average, nm) and polydispersity index (PDI, dimensionless) in both ultrapure water and culture water with a detection angle of 173° at 25 °C using a Zetasizer Nano-Zs Laser Light Scattering Instrument (Malvern Instruments, UK). AgNPs were characterized through transmission electron microscopy (TEM) and scanning electron microscopy (SEM) in both ultrapure and culture water. The Ag+ release from AgNPs was followed by analyzing the dissolved fraction during 96 h in culture water with the three algae concentrations employed in the bioassays (A0 = 0, A1 = 10 × 104, and A2 = 50 × 104 cel/mL) and a concentration of 44 µg/L AgNPs. Briefly, 1 mL of the solution was placed in the upper chamber of Vivaspin® 20 centrifugal concentrator (30 kDa molecular weight cutoff, Sartorius Stedim Biotech GmbH, Göttingen, Germany) and was then centrifuged at 5000 rpm for 15 s at 25 °C. The nanoparticles remained in the upper chamber, while the aqueous filtrate contained the dissolved fraction. The concentration of Ag in the filtrate was measured consecutively in time for 96 h by atomic absorption. Cumulative doses were calculated using a standard curve and expressed as a function of time. In all cases, results were expressed as mean ± SD from triplicate experiments.
Algae flocculation test
In order to assess the effect of AgNPs on the algae flocculation, which may have influenced their availability for cladocerans, T. obliquus flocculation was assessed at the same AgNP concentrations tested in the chronic toxicity test (C1, C2, and C3) after 48 h of exposure (chronic assay renewal time). Algae flocculation was determined by measuring the absorbance of the supernatant at 750 nm (Griffiths et al. 2011) both at the beginning and final time following Beuckels et al. (2013):
where OD = optical density of the supernatant (750 nm), t0 = initial time (0 h), and tf = final time (48 h).
Acute toxicity tests
In order to obtain the LC50 of AgNPs on C. reticulata, three acute toxicity tests were developed with different food conditions: without algae (A0) and with two concentrations of T. obliquus: 10 × 104 and 50 × 104 cel/mL (A1 and A2, respectively). These concentrations were based on a preliminary growing and reproduction experiment (Supplementary Material 1) and bibliographic information (Savaş and Erdoğan 2006; Rodgher and Espíndola 2008). The acute toxicity of AgNPs was tested following APHA (2017) guidelines with some modifications by exposing 20 neonates (> 24 h) (four replicates, with five neonates each) during 48 h to five concentrations of AgNPs (shown in Table 1) (dilution factor: 1.3) plus a control for each algae concentration (A0, A1, and A2). AgNP concentrations were chosen based on preliminary exploratory toxicity tests and bibliographic information. Fresh stock solutions (400 µg/L) were prepared in ultrapure water before each bioassay and stored in the dark to prevent any prior transformation (e.g., aggregation, agglomeration, or dissolution). The nominal detected Ag concentration correlated with the product description. We used 50 mL beakers for each replicate with dechlorinated and aerated tap water at 21 ± 1 °C. The acute toxicity tests were performed in darkness to avoid algae growth and AgNP degradation (Li et al. 2013). Mortality, determined as immobility after a gentile stimulus, was assessed at 24 and 48 h of exposure. Conductivity (µs/cm), dissolved oxygen (DO, mg/L), and pH were measured (Hanna multi-parameter portable meter) at the beginning and the end of each bioassay.
In addition to mortality, two other biological variables were measured at 48 h of exposure: body size and heart rate. Body size was measured on three surviving individuals per replicate (when possible) by a micro-scale attached to the microscope eyepiece (Nikon E100) to identify potential effects of the AgNPs on growth. The methodology to assess heart rate (beats per minute -bpm-) was adapted from Baylor (1942). Briefly, three surviving individuals per replicate (when possible) were individually placed on a microscope slide, and most of the water was withdrawn; thereby, the animals were kept in the microscope’s field by the surface tension of the remaining fluid. Organisms were recorded with an iPhone 7 (1080p, 30 fps) mounted on an optical microscope (Nikon E100) for 10 s. For greater precision, each video was cut to a length of 6 s (MKVToolNix 66.0.0), and heartbeats were recorded with a manual counter at a low video speed (0.12x) (Villegas-Navarro et al. 2003; Borase et al. 2019).
Additional experiment with AgNO3
The acute toxicity of AgNO3 was also tested under the three algae concentrations (A0, A1, and A2) (Table 1) to identify the toxicity of dissolved silver ions and elucidate whether the toxicity of AgNPs is due to the Ag+ release or the AgNPs themselves. This experiment was developed as described for AgNPs, but only mortality was assessed.
Chronic toxicity test
Chronic toxicity test followed APHA (2017) guidelines with some modifications. Briefly, C. reticulata < 24 h neonates were exposed to three AgNP concentrations plus a control (C0) for ten days in order to cover three broods of neonates according to the life cycle of C. reticulata (Mount and Norberg 1984). Exposure concentrations were based on the A1 48 h-LC50, thus representing the 10, 50, and 75%: C1, C2, and C3, respectively (Table 1). In each treatment, five replicates were performed. Each replicate corresponded to one neonate, which was placed individually in 50 mL beakers with dechlorinated and aerated tap water. Laboratory conditions were the same as described for the acute toxicity test. Culture water (control and treatments) was completely renewed every 48 h. This methodology was replicated simultaneously with two algae concentrations (A1 and A2) as described for the acute toxicity test.
Every 24 h, molts and neonates were quantified, measured as described for the acute test, and removed. In addition, the adult body size and heart rate were also measured at the end of the experiment, as described before.
Data analysis
The dissolved fraction of AgNPs was compared among each algae concentration (A0, A1, and A2) through analysis of variance (ANOVA, Tukey post-test). The mean flocculation percentages of algae in AgNPs treatments were compared to control through ANOVA (Dunnett post-test) or Kruskal–Wallis test (KW), as appropriate, in both algae concentrations (A1 and A2). Mean physicochemical variables (conductivity, DO, and pH) were compared between treatments by ANOVA (Tukey post-test) and through time by paired t-test.
With the mortality of the acute exposure assay, probit analyses (Finney 1971) were performed to obtain the 24 and 48 h LC50. The mean size and heart rate of treatments in acute assays were compared to control through ANOVA (Dunnett post-test).
The molt sizes of each replicate of the chronic assay were plotted in relation to time, and their linear regression slopes were considered as growth rates. The neonate relative size and number were calculated per each replicate by dividing the mean neonate size and mean brood number by mother size, respectively. The neonate size:number ratio was calculated for each replicate by dividing the mean neonate size by the mean brood number. The mean of these variables from treatments, in addition to molt number and heart rate means, was compared to control through ANOVA (Dunnett post-test).
Data analysis was performed with R Studio (version 1.2.5042), packages “drc” (Ritz et al. 2015), and “rstatix” (Kassambara 2020).
Results
Exposure conditions
Nanoparticle characterization
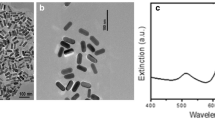
The characteristics of AgNPs were analyzed in ultrapure water through FTIR and UV–visible spectroscopy. FTIR spectra of the AgNPs stabilized with PVP showed a wide band at 3246 cm−1 (H-bonded OH), a peak at 1288 cm−1 (N–OH complex), and a strong peak at 1060 cm−1 (C-N of pyrrolidone) (Wang et al. 2005) (Fig. 1a). The UV–visible absorption spectrum presented the typical surface plasmon of AgNPs, with a maximum peak close to 410 nm (Fig. 1b). The observed asymmetry in the plasmon has been reported by several authors and is due to the presence of nanoparticles that are not spherical, but have triangular or elongated shapes (Tak et al. 2015), as can be seen in TEM and SEM analyses (Fig. 2a and c). TEM analysis showed that the average size of the spherical AgNPs was 24 ± 7 nm, while for the non-spherical shapes, it was 80 ± 13 nm. This correlates with what was observed in the DLS (Table 2 and Fig. 2e).
The AgNPs tended to agglomerate in culture water, but the z-potential remained constant (-13.7), as can be observed in TEM, SEM (Fig. 2b and d), and DLS analyses (Table 2 and Fig. 2f).
The dissolved fraction of AgNPs increased significantly at higher algae concentration (ANOVA F = 59.46 p = 0.004) (Fig. 1c). At 48 h, the mean dissolved fraction was 11.17 ± 0.61% in absence of algae (A0) and increased to 15.95 ± 1.18% (p < 0.05) and 23.35 ± 0.15% (p < 0.01) at A1 and A2 algae concentrations, respectively.
Algae flocculation test
The AgNP treatments did not induce algae flocculation (A1: KW H = 6.83, p = 0.078, A2: ANOVA F = 0.06, p = 0.981). The percentage of algal flocculation was significantly lower in A2 control compared to A1 control (ANOVA F = 14.65, p = 0.009) (Supplementary Material 2).
Physicochemical variables
Physicochemical variables did not vary significantly neither between treatments (ANOVA F = 2.07, 2.78, and 1.09, p = 0.21, 0.14, and 0.39, for conductivity, DO, and pH, respectively) nor through time (paired t-test t = 2.06, 1.27, and 1.51, p = 0.1, 0.33, and 0.19, for conductivity, DO, and pH, respectively); conductivity: 1018 – 1089 µs/cm, DO: 7.7 – 8.6 mg/L, and pH: 7.1 – 7.7.
Acute toxicity tests
A reduction in both AgNPs and AgNO3 acute toxicity on C. reticulata was observed; meanwhile, algae concentration increased. AgNO3 was less toxic than AgNPs in all treatments (Table 3 and Fig. 3a and b).
Dose response curves of acute exposure of C. reticulata to (a) AgNPs and (b) AgNO3. A0: absence of algae, A1: low algae concentration, and A2: high algae concentration. Mean values ± SD of (c) size (µm) and (d) heart rate (bpm) of surviving neonates of the acute toxicity test with AgNPs (48 h). A0: absence of algae, A1: low algae concentration, and A2: high algae concentration. Letters: significative differences between controls, *Significative differences between treatments and control
C. reticulata body size increased significantly in controls with algae (A1 and A2) in comparison with the control without algae (A0) (ANOVA F = 115.04, p < 0.001). The organism size was significantly reduced at higher AgNP concentration (i.e., ≥ 1 µg/L) compared to control in the presence of algae (ANOVA A1: F = 8.8, p = 0.001; A2 F = 7.46, p = 0.002) (Fig. 3c). The heart rate was significantly higher in the controls with algae (A1 and A2) than the control without algae (A0) (ANOVA F = 115.04, p < 0.001). In both treatments with algae (A1 and A2), a significative decrease in heart rate was observed at higher AgNP concentrations compared to control (i.e., ≥ 0.78 and 1 µg/L for A1 and A2, respectively) (ANOVA A1: F = 6.13, p = 0.006; A2 F = 12.67, p < 0.001) (Fig. 3d).
Chronic toxicity test
The growth rate decreased significantly in the lower algae concentration (A1) in the three AgNP treatments compared to control (ANOVA F = 7.34, p = 0.004) (Fig. 4a). Also, a smaller number of molts were observed at 0.07 and 0.56 µg/L AgNPs compared to control in the lower algae concentration (A1) (ANOVA F = 3.83, p = 0.032) (Fig. 4b). Under the higher algae concentration (A2), a significant increase in growth rate was observed at 0.07 and 0.56 µg/L AgNPs compared to control (ANOVA F = 21.46, p < 0.001) (Fig. 4a).
Chronic toxicity test with AgNPs results: C. reticulata: (a) growth rate (µm/day), (b) molt number, (c) heart rate (bpm), (d) neonate relative size (dimensionless: mean neonate size µm/mother size µm), (e) neonate relative number (dimensionless: mean brood number/mother size µm), and (f) neonate size:number ratio (dimensionless: mean neonate size µm /mean brood number)
At the end of the experiment, the heart rate of adults was significantly lower in the three AgNP treatments compared to control in the case of the lower algae concentration (A1) (ANOVA F = 10.29, p = 0.001). Under the higher algae concentration (A2), no significative effects of the AgNP treatments were observed (ANOVA F = 2.44, p = 0.111) (Fig. 4c).
The neonate relative size significantly increased in the three AgNP treatments compared to control at the lower algae concentration (A1) (ANOVA F = 7.98, p = 0.002). In the case of the higher algae concentration (A2), this relation was significantly higher at 0.37 and 0.56 µg/L AgNPs than control (ANOVA F = 5.06, p = 0.013) (Fig. 4 d). Also, under both algae concentrations (A1 and A2), the neonate relative number was significantly higher at 0.37 and 0.56 µg/L AgNPs compared to control (ANOVA A1: F = 10.58, p = 0.001, A2: F = 13.78, p < 0.001) (Fig. 4e).
The neonate size:number ratio was lower at the higher algae concentration (A2) control (i.e., many smaller neonates) compared to the lower algae concentration (A1) control (ANOVA F = 12.93, p = 0.016). In A1, a significant increase in this relation was observed at 0.07 µg/L (i.e., fewer bigger neonates) compared to control, while at 0.37 and 0.56 µg/L AgNPs, this relation decreased significantly (i.e., many smaller neonates) (ANOVA F = 27.76, p < 0.001). In A2, no significative effects of the AgNPs treatments were observed (ANOVA F = 1, p = 0.421) (Fig. 4f).
Discussion
Exposure conditions
Nanoparticle characterization
Although no agglomeration was observed in ultrapure water, AgNPs tended to form medium-sized agglomerates in the culture water. This may be a consequence of the interaction with the salts present in the media, as observed in numerous studies (Griffitt et al. 2008; Li et al. 2013; Borase et al. 2019).
Several authors reported that algae might induce AgNP dissolution by increasing Ag+ release, given that algae exudates and reactive oxygen species (ROS) metabolites—such as hydrogen peroxide—can destabilize AgNPs (Navarro et al. 2015; Sigg and Lindauer 2015; Chen et al. 2019; Ponton et al. 2019). This process might occur in the algae cell boundary layer since, in general, algal wall pores have an average diameter of 5—20 nm; therefore, only smaller nanoparticles could enter cells (Chen et al. 2019). Moreover, Tetradesmus obliquus has the typical cell wall of the Chlorococcales, which is more resistant due to the trilaminar structure (Burczyk 1973; Allard and Templier 2001), which would make it even more difficult for the AgNPs to enter the cells. Thus, in our case, it is also likely that the mechanism of Ag+ release occurred in the algae wall.
Algae flocculation test
AgNPs did not affect algae flocculation in the chronic exposure concentrations used in the present study (up to 0.56 µg/L). Therefore, algae were equally available for Ceriodaphnia reticulata in treatments and control. This agrees with the toxicological data on the effect of AgNPs on T. obliquus, as the reported EC50 growth inhibition concentrations are several orders of magnitude higher (between 38.5 and 1000 µg/L (Zouzelka et al. 2016; Pham 2018).
Acute toxicity tests
The AgNPs were highly toxic for C. reticulata even under the presence of food (LC50 0.44 – 1.1 µg/L). Although the AgNP toxicity has not been previously evaluated for this species, the LC50 values found in the present study are among the lowest toxicity values reported for C. dubia: LC50 between 0.15 and 67 µg/L (Griffitt et al. 2008; McLaughlin and Bonzongo 2012; Angel et al. 2013; Kennedy et al. 2015; Harmon et al. 2017) and Daphnia spp.: LC50 between 0.26 to 30 µg/L (Hoheisel et al. 2012; Zhao and Wang 2012; Silva et al. 2014; Becaro et al. 2015; Assis da Silva et al. 2022). Moreover, the LC50 informed in the present study is below the estimated exposure scenarios for freshwater bodies (8.8 × 10–5 – 10 μg/L) (Nowack and Mueller 2008; Gottschalk et al. 2013; Maurer-Jones et al. 2013), which may imply a high environmental risk for cladocerans. The AgNPs imposed higher toxicity than AgNO3 for C. reticulata; therefore, the nanoparticles themselves showed to be more toxic than the Ag+ ions they release. This constitutes a relevant result as is opposite to previous studies (e.g., Angel et al. 2013; Hu et al. 2017; Ribeiro et al. 2014; Zhao and Wang 2011), which tend to subordinate the toxicity of AgNPs to the current regulation for silver and may underestimate the toxic effects and the different mechanisms of action of nanoparticles. The higher toxicity of AgNPs could have been due to different aspects related to their nanometric properties. Several studies reported that their greater reactivity could promote their permeability through biological membranes (McShan et al. 2014), and then, AgNPs can release Ag+ into organism cells, generating oxidative stress, a process called “Trojan horse” mechanism (Ulm et al. 2015; de Souza et al. 2019; Galhano et al. 2022). Also, several reports showed that AgNPs can impose mechanical effects such obstruction of filter setae and digestive system and adhesion to antennas and carapace (Zhao and Wang 2010; Asghari et al. 2012).
The lethal toxicity of AgNPs in C. reticulata decreased when algae concentration increased. Some authors have attributed this toxicity reduction to two main factors: better nutritional condition and the interaction algae-AgNPs (Allen et al. 2010; Ribeiro et al. 2014; Harmon et al. 2017). In this sense, Conine and Frost (2017) tested the AgNP toxicity under the presence of T. obliquus with different phosphorus content and concluded that the decrease of AgNP toxicity was primarily due to the algae binding and uptake abilities and, to a lesser extent, to their effects on Daphnia magna nutrition. Although both factors (nutritional condition and AgNPs-algae interaction) can act simultaneously, the observed increase in Ag+ release in relation to the algae concentration in the present study indicated that algae actually interacted with the AgNPs. Therefore, as AgNPs were more toxic than Ag+ for C. reticulata, the AgNP-algae interaction could have been one of the main factors conditioning the particle toxicity.
The observed increase in C. reticulata size in relation to food concentration in controls was expectable due to the increase in energy supply (Savaş and Erdoğan 2006). However, AgNP concentrations ≥ 1 µg/L caused development inhibition (i.e., decreased final size) together with a decreased heart rate. As AgNPs did not affect algal flocculation, food availability for C. reticulata was not affected in treatments. Nevertheless, several studies reported that algae can uptake Ag+ or adsorb AgNPs; therefore, they may have less nutritional quality and constitute another Ag uptake route for cladocerans, which could have affected their metabolism and growth (Zhao and Wang 2010; Yoo-iam et al. 2014; Wang et al. 2019a; Dang et al. 2021). In addition, the potential mechanical effects of AgNPs on micro-crustaceans previously mentioned (obstruction of filter setae and digestive system, and adhesion to antennas and carapace) can affect their feeding and locomotion (Zhao and Wang 2010; Asghari et al. 2012).
Chronic toxicity test
In the case of low algae concentration, C. reticulata heart rate, number of molts, and growth rate were lower in all AgNP treatments. However, these effects were not observed at the highest algae concentration. Furthermore, an opposite trend was observed as the growth rate increased in the highest algae concentration treatments compared to control; thus, the underlying mechanisms of this trend remain unclear and need to be further explored. Our findings clearly show a mitigation on AgNP toxicity when the algae concentration increased, which, as discussed above, might be due to both nutritional condition and algae-AgNP interaction (Harmon et al. 2017). Concomitantly, Ribeiro et al. (2014) reported no effects of AgNPs on D. magna growth exposed to up to 5 µg/L AgNPs (21 d). Conversely, Zhao and Wang (2011) registered an inhibition of D. magna growth when exposed to higher AgNP concentrations (over 5 µg/L, 21 d) and attributed these results to a poor food quality because algae can adsorb the particles or uptake Ag+ ions (Wang et al. 2019b; Dang et al. 2021).
Cladocera heart rate is considered a sensitive physiological indicator (Villegas-Navarro et al. 2003; Corotto et al. 2010), and recent studies showed negative effects of AgNPs on this variable. For instance, Borase et al. (2019) reported that Moina macrocopa heart rate decreased when exposed for 15 min to a high concentration (500 µg/L) of AgNPs. Park et al. (2022) found a significant decrease in D. magna heart rate after a 3 h exposure to a lower concentration (10 µg/L) of AgNPs.
Regarding reproductive parameters, an increase in neonate relative size and number in relation to the mother size was observed in AgNP treatments under both low and high algae concentrations. These results may indicate that C. reticulata has suffered energy allocation compromises, investing its energy in reproduction instead of growth. In accordance, Li et al. (2011) reported energy allocation compromises in C. dubia exposed to titanium oxide and aluminum oxide nanoparticles due to a reduction in energy assimilation. Also, Sun et al. (2022) found that zinc oxide nanoparticles affect energy allocation on reproduction and growth of D. magna under different food (Chlorella pyrenoidosa) concentrations.
In the present study, energy allocation compromises were also observed in the offspring characteristics. At low algae concentration, the neonate size:number ratio was variable depending on AgNP concentration: at 0.075 µg/L AgNPs, fewer bigger neonates were observed compared to control, whereas the opposite was registered at concentrations ≥ 0.373 µg/L AgNPs. At high algae concentration, this ratio was lower (i.e., many smaller neonates) but AgNPs did not change this pattern, indicating that this effect was mitigated by the algae. Several studies reported a decrease in D. magna offspring when exposed to AgNPs concentrations ≥ 1 µg/L (Ribeiro et al. 2014) and 50 µg/L (Zhao and Wang 2011). Those effects were attributed to reduced food consumption and nutrient absorption (Park et al. 2021).
Conclusions
AgNPs negatively affected C. reticulata life-history traits, including mortality, growth, reproduction, and heart rate. The presence of algae mitigated most of these negative effects in a concentration-dependent manner. Nevertheless, even in presence of algae, negative effects of AgNPs on C. reticulata were imposed below the predicted environmental concentrations. Our study demonstrated that algae promote the release of Ag+ from AgNPs, which could negatively affect other organisms and ecological processes. This study highlights the importance of assessing realistic exposure scenarios considering potential environmental effects on AgNP behavior and toxicity.
Data availability
Data associated to this document are available from the corresponding author (vandrade@inali.unl.edu.ar).
References
Ahmad SA, Das SS, Khatoon A et al (2020) Bactericidal activity of silver nanoparticles: a mechanistic review. Mater Sci Energy Technol 3:756–769. https://doi.org/10.1016/j.mset.2020.09.002
Ale A, Galdopórpora JM, Mora MC, et al (2021) Mitigation of silver nanoparticle toxicity by humic acids in gills of Piaractus mesopotamicus fish. Environ Sci Pollut Res 1https://doi.org/10.1007/s11356-021-12590-w
Allard B, Templier J (2001) High molecular weight lipids from the trilaminar outer wall ( TLS ) -containing microalgae Chlorella emersonii, Scenedesmus communis and Tetraedron minimum. Phytochemistry 57:459–467. https://doi.org/10.1016/S0031-9422(01)00071-1
Allen HJ, Impellitteri CA, Macke DA et al (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750. https://doi.org/10.1002/etc.329
Angel BM, Batley GE, Jarolimek CV, Rogers NJ (2013) The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 93:359–365. https://doi.org/10.1016/j.chemosphere.2013.04.096
Antezana PE, Municoy S, Pérez CJ, Desimone MF (2021) Collagen hydrogels loaded with silver nanoparticles and Cannabis sativa oil. Antibiotics 10https://doi.org/10.3390/antibiotics10111420
APHA (2017) Baird RB, Eaton AD, Rice EW (eds) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, American Water Works Federation. Water Environment Association, Washington, D.C.
Asghari S, Johari SA, Lee JH et al (2012) Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J Nanobiotechnology 10:1–11. https://doi.org/10.1186/1477-3155-10-14
Assis da Silva C, Ribeiro BM, do Trotta CV et al (2022) Effects of mycogenic silver nanoparticles on organisms of different trophic levels. Chemosphere 308:136540. https://doi.org/10.1016/j.chemosphere.2022.136540
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17:387–395. https://doi.org/10.1007/s10646-008-0208-y
Baylor ER (1942) Cardiac pharmacology of the cladoceran, Daphnia Author ( s ): E. R. Baylor Published by : Marine Biological Laboratory. Biol Bull 83:165–172. https://doi.org/10.2307/1538141
Becaro AA, Jonsson CM, Puti FC et al (2015) Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ Nanotechnol Monit Manag 3:22–29. https://doi.org/10.1016/j.enmm.2014.11.002
Beuckels A, Depraetere O, Vandamme D et al (2013) Influence of organic matter on flocculation of Chlorella vulgaris by calcium phosphate precipitation. Biomass Bioenerg 54:107–114. https://doi.org/10.1016/j.biombioe.2013.03.027
Borase HP, Patil SV, Singhal RS (2019) Moina macrocopa as a non-target aquatic organism for assessment of ecotoxicity of silver nanoparticles: effect of size. Chemosphere 219:713–723. https://doi.org/10.1016/j.chemosphere.2018.12.031
Bour A, Mouchet F, Silvestre J et al (2015) Environmentally relevant approaches to assess nanoparticles ecotoxicity: a review. J Hazard Mater 283:764–777. https://doi.org/10.1016/j.jhazmat.2014.10.021
Burczyk J (1973) The chemical composition of the cell wall of Scenedesmus obliquus. I. General Chemical Characteristics. Folia Histochem Cytochem (krakow) 11:119–133
Chen F, Xiao Z, Yue L et al (2019) Algae response to engineered nanoparticles: current understanding, mechanisms and implications. Environ Sci Nano 6:1026–1042. https://doi.org/10.1039/c8en01368c
Conine AL, Frost PC (2017) Variable toxicity of silver nanoparticles to Daphnia magna: effects of algal particles and animal nutrition. Ecotoxicology 26:118–126. https://doi.org/10.1007/s10646-016-1747-2
Corotto F, Ceballos D, Lee A, Vinson L (2010) Making the most of the daphnia heart rate lab: optimizing the use of ethanol, nicotine & caffeine. Am Biol Teach 72:176–179. https://doi.org/10.1525/abt.2010.72.3.9
Corsi I, Desimone MF, Cazenave J (2022) Building the bridge from aquatic nanotoxicology to safety by design silver nanoparticles. Front Bioeng Biotechnol 10:1–28. https://doi.org/10.3389/fbioe.2022.836742
Dang F, Huang Y, Wang Y et al (2021) Transfer and toxicity of silver nanoparticles in the food chain. Environ Sci Nano 8:1519–1535. https://doi.org/10.1039/d0en01190h
de Souza TAJ, Souza LRR, Franchi LP (2019) Ecotoxicology and environmental safety silver nanoparticles : an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Saf 171:691–700. https://doi.org/10.1016/j.ecoenv.2018.12.095
Devasena T, Iffath B, Renjith Kumar R, et al (2022) Insights on the dynamics and toxicity of nanoparticles in environmental matrices. Bioinorg Chem Appl 2022https://doi.org/10.1155/2022/4348149
Ferdous Z, Muktadir A (2009) A review : potentiality of zooplankton as bioindicator. Am J Appl Sci 6:1815–1819
Finney D (1971) Probit analysis. Cambridge University Press, London, U.K.
Gaiser BK, Biswas A, Rosenkranz P et al (2011) Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J Environ Monit 13:1227–1235. https://doi.org/10.1039/c1em10060b
Galhano V, Zeumer R, Monteiro MS et al (2022) Effects of wastewater-spiked nanoparticles of silver and titanium dioxide on survival, growth, reproduction and biochemical markers of Daphnia magna. Sci Total Environ 839:156079. https://doi.org/10.1016/j.scitotenv.2022.156079
Gottschalk F, Sun T, Nowack B (2013) Environmental concentrations of engineered nanomaterials : review of modeling and analytical studies. Environ Pollut 181:287–300. https://doi.org/10.1016/j.envpol.2013.06.003
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85:119–123. https://doi.org/10.1016/j.mimet.2011.02.005
Griffitt RJ, Luo J, Gao J et al (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978. https://doi.org/10.1897/08-002.1
Gutierrez MF, Ale A, Andrade V et al (2021) Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: an update and basis for the use of new test species. Water Environ Res 93:2505–2526. https://doi.org/10.1002/wer.1637
Harmon AR, Kennedy AJ, Laird JG et al (2017) Comparison of acute to chronic ratios between silver and gold nanoparticles, using Ceriodaphnia dubia. Nanotoxicology 11:1127–1139. https://doi.org/10.1080/17435390.2017.1399219
Hoheisel SM, Diamond S, Mount D (2012) Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas. Environ Toxicol Chem 31:2557–2563. https://doi.org/10.1002/etc.1978
Hu Y, Chen X, Yang K, Lin D (2017) Distinct toxicity of silver nanoparticles and silver nitrate to Daphnia magna in M4 medium and surface water. Sci Total Environ 618:838–846. https://doi.org/10.1016/j.scitotenv.2017.08.222
Islam MA, Jacob MV, Antunes E (2021) A critical review on silver nanoparticles: from synthesis and applications to its mitigation through low-cost adsorption by biochar. J Environ Manage 281:111918. https://doi.org/10.1016/j.jenvman.2020.111918
Jaser W, Severin GF, Jütting U, Jüttner I, Schramm KW, Kettrup A (2003) Effects of 17α-ethinylestradiol on the reproduction of the cladoceran species Ceriodaphnia reticulata and Sida crystallina. Environ Int 28(7):633–638. https://doi.org/10.1016/S0160-4120(02)00101-0
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Jones RS, Draheim RR, Roldo M (2018) Silver nanowires: synthesis, antibacterial activity and biomedical applications. Appl Sci 8https://doi.org/10.3390/app8050673
Jung Y, Metreveli G, Park CB et al (2018) Implications of pony lake fulvic acid for the aggregation and dissolution of oppositely charged surface-coated silver nanoparticles and their ecotoxicological effects on Daphnia magna. Environ Sci Technol 52:436–445. https://doi.org/10.1021/acs.est.7b04635
Kansara K, Bolan S, Radhakrishnan D et al (2022) A critical review on the role of abiotic factors on the transformation, environmental identity and toxicity of engineered nanomaterials in aquatic environment. Environ Pollut 296:118726. https://doi.org/10.1016/j.envpol.2021.118726
Kassambara A (2020) rstatix: pipe-friendly framework for basic statistical tests. R Packag version 060
Kennedy AJ, Hull MS, Diamond S et al (2015) Gaining a critical mass: a dose metric conversion case study using silver nanoparticles. Environ Sci Technol 49:12490–12499. https://doi.org/10.1021/acs.est.5b03291
Kotov A, Forró L (2019) World checklist of freshwater Cladocera species in the Catalogue of Life
Li M, Czymmek KJ, Huang CP (2011) Responses of Ceriodaphnia dubia to TiO2 and Al2O3 nanoparticles: a dynamic nano-toxicity assessment of energy budget distribution. J Hazard Mater 187:502–508. https://doi.org/10.1016/j.jhazmat.2011.01.061
Li Y, Zhang W, Niu J, Chen Y (2013) Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ Sci Technol 47:10293–10301. https://doi.org/10.1021/es400945v
Mangas-Ramirez E, Sanchez MM, Garcia-Martinez YG, Rodriguez OA, Espinoza SG, Luna-Ramirez R, Molina HA (2007) Effect of benomile fungicide in the demographic parameters of Ceriodaphnia reticulata Jurine, 1820 (Crustacea: Cladocera). J Environ Sci Health, Part A 42(10):1461–1466. https://doi.org/10.1080/10934520701480870
Mano H, Tanaka Y (2016) Mechanisms of compensatory dynamics in zooplankton and maintenance of food chain efficiency under toxicant stress. Ecotoxicology 25:399–411. https://doi.org/10.1007/s10646-015-1598-2
Mano H, Sakamoto M, Tanaka Y (2010) A comparative study of insecticide toxicity among seven cladoceran species. Ecotoxicology 19(8):1620–1625. https://doi.org/10.1007/s10646-010-0547-3
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanoparticles in the environment. Anal Chem 85:3036–3049. https://doi.org/10.1021/ac303636s
McLaughlin J, Bonzongo JCJ (2012) Effects of natural water chemistry on nanosilver behavior and toxicity to Ceriodaphnia dubia and Pseudokirchneriella subcapitata. Environ Toxicol Chem 31:168–175. https://doi.org/10.1002/etc.720
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22:116–127. https://doi.org/10.1016/j.jfda.2014.01.010
Mount DI, Norberg UT (1984) A seven-day life cycle cladoceran toxicity test. Environ Toxicol Chem: an Int J 3(3):425–434. https://doi.org/10.1002/etc.5620030307
Municoy S, Antezana PE, Pérez CJ et al (2021) Tuning the antimicrobial activity of collagen biomaterials through a liposomal approach. J Appl Polym Sci 138:1–13. https://doi.org/10.1002/app.50330
Navarro E, Wagner B, Odzak N et al (2015) Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ Sci Technol 49:8041–8047. https://doi.org/10.1021/acs.est.5b01089
Nowack B, Mueller NC (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453. https://doi.org/10.1021/es7029637
Park HS, Behzadi Tayemeh M, Yu IJ, Johari SA (2021) Evaluation of silver nanowires (AgNWs) toxicity on reproductive success of Daphnia magna over two generations and their teratogenic effect on embryonic development. J Hazard Mater 412:1–9. https://doi.org/10.1016/j.jhazmat.2021.125339
Park J, Park C, Lee Y et al (2022) Acute adverse effects of metallic nanomaterials on cardiac and behavioral changes in Daphnia magna. Environ - MDPI 9:1–12. https://doi.org/10.3390/environments9020026
Pham T (2018) Toxicity of silver nanoparticles to tropical microalgae Scenedesmus acuminatus, Chaetoceros gracilis and Crustacean Daphnia lumholtzi. Turkish J Fish Aquat Sci 19:1009–1016. https://doi.org/10.4194/1303-2712-v19_12_03
Ponton DE, Croteau MN, Luoma SN et al (2019) Three-layered silver nanoparticles to trace dissolution and association to a green alga. Nanotoxicology 13:1149–1160. https://doi.org/10.1080/17435390.2019.1640912
Resh VH (2008) Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ Monit Assess 138:131–138. https://doi.org/10.1007/s10661-007-9749-4
Ribeiro F, Gallego-Urrea JA, Jurkschat K et al (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466–467:232–241. https://doi.org/10.1016/j.scitotenv.2013.06.101
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS One 10:1–13. https://doi.org/10.1371/journal.pone.0146021
Rodgher S, Espíndola ELG (2008) The influence of algal densities on the toxicity of chromium for Ceriodaphnia dubia Richard (Cladocera, Crustacea). Brazilian J Biol 68:341–348. https://doi.org/10.1590/S1519-69842008000200015
Rogers DC, Cohen RG, Hann BJ (2020) Class Branchiopoda. In Thorp and Covich's Freshwater Invertebrates. Academic Press, pp 585–630
Savaş S, Erdoğan Ö (2006) The effect of food (Scenedesmus acuminatus (von Lagerheim ) R. H. Chodat ) densities and temperature on the population growth of the cladoceran Ceriodaphnia quadrangula (O. F. Muller, 1785). J Fish Aquat Sci 23:113–116
Sigg L, Lindauer U (2015) Silver nanoparticle dissolution in the presence of ligands and of hydrogen peroxide. Environ Pollut 206:582–587. https://doi.org/10.1016/j.envpol.2015.08.017
Silva T, Pokhrel LR, Dubey B et al (2014) Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ 468–469:968–976. https://doi.org/10.1016/j.scitotenv.2013.09.006
Skjolding LM, Sørensen SN, Hartmann NB et al (2016) Aquatic ecotoxicity testing of nanoparticles—the quest to disclose nanoparticle effects. Angew Chemie - Int Ed 55:15224–15239. https://doi.org/10.1002/anie.201604964
Sun Y, Liu Q, Huang J et al (2022) Food abundance mediates the harmful effects of ZnO nanoparticles on development and early reproductive performance of Daphnia magna. Ecotoxicol Environ Saf 236:113475. https://doi.org/10.1016/j.ecoenv.2022.113475
Tak YK, Pal S, Naoghare PK et al (2015) Shape-dependent skin penetration of silver nanoparticles: does it really matter? Sci Rep 5:1–11. https://doi.org/10.1038/srep16908
Turan NB, Erkan HS, Engin GO, Bilgili MS (2019) Nanoparticles in the aquatic environment: usage, properties, transformation and toxicity—a review. Process Saf Environ Prot 130:238–249. https://doi.org/10.1016/j.psep.2019.08.014
Ulm L, Krivohlavek A, Jurašin D et al (2015) Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver nanoparticles. Environ Sci Pollut Res 22:19990–19999. https://doi.org/10.1007/s11356-015-5201-4
Villegas-Navarro A, Rosas-L E, Reyes JL (2003) The heart of Daphnia magna: effects of four cardioactive drugs. Comp Biochem Physiol - C Toxicol Pharmacol 136:127–134. https://doi.org/10.1016/S1532-0456(03)00172-8
Völker C, Boedicker C, Daubenthaler J, et al (2013) Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS One 8https://doi.org/10.1371/journal.pone.0075026
Wang H, Qiao X, Chen J et al (2005) Mechanisms of PVP in the preparation of silver nanoparticles. Mater Chem Phys 94:449–453. https://doi.org/10.1016/j.matchemphys.2005.05.005
Wang Z, Quik JT, Song L et al (2015) Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ Toxicol Chem 34:1239–1245. https://doi.org/10.1002/etc.2936
Wang F, Guan W, Xu L et al (2019a) Effects of nanoparticles on algae: adsorption, distribution, ecotoxicity and fate. Appl Sci 9:1–15. https://doi.org/10.3390/app9081534
Wang P, Zhang B, Zhang H et al (2019b) Metabolites change of Scenedesmus obliquus exerted by AgNPs. J Environ Sci 76:310–318. https://doi.org/10.1016/j.jes.2018.05.017
Watanabe A (1960) List of algal strains in collection at the Institute of Applied Microbiology, University of Tokyo. J Gen Appl Microbiol 6:283–292. https://doi.org/10.2323/jgam.6.283
Wynne MJ, Hallan JK (2015) Reinstatement of Tetradesmus GM Smith (Sphaeropleales, Chlorophyta). Feddes Repertorium 126(3–4):83–86. https://doi.org/10.1002/fedr.201500021
Yoo-iam M, Chaichana R, Satapanajaru T (2014) Toxicity, bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem Speciat Bioavailab 26:257–265. https://doi.org/10.3184/095422914X14144332205573
Zahoor M, Nazir N, Iftikhar M et al (2021) A review on silver nanoparticles: classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 13:2216. https://doi.org/10.3390/w13162216
Zhang W, Ke S, Sun C et al (2019) Fate and toxicity of silver nanoparticles in freshwater from laboratory to realistic environments: a review. Environ Sci Pollut Res 26:7390–7404. https://doi.org/10.1007/s11356-019-04150-0
Zhao CM, Wang WX (2010) Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ Sci Technol 44:7699–7704. https://doi.org/10.1021/es101484s
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892. https://doi.org/10.1002/etc.451
Zhao CM, Wang WX (2012) Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 6:361–370. https://doi.org/10.3109/17435390.2011.579632
Zouzelka R, Cihakova P, Rihova Ambrozova J, Rathousky J (2016) Combined biocidal action of silver nanoparticles and ions against Chlorococcales (Scenedesmus quadricauda, Chlorella vulgaris) and filamentous algae (Klebsormidium sp.). Environ Sci Pollut Res 23:8317–8326. https://doi.org/10.1007/s11356-016-6361-6
Acknowledgements
We thank Andrea S. Rossi and Alicia E. Loteste (INALI-CONICET-UNL) for their collaboration and technical support.
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Técnica (grant number PICT 2018–01271, PI: Jimena Cazenave; and grant number PICT-2020–01206, PI: Analía Ale).
Author information
Authors and Affiliations
Contributions
Victoria S. Andrade: conceptualization, formal analysis, investigation, methodology, visualization, and writing—original draft. Analía Ale: investigation, funding acquisition, methodology, and writing—review and editing. Pablo E. Antezana: formal analysis, investigation, and writing—review and editing. Martín F. Desimone: methodology, resources, and writing—review and editing. Jimena Cazenave: conceptualization, funding acquisition, methodology, project administration, resources, and writing—review and editing. María F. Gutierrez: conceptualization, methodology, project administration, resources, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrade, V.S., Ale, A., Antezana, P.E. et al. Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ Sci Pollut Res 30, 27137–27149 (2023). https://doi.org/10.1007/s11356-022-24154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24154-7