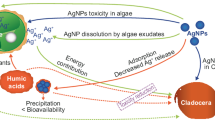
Abstract
Aquatic environments vary widely in aspects other than their physicochemical properties that could alter the toxicity of novel contaminants. One factor that could affect chemical toxicity to aquatic consumers is their nutritional environment as it can strongly affect their physiology and life history. Nutrition has the potential to alter an organism’s response to the toxin or how the toxin interacts with the consumer through its food. Here we determined how growth and survival responses of Daphnia to an emerging contaminant, silver nanoparticles (AgNPs), are affected by the presence of food and its stoichiometric food quality. We used a series of survival tests, each slightly modified, to determine whether variable toxicity in different nutritional environments resulted from algal sequestration of AgNPs in a nontoxic form or from changes to the nutritional status of the test animals. We found that the presence of algae, of good or poor quality, reduced the toxicity of AgNPs on animal growth and survival. However, the decrease in AgNP toxicity was greater for animals consuming P-rich compared to P-poor food. We found evidence that this effect of food quality was due to greater algal uptake of AgNPs by P-rich than by P-stressed algae. However, we also found animal nutrition, in the absence of algal AgNP binding, could affect toxicity with P-nourished animals surviving slightly better when exposed to AgNPs compared to their P-stressed counterparts. Our results show an important role for algal particles and their P content in determining the toxicity of AgNPs in natural waters primarily due to their binding and uptake abilities and, less so, to their effects on animal nutrition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silver nanoparticles (AgNPs) are a class of an emerging contaminants that are used as an antimicrobial agent in commercial products and have recently received much study to determine how toxic they are to aquatic consumers. While AgNP toxicity has been found for many types of organisms (e.g., bacteria, algae, and fish), filter feeding invertebrates such as Daphnia are among the most sensitive (Griffitt et al. 2008; Das et al. 2012b, 2014). However, the concentration of AgNPs needed to cause median lethal effects (LC50) of Daphnia is quite variable as it ranges from ng L−1 to mg L−1 (Kennedy et al. 2010; Pokhrel et al. 2013). This variation may partly result from different types of AgNPs, which vary in size and capping agents; (Hoheisel et al. 2012; Blinova et al. 2013). Even identical AgNPs can have very different toxicity. Environmental factors such as dissolved organic carbon (Kennedy et al. 2012), solar radiation (Shi et al. 2012), dissolved oxygen (Xiu et al. 2011), pH (Stebounova et al. 2010), and the presence of certain ligands including chlorides, sulfides, and phosphates all alter the toxicity of silver nanoparticles by changing rates of agglomeration and sedimentation (Choi et al. 2009; Xiu et al. 2011). While these factors all can influence the toxicity of AgNPs on zooplankton, another important stressor, the presence and nutritional quality of food sources, such as algae, remains largely under studied.
The presence of algae in AgNP toxicity experiments has been noted to increase animal survival (Allen et al. 2010; Ribeiro et al. 2014), but the mechanism behind this result remains largely unknown. One of the pathways that algae could influence AgNP toxicity is through their uptake and binding of AgNPs. AgNPs may bind directly to the alga’s surface (Oukarroum et al. 2012) or be moved into the cell (Leclerc and Wilkinson 2014). This type of algal mediated AgNP-removal appears to operate in natural lake phytoplankton communities (Das et al. 2014). Regardless of whether this removal is through cell surface attachment or via internal accumulation, the overall concentration of free AgNPs remaining in the water would presumably be reduced, which may lower the toxicity of AgNPs to Daphnia. This removal-dependent reduction in toxicity is contingent on the algal-sequestered AgNPs having less toxicity to the Daphnia (Zhao and Wang 2011).
A different pathway for algal mediated changes in AgNP toxicity is through its nutritional effects on exposed animals. When included in toxicity experiments, food provides energy and materials needed for growth and maintenance metabolism and can strongly affect chemical toxicity (Spadaro et al. 2008; Doke et al. 2014). Algal food can vary substantially in its nutritional quality to aquatic consumers including Daphnia (Frost et al. 2005). In general, nutrient limitation (e.g., low phosphorus supply) reduces food quality of algae by altering their elemental content (Sterner 1993). Nutritional imbalances with their food strongly affect Daphnia performance (e.g., growth rates (Sterner 1993)) and alters its responses to chemical toxins (Hansen et al. 2008). For example, Daphnia consuming P-poor food showed increased survival in the presence of imidicloprid and fluoxetine, and no change in survival in the presence of the pesticide, Round Up (Hansen et al. 2008; Lessard and Frost 2012; Ieromina et al. 2013). One explanation for variable toxicity in differentially nourished Daphnia is that there is less incorporation of the toxin into body tissues when animals are growing at a slower rate. Alternatively, P limitation may increase toxicity in Daphnia because poor nutrition may place limits on animal maintenance and repair metabolism. Algae having different P content could also differentially sequester the contaminant through binding or uptake. Altogether, it seems likely that the toxicity of AgNPs to Daphnia could be altered by the P content of its algal food.
In this study, we examined how algal particles affect the toxicity of AgNPs on the growth and survival of Daphnia magna. To do this, we exposed animal neonates to a range of AgNP concentrations with and without food particles of different P content. We also examined the large scale mechanism of particle-mediated toxicity by determining whether it is caused by AgNPs binding to algae or whether it results from algal effects on the nutritional status of the Daphnia. Our study thus provides a thorough examination of the effects of food and its nutritional effects on AgNP toxicity to an aquatic herbivore.
Materials and methods
Organisms and culture conditions
For all toxicity experiments, we used the zooplankter, Daphnia magna (clone DG-106, originally isolated in Germany). Prior to toxicity experiments, groups of about 10 Daphnia were kept in 250 mL of COMBO media (Kilham et al. 1998) and fed non-limiting quantities of P-rich Scenedesmus obliquus (purchased as S. acutus from University of Toronto Culture Collection Strain 10). Media and food were refreshed and neonates were removed from each jar every other day. On the day of each experiment, neonates less than 24 h old were collected from the jars and used for the experiment. All toxicity experiments were based on EPA guidelines for toxicity testing, adjustments were made to test the algal effects (US Environmental Protection Agency 2002). For example, COMBO media, a media containing essential trace elements, trace metals, essential nutrients with a controlled pH, was used in order to promote growth and health of both algae and Daphnia (Kilham et al. 1998). All other deviations from this protocol are described below.
Scenedesmus obliquus was grown in COMBO media (Kilham et al. 1998) with different media P concentrations to alter the P-content of algal cells. Cultures were held in a growth chamber at 20 °C and on a 16 h:8 h light:dark cycle. On a daily basis, algae were removed and new media was added at a fixed dilution rate. Collected algal suspensions were centrifuged at 5000 rpm for 20 min to separate cells from growth media. The resulting algal pellet was resuspended in P- and Ag-free Daphnia media so that any differences in P in experiments were those associated with the algae. Subsamples of this algal suspension were collected on GFC filters. We measured the P content of these filtered samples using the potassium persulfate digestion molybdate blue colorimetric method (APHA 1992). Based on the P-content of each suspension, we created food mixes that contained a target carbon to phosphorus (C:P) ratios of 100 for P-rich and 700 for P-poor food treatments to represent food found in eutrophic and oligotrophic lakes respectively. Additional samples of the mixed food were collected and C:P ratios was determined after the experiment to verify food conditions provided to experimental animals. For all experiments algae was added to the test system immediately following the creation of AgNP suspensions and Daphnia were added immediately following the algae unless otherwise stated.
Silver nanoparticles
AgNP suspensions were made by diluting a concentrated carboxy-functionalized polyacrylate capped AgNP suspension purchased from ViveNano (Toronto, CA; PN3010L) with Daphnia COMBO media. The stock suspension was shaken for at least 5 min prior to dilution for all experiments. These AgNPs, based on data obtained from the manufacturer, are approximately 10 nm in diameter. ICP-MS measurements, following previously described methods (Das et al. 2012b), found total silver concentrations to be about 20 % of the nominal starting concentration of AgNPs due to the relative mass of the capping agent. Additional characterization including size and shape using DLS and TEM of AgNP stock suspension and ultracentrifugation and flow field fractionation of for dissolved Ag concentrations has also been previously described as this AgNP stock has been used for other studies (Das et al. 2012b; Hoque et al. 2012). Dissolved silver including silver ions and silver salts was always found to be less than 3 % of the total silver with this stock suspension and subsequent dilutions (Das et al. 2012b). Due to the large number of replicate tubes used in all of our experiments, we were did not measure silver concentrations in every replicate. Instead we created a silver gradient using the growth media and the nanosilver stock and measured the Ag concentrations across the nominal concentrations used in our experiment (y = 0.2009x−1.991, r 2 = 0.99 Supplemental Figure 1). We then used this curve like a standard curve to convert nominal concentrations (µg AgNP L−1) to measured concentrations (µg Ag L−1). For this study, we created AgNP concentrations that nominally ranged between 0 and 160 µg AgNP L−1 and were converted using this equation to total silver concentrations (0–32 µg Ag L−1) that are reported through the remainder of the manuscript. As the slope of the curve was 0.2, this conversion is highly consistent with manufacturer claims the AgNPs are 20 % Ag and indicate we produced a AgNP exposure gradient as desired.
Effects of algal presence
In order to confirm the mitigating effect of algal presence on a standard toxicity test system, we performed LC50 tests on Daphnia with and without algae (Supplementary Figure 2). We created seven concentrations of AgNPs in COMBO media across the range of 0 to 20 µg Ag L−1 in two separate experimental runs. For each of the seven concentrations, there was an algae treatment (4 mg C L−1) and a no algae treatment. In these two experimental runs, we exposed 25 neonates held individually in 20 mL of each test solution and survival was monitored for 2 days to determine the probability of survival at each AgNP concentration. As algae are generally found to experience toxic effects above 100 µg Ag L−1,we did not assess direct toxicity to algae and did not consider this possibility as a confounding variable in our study (Griffitt et al. 2008; Baptista et al. 2015).
Effects of algae as a food source on toxicity
We completed a standard 48 h toxicity test using 6 AgNP concentrations (0–20 µg Ag L−1) and 20 individual neonates per treatment. For two hours during each day of this experiment, we transferred Daphnia either into AgNP free media with 4 mg C L−1 algae and allowed them to feed or transferred test animals into AgNP free media with no food. For the other 22 h, animals were exposed to AgNPs in the absence of food particles. This allowed us to compare nourished and starved animals without the confounding interaction of algae and AgNPs. We completed this experiment only once due to the absence of significant differences discussed below.
Effects of algae uptake/binding on toxicity
In this experiment that we repeated twice, we used plastic bags containing 500 mL of seven concentrations of AgNP suspension in COMBO (0–25 µg Ag L−1) to which high P algae were added at a concentration of 4 mg C L−1 (Supplementary Figure 2). Algal assays were incubated with the AgNP suspension at room temperature (20 °C) for six hours in the absence of Daphnia. After this time period, water from each bag was filtered through 0.2 µm polycarbonate filters. This filtrate, containing materials smaller than ~0.2 µm and not attached to algae, was used for subsequent toxicity tests and samples of it were saved and acidified to a final concentration of 4 % HNO3 to measure total silver (TAg). All TAg samples were refrigerated until they were analyzed using ICP-MS following Das et al. (Das et al. 2012b). Two bags with 20 µg Ag L−1 and no algae were also measured in the same way and it was determined that Ag loss due to bags and filtration was less than 2 % of the total silver.
Algae retained on filters during this test were used to assess the toxicity of algal-bound silver. To do so, we resuspended AgNP-exposed algae in AgNP free media at the same concentration as it had been when it was incubating in the bag with AgNPs (4 mg C L−1). We subsequently exposed Daphnia to both the resuspended algae and to the filtrate with individuals placed in 20 mL of each respective test solution for 48 h. Survival was monitored daily to determine how AgNP toxicity was altered by algal uptake and/or binding. Silver was not directly measured in the algal fraction and instead this particle-bound fraction was determined by subtracting the amount remaining in the filtrate from the initial amount added to the system.
Influence of algal P content on toxicity
Survival experiments with different algal food quality were completed where we exposed 25 neonates, held individually, at 7 AgNP concentrations (0–20 µg Ag L−1) in 20 mL of solution. In two repeated experiments, each animal was fed saturating quantities (4 mg C L−1) of either P-rich or P-poor algae. The number of surviving Daphnia in each Ag level was recorded daily for four days after exposure. To measure animal mass specific growth rate (MSGR), we cultured 10 neonates individually in each AgNP suspension with the two different foods as in the survival assays. Additionally, three replicates of 15 to 20 neonates were dried at 60 °C and weighed for an average starting mass. Neonates were allowed to grow for 6 days, and were fed either high P or low P algae every other day with AgNP solution being refreshed on the fourth day. This experiment was repeated twice. After 6 days, each individual animal was dried overnight at 60 °C and weighed. MSGR was calculated using the following equation:
We further determined if differences in toxicity at the two P levels were due to the presence and absence of food, change in the nutritional status of the animal or differences in the algae’s ability to bind AgNPs. The influence of binding was examined using bag experiments, similar to the presence/absence mechanism experiments (section 2.5) except using both high and low P algae. The Daphnia neonates were cultured in the filtrate, which was measured for TAg. We used these data to assess survival against TAg remaining in the water after the incubation instead of the starting concentration added to the system.
To isolate the effect of nutrition in the absence of algal AgNP removal, Daphnia neonates were grown for 4 days prior to exposure to AgNP. Animals were grown on food of high P or low P content to determine if food nutrient content would alter subsequent AgNP toxicity. On day 4, we made seven AgNP suspensions in COMBO media (nominal concentrations 0–20 µg Ag L−1) and 25 daphnids fed each algae type was transferred individually into each of these Ag suspensions. Survival was monitored for 2 days with no food present after the Daphnia were transferred to the AgNP solutions and the experiment was repeated 3 times.
Statistical analysis
Survival data was plotted and LC50 values were calculated using 3 parameter sigmoidal curve fitting. The equation for this sigmoidal curve is:
In this equation, a is the maximum value of the curve, b is minimum value of the curve and X0 is the LC50 value. Logistic regression analysis of survival curves and their 95 % confidence intervals was used to compare differences between treatments. Error bars in the survival curves were calculated as the standard deviation of the binomial probability for each point (Harnett 1982). Growth data was compared using a 2-way ANOVA. All statistical analysis was done using SigmaPlot 12.5 statistical software.
Results and discussion
Presence of algae
When we compared standard toxicity tests with and without food present, we found that animals held without algae experienced greater toxicity from AgNPs than those that included algae (Fig. 1). The LC50 values shifted from 5.2 + /−0.1 µg Ag L−1 with no food present to 17+/−0.4 µg Ag L−1 with algal particles present, which indicates that the presence of algae reduced the toxicity experienced by the Daphnia. Adding algae to the system increased survival of the Daphnia just over three fold, and in the direction of the 8 fold increase observed by Allen et al. (2010) and about 6 fold increase of Ribeiro et al. (2014) when they added food to their experimental systems. Differences between toxicity shifts seen among these studies may be due to differences in the food concentration and species of algae used in each experiment. Nonetheless, the considerable shift in AgNP toxicity seen in these studies has interesting implications for natural ecosystems because the highest estimates of environmental concentrations of AgNPs have been reported to be ~1.3 µg AgNP L−1. These estimates are predicted to increase considerably if the use of AgNPs in consumer products continues to rise as it has over the past few years (Massarsky et al. 2014). The shift of Daphnia-AgNP LC50 values from 5.2 µg Ag L−1 to over 17 µg Ag L−1 by algal particles would greatly reduce the possibility of AgNPs toxicity in coming years in particle-rich, natural environments.
Impact of nourishment on toxicity
To determine whether malnourishment alters Daphnia survival during toxicity tests, we performed a test in which we transferred exposed animals to AgNP free media either with or without food for 2 h each day. There was no difference in the survival of Daphnia that received the supplemental nourishment and those that did not have access to food (LC50 values of 3.9+/−0.1 and 4.5+/−0.2 µg Ag L−1 respectively) when exposed to AgNPs over two days (Fig. 2). These LC50 values matched those found in our first experiment for animals with no access to food. The lack of a difference between the two feeding regimes may be due to the short experimental period (2 days) and/or short feeding duration (2 h day−1), which may have limited the nutritional differences among test animals. In addition, most death in experimental animals exposed to AgNPs typically occurs within the first 24 h of exposure, when nutritional differences would be even more minimal. Nonetheless, this result shows that the presence of animal nourishment alone does not necessarily reduce AgNP toxicity. The considerable reduction in AgNP toxicity created by algal particles thus appears to result from the removal of AgNPs from the water and less so from altered animal nutrition.
LC50 curves of Daphnia exposed to AgNP for 2 days. For 2 h daily, Daphnia were either transferred to AgNP free media with algae to feed (solid line, R 2 = 0.99) or without algae to remain starved (dashed line, R 2 = 0.99). This curve is the result of one experiment, each point represents 25 individual Daphnia
Impact of algal binding on toxicity
We incubated high P algae together with AgNPs for 6 h. Subsequently we separated algal particles from the media and exposed Daphnia to both the filtrate and the resuspended algae that contained AgNPs. We found that algal bound AgNPs were not toxic to Daphnia at any tested concentration (Fig. 3). This result mirrors Zhao and Wang (2011) who used a different type of AgNP, a different species of algae, and tested up to 1 mg Ag L−1. As we did not examine concentrations above 30 µg Ag L−1 which is above environmentally relevant concentrations, we are hesitant to conclude that higher concentrations would also be non-toxic. Nonetheless, our results are strong evidence that algal removal of AgNP either renders it inaccessible and/or chemically modifies it, which thereby reduces its toxicity to Daphnia. This is consistent with recent observations of the green alga, Coccomxya actinobiotis, which has been shown at low AgNP concentrations to internally stabilize silver ions derived from AgNPs with cytosolic S bearing compounds that reduces the toxicity of Ag (Leonardo et al. 2016). At higher Ag concentrations, this alga transports released silver ions to organelles, especially the chloroplasts, where they are reorganized into small, stable nanoparticles that apparently do not interact with other cellular components (Leonardo et al. 2016). It seems likely that these types of chemical modification that detoxify silver in alga cells also render it less biologically active after ingestion by zooplankton consumers, such as Daphnia.
Effect of AgNPs binding to algae after 6 h incubations and then separation by filtration. Daphnia were exposed to the filtrate (solid line, R 2 = 0.96) or the algae in the resuspended retentate (no line) and survival was monitored for 2 days. Due to the lack of death in the algal treatment, we were unable to fit a survival curve to those data. Each data set is the result of two experiments with each point representing 20 individual Daphnia
When the filtrate from the algae-AgNP incubations was analyzed, it was found that algae reduced the concentrations of AgNPs in the test media, but that these remaining AgNPs retained their toxicity to Daphnia survival (Table 1). Whenever algae is present in the test, higher concentrations of AgNP are needed to see the same amount of toxicity without algae present. This shift in LC50 is consistent with the reduction of AgNP by its absorption to algae from the media prior to its exposure to the Daphnia. It also demonstrates that interactions between algae and AgNPs that can alter toxicity to other organisms relatively quickly because it is a process appears to be largely mediated by cellular binding.
Future studies should determine exactly how algal cells interact with AgNPs to reduce toxicity to invertebrate consumers. In our experiments, we broadly use the term “binding” to mean any interaction that attaches the AgNPs to an algal cells or their derived material that is greater than 0.2 µm and is therefore removed through filtration. It is unknown whether AgNPs are attaching to the surface of the algae cells, being incorporated inside the cells, or binding to exudates or other detrital material released by the algae in response to the toxin. Due to our interest in the biological consequences of particle binding and the small volumes of suspension used to culture Daphnia, we did not measure rates of sedimentation, aggregation, and dissolution of the AgNPs themselves. It is possible that the presence of algae changes these dynamics, but it seems unlikely that they would be primary mechanism accounting for decreased toxicity on Daphnia observed here. Nevertheless, these mechanisms should be examined in future studies to further increase our understanding of algal-mediated reductions in AgNP toxicity.
The mitigation of AgNP toxicity to Daphnia by suspended particles would seemingly occur in all aquatic environments, where there is a normally a diversity and high quantity of particulate material. This suspended matter includes phytoplankton and bacterioplankton communities, both of which have previously been reported to remove AgNPs from the water in a dose dependent manner (Das et al. 2012b, 2014). The ability of particulate organic matter to remove AgNPs is consistent with its ability to bind dissolved trace metals such as silver, cadmium, nickel, aluminum, zinc, copper, manganese, cobalt and lead in aquatic ecosystems (Tang et al. 2002; Adam and Garnier-Laplace 2003; Upadhyay et al. 2003; Turner et al. 2004). Altogether, our results strongly indicate that particulate binding of AgNPs would play an important role in mediating the toxic effects caused by nanoparticle contaminants in lakes, rivers, and streams.
Growth and survival at varying algal P
While algal particles have the potential to mitigate toxicity to higher trophic levels in natural ecosystems, it remains to be seen whether properties of phytoplankton themselves will modify the toxicity caused by AgNPs. One important property that is known to vary widely within and among aquatic ecosystems is algal nutritional quality (Elser et al. 2000; Sterner et al. 2008). For example, Hecky et al. (1993) surveyed a number lakes in different biomes and found sestonic C:P molar ratios ranging between 100 and 550. When we exposed Daphnia to AgNP and food with different P levels, Daphnia fed the more P-rich algae (C:P 100, typical of nutrient-rich lakes) demonstrated higher survival in response to AgNP exposure than those fed low P content algae (C:P 700, found in oligotrophic lakes) (Fig. 4a). As Daphnia fed high P food grow faster than those fed low P food, one possible explanation for this different toxicity is that smaller animals in the low P treatment received a higher dose of AgNPs due to their higher surface area to volume ratio. There was also a significant interaction between the concentration of AgNPs and the P quality of food on growth rate of Daphnia (F = 7.425, d.f. = 9, p < 0.001). In this experiment, low food P content and higher AgNP concentrations reduced growth rates in the Daphnia over the course of 6 days but this effect differed among AgNP concentrations (Fig. 4b). Despite the significant interaction term, there was no over-riding pattern to the AgNP × Food P content effect, which indicates this interactive effect may have been idiosyncratic and due to experimental error. Other contaminants including fluoxetine, RoundUp, and imidacloprid, also exhibit variable toxicity on growth and survival of Daphnia consuming different food qualities. While food quality affects the toxicity of all of these contaminants, it is likely that the mechanism underlying food quality-toxicity interactions depends strongly on the class of contaminant under consideration (Hansen et al. 2008; Lessard and Frost 2012; Ieromina et al. 2013). This underscores the necessity of further determining the mechanisms behind how food quality effects on the toxicity of contaminants including AgNP.
a Survival and b mass specific growth rate (MSGR) of Daphnia under high (solid line, black circles R 2 = 0.86) and low algae P content (dashed line, open circles, R 2 = 0.88) and different AgNP concentrations after 6 days. Both graphs are the result of two repeated experiments, points represent 25 individual Daphnia in the survival graph and 10 individual Daphnia in the growth experiments
Influence of algal P on binding and nutritional effects
Similar to algal experiments above, we examined whether variable toxicity resulted from changing the P content of algae (and daphnid nutrition) or whether it was due to AgNP binding to algae. In our second nutritional experiment, Daphnia that were raised for 4 days on high P food prior to AgNP exposure had slightly, but significantly, higher survival than Daphnia raised for 4 days on low P food when subsequently exposed to the same concentrations of AgNPs (Fig. 5a). The LC50 for P-nourished Daphnia was 6.7 ± 0.3 µg Ag L−1 and the LC50 for P-stressed Daphnia was 5.4 ± 0.5 µg Ag L−1. Given this small treatment effect, this change in toxicity may be of limited ecological relevance. Nonetheless, this result indicates that changes in animal metabolism, due to altered nutrition, can change AgNP toxicity and thereby should be accounted for in future risk assessments.
Influence of P on nutritional and binding impacts on toxicity. a LC50 curves of Daphnia that had been previously fed for four days on high P (solid line, R 2 = 0.95) and low P (dashed line, R 2 = 0.90) algae before transferring to different AgNP concentrations for 2 days with no food. The results represent 3 repeated experiments with 25 Daphnia per concentration b LC50 curves of Daphnia cultured in the filtrate that remained after high P (solid line, R 2 = 0.99) and low P (dashed line, R 2 = 0.94) algae were incubated with different AgNP concentrations for 6 h then filtered to remove algal bound silver, using Ag concentrations measured in the filtrate after removal of the algae, compared to an LC50 experiment with no algae present (dotted line, R 2 = 0.99). These curves are the results of two repeated experiments with each point representing 20 individual Daphnia
We also found that algal removal of AgNP varied between P-rich algae and P-poor algae, and probably accounts for most of the differences seen in toxicity between the two types of algae (Fig. 4a). When the amount of total silver in the media was measured after the incubation of AgNPs with high P and low P algae and used to create the LC50 curves, there was no difference in toxicity of AgNP remaining after exposure to high P algae and low P algae (Fig. 5b). This is in contrast to the survival curves made with the starting concentrations (not shown), which showed a difference between high P and low P algae, with LC50 values of 22.8 ± 0.5 and 18.4 ± 0.5 µg Ag L−1 respectively. Based on the amount of silver remaining in the filtrate and the starting concentration, it was determined that high P algae may retain more Ag (up to 45 % of starting Ag) than low P algae (up to 35 % of initial Ag) (Table 1). Together these results are further indication that the amount of free AgNPs in the water is the driving factor behind toxicity experienced by the Daphnia.
Our results suggest algal-mediated reductions in toxicity largely result from differential binding and uptake of these particles into a nontoxic form after algal removal. AgNPs remaining in the media after algal exposure to both P treatments retained the same toxicity seen in the system without any algal incubation (Fig. 5b). Our binding experiments also produced results that matched those from the standard toxicity tests with food, which led us to conclude that binding is the primary mechanism for reducing toxicity in the system. However, we also found that P-replete Daphnia were able to survive slightly, but significantly better, than P-stressed Daphnia in the presence of AgNPs, which indicates that the overall health and nutrition of the animal plays a role, albeit a minor one, in its response to AgNPs. There are some limitations to these results as we did not measure the dynamics of silver in our system including dissolution, aggregation, sedimentation, and whether silver is attached to the surface of cells or internalized. These kinds of data would be necessary to complete the understanding of our results because it would give a chemical mechanism for how algae is interacting with AgNPs or silver ions to mitigate toxicity.
Nevertheless, our results are a further demonstration that environmental conditions are likely to mediate future impacts of AgNPs in natural water bodies. Suspended particles are ubiquitous in aquatic ecosystems, but they can vary greatly both spatially and temporally. Algae are not the only type of particle that has the potential to bind AgNPs in aquatic ecosystems. Bacterioplankton, viruses, protists, detritus, and suspended sediments are all common particles found in freshwater that could serve as AgNP binding sites. Both natural phytoplankton and bacterioplankton have been found to bind AgNPs and together they are likely to be a significant sink for AgNPs within aquatic ecosystems (Das et al. 2012a, 2014). It will be important to understand how different types of particles will alter the toxicity of AgNPs within and between ecosystems. For example, bacterioplankton abundance varies among lakes with as few as 0.5 × 106 cells mL−1 in oligotrophic lakes and as much as 6 × 106 cells mL−1 in eutrophic lakes (Wetzel 1975). By changing the presence and P content of algae, we were able to increase LC50 values by almost 50 % across a range that either places AgNP toxicity as potentially having an impact on Daphnia in the future or not being of any concern as a contaminant in terms of zooplankton communities. Given this, the impact of the presence and quality of food should be given careful consideration when attempting to determine the impact of emerging contaminants such as AgNPs on aquatic ecosystems.
Conclusion
Overall, our results show that algae can mitigate the amount of toxicity Daphnia experience when exposed to AgNPs. Further, increasing the P content in algae reduced AgNP toxicity, which indicates eutrophic ecosystems could experience less AgNP toxicity than oligotrophic ecosystems. The mitigation of toxicity appears to result largely from AgNPs attaching to algae whereas nutrition was found to be less effective at mitigating toxicity in the absence of binding mechanisms. Altogether we find strong evidence that the presence and P content of algae helps determine AgNP toxicity in natural ecosystems, but more work needs to be done to determine the chemical mechanism of how this decrease in toxicity occurs.
References
Adam C, Garnier-Laplace J (2003) Bioaccumulation of silver-100m, cobalt-60, cesium-137, and manganese-54 by the freshwater algae Scenedesmus obliquus and Cyclotella meneghiana and by suspended matter collected during a summer bloom event. Limnol Oceanogr 48:2303–2313
Allen HJ, Impellitteri CA, Macke DA, et al. (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750. doi:10.1002/etc.329
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn.. American Public Health Association, Washington DC
Baptista MS, Miller RJ, Halewood ER, et al. (2015) Impacts of silver nanoparticles on a natural estuarine plankton community. Environ Sci Technol 49:12968–12974. doi:10.1021/acs.est.5b03285
Blinova I, Niskanen J, Kajankari P, et al. (2013) Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environ Sci Pollut Res Int 20:3456–3463. doi:10.1007/s11356-012-1290-5
Choi O, Clevenger TE, Deng B, et al. (2009) Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res 43:1879–1886. doi:10.1016/j.watres.2009.01.029
Das P, Metcalfe CD, Xenopoulos MA (2014) Interactive effects of silver nanoparticles and phosphorus on phytoplankton growth in natural waters. Environ Sci Technol 48:4573–4580
Das P, Williams CJ, Fulthorpe RR, et al. (2012a) Changes in bacterial community structure after exposure to silver nanoparticles in natural waters. Environ Sci Technol 46:9120–9128. doi:10.1021/es3019918
Das P, Xenopoulos MA, Williams CJ, et al. (2012b) Effects of silver nanoparticles on bacterial activity in natural waters. Environ Toxicol Chem 31:122–130. doi:10.1002/etc.716
Doke DA, Hudson SL, Dawson JA, Gohlke JM (2014) Effects of early life exposure to methylmercury in Daphnia pulex on standard and reduced food ration. Reprod Toxicol 49:219–225. doi:10.1016/j.reprotox.2014.09.006
Elser JJ, Fagan WF, Denno RF, et al. (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580. doi:10.1038/35046058
Frost PC, Evans-White MA, Finkel ZV, et al. (2005) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 1:18–28
Griffitt RJ, Luo J, Gao J, et al. (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978. doi:10.1897/08-002.1
Hansen LK, Frost PC, Larson JH, Metcalfe CD (2008) Poor elemental food quality reduces the toxicity of fluoxetine on Daphnia magna. Aquat Toxicol 86:99–103. doi:10.1016/j.aquatox.2007.10.005
Harnett D (1982) Statistical methods, 3rd edn.. Addison-Wesley Publishing Company, Reading, MA
Hecky RE, Campbell P, Hendzel LL (1993) The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol Oceanogr 38:709–724. doi:10.4319/lo.1993.38.4.0709
Hoheisel SM, Diamond S, Mount D (2012) Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas. Environ Toxicol Chem 31:2557–2563. doi:10.1002/etc.1978
Hoque ME, Khosravi K, Newman K, Metcalfe CD (2012) Detection and characterization of silver nanoparticles in aqueous matrices using asymmetric-flow field flow fractionation with inductively coupled plasma mass spectrometry. J Chromatogr A 1233:109–15. doi:10.1016/j.chroma.2012.02.011
Ieromina O, Peijnenburg WJGM, de Snoo G, et al. (2013) Impact of imidacloprid on Daphnia magna under different food quality regimes. Environ Toxicol Chem. 10.1002/etc.2472.
Kennedy AJ, Chappell MA, Bednar AJ, et al. (2012) Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ Sci Technol 46:10772–10780. doi:10.1021/es302322y
Kennedy AJ, Hull MS, Bednar AJ, et al. (2010) Fractionating nanosilver: importance for determining toxicity to aquatic test organisms. Environ Sci Technol 44:9571–9577. doi:10.1021/es1025382
Kilham SS, Kreeger DA, Lynn SG, et al. (1998) COMBO : a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Leclerc S, Wilkinson KJ (2014) Bioaccumulation of nanosilver by Chlamydomonas reinhardtii—nanoparticle or the free ion?. Environ Sci Technol 48:358–364. doi:10.1021/es404037z
Leonardo T, Farhi E, Pouget S, et al. (2016) Silver accumulation in the green microalga coccomyxa actinabiotis: toxicity, in situ speciation, and localization investigated using synchrotron XAS, XRD, and TEM. Environ Sci Technol 50:359–367. doi:10.1021/acs.est.5b03306
Lessard CR, Frost PC (2012) Phosphorus nutrition alters herbicide toxicity on Daphnia magna. Sci Total Environ 421–422:124–128. doi:10.1016/j.scitotenv.2012.01.040
Massarsky A, Trudeau VL, Moon TW (2014) Predicting the environmental impact of nanosilver. Environ Toxicol Pharmacol 38:861–873. doi:10.1016/j.etap.2014.10.006
Oukarroum A, Sebastien B, Perrault F, Popovic R (2012) Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–85. doi:10.1016/j.ecoenv.2011.11.012
Pokhrel LR, Dubey B, Scheuerman PR (2013) Impacts of select organic ligands on the colloidal stability, dissolution dynamics, and toxicity of silver nanoparticles. Environ Sci Technol 47:12877–12885. doi:10.1021/es403462j
Ribeiro F, Gallego-urrea JA, Jurkschat K, et al. (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466–467:232–241. doi:10.1016/j.scitotenv.2013.06.101
Shi J-P, Ma C-Y, Xu B, et al. (2012) Effect of light on toxicity of nanosilver to Tetrahymena pyriformis. Environ Toxicol Chem 31:1630–1638. doi:10.1002/etc.1864
Spadaro DA, Micevska T, Simpson SL (2008) Effect of nutrition on toxicity of contaminants to the epibenthic amphipod Melita plumulosa. Arch Environ Contam Toxicol 55:593–602. doi:10.1007/s00244-008-9153-2
Stebounova LV, Guio E, Grassian VH (2010) Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J Nanoparticle Res 13:233–244. doi:10.1007/s11051-010-0022-3
Sterner RW (1993) Daphnia growth on varying quality of Scenedesmus: mineral limitation of zooplankton. Ecology 74:2351–2360. doi:10.2307/1939587
Sterner RW, Andersen T, Elser JJ, et al. (2008) Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnol Oceanogr 53:1169–1180. doi:10.4319/lo.2008.53.3.1169
Tang D, Warnken KW, Santschi PH (2002) Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters. Mar Chem 78:29–45. doi:10.1016/S0304-4203(02)00007-5
Turner A, Millward GE, Le Roux SM (2004) Significance of oxides and particulate organic matter in controlling trace metal partitioning in a contaminated estuary. Mar Chem 88:179–192. doi:10.1016/j.marchem.2004.03.008
Upadhyay S, Liss PS, Jickells TD (2003) Sorption model for dissolved aluminium in freshwaters. Aquat Geochem 8:255–275
US Environmental Protection Agency (2002) US EPA: methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Environ Prot 232:266
Wetzel R (1975) Limnology: Lake and river ecosystems, 3rd edn. Elsevier Academic Press Environmental Science and Technology, San Diego, California, USA
Xiu Z-M, Ma J, Alvarez PJJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45:9003–9008. Houston Texas doi:10.1021/es201918f
Zhao C-M, Wang W-X (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892. doi:10.1002/etc.451
Acknowledgments
We thank Nicole Wagner, Andrew Scott, and Clay Prater for advice and help with Daphnia experiments and Daniel Rearick for assistance with silver analysis. Funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada and by Trent University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Conine, A.L., Frost, P.C. Variable toxicity of silver nanoparticles to Daphnia magna: effects of algal particles and animal nutrition. Ecotoxicology 26, 118–126 (2017). https://doi.org/10.1007/s10646-016-1747-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1747-2