Abstract
Gold nanorods (AuNRs) are rod-shaped nanoparticles (NPs) with special optical properties that allow their application in several areas including photothermal therapy, diagnosis, drug and gene delivery, cellular imaging, and biosensors. Their high potential for many applications increases the possibility of release in aquatic environments, which can cause risks to organisms. In this study, we evaluated toxic effects of AuNRs on cladoceran and fish (Ceriodaphnia dubia and Danio rerio) and their recovery after post-exposure periods. The EC50 of 0.03 mg L−1 was found for C. dubia in the acute exposure. There was a significant decrease in the number of neonates produced and in the filtration rate of C. dubia after sub-lethal exposure to AuNRs. The toxic mechanism of these NPs to cladocerans was attributed to increases in the reactive oxygen species (ROS) generation. After 4 h of recovery in clean medium, C. dubia were able to reestablish the filtration rate. Enzymatic biomarkers for D. rerio showed significant increases in the activity of superoxide dismutase, catalase, and lipid peroxidation after sub-lethal exposure to AuNRs. These biomarkers were recovered after 168 h in clean water. These results are pivotal on the comprehension of AuNR toxicity to aquatic organisms and are useful in assessing this novel nanomaterial impacts on aquatic biota.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold nanoparticles (AuNPs) are of great interest in nanotechnology. The advances in the synthesis methods have resulted in a wide variety of structures with controlled geometry and properties (Marangoni et al. 2016). Gold nanorods (AuNRs) are rod-shaped AuNPs that present two surface plasmon resonance (SPR) bands (Perez-Juste et al. 2005). The transversal band occurs in the visible region at around 520 nm, while the longitudinal band is located at the near-infrared region and depends on the aspect ratio of the particle (Perez-Juste et al. 2005). Due to these tunable optical properties and controlled shape and size, AuNRs have potential for application in several fields including biomedical areas, as in photothermal therapy and detection of cancer, drug and gene delivery, cellular imaging, and biosensors (Huang et al. 2006; Huang et al. 2009; Smith et al. 2009; Congur et al. 2013; Okuno et al. 2013).
The wide range for application of AuNPs is a concern from an environmental point of view. The spread use of this nanomaterial could lead to a release in environment compartments where the risks, effects, and toxicity mechanisms for organisms are not well understood (García-Cambero et al. 2013). Some studies have pointed out for the in vivo toxic effects of AuNPs and related them to physico-chemical properties such as surface functionalization, shape, and size (Bozich et al. 2014; Dominguez et al. 2015; Wang et al. 2016). In Daphnia magna, it was observed that positively charged AuNPs induced higher reactive oxygen species (ROS) generation and expression of genes related to oxidative stress, compared with negatively charged AuNPs (Dominguez et al. 2015). In another study, positively charged AuNPs presented higher toxicity than the negatively charged ones for D. magna in acute exposures, but in chronic toxicity evaluations, both positively and negatively charged AuNPs brought impacts on reproductive parameters (Bozich et al. 2014). In zebrafish embryos, a shape-dependent toxicity was observed, where gold nanospheres exhibited higher lethality rates compared with nanorods and nanopolyhedrons (Wang et al. 2016).
Exposure of aquatic organisms to AuNPs has been studied mainly due to evidences for NP accumulation and trophic transfer in the aquatic food chain (Ferry et al. 2009). Aquatic organisms can be in contact with AuNPs, either by direct uptake from water or by ingestion of food contaminated with NPs, which could result in adverse consequences to the organisms, such as acute effects (mortality), and sub-lethal effects, including inhibition of reproduction, decrease on growth rate and food ingestion, oxidative stress, pathologies on tissues, and other physiological parameters like apoptosis and necrosis (Bozich et al. 2014; Dominguez et al. 2015; Chupani et al. 2018; Mansano et al. 2018). However, studies are not conclusive regarding the fate of AuNPs in the aquatic environment (García-Cambero et al. 2013). Moreover, complete studies including acute toxicity, sub-lethal effects, toxicity mechanisms, and ability of recovery on aquatic organisms are rare in the literature, and they are of crucial importance in supporting environmental regulation processes and environmental impact studies.
Parameters like mortality, reproduction rates, feed activity, and biochemical endpoints including enzymatic activity and recovery are critical in ecotoxicological evaluations since they can determine how NPs impact the survival and maintenance of organisms in aquatic environments. Ceriodaphnia dubia and Danio rerio are standard organisms for ecotoxicological assessments (OECD 1992; ABNT 2017). These animals are representative of aquatic food chains, and they are easy to maintain in laboratory conditions. The main goals of this study were to evaluate the toxic effects of AuNRs on aquatic organisms (Ceriodaphnia dubia and Danio rerio) after sub-lethal exposures and to investigate the toxic mechanisms through reactive oxygen species generation and enzymatic biomarkers. The same biomarkers were evaluated after a recovery period in clean water, providing a wide panorama of these organisms’ responses to AuNR contact.
Materials and methods
Synthesis and characterization of AuNRs
AuNRs were synthesized by the seed-mediated method, using hexadecyltrimethylammonium bromide (CTAB) in aqueous medium (Sau and Murphy 2004). Synthesis was performed from the production of seeds and then the growth of particles in rod shape. In the first step, seed solution was prepared by adding 0.25 mL of HAuCl4·3H2O 0.01 M to 7.5 mL of CTAB solution 0.1 M, stirring slowly for few seconds. Then, 0.6 mL of NaBH4 solution freshly prepared and cooled was quickly added. The solution was stirred for 10 min and its color changed from yellow to brown. It was maintained at 25 °C during 2 h before using. In the second step, growth solution was prepared by mixing 3 mL of HAuCl4·3H2O 0.01 M and 47 mL of CTAB 0.1 M followed by 0.4 mL of AgNO3 0.01 M and 0.48 mL of ascorbic acid 0.1 M. Finally, 0.1 mL of the seed solution was added under gentle stirring at room temperature. The dispersion color changed gradually to blue. This mixture was left undisturbed for 24 h at room temperature, protected from light. For purification, AuNRs were centrifuged at 4000 rpm for 2 min and the supernatant was washed twice (7000 rpm, 5 min). UV-Vis absorbance spectra were recorded using a spectrophotometer Hitachi U-2900. Transmission electron microscopy (TEM) images were acquired using a JEOL JEM-2100 operating at 200 kV. AuNR dispersions were obtained at the concentrations used for exposure by diluting from a stock solution. These dilutions were submitted to bath sonication (Elmasonic P30H; 37 kHz) for 15 min before use under controlled temperature. Hydrodynamic size and zeta potential were measured in Milli-Q water and exposure medium (reconstituted water) at 0, 24, and 48 h using a Malvern Nano ZS90 spectrometer.
Zooplankton and zebrafish cultures
Individuals of Ceriodaphnia dubia were kept in the laboratory in reconstituted water, which was prepared by adding CaSO4·2H2O (1.2 × 10−4 mol L−1), KCl (3 × 10−5 mol L−1), NaHCO3 (6 × 10−4 mol L−1), and MgSO4·7H2O (4 × 10−4 mol L−1). The parameters for reconstituted water were 40–48 mg CaCO3 L−1 of hardness, pH 7.2–7.6, temperature of 22 ± 1 °C. The reconstituted water was 50% renewed every 2 days. Organisms were maintained at photoperiod of 16:8 h light/dark cycle. C. dubia were fed daily on 1 × 105 cells mL−1 suspensions of algae Pseudokirchneriella subcapitata (ABNT 2017).
Male and female adult zebrafish (Danio rerio; 4–6 months old, body weight 0.20 ± 0.07 g, body size 3.20 ± 0.35 cm) were purchased from a local commercial source and maintained in stock aquaria with dechlorinated tap water and aeration for 2 weeks before to the assays. Fish were housed under standardized temperature (22.0 ± 2 °C), pH (7.0 ± 1.0), dissolved oxygen (60% air saturation, maintained in constant aeration), and photoperiod (12:12 h light/dark cycle) conditions. These parameters were measured in the stock aquaria during acclimatization (OECD 1992). The animals were fed with fish meal once a day.
The evaluations performed with C. dubia and D. rerio are described as follows. The endpoints were chosen based on organism’s characteristics that are affected by NP exposure, and in the attempt to assess the AuNR toxicity mechanisms for cladocerans and fish.
Survival and reproduction of C. dubia
C. dubia (< 24 h old) were submitted to AuNRs in acute and chronic experiments. Acute experiments occurred in polyethylene containers of 10 mL, on AuNR concentrations of 0.0 (control), 0.008, 0.015, 0.030, 0.060, and 0.120 mg L−1 during 48 h. The acute assay was achieved in four replicates in each concentration and five organisms per replicate (n = 4). As endpoint, we used mortality of organisms, evidenced by no movement of body or appendages on gentle prodding (ABNT 2017).
After acute experiments, we achieved a chronic test with neonates of C. dubia (< 24 h old), which were submitted to AuNRs in sub-lethal concentrations of 0.0 (control), 0.0013, 0.0025, 0.0050, 0.0100, and 0.0200 mg L−1 for 7 days, which were observed effects on reproduction. This experiment was achieved in polyethylene containers on 10 replicates and one organism per concentration (n = 10) in a volume of 20 mL. Experiment occurred in semi-static system, with restoration of suspension medium every 48 h. The reproduction effect was measured by number of neonates produced during the period (ABNT 2017).
Reactive oxygen species generation on C. dubia
Tests for reactive oxygen species generation were performed using 2,7′dichlorofluorescein diacetate (H2DCF-DA) (Sigma). This compound is non-fluorescent, but it is changed to the 2,7′dichlorodihydrofluorescein (DCF) that is fluorescent when intracellular ROS production occurs. C. dubia (2 days old) were submitted to concentrations of 0.0 (control), 0.0013, 0.0050, and 0.200 mg L−1 (lower, intermediate, and higher sub-lethal concentrations) for 48 h. We choose 2-day-old organisms after preliminary tests. Assay was achieved on 24 replicates containing one organism per replicate (n = 24). After experiment, each living organism was transferred to a 96-well plate containing 200 μL of 10 μM H2DCFDA diluted with reconstituted water. The plate was then incubated for 4 h in the dark, at 22 °C. Fluorescence was measured through a fluorescence plate reader (SpectraMax M3), excitation wavelength of 485 nm, and emission wavelength of 535 nm. A positive control with a subgroup of organisms was treated with hydrogen peroxide solution (0.025%). We used reconstituted water plus AuNRs (without organisms) as blank. ROS levels were expressed in terms of the percentage of fluorescence intensity compared with the control group.
Filtration rate and recovery of C. dubia
The filtration rate on microalgae P. subcapitata in the presence of AuNRs was used to measure the effects on C. dubia feeding. Adult individuals (2 days old) were submitted to AuNRs in four replicates containing five organisms per replicate (n = 4). P. subcapitata microalgae (1.0 × 106 cells mL−1) were furnished to organisms in the presence of sub-lethal AuNR nominal concentrations: 0.0 (control), 0.0013, 0.0050, and 0.0200 mg L−1 along 24 h in the dark (exposure period). After 24 h, organisms were transferred with a plastic pipette to vials containing only reconstituted water with P. subcapitata microalgae suspension, at a concentration of 1.0 × 106 cells mL−1, and allowed to feed for 4 h in the absence of AuNRs (recovery period). This period for recovery is suitable for the organisms and is in accordance with McWillian and Bair (2002) experiments. After 4 h, organisms were removed from the containers, and the number of microalgae cells was measured, and filtration rates calculated for each period (exposure and recovery) using a Fuchs-Rosenthal chamber under optical microscope (Nikon Eclipse 50i).
The filtration rate (F) (mL animal−1 h−1) was calculated by the equation adapted from Gauld (1951):
where C0 and Ct mean the initial and final microalgae cell number (cells mL−1), t means the assay period (h), and v means the incubation volume (mL) per animal (n). An additional replication was achieved as a correction factor (A) using just exposure medium and microalgae cells, without organisms, to ensure that AuNRs did not interfere in the final concentration C′t of microalgae cells after time t.
Sub-lethal exposure: enzymatic activity and recovery of zebrafish
Enzymatic activity and recovery experiments were executed according to our previous study with another NPs (Souza et al. 2019). Zebrafish were exposed to 0.0 (control) and 0.075 mg L−1 of AuNRs (sub-lethal nominal concentration). This AuNR sub-lethal concentration was selected based on preliminary tests, which estimated that concentrations higher than 0.3 mg L−1 of AuNRs induced 100% of mortality to zebrafish. The exposure was performed in containers filled with 4 L of dechlorinated tap water with constant aeration and without food during 48 h (exposure) and 168 h (recovery). During the recovery period, the water was renewed every 48 h. The recovery phase duration was chosen to ensure the restoration of homeostasis after the chemical stressor had been withdrawn from the test environment, as it has been reported by Sancho et al. (2009), Venturini et al. (2015, 2019), and Souza et al. (2019). Recent papers also indicate that zebrafish is already able to express antioxidant enzymes after 48 h (Craigh et al. 2007; Hirayama et al. 2007; Feng et al. 2016). After 48 h of AuNR exposure, organisms of recovery group were moved to vessels filled with 4 L of dechlorinated tap water free of AuNRs with constant aeration and without food. Experiments were performed in four replicates, and in each replicate, five zebrafish from both sexes randomly chosen were used (exposure: n = 4; recovery: n = 4). After both experimental periods, fish were collected and anesthetized in cold water (4 °C); gills were harvested and immediately frozen at − 80 °C until analysis. All fish assays were submitted and approved by the Ethics Committee for Animal Research of the Physics Institute of São Carlos of the University of São Paulo, under protocol number 01/2014.
Gills harvested were homogenized in ice-cold 20 mM HEPES buffer, pH 7.2, containing 1 mM EDTA, 210 mM D-mannitol, and 70 mM sucrose in a proportion of 1:1 (tissue mass/buffer volume). This homogenization ratio had been chosen after preliminary studies and in accordance with Souza et al. (2019). The homogenates were centrifuged at 3000 rpm for 5 min, at 4 °C. Determination of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were achieved on supernatant. Lowry method (1951) was used for protein concentration (mg mL−1) using bovine serum albumin as standard.
SOD activity was determined by a commercial kit (Cayman Chemicals). The Superoxide Dismutase Assay Kit uses a tetrazolium salt for detection of superoxide radicals produced by xanthine oxidase, read at 540 nm. One unit (U) of SOD activity was established as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical (manufacturer information).
CAT activity analysis was achieved according to Beers and Sizer (1952) method. In brief, H2O2 was used as a substrate, and his decomposition by CAT was observed using UV-Vis spectrophotometer (Hitachi U-2900), at 240 nm during 3 min. The reaction happened on 20 μL of supernatants and 980 μL of H2O2 10 mM (volume of 1 mL). CAT activity was established using the molar extinction coefficient of H2O2 (0.043 mmol−1 cm−1). Data were expressed as micromoles (μM) of H2O2 consumed per min per mg of protein.
GPx was quantified accordingly to Beutler (1984) method, though of t-butyl hydroperoxide as substrate. This was observed by measurements of the decrement of the concentration of nicotinamide adenine dinucleotide phosphate (NADPH) in an assay coupled to glutathione reductase (GR), which catalyzed NADPH oxidation at 340 nm, for 1 min. The reaction mixture consisted of 100 μL of buffer (1 M Tris-HCl, 5mM EDTA pH 7.7), 250 μL Milli-Q H2O, 20 μL of 0.1 M glutathione reduced, 100 μL of glutathione reductase (10 U mL−1), 100 μL of 2 mM NADPH, 380 μL of 2.6 μM sodium azide, 20 μL of sample supernatant, and finally, 30 μL of 7 mM t-butyl hydroperoxide to initiate the reaction. The activity of the enzyme was established using the molar extinction coefficient of NADPH (6.22 mmol−1 cm−1). Data were expressed as μM of NADPH min−1 mg−1 of protein.
Lipid peroxidation was estimated through thiobarbituric acid-reactive species (TBARS) generation, achieved by malondialdehyde reaction with 2-thiobarbituric acid, which was observed according to Ohkawa et al. (1979) and Bird and Draper (1984). Briefly, homogenate of the gills (150 μL), in a proportion of 1:1 (tissue mass/buffer volume) in phosphate buffer 0.1 M, pH 7.4, was added to falcon tubes, to which 2.5 μL of butylated hydroxytoluene (4%) was added (avoiding undesired further oxidation of samples), 500 μL of trichloroacetic acid 12%, 400 μL of Tris-HCl 60 mM and EDTA 0.1 M, and 500 μL of thiobarbituric acid 0.73%. The mixture was heated at 100 °C in bath along 1 h. Following, samples were refreshed to room temperature and centrifuged at 11,500 rpm, during 20 min. The absorbances were read at 532 nm. A calibration curve was established using 1,1,3,3-tetraethoxypropane (nmol/mL) as standard. Data were expressed as nmol TBARS mg−1 protein.
Statistical analysis
Median effective concentration (EC50) for C. dubia was determined through linear regression analysis after logarithmic transformation of AuNR concentrations in relation to percentage of effect on organisms (OECD 1992, 2004). The confidence limit used in the EC50 determination was 95%. The data were evaluated for normality (Shapiro–Wilk test) and homogeneity of variance (Levene test). The data were also analyzed using ANOVA and the Tukey test at 5% of significance level to detect significant differences among control vs. treatments. Statistical tests were performed using Paleontological Statistics (Past) version 2.08 for Windows.
Results
Synthesis and characterization of AuNRs
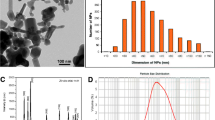
Transmission electron microscopy (TEM) images show AuNRs with a well-defined size and shape (Fig. 1a, b); the average transversal and longitudinal sizes were 20 and 50 nm, respectively. AuNRs exhibited two surface plasmon resonance bands, which come from the coherent motion of the conduction band electrons along the long and short axes of the particle. The transversal band occurs in the visible region (512 nm), while the longitudinal band occurs in the near-infrared region (NIR) (660 nm) (Fig. 1c). Mean hydrodynamic size and zeta potential values in Milli-Q water and in exposure medium are shown in Table 1. There was no change in the transversal or longitudinal size of AuNRs during 48 h in Milli-Q water. On the other hand, in exposure medium (reconstituted water), there was an increase in the longitudinal size during 24 h and 48 h. Zeta potential values in Milli-Q water and exposure medium were constant during 48 h.
Survival and reproduction of C. dubia
The EC50 for C. dubia after 48 h of exposure to AuNRs was 0.030 mg L−1 (Fig. 2a). The values for EC10 and EC20 were 0.011 and 0.015 mg L−1, respectively. Sub-lethal exposure to AuNRs diminished neonate production of C. dubia (Fig. 2b). The concentrations of 0.010 and 0.020 mg L−1 caused significant decrease in the neonate number after sub-lethal exposure. The reductions on neonate production in these concentrations were 62.5% and 72.4%, respectively, lower than control group.
a Linear regression equation after logarithmic transformation for the estimative of median effective concentration (EC50) of Ceriodaphnia dubia after exposure to AuNRs. The EC50 estimated was 0.03 mg L−1 with confidence intervals between 0.010 and 0.120 mg L−1 represented by dotted lines. b Mean ± standard deviation of neonates produced by Ceriodaphnia dubia after sub-lethal exposure to AuNRs. (*) Significant difference (p < 0.05 Tukey test) when compared with control
Reactive oxygen species generation on C. dubia
AuNR exposure induced significant ROS generation in C. dubia after 48 h. This ROS induction was dependent on concentration (Fig. 3).
Filtration rate and recovery of C. dubia
Filtration rate (percentage in relation to control) on microalgae cells was significantly decreased in concentrations 0.005 and 0.020 mg L−1 of AuNRs after 24 h of exposure. After the transferring of the organisms to an AuNR-free medium, the filtration rate was reestablished (Fig. 4).
Median ± standard deviation of filtration rate of Ceriodaphnia dubia on microalgae cells after 24 h of AuNR exposure and 4 h of recovery in AuNR-free medium. Control filtration rate (exposure phase) is 100% and indicated by the dotted line. (*) Means significant difference (p < 0.05 Tukey test) compared with the control in the same phase
Sub-lethal exposure: enzymatic activity and recovery of zebrafish
Enzymatic biomarkers showed a significant increase in SOD and CAT activities and in lipid peroxidation after 48 h of AuNR exposure, which were, respectively, 27.2%, 41.7%, and 139.6% higher than control group (Fig. 5a–d). The activity of GPx remained unchanged after the exposure period.
After 168 h of recovery in the absence of AuNRs, SOD activity was not significantly different from SOD activity in the control, CAT activity remained augmented (43.8%), and GPx activity and lipid peroxidation were significantly lower (20.0% and 35.0%, respectively) (Fig. 5a–d).
Discussion
AuNRs can cause acute and sub-lethal adverse effects to the cladoceran and zebrafish. This can be a concern, since gold nanomaterials have potential for application in several areas, and they can eventually be released in aquatic environments. The concentration 0.030 mg L−1 of AuNRs was enough to cause mortality on 50% of C. dubia population in acute exposure. In addition, the concentrations 0.010 and 0.020 mg L−1 were not lethal to these organisms but were sufficient to induce significant inhibition on the reproductive parameters into sub-lethal exposure. The same concentration (0.010 mg L−1) of AuNRs caused lethality to D. magna in acute exposure (Bozich et al. 2014). The authors attributed the toxicity to the surface charge of gold nanoparticles, since the positively charged were more toxic than negatively charged ones (Bozich et al. 2014). This is attributed to the higher affinity of positively charged to cellular membranes (Verma and Stellacci 2010). This affinity could facilitate the uptake of AuNRs in our study by phagocytosis and pinocytosis, inducing acute and sub-lethal effects. The impact of AuNRs on C. dubia reproduction after the sub-lethal exposure is a crucial result, since effects on reproductive outputs pose significant consequences on population endpoints, such as population size and growth (Arndt et al. 2013). When reproductive parameters are affected, there is the possibility of compromising the population maintenance and survival. Moreover, effects on neonate’s production, when held for long periods of time, could cause risk to organisms of higher trophic levels in the aquatic food chain, such as fish (Larguinho et al. 2014). These acute and chronic effects brought by our study are critical in predicting how AuNRs impact cladoceran population fitness, mainly after sub-lethal exposures.
The toxicity mechanism attributed to AuNRs is possibly related to the ROS generation. The presence of AuNRs in the exposure medium increased the ROS generation in C. dubia, in a concentration-dependent manner (Fig. 3). ROS generation has been reported as the main mechanism of toxicity attributed to NPs (Dominguez et al. 2015; Iswarya et al. 2016; Souza et al. 2018). Another outcome observed in our study was a decrease in the food ingestion on microalgae cells after AuNR exposure on C. dubia (Fig. 4). Daphniidae exposed to nanomaterials and other toxic substances can present impairments on food ingestion (McWillian and Bair 2002; Lovern et al. 2008). The latter may occur because of the selectivity of daphnids to uncontaminated microalgae cells. As a consequence, organisms can suffer reduction on growth rate and reproduction, due to the starvation (Wang et al. 2019). Moreover, it is likely that AuNRs could be adhered to the filtering apparatus of organisms, which could prevent the catch of small particles, including microalgae cells. This loss of food intake can generate metabolic costs to the organisms, since the absorption of nutrients was prejudiced (Lovern et al. 2008). It is also important to consider the possibility of physical interactions between the microalgae cells and AuNRs, which may result in agglomeration of microalgae cells, making them unavailable for organisms (Wang et al. 2019). The final consequence attributed to the decrease in food ingestion could be an impact on essential physiological functions, such as reproductive parameters, as observed in other studies (McWillian and Bair 2002; Arndt et al. 2013; Souza et al. 2018). In addition, detoxification processes rely heavily on energy expenditure for proper functioning, and such procedures might be affected by the observed food intake lessening. Therefore, AuNR presence in freshwater systems is able to cause physiological damages on Daphniidae species, including oxidative stress, inhibition on food ingestion, and reproduction decreases.
A surprising finding was that when organisms were transferred to containers containing just reconstituted water and microalgae suspension and in the absence of AuNRs, food ingestion was reestablished to normal levels (Fig. 4). Even after 24 h of contact with AuNRs, a recovery could occur. Khan et al. (2014) observed that AuNPs can be eliminated of organism’s body in two phases in Daphnia magna; in the first phase occurs a fast elimination, where about 75% of ingested AuNPs are eliminated in 1 h; and in the second phase, there is a slower elimination rate, where about 25% of ingested AuNPs are eliminated in 21 h. Our results indicated that, after 4 h in medium without NPs, the food ingestion of C. dubia was normal, suggesting the feasibility of the organisms to adapt to different situations. When conditions of clean water were provided, organisms were able to keep a filtration rate between 150 and 200% higher than control group during exposure phase (dotted line Fig. 4). It indicates that when organisms are in suitable conditions, the toxic effects can be overcome, and nanoparticles can be eliminated of organism’s body. The decreasing in the food ingestion in the presence of AuNRs could be an attempt to avoid NP ingestion through contaminated food, probably due to changes in taste, size, or shape of microalgae (Bern 1994), or as we suggested before, because the nanoparticle could be adhered to the filtering apparatus of the organisms, impairing food collection (Lovern et al. 2008). On the other hand, in the absence of AuNRs, the organisms reestablished the food intake, probably in an attempt to recover from toxic effects of oxidative stress, to detoxification, and to restoration of physiological processes that are crucial for growth and reproduction. These parameters allow the maintenance and survival of organisms after perturbation events like exposure to pollutants, including nanoparticles. Therefore, this result is critical when assessing AuNP contamination, as it indicates cladoceran population trend towards recovery.
The exposure of zebrafish to AuNRs resulted in toxicity. The 48 h of exposure to the sub-lethal concentration (0.075 mg L−1) of AuNRs resulted in toxic effects on the gill cells of zebrafish. The toxicity mechanism of AuNRs to the fish was the induction of oxidative stress, evidenced by the increase in the activity of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and lipid peroxidation. Briefly, according to Lushchak (2011a), SOD is the first enzymatic defense of organisms; it acts directly on the antioxidant system through reactions of dismutation of free radical superoxide (O2·−), which generates hydrogen peroxide and oxygen (H2O2 and O2). However, these molecules can also cause damage to the cells by generating other free radicals, such as hydroxyl radical (·OH) via Haber-Weiss reaction (Lushchak 2011a). Therefore, CAT metabolizes the sub-products of SOD, converting H2O2 in H2O and O2, which are notably not hazardous to cells. Glutathione peroxidase (GPx), a glutathione-dependent enzyme, also plays a critical role in the antioxidant defense; its activity is responsible for reducing H2O2, preventing the chain reactions that cause lipid peroxidation (Di Giulio et al. 1989). When these antioxidant responses are efficient, the organism is able to overcome oxidative stress (Lushchak 2011a). However, when these responses are not enough, lipid peroxidation can occur (Lushchak 2011a). This means that the imbalance between oxidants and antioxidant defenses is so severe that induces disturbances on membrane integrity, with degradation of the lipids present in the cell membranes.
In our study, there was an increase in the lipid peroxidation of zebrafish gills exposed to AuNRs. Other studies have reported toxic effects in zebrafish after NP exposure. Dedeh et al. (2015) observed activation of genes related to oxidative stress in zebrafish exposed to AuNPs. Antioxidant responses were also observed in the marine fish Sparus aurata after AuNP exposure (Teles et al. 2016) and after exposure to other metallic NPs (Hao and Chen 2006; Kaya et al. 2015; Valerio-García et al. 2017). The activation of the enzymatic antioxidant system (e.g., SOD and CAT increases) is an attempt of preventing the damages in organisms exposed to AuNRs. GPx did not depict any changes after exposure, which might have contributed for the observed gills lipid peroxidation. It means that in the absence of GPx activation, lipid peroxidation mechanisms occurred, probably inducing damages on gill cells. The central function of antioxidant system is to protect the fish of oxidative damage on cells and molecules. However, depending on the severity of the damage and the exposure time, it is possible that some important cellular mechanisms are affected, including loss of transmembrane ion gradients, irreversible changes to mitochondrial or nuclear DNA, and induction of apoptosis and necrosis processes (Lushchak 2011b). Such effects could affect the survival and suitable responses to oxidative insults.
After the recovery period, in the absence of NPs, zebrafish were able to restore homeostasis, regarding SOD activities. In this condition, there was a significant reduction on lipid peroxidation, indicating that the antioxidant system, activated during the exposure period, was able to cope with AuNR intoxication. The lessening in GPx activity seen after recovery might be due to a later recruitment of this enzyme, or even depletion of glutathione molecules, pivotal in xenobiotic detoxification pathways (Halliwell 2006; Di Giulio and Meyer 2008). Moreover, SOD and CAT increased activities after exposure were enough to convert the toxic H2O2 to H2O, and there was a lack of substrate for GPx activity, contributing to its decreased activity after recovery. Chupani et al. (2018) observed decreases in lipid peroxidation in Cyprinus carpio submitted to ZnO NPs (zinc oxide nanoparticles) after a 2-week recovery period. The authors attributed the results to the adaptive responses that resulted in an improvement of the antioxidant defense system. The reductions on LPO levels during the recovery phase are indicatives that the organisms were able to surpass the damages suffered during the exposure phase on gill cells. As stated by Lushchak (2011b), organisms can present adaptive responses depending on the oxidative stress level. This means that organisms can adapt to the H2O2 generated in the cells using cellular mechanisms, such as H2O2 cell permeability regulation, facilitating the tolerance to presence of ROS (Lushchak 2011b). In addition, the cell machinery is able to quickly reduce the toxicant penetration, inactivating ROS toxicity by the antioxidant system defense. This occurs in two ways: the first, not too costly for the cell, is a rapid change of physiological properties of the membrane, and second, more metabolically expensive, some genes are expressed, and their products are responsible for the reduction of membrane permeability (Lushchak 2011b). The first way is useful to the animal survival in the acute exposure and strong oxidative damage, and the second assists at chronic exposures, at enhanced ROS levels. Additionally, the equilibrium between ROS production and ROS elimination depends on the physiological state, intensity, and nature of stress (Lushchak 2011b). We suggested that zebrafish would be able to develop adaptive responses, which could occur via restoration of enzymatic activity, activating the defense system and overcoming the oxidative stress effects.
The toxic effects of metal ions and bulk metals on different aquatic organisms are extensively described in the literature (Shaw and Handy 2011; García-Cambero et al. 2013; Mansano et al. 2018). Along these lines, the exposure of organisms to salt of gold is beyond the scope of our study since we would like to characterize the effects of gold in the form of nanorods. The values of EC50 of gold III (HAuCl4·3H2O) for cladocerans (D. magna) and fish (D. rerio) were 1.34 mg L−1 and higher than 2.0 mg L−1, respectively (García-Cambero et al. 2013), being two and one order of magnitude higher than values obtained in our study with C. dubia and D. rerio (0.030 mg L−1 and 0.19 mg L−1, respectively). However, AuNPs can be many times more toxic than bulk material (García-Cambero et al. 2013; Mansano et al. 2018). In the ecotoxicological point of view, it is paramount to characterize the effects of NPs on organisms, since bulk materials are already well characterized. It is known that many toxic effects can occur immediately after NP addition in the exposure medium. AuNPs present specific properties that allow their use in several areas, and these AuNPs will inevitably be released in aquatic environments where, as demonstrated in our article, can cause acute and sub-lethal effects in cladocerans and fish species. In addition, the toxic effects observed on cladocerans and zebrafish were the aftermath of very low concentrations (less than 0.1 mg L−1), so we believe that before the nanoparticles are accumulated by the organisms, they have already caused effects that can compromise the viability of populations. Therefore, environmental risk assessment should include the toxic effects caused by NPs.
Conclusion
The exposure of C. dubia and D. rerio to AuNRs resulted in several toxic effects to these organisms. We observed acute toxicity to C. dubia with the concentration of 0.030 mg L-1. The cladocerans undergo sub-lethal toxic effects, including decrease in the reproduction and filtration rates and increase in the ROS generation. The fish also suffered sub-lethal toxic effects, including increases in the activity of SOD and CAT, and lipid peroxidation. These results allowed us to conclude that the exposure to the AuNRs can threaten cladocerans and fish. It was also observed that both organisms are able to reestablish some parameters after recovery periods in the absence of AuNRs. Cladocerans were able to recover the filtration rate and the fish did not present lipid peroxidation, which was noted right after exposure. Therefore, AuNRs are toxic to cladocerans and fish; nonetheless, these organisms are able to overcome the toxic effects when this contaminant is removed from the exposure medium. Our results are important to support the regulation environmentally safe concentrations of AuNRs, in addition to developing strategies for recovery and protection of freshwater organisms.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
ABNT (2017) Associação Brasileira de Normas Técnicas. Ecotoxicologia Aquática – Toxicidade Crônica – Método de ensaio com Ceriodaphnia spp (Crustacea, Cladocera) NBR 13373:2017. (Brazilian Association of Technical Standards. Aquatic Ecotoxicology - Chronic toxicity – Test method with Ceriodaphnia spp). https://www.abntcatalogo.com.br/norma.aspx?ID=359921
Arndt DA, Moua M, Chen J, Klaper RD (2013) Core structure and surface functionalization of carbon nanomaterials alter impacts to Daphnid mortality, reproduction, and growth: acute assays do not predict chronic exposure impacts. Environ Sci Technol 47:9444–9452 https://pubs.acs.org/doi/abs/10.1021/es4030595
Beers RF, Sizer JW (1952) A spectrophotometric method of measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140 https://pdfs.semanticscholar.org/f824/22e2e58932030184e840cdceed0e6b3ae8df.pdf
Bern L (1994) Particle selection over a broad size range by crustacea zooplankton. Freshw biol 35:105–112. https://doi.org/10.1111/j.1365-2427.1994.tb00870.x
Beutler E (1984) Red cell metabolism: manual of biochemical methods. Grune & Stratton INC, Michigan
Bird RP, Draper HH (1984) Comparative studies on different methods of malonaldehyde determination. Methods Enzymol 105:299–305. https://doi.org/10.1016/S0076-6879(84)05038-2
Bozich JS, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Klaper RD (2014) Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ Sci Nano 1:260–270 http://pubs.rsc.org/en/content/articlepdf/2014/en/c4en00006d
Chupani L, Niksirat H, Velíšek J, Stará A, Hradilová Š, Kolařík J, Panáček A, Zusková E (2018) Chronic dietary toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio L.): tissue accumulation and physiological responses. Ecotoxicol Environ Saf 147:110–116. https://doi.org/10.1016/j.ecoenv.2017.08.024
Congur G, Sayar F, Erdem A, Piskin E (2013) Voltammetric and impedimetric DNA detection at single-use graphite electrodes modified with gold nanorods. Colloids Surf B Biointerfaces 112:61–66. https://doi.org/10.1016/j.colsurfb.2013.07.040
Craigh PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am J Physiol Regul Integr Comp Physiol 293:1882–1892. https://doi.org/10.1152/ajpregu.00383.2007
Dedeh A, Ciutat A, Treguer-Delapierre M, Bourdineaud JP (2015) Impact of gold nanoparticles on zebrafish exposed to a spiked sediment. Nanotoxicology 9:71–80 https://www.ncbi.nlm.nih.gov/pubmed/24559428
Di Giulio RT, Meyer JN (2008) Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE (eds) The Toxicology of fishes. CRC Press, Boca Raton, pp 273–326
Di Giulio RT, Washburn PC, Wenning RJ (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123 https://setac.onlinelibrary.wiley.com/doi/pdf/10.1002/etc.5620081203
Dominguez GA, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Orr G, Klaper RD (2015) Effects of charge and surface ligand properties of nanoparticles on oxidative stress and gene expression within the gut of Daphnia magna. Aquat Toxicol 162:1–9 https://www.ncbi.nlm.nih.gov/pubmed/25734859
Feng J, Guo Y, Gao Y, Zhu L (2016) Effects of hypoxia on the physiology of zebrafish (Danio rerio): initial responses, acclimation and recovery. Bull Environ Contam Toxicol 96:43–48. https://doi.org/10.1007/s00128-015-1668-4
Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott GI, Decho AW, Kashiwada S, Murphy CJ, Shaw TJ (2009) Transfer of gold nanoparticles from the water column to the estuarine food web. Nat Nanotechnol 4:441–444 https://www.nature.com/articles/nnano.2009.157
García-Cambero JP, García MN, López GD, Herranz AL, Cuevas L, Pérez-Pastrana E, Cuadal JS, Castelltort MR, Calvo AC (2013) Converging hazard assessment of gold nanoparticles to aquatic organisms. Chemosphere 93:1194–1200. https://doi.org/10.1016/j.chemosphere.2013.06.074
Gauld DT (1951) The grazing rate of planktonic copepods. J Mar Biol Assoc UK 29:695–705. https://doi.org/10.1017/S0025315400052875
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322. https://doi.org/10.1104/pp.106.077073
Hao L, Chen L (2006) Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol Environ Saf 80:103–110. https://doi.org/10.1016/j.ecoenv.2012.02.017
Hirayama J, Cho S, Sassone-Corsi P (2007) Circadian control by the reduction/oxidation pathway: catalase represses light-dependent clock gene expression in the zebrafish. PNAS 140:15747–15752. https://doi.org/10.1073/pnas.0705614104
Huang XH, El-Sayed IH, Qian W, El-Sayed MA (2006) Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 128:2115–2120 https://pubs.acs.org/doi/abs/10.1021/ja057254a
Huang HC, Barua S, Kay DB, Rege K (2009) Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes. ACS Nano 3:2941–2952 https://pubs.acs.org/doi/abs/10.1021/nn900947a
Iswarya V, Manivannan J, De A, Paul S, Roy R, Johnson JB, Kundu R, Chandrasekaran N, Mukherjee A, Mukherjee A (2016) Surface capping and size-dependent toxicity of gold nanoparticles on different trophic levels. Environ Sci Pollut Res 23:4844–4858 https://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs11356-015-5683-0
Kaya H, Aydın F, Gürkan M, Yılmaz S, Ates M, Demir V, Arslan Z (2015) Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus). Environ Toxicol Pharmacol 40:936–947. https://doi.org/10.1016/j.etap.2015.10.001
Khan FR, Kennaway GM, Croteau MN, Dybowska A, Smith BD, Nogueira AJA, Rainbow PS, Luoma SN, Valsami-Jones E (2014) In vivo retention of ingested Au NPs by Daphnia magna: no evidence for trans-epithelial alimentary uptake. Chemosphere 100:97–104. https://doi.org/10.1016/j.chemosphere.2013.12.051
Larguinho M, Correia D, Diniz MS, Baptista PV (2014) Evidence of one-way flow bioaccumulation of gold nanoparticles across two trophic levels. J Nanopart Res 16:2549–2560 https://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs11051-014-2549-1
Lovern SB, Owen HA, Klaper R (2008) Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna. Nanotoxicology 2:43–48. https://doi.org/10.1080/17435390801935960
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 https://www.ncbi.nlm.nih.gov/pubmed/14907713
Lushchak VI (2011a) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak VI (2011b) Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comp Biochem Physiol-C 153:175–190. https://doi.org/10.1016/j.cbpc.2010.10.004
Mansano AS, Souza JP, Cancino-Bernardi J, Venturini FP, Marangoni VS, Zucolotto V (2018) Toxicity of copper oxide nanoparticles to Neotropical species Ceriodaphnia silvestrii and Hyphessobrycon eques. Environ Pollut 243:723–733. https://doi.org/10.1016/j.envpol.2018.09.020
Marangoni VS, Cancino-Bernardi J, Zucolotto V (2016) Synthesis, physico-chemical properties, and biomedical applications of gold nanorods - a review. J Biomed Nanotechnol 12:1136–1158 https://www.ncbi.nlm.nih.gov/labs/articles/27319210
McWillian RA, Bair DJ (2002) Postexposure feeding depression: a new toxicity endpoint for use in laboratory studies with Daphnia magna. Environ Toxicol Chem 21:1198–1205. https://doi.org/10.1002/etc.5620210612
OECD (1992) Guideline for Testing of Chemicals, Fish, Acute Toxicity Test 203. OECD Publishing. http://www.oecd.org/chemicalsafety/risk-assessment/1948241.pdf.
OECD (2004) Guideline for testing of chemicals, Daphnia sp., acute imobilisation test 202. OECD Publishing. https://www.oecd-ilibrary.org/docserver/9789264069947-en.pdf
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Okuno T, Kato S, Hatakeyama Y, Okajima J, Maruyama S, Sakamoto M, Mori S, Kodama T (2013) Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light. J Control Release 172:879–884. https://doi.org/10.1016/j.jconrel.2013.10.014
Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P (2005) Gold nanorods: synthesis, characterization and applications. Coord Chem Rev 249:1870–1901. https://doi.org/10.1016/j.ccr.2005.01.030
Sancho E, Fernández-Vega C, Villarroel MJ, Andreu-Moliner E, Ferrando DM (2009) Physiological effects of tricyclazole on zebrafish (Danio rerio) and post-exposure recovery. Comp Biochem Physiol C: Toxicol Pharmacol 150:25–32. https://doi.org/10.1016/j.cbpc.2009.02.004
Sau TK, Murphy CJ (2004) Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 20:6414–6420 https://pubs.acs.org/doi/abs/10.1021/la049463z
Shaw BJ, Handy RD (2011) Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal íons. Environ Internat 37:1083–1097 https://www.ncbi.nlm.nih.gov/pubmed/21474182
Smith AM, Mancini MC, Nie SM (2009) Bioimaging second window for in vivo imaging. Nat Nanotechnol 4:710–711 https://www.nature.com/articles/nnano.2009.326
Souza JP, Venturini FP, Santos F, Zucolotto V (2018) Chronic toxicity in Ceriodaphnia dubia induced by graphene oxide. Chemosphere 190:218–224. https://doi.org/10.1016/j.chemosphere.2017.10.018
Souza JP, Mansano AS, Venturini FP, Santos F, Zucolotto V (2019) Antioxidant metabolism of zebrafish after sub-lethal exposure to graphene oxide and recovery. Fish Physiol Biochem 45:1289–1297. https://doi.org/10.1007/s10695-019-00678-7
Teles M, Fierro-Castro C, Na-Phatthalung P, Tvarijonaviciute A, Trindade T, Soares AMVM, Tort L, Oliveira M (2016) Assessment of gold nanoparticle effects in a marine teleost (Sparus aurata) using molecular and biochemical biomarkers. Aquat Toxicol 177:125–135. https://doi.org/10.1016/j.aquatox.2016.05.015
Valerio-García RC, Carbajal-Hernández AL, Martínez-Ruíz EB, Jarquín-Díaz VH, Haro-Pérez C, Martínez-Jerónimo F (2017) Exposure to silver nanoparticles produces oxidative stress and affects macromolecular and metabolic biomarkers in the goodeid fish Chapalichthys pardalis. Sci Total Environ 583:308–318. https://doi.org/10.1016/j.scitotenv.2017.01.070
Venturini FP, Moraes FD, Cortella LRX, Rossi PA, Cruz C, Moraes G (2015) Metabolic responses of trichlorfon (Masoten) on the neotropical freshwater fish pacu (Piaractus mesopotamicus). Fish Physiol Biochem 41:299–309 https://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs10695-014-9983-y
Venturini FP, Moraes FD, Rossi PA, Avilez IM, Shiogiri NS, Moraes G (2019) A multi-biomarker approach to lambda-cyhalothrin effects on the freshwater teleost matrinxã Brycon amazonicus: single-pulse exposure and recovery. Fish Physiol Biochem 45:341–353. https://doi.org/10.1007/s10695-018-0566-1
Verma A, Stellacci F (2010) Effect of surface properties on nanoparticle-cell interactions. Small 6:12–21. https://doi.org/10.1002/smll.200901158
Wang Z, Xie D, Liu H, Bao Z, Wang Y (2016) Toxicity assessment of precise engineered gold nanoparticles with different shapes in zebrafish embryos. RSC Adv 6:33009–33013 http://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra00632a#!divAbstract
Wang F, Guan W, Xu L, Ding Z, Ma H, Ma A, Terry N (2019) Effects of nanoparticles on algae: adsorption, distribution, ecotoxicity and fate. Appl Sci 9:1534–1547. https://doi.org/10.3390/app9081534
Acknowledgments
Centro Nacional de Pesquisa em Energia e Materiais - Brazilian Nanotechnology National Laboratory CNPEM-LNNano (TEM-18453).
Funding
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (Proc. No. 2014/13493-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (Proc. No. 150555/2018-0).
Author information
Authors and Affiliations
Contributions
ASM analyzed the reactive oxygen species generation on C. dubia. FPV analyzed the enzymatic activity of zebrafish and performed the English review of the manuscript. VSM synthesized the AuNRs and performed images of transmission electron microscopy. PMPL characterized the AuNRs including hydrodynamic size and zeta potential in Milli-Q water and exposure media. BPCS cultivated and maintained C. dubia in laboratory and performed experiments of survival and reproduction. BD cultivated zebrafish in laboratory and achieved sub-lethal experiments. JPS performed all analyses on C. dubia and zebrafish, proposed the experimental design, discussed and interpreted the results, and was a major contributor in writing the manuscript. VZ proposed to experimental design, reviewed, and discussed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All zebrafish experiments were approved by the Ethics Committee for Animal Research of the Physics Institute of São Carlos of the University of São Paulo, under protocol number 01/2014.
Consent to publication
Not applicable.
Consent to participate
Not applicable.
Competing interest
The authors declare that they have no competing interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souza, J.P., Mansano, A.S., Venturini, F.P. et al. Toxicity of gold nanorods on Ceriodaphnia dubia and Danio rerio after sub-lethal exposure and recovery. Environ Sci Pollut Res 28, 25316–25326 (2021). https://doi.org/10.1007/s11356-021-12423-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12423-w