Abstract
We investigated the interspecific variation of silver nanoparticle (SNP) sensitivity in common cladocerans (Daphnia magna, D. galeata, and Bosmina longirostris) and the exact cause of both acute and chronic toxicity focusing on the form of silver (NPs and ions). Materials tested were non-surface-coated silver nanocolloids (SNCs) and AgNO3. The results of the acute toxicity tests support the theory that the effects of SNPs on aquatic organisms is mainly due to Ag+ released from SNPs. Among the three cladocerans, D. galeata was more sensitive to silver (as Ag+) than both D. magna and B. longirostris. Moreover, the chronic toxicity of SNCs was also derived from dissolved silver (especially Ag+). SNCs (as total silver concentration) showed far lower chronic compared with acute toxicity to daphnids because the amount of dissolved silver decreased in the presence of prey algae. The chronic end-point values (EC10 values for net reproductive rate and the probability of survival to maturation) did not differ largely from acute ones (48-h EC50 obtained from acute toxicity tests and 48-h LC50 estimated by the biotic ligand model) when the values were calculated based on Ag+ concentration. The α value (concentration at which intrinsic population growth rate is decreased to zero) estimated by a power function model was a reliable parameter for assessing the chronic toxicity of silver.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
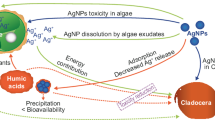
Despite rapid nanotechnology development, biochemical mechanisms on acute and chronic toxicity of nanomaterials are still uncertain. Nano-silver is one of the most frequently used nanomaterials because of its antimicrobial activity. Fabrega et al. (2011) reviewed the behavior and effects of silver nanoparticles (SNPs) in aquatic environments and pointed out the necessity to develop analytical and metrological methods to detect, quantify, and characterize SNPs under ambient conditions. Despite the lack of experimental validation, the predicted environmental concentrations of SNPs (e.g., surface water, sludge treatment plant effluents, and sludge-treated soil) are estimated in the range of ng L−1 and mg kg−1 (Gottschalk et al. 2009).
In aquatic environments, SNPs can affect prokaryotes, invertebrates, and fish at a few ng L−1 (see Fabrega et al. 2011 for review). However, the potential toxicity of SNPs differs depending on their own properties (e.g., particle size, presence/absence and type of capping agent, purity, and dissolution rate) and environmental conditions (e.g., water chemistry and temperature). For instance, small particles tend to show high toxicity to aquatic organisms (Kennedy et al. 2012). SNPs penetrate into the algal cell if the particle size is smaller than the cell wall pore (5–20 nm) (Navarro et al. 2008a). The depositions of SNPs have also been observed in fish eggs and embryos (Asharani et al. 2008; Kashiwada et al. 2012). Starch-capped and bovine serum albumin-capped SNPs and released free ions (Ag+) exert oxidative stress, DNA damage, and cellular proliferation delay (Asharani et al. 2008). The expression of six embryogenesis- and morphogenesis-related genes (ctsL, tpm1, rbp, mt, atp2a1, and hox6b6) in fish were significantly affected by SNP (non-surface coating, particle size = 3.8 ± 1.0 nm) exposure (Kashiwada et al. 2012). SNP-specific toxicity has also been observed in an invertebrate (Daphnia magna), in which mitochondrial function (proton efflux) of the embryo was more severely affected by SNP exposure than that of AgNO3 (Stensberg et al. 2014). Poynton et al. (2012) reported that polyvinylpyrrolidone (PVP)-coated SNPs (particle size = 35 nm) and AgNO3 affected the different gene expressions of D. magna: the former disrupted protein metabolism- and signal transduction-related gene expression profiles, and the latter caused a downregulation of developmental processes, particularly in sensory development.
In contrast, the results of laboratory bioassays have indicated that toxicity to algae and cladocerans is attributed mainly to dissolved silvers released from SNPs, especially Ag+ (Navarro et al. 2008b; Burchardt et al. 2012; Kennedy et al. 2012; Völker et al. 2013; Newton et al. 2013; Ribeiro et al. 2014). The most likely mechanism causing acute toxicity of Ag+ to aquatic animals is the inhibition of Na+ uptake by the gills as a result of the impact on Na+, K+-ATPase activity (Hogstrand and Wood 1998; Bianchini and Wood 2003). Dissolution of Ag+ from SNPs should be dependent on the properties of the test media (chemical composition, pH, and temperature) and SNPs (size, shape, and capping agent) (Fabrega et al. 2011). In fact, the acute toxicity results (48-h LC50) for D. magna as dissolved silver concentration are comparable for gum arabic-coated, polyethylene glycol-coated and PVP-coated SNPs and AgNO3 solutions (Newton et al. 2013). Thus, the 48-h LC50 of SNPs for Ceriodaphnia dubia were remarkably consistent with the value predicted by the biotic ligand model (BLM; Niyogi and Wood 2004) when expressed on the basis of dissolved silver (Kennedy et al. 2012). Although the BLM has not yet been calibrated to predict SNP toxicity, existing metal speciation and toxicity models might be effective tools for this purpose.
For ecological risk assessment, extrapolation from toxicological data obtained at the individual level into effects at the population level is inevitable (Tanaka and Nakanishi 2001). Population level effects can be evaluated by life-history data obtained from chronic toxicity tests; however, only a few studies addressing chronic SNP toxicity have been performed on aquatic animals (Daphnia: Zhao and Wang 2011; Blinova et al. 2013; Völker et al. 2013). According to Zhao and Wang (2011), the lowest-observable effect concentration of carbonate-coated SNPs for reproduction in D. magna was <1/10 of the 48-h EC50. As a result, they concluded that the mechanisms of chronic effects were caused by the low food quality of algae associated with SNPs and the low depuration of ingested SNPs. Völker et al. (2013) showed that PVP-coated SNPs (particle size < 20 nm) affected reproduction in three Daphnia species at far lower concentrations than the 48-h EC50. The investigators also showed that SNP toxicity on Daphnia increased with multigeneration exposures. These empirical data suggest that ecological risk assessment based on acute toxicity data (e.g., 48-h EC50 values) is not yet sufficient to explain the impacts of SNPs on aquatic ecosystems. Contrary to those reports, Blinova et al. (2013) recorded lower chronic than acute toxicity of PVP-coated and protein-coated SNPs (particle size 6–12.5 nm) to D. magna. Note, however, that the information about chronic SNP toxicity is limited. Moreover, interspecific variation of sensitivity in cladocerans has seldom been investigated, except for standard test organisms (D. magna, D. pulex, and C. dubia), even for dissolved silver.
The aims of the present study were to determine (1) the interspecific variation of SNP sensitivity in common cladocerans and (2) the exact cause of both acute and chronic toxicity focusing on the form of silver (nanoparticles and ions). We evaluated the sensitivity of three cladocerans (D. magna, D. galeata, and Bosmina longirostris) to SNCs (a mixture of colloidal and ionic silver) compared with AgNO3 by acute toxicity tests. The selected organisms (D. galeata and B. longirostris) are common lake species distributed in Eurasia and North America and often dominate the zooplankton community (Alonso 1991). Furthermore, we obtained the whole life-table data sets of two daphnids (D. magna and D. galeata) by chronic toxicity tests to evaluate the net reproductive rate (R 0) and intrinsic population growth rate (r) under different silver concentrations. With the above-mentioned data sets, we estimated the EC10 for R 0 and the probability of survival to maturation. In addition, we evaluated the silver concentration (α) at which r reduces to 0 by fitting the concentration-r data to a power function model (Tanaka and Nakanishi 2001). The response of the r value to pollutant concentration approximates was in many cases nearly quadratic (Tanaka and Nakanishi 2001). By comparing the silver concentrations (total silver and Ag+) that affect immobilization (48-h EC50) and reproduction (EC10 values and α) in daphnids, we re-examined the hypothesis that toxicity is mainly attributed to dissolved silvers released from SNPs.
Materials and Methods
Test Animals
Three cladoceran species (D. magna, D. galeata, and B. longirostris) were used in the present study. Single clones of D. magna and D. galeata were obtained from the National Institute for Environmental Studies, Japan. These stock cultures have been maintained for 30 years at the institute. The B. longirostris clonal culture was established from animals collected in Lake Suwa (36°2′N, 138°5′E), Japan. We cultured these cladocerans in 1-L glass beakers. COMBO medium (Kilham et al. 1998) containing Chlorella vulgaris (Chlorella Industry Co. Ltd, Fukuoka, Japan) was used as the culture medium. The medium was changed once a week. The food concentrations were 5 × 105 cells mL−1 in the D. magna and D. galeata cultures and 1 × 105 cells mL−1 in the B. longirostris culture. We added C. vulgaris to the culture medium every second or third day. Stock cultures were kept under constant laboratory conditions (20 ± 1 °C; 16 h of light to 8 h of darkness).
SNCs and AgNO3
Purified SNCs (99.99 % purity, non-surface coating, suspended in deionized water) were purchased from Utopia Silver Supplements (Utopia, Texas, USA). Diluted SNC solutions (a mixture of colloidal and ionic silver) for exposure tests were prepared with ultrapure water. The silver purity and concentration were validated by inductively coupled plasma mass spectrometry (ICP-MS) analysis using a Thermo Scientific X Series 2 (Thermo Scientific, Pittsburgh, Pennsylvania, USA): actual concentration and average diameter were 24.46 mg/L and 79.9 nm, respectively. The toxicity of SNCs was compared with dissolved Ag from reagent-grade AgNO3 (Wako Pure Chemical Industries Ltd., Osaka, Japan). A stock solution of AgNO3 (20 mg L−1) was prepared by dissolving the compounds in distilled water.
Bioassays
The laboratory conditions (20 ± 1 °C, 16 h of light to 8 h of darkness) were the same as those for the stock cultures. Procedure for the acute toxicity tests for daphnids (D. magna and D. galeata) were performed according to Organisation for Economic Co-operation and Development (OECD) guideline no. 202 (OECD 2004) and with slight modifications of the guideline for B. longirostris. Female neonates (<24 h old) from the third or later broods were used in all tests. For the tests on D. magna and D. galeata, the nominal concentrations of SNCs and AgNO3 were 5.92–28.56 µg L−1 (common rate = 1.3; no. of treatments = 7) and 1.01–3.73 µg L−1 (common rate = 1.3; no. of treatments = 6), respectively. For B. longirostris, SNCs and AgNO3 were 3.85–8.45 µg L−1 (common rate = 1.3; no. of treatments = 4) and 0.78–3.73 µg L−1 (common rate = 1.3; no. of treatments = 7), respectively. A control (0 µg L−1) was also prepared for each assay. For all of the test media, pH was adjusted to 7.0 with minimal drop additions of 10 % (v v−1) HCl as required. Tests on daphnids were performed in four 50-mL glass beakers containing 50 mL of each test solution, and five neonates were introduced into a beaker. The procedure for B. longirostris was the same as that in our previous assay (Sakamoto et al. 2005). Ten glass bottles (12 mL) were filled with each test solution. One bosminid individual was placed into each bottle, and the exposure test was started. The mouth of each bottle was covered with a cover glass to exclude air from the test water so as to avoid trapping the animals at the water surface. Test individuals were not fed during the assays. At 48 h after the exposure began, the numbers of immobilized individuals were counted. Dissolved oxygen (DO) and pH were measured at the beginning and end of the tests in the controls and the highest test substance concentrations. The physicochemical conditions met the criteria: Values at the start and end were 8.70 ± 0.54 mg L−1 (mean ± SD) and 9.04 ± 0.69 mg L−1 for DO and 7.04 ± 0.03 and 7.35 ± 0.14 for pH.
Chronic toxicity tests were performed for D. magna and D. galeata according to OECD guideline no. 211 (OECD 2012). Nominal concentrations of SNC and AgNO3 were 11.6–93.52 µg L−1 (common rate = 2.0; no. of treatments = 4) and 2.18–17.44 µg L−1 (common rate = 2.0; no. of treatments = 4) for D. magna, and 7.55–60.40 µg L−1 (common rate = 2.0; no. of treatments = 4) and 1.55–12.40 µg L−1 (common rate = 2.0; no. of treatments = 4) for D. galeata, respectively. The lowest dose in each assay was equal to the 48-h EC50 calculated using nominal silver concentrations. A control (0 µg L−1) was also prepared for each assay. A female neonate was put into a 50-mL glass beaker containing 50 mL of the each test solution with food (C. vulgaris, 5 × 105 cells mL−1). The replicate number of individuals at the start of the experiment was 10/treatment. Each animal was transferred to a clean beaker containing new test solution with food every second day, at which point neonates born to the experimental animal were counted and removed. Parturition, mortality, and number of newborns were checked daily until all of the individuals had died.
Water-Chemistry Analysis
To measure the absolute concentrations of cation (Na+, Mg2+, K+ and Ca2+), anion (Cl−, NO2 −, NO3 −, PO4 3− and SO4 2−), dissolved organic carbon (DOC), and dissolved inorganic carbon (DIC), 100 mL water was collected from each test solution at the start of both the acute and the chronic toxicity tests. Each water sample was filtered through a Whatman GF/C filter. The concentrations of cations and anions were measured using ion chromatography (ICS-1600; Thermo Fisher Scientific, Waltham, Massachusetts, USA), and DOC and DIC were evaluated with a TOC analyzer (multi N/C 3100, Analytik Jena AG, Jena, Germany).
Silver concentration was quantified from a 1.5-mL sample collected at the start of the bioassays. The water samples were centrifuged at 3,500×g for 10 min at 4 °C to remove the large solids (algal cells) before instrumental analysis. Total silver concentration was quantified from the supernatant. Dissolved silver (size < 1.5 nm) was separated from the particles through a 3-kDa membrane filter tube (Amicon Ultra-0.5, EMD Millipore Corporation, Billerica, Massachusetts, USA) where 0.5 mL supernatant was centrifuged at 14,000×g at 4 °C for 10 min. Two milliliters of HNO3 (Ultrapur-100, specific gravity 1.42, Kanto Chemical, Tokyo, Japan) was added to the samples (0.4 mL supernatant for the total Ag or 0.1 mL filtrate for the dissolved Ag) in a 50-mL Teflon beaker (Sanplatec, Osaka, Japan). The mixture was heated at 110 °C until just before it dried out. Then 2.0 mL of ultrapure nitric acid and 0.5 mL of hydrogen peroxide (for atomic absorption spectrometry, Kanto Chemical) were added to the beaker and heated until just before the mixture dried out. The residue was dissolved with 1.0 % ultrapure nitric acid solution to a volume of 12.0 mL and then subjected to ICP-MS analysis (determination limit 0.03–1 µg/L).
Free-ion (Ag+) concentrations in the test media were estimated using the freeware program Visual MINTEQ version 3.0 (http://www.lwr.kth.se/English/OurSoftware/vminteq) and then used to calculate the silver toxicities (EC50). Input data sets are listed in Table 1. Binding of metal ions to dissolved organic matter was modeled using the NICA-Donnan formulation (Milne et al. 2001; Milne et al. 2003).
Data Analysis
Values of 48-h EC50 with 95 % confidence intervals (CIs) were estimated by fitting acute toxicity data to a two-parameter log-logistic model using the drc package (Ritz and Streibig 2005) in R version 2.15.2 [R Development Core Team, Vienna, Austria (http://www.R-project.org/)]:
where f(x) is the probability of immobilization, x is the measured silver concentration (total Ag or Ag+), b is the relative slope at the inflection point, and e is the inflection point of the fitted line (equivalent to the dose required to cause a 50 % response).
Life table data obtained from the chronic toxicity tests were used to estimate the parameters related to population growth. Net reproductive rate (R 0) was calculated as
where l x and m x are the probability of surviving from birth to age class x and number of offspring for a female in age class x, respectively (Stearns 1992). EC10 values for R 0 with 95 % CIs were estimated by a three-parameter log-logistic model using the drc package in R version 2.15.2:
where f(x) is the R 0 value, x is the silver concentration (total Ag or Ag+), b is the relative slope at the inflection point, d is the maximum value of R 0, and e is the inflection point of the fitted line. The EC10 values for the probability of survival to maturation (first parturition) were estimated by the above-mentioned two-parameter log-logistic model. Intrinsic population growth rate (r) was estimated using the dominant eigenvalue (λ) of the Leslie matrix for each treatment (Case 2000).
The silver concentration (α) at which r reduces to 0 was estimated by a power function model (Tanaka and Nakanishi 2001),
where x is the exposure concentration, r max is the r in control, and α and β are parameters. Respectively, α and β are associated, with the magnitude of toxicity and the curvature of responses in r to exposure concentration, x.
As a reference value, we estimated the 48-h LC50 value of Ag+ for D. magna by the BLM for each test condition using the freeware program BLM version 2.2.3 [HydroQual (http://www.hydroqual.com/wr_blm.html)].
Results
Water-Chemistry Analysis
Hardness of the test media was approximately 39 mg (as CaCO3). Because there was no marked variation of the cation and anion concentrations, the BLM estimated similar 48-h LC50 values (Ag+ for D. magna) in the three media (Table 1). Measured silver concentrations in the SNC and AgNO3 solutions were far lower than the nominal ones. The percentage recoveries were 6–66 % for SNCs and 5–38 % for AgNO3. By employing the metal speciation results, approximately 48 % of the dissolved silver was free ion (Ag+), and the rest was AgCl(aq) (49 %) and AgCl2 − (2 %) in the acute toxicity tests. In the chronic toxicity tests, Ag+, AgCl(aq), and AgCl2 − presented in the media were approximately 38, 58, and 4 %, respectively. Other silver species (e.g., AgSO4 −, AgNO2(aq), and AgNO3(aq)) were <0.5 % of the total dissolved silver. Although the COMBO medium contained ethylene diamine tetraacetic acid (EDTA) (3.4 mg L−1, 1.4 mg C L−1) as a chelating agent, the concentrations of chelated silver (AgEDTA3− and AgHEDTA2−) were negligibly low (<0.01 %).
Bioassays
The 48-h EC50 value of SNCs based on the measured total silver concentrations did not differ largely among the three cladocerans (Table 2). However, the sensitivity of D. galeata to SNCs was greater than that of the other two species when the values were calculated with the Ag+ concentrations. A similar trend was observed for AgNO3. The 48-h EC50 value of SNCs (0.90 µg L−1, for Ag+) and AgNO3 (0.25 µg L−1) for D. magna were comparable with the 48-h LC50 value (0.56 µg L−1) estimated by the BLM (Table 1).
In each chronic toxicity test, daily observation was continued until all of the test individuals had died, and thus the experimental period differed between treatments. In the controls, D. magna reached greater net reproductive rates (R 0 = 71.9 in SNCs experiment and 68.8 in AgNO3 exp.) than D. galeata (R 0 = 44.5 in SNCs exp. and 53.0 in AgNO3 exp.) (open circles in Fig. 1). SNCs did not affect the daphnids’ R 0 when the total silver concentrations were <14.5 µg L−1 (Fig. 1a, c). The EC10 values of SNCs (as total silver) for R 0 were far greater than the 48-h EC50 value, although the broad range of 95 % CIs indicate that the estimation is highly uncertain (Table 2). In contrast, AgNO3 was highly toxic to daphnids: All of the D. magna neonates died within 1 day at 1.93 µg L−1. Although SNC toxicity was lower than that of AgNO3, the dose ranges did not differ between the chemicals in Ag+ concentration (Fig. 2a, b). The SNC EC10 values based on Ag+ concentrations (1.20 µg L−1 for D. magna and 1.00 µg L−1 for D. galeata) for R 0 did not differ largely from the 48-h EC50 values. The AgNO3 EC10 values for R 0 (0.09 µg L−1 for D. magna and 0.02 µg L−1 for D. galeata) were 1 to 50 times lower than those of SNCs (as Ag+), but the 95 % CIs were very wide. A similar trend was observed in the probability of survival to maturation (Table 2; Fig. 2c, d). Calculated SNC EC10 values for maturation tended to be lower than those for R 0 and were comparable with the 48-h EC50 values.
The predicted SNC concentrations (α) at which the intrinsic growth rate (r) reaches 0 was approximately 26 µg L−1 for D. magna and 20 µg L−1 for D. galeata (Table 3; Fig. 3). The estimated α value of AgNO3 for D. magna was 0.8 µg L−1. We could not estimate the AgNO3 value for D. galeata because r (0.22 day−1) at the highest dose was very high [only 25 % lower than the control (Fig. 3b)]. Despite the fact that SNCs were less toxic to daphnids than AgNO3, the α Ag+ values in SNCs were very low (1.9 µg L−1 for D. magna and 1.3 µg L−1 for D. galeata).
Discussion
The present results do not support the view that SNCs (as total silver concentration) are more toxic to cladocerans than AgNO3 (as Ag+) when survival rate, swimming behaviour, and reproductivity are used as end points. Likewise, laboratory studies suggest that acute toxicity of SNPs to zooplankton and phytoplankton is attributed mainly to dissolved silvers released from SNPs (Burchardt et al. 2012; Kennedy et al. 2012; Völker et al. 2013; Newton et al. 2013; Ribeiro et al. 2014).
All three species (D. magna, D. galeata, and B. longirostris) exhibited a 48-h EC50 values ten times greater for SNCs than AgNO3 (Table 2). The SNC 48-h EC50 values based on Ag+ concentrations were comparable with those of AgNO3 and the estimated 48-h LC50 value by BLM (Table 1), although the SNC values tended to be greater. In our acute toxicity tests, sensitivity to Ag+ was D. galeata > B. longirostris ≥ D. magna. Völker et al. (2013) also reported that the 48-h EC50 value of PVP-coated SNPs (particle size < 20 nm) for D. galeata (13.9 µg L−1) was approximately ten times lower than that of D. magna (121 µg L−1). They also showed that D. magna was more sensitive to AgNO3 than D. galeata (48-h EC50 = 1.1 µg L−1 for D. magna and 2.1 µg L−1 for D. galeata). However, our results contradict this: D. galeata was slightly more sensitive to AgNO3 than D. magna. Although we have found no other report addressing silver toxicity to D. galeata, this species may generally show greater sensitivity to heavy metals [e.g., copper (Bossuyt and Janssen 2005) and zinc (Vesela and Vijverberg 2007)]. In addition, this is the first report on the toxicity of silver to B. longirostris. B. longirostris is more sensitive to copper than either D. galeata or D. magna (Koivisto et al. 1992). Vesela and Vijverberg (2007) reported that the sensitivity of neonates of four Daphnia spp. to zinc was positively correlated with body size (the sensitivity was D. galeata > D. pulex > D. pulicaria > D. magna). The metabolic rate and the amount of accumulated metal ions per unit of body volume are greater in small-sized than large-sized species (Grosell et al. 2002; Bianchini et al. 2002). In the present study, approximate neonate sizes were B. longirostris (250 µm) < D. galeata (450 µm) < D. magna (1,100 µm), meaning that size-dependent sensitivity is inapplicable to all cladoceran species.
Copper and silver ions are thought to render their effect through a similar physiological mechanism whereby they inhibit Na+ uptake by the gills of aquatic animals (Niyogi and Wood 2004). Therefore, the mechanism causing the contradiction observed in bosminid sensitivity might be elucidated by the investigation of whole-body sodium uptake and Na+, K+-ATPase activity (Bianchini and Wood 2003). In addition, because downsizing vessels and volumes enlarges the surface/volume ratio, which in turn affects the toxicity of potentially adsorbing substances such as SNPs (Baumann et al. 2013), the smaller test vessels used for the Bosmina (12-mL bottles) tests compared with those used for the Daphnia (50-L beaker) tests could have caused decreased toxicity of the silver.
Population-level effects of chemicals are generally evaluated based on both the survival and reproductive rate of the individuals. However, some researchers have found that mortality during chronic toxicity tests is a more critical end point for silver than per-capita fecundity (Nebeker et al. 1983; Blinova et al. 2013). Here, we show the effects of SNCs on the net reproductive rate (R 0), the probability of survival of tested individuals to maturation, and the intrinsic population growth rate (r) (Figs. 2 and 3). The 95 % CIs of EC10 values for R 0 and maturation tended to have a broad range, thus implying high uncertainty of the estimation (Table 2). However, we found that the EC50 (acute toxicity) and EC10 (chronic toxicity) values did not vary largely when they were calculated using Ag+ concentrations. The α values (silver concentrations at r = 0) were greater than the EC10 for R 0 and maturation (Table 3). Mortality, especially during juvenile stages, exerted a strong influence on the total offspring number. Among the three chronic toxicity end points compared, probability of survival to maturation was the most sensitive to SNCs, which supports the conclusion by Blinova et al. (2013) that mortality is a convenient end point to assess the chronic toxicity of SNPs.
There are two contrasting laboratory findings on the relationship between acute and chronic toxicity of SNPs. The first is that SNPs influence daphnid reproduction at concentrations lower than the 48-h EC50 (Zhao and Wang 2011; Völker et al. 2013). A chronic effect (low offspring number) results from altered energy-reserve fractions (e.g., lipids) (Muyssen and Janssen 2001). Thus, Völker et al. (2013) concluded that the lower 21-day EC10 compared with the 48-h EC50 (and EC10) recorded in their experiments was due to the inhibition of algal food ingestion by accumulated PVP-coated SNPs in the daphnids’ gut. In the present study, however, SNCs did not affect daphnid reproduction even at concentrations five to six times greater (as total silver) than the 48-h EC50 value (Fig. 1a, c). The contradictory results of those reports may be explained by the size-dependent influx rates of the SNPs (Zhao and Wang 2012). The influx rate (ingestion is the dominant uptake pathway) of SNPs into daphnids is size dependent with smaller particles exhibiting high values. The average particle sizes of silver used by Völker et al. (2013) and the present study were < 20 nm (58 nm in the test medium) and 80 nm, respectively. Unfortunately, we did not measure the particle size or hydrodynamic diameter in the COMBO medium; however, we assumed that particles formed large aggregates resulting in the low influx rates.
The second study, by Blinova et al. (2013), showed a lower chronic than acute toxicity to D. magna. This is consistent with our results. The toxicity of SNPs to D. magna decreases with the addition of algae (Allen et al. 2010), in which organic materials may either alter the availability of ionic Ag or stimulate active sodium uptake by Na+, K+-ATPase (Bianchini and Wood 2003; Glover and Wood 2004). Moreover, Stevenson et al. (2013) elucidated that extracellular DOC compounds produced by algal cells mitigate the citrate-coated SNP toxicity to Chlamydomonas reinhardtii. In the present study, chronic SNC end point values as total silver concentration for D. magna (EC10 for R 0 = 20.88 µg L−1, α = 25.99 µg L−1) were approximately ten times greater than that of acute values (48-h EC50 = 2.43 µg L−1) (Tables 2 and 3). Similar acute/chronic ratios were also observed in D. galeata. Such low chronic toxicity of SNPs might be explained by the low dissolved silver/total silver concentrations in the chronic toxicity tests. The metal speciation results showed that the relative abundance of Ag+ in the dissolved silver did not differ largely among the toxicity tests (40–50 %) despite slightly greater DOC concentrations in the chronic ones. For instance, Ag+/total silver concentration in SNC test media in the acute and chronic toxicity tests for D. magna were 35–68 % and 6–9 %, respectively. This resulted in the high acute/chronic toxicity SNC ratios. In fact, the dose ranges in the chronic toxicity tests did not differ between SNCs and AgNO3 in Ag+ concentrations (Figs. 2 and 3). Moreover, similar EC10 values and 48-h SNCs and AgNO3 EC50 were obtained when they were calculated using Ag+ concentrations (Table 2). The estimated α value was only two to five times greater than the 48-h EC50 value based on the Ag+ concentrations (Table 3). These results indicate that Ag+ was the main toxic substance for the cladocerans. However, as is also shown in the 48-h EC50 value, the EC10 value, and the α of AgNO3 as Ag+ for daphnids tended to be lower than those of SNCs, suggesting that the exact cause of SNP toxicity is still uncertain. Nevertheless, similar BLM-estimated 48-h LC50 values for D. magna [Table 1 (0.49–0.56 µg L−1)] suggest that the chemical composition of the media affecting the interaction between daphnid biotic ligands and bioavailable silver did not differ among the assays.
The low dissolved:total silver ratio in the chronic toxicity tests implied that a large amount of dissolved silver went missing from the SNC test media. The instrumental analysis was performed after two centrifugal separation processes. First, the water samples were centrifuged to remove the algal cells. Afterward, dissolved silver (<1.5 nm) in the supernatant was separated from the particles with a 3-kDa membrane filter tube. Therefore, it is conceivable that the dissolved silver was absorbed by the algal cells and/or other organic matter (>1.5 nm), which decreased the toxicity of SNCs to daphnids (Allen et al. 2010). Chronic toxicity tests can be expressed as bitrophic level experiments in another way. In lake ecosystems, cladocerans always exist with their prey algae, and thus our findings might be useful for SNP ecological risk assessment.
The impact of anthropogenic stress on ecosystems often depends not only on the level of pollution but also on the community structure of the particular ecosystem (Sakamoto and Tanaka 2013). However, we still have insufficient knowledge on how SNPs affect aquatic ecosystems through the modification of food web structure with species-specific sensitivities. Our results forecast that zooplankton communities dominated by D. galeata are relatively vulnerable to SNP pollution. Moreover, we found that acute and chronic SNC toxicity (composed of non-surface-coated silver nanocolloids and ionic silver) to cladocerans was mainly derived from dissolved silver (especially Ag+) in the test medium. Chronic SNC toxicity was lower than acute toxicity because of low dissolved/total silver in the former. The chronic end point values (SNC EC10 values for R 0 and probability of survival to maturation) did not differ largely from acute ones (48-h EC50 and BLM-estimated 48-h LC50 value) when the values were calculated based on Ag+ concentration. However, very wide 95 % CIs of EC10 values indicate that the estimation is highly uncertain. The α values estimated by fitting r to a power function model were reliable parameters for assessing the chronic toxicity of silver. In the present study, all of the end point values tended to be greater for SNCs than for AgNO3 even if they had been calculated using Ag+ concentrations. Further study is needed to clarify this contradiction.
References
Allen HJ, Impellitteri CA, Macke DA, Heckman JL, Poynton HC, Lazorchak JM et al (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750
Alonso M (1991) Review of Iberian Cladocera with remarks on ecology and biogeography. Hydrobiologia 225:37–43
Asharani PV, Wu YL, Gong Z, Valiyaveettil S (2008) Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19:255102
Baumann J, Sakka Y, Bertrand C, Köser J, Filser J (2013) Adaptation of the Daphnia sp. acute toxicity test: miniaturization and prolongation for the testing of nanomaterials. Environ Sci Pollut Res 21:2201–2213
Bianchini A, Wood C (2003) Mechanism of acute silver toxicity in Daphnia magna. Environ Toxicol Chem 22:1361–1367
Bianchini A, Grosell M, Gregory SM, Wood CH (2002) Acute silver toxicity in aquatic animals is a function of sodium uptake rate. Environ Sci Technol 36:1763–1776
Blinova I, Niskanen J, Kajankari P, Kanarbik L, Käkinen A, Tenhu H, Penttinen OP, Kahru A (2013) Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environ Sci Pollut Res 20:3456–3463
Bossuyt BTA, Janssen CR (2005) Copper toxicity to different field-collected cladoceran species: intra- and inter-species sensitivity. Environ Pollut 136:145–154
Burchardt AD, Carvalho RN, Valente A, Nativo P, Gilliland D, Garcìa CP et al (2012) Effects of silver nanoparticles in diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp. Environ Sci Technol 46:11336–11344
Case TJ (2000) An illustrated guide to theoretical ecology. Oxford University Press, New York
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531
Glover CN, Wood CM (2004) Physiological interactions of silver and humic substances in Daphnia magna: effects on reproduction and silver accumulation following an acute silver challenge. Comp Biochem Physiol C 139:273–280
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43:9216–9222
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol C 133:287–303
Hogstrand C, Wood CM (1998) Towards a better understanding of the bioavailability, physiology and toxicity of silver to fish: implications for water quality criteria. Environ Toxicol Chem 17:572–578
Kashiwada S, Ariza ME, Kawaguchi T, Nakagame Y, Jayasinghe BS, Gärtner K et al (2012) Silver nanocolloids disrupt Medaka embryogenesis through vital gene expressions. Environ Sci Technol 46:6278–6287
Kennedy AJ, Chappell MA, Bednar AJ, Ryan AC, Laird JG, Stanley JK et al (2012) Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ Sci Technol 46:10772–10780
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377:147–159
Koivisto S, Ketola M, Walls M (1992) Comparison of five cladoceran species in short- and long-term copper exposure. Hydrobiologia 248:125–136
Milne CJ, Kinniburgh DG, Tipping E (2001) Generic NICA-Donnan model parameters for proton binding by humic substances. Environ Sci Technol 35:2049–2059
Milne CJ, Kinniburgh DG, Van Riemsdijk WH, Tipping E (2003) Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environ Sci Technol 37:958–971
Muyssen BTA, Janssen CR (2001) Multigeneration zinc acclimation and tolerance in Daphnia magna: implications for water-quality guidelines and ecological risk assessment. Environ Toxicol Chem 20:2053–2060
Navarro E, Baun A, Behra R, Hartmann N, Filser J, Miao AJ et al (2008a) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N et al (2008b) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–8964
Nebeker AV, McAuliffe CK, Mshar R, Stevens DG (1983) Toxicity of silver to steelhead and rainbow trout, fathead minnows and Daphnia magna. Environ Toxicol Chem 2:95–104
Newton KM, Puppala H, Kitchens CL, Colvin VL, Klaine SJ (2013) Silver nanoparticles toxicity to Daphnia magna is a function of dissolved silver concentration. Environ Toxicol Chem 32:2356–2364
Niyogi S, Wood CM (2004) Biotic ligand model, a flexible tool for developing site-specific water quality guidelines for metals. Environ Sci Technol 38:6177–6192
Organisation for Economic Co-operation and Development (OECD) (2004) OECD guidelines for testing of chemicals, no. 202: Daphnia sp., acute immobilization test. OECD, Paris
Organisation for Economic Co-operation and Development (OECD) (2012) OECD guidelines for testing of chemicals, no. 211: Daphnia magna reproduction test. OECD, Paris
Poynton HC, Lazorchak JM, Impellitteri A, Blalock BJ, Rogers K, Allen HJ, Loguinov A, Heckman JL, Govidnaswamy S (2012) Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46:6288–6296
Ribeiro F, Gallego-Urrea JA, Jurkschat K, Crossley A, Hassellöv M, Taylor C et al (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466–467:232–241
Ritz C, Streibig JC (2005) Bioassay analysis using R. J Stat Softw 12:1–22
Sakamoto M, Chang KH, Hanazato T (2005) Differential sensitivity of a predacious cladoceran (Leptodora) and its prey (the cladoceran Bosmina) to the insecticide carbaryl: results of acute toxicity tests. B Environ Contam Tox 75:28–33
Sakamoto M, Tanaka Y (2013) Different tolerance of zooplankton communities to insecticide application depending on the species composition. J Ecol Environ 36:141–150
Stearns SC (1992) The evolution of life histories. Oxford University Press, New York
Stensberg MC, Madangopal R, Yale G, Wei Q, Ochoa-Acuña H, Wei A et al (2014) Silver nanoparticles-specific mitotoxicity in Daphnia magna. Nanotoxicology 8:833–842
Stevenson LM, Dickson H, Klanjscek T, Keller AA, McCauley E, Nisbet RM (2013) Environmental feedbacks and engineered nanoparticles: mitigation of silver nanoparticle toxicity to Chlamydomonas reinhardtii by algal-produced organic compounds. PLoS ONE 8:e74456
Tanaka Y, Nakanishi J (2001) Model selection and parameterization of the concentration-response function for population-level effects. Environ Toxicol Chem 20:1857–1865
Vesela S, Vijverberg J (2007) Effect of body size on toxicity of zinc in neonates of four differently sized Daphnia species. Aquat Ecol 41:67–73
Völker C, Boedicker C, Daubenthaler J, Oetken M, Oehlmann J (2013) Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS One 8:e75026
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892
Zhao CM, Wang WX (2012) Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ Sci Technol 46:11345–11351
Acknowledgments
The authors thank N. Watanabe for kind assistance during the experiments. We are grateful to T. Kusui for helpful comments on this study. This study was supported by Grants-in-Aid for Scientific Research to M. Sakamoto (Grant No. 23510031) and to S. Kashiwada (Grant No. 23310026) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakamoto, M., Ha, JY., Yoneshima, S. et al. Free Silver Ion as the Main Cause of Acute and Chronic Toxicity of Silver Nanoparticles to Cladocerans. Arch Environ Contam Toxicol 68, 500–509 (2015). https://doi.org/10.1007/s00244-014-0091-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0091-x