Abstract
As a class I carcinogen, arsenic has been reported to cause diseases accompanied by circRNAs regulating proliferation and apoptosis at the molecular level, but whether circP50 (circBase ID: hsa_circ_0008012) does the same has not been demonstrated. The aim of this study is to provide the basis for anti-lung cancer mechanism research, by studying the expression of circP50 under arsenic-induced conditions, and the effect and mechanism on the proliferation and apoptosis of A549 cells based on the circP50 knockdown models. To explore whether the circP50 is responsive to arsenic exposure, the qRT-PCR was applied to discover that the relative expression of circP50 in A549 cells increased only with increasing NaAsO2 dose and independent of its metabolites. We further determined the mechanism of circP50 by establishing circP50 knockdown models. The results of cell viability and EdU assays indicated the proliferation of A549 cells. According to the western blotting, phosphorylation of p53 at Ser15, Ser376, and Ser392 and acetylation of p53 at Lys370 and Lys382 were inhibited, resulting in the deficiency of p53 expression. Subsequently, the expression of genes downstream of p53 was reduced, including p21, PUMA, Caspase3, and Bcl-xS. Furthermore, the expressions of IKB-α, p65, and p50 decreased, but C-myc expression did not change significantly, referring to the NF-κB pathway was not dominant. The results suggest that circP50 mainly functions through the p53 pathway to mediate apoptosis in response to arsenic exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is present in the form of inorganic arsenic in nature and usually accumulates in various forms after oxidation. Inorganic arsenic can convert into monomethylarsenic acid (MMA) and dimethylarsenic acid (DMA) by methylation metabolism in the human body and excrete through the kidney (Bozack et al. 2018). Arsenic exposure diseases are typical biogeochemical diseases. According to statistics, about 6 to 10 million people worldwide are exposed to high arsenic levels (Rahman et al. 2019). Long-term exposure to arsenic enhances the risk of lung, liver, kidney, skin, and bladder cancer (Chen and Costa 2021; Ferragut Cardoso et al. 2020; Islam and Takeyama 2021). Arsenic has been classified as a class I carcinogen, but its carcinogenic mechanism has not been elucidated.
Some scholars propose that arsenic may induce tumorigenesis and development by participating in cell proliferation, apoptosis, oxidative stress, DNA damage, and chromosomal aberrations (Mar Wai et al. 2019; Medda et al. 2021; Wang et al. 2020; Wu et al. 2019; Zang et al. 2020). As an essential tumor suppressor regulatory gene, the p53 regulatory network is closely related to the known mechanisms of arsenic toxicity. The p53 is subject to complex regulation, and it is currently accepted that post-translational modifications of p53 play significant roles in the regulation of its activity, including phosphorylation and acetylation modifications and so on (Chen et al. 2020). It has been shown that sodium arsenite (NaAsO2) can activate p53 phosphorylation and acetylation through PUMA overexpression and enhance p53 activity, thereby promoting the apoptosis of arsenite-treated A549 cells (Zhou et al. 2022). Besides, in tumor studies, it is generally assumed that the NF-κB pathway promotes tumorigenesis, proliferation, invasion, and metastasis through the transcriptional regulation of related genes after activation (Mitchell et al. 2016). NF-κB is an important family of transcription factors in mammals, with five members: Rel (cRel), p65 (RelA), RelB and p50 (NF-κB 1), and p52 (NF-κB 2) (Williams and Gilmore 2020). It has been proved that the NF-κB family also has a tumor-suppressive effect (Taniguchi and Karin 2018). The role of p50 depends largely on its dimerization partners, the co-factors, cell types, and cancer types so that gene expression can either be suppressed or activated, hindering or driving tumorigenesis (Concetti and Wilson 2018).
Recently, the role and the mechanism of linear RNA and circRNA in disease development have attracted much attention. CircRNAs are a special class of non-coding RNA with a covalent bond closed loop structure (SMeng et al. 2017, Zhang et al. 2018), which participate in the development of environmental chemical exposure-related diseases, which can regulate proliferation, apoptosis, metastasis, and inflammation at the molecular level (Nan et al. 2017; Xiao et al. 2018; Xue et al. 2018; Yang et al. 2018). CircRNA biological functions include serving as a miRNA sponge, regulating gene splicing and transcription, serving as an RNA-binding protein sponge, regulating protein translation, etc. (Chen 2020, Du et al. 2017, Huang et al. 2021, Prats et al. 2020, Zhang et al. 2020). The circRNA is formed by precursor RNA by shear, followed by head-to-tail ligation of the linear RNA. Some studies believe that linear RNA can affect the expression of proteins associated with lung cancer, and circRNA can be used as a feasible and important biomarker for the diagnosis, prognostic judgment, and clinicopathological features of lung cancer (Dong et al. 2021; Van Der Steen et al. 2020). Many stimuli such as cytokines, protein kinase C activators, oxidants, etc. can activate the transcription of p50 gene, and its activation can be involved in the regulation of genes such as inflammation, cell proliferation, and apoptosis. It has been reported that p50 cooperates with the promoter-binding protein of inflammatory cytokines to promote the growth of tumor cells in lung cancer (Dai et al. 2019). However, the exact mechanism by which p50 gene-spliced circP50 (circBase ID: hsa_circ_0008012) expression in lung cancer has not been illustrated.
As a result, we explored the expression of circP50 under arsenic-induced conditions in the research. Lung cancer caused by arsenic compounds has been listed as a legal occupational disease in China (Sun et al. 2021), and many scholars have studied the effect of arsenic on lung cancer (Pietrzak et al. 2021). We targeted the human lung adenocarcinoma cell line to explore its effect on A549 cell proliferation and apoptosis and its mechanisms by interfering with the expression of circP50. The aim is to provide the basis for anti-lung cancer mechanism research by observing and analyzing the experimental results.
Materials and methods
Introduction to circP50
In combination with the circBase database, we selected hsa_circ_0008012 (circP50) as a subject spliced from the p50 (NF-κB 1) gene (Glazar et al. 2014), and its sequence length is 265 bp which is located at chr4:103446668-103459113. For more information, please visit: http://www.circbase.org/.
Cell treatment and culture
We consult when purchasing cell lines and cultivate in the laboratory, RPMI 1640 medium is suitable for A549 cell growth (Sun et al. 2020). Therefore, human lung adenocarcinoma cell line A549 cells from Kunming Institute of Zoology, Chinese Academy of Sciences were seeded in RPMI 1640 containing 10% FBS in a 37 °C, 5% CO2 incubator, and the medium was replaced every 2 days. Logarithmic growth stage A549 cells were cultured into 6-well plates at 9 × 104 cells/well. The concentration of NaAsO2 (CAS 7784-46-5; purity ≥ 90.0%) was adjusted to 0, 20, 40, and 60 μM with fresh mediums for another 22 h. Furthermore, in 6-well plates seeded with the same number of cells, we replaced the culture medium with 60 μmol/L NaAsO2, dimethylarsenic acid (DMA) (MF: C2H7AsO2; CAS 75-60-5; purity ≥ 99.0%), and monomethylarsenic acid (MMA) (MF: CH5AsO3; CAS 124-58-3; purity ≥ 99.0%). Only fresh medium was added into cells from the control group without any treatment. Total RNAs were extracted for analysis after another 48 h of incubation.
RNA preparation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from A549 cells by the Trizol method, and the Roche Reverse Transcription kit (Roche, German) was applied to cDNA synthesis. The relative expression of circP50 mRNA was detected by the LightCyler®96 real-time PCR instrument (Roche, German). Reaction conditions were preincubation at 95 °C for 120 s, followed by 45 cycles of 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 10 s. β-actin is accurate and stable as an internal reference (Januszyk et al. 2020), and is highly expressed in A549 cells and can be easily detected. Therefore, for the internal reference, we used β-actin. All experiments were repeated in triplicate, and the results were evaluated by 2−∆∆ CT method. CircP50 primers: Forward: GACTACCTGGTGCCTCTAGT Reverse: GCAGTGCCATCTGTGGTTG; β-actin primers: Forward: GCCGAGGACTTTGATTGCAC Reverse: TGGACTTGGGAGAGGACTGG.
Cell transfection
Logarithmic growth stage A549 cells with a density of 9 × 104 cells/well were seeded into 6-well plates for 19 h after incubation in Penicillin-Streptomycin-free medium. Cells were transfected with RFect (Changzhou, China) and circP50 siRNA (Shanghai, China), formulation of RFect and siRNA in serum- and Penicillin-Streptomycin-free medium, and the medium containing serum and Penicillin-Streptomycin solution was replaced after 24 h incubation according to instructions. The NC, siRNA-1, and siRNA-2 three groups were the grouping of cells. The NC sequence is sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. The siRNA-1 sequence is sense: 5′-CCUCAGGUCAAACUUCAGATT-3′; antisense: 5′-UCUGAAGUUUGACCUGAGGTT-3′. The siRNA-2 sequence is sense: 5′-AGGUCAAACUUCAGAAUGGTT-3′; antisense: 5′-CCAUUCUGAAGUUUGACCUTT -3′. Transfection efficiency was determined by fluorescence microscopy at 6 h and qRT-PCR detection for the expression of circP50 at 72 h after transfection, respectively. After 72 h transfection, cells were harvested for western blotting analysis.
Detection of cell viability
Cell Counting Kit-8 (CCK-8, CAS 193149-74-5) (MedChemExpress, China) was used for the cell viability assay according to the manufacturer’s protocol. A549 cells were seeded into 96-well plates at a concentration of 2500 cells/well, followed by maintained in 100 μl buffer involving 10 μl CCK-8 for 1–4 h after 72 h transfection. Grouping and siRNA sequences refer to the “Cell transfection” section, 4-6 replicate wells for each treatment group. The optical density (OD) value was detected at 450 nm by the enzyme mark instrument (Bio-Rad, USA). The test was repeated three times and the calculation formula of cell viability is:
Cell proliferation assay
A549 cells were seeded into 6-well plates at a density of 6 × 104 cells/well. Grouping and siRNA sequences refer to the “Cell transfection” section, 72 h after transfection, the single proliferating cell was detected using the BeyoClickTM EdU-555 kit (Beyotime, Shanghai, China). According to the manufacturer’s protocol, cells were stained within azide-555 and Hoechst-33342 and visualized by inverted microscope with red and blue fluorescence, respectively, and after fluorescence microscopy analysis, the percentage of EdU-positive cells was counted from three random fields in three wells.
Protein preparation and western blotting
Total protein samples were extracted from cells transfected with circP50-siRNA for 72 h; cells were lysed with RIPA buffer (Thermo Fisher Scientific lnc. USA) involving protease inhibitors determined the concentration of protein through the BCA Protein Assay kit (Beijing Biotechnology Co., China). Proteins were electrophoresis in 10% SDS-PAGE (30 μg/well) and transferred to PVDF membranes (Roche, German). After blocking for 20 min, the membranes were maintained with the primary antibody overnight in a 4 °C fridge and then maintained with secondary antibodies for 2 h at RT. Bands were visualized using a BeyoECLPlus chromorendering substrate, and band intensities were assessed utilizing the Gel-Pro Analyzer software (Media Contronetics). The band intensity of western blotting was quantified by ImageJ software. Primary antibodies included in this study are available in Table 1.
Statistical analysis
All experiments were repeated in triplicate. Data analysis was carried out using the GraphPad Prism 6.0 and ImageJ software. Experimental data met a normal distribution, and the statistical analysis was performed by student’s t-test. Mean ± standard deviation (SD) is the final form to present the outcomes. P < 0.05 was considered as significant differences.
Results
Expression of circP50 in A549 cells responding to NaAsO2, DMA, and MMA
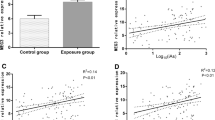
We analyzed whether inorganic arsenic affected circP50 expression in A549 cells by qRT-PCR. The NaAsO2 treatment (40, 60 μM) significantly increased the circP50 expression than in control and 20 μM. Compared with the control, it was 4.8-fold increase in 40 μM; 9.3-fold increase in 60 μM, p < 0.05 (Fig. 1a), implying that the expression of circP50 in A549 cells was increased with the increasing NaAsO2 concentration. Figure 1b shows that the relative expression of circP50 was significantly decreased in the DMA and MMA as compared to the NaAsO2 group (73% in DMA, p < 0.01; 85% in MMA, p < 0.01). On the other hand, the expression of circP50 elevated 7.2-fold with NaAsO2 treatment (p < 0.0001), while no significant differences existed among the control, MMA, and DMA groups.
Expression of circP50 was detected after infection of A549 cells with NaAsO2, DMA, and MMA. (a) A549 cells were treated with the indicated concentration of NaAsO2 for 72 h. (b) A549 cells were treated with DMA, MMA, and NaAsO2 for 72 h. The control group set the same as others without arsenic exposure. Compared with the control group, ****P < 0.0001, *P < 0.05
Knockdown of circP50 in A549 cells
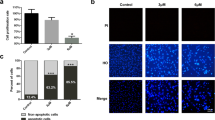
The circP50 expression in the siRNA-1 group and siRNA-2 group was reduced by 79% and 83%, respectively, with a significant difference when compared to the NC group, following the qRT-PCR results (Fig. 2a). It is suggested that the siRNA can successfully knock down the circP50. The results in Fig. 2b and c showed that the RFect transfection reagent successfully transferred siRNA into A549 cells, and the cells had a good growth state. It was suggested that RFect and siRNA showed no obvious cytotoxicity.
Knockdown of circP50 in A549 cells. (a) At 72 h after transfection with siRNA-circP50 successfully knocked down circP50 expression. (b, c) At 6 h after the transfection of A549 cells with FAM-siRNA, the cell transfection efficiency was measured by fluorescence microscopy. Compared with the NC group, **p < 0.01
Effect of circP50 knockdown on cell viability
We knocked down the circP50 in the A549 cells and examined cell viability changes. The results showed that the cell viability was higher in the siRNA-1 and siRNA-2 groups than in the NC group, and the difference was statistically significant (19% in siRNA-1, p < 0.01; 29% in siRNA-2, p < 0.0001). It is suggested that circP50 may be involved in regulating cell proliferation and apoptotic behavior (Fig. 3a). We observed cell proliferation in the NC and circP50-siRNA groups by staining with the BeyoClickTM EdU-555 cell proliferation assay. The results reflected that the low circP50 expression of the siRNA-1 and siRNA-2 groups significantly increased A549 cell proliferation when compared to the NC group (Fig. 3b). A statistically significant difference in the percentage of EdU-positive cells of the siRNA-1 and the siRNA-2 groups, as compared to the NC group (36% higher for siRNA-1, p < 0.05; 56% higher for siRNA-2, P < 0.01), following results in Fig. 3c. The above results demonstrated that knockdown of circP50 expression could significantly promote the viability of human lung cancer A549 cells.
Detection of cell viability and proliferation after circP50 knockdown in A549 cells for 72 h. (a) The results of the CCK-8 assay revealed that the cell viability was elevated significantly after circP50-siRNA transfection. (b) Cell proliferation assay showed that knockdown of circP50 significantly promoted cell proliferation, assessed by fluorescence microscope. A blue light represents all cells in the observation field, and red represents cells in the proliferative phase. (c) Knockdown of circP50 significantly promoted cell proliferation efficiency by counting the percentage of EdU-positive cells. Compared with the NC group, *p < 0.05, **p < 0.01 and ****p < 0.0001
The protein expression of IKB-α, p65, p50, and C-myc in A549 cells after knockdown of circP50
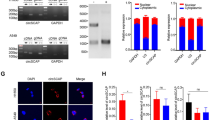
We found that expressions of IKB-α (2.5-fold in siRNA-1; 1.5-fold in siRNA-2), p65 (1.8-fold in siRNA-1; 1.2-fold in siRNA-2), and p50 (1.2-fold in siRNA-1; 1.5-fold in siRNA-2) were decreased, and C-myc expression did not change significantly in circP50 knockdown cells between the siRNA-1 and siRNA-2 compared with the NC group, p < 0.05 (Fig. 4a, b).
The protein expression of p53 phosphorylation, acetylation, and the genes downstream of p53, as well as IKB-α, p65, p50, and C-myc in A549 cells after knockdown of circP50. (a, b) The expression of IKB-α, p65, p50, C-myc in A549 cells. (c, d) The expression of phosphorylation of p53 at Ser15, 376, 392, as well as acetylation of p53 at Lys370, 382. (e, f) The detection of protein expression levels, including p21, PUMA, Caspase3, Bcl-xS
Knockdown of circP50 inhibited the activity of the p53 pathway
In A549 cells, p53 (2.8-fold in siRNA-1; 6-fold in siRNA-2), p53 phosphorylated at Ser15 (2.9-fold in siRNA-1; 11.1-fold in siRNA-2), 376 (2.9-fold in siRNA-1; 2.5-fold in siRNA-2), and 392 (1.3-fold in siRNA-1; 1.2-fold in siRNA-2), as well as p53 acetylated at Lys370 (5.3-fold in siRNA-1; 3.2-fold in siRNA-2) and 382 (1.2-fold in siRNA-1; 1.3-fold in siRNA-2), were downregulated after circP50 knockdown, p < 0.05 (Fig. 4c, d). In addition, the protein expression of the pro-apoptotic genes, including p21 (1.4-fold in siRNA-1; 1.6-fold in siRNA-2), PUMA (1.8-fold in siRNA-1; 2.3-fold in siRNA-2), Caspase3 (2.7-fold in siRNA-1; 2.7-fold in siRNA-2), and Bcl-xS (3.2-fold in siRNA-1; 3.3-fold in siRNA-2), was significantly decreased in the siRNA-1 and siRNA-2 groups when compared to the NC group, p < 0.05 (Fig. 4e, f).
Discussion
Arsenic lung cancer was included in the occupational disease in China in 2013 (He et al. 2020). Arsenic and its compounds often enter the body through drinking water, air, or food and induce acute and chronic arsenic poisoning. As a natural metalloid, arsenic can be metabolized in the human body and can cause systemic multisystem, multiple organ damage, and even cancer (Bjorklund et al. 2020; Chen and Costa 2021). Arsenic has “two sides” from toxicity to drug nature. On the other hand, it can induce tumors by causing abnormal cell proliferation. In cancer, the coordinating role between uncontrolled cellular metabolism, proliferation, and apoptosis is crucial for tumorigenesis (Martinez-Reyes and Chandel 2021). Furthermore, diseases associated with exposure to environmental chemicals follow the aberrant expression of specific circRNA. Increasing studies suggest that the expression of circRNAs perhaps be an integral part of the mechanism of lung cancer (Fan et al. 2017). Several reports have demonstrated that aberrant circRNAs expression can promote or inhibit the occurrence and progression of lung cancer. For instance, functional experiments showed that circITCH has inhibitory effects on lung cancer cell proliferation and circHIPK3 promotes the proliferation of lung cancer cells by its interaction with miR-379, IGF1 (Tian et al. 2017). In addition, hsa_circ_0013958 promotes lung adenocarcinoma cell proliferation and invasion (Zhu et al. 2017). Therefore, it is feasible to explain the arsenic carcinogenesis mechanism from the perspective of circRNA; however, whether circP50 expression after inorganic arsenic exposure can directly act on cells and affect lung cancer progression is unclear.
According to the results of qRT-PCR, the expression of circP50 was independent of MMA and DMA. Still, the expression level of circP50 gradually increased in cells treated with different doses of NaAsO2 and showed a certain dose-response relationship. What’s more, it has been shown that arsenic exposure can directly affect the expression of circRNAs, which can regulate the relevant signaling pathways alone or together with other factors to induce disease. For example, the expression of circ100284 (Dai et al. 2018; Xue et al. 2017), circLRP (Xue et al. 2018) was significantly increased in arsenite-treated cells, which is similar to our results. However, it has also been found that circ008913 is reduced by arsenic exposure in HaCaT cells (Xiao et al. 2018) since circRNA can act as a miRNA sponge to regulate cell growth by absorbing multiple miRNAs; meanwhile, arsenic exposure can regulate the direct target level of miRNA, leading to the circRNA expression being increased or downregulated under arsenic exposure due to differences with the miRNA binding sites.
Based on this, we explored whether circP50 has a function to regulate human lung cancer A549 cells proliferation. Our results showed that the knockdown of circP50 promoted the A549 cells proliferation, suggesting that circP50 may be involved in the regulation of cell proliferation. When it comes to proliferation, it has to be mentioned that in many cancer cells, the NF-κB pathway can protect cancer cells from apoptosis by upregulating the expression of genes that promote cell migration and invasion and apoptosis-repressed or inhibiting pro-apoptotic factors or activating persistent growth signaling pathway molecules (Giridharan and Srinivasan 2018). Our research found that the expression of IKB-α, p50, p65 was reduced, but the expression of C-myc did not change significantly. We believe that the NF-κB pathway may not be suppressed and needs to be regulated with other mechanisms, so we mainly studied the p53 pathway in subsequent experiments.
The p53 is an important tumor suppressor that acts as a transcription factor that transcribes its downstream genes, regulates multiple cellular stress responses, and exerts its tumor suppressor function. When cellular stress, p53 undergoes serial post-translational modifications and altered protein levels and activity, acting mainly as transcription factors to regulate the expression of multiple downstream target genes, thus initiating cytological effects such as cell cycle arrest, apoptosis, senescence, and differentiation (Liu et al. 2019). Post-translational modifications of p53, including phosphorylation and acetylation, are the most extensive and effective types of regulating p53 function and are essential for regulating p53 stability and activity (Chung et al. 2014). Different roles were reported for the different p53 phosphorylation and acetylation sites; for example, Ser15 can slow down inhibition or degradation of p53, leading to stabilization and activation of p53 (Han et al. 2021); Ser376, Lys370 (Kon et al. 2021) and Lys382 (Lin et al. 2020) can affect nonspecific DNA binding (Appella and Anderson 2000); meanwhile, Ser392 can enhance the ability of p53 to bind to DNA (Pospisilova et al. 2004). According to the western blotting assay, knockdown of circP50 inhibited p53 phosphorylated at Ser15, 376, 392, and p53 acetylated at Lys370, 382, ultimately reducing p53 expression. In the meantime, our data showed that knockdown of circP50 inhibited related genetic changes in the p53 pathway, including knockdown of protein levels like p21, PUMA, Caspase3, and Bcl-xS. In these downstream genes of p53, p21 can arrest cell cycle progression (Lai et al. 2020), the protein encoded by PUMA is important in the mitochondrial apoptosis pathway (Ma et al. 2016). Caspase3 is a cleaved housekeeping protein, and DNA fragments are considered a key effector molecule involved in the apoptotic pathway (Jiang et al. 2020); excessive expression of Bcl-xS can accelerate the progression of apoptosis (Stevens and Oltean 2019). Therefore, our results suggest that knockdown of circP50 may inhibit the p53-dependent apoptotic signaling pathway, which is mediated by p53 phosphorylation and acetylation and promotes A549 cells proliferation.
In conclusion, our results indicate that inorganic arsenic increases circP50 expression in A549 cells, and knockdown of circP50 inhibits p53 phosphorylation, acetylation as well as downstream target genes, thereby promoting A549 cell proliferation, suggesting that circP50 functions as a stimulus to the p53 pathway in response to inorganic arsenic exposure. Moreover, the contribution of circP50 to the response of arsenic exposure to the NF-κB pathway has been elusive, and it does not exclude its potential as a specific therapeutic target for lung cancer.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Appella E, Anderson CW (2000) Signaling to p53: breaking the posttranslational modification code. Pathol Biol (Paris) 48:227–245
Bjorklund G, Oliinyk P et al (2020) Arsenic intoxication: general aspects and chelating agents. Arch Toxicol 94:1879–1897. https://doi.org/10.1007/s00204-020-02739-w
Bozack AK, Saxena R et al (2018) Nutritional influences on one-carbon metabolism: effects on arsenic methylation and toxicity. Annu Rev Nutr 38:401–429. https://doi.org/10.1146/annurev-nutr-082117-051757
Chen LL (2020) The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol 21:475–490. https://doi.org/10.1038/s41580-020-0243-y
Chen QY, Costa M (2021) Arsenic: a global environmental challenge. Annu Rev Pharmacol Toxicol 61:47–63. https://doi.org/10.1146/annurev-pharmtox-030220-013418
Chen L, Liu S et al (2020) Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther 5:90. https://doi.org/10.1038/s41392-020-0196-9
Chung SK, Zhu S et al (2014) Functional analysis of the acetylation of human p53 in DNA damage responses. Protein Cell 5:544–551. https://doi.org/10.1007/s13238-014-0048-x
Concetti J, Wilson CL (2018) NFKB1 and cancer: friend or foe? Cells 7:133. https://doi.org/10.3390/cells7090133
Dai X, Chen C et al (2018) Exosomal circRNA_100284 from arsenite-transformed cells, via microRNA-217 regulation of EZH2, is involved in the malignant transformation of human hepatic cells by accelerating the cell cycle and promoting cell proliferation. Cell Death Dis 9:454. https://doi.org/10.1038/s41419-018-0485-1
Dai M, Hu S et al (2019) BPTF cooperates with p50 NF-kappaB to promote COX-2 expression and tumor cell growth in lung cancer. Am J Transl Res 11:7398–7409
Dong H, Zhou J et al (2021) Biogenesis, functions, and role of CircRNAs in lung cancer. Cancer Manag Res 13:6651–6671. https://doi.org/10.2147/CMAR.S324812
Du WW, Zhang C et al (2017) Identifying and characterizing circRNA-protein interaction. Theranostics 7:4183–4191. https://doi.org/10.7150/thno.21299
Fan X, Weng X et al (2017) Circular RNAs in cardiovascular disease: an overview. Biomed Res Int 2017:5135781. https://doi.org/10.1155/2017/5135781
Ferragut Cardoso AP, Udoh KT et al (2020) Arsenic-induced changes in miRNA expression in cancer and other diseases. Toxicol Appl Pharmacol 409:115306. https://doi.org/10.1016/j.taap.2020.115306
Giridharan S, Srinivasan M (2018) Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J Inflamm Res 11:407–419. https://doi.org/10.2147/JIR.S140188
Glazar P, Papavasileiou P et al (2014) circBase: a database for circular RNAs. RNA 20:1666–1670. https://doi.org/10.1261/rna.043687.113
Han CY, Patten DA et al (2021) Nuclear HKII-P-p53 (Ser15) Interaction is a prognostic biomarker for chemoresponsiveness and glycolytic regulation in epithelial ovarian cancer. Cancers (Basel) 13:3399. https://doi.org/10.3390/cancers13143399
He W, Zhang Y et al (2020) LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis. Thorac Cancer 11:549–560. https://doi.org/10.1111/1759-7714.13280
Huang J, Yu S et al (2021) The dual role of circular RNAs as miRNA sponges in breast cancer and colon cancer. Biomedicines 9:1590. https://doi.org/10.3390/biomedicines9111590
Islam MM, Takeyama N (2021) Inorganic arsenic administration suppresses human neutrophil function in vitro. Hum Exp Toxicol 40:725–734. https://doi.org/10.1177/0960327120966040
Januszyk K, Januszyk P et al (2020) The influence of salinomycin on the expression profile of mRNAs encoding selected caspases and MiRNAs regulating their expression in endometrial cancer cell line. Curr Pharm Biotechnol 21:1505–1515. https://doi.org/10.2174/1389201021666200514095043
Jiang M, Qi L et al (2020) The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Dis 6:112. https://doi.org/10.1038/s41420-020-00349-0
Kon N, Churchill M et al (2021) Robust p53 stabilization is dispensable for its activation and tumor suppressor function. Cancer Res 81:935–944. https://doi.org/10.1158/0008-5472.CAN-20-1804
Lai L, Shin GY et al (2020) The role of cell cycle regulators in cell survival-dual functions of cyclin-dependent kinase 20 and p21(Cip1/Waf1). Int J Mol Sci 21:8504. https://doi.org/10.3390/ijms21228504
Lin XL, Li K et al (2020) Dulcitol suppresses proliferation and migration of hepatocellular carcinoma via regulating SIRT1/p53 pathway. Phytomedicine 66:153112. https://doi.org/10.1016/j.phymed.2019.153112
Liu Y, Tavana O et al (2019) p53 Modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol 11:564–577. https://doi.org/10.1093/jmcb/mjz060
Ma J, Feng Y et al (2016) PUMA and survivin are involved in the apoptosis of HepG2 cells induced by microcystin-LR via mitochondria-mediated pathway. Chemosphere 157:241–249. https://doi.org/10.1016/j.chemosphere.2016.05.051
Mar Wai K, Umezaki M et al (2019) Arsenic exposure through drinking water and oxidative stress status: a cross-sectional study in the Ayeyarwady region, Myanmar. J Trace Elem Med Biol 54:103–109. https://doi.org/10.1016/j.jtemb.2019.04.009
Martinez-Reyes I, Chandel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21:669–680. https://doi.org/10.1038/s41568-021-00378-6
Medda N, De SK et al (2021) Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol Environ Saf 208:111752. https://doi.org/10.1016/j.ecoenv.2020.111752
Meng S, Zhou H et al (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16:94. https://doi.org/10.1186/s12943-017-0663-2
Mitchell S, Vargas J et al (2016) Signaling via the NFkappaB system. Wiley Interdiscip Rev Syst Biol Med 8:227–241. https://doi.org/10.1002/wsbm.1331
Nan A, Chen L et al (2017) A novel regulatory network among LncRpa, CircRar1, MiR-671 and apoptotic genes promotes lead-induced neuronal cell apoptosis. Arch Toxicol 91:1671–1684. https://doi.org/10.1007/s00204-016-1837-1
Pietrzak S, Wojcik J et al (2021) Influence of the levels of arsenic, cadmium, mercury and lead on overall survival in lung cancer. Biomolecules 11:1160. https://doi.org/10.3390/biom11081160
Pospisilova S, Brazda V et al (2004) Activation of the DNA-binding ability of latent p53 protein by protein kinase C is abolished by protein kinase CK2. Biochem J 378:939–947. https://doi.org/10.1042/BJ20030662
Prats AC, David F et al (2020) Circular RNA, the key for translation. Int J Mol Sci 21:8591. https://doi.org/10.3390/ijms21228591
Rahman M, Sohel N et al (2019) Arsenic exposure and young adult's mortality risk: a 13-year follow-up study in Matlab, Bangladesh. Environ Int 123:358–367. https://doi.org/10.1016/j.envint.2018.12.006
Stevens M, Oltean S (2019) Modulation of the apoptosis gene Bcl-x function through alternative splicing. Front Genet 10:804. https://doi.org/10.3389/fgene.2019.00804
Sun DZ, Song CQ et al (2020) Role of the MAPK pathway in human lung epithelial-like A549 cells apoptosis induced by paraquat. Genet Mol Biol 43:e20190137. https://doi.org/10.1590/1678-4685-GMB-2019-0137
Sun M, Tan J et al (2021) Inorganic arsenic-mediated upregulation of AS3MT promotes proliferation of nonsmall cell lung cancer cells by regulating cell cycle genes. Environ Toxicol 36:204–212. https://doi.org/10.1002/tox.23026
Taniguchi K, Karin M (2018) NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 18:309–324. https://doi.org/10.1038/nri.2017.142
Tian F, Wang Y et al (2017) Circular RNA CircHIPK3 promotes NCI-H1299 and NCI-H2170 cell proliferation through miR-379 and its target IGF1. Zhongguo Fei Ai Za Zhi 20:459–467. https://doi.org/10.3779/j.issn.1009-3419.2017.07.04
Van Der Steen N, Lyu Y et al (2020) The circular RNA landscape of non-small cell lung cancer cells. Cancers (Basel) 12:1091. https://doi.org/10.3390/cancers12051091
Wang L, Lu YF et al (2020) HB-EGF activates the EGFR/HIF-1alpha pathway to induce proliferation of arsenic-transformed cells and tumor growth. Front Oncol 10:1019. https://doi.org/10.3389/fonc.2020.01019
Williams LM, Gilmore TD (2020) Looking down on NF-kappaB. Mol Cell Biol 40:e00104–e00120. https://doi.org/10.1128/MCB.00104-20
Wu J, Ferragut Cardoso AP et al (2019) Overexpression of hsa-miR-186 induces chromosomal instability in arsenic-exposed human keratinocytes. Toxicol Appl Pharmacol 378:114614. https://doi.org/10.1016/j.taap.2019.114614
Xiao T, Xue J et al (2018) Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Metallomics 10:1328–1338. https://doi.org/10.1039/c8mt00207j
Xue J, Liu Y et al (2017) Circ100284, via miR-217 regulation of EZH2, is involved in the arsenite-accelerated cell cycle of human keratinocytes in carcinogenesis. Biochim Biophys Acta Mol basis Dis 1863:753–763. https://doi.org/10.1016/j.bbadis.2016.12.018
Xue J, Chen C et al (2018) CircLRP6 regulation of ZEB1 via miR-455 is involved in the epithelial-mesenchymal transition during arsenite-induced malignant transformation of human keratinocytes. Toxicol Sci 162:450–461. https://doi.org/10.1093/toxsci/kfx269
Yang X, Wang J et al (2018) Silica-induced initiation of circular ZC3H4 RNA/ZC3H4 pathway promotes the pulmonary macrophage activation. FASEB J 32:3264–3277. https://doi.org/10.1096/fj.201701118R
Zang J, Lu D et al (2020) The interaction of circRNAs and RNA binding proteins: an important part of circRNA maintenance and function. J Neurosci Res 98:87–97. https://doi.org/10.1002/jnr.24356
Zhang HD, Jiang LH et al (2018) CircRNA: a novel type of biomarker for cancer. Breast Cancer 25:1–7. https://doi.org/10.1007/s12282-017-0793-9
Zhang AL, Chen L et al (2020) Role of H3K18ac-regulated nucleotide excision repair-related genes in arsenic-induced DNA damage and repair of HaCaT cells. Hum Exp Toxicol 39:1168–1177. https://doi.org/10.1177/0960327120903482
Zhou Q, Yin J et al (2022) Up-regulation of PUMA caused the activation of p53 phosphorylation and acetylation, enhancing the interaction between PUMA and Bcl-X and mediating arsenic-induced apoptosis. Toxicol Appl Pharmacol 434:115800. https://doi.org/10.1016/j.taap.2021.115800
Zhu X, Wang X et al (2017) hsa_circ_0013958: a circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J 284:2170–2182. https://doi.org/10.1111/febs.14132
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.82160607), Yunnan Applied Basic Research Projects-Union Foundation, Yunnan Provincial Science and Technology Department, and Kunming Medical University, China (Grant No.202101AY070001-054).
Author information
Authors and Affiliations
Contributions
Yizhu Mao wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript; Yizhu Mao and Qian Zhou analyzed and interpreted the present study experiment and data; Jinhua Wang and Ruihuan Zhao performed material preparation; Xuefei Yang, Ya Shi and Jinyao Yin collected and analyzed data; Chenglan Jiang prepared the instrument for the experiment; Yuefeng He had the idea for the article and revised the first version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mao, Y., Zhou, Q., Wang, J. et al. CircP50 functions through the phosphorylation- and acetylation-activated p53 pathway to mediate inorganic arsenic-induced apoptosis in A549 cells. Environ Sci Pollut Res 29, 91232–91240 (2022). https://doi.org/10.1007/s11356-022-22094-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22094-w