Abstract
Arsenic is a wildly distributed carcinogen in the environment. Arsenic-induced apoptosis has been extensively studied in therapeutics and toxicology. LncRNA MEG3 has been extensively studied as apoptosis regulatory gene in recent years. However, it stays unclear regarding how the mechanism of MEG3 regulates arsenic-induced apoptosis. Our focus was to explore the effects of MEG3 on arsenic-induced apoptosis. MTS assay was used to test cell viability, and qRT-PCR was for the examination of gene expressions. The effect of the apoptosis and necrosis after knockdown MEG3 was detected with double staining. Our results demonstrated that MEG3 expression was positively correlated with the concentration of three arsenic species (inorganic arsenic (iAs), monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA)) (p < 0.05). The ability of iAs to induce MEG3 expression was much higher compared with that induced by MMA and DMA. In addition, our experiments confirmed that MEG3 knockdown increased cell viability and arsenic-induced apoptosis, but cell viability decreased after iAs treatment. Moreover, LncRNA MEG3 regulated apoptosis via down-regulate API5 while up-regulate CASP7, CCND3 and APAF1. It is further proved that arsenic-induced apoptosis increased after the knockdown of MEG3, which regulates these genes. These findings provide experimental evidence and possible mechanisms for subsequent research on the effects of arsenic on health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maternally expressed (MEG3) is a Long non-coding RNAs (LncRNA) with the length of more than 1.6 kb and has no function of encoding proteins (Yang et al. 2020). MEG3 is recognized as a gene which suppresses tumor. A large number of studies demonstrates that MEG3 regulates the expression of downstream genes such as BCL2, BAX, CASP3, TP53 in several tumors, including glioma, lung cancer, gastric cancer, breast cancer, etc. (Deng et al. 2020; Wu et al. 2018; Ghafouri-Fard et al. 2019) Dong et al. (2018). found that knockdown of MEG3 increases SPHK1 and TGFβ1 to inhibit cell apoptosis. A study by Zhang et al. (2019) showed that over-expression of MEG3 increases APAF1 expression and activates CASP9 and CASP3 to promote cell apoptosis. It has been suggested that MEG3 exerts tumor suppressive function by regulating the expression of tumor-related genes, and therefore regulating apoptosis. Inorganic arsenic (iAs) is well known and identified as human carcinogens (Zhang et al. 2019). Through various ways, humans gain exposure to arsenic, such as respiratory tract, digestive tract and skin. There is considerable evidence coming from the epidemiological study which reveals that arsenic exposure increases the incidence of clinical complications, such as related blood diseases, skin lesions, bronchitis and internal organ malignancies (Smith et al. 2018; Sinha et al. 2020).
The metabolism of inorganic arsenic happens in the liver, and then, it is converted to monomethylarsine acid (MMA) and dimethylarsine acid (DMA) (Bozack et al. 2018). Furthermore, MMA is more toxic with regard to inducing in some cancer (Zhang et al. 2014). In addition, high MMA% is associated with cancer and cardiovascular outcomes, while low MMA% and high DMA% are commonly associated with diabetes and metabolic syndrome (Kuo et al. 2017).
Arsenic has been confirmed to induce apoptosis in many tumors such as breast cancer, lung cancer, liver cancer and ovarian cancer (Jiang et al. 2018; Luo et al. 2018). The underlying mechanism of carcinogenesis by arsenic is linked to regulating genes related to proliferation and apoptosis. Evidence has shown that arsenic promotes the apoptosis by down-regulating anti-apoptotic genes BCL2, BBC3 and STAT6 expression levels, and up-regulating expression levels of pro-apoptotic genes BAX and CASP3 and 9 (Yin et al. 2018; Zhang et al. 2019a, b, c). Fan et al. indicated that arsenic positively regulates MEG3 to inhibit migration in hepatocellular carcinoma (Fan et al. 2019). We are interested in the mechanism and effect of how MEG3 regulates apoptosis in A549 cells.
Therefore, through the study, the effect of arsenic exposure on MEG3 expression has been explored, and the possible mechanism of arsenic-induced apoptosis in A549 cells has been further investigated.
Materials and methods
Study population
Base on this case–control study, we used questionnaires to obtain demographic information and collect blood samples, urine of each subject. The exposed group was composed of 73 smelter workers. Working in the factory for no less than 3 months is adopted as the inclusion criterion for exposed workers. In addition, those who had severe diseases, renal tumors and had consumed fishery during the past 7 days before the blood sampling were excluded. The control group was made up of 22 residents, who lived at least 10 km away from the arsenic smelter and had no occupational history of arsenic exposure. Residents who have been exposed to pesticide or have taken traditional Chinese medicine during the past 3 months would be excluded. The exposed group and the control group appeared to be similar except for the exposed factors. Subjects who had diabetes, coronary heart diseases and other blood pressure-influencing pathologies in both groups were excluded. Theoretical Committee of Kunming Medical University has approved our study.
Measurement of urinary arsenic metabolites
Based on the theory of low-temperature separation of liquid nitrogen and hydride to produce volatile arsenic, 2 mol/L of the reaction solution was added to one milliliter of urine and heated to 100 °C for 3 h. Samples and blank controls were placed on an atomic absorption spectrophotometer to detect DMA, MMA and iAs, respectively. The detection limit of low detection was 1 ng/L, and RSD < 5% (He et al. 2018; 2020). The total arsenic concentration (tAs), urine arsenic metabolite percentage and the calculation of two methylation indices were done as follows:
tAs = iAs + MMA + DMA.
Inorganic arsenic percentage (iAs%) = iAs/total As;
Monomethylarsine acid percentage (MMA%) = MMA/total As.
Dimethylarsine acid percentage (DMA%) = DMA/total As,
Primary methylation index (PMI) = (MMA + DMA)/total As.
Secondary methylation index (SMI) = DMA/(MMA + DMA)
RNA extraction and quantitative real-time PCR analysis
The total RNA was obtained from cells via TRNzol (Tiangen Biotech, China), and TIANScriptII RT kit (Tiangen Biotech, China) was used to reverse transcription into complementary DNA according to instructions.
The LightCycler (LC)® 96 was used for PCR amplification. SYBR Green PCR kit (Roche, Germany) was used to analyze real-time PCR. Reaction conditions were: pre-denaturation at 95 °C for 1 min, followed by 45 cycles of 95 °C for 10 s, 60 °C for10 s and 72 °C for 15 s. Ct values were analyzed using the 2−ΔΔCT method. Primer sequences and internal reference (β-actin) used in qRT-PCR are listed in Table 1. All the presented experiments were performed 3 times (He et al. 2020; Wen et al. 2016).
Cell culture and transfection
A549 were obtained from the Kunming Institute of Zoology, Chinese Academy of Sciences. Cells were cultured in RPMI 1640 medium containing 10% FBS at 37 °C, 5% CO2 incubator and were collected in the logarithmic growth stage. A549 cells were plated in 96-well plates, 12-well plates and 6-well plates, with medium changed every 2 days (Fan et al. 2019; Gong et al. 2018). Small-interfering RNA duplexes (siRNAs) carrying non-targeting sequences are served as the negative control (NC). Three siRNA groups and negative control group were transfected with RFect transfection reagent (Bio-generating Biotechnology, China) according to instructions. The medium was changed after transfected with NC and MEG3 siRNAs for 6 h. All the experiments were done independently and performed in triplicate.(Dong et al. 2018; Gong et al. 2018). The sequences of three MEG3-siRNAs and negative control in this study are listed in Table 2.
Detection of cell viability through MTS assay
A549 cells were cultured in 96-well plates, which were applied with arsenic after the knockdown of MEG3. Then, 20 µl/well of CellTiter 96® AQueous One Solution Reagent was added (Cheng et al. 2010; Gholizadeh et al. 2020). The absorbance at 490 nm was recorded using an ELISA plate reader after maintained in the humidified incubator for 20 min. Each point represents the mean ± SD of 4 replicates.
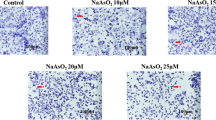
Double staining with hoechst 33,342 and propidium iodide (PI) in A549 cells
Fluorescence staining of apoptotic A549 cells was performed using an Apoptosis and Necrosis Assay Kit (Beyotime, China). After being washed with PBS, cells were stained by Hoechst 33,342 and propidium iodide (PI) and then incubated for 15 min at the temperature around 20 °C in darkness. The cell conditions of each group were recorded, observed and photographed under a fluorescence microscope in different perspectives. Apoptotic cells appeared the bright blue color in the nucleus and necrotic cells were red, while the normal cells showed the dark blue color. Photographs were taken 3 times at random, and the apoptosis rate of each group was calculated (Wang et al. 2019a, b; Zhou et al. 2020).
Statistical analysis
Data were transformed by logarithm before being analyzed, and SPSS 22.0 (SPSS, Inc., Chicago, IL, the USA) was used.
Student's t-test was used to compare the data between the two groups, while the mean and standard deviation were used to present the results. The analysis of variance was performed to compare the data among different groups. The associations between concentrations of three arsenic species and gene expression were demonstrated by Pearson correlation coefficient. p value < 0.05 was used for statistical significance.
Results
General information of workers and the urinary arsenic species levels
There were no significant differences in age, sex, cigarette smoking and alcohol consumption between two groups. The species of urinary arsenic are shown in Table 3. In comparison with the control group, the exposed group showed increase in the MMA% and decline in PMI and SMI. (p < 0.05).
Arsenic exposure and the level of MEG3 expression
The correlation between arsenic exposure and MEG3 expression level with regard to peripheral blood lymphocytes is demonstrated in Table 4 and Fig. 1. The data of urinary arsenic species were normally distributed after being transformed by logarithm. The level of MEG3 expression was positively correlated with the three arsenic species level and MMA%.
Correlation analysis of MEG3 and urinary arsenic species in all subjects a MEG3 expression in peripheral blood lymphocytes between exposure group and control group. b, c, d The X-axis shows the concentration of Log (iAs), Log (MMA) and Log (DMA), the Y-axis shows the level of MEG3 expression in workers. Black dots represent a 95% confidence interval. 95% confidence interval was represented by black dots. Data are expressed as means ± SE. **indicated that compared with the control group, p < 0.01
Association between PMI and SMI with the MEG3 expression level
As can be seen in Tables 5 and 6, the workers were put into two groups based on PMI and SMI. The expression level of MEG3 was lower in high PMI or high SMI group (p < 0.05).
Arsenic promotes the expression level of MEG3 in A549 cells
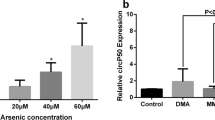
A549 cells were applied with iAs for 48 h (0, 30, 60 and 90 μmol/L), and qRT-PCR was adopted for the detection of MEG3 expression. As it is shown in Fig. 2, in iAs group, the expression level of MEG3 was higher than it was in the control group (p < 0.05). Cells were treated with three arsenic species and the expression of MEG3 increased greatly. However, the expression of MEG3 induced by MMA and DMA was lower than that induced by iAs. S-adenosy-L-methionine (SAM) and reducing agent glutathione (GSH) were required in iAs metabolism. SAM in combination with iAs decreased the expression of MEG3. However, GSH in combination with iAs did not change the expression of MEG3.
The effects of arsenic metabolism on the expression of MEG3 in A549 cells. a A549 cells were exposed to iAs for 48 h (0, 30, 60 and 90 μmol/L). b Comparison of the expression of MEG3 in different arsenic species groups. c SAM in combination with iAs decreased the expression of MEG3. GSH in combination with iAs did not change the expression of MEG3.* indicated that compared with the control group, p < 0.05. ** indicated that compared with the control group, p < 0.01.# indicated that compared with the iAs group, p < 0.05
Knockdown of MEG3 increases cell viability and arsenic-induced apoptosis in A549 cells
A549 cells were transfected with NC and MEG3-siRNAs. As it is displayed in Fig. 3, MEG3 showed significantly reduced expression in three MEG3-siRNA groups (p < 0.05). This result indicated that the transfection with MEG3-siRNA successfully knocked down MEG3 expression.
Detection of MEG3 expression after MEG3 knockdown in A549 cells. a The transfection with MEG3-siRNA successfully knocked down MEG3 expression. b A549 cells were transfected with FAM-siRNA. Fluorescent microscope was used to test the transfection efficiency. * indicated that compared with the NC group, p < 0.05
As can be seen in Fig. 4, the cell viability increased in three MEG3-siRNA groups. After arsenic treatment, the cell viability of the MEG3 knockdown groups was less than it was in the NC group (p < 0.05). The effect of the apoptosis and necrosis after MEG3 knockdown was detected with double staining. As it is shown in Fig. 5, the number of apoptosis and necrosis remarkably increased in MEG3 knockdown groups.
Detection of cell viability after MEG3 knockdown in A549 cells. The cell viability was significantly increased after MEG3-siRNA transfection in A549 cells. In addition, A549 cells were incubated with 90 μmol/L iAs for 48 h and the cell viability of the MEG3 knockdown groups was lower than that of the NC group. * indicated that compared with the NC group, p < 0.05. ** indicated that compared with the NC group, p < 0.01
Detection of apoptosis and necrosis after MEG3 knockdown in A549 cells. Apoptosis and necrosis level detected through double staining with Hoechst 33,342 and PI. A549 cells were exposed to inorganic arsenic after transfected with NC and three MEG3-siRNAs for 72 h. Apoptotic cells appeared bright blue color in the nucleus, and necrotic cells were red. The number of apoptosis and necrosis remarkably increased in MEG3 knockdown groups
Change of downstream gene after the knockdown of MEG3
As can be seen in Fig. 6, knockdown of MEG3 for 72 h down-regulated the expression of BCL2A1 and AIP5 in A549 cells (p < 0.05) and up-regulated the expression of CASP7, CCND3, APAF1 (p < 0.05).
MEG3 knockdown regulated the expressions of apoptosis related genes. A549 cells were transfected with NC and three MEG3 siRNAs for 72 h and genes expression detected by qRT-PCR. a Knockdown of MEG3 inhibited the expression levels of anti-apoptotic genes BCL2A1 and API5. b Knockdown of MEG3 increased expression of CASP7, CCND3 and APAF1. * indicated that compared with the NC group, p < 0.05
Discussion
Arsenic brings influence to human health via various ways. A huge number of findings highlighted that chronic arsenic exposure increases risks, including type 2 diabetes, chronic obstructive pulmonary disease and peripheral arterial disease (Ashley-Martin et al. 2018; Smith et al. 2018). Health problems caused by arsenic exposure are related to regulated gene expression in humans (Cheng et al. 2018; Rasheed et al. 2018). In this research, we found the urine arsenic species levels of iAs, MMA, DMA, tAs and MMA% were meaningfully lower in the control group than they were in the exposed group. Arsenic exposure increased expression level of MEG3 and was positively correlated with the concentration of three different arsenic species. The result points out that the urine arsenic species level has the effect of up-regulating MEG3, which is consistent with the results of Fan (2019) and Wen et al. (2016). Three arsenic species in urine were considered as metabolic markers. Compared with that of iAs, MMA and DMA are more water soluble and have a shorter period of biological half-value. (Bozack et al. 2018; Gribble et al. 2015). Three arsenic species have different signaling pathways and biological effects (Mochizuki et al. 2019; Dodmane et al. 2013). For example, DMA causes the generation of ROS and stimulates cell proliferation (Kinoshita et al. 2007). Furthermore, MMA and DMA inhibit the activity of many enzymes and are much more toxic than iAs (Khairul et al. 2017). We investigated the effects of iAs and arsenic metabolites on MEG3 RNA expression. Our experiment showed that the expression of MEG3 induced by MMA and DMA was lower than that induced by iAs. The data support that gene expression was weakened after inorganic arsenic metabolism.
Arsenic metabolism requires the participation of methyl donor SAM and GSH (Hayakawa et al. 2005; Naranmandura et al. 2006). Du et al. (2012) identified that SAM causes the reduction in the degree of arsenic-induced gene expression and demethylation. Cheng et al. (2010) determined that GSH has resistance to the cell apoptosis induced by arsenic. However, there is also evidence which shows that SAM has no effect on arsenic-induced mRNA up-regulation (He et al. 2018). Therefore, we are interested in the effect of SAM and GSH on the MEG3 expression by arsenic-induced. Our data showed that SAM in combination with iAs decreased the expression of MEG3, but GSH did not.
MEG3, as a tumor suppressor, has low expression in many human tumors (Ma et al. 2018; Wang et al. 2019a, b; Liu et al. 2020). Over-expression of MEG3 promotes apoptosis and decreases cell proliferation ability (Zhu et al. 2019). In addition, knockdown of MEG3 to activate the PI3K/AKT/FOXO3a signaling pathway inhibits apoptosis (Zou et al. 2020). Showing compatible results with existing studies, our study reveals that the cell viability of A549 increased after knockdown of MEG3. After arsenic treatment, the cell viability were lower in the MEG3 knockdown groups than those values in the NC group. Cell viability is influenced by the proportion of apoptosis and proliferation. Arsenic induces apoptosis. Our result showed that arsenic-induced apoptosis remarkably increased in MEG3 knockdown groups. It has been proven that iAs inhibits cell viability by promoting apoptosis. This result demonstrated that arsenic-induced apoptosis increases after Knockdown of MEG3.
Apoptosis requires a large number of gene expressions to participate in the complex process. Previous evidences show that knockdown of MEG3 has a protective effect to reduce cellular damage by regulating related-genes of proliferation and apoptosis, like MMP7, CCND1, CDKN1A and CASP1 (Deng et al. 2020; Wu et al. 2018; Ghafouri-Fard et al. 2019; Gong et al. 2018). We testified that MEG3 regulates cell apoptosis by up-regulating the expression of apoptotic genes, CASP7, CCND3 and APAF1, and down-regulating the expression of BCL2A1 and apoptosis inhibitor 5 (API5) in A549 cells. API5 function being anti-apoptotic, the low expression of API5 enhances the transcription factor E2F1-induced apoptosis. Compared with previous experiments, our experiments reported that API5 is regulated by MEG3 for the first time.
Conclusion
In summary, our data indicated that arsenic exposure has effects on the expression of lncRNA MEG3. MEG3 regulates apoptosis by regulating downstream target genes such as API5, CASP7, CCND3 and APAF1 in A549 cells. Our work provides experimental evidence and the possible mechanism for subsequent research on the effects of arsenic on health.
References
Ashley-Martin, J., Dodds, L., Arbuckle, T. E., Bouchard, M. F., Shapiro, G. D., Fisher, M., et al. (2018). Association between maternal urinary speciated arsenic concentrations and gestational diabetes in a cohort of Canadian women. Environment International, 121(Pt 1), 714–720.
Basset, C., Bonnet-Magnaval, F., Navarro, M. G., Touriol, C., Courtade, M., Prats, H., et al. (2017). Api5 a new cofactor of estrogen receptor alpha involved in breast cancer outcome. Oncotarget, 8(32), 52511–52526.
Bozack, A. K., Saxena, R., & Gamble, M. V. (2018). Nutritional influences on one-carbon metabolism: Effects on arsenic methylation and toxicity. Annual Review of Nutrition, 38, 401–429.
Cheng, B., Yang, X., An, L., Gao, B., & Liu, X. (2010). Arsenic trioxide-induced apoptosis of Hep-2 cell line through modulating intracellular glutathione (GSH) level. Auris, Nasus, Larynx, 37(1), 89–94.
Cheng, H., Qin, M., Chen, Y., Dong, H., Shao, Y., Ren, S., et al. (2018). Expression changes of tumor-related gene mrna in workers who smelt arsenic. Journal of Hygiene Research, 47(4), 577–587.
Deng, D., & Liang, H. (2020). Silencing meg3 protects PC12 cells from hypoxic injury by targeting miR-21. Artificial Cells, Nanomedicine, and Biotechnology, 48(1), 610–619.
Dodmane, P. R., Arnold, L. L., Kakiuchi-Kiyota, S., Qiu, F., Liu, X., Rennard, S. I., et al. (2013). Cytotoxicity and gene expression changes induced by inorganic and organic trivalent arsenicals in human cells. Toxicology, 312, 18–29.
Dong, X., Wang, J., Li, T., Xu, Y. P., & Li, S. Y. (2018). Down regulation of lncRNA meg3 promotes colorectal adenocarcinoma cell proliferation and inhibits the apoptosis by up-regulating TGF-β1 and its downstream sphingosine kinase 1. European Review for Medical and Pharmacological Sciences, 22(23), 8265–8272.
Du, J., Zhou, N., Liu, H., Jiang, F., Wang, Y., Hu, C., et al. (2012). Arsenic induces functional re-expression of estrogen receptor α by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS ONE, 7(4), e35957.
Fan, Z., He, J., Fu, T., Zhang, W., Yang, G., Qu, X., et al. (2019). Arsenic trioxide inhibits EMT in hepatocellular carcinoma by promoting lncRNA meg3 via PKM2. Biochemical and Biophysical Research Communications, 513(4), 834–840.
Ghafouri-Fard, S., & Taheri, M. (2019). Maternally expressed gene 3 (meg3): A tumor suppressor long non coding RNA. Biomedicine & Pharmacotherapy, 118, 109129.
Gholizadeh, M. A., Shamsabadi, F. T., Yamchi, A., Golalipour, M., Jhingan, G. D., & Shahbazi, M. (2020). Identification of hub genes associated with RNAi-induced silencing of XIAP through targeted proteomics approach in MCF7 cells. Cell & Bioscience, 10, 78.
Gong, L., Xu, H., Chang, H., Tong, Y., Zhang, T., & Guo, G. (2018). Knockdown of long non-coding RNA MEG3 protects H9c2 cells from hypoxia-induced injury by targeting microRNA-183. Journal of Cellular Biochemistry, 119(2), 1429–1440.
Gribble, M. O., Voruganti, V. S., Cole, S. A., Haack, K., Balakrishnan, P., Laston, S. L., et al. (2015). Linkage analysis of urine arsenic species patterns in the strong heart family study. Toxicological Sciences : An Official Journal of the Society of Toxicology, 148(1), 89–100.
Hayakawa, T., Kobayashi, Y., Cui, X., & Hirano, S. (2005). A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Archives of Toxicology, 79(4), 183–191.
He, Y., Zhang, R., Song, X., Shang, L., Wu, X., & Huang, D. (2018). Inorganic arsenic exposure increased expression of fas and bax gene in vivo and vitro. Gene, 671, 135–141.
He, Y., Zhang, R., Chen, J., Tan, J., Wang, M., & Wu, X. (2020). The ability of arsenic metabolism affected the expression of lncRNA PANDAR, DNA damage, or DNA methylation in peripheral blood lymphocytes of laborers. Human & Experimental Toxicology, 39(5), 605–613.
Jiang, X., Chen, C., Gu, S., & Zhang, Z. (2018). Regulation of ABCG2 by nuclear factor kappa B affects the sensitivity of human lung adenocarcinoma A549 cells to arsenic trioxide. Environmental Toxicology and Pharmacology, 57, 141–150.
Khairul, I., Wang, Q. Q., Jiang, Y. H., Wang, C., & Naranmandura, H. (2017). Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget, 8(14), 23905–23926.
Kinoshita, A., Wanibuchi, H., Wei, M., Yunoki, T., & Fukushima, S. (2007). Elevation of 8-hydroxydeoxyguanosine and cell proliferation via generation of oxidative stress by organic arsenicals contributes to their carcinogenicity in the rat liver and bladder. Toxicology and Applied Pharmacology, 221(3), 295–305.
Kuo, C. C., Moon, K. A., Wang, S. L., Silbergeld, E., & Navas-Acien, A. (2017). The Association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environmental Health Perspectives, 125(8), 087001.
Liu, F., Chen, Y., Liu, R., Chen, B., Liu, C., & Xing, J. (2020). Long noncoding RNA (meg3) in urinal exosomes functions as a biomarker for the diagnosis of hunner-type interstitial cystitis (HIC). Journal of Cellular Biochemistry, 121(2), 1227–1237.
Luo, D., Zhang, X., Du, R., Gao, W., Luo, N., Zhao, S., et al. (2018). Low dosage of arsenic trioxide (As2O3) inhibits angiogenesis in epithelial ovarian cancer without cell apoptosis. Journal of Biological Inorganic Chemistry, 23(6), 939–947.
Ma, L., Wang, F., Du, C., Zhang, Z., Guo, H., Xie, X., et al. (2018). Long non-coding RNA meg3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncology Reports, 39(3), 1132–1140.
Mochizuki, H. (2019). Arsenic Neurotoxicity in Humans. International Journal of Molecular Sciences, 20(14), 3418.
Naranmandura, H., Suzuki, N., & Suzuki, K. T. (2006). Trivalent arsenicals are bound to proteins during reductive methylation. Chemical Research in Toxicology, 19(8), 1010–1018.
Rasheed, H., Kay, P., Slack, R., & Gong, Y. Y. (2018). The effect of association between inefficient arsenic methylation capacity and demographic characteristics on the risk of skin lesions. Toxicology and Applied Pharmacology, 339, 42–51.
Sinha, D., & Prasad, P. (2020). Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. Journal of Applied Toxicology, 40(1), 87–131.
Smith, A. H., Marshall, G., Roh, T., Ferreccio, C., Liaw, J., & Steinmaus, C. (2018). Lung, bladder, and kidney cancer mortality 40 years after arsenic exposure reduction. Journal of the National Cancer Institute, 110(3), 241–249.
Wang, H., Cheng, J., Wang, H., Wang, M., Zhao, J., & Wu, Z. (2019a). Protective effect of apple phlorizin on hydrogen peroxide-induced cell damage in HepG2 cells. Journal of food Biochemistry, 43(12), e13052.
Wang, W., Xie, Y., & Chen, F. (2019b). LncRNA meg3 acts a biomarker and regulates cell functions by targeting ADAR1 in colorectal cancer. World Journal of Gastroenterology, 25(29), 3972–3984.
Wen, W., Lu, L., He, Y., Cheng, H., He, F., Cao, S., et al. (2016). LincRNAs and base modifications of p53 induced by arsenic methylation in workers. Chemico-biological Interactions, 246, 1–10.
Wu, J. L., Meng, F. M., & Li, H. J. (2018). High expression of lncRNA meg3 participates in non-small cell lung cancer by regulating microRNA-7-5p. European Review for Medical and Pharmacological Sciences, 22(18), 5938–5945.
Yang, Z., Bian, E., Xu, Y., Ji, X., Tang, F., Ma, C., et al. (2020). Mega3 induces EMT and invasion of glioma cells via autophagy. Onco Targets and Theraphy, 13, 989–1000.
Yin, L., & Yu, X. (2018). Arsenic-induced apoptosis in the p53-proficient and p53-deficient cells through differential modulation of NFkB pathway. Food and Chemical Toxicology: An International Journal Published for British Industrial Biological Research Association, 118, 849–860.
Zhang, Q., Li, Y., Liu, J., Wang, D., Zheng, Q., & Sun, G. (2014). Differences of urinary arsenic metabolites and methylation capacity between individuals with and without skin lesions in Inner Mongolia, Northern China. International Journal of Environmental Research and Public Health, 11(7), 7319–7332.
Zhang, W., Cui, X., Gao, Y., Sun, L., Wang, J., Yang, Y., et al. (2019a). Role of pigment epithelium-derived factor (PEDF) on arsenic-induced neuronal apoptosis. Chemosphere, 215, 925–931.
Zhang, X., Wu, N., Wang, J., & Li, Z. (2019b). LncRNA meg3 inhibits cell proliferation and induces apoptosis in laryngeal cancer via miR-23a/APAF-1 axis. Journal of Cellular and Molecular Medicine, 23(10), 6708–6719.
Zhang, Y., Li, Y., Luo, L., He, Q., Gao, Y., Feng, H., et al. (2019c). Factors affecting differential methylation of dna promoters in arsenic-exposed populations. Biological Trace Element Research, 189(2), 437–446.
Zhou, J., Li, G., Han, G., Feng, S., Liu, Y., Chen, J., et al. (2020). Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Investigational New Drugs, 38(1), 50–59.
Zhu, M., Wang, X., Gu, Y., Wang, F., Li, L., & Qiu, X. (2019). Meg3 overexpression inhibits the tumorigenesis of breast cancer by downregulating miR-21 through the PI3K/Akt pathway. Archives of Biochemistry and Biophysics, 661, 22–30.
Zou, D. M., Zhou, S. M., Li, L. H., Zhou, J. L., Tang, Z. M., & Wang, S. H. (2020). Knockdown of long noncoding rnas of maternally expressed 3 alleviates hyperoxia-induced lung injury via inhibiting thioredoxin-interacting protein-mediated pyroptosis by binding to mir-18a. The American Journal of Pathology, 190(5), 994–1005.
Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (Grant No. 81860572).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
No potential conflicts of interest were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Tan, J., Jiang, C. et al. Inorganic arsenic influences cell apoptosis by regulating the expression of MEG3 gene. Environ Geochem Health 43, 475–484 (2021). https://doi.org/10.1007/s10653-020-00740-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00740-x